Abstract
PURPOSE
A randomized, double-blind, phase III study of idelalisib (IDELA) plus rituximab versus placebo plus rituximab in patients with relapsed chronic lymphocytic leukemia (CLL) was terminated early because of superior efficacy of the IDELA-plus-rituximab (IDELA/R) arm. Patients in either arm could then enroll in an extension study to receive IDELA monotherapy. Here, we report the long-term efficacy and safety data for IDELA-treated patients across the primary and extension studies.
PATIENTS AND METHODS
Patients were randomly assigned to receive rituximab in combination with either IDELA 150 mg twice daily (IDELA/R; n = 110) or placebo (placebo/R; n = 110). Key end points were progression-free survival (PFS), overall response rate (ORR), overall survival (OS), and safety.
RESULTS
The long-term efficacy and safety of treatment with IDELA was assessed in 110 patients who received at least one dose of IDELA in the primary study, 75 of whom enrolled in the extension study. The IDELA/R-to-IDELA group had a median PFS of 20.3 months (95% CI, 17.3 to 26.3 months) after a median follow-up time of 18 months (range, 0.3 to 67.6 months). The ORR was 85.5% (94 of 110 patients; n = 1 complete response). The median OS was 40.6 months (95% CI, 28.5 to 57.3 months) and 34.6 months (95% CI, 16.0 months to not reached) for patients randomly assigned to the IDELA/R and placebo/R groups, respectively. Prolonged exposure to IDELA increased the incidence of all-grade, grade 2, and grade 3 or greater diarrhea (46.4%, 17.3%, and 16.4%, respectively), all-grade and grade 3 or greater colitis (10.9% and 8.2%, respectively) and all-grade and grade 3 or greater pneumonitis (10.0% and 6.4%, respectively) but did not increase the incidence of elevated hepatic aminotransferases.
CONCLUSION
IDELA improved PFS and OS compared with rituximab alone in patients with relapsed CLL. Long-term IDELA was effective and had an expected safety profile. No new IDELA-related adverse events were identified with longer exposure.
INTRODUCTION
Chronic lymphocytic leukemia (CLL) is most prevalent in patients age 65 years or older, who often have comorbidities that include decreased renal or bone marrow function. 1-4 Treating such patients with chemotherapy is challenging because of an increased risk of toxicity that may complicate disease management. 2-4
Idelalisib (IDELA) is a small molecule that targets the delta isoform of phosphoinositol 3-kinase (PI3Kδ) and inhibits PI3Kδ-dependent signaling, which leads to decreased activity of the AKT and mammalian target of rapamycin pathways and reduced survival of malignant B cells. 5,6 A randomized, double-blind, placebo-controlled phase III study evaluated the efficacy and safety of rituximab in combination with IDELA or placebo in patients with relapsed and refractory CLL who had notable comorbidities (cumulative illness rating scale score > 6, renal insufficiency, and/or poor bone marrow reserve) that precluded use of standard chemoimmunotherapy. 7 In August 2013, at the first prespecified interim analysis, the study was terminated because of the superior efficacy of IDELA plus rituximab (IDELA/R). 7 These results led to approval of IDELA in combination with rituximab for the treatment of patients with relapsed CLL. 8,9
Upon primary study termination and unblinding, patients could transition to the extension study to receive open-label IDELA monotherapy. The focus of this report is the long-term efficacy and safety of IDELA in patients who received IDELA/R in the primary study and continued IDELA treatment in the extension study (IDELA/R-to-IDELA arm). In addition, we provide the final results from the primary, double-blinded study as an update to the previously published article. 7
PATIENTS AND METHODS
Study Design and Treatments
The primary study was a randomized, two-arm, double-blind, placebo-controlled, phase III study (ClinicalTrials.gov identifier: NCT01539512). Random assignment (1:1) was stratified by the presence of deletion 17p (del[17p]) and/or TP53 mutation status and immunoglobulin heavy-chain variable region gene (IGHV) mutation status. 7 Rituximab was administered intravenously at a dose of 375 mg/m2 on day 1, week 0, and at 500 mg/m2 on day 1 of weeks 2, 4, 6, 8, 12, 16, and 20 for a total of eight infusions. IDELA 150 mg twice daily was administered orally. 7 IDELA or placebo were given until progressive disease (PD), drug-related toxicity, or study discontinuation occurred.
The extension study (ClinicalTrials.gov identifier: NCT01539291) was designed as a blinded study in which patients who experienced PD on placebo/R could receive oral IDELA 150 mg and matched placebo (two tablets) twice daily, whereas patients who experienced PD not as a result of transformation on IDELA/R received dose-intensified treatment with IDELA 300 mg (two tablets) twice daily. After termination of the primary study, the extension study was unblinded and patients who remained on the primary study transitioned to the extension study to receive open-label IDELA monotherapy 150 mg twice daily (Fig 1A).
FIG 1.
