Abstract
Objectives:
Given that deficiency in B vitamins can lead to the accumulation of homocysteine (Hcy), and hyperhomocysteinemia may have a role in migraine pathogenesis, the present prospective randomized double-blinded placebo-controlled trial aimed to evaluate the effect of vitamin B-complex supplementation on the alleviation of migraine in children through a possible reduction in Hcy levels.
Materials & Methods:
Ninety children under 15 years of age suffering from typical migraine were included in the present trial. They were randomly assigned into two groups (forty-five patients in each group) to receive either vitamin B-complex or a matching placebo for six months. Serum Hcy levels and headache characteristics were evaluated and compared before and after administering vitamin B-complex or placebo.
Results:
Unlike the placebo group, the monthly headache frequency, severity of headache, headache disability, and serum Hcy levels were significantly decreased after the vitamin administration. The headache duration was not significantly different before and after the treatment. In the vitamin group, there were significant positive correlations between the frequency and severity, frequency and disability, and severity and disability of headaches. Hcy also had significant positive correlations with the frequency and disability of headaches. In the placebo group, the only found significant correlation was between headache frequency and disability.
Conclusion:
The administration of vitamin B-complex might effectively relieve migraine severity in children by reducing serum Hcy. However, further studies are needed to confirm the results.
Key Words: B Vitamins, Children, Homocysteine, Migraine
Introduction
Pediatric migraine is a debilitating headache in children that, like other pain disorders, can be associated with sleep, mood, and cognitive problems. This disorder can interrupt daily activities needed at both home and school. Therefore, it requires the appropriate diagnosis and treatment (1). According to the International Headache Society (IHS), migraine can be defined as moderate to severe recurrent headaches lasting up to 72 hours. They are one-sided and throbbing, intensified by daily routine activities, and are often accompanied by anorexia, nausea, and vomiting (2). Approximately 10-20% of individuals may suffer from migraine in their lifetime (3). It is two to three times more prevalent in women than men (4). There are two types of migraine, with and without aura. In migraine with aura, there is a vision problem known as “aura,” which affects about one in four migraine sufferers (5). Migraines in children are often bilateral, without aura, and the duration of headaches is shorter than in adults (6). About 5% of children under 12 years of age suffer from migraine. However, its prevalence usually rises (5-10%) after puberty(7). Persistent headaches in patients have adverse economic effects due to the medication cost and repeated treatments and can cause emotional and social problems (8).
Various compounds with different efficacy, such as beta-blockers (propranolol), antidepressants, calcium blockers, antiepileptic drugs, serotonin antagonists, and monoamine oxidase inhibitors, have been therapeutically used to alleviate migraine headaches (9). In recent years, vitamin supplements have also shown positive effects in reducing the severity of migraines. B vitamins are involved in many metabolic pathways. One of these pathways is the methionine-homocysteine metabolism cycle (10). Homocysteine (Hcy) is an intermediate amino acid formed during the metabolism of methionine and its conversion to cysteine (11). Hyperhomocysteinemia (HHcy) is not only associated with cardiovascular disease but may also have a role in migraine pathogenesis (12).
Given that a deficiency of B vitamins can lead to the accumulation of Hcy, and HHcy may be associated with migraine; the present study aimed to evaluate the effect of vitamin B-complex supplementation on the alleviation of migraine in children through a possible reduction in Hcy levels.
Materials & Methods
Patients
In the present prospective randomized, double-blinded, placebo-controlled trial, ninety children under 15 years of age suffering from typical migraine, with at least one attack per week, were randomly recruited from the patients referred to (removed for blind peer review) after obtaining informed consent from their parents (the inclusion criteria). Patients with congenital anomalies, cardiovascular problems, malignancies, epilepsy, metabolic diseases, taking any vitamins or food supplements one month before the study, and participating in a previous investigational drug study within the three months preceding screening were excluded.
Data collection
At first, the characteristics of the disease and its severity were evaluated and recorded in a questionnaire. Headache severity and headache disability index were assessed by the visual analog scale for pain (VAS-pain) (13) and the pediatric migraine disability assessment score (pedMIDAS) (14), respectively.
Sample size calculation
The sample size was calculated using the PASS® version 11 program, setting the type-1 error (α) at 0.05 and the power (1-β) at 0.8. According to the literature (15), a sample size of 90 (45 cases in each group) was determined after considering a 10% dropout rate. The analyses were conducted based on the intention-to-treat (ITT) principle.
Randomization, Sampling, and Design
One hundred thirty-eight patients with migraine were screened for eligibility. Twenty-one patients did not meet the inclusion criteria, 11 declined to participate, 16 were excluded, and ninety patients were enrolled (Figure 1). The administrations were according to a predetermined schedule generated from random numbers in a 1:1 manner based on a computer-generated randomization sequence maintained within the investigational drug pharmacy, with allocation concealment by opaque sequentially numbered sealed envelopes. The ninety patients were randomly assigned into two groups (forty-five patients in each group) to receive either vitamin B-complex or a matching placebo. The patients, data collectors, and analyzers were blinded about the grouping. Venous blood samples were taken from the patients and centrifuged at 3000 g for ten minutes. The sera were separated and stored in a freezer at -70 °C until the analysis was performed. The vitamin group received oral vitamin B-complex capsules (Eurovital, Germany) in a dose of one capsule once daily. Each capsule contains 9.3 mg of vitamin B1, 9.2 mg of vitamin B2, 31 mg of vitamin B3, 9.2 mg of Pantothenic acid (vitamin B5), 10 mg of vitamin B6, 411 μg of Biotin (vitamin B7), 416 μg of Folic acid (vitamin B 9), and 9 μg of vitamin B12. In the control group, a placebo was similarly administered to the patients.
Figure 1.
Study flowchart
Follow-up and endpoints
The administration period was six months in both groups. All patients were followed up by phone call weekly during the study period to evaluate their compliance to both placebo and vitamin B-complex and monitor signs of any potential adverse effect. At the end of the treatment, compliance was assessed based on the capsule count of dispensed and returned medication, and non-compliance was considered if <80% of the study medication had been taken. After the intervention period, the headache characteristics were re-evaluated. The blood sampling and serum separation were also performed and stored at -70 °C.
Homocysteine measurement
After the collection, all serum samples were taken out of the freezer and thawed at room temperature. According to the manufacturer’s instructions, the amount of Hcy was measured by an enzyme-linked immunosorbent assay (ELISA) kit (Axis-Shield Diagnostics Ltd, UK).
Ethics approval
The study was approved by the Research Ethics Committee of Tabriz University of Medical Sciences (IR.TBZMED.REC.1397.544) and registered in the Iranian Registry of Clinical Trials (www.irct.ir; IRCT Registration Number: IRCT20131012014988N6).
Statistical analyses
The data were analyzed by SPSS software (version 16.0; SPSS, Inc., Chicago, IL). Initially, the variables were statistically checked for normality by one-sample Kolmogorov–Smirnov test. All variables except headache severity, duration, and the parameters changes had normal distributions and therefore were shown as mean ± standard deviation and compared by independent samples t-test between two study groups. Non-parametric variables, including sex, type of migraine, and positive family history, were compared by Chi-Square Test. The headache severity, duration, and parameters changes were shown as median with interquartile range and compared by Mann–Whitney U-test between the study groups. Paired t-test and Wilcoxon test were applied to compare normally and non-normally distributed data before and after the treatments. The correlation was analyzed by the Spearman correlation coefficient method. A p-value of less than 0.05 (p<0.05) was considered statistically significant.
Results
In the present study, the migraine characteristics and serum Hcy levels were evaluated in ninety children with typical migraine and compared before and after the six months of taking vitamins or a placebo. No adverse effect was reported during the study period. At the end of the study, no patient dropped out, and all were included in the final analysis. The studied children comprised forty-nine (54.4%) boys and forty-one (45.6%) girls with a mean age of 10.91 ± 2.65. The changes in the measured parameters before and after the administration were also evaluated and compared between the groups. Table 1 shows no significant difference between the vitamin and placebo groups in terms of mean age, sex distribution, age of onset, migraine type, and family history before the administration (p>0.05). The data relating to the headache frequency, severity, duration, headache disability, and Hcy levels were also not significantly different between the groups (p>0.05). The comparison of headache characteristics and Hcy levels before and after vitamin and placebo administrations are depicted in Table 2. The monthly headache frequency (14.69 ± 5.06), the severity of headache [5 (5-6)], headache disability (32.69 ± 9.23), and serum Hcy level (13.49 ± 5.19, µmol/L) were significantly (p<0.05) decreased after the vitamin administration [12.42 ± 3.67, 4 (4-5), 28.31 ± 7.95, and 11.56 ± 3.07, respectively]. The duration of the headache was not significantly different before [3 (2 - 3.5), hour] and after [3 (2-3), hour] the treatment (p = 0.168). However, monthly headache frequency, the severity of headache, headache duration, headache disability, and Hcy levels were not significantly different before and after the placebo administration [15.29 ± 4.23 vs. 14.56 ± 3.35, p=0.070; 6 (5-7) vs. 5 (5-6), p=0.055; 3 (2-4) vs. 3 (2-4), p=0.155; 34.36 ± 9.63 vs. 33.60 ± 8.47, p=0.078; 13.33 ± 4.21 vs. 13.25 ± 3.99, µmol/L, p=0.814; respectively].
Table 1.
Demographic characteristics of children with migraine before intervention in the study groups
| Parameter | Vitamin Group (n=45) |
Placebo Group (n=45) |
p-value |
|---|---|---|---|
| Age (year) | 11.09 ± 2.79 | 10.73 ± 2.53 | 0.528a |
| Sex (F/M) | 21/24 | 20/25 | 0.832b |
| Age of Onset (Year) | 8.71 ± 1.53 | 8.58 ± 1.73 | 0.699a |
| Type of Migraine (With/Without Aura) | 16/29 | 14/31 | 0.655b |
| Positive Family History [n (%)] | 29 (64.44) | 31 (68.89) | 0.655b |
| Monthly Headache Frequency | 14.69 ± 5.06 | 15.29 ± 4.23 | 0.543a |
| Severity of Headache (VAS-pain) | 5 (5-6) | 6 (5-7) | 0.279c |
| Headache Duration (Hour) | 3 (2-3.5) | 3 (2-4) | 0.125c |
| Headache Disability (pedMIDAS) | 32.69 ± 9.23 | 34.36 ± 9.63 | 0.404a |
| Homocysteine (μmol/L) | 13.49 ± 5.19 | 13.33 ± 4.21 | 0.874a |
F, female; M, male; pedMIDAS, pediatric migraine disability assessment score; VAS-pain, visual analog scale for pain.
a Statistical comparison was done by independent samples t-test.
b Statistical comparison was done by Chi Square test.
c Statistical comparison was done by Mann–Whitney U-test.
Table 2.
The homocysteine levels and headache characteristics before and after treatment in the
| Parameter | Before | After | p-value |
|---|---|---|---|
| Vitamin group | |||
| Monthly Headache Frequency | 14.69 ± 5.06 | 12.42 ± 3.67 | <0.001a, * |
| Severity of Headache (VAS-pain) | 5 (5-6) | 4 (4-5) | <0.001b, * |
| Headache Duration (Hour) | 3 (2-3.5) | 3 (2-3) | 0.168b |
| Headache Disability (pedMIDAS) | 32.69 ± 9.23 | 28.31 ± 7.95 | <0.001a, * |
| Homocysteine (μmol/L) | 13.49 ± 5.19 | 11.56 ± 3.07 | 0.004a, * |
| Placebo Group | |||
| Monthly Headache Frequency | 15.29 ± 4.23 | 14.56 ± 3.35 | 0.070a |
| Severity of Headache (VAS) | 6 (5-7) | 5 (5-6) | 0.055b |
| Headache Duration (Hour) | 3 (2-4) | 3 (2-4) | 0.155b |
| Headache Disability (pedMIDAS) | 34.36 ± 9.63 | 33.60 ± 8.47 | 0.078a |
| Homocysteine (μmol/L) | 13.33 ± 4.21 | 13.25 ± 3.99 | 0.814a |
PedMIDAS, pediatric migraine disability assessment score; VAS-pain, visual analog scale for pain.
a Statistical comparison was done by paired t-test.
b Statistical comparison was done by Wilcoxon test.
*Statistically significant (p<0.05).
The changes (Δ = after − before) in Hcy levels and headache characteristics were also evaluated and compared between the study groups. As shown in Table 3, the changes in monthly headache frequency, severity of headache, and headache disability before and after the treatment in the vitamin group were significantly more than those in the placebo group [-3 (-4.5– 0.5) vs. -1 (-3 – 2), p=0.014; -1 (-2 – 0) vs. 0 (-1 – 1), p=0.001; -4 (-8.5 – 2) vs. -1 (-2 – 2), p=0.002; respectively]. The headache duration change was not significantly different between the study groups [0 (-1 – 0.5) vs. 0 (-1 – 0), hours; p=0.973]. The change of serum Hcy levels before and after the treatment in the vitamin group [-2.45 (-5.34 – 1.72), (µmol/L)] was also significantly (p=0.040) higher than that in the placebo group [0.1 (-1.86 – 1)].
Table 3.
The homocysteine levels and headache characteristics changes (after − before) in the study groups
| Parameter | Vitamin Group | Placebo Group | p-value |
|---|---|---|---|
| Monthly Headache Frequency | -3 (-4.5 – 0.5) | -1 (-3 – 2) | 0.014* |
| Headache Severity (VAS) | -1 (-2 – 0) | 0 (-1 – 1) | 0.001* |
| Headache Duration (Hour) | 0 (-1 – 0.5) | 0 (-1 – 0) | 0.973 |
| Headache Disability (pedMIDAS) | -4 (-8.5 – 2) | -1 (-2 – 2) | 0.002* |
| Homocysteine (μmol/L) | -2.45 (-5.34 – 1.72) | 0.1 (-1.86 – 1) | 0.040* |
PedMIDAS, pediatric migraine disability assessment score; VAS-pain, visual analog scale for pain.
Statistical comparisons were done by Mann–Whitney U-test.
*Statistically significant (p<0.05).
The study groups also evaluated the correlations among the Hcy and headache characteristics changes. In the vitamin group, among the headache characteristics changes, there were significant positive correlations between the frequency and severity (r=0.629, p<0.001), frequency and disability (0.832, p<0.001), and severity and disability (r=0.711, p<0.001) of headache. Hcy also had significant positive correlations with the frequency (r=0.329, p=0.027) and disability (r=0.342, p=0.021) of headaches. However, in the placebo group, the only found significant correlation was between headache frequency and disability (r=0.690, p<0.001). No other significant correlation was found among the evaluated parameters.
Discussion
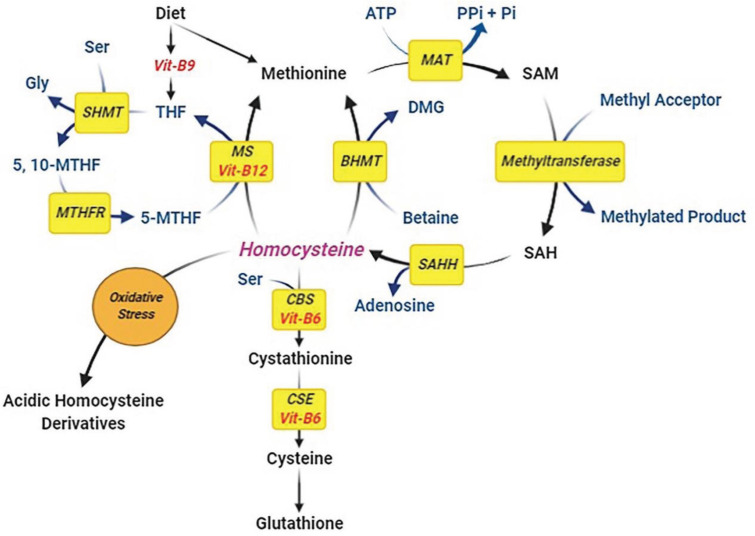
Migraine is due to a complex cycle of neurotransmitter release, neuronal activation, cortical spreading depression, and release of inflammatory markers leading to vasodilation of cerebral and meningeal vessels and eventually pain sensation (headache). Various factors may play a role in triggering migraine. Several studies, such as Liampas et al. (16) and Moschiano et al. (17), have shown that there may be a direct relationship between serum Hcy levels and migraine. This intermediate amino acid is produced from the demethylation of methionine (11). It can be transsulfurated to cysteine, which requires vitamin B6 as a cofactor. In an alternative pathway, Hcy can be re-methylated to methionine, which needs vitamins B9 (folic acid) as a co-substrate, and B12 as a cofactor (Figure 2). Not only enzymatic deficiencies such as methylenetetrahydrofolate reductase (MTHFR) or cystathionine-b-synthase, but deficiencies in B vitamins can also lead to impaired conversion of Hcy to cysteine or methionine, and it eventually accumulates in the blood (HHcy). According to previous studies, patients with migraines may also suffer from vitamin B deficiencies (18-20). İpçioğlu et al. (19) found a functional vitamin B12 deficiency represented by elevated methylmalonic acid levels in urine samples of patients with migraines without aura. Aydin et al. (18) found that the serum B12 and B9 (folic acid) levels of pediatric patients with migraine were significantly lower than those of the healthy controls. Nelson et al. (20) also revealed higher serum Hcy and lower levels of vitamin B12 and folic acid in children with recurrent headaches than those without headaches.
Figure 2.
The roles of B-vitamins in homocysteine metabolism. Homocysteine (Hcy) is produced from the demethylation of methionine. It can be transsulfurated to cysteine which requires vitamin B6 as a cofactor that eventually can produce glutathione. In an alternative pathway, Hcy can be re-methylated to methionine, which needs vitamins B9 (folic acid) as a co-substrate and B12 as a cofactor. Deficiencies in B vitamins can lead to impaired conversion of Hcy to cysteine (and glutathione) or methionine, and it eventually accumulates in the blood. Impairment in glutathione production can lead to elevated oxidative stress, resulting in the Hcy oxidation and production of its acidic derivatives such as Hcy sulfinic acid and homocysteic acid
Considering the role of B vitamins in Hcy metabolism and the possible association of this intermediate amino acid in migraine pathogenesis, the study evaluated the effect of vitamin B-complex administration on migraine severity in children. The results showed that serum Hcy levels significantly decreased after a six-month administration of vitamin B-complex. However, no significant reduction was observed in the placebo group. Unlike the placebo group, the values of headache indices, except for headache duration, were significantly diminished after the vitamin administration. Furthermore, there were significant positive correlations between the reduction of Hcy and the reductions in headache frequency and disability indices. Askari et al. (21) showed that supplementation of folic acid with pyridoxine could reduce the characteristics of migraine attacks, including headache severity, attack frequency, and headache diary result (HDR) in ninety-five migraine patients compared to the placebo group. In a study, Lea et al. (22) showed that the serum levels of Hcy were reduced in migraine patients after a six-month supplementation of folic acid (B9), B6, and B12. The reduction was significantly greater than in the placebo group. The headache frequency, pain severity, and migraine disability were also considerably reduced by vitamin supplementation. No reduction was found in the placebo group. A study by Menon et al. (23) also reported that vitamin (B6, B9, and B12) supplementation significantly reduced Hcy levels and the severity and disability of migraine in Australian female patients compared to individuals receiving a placebo.
Although any significant relationship between Hcy levels and characteristics of migraine attacks was not found in a study by Sadeghi et al. (22), after adjustment of confounding variables, they found a significant positive relationship between Hcy levels and HDR among men. Some possible differences in the results of these studies may be due to variations in the form and dose of the prescribed vitamins. Unlike the other mentioned studies, B vitamins were administered separately; in the present study, B1, B2, B3, B5, B6, B7, B9, and B12 vitamins were administered as a B-complex capsule. Differences in mean age, sex ratio, and race of the study groups might also affect the results. These results suggest a possible role of B vitamins supplementation in alleviating migraine by lowering serum Hcy.
Although the mechanisms of how HHcy can induce migraine have not yet been completely elucidated, some possible mechanisms have been introduced. According to vascular theory, HHcy may cause initial intracranial arterial vasoconstriction. It then leads to reduced blood flow to the visual cortex, followed by extracranial dilatation and pain. Based on the neurogenic theory, trigeminal vascular neurons may release the substance P in response to triggers such as HHcy, resulting in vasodilation, edema of the meninges, and pain (23). According to other proposed mechanisms, glutathione production can be reduced by HHcy, resulting in oxidative stress. Elevated oxidative stress can subsequently lead to Hcy oxidation and the production of Hcy thiolactone, S-sulfocysteine, Hcy sulfinic acid, and homocysteic acid. It has been shown that the acidic Hcy derivatives could sensitize the dura mater and/or cerebral arteries promoting the activation of the trigeminovascular system, which can eventually predispose to migraine (23). They can also act as a gamma-aminobutyric acid (GABA)-A receptor antagonist (24). Additionally, it has been shown that Hcy and its acidic derivatives may not only act as agonists at the glutamate binding site of the N-methyl-D-aspartate (NMDA) receptor but also as a partial antagonist of the glycine coagonist site causing neuronal damage derived from excessive Ca2+ influx and oxidative stress; which can be considered as a “trigger” for migraine onset (Figure 3) (23-26).
Figure 3.
The association of hyperhomocysteinemia with migraine (the possible mechanisms). Hyperhomocysteinemia (HHcy) may cause initial intracranial arterial vasoconstriction. It leads to reduced blood flow to the visual cortex followed by extracranial dilatation and pain (vascular theory). The substance P may be released by trigeminal vascular neurons in response to HHcy, which then results in vasodilation, oedema of the meninges, and pain (neurogenic theory). Glutathione production can be reduced by HHcy resulting in oxidative stress. Elevated oxidative stress can subsequently lead to Hcy oxidation and production of the acidic Hcy derivatives. They could sensitise the dura mater and/or cerebral arteries promoting the activation of the trigeminovascular system, which can eventually predispose to migraine. These acidic derivatives can also behave as agonists and antagonists of the N-methyl-D-aspartate (NMDA) and gamma-aminobutyric acid (GABA)-A receptors, respectively, causing neuronal damage derived from excessive Ca2+ influx triggering migraine onset
Although migraine is two to three times more prevalent in women than men,(4) it is approximately the same between the sexes in children before puberty (27). In the present study, according to the mean age of 10.91 ± 2.65, the subjects were primarily preadolescent children, which consisted of forty-nine (54.4%) boys and forty-one (45.6%) girls. The higher prevalence of migraine in women compared to men after puberty is most likely due to the increase in female hormones and the association of these hormones with increasing susceptibility to migraine (27). Besides, it is specified that females compared to males have higher gray matter in the primary somatosensory cortex, supplementary motor area, precuneus, basal ganglia, and amygdala. They have greater precuneus resting-state functional connectivity to the thalamus, amygdala, and basal ganglia and greater amygdala resting-state functional connectivity to the thalamus, anterior midcingulate cortex, and supplementary motor area. These differences in adults probably make the female brain more prone to migraine and pain than men (28). However, more studies are needed to evaluate the exact roles of sex hormones on migraine.
In the present study, the prescribed supplement also contained vitamins B1, B2, B3, B5, and B7, which may affect migraine severity by mechanisms other than Hcy reduction. However, these possible mechanisms were not evaluated, which can be considered a limitation of the present study. Therefore, it is suggested that other possible mechanisms and related factors other than Hcy be studied in the future.
In conclusion, migraine is one of the most common headaches in children. Like other pain disorders, it can lead to interruptions in daily activities and reduce the quality of life. Reducing the number of headache attacks can increase learning ability in children. Therefore, pediatric migraine must be diagnosed and treated appropriately. Although various drugs with different efficacy have been introduced and used to alleviate migraine headaches, taking vitamin supplements through different mechanisms can also help reduce the severity of migraine. The present study showed that the six-month administration of vitamin B-complex might effectively relieve migraine severity in children by lowering serum Hcy. However, further studies with larger sample sizes are needed to confirm the obtained results.
Authorʼs contribution
All the authors have read and approved the final submitted manuscript.
Conflict of interest
The authors have declared no competing or potential conflicts of interest
Acknowledgment
Pediatric Health Research Center, Tabriz University of Medical Sciences, Tabriz, Iran, supported this work. The authors want to thank all the participants who agreed to participate in this study.
The authors declare that there is no conflict of interest.
The data supporting this study’s findings are available from the corresponding author (SR) upon reasonable request.
The study was approved by the Research Ethics Committee of Tabriz University of Medical Sciences (IR. TBZMED. REC. 1397. 544) and registered in the Iranian Registry of Clinical Trials (www.irct.ir; IRCT Registration Number: IRCT20131012014988N6).
References
- 1.Kacperski J, Green A, Qaiser S. Management of chronic migraine in children and adolescents: a brief discussion on preventive therapies. Pediatric Drugs. 2020:1–9. doi: 10.1007/s40272-020-00418-y. [DOI] [PubMed] [Google Scholar]
- 2.Barnes NP. Migraine headache in children. BMJ clinical evidence. 2011:2011. [PMC free article] [PubMed] [Google Scholar]
- 3.Schürks M, Rist PM, Bigal ME, Buring JE, Lipton RB, Kurth T. Migraine and cardiovascular disease: systematic review and meta-analysis. Bmj. 2009;339:b3914. doi: 10.1136/bmj.b3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martelletti P, Haimanot RT, Láinez MJ, Rapoport AM, Ravishankar K, Sakai F, et al. The global campaign (GC) to reduce the burden of headache worldwide The international team for specialist education (ITSE) The journal of headache and pain. 2005;6(4):261–3. doi: 10.1007/s10194-005-0202-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olesen J, Steiner T. The International classification of headache disorders, 2nd edn (ICDH-II) BMJ Publishing Group Ltd. 2004 doi: 10.1136/jnnp.2003.031286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ayatollahi S, Khosravi A. A case-control study of migraine and tension-type headache’s risk factors among Shiraz schoolchildren. Scientific Journal of Hamadan University of Medical Sciences. 2005;11(4):37–42. [Google Scholar]
- 7.David R. In: Headache: Acute recurrent headaches. 3rd ed . Swiaman Kenneth Ashwal Stephen., editor. St Louis: Mosby. ; 1991. pp. 753–4. [Google Scholar]
- 8.Piña-Garza JE, Fenichel GM. Fenichel’s clinical pediatric neurology: a signs and symptoms approach. Elsevier Health Sciences; 2013. [Google Scholar]
- 9.Pakalnis A. Preventive therapies of pediatric migraine. Drug Development Research. 2007;68(6):350–4. [Google Scholar]
- 10.Chambers JC, Ueland PM, Wright M, Doré CJ, Refsum H, Kooner JS. Investigation of relationship between reduced, oxidized, and protein-bound homocysteine and vascular endothelial function in healthy human subjects. Circulation research. 2001;89(2):187–92. doi: 10.1161/hh1401.093459. [DOI] [PubMed] [Google Scholar]
- 11.Nekrassova O, Lawrence NS, Compton RG. Analytical determination of homocysteine: a review. Talanta. 2003;60(6):1085–95. doi: 10.1016/S0039-9140(03)00173-5. [DOI] [PubMed] [Google Scholar]
- 12.Baszczuk A, Kopczyński Z. Hyperhomocysteinemia in patients with cardiovascular disease. Postepy higieny i medycyny doswiadczalnej (Online). 2014;68:579–89. doi: 10.5604/17322693.1102340. [DOI] [PubMed] [Google Scholar]
- 13.Ung D, De Nadai AS, McBride NM, Haney B, Huszar P, Hart D, et al. The association between quality of life and clinical characteristics youth with headaches. Children’s health care. 2019;48(1):1–17. [Google Scholar]
- 14.Hershey A, Powers S, Vockell A-L, LeCates S, Kabbouche M, Maynard M. PedMIDAS: development of a questionnaire to assess disability of migraines in children. Neurology. 2001;57(11):2034–9. doi: 10.1212/wnl.57.11.2034. [DOI] [PubMed] [Google Scholar]
- 15.Elbarbary NS, Ismail EAR, Zaki MA, Darwish YW, Ibrahim MZ, El-Hamamsy M. Vitamin B complex supplementation as a homocysteine-lowering therapy for early stage diabetic nephropathy in pediatric patients with type 1 diabetes: A randomized controlled trial. Clinical Nutrition. 2020;39(1):49–56. doi: 10.1016/j.clnu.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Liampas I, Siokas V, Mentis AFA, Aloizou AM, Dastamani M, Tsouris Z, et al. Serum Homocysteine, Pyridoxine, Folate, and Vitamin B12 Levels in Migraine: Systematic Review and Meta‐Analysis. Headache: The Journal of Head and Face Pain. 2020;60(8):1508–34. doi: 10.1111/head.13892. [DOI] [PubMed] [Google Scholar]
- 17.Moschiano F, D’Amico D, Usai S, Grazzi L, Di Stefano M, Ciusani E, et al. Homocysteine plasma levels in patients with migraine with aura. Neurological Sciences. 2008;29(1):173–5. doi: 10.1007/s10072-008-0917-2. [DOI] [PubMed] [Google Scholar]
- 18.Aydin H, Bucak IH, Geyik M. Vitamin B12 and folic acid levels in pediatric migraine patients. Acta Neurologica Belgica . 2020:1–4. doi: 10.1007/s13760-020-01491-3. [DOI] [PubMed] [Google Scholar]
- 19.İpçioğlu OM, Özcan Ö, Gültepe M, Tekeli H, Şenol MG. Functional vitamin B12 deficiency represented by elevated urine methylmalonic acid levels in patients with migraine. Turkish Journal of Medical Sciences. 2008;38(5):409–14. [Google Scholar]
- 20.Nelson KB, Richardson AK, He J, Lateef TM, Khoromi S, Merikangas KR. Headache and biomarkers predictive of vascular disease in a representative sample of US children. Archives of pediatrics & adolescent medicine. 2010;164(4):358–62. doi: 10.1001/archpediatrics.2010.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Askari G, Nasiri M, Mozaffari-Khosravi H, Rezaie M, Bagheri-Bidakhavidi M, Sadeghi O. The effects of folic acid and pyridoxine supplementation on characteristics of migraine attacks in migraine patients with aura: A double-blind, randomized placebo-controlled, clinical trial. Nutrition. 2017;38:74–9. doi: 10.1016/j.nut.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 22.Sadeghi O, Maghsoudi Z, Askari G, Khorvash F, Feizi A. Association between serum levels of homocysteine with characteristics of migraine attacks in migraine with aura. Journal of research in medical sciences: the official journal of Isfahan University of Medical Sciences. 2014;19(11) [PMC free article] [PubMed] [Google Scholar]
- 23.Cacciapuoti F. Migraine homocysteine‐related: Old and new mechanisms. Neurology and Clinical Neuroscience. 2017;5(5):137–40. [Google Scholar]
- 24.Lippi G, Mattiuzzi C, Meschi T, Cervellin G, Borghi L. Homocysteine and migraine A narrative review. Clinica Chimica Acta. 2014;433:5–11. doi: 10.1016/j.cca.2014.02.028. [DOI] [PubMed] [Google Scholar]
- 25.Deep SN, Mitra S, Rajagopal S, Paul S, Poddar R. GluN2A-NMDA receptor–mediated sustained Ca2+ influx leads to homocysteine-induced neuronal cell death. Journal of Biological Chemistry. 2019;294(29):11154–65. doi: 10.1074/jbc.RA119.008820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lipton SA, Kim W-K, Choi Y-B, Kumar S, D’Emilia DM, Rayudu PV, et al. Neurotoxicity associated with dual actions of homocysteine at the N-methyl-D-aspartate receptor. Proceedings of the National Academy of Sciences. 1997;94(11):5923–8. doi: 10.1073/pnas.94.11.5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vetvik KG, MacGregor EA. Sex differences in the epidemiology, clinical features, and pathophysiology of migraine. The Lancet Neurology. 2017;16(1):76–87. doi: 10.1016/S1474-4422(16)30293-9. [DOI] [PubMed] [Google Scholar]
- 28.Faria V, Erpelding N, Lebel A, Johnson A, Wolff R, Fair D, et al. The migraine brain in transition: girls vs boys. Pain. 2015;156(11):2212–21. doi: 10.1097/j.pain.0000000000000292. [DOI] [PubMed] [Google Scholar]