Abstract
PURPOSE
BRAF/MEK inhibition is a standard of care for patients with BRAF V600E/K–mutated metastatic melanoma. For patients with less frequent BRAF mutations, however, efficacy data are limited.
METHODS
In the current study, 103 patients with metastatic melanoma with rare, activating non-V600E/K BRAF mutations that were treated with either a BRAF inhibitor (BRAFi), MEK inhibitor (MEKi), or the combination were included. BRAF mutation, patient and disease characteristics, response, and survival data were analyzed.
RESULTS
Fifty-eight patient tumors (56%) harbored a non-E/K V600 mutation, 38 (37%) a non-V600 mutation, and seven had both V600E and a rare BRAF mutation (7%). The most frequent mutations were V600R (43%; 44 of 103), L597P/Q/R/S (15%; 15 of 103), and K601E (11%; 11 of 103). Most patients had stage IV disease and 42% had elevated lactate dehydrogenase at BRAFi/MEKi initiation. Most patients received combined BRAFi/MEKi (58%) or BRAFi monotherapy (37%). Of the 58 patients with V600 mutations, overall response rate to BRAFi monotherapy and combination BRAFi/MEKi was 27% (six of 22) and 56% (20 of 36), respectively, whereas median progression-free survival (PFS) was 3.7 months and 8.0 months, respectively (P = .002). Of the 38 patients with non-V600 mutations, overall response rate was 0% (zero of 15) to BRAFi, 40% (two of five) to MEKi, and 28% (five of 18) to combination treatment, with a median PFS of 1.8 months versus 3.7 months versus 3.3 months, respectively. Multivariable analyses revealed superior survival (PFS and overall survival) with combination over monotherapy in rare V600 and non-V600 mutated melanoma.
CONCLUSION
Patients with rare BRAF mutations can respond to targeted therapy, however, efficacy seems to be lower compared with V600E mutated melanoma. Combination BRAFi/MEKi seems to be the best regimen for both V600 and non-V600 mutations. Yet interpretation should be done with care because of the heterogeneity of patients with small sample sizes for some of the reported mutations.
INTRODUCTION
The discovery of BRAF V600E as a therapeutic target has been a milestone in the modern treatment of advanced melanoma. Approximately 35% to 50% of melanomas harbor an activating BRAF mutation that promotes tumor proliferation through the mitogen-activated protein kinase (MAPK)/ERK signaling pathway.1,2 Of these, 70% to 80% account for the V600E mutation and another 10% to 20% for the less active V600K.3-8 Genetic analyses revealed rarer BRAF mutations in 3.4% to 14% of melanomas2,5,8-10
The drug registration studies Combi-D (dabrafenib/trametinib)4 and Columbus (encorafenib/binimetinib)3 excluded patients with melanoma with mutations other than V600E/K, and the CoBRIM study (vemurafenib/cobimetinib)11 relied on the Cobas test, which mainly detects V600E mutations. Here, combined BRAF inhibitor (BRAFi)/MEK inhibitor (MEKi) therapy produced an improvement in overall response rate (ORR) from 50% to 70%, prolongation of progression-free survival (PFS) from a median of 7 months to 12 months, and overall survival (OS) from 17 months to approximately 2 years compared with BRAFi monotherapy.3,12,13 Results from the METRIC (ClinicalTrials.gov identifier: NCT01245062) study with trametinib monotherapy in patients with BRAF V600E/K–mutated melanoma show that patients with activating BRAF mutations respond to downstream MEK inhibition.14 Hence, the use of MEKi with or without BRAFi is rational for melanomas with likely activating BRAF mutations, including those with rare V600 or non-V600 mutations; however, clinical experience in such patients is based on anecdotal case reports.
This international retrospective study assessed the therapeutic response to treatment with BRAFi or MEKi monotherapy, or the combination, in patients with metastatic melanoma harboring either activating BRAF mutations other than class I and II V600E/K or kinase-impaired class III mutations and translocations leading to MAPK pathway activation via upstream mechanisms.14 The aim of the current study was to share the therapeutic experience of BRAFi/MEKi in patients with these rare melanomas to give treating physicians a foundation on which to make informed discussions about possible targeted therapy options.
METHODS
Retrospective data from patients with advanced melanoma who were treated with BRAFi, MEKi, or the combination between November 2009 and April 2018 and who harbored BRAF mutations other than V600E/K or translocations that are known or likely to activate the MAPK pathway were collected for this study.5,14-16 BRAF sequencing was performed locally as clinical routine in the respective cancer center and hence the methods used varied (Appendix, online only). Data were extracted from medical records from 20 participating institutions from four countries (Germany, Australia, the Netherlands, and the United States) and Novartis (three patients from Combi-MB study). The data cutoff date was September 27, 2018. The history of three patients—V600M, V600G, and D594G—had been published, in part, previously.17-19 Retrospective analyses of patient data were approved by the ethics committee of the University Hospital Heidelberg (S-454/2015).
Statistical Analysis
Patients were grouped for V600 and non-V600 BRAF mutations and for the treatment regimen used. Univariable analyses to compare V600 with non-V600 groups consisted of Fisher’s exact test for qualitative data and the Wilcoxon rank-sum test for quantitative data. Melanoma stages were determined according to the American Joint Committee on Cancer classification (eight edition).20 PFS and OS were measured from BRAFi/MEKi initiation to the date of first progression or death, respectively, or censored at the date of last contact when patients were still on therapy. The Kaplan-Meier method was used for their estimation, and differences between groups were assessed using the log-rank test. Lactate dehydrogenase (LDH) at treatment start was dichotomized as normal or elevated according to the institutional upper limit of normal. This variable was missing for nine patients and multiple imputation was used for multivariable analyses. Multivariable logistic regression was used to model response to treatment, including the most relevant factors—BRAF genotype, therapy regimen, age, sex, and LDH. Multivariable Cox proportional hazards regression served to model PFS and OS. As the proportional hazards assumption was violated for the factor BRAF genotype, separate analyses were performed for V600 and non-V600 patients, including the following factors: therapy regimen, age, sex, and LDH. To investigate the effect of therapy in this observational study, additional Cox proportional hazards regression analysis with inverse probability of treatment weighting (IPTW), including the covariables age, sex, and LDH, was performed to account for the observational nature of the data (Appendix Tables A1 and A2, online only).21 Two analyses were performed for non-V600 patients, one for each comparison of mono- and combination therapy. A P value of ≤ .05 was considered statistically significant. All computations were performed using R (The R Foundation for Statistical Computing, version 3.4.3) with packages survival, survminer, prodlim, mice, and glm.
RESULTS
In total, 103 patients with advanced melanoma and rare activating BRAF mutations or translocations were identified. A cohort of seven patients with a V600E/K plus another rare BRAF mutation (Appendix Table A3, online only) were excluded from statistical analyses, as the cohort was small and suspicion is high that the V600E/K mutation had a greater influence on the outcome of these patients. Median age for the full cohort was 62 years and 74% were male. At the time of targeted therapy commencement, 96% of patients had stage IV melanoma (M1c, 45%; M1d, 29%) and 42% had elevated LDH. More than one half of patients (n = 60; 58%) were treated with combined BRAFi/MEKi, 38 (37%) with BRAFi monotherapy, and five (5%) with MEKi monotherapy. The latter group included one patient who was treated with trametinib plus the multikinase inhibitor sorafenib. A majority of patients (76%) were treatment naïve. Those with prior treatment had received immunotherapies with either ipilimumab and/or programmed death-1 antibodies (20 of 26; 77%) or chemotherapy with mostly dacarbazine (11 of 26; 42%). Several factors, such as the physician’s assessment of possible therapeutic benefit and area-specific availability and drug approval status at the time of therapy, influenced whether BRAFi or MEKi monotherapy or the combination were administered. As time progressed, more patients received the BRAFi/MEKi combination.3,12,13
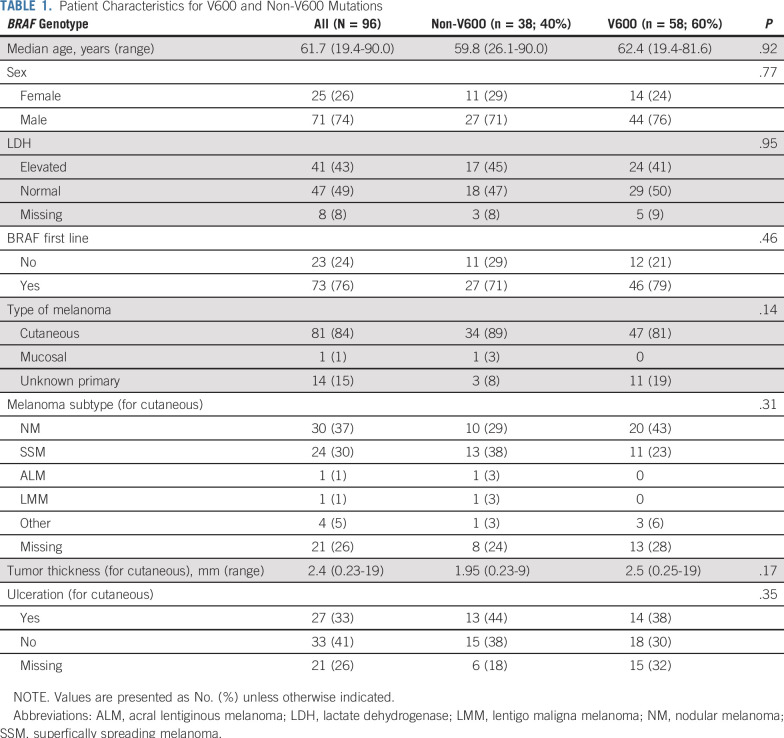
Assessed patients (n = 96) consisted of two groups: BRAF mutations in codon 600 (V600; n = 58; 56%; class I mutation) and BRAF mutations or translocations affecting other codons nearby (non-V600; n = 38; 37%; class II and III mutations; Table 1), with similar characteristics concerning age, sex, elevated LDH, and line of treatment (Table 1).
TABLE 1.
Patient Characteristics for V600 and Non-V600 Mutations
Melanoma Characteristics
The majority of included melanomas were of cutaneous origin (non-V600, 89%; V600, 81%), with nodular melanomas and superficially spreading melanomas the most frequent subtypes. Although not significant, the V600 group contained more melanomas with unknown primary compared with the non-V600 group (19% v 8%; P = .14). Median Breslow thickness was slightly higher in V600-mutated melanomas (2.5 mm v 1.95 mm; P = .17; Table 1).
BRAF Mutations
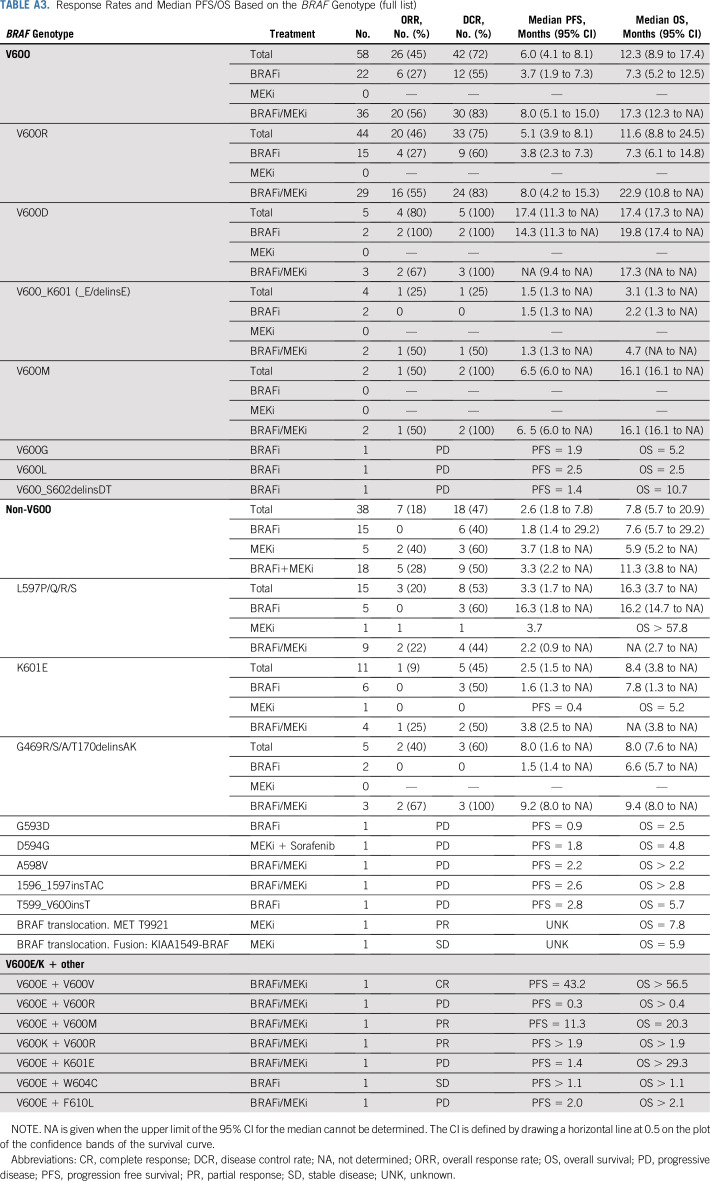
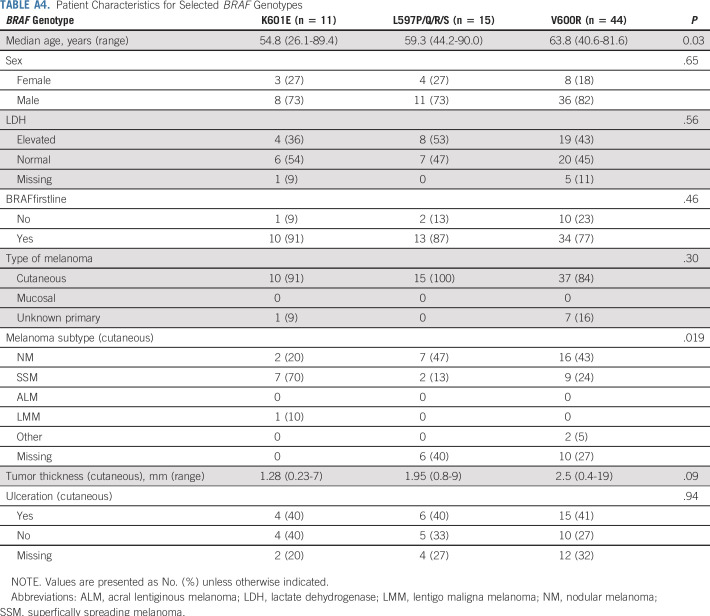
V600 mutations included V600R (44 of 58; 76%), V600D (five of 58; 9%), V600M (two of 58; 3%), V600_K601 (four of 58; 7%), and unique mutations (V600G, V600L, and V600_S602delinsDT). Non-V600 mutations included mainly class II kinase-activating mutations L597P/Q/R/S (15 of 38; 39%), K601E (11 of 38; 29%), G469R/S/A (five of 38; 13%), and unique mutations, including kinase-impaired class III mutations leading to MAPK pathway activation via upstream coalterations (A598V, 1596_1597insTAC, T599_V600insT, D594G, and G593D). Subgroup demographics for BRAF mutations with more than 10 patients are provided in Appendix Table A4, online only.
Therapeutic Response
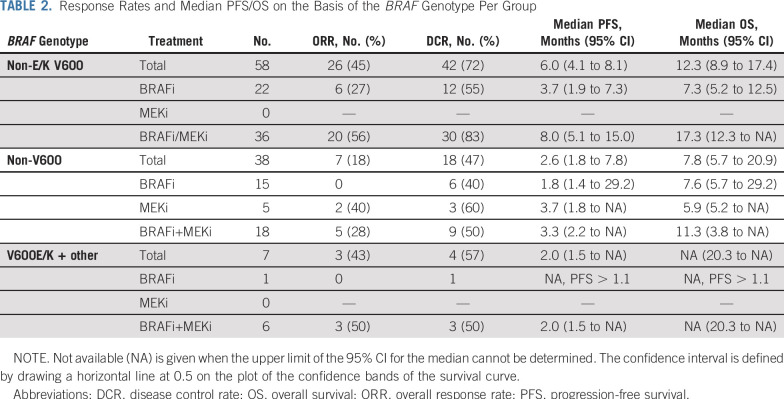
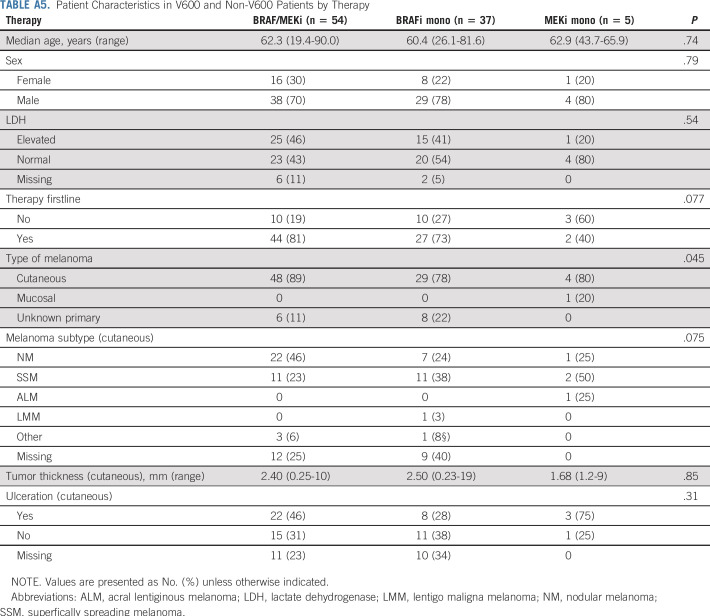
Of 96 patients, 56% received BRAFi/MEKi combination treatment, 39% BRAFi monotherapy, and 5% MEKi monotherapy (Table 2). Most patients received the targeted treatment in the first-line setting, with the exception of patients with MEKi monotherapy (Appendix Table A5, online only).
TABLE 2.
Response Rates and Median PFS/OS on the Basis of the BRAF Genotype Per Group
ORR of rare BRAF-mutated melanomas to MAPK pathway inhibition was 34% (33 of 96) and was higher in V600 than non-V600 melanomas (45% v 18%; P = .009; Table 2). Response rates to BRAFi/MEKi combinations were 56% (20 of 36) in V600 and 28% (five of 18) in non-V600 melanomas (P = .082). Non-V600 mutations showed no response with BRAFi monotherapy, but only with (additional) MEKi (MEKi, 40% [two of five]; BRAFi/MEKi combination, 28% [five of eight]; P = .026). In V600, the response rate was also higher with combination treatment compared with BRAFi monotherapy (56% [20 of 36] v 27% [six of 22]; P = .056), with superior disease control rate (DCR, 83% [30 of 36] v 55% [12 of 22]; P = .032). In non-V600 melanoma, DCR did not differ significantly between combined treatment and the respective monotherapies (Table 2; P = .74). Again, DCR was higher in patients with V600 mutation compared with non-V600 (P = .018).
In multivariable analysis, response rate was significantly dependent on the BRAF genotype and treatment regimen, with a better response in V600-mutated melanomas (P = .006) and with BRAFi/MEKi combination treatment (P = .005) compared with BRAFi monotherapy. Response rates for the different BRAF genotypes are provided in Appendix Table A3. Of note, for many unique BRAF genotypes no responses were observed.
Patient Survival
Although median PFS was generally lower in patients with non-V600 melanoma, both groups demonstrated no significant difference in overall PFS and OS, likely because of small patient numbers. Median PFS was 6.0 months for V600 and 2.6 months for non-V600 patients with melanoma (P = .63). Median OS was 12.3 months for V600 and 7.8 months for non-V600 patients with melanoma (P = .74; Table 2 and Appendix Fig A1, online only). However, as combination treatment in V600E/K-mutated melanoma leads to better survival compared with monotherapy, different treatment groups were evaluated separately. In the combination therapy group, median PFS/OS was numerically higher in V600-mutated melanomas (Table 2 and Figs 1A and 1C). There was no difference between the groups treated with BRAFi monotherapy (Figs 1B and 1D). As expected, BRAFi/MEKi combination was superior to BRAFi alone for rare V600 mutations with a median PFS of 8 months versus 3.7 months and OS of 17.3 months versus 7.3 months (P ≤ .05; Figs 2A and 2C). In the non-V600 group, no difference was observed (Figs 2B and 2D). However, median PFS tended to be greater for BRAFi/MEKi combination and MEKi monotherapy compared with BRAFi monotherapy (Table 2).
FIG 1.
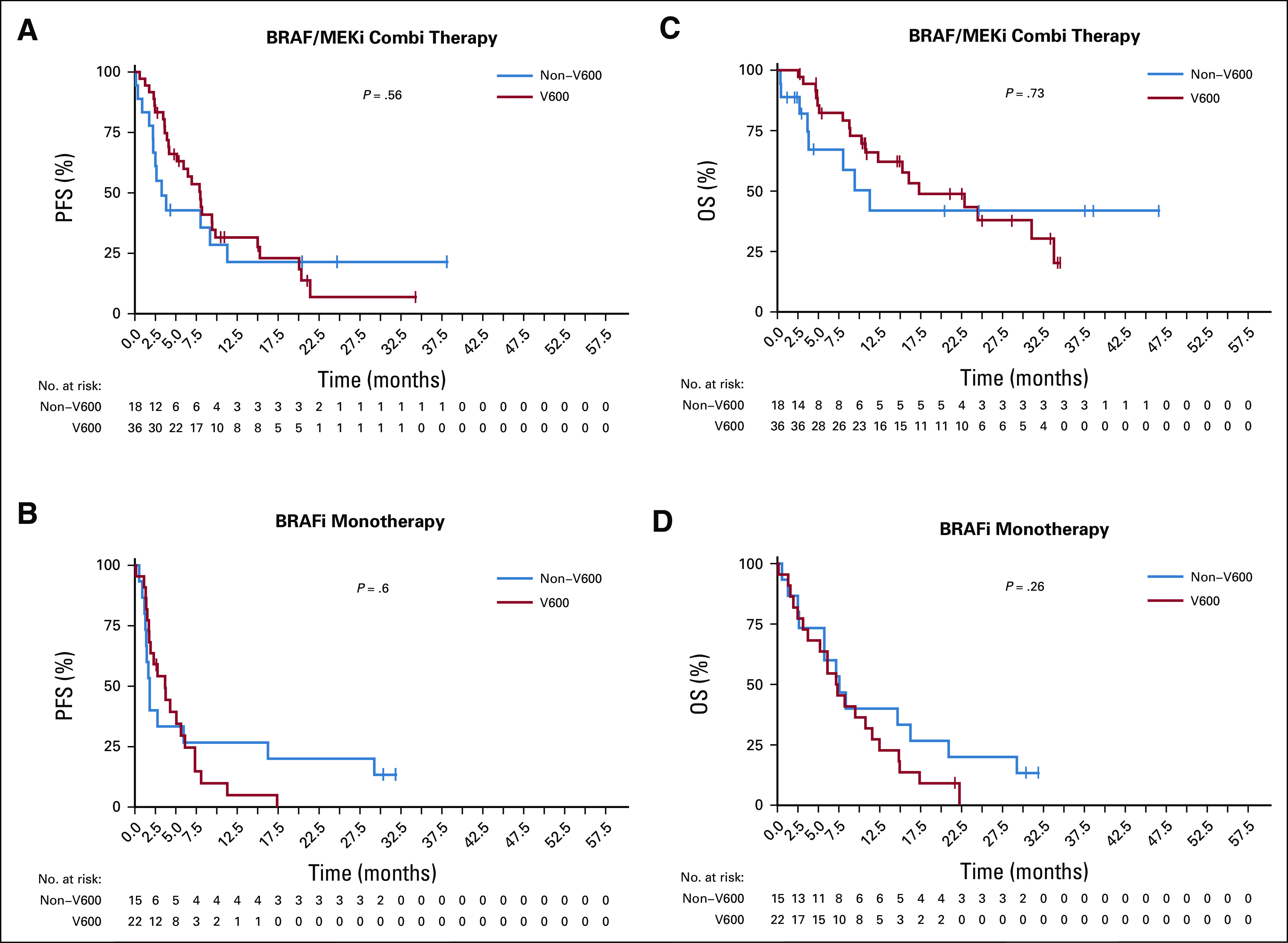
Kaplan-Meier estimates of (A and B) progression-free survival (PFS) and (C and D) overall survival (OS) for the different therapies BRAF inhibitor (BRAFi)/MEK inhibitor (MEKi) combination (A and C) and BRAFi monotherapy (B and D) in rare V600 (red line) and non-V600 mutations (blue line). In the combination therapy group, median PFS/OS was numerically higher in V600-mutated melanomas (A and C). There was no difference between the groups treated with BRAFi monotherapy (B and D).
FIG 2.
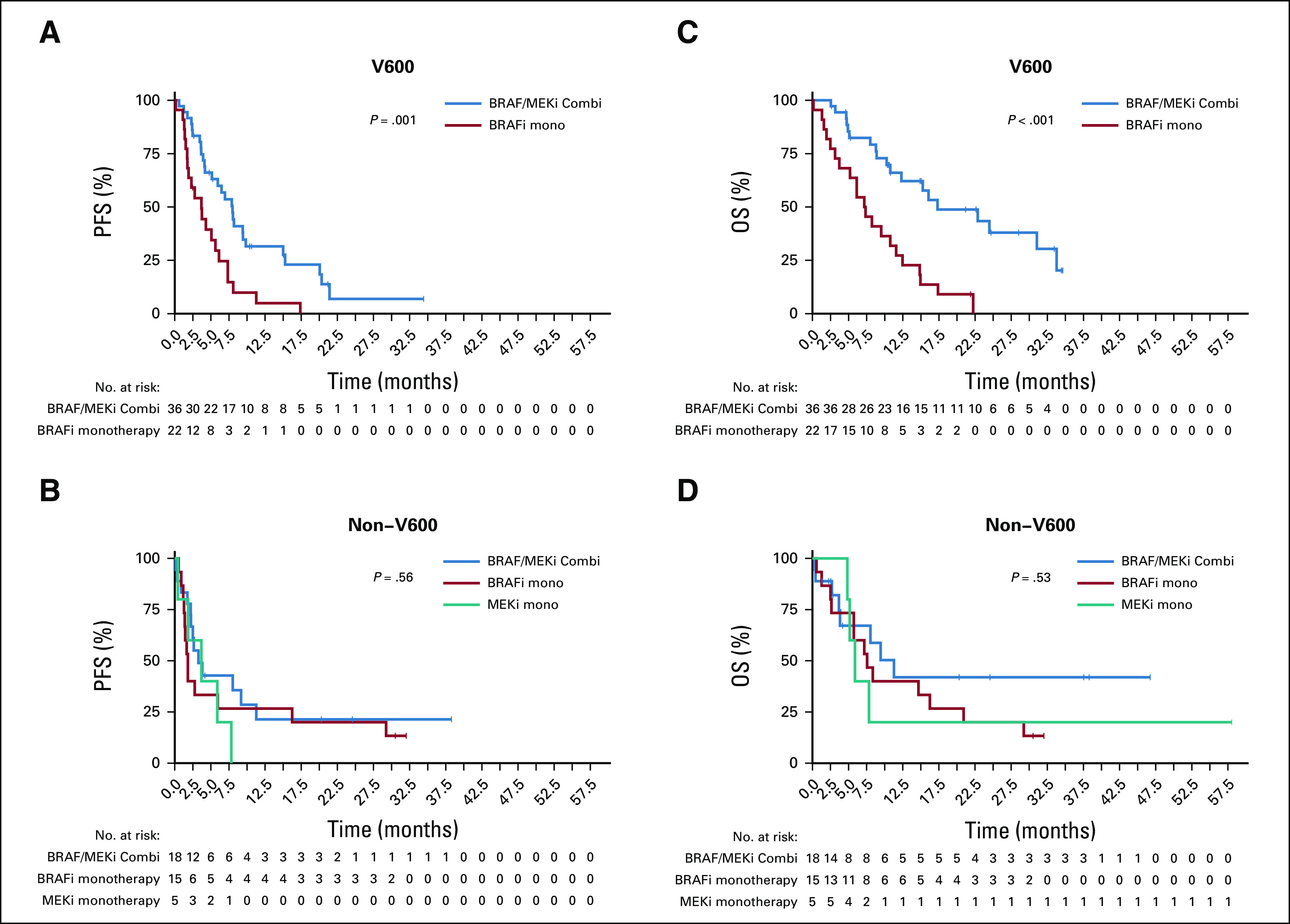
Kaplan-Meier estimates of (A and B) progression-free survival (PFS) and (C and D) overall survival (OS) for the different BRAF genotypes V600 (A and C) and non-V600 (B and D) in reference to their treatment (BRAF inhibitor [BRAFi]/MEK inhibitor [MEKi] combination [blue line], BRAFi monotherapy [red line], and MEKi monotherapy [green line]). In BRAFi/MEKi versus BRAFi alone, there was a significant difference in median PFS for rare V600 mutations (8 months v 3.7 months) and OS (17.3 months v 7.3 months; P ≤ .05; A and C). In the non-V600 group, no difference was observed for BRAFi/MEKi combination or monotherapy (B and D). Median PFS was longer for BRAFi/MEKi combination and MEKi monotherapy compared with BRAFi monotherapy.
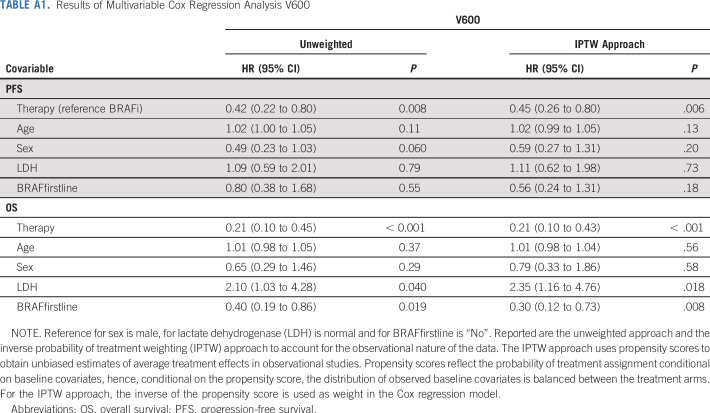
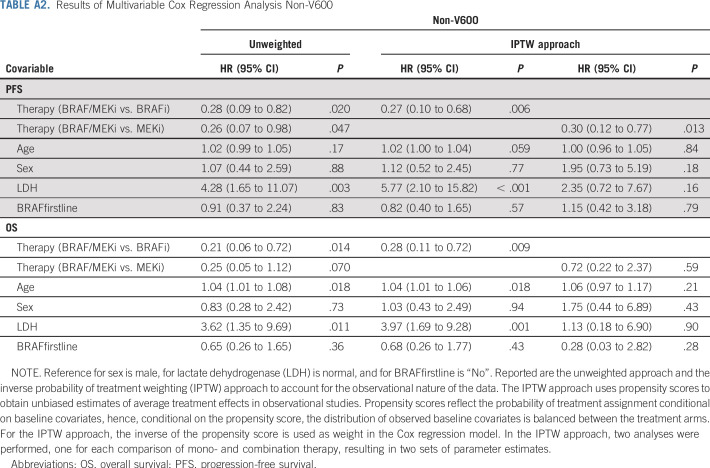
Multivariate Cox proportional hazards regression analysis demonstrated that patients with V600 who received combination treatment had superior PFS compared with BRAFi monotherapy (hazard ratio [HR], 0.42; 95% CI, 0.22 to 0.80; P = .008; age: P = .11; sex: P = .06; LDH: P = .79; line of treatment: P = .55; Table A1). Similarly, for non-V600-patients combination treatment had superior PFS compared with BRAFi monotherapy (HR, 0.28; 95% CI, 0.09 to 0.82; P = .02) and MEKi monotherapy (HR, 0.26; 95% CI, 0.07 to 0.98; P = .047; age: P = .17; sex: P = .88; LDH: P = .003; line of treatment: P = .83; Table A2). IPTW analysis—used to account for the observational nature of the data—qualitatively resulted in similar estimates for therapy effect for both patient cohorts.
As for OS in patients with V600, those with combination treatment had a superior outcome (HR, 0.21; 95% CI, 0.10 to 0.45; P < .001) compared with BRAFi monotherapy (age: P = .37; sex: P = .29; LDH: P = .04; line of treatment: P = .019; Table A1). Non-V600 patients receiving combination treatment also had superior OS compared with BRAFi monotherapy (HR, 0.21; 95% CI, 0.06 to 0.72; P = .014) and MEKi monotherapy (HR, 0.25; 95% CI, 0.05 to 1.12; P = .07; age: P = .018; sex: P = .73; LDH: P = .011; line of treatment: P = .36; Table A2). IPTW analysis resulted in similar estimates for therapy effect for V600 patients; however, for non-V600 patients, combination therapy was superior to BRAFi monotherapy but not to MEKi therapy.
The most common non-V600E/K mutation was the V600R mutation (44 of 58; 76%; Data Supplement). Results from this cohort resembled the overall V600 group with a significant advantage of the combination therapy for PFS (median, 8.0 months v 3.8 months; P = .002) and OS (median, 22.9 months v 7.3 months; P = .002; Appendix Fig A2, online only). For non-V600 patients with melanoma, the two most frequent mutations were L597P/Q/R/S (15 of 38; 40%) and K601E (11 of 38; 29%). In these, no significant differences were detected between the different treatment groups, comparable to the full non-V600 cohort (PFS: L597 [P = .81]; K601E [P = .004]; OS: L597 [P = .57]; K601E [P = .36]; Appendix Fig A3, online only). Of note, of three patients with G469R/S/A mutations who were treated with BRAFi/MEKi, two responded and all three showed controlled disease.
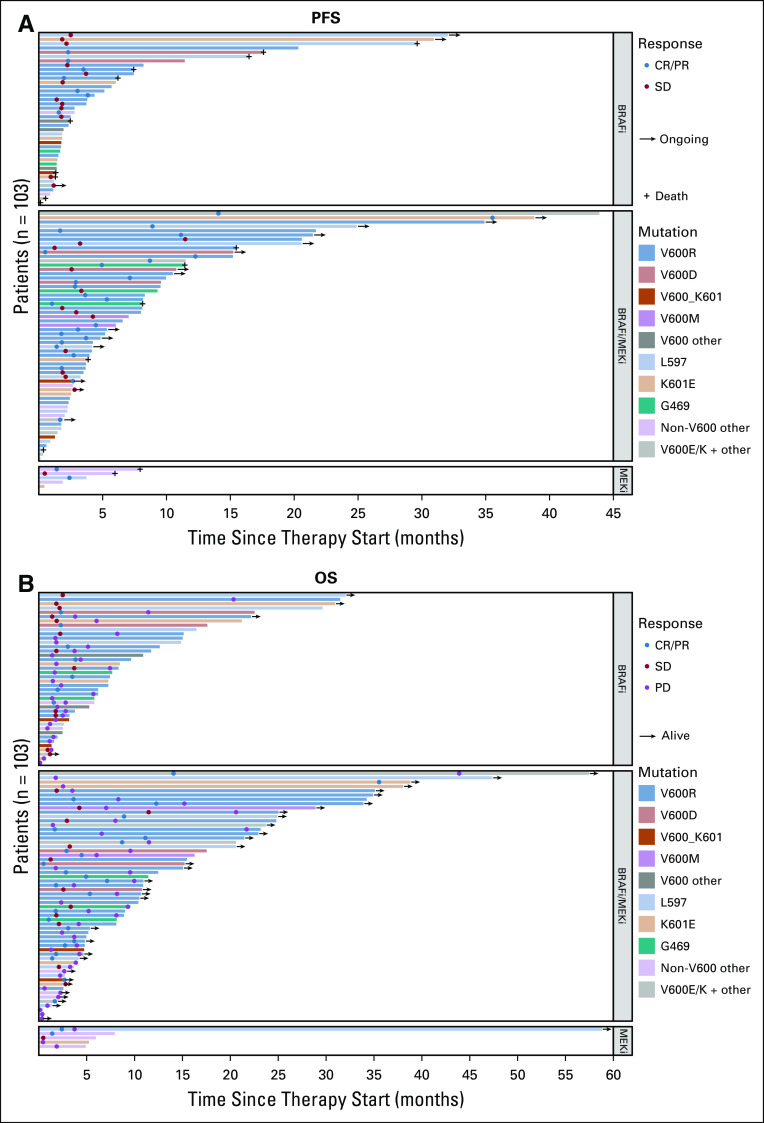
However, patients with the longest PFS (> 22 months) had V600R-, K601E-, or L597P/S -mutated melanoma (Fig 3). PFS and OS data for all and single rare BRAF mutations are provided in Appendix Table A3. In general, none of these single cases achieved durable PFS.
FIG 3.
Swimmer plots demonstrating (A) progression-free survival (PFS) and (B) overall survival (OS) with corresponding therapy (BRAF inhibitor [BRAFi] monotherapy, BRAFi/MEK inhibitor [MEKi] combination, or MEKi monotherapy) for each patient with rare BRAF mutation included in this study. Patients with the longest PFS (> 22 months) had a V600R-, K601E-, or L597P/S-mutated melanoma (A). In general, none of the single cases achieved durable PFS. CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease.
DISCUSSION
BRAFi/MEKi combinations have shown a high ORR and significantly prolonged PFS and OS for V600E and, to a lesser extent, V600K-mutated melanomas.3,4,11,22,23 As patients with melanoma harboring less frequent BRAF mutations have been excluded from the controlled clinical registration trials, there are no evidence-based data available on response and survival benefit with BRAFi/MEKi for these patients.
The most frequent non-V600E/K BRAF mutations reported in this study were V600R (43%), L597P/Q/R/S (15%), and K601E (11%). All of these are activating mutations with high kinase activity responsible for strong MAPK pathway activation.24
V600R mutations are reported in 3% to 7% of BRAF-mutated melanomas, making it the third most common BRAF mutation.25 ORRs of up to 83% have been reported for treatment with BRAFi monotherapy,25,26 and with the MEKi trametinib a partial response could be achieved.27,28 In our cohort of 44 V600R patients with melanoma, the combination of BRAFi/MEKi showed an advantage for response compared with BRAFi monotherapy (55% v 27%; P = 0.11) and superior PFS (median, 8.0 months v 3.8 months; P = .002) and OS (median, 22.9 months v 7.3 months; P = .002). Considering this, ORR, as it is for the whole V600 group, is slightly lower than the ORR of 67% to 76% reported for combined BRAFi/MEKi in BRAF V600E/K-mutated melanomas,3,12,13 although the 50% inhibitory concentrations in vitro are similar to vemurafenib (9 nM for V600R v 10 nM for V600E v 7 nM for V600K).29 However, clinical outcome, especially PFS, seemed to be inferior (median, 8.0 months for V600R v 12.1 to 14.9 months for V600E/K in the respective registration trials; Appendix Fig A4, online only).3-5 Of note, our cohort had a similar proportion of patients with elevated LDH (our cohort, 43%; CoBRIM, 46%; Combi-D, 34%) and 75% of patients were treated first line. In a pooled subgroup analysis of the Combi-D and -V trials, patients with a V600K mutation revealed significantly lower PFS and OS in multivariable analysis compared with V600E.22 This might be a result of differences in gene expression between V600E and V600K melanomas, with V600E depending mostly on the MEK/ERK pathway, whereas in V600K alternative pathways, such as phosphoinositide (PI) 3-kinase (PIK3-AKT), gain a more important role.23 In addition, V600E and V600D-mutated BRAF have been shown to be two- to four-fold more active than V600R or V600K.2,6,30-33
Non-V600 mutations have been reported to demonstrate responsiveness to BRAFi/MEKi as well. L597Q/R/S are neighboring oncogenic mutations on the BRAF gene with a 138-fold increased kinase activity (compared with a 500-fold activity increase in V600E).34 L597P has not been functionally or clinically validated, but is considered likely oncogenic as a result of its vicinity to L597Q/R/S.35-37 Ectopic expression of L597Q/R/S has shown elevated phospho-MEK and -ERK, and tumor regression was shown for L597S and L597Q in vitro and in vivo when treated with MEKi.24,35 This finding was clinically supported in two case reports in patients with L597R- and L597S-mutated melanoma.24 One report showed a response of L597R to BRAFi with vemurafenib.38 On the contrary, Kim et al39 could not confirm efficacy for either vemurafenib or trametinib in two patients with L597R-mutated melanoma. Finally, Dankner et al40 generated patient-derived xenografts bearing an L597S mutation that responded to BRAFi, MEKi, and BRAFi/MEKi combination with superiority for the combination. The same group reported a response to treatment with BRAFi/MEKi combination in two patients with L597S-mutated melanoma.40 In our study, we included 15 patients with L597P/Q/R/S mutation with no response to BRAFi monotherapy, one (L597Q) to MEKi monotherapy, and only two of nine (both L597S) to combination treatment (Appendix Table A3). No significant advantage for PFS or OS was observed for the combination over BRAFi or MEKi monotherapy in Kaplan-Meier analysis (Appendix Fig A3, online only), although this comparison is limited by low numbers.
In several case reports, patients with BRAF K601E-mutated melanoma did not benefit from either BRAFi or BRAFi/MEKi combination therapy39,41; however, a response to the MEKi trametinib was reported by Kim et al39 in one patient and by Bowyer et al36 in three of four patients.28,36 We included 11 patients with a K601E mutation. Here, response rates were low, with none of the patients treated with BRAFi (zero of six) or MEKi (zero of one) monotherapy experiencing a response and only one of four treated with combination therapy (Appendix Table A3). With a median PFS of only 3.3 months for combination treatment, clinical efficacy for non-V600-mutated melanoma has to be stated as low and hence targeted therapy should be considered with care.
Of interest, five patients with a G469 mutation were included in the study. Two of two did not experience a response to BRAFi monotherapy, but two of three treated with BRAFi/MEKi experienced a response. Of note, these are the first reports on responses to BRAFi/MEKi in patients harboring this mutation. In vitro, G469V/A-mutated cell lines from melanoma and non–small-cell lung cancer, respectively, were growth inhibited by MEKi with or without BRAFi, but not by BRAFi monotherapy.33 As G469 mutations are the second most frequent BRAF mutation in non–small-cell lung cancer,14 this might be of great interest for the treatment of such patients.
In intermediate activating mutants, or class II mutations, such as the V600 neighboring mutants K601E, L597, and G469A, suboptimal catalytic kinase efficiency has been demonstrated. Differences in activity to high-activity mutations of class I may rely on the bulkiness of the amino acids that occur as substitutes in the activation segments of the protein.16 In these mutations, activation of wild-type C-RAF and the subsequent elevation of ERK activity seem to play an important role in pathogenesis. Reports that elevated C-RAF protein levels are associated with drug resistance to BRAFi in melanoma support this hypothesis.42
Limitations of this study are the retrospective collection of data and the heterogeneity of patients as from 20 different cancer centers worldwide over a time period of more than 8 years. In addition, some mutations are rare, leading to small sample sizes for some of the reported mutations. The locally performed BRAF testing resulted in a variety of genomic sequencing methods with a variance in sensitivity and specificity for BRAF mutation detection; however, all methods used show a sensitivity of more than 90% for the detection of BRAF mutants and can be considered reliable in the diagnosis of BRAF single mutants. For the double BRAF mutations (V600E/K plus another mutation), there is a risk of detection failure on the basis of misleading next-generation sequencing mutation calling software.
To our knowledge, this is to date the largest collection of data on the clinical experience with the treatment of BRAFi and/or MEKi therapy in patients carrying a BRAF mutation other than V600E/K. In summary, as the BRAFi/MEKi drug combination results in less toxicity than either BRAFi or MEKi alone, its use in patients with advanced melanoma with rare V600 BRAF mutations should be considered. High response rates were observed with rare BRAF V600-mutated melanomas but, as previously reported for V600K mutations,6,23 median PFS was shorter compared with that reported for V600E.11,43,44 Non-V600-mutated melanomas do not respond to BRAFi monotherapy and reveal a short median PFS to combination treatment. However, considering the still small sample size for mutations in other locations than V600, results from this study should be interpreted with caution.
ACKNOWLEDGMENT
The authors thank Albrecht Stenzinger (University Hospital Heidelberg) and Petra Nederlof (NKI Amsterdam) for contributions to the BRAF testing of the respective center patients. The authors also thank Novartis Pharma for providing patient treatment histories for three patients from the Combi-MB trial.
Appendix
BRAF Sequencing
As patients from cancer centers around the world were included, sequencing methods to detect BRAF mutations differed, comprising deep sequencing (next generation sequencing [NGS]; 21%, 22/103), high resolution melting (HRM; 9%, 9/103), pyrosequencing (3%; 3/103), real time polymerase chain reaction (PCR; 21%, 22/103), and Sanger sequencing (33%; 34/103; method not specified in 13 subjects). We did not reconfirm the BRAF mutation results from the centers centrally. The capability to activate the MAPK pathway of different BRAF mutants and translocations was determined by and their outcome compared to the current literature.14,15,19
FIG A1.
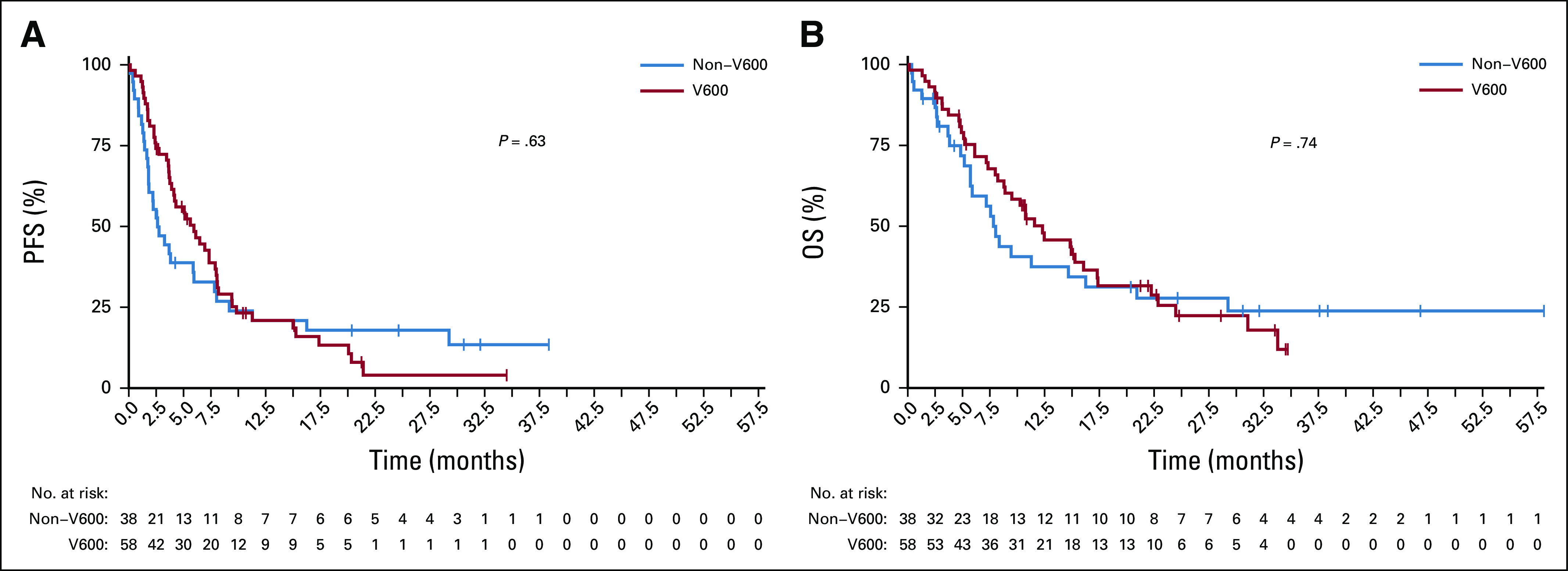
Kaplan Meier estimates for the whole patient group (V600 and non-V600) for (A) progression-free survival (PFS) and (B) overall survival (OS). Median PFS was generally lower in patients with non-V600 melanoma. Both groups showed no significant difference in overall PFS and OS likely due to small numbers of patients. Median PFS was 6.0 months for patients with V600 and 2.6 months for non-V600 mutated melanoma (P = .63); median OS was 12.3 months for V600 and 7.8 months for non-V600 melanomas (P = .74)
FIG A2.
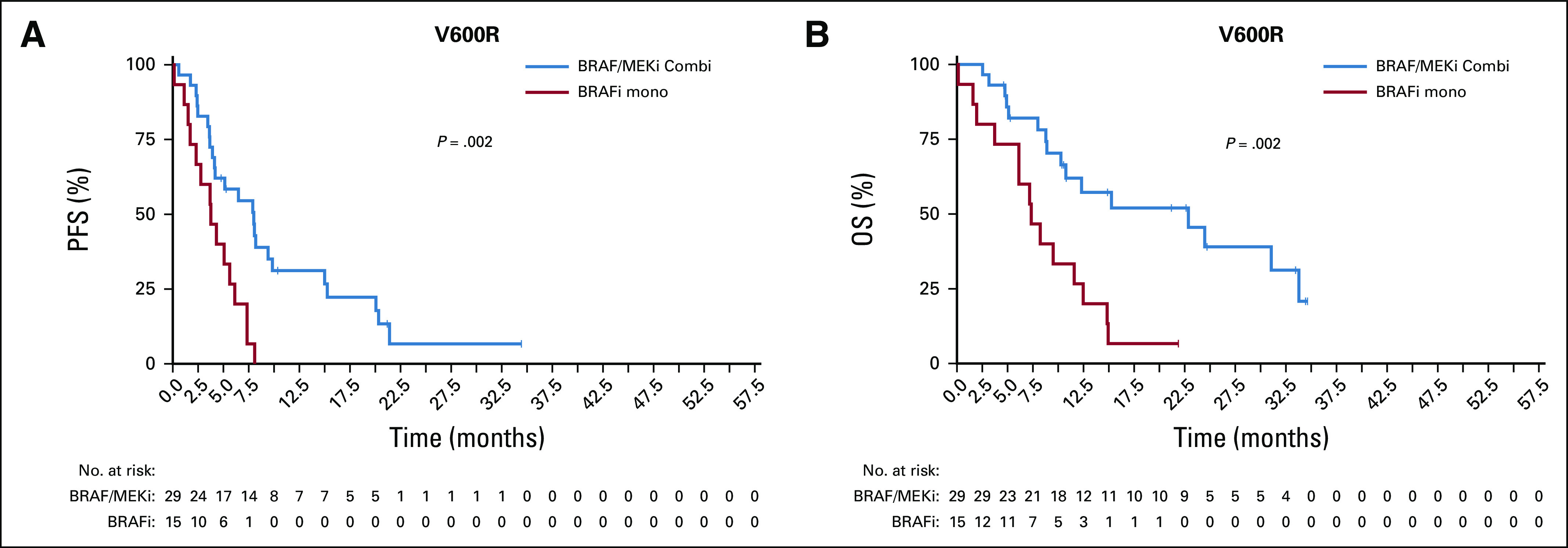
Kaplan Meier estimates for V600R mutated patients with BRAFi/MEKi combination and BRAFi monotherapy for (A) progressiom-free survival (PFS) and (B) overall survival (OS). Results from this cohort resembled the overall V600 group with a significant advantage of the combination therapy compared with monotherapy for PFS (median 8.0 months v 3.8 months; P = .002) and OS (median 22.9 months v 7.3 months, P = .002).
FIG A3.
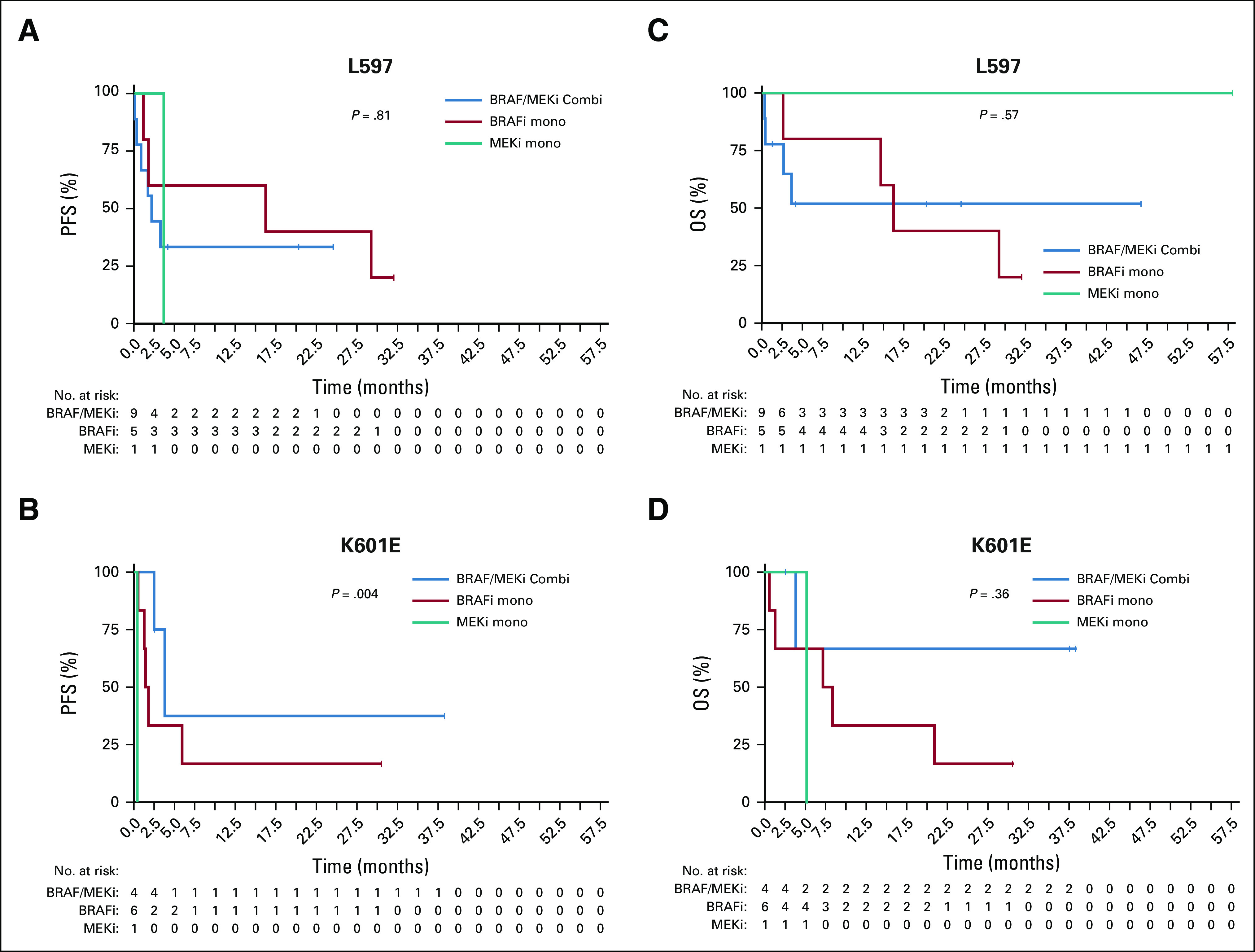
Kaplan Meier estimates for (A, C) L597 and (B, C) K601E mutated patients with BRAFi/MEKi combination and BRAFi or MEKi monotherapy for (A, B) progression-free survival (PFS) and (C, D) overall survival (OS). For non-V600 melanoma the two most frequent mutations were L597P/Q/R/S (15/38, 40%) and K601E (11/38, 29%). No significant differences were detected between the outcome for these unique non-V600 mutations compared with the full non-V600 cohort (PFS: L597 (P = .81); K601E (P = .004); OS: L597 (P = .57); K601E (P = .36).
FIG A4.
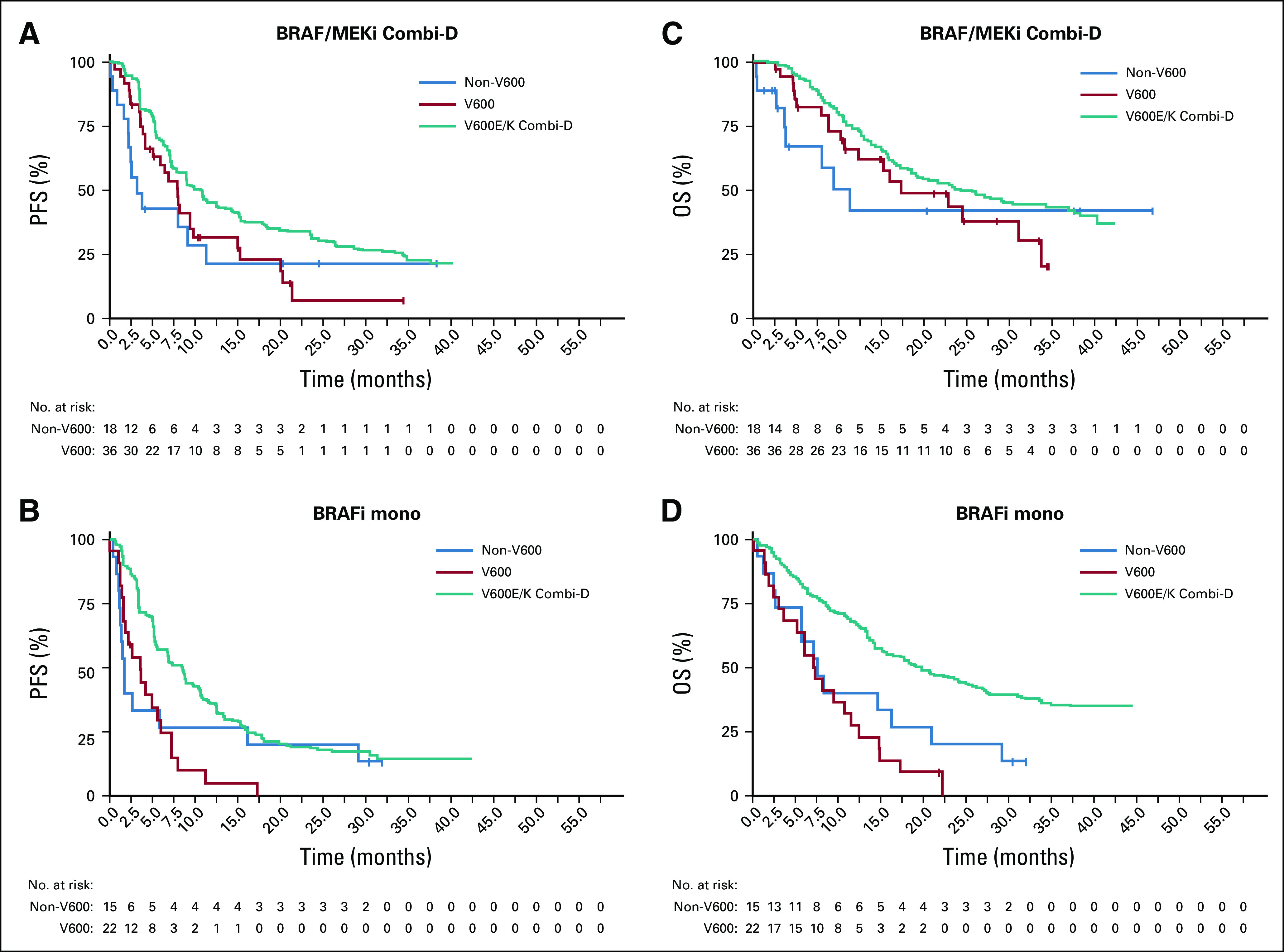
Overlay of Kaplan-Meier curves from this study (V600, red line; non-V600, blue line) and from Combi-D study (Novartis; V600E/K, green line) for orientating comparison of survival outcomes of rare BRAF mutations compared to V600E/K: (A,C) Kaplan Meier estimates for BRAFi/MEKi combination; (B,D) BRAFi monotherapy. Clinical outcome of patients with rare BRAF mutations appeared inferior to patients with BRAF V600E/K mutations. For overall survival (OS) it has to be noted that Combi-D allowed for cross-over to BRAFi/MEKi combination after progression on BRAFi monotherapy. PFS, progression-free survival.
TABLE A1.
Results of Multivariable Cox Regression Analysis V600
TABLE A2.
Results of Multivariable Cox Regression Analysis Non-V600
TABLE A3.
Response Rates and Median PFS/OS Based on the BRAF Genotype (full list)
TABLE A4.
Patient Characteristics for Selected BRAF Genotypes
TABLE A5.
Patient Characteristics in V600 and Non-V600 Patients by Therapy
Footnotes
Part of the data of this study was presented at the ASCO conference in Chicago, June 2018.
AUTHOR CONTRIBUTIONS
Conception and design: Christian Menzer, Alexander Menzies, Christian Blank, Jessica C. Hassel
Administrative support: Christian Menzer, Alexander Menzer, Annette Kopp-Schneider
Provision of study materials or patients: Alexander M. Menzies, Matteo S. Carlino, Emma J. Groen, Astrid A.M. van der Veldt, Frank Meiss, Hans M. Westgeest, Ralf Gutzmer, Friedegund Meier, Lisa Zimmer, Karin Herbschleb, Georgina V. Long, Richard Kefford, Jessica C. Hassel
Collection and assembly of data: Christian Menzer, Alexander M. Menzies, Matteo S. Carlino, Irene Reijers, Emma J. Groen, Thomas Eigentler, Jan Willem B. de Groot, Astrid A.M. van der Veldt, Douglas B. Johnson, Frank Meiss, Max Schlaak, Bastian Schilling, Hans M. Westgeest, Ralf Gutzmer, Claudia Pföhler, Friedegund Meier, Lisa Zimmer, Karijn P.M. Suijkerbuijk, Thomas Haalck, Kai-Martin Thoms, Karin Herbschleb, Georgina V. Long, Richard Kefford, Christian U. Blank, Jessica C. Hassel
Data analysis and interpretation: Christian Menzer, Alexander M. Menzies, Matteo S. Carlino, Jonas Leichsenring, Alexander Menzer, Annette Kopp-Schneider, Georgina V. Long, Richard Kefford, Alexander Enk, Christian U. Blank, Jessica C. Hassel
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Targeted Therapy in Advanced Melanoma With Rare BRAF Mutations
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Christian Menzer
Travel, Accommodations, Expenses: Bristol-Myers Squibb, MSD Oncology, Merck, Amgen, Novartis
Alexander M. Menzies
Consulting or Advisory Role: MSD Oncology, Novartis, Pierre Fabre, Bristol-Myers Squibb, Roche
Matteo S. Carlino
Honoraria: Bristol-Myers Squibb, MSD Oncology, Novartis
Consulting or Advisory Role: Bristol-Myers Squibb, MSD Oncology, Amgen, Novartis, Pierre Fabre, Roche, IDEAYA Biosciences
Thomas Eigentler
Consulting or Advisory Role: Bristol-Myers Squibb, Roche, MSD Oncology, Pierre Fabre, LEO Pharma, Sanofi, Regeneron, Novartis
Speakers' Bureau: Roche, MSD Oncology, Bristol-Myers Squibb, Novartis
Jan Willem B. de Groot
Consulting or Advisory Role: Novartis, Bristol-Myers Squibb, Pierre Fabre, MSD Oncology, Servier
Astrid A.M. van der Veldt
Consulting or Advisory Role: Ipsen (Inst), Roche (Inst), Bristol-Myers Squibb (Inst), Pfizer (Inst), MSD Oncology (Inst), Sanofi (Inst), Pierre Fabre (Inst), Novartis (Inst), Eisai (Inst)
Research Funding: Bayer (Inst)
Travel, Accommodations, Expenses: Roche, MSD Oncology, Novartis, Roche, Bayer
Douglas B. Johnson
Consulting or Advisory Role: Bristol-Myers Squibb, Merck, Novartis, Incyte, Array Biopharma
Research Funding: Incyte, Bristol-Myers Squibb
Patents, Royalties, Other Intellectual Property: Intellectual property and patents pending surrounding use of MHC-II and response to immune therapy
Travel, Accommodations, Expenses: Genentech
Frank Meiss
Honoraria: Bristol-Myers Squibb, Novartis, Pierre Fabre
Consulting or Advisory Role: Bristol-Myers Squibb, Novartis, Pierre Fabre, Roche
Speakers' Bureau: Bristol-Myers Squibb
Travel, Accommodations, Expenses: Novartis, Bristol-Myers Squibb, Pierre Fabre
Max Schlaak
Honoraria: Bristol-Myers Squibb, MSD Oncology, Roche, Pierre Fabre
Consulting or Advisory Role: Novartis, Roche, MSD Oncology, Pierre Fabre
Bastian Schilling
Honoraria: Bristol-Myers Squibb, MSD Oncology, Roche, Incyte, Novartis
Consulting or Advisory Role: Bristol-Myers Squibb, MSD Oncology, Roche, Incyte, Novartis
Research Funding: MSD Oncology (Inst), Pierre Fabre (Inst), Bristol-Myers Squibb (Inst)
Travel, Accommodations, Expenses: Amgen, Novartis, Roche, Bristol-Myers Squibb, Pierre Fabre, MSD Oncology
Hans M. Westgeest
Honoraria: Roche
Travel, Accommodations, Expenses: Ipsen
Ralf Gutzmer
Honoraria: Bristol-Myers Squibb, Merck Sharp & Dohme, Genentech, Novartis, Merck Serono, Almirall Hermal, Amgen, AstraZeneca, Sun Pharma, Pierre Fabre, Sanofi, Regeneron
Consulting or Advisory Role: Bristol-Myers Squibb, Merck Sharp & Dohme, Genentech, Novartis, Almirall Hermal, 4SC, Amgen, Pierre Fabre, Merck Serono, Incyte, Sun Pharma, Takeda, Sanofi
Research Funding: Pfizer (Inst), Novartis (Inst), Johnson & Johnson (Inst), Amgen (Inst), Merck Serono (Inst)
Travel, Accommodations, Expenses: Bristol-Myers Squibb, Roche, Merck Serono, Pierre Fabre, Sun Pharma
Claudia Pföhler
Honoraria: Novartis, Bristol Myers Squibb, Roche, Merck Serono, MSD Oncology, Celgene, AbbVie, LEO Pharma
Consulting or Advisory Role: Novartis, Roche, Merck Serono
Travel, Accommodations, Expenses: Novartis, Bristol-Myers Squibb, Roche, Merck Serono, MSD Oncology, Celgene, AbbVie, LEO Pharma
Friedegund Meier
Honoraria: Roche, Bristol-Myers Squibb, Novartis, MSD Oncology, Amgen, Merck, Sanofi
Consulting or Advisory Role: Roche, Bristol-Myers Squibb, Novartis, MSD Oncology, Amgen, Merck, Sanofi
Research Funding: Novartis
Travel, Accommodations, Expenses: Novartis, Roche, Bristol-Myers Squibb, MSD Oncology, Amgen, Merck, Sanofi
Lisa Zimmer
Honoraria: Merck Sharp & Dohme, Bristol-Myers Squibb, Novartis, Roche, Pierre Fabre
Consulting or Advisory Role: Merck Sharp & Dohme, Roche, Bristol-Myers Squibb, Novartis, Sanofi, Pierre Fabre
Travel, Accommodations, Expenses: Amgen, Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis, Pierre Fabre
Karijn P.M. Suijkerbuijk
Consulting or Advisory Role: Bristol-Myers Squibb (Inst), Novartis (Inst), MSD Oncology (Inst), Pierre Fabre (Inst)
Speakers' Bureau: Roche, Novartis
Travel, Accommodations, Expenses: Roche, MSD Oncology
Thomas Haalck
Consulting or Advisory Role: Bristol-Myers Squibb, Novartis, Roche, MSD Oncology
Travel, Accommodations, Expenses: Bristol-Myers Squibb, Novartis
Kai-Martin Thoms
Honoraria: Bristol-Myers Squibb, Roche, Novartis, MSD Oncology, LEO Pharma, Pierre Fabre, AbbVie, Galderma
Consulting or Advisory Role: Bristol-Myers Squibb, Roche, Novartis, MSD Oncology, LEO Pharma, AbbVie
Travel, Accommodations, Expenses: Bristol-Myers Squibb, Novartis, LEO Pharma, Pierre Fabre
Karin Herbschleb
Honoraria: Roche
Travel, Accommodations, Expenses: Roche
Jonas Leichsenring
Honoraria: AstraZeneca
Expert Testimony: AstraZeneca
Georgina V. Long
Honoraria: Bristol-Myers Squibb, Merck, Pierre Fabre
Consulting or Advisory Role: Bristol-Myers Squibb, Merck, Novartis, Array BioPharma, Pierre Fabre, Aduro Biotech, OncoSec, Roche, Amgen
Richard Kefford
Consulting or Advisory Role: Amgen (Inst), Teva (Inst)
Travel, Accommodations, Expenses: Bristol-Myers Squibb, Amgen
Alexander Enk
Honoraria: MSD Oncology, Bristol-Myers Squibb, Roche, Biotest, Janssen-Cilag, Novartis, Celgene, Galderma
Consulting or Advisory Role: Bristol-Myers Squibb, MSD Oncology, Janssen-Cilag, Biotest, Novartis
Travel, Accommodations, Expenses: Bristol-Myers Squibb, MSD Oncology, Novartis, Biotest, Roche
Christian U. Blank
Consulting or Advisory Role: Genentech (Inst), MSD Oncology (Inst), Bristol-Myers Squibb (Inst), Novartis (Inst), GlaxoSmithKline (Inst), Pfizer (Inst), AstraZeneca (Inst), Eli Lilly (Inst), Pierre Fabre (Inst), GenMab (Inst)
Research Funding: Bristol-Myers Squibb (Inst), Novartis (Inst), NanoString Technologies (Inst)
Travel, Accommodations, Expenses: Bristol-Myers Squibb
Jessica C. Hassel
Honoraria: Bristol-Myers Squibb, MSD Oncology, Roche, Novartis, Pfizer, Sanofi
Consulting or Advisory Role: MSD Oncology, Pierre Fabre
Research Funding: Bristol-Myers Squibb (Inst), Novartis (Inst), Roche (Inst), Immunocore (Inst), BioNTech (Inst), Amgen (Inst), 4SC (Inst), Philogen (Inst), Idera (Inst)
Travel, Accommodations, Expenses: Pierre Fabre
No other potential conflicts of interest were reported.
REFERENCES
- 1.Curtin JA, Fridlyand J, Kageshita T, et al. : Distinct sets of genetic alterations in melanoma. N Engl J Med 353:2135-2147, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Cancer Genome Atlas Network : Genomic classification of cutaneous melanoma. Cell 161:1681-1696, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dummer R, Ascierto PA, Gogas HJ, et al. : Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol 19:603-615, 2018 [DOI] [PubMed] [Google Scholar]
- 4.Long GV, Stroyakovskiy D, Gogas H, et al. : Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: A multicentre, double-blind, phase 3 randomised controlled trial. Lancet 386:444-451, 2015 [DOI] [PubMed] [Google Scholar]
- 5.Leichsenring J, Stögbauer F, Volckmar AL, et al. : Genetic profiling of melanoma in routine diagnostics: Assay performance and molecular characteristics in a consecutive series of 274 cases. Pathology 50:703-710, 2018 [DOI] [PubMed] [Google Scholar]
- 6.Menzies AM, Haydu LE, Visintin L, et al. : Distinguishing clinicopathologic features of patients with V600E and V600K BRAF-mutant metastatic melanoma. Clin Cancer Res 18:3242-3249, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Lee JH, Choi JW, Kim YS: Frequencies of BRAF and NRAS mutations are different in histological types and sites of origin of cutaneous melanoma: A meta-analysis. Br J Dermatol 164:776-784, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Heinzerling L, Kühnapfel S, Meckbach D, et al. : Rare BRAF mutations in melanoma patients: Implications for molecular testing in clinical practice. Br J Cancer 108:2164-2171, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lyle M, Haydu LE, Menzies AM, et al. : The molecular profile of metastatic melanoma in Australia. Pathology 48:188-193, 2016 [DOI] [PubMed] [Google Scholar]
- 10.Long GV, Menzies AM, Nagrial AM, et al. : Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol 29:1239-1246, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Larkin J, Ascierto PA, Dréno B, et al. : Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med 371:1867-1876, 2014 [DOI] [PubMed] [Google Scholar]
- 12.Long GV, Flaherty KT, Stroyakovskiy D, et al. : Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: Long-term survival and safety analysis of a phase 3 study. Ann Oncol 28:1631-1639, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ascierto PA, McArthur GA, Dréno B, et al. : Cobimetinib combined with vemurafenib in advanced BRAF(V600)-mutant melanoma (coBRIM): Updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol 17:1248-1260, 2016 [DOI] [PubMed] [Google Scholar]
- 14.Dagogo-Jack I, Martinez P, Yeap BY, et al. : Impact of BRAF mutation class on disease characteristics and clinical outcomes in BRAF-mutant lung cancer. Clin Cancer Res 25:158-165, 2019 [DOI] [PubMed] [Google Scholar]
- 15.Heidorn SJ, Milagre C, Whittaker S, et al. : Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell 140:209-221, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wan PT, Garnett MJ, Roe SM, et al. : Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell 116:855-867, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Lang N, Weisser A, Enk A, et al. : Vemurafenib therapy for stage IV melanoma with V600G-mutation. J Dtsch Dermatol Ges 11:1198-1199, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Shiuan E, Beckermann KE, Ozgun A, et al. : Thrombocytopenia in patients with melanoma receiving immune checkpoint inhibitor therapy. J Immunother Cancer 5:8, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoefflin R, Geißler A-L, Fritsch R, et al. : Personalized clinical decision making through implementation of a molecular tumor board: A German single-center experience. JCO Precis Oncol 10.1200/PO.18.00105, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gershenwald JE, Scolyer RA: Melanoma staging: American Joint Committee on Cancer (AJCC) 8th edition and beyond. Ann Surg Oncol 25:2105-2110, 2018 [DOI] [PubMed] [Google Scholar]
- 21.Austin PC: The use of propensity score methods with survival or time-to-event outcomes: Reporting measures of effect similar to those used in randomized experiments. Stat Med 33:1242-1258, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schadendorf D, Long GV, Stroiakovski D, et al. : Three-year pooled analysis of factors associated with clinical outcomes across dabrafenib and trametinib combination therapy phase 3 randomised trials. Eur J Cancer 82:45-55, 2017 [DOI] [PubMed] [Google Scholar]
- 23.Pires da Silva I, Wang KYX, Wilmott JS, et al. : Distinct molecular profiles and immunotherapy treatment outcomes of V600E and V600K BRAF-mutant Q:9 melanoma. Clin Cancer Res 25:1272-1279, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richtig G, Hoeller C, Kashofer K, et al. : Beyond the BRAFV600E hotspot: Biology and clinical implications of rare BRAF gene mutations in melanoma patients. Br J Dermatol 177:936-944, 2017 [DOI] [PubMed] [Google Scholar]
- 25.Klein O, Clements A, Menzies AM, et al. : BRAF inhibitor activity in V600R metastatic melanoma. Eur J Cancer 49:1073-1079, 2013 [DOI] [PubMed] [Google Scholar]
- 26.Casadevall D, Vidal J, Gallardo F, et al. : Dabrafenib in an elderly patient with metastatic melanoma and BRAF V600R mutation: A case report. J Med Case Reports 10:158, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van den Brom RR, de Vries EG, Schröder CP, et al. : Effect of vemurafenib on a V600R melanoma brain metastasis. Eur J Cancer 49:1795-1796, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Kim KB, Kefford R, Pavlick AC, et al. : Phase II study of the MEK1/MEK2 inhibitor trametinib in patients with metastatic BRAF-mutant cutaneous melanoma previously treated with or without a BRAF inhibitor. J Clin Oncol 31:482-489, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Genentech : Zelboraf (vemurafenib) prescribing information. http://www.gene.com/download/pdf/zelboraf_prescribing.pdf
- 30.Cheng L, Lopez-Beltran A, Massari F, et al. : Molecular testing for BRAF mutations to inform melanoma treatment decisions: A move toward precision medicine. Mod Pathol 31:24-38, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cardarella S, Ogino A, Nishino M, et al. : Clinical, pathologic, and biologic features associated with BRAF mutations in non-small cell lung cancer. Clin Cancer Res 19:4532-4540, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poulikakos PI, Zhang C, Bollag G, et al. : RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature 464:427-430, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yao Z, Yaeger R, Rodrik-Outmezguine VS, et al. : Tumours with class 3 BRAF mutants are sensitive to the inhibition of activated RAS. Nature 548:234-238, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zehir A, Benayed R, Shah RH, et al. : Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med 23:703-713, 2017. [Erratum: Nat Med 23:1004, 2017] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dahlman KB, Xia J, Hutchinson K, et al. : BRAF(L597) mutations in melanoma are associated with sensitivity to MEK inhibitors. Cancer Discov 2:791-797, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bowyer SE, Rao AD, Lyle M, et al. : Activity of trametinib in K601E and L597Q BRAF mutation-positive metastatic melanoma. Melanoma Res 24:504-508, 2014 [DOI] [PubMed] [Google Scholar]
- 37.Voskoboynik M, Mar V, Mailer S, et al. : Clinicopathological characteristics associated with BRAF(K601E) and BRAF(L597) mutations in melanoma. Pigment Cell Melanoma Res 29:222-228, 2016 [DOI] [PubMed] [Google Scholar]
- 38.Bahadoran P, Allegra M, Le Duff F, et al. : Major clinical response to a BRAF inhibitor in a patient with a BRAF L597R-mutated melanoma. J Clin Oncol 31:e324-e326, 2013 [DOI] [PubMed] [Google Scholar]
- 39.Kim DW, Haydu LE, Joon AY, et al. : Clinicopathological features and clinical outcomes associated with TP53 and BRAFNon-V600 mutations in cutaneous melanoma patients. Cancer 123:1372-1381, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dankner M, Lajoie M, Moldoveanu D, et al. : Dual MAPK inhibition is an effective therapeutic strategy for a subset of class II BRAF mutant melanomas. Clin Cancer Res 24:6483-6494, 2018 [DOI] [PubMed] [Google Scholar]
- 41.Falchook GS, Long GV, Kurzrock R, et al. : Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: A phase 1 dose-escalation trial. Lancet 379:1893-1901, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cope N, Candelora C, Wong K, et al. : Mechanism of BRAF activation through biochemical characterization of the recombinant full-length protein. ChemBioChem 19:1988-1997, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Long GV, Stroyakovskiy D, Gogas H, et al. : Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med 371:1877-1888, 2014 [DOI] [PubMed] [Google Scholar]
- 44.Robert C, Karaszewska B, Schachter J, et al. : Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med 372:30-39, 2015 [DOI] [PubMed] [Google Scholar]