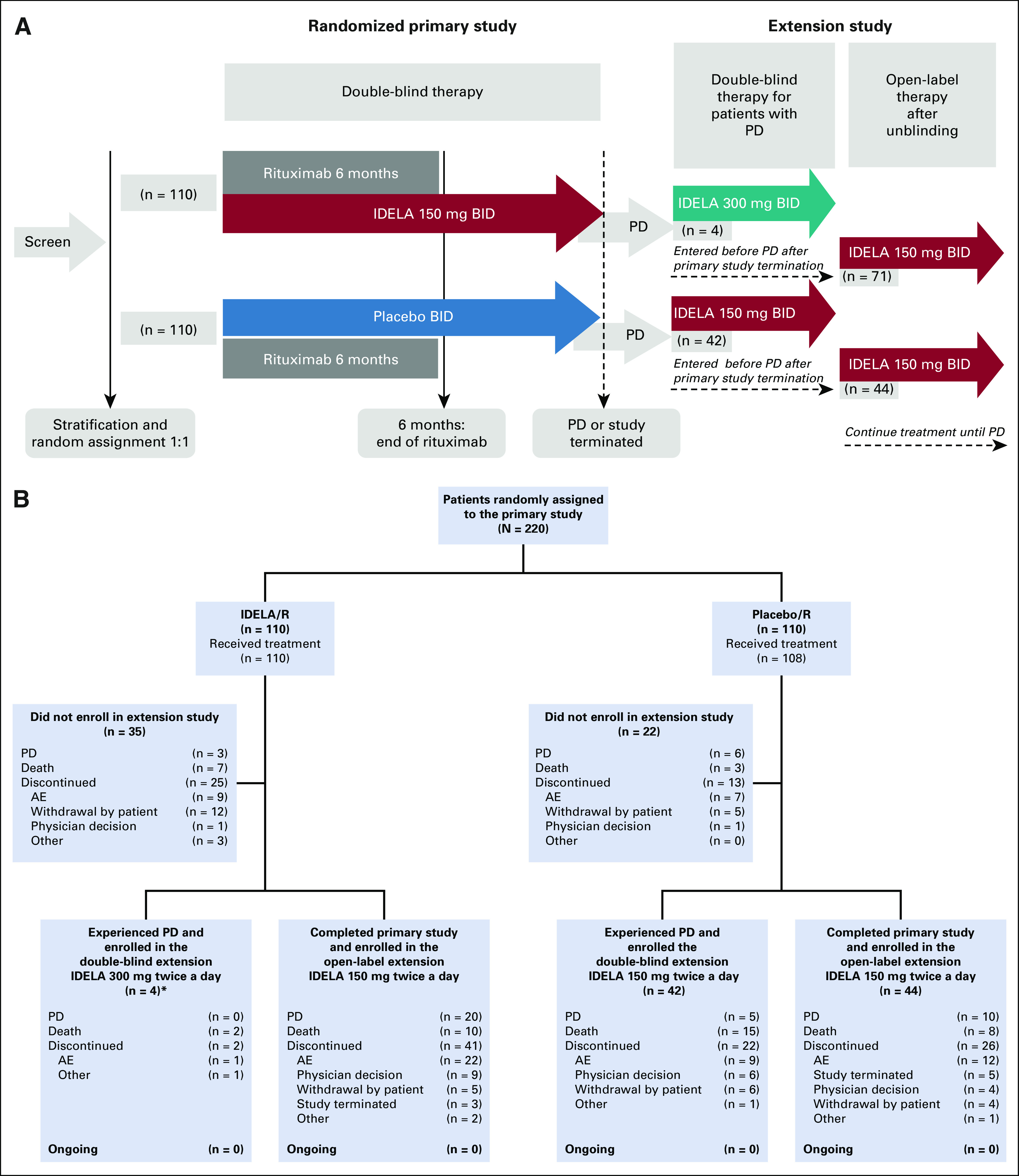
(A) Study flow. (B) Patient flow. (*)For all analyses in this report, the four patients who experienced PD during treatment with IDELA/R and received single-agent IDELA 300 mg twice a day in the double-blind part of the extension study were pooled together with patients treated with IDELA/R who did not experience PD, and, at study termination, transitioned to open-label IDELA 150 mg twice a day in the extension study. AE, adverse event; IDELA, idelalisib; PD, progressive disease; R, rituximab.
All study protocols were approved by the institutional review board at each participating center. Studies were conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization guidelines for Good Clinical Practice.
Patient Eligibility
Detailed patient eligibility criteria for the primary study have been reported previously. 7 Briefly, eligible patients had recurrent, previously treated CLL; experienced PD within 24 months of completion of the last line of therapy; and were unable to receive cytotoxic therapies on the basis of cumulative illness rating scale scores greater than 6 points, decreased renal function, or cumulative marrow toxicity from prior therapy. Prior therapies must have included either a CD20 antibody–based regimen or at least two previous cytotoxic regimens. 7 Patients who either experienced centrally confirmed PD during the study or were actively participating at the time of primary study termination could transition to a companion extension study. Patients with confirmed malignant transformation from CLL to an aggressive lymphoma during the primary study were excluded.
Study Objectives and Assessments
The primary end point of the randomized trial was progression-free survival (PFS). Key secondary objectives were overall response rate (ORR), overall survival (OS), efficacy in subgroups, and safety. 7 The objectives of the extension study were to evaluate the long-term efficacy and safety of IDELA maintenance. Thus, in the extension study, no formal comparisons of outcomes between groups were made.
Safety and treatment response assessments were performed as previously described. 7 Determination of CLL response and progression was based on International Workshop on Chronic Lymphocytic Leukemia 2008 criteria, 10 modified to reflect current recommendations that pertain to novel target agents in treatment of CLL. 11 Disease progression was adjudicated by a blinded independent review committee. Adverse events (AEs) were graded using the Common Terminology Criteria for Adverse Events, version 4.03. Diarrhea and colitis were diagnosed by the investigator according to the Common Terminology Criteria for Adverse Events. The use of colonoscopy and/or biopsy was performed according to investigator discretion.
Statistical Analysis
Results from the randomized study were presented for the two treatment arms (IDELA/R and placebo/R) using the intent-to-treat (ITT) analysis set, which included all patients randomly assigned in the primary study. The safety analysis set included data from patients who received at least one dose of study treatment; treatment assignments were designated according to the actual treatment received. The full analysis set was used for the long-term efficacy and safety analyses of treatment with IDELA and included all patients in the ITT set who received at least one dose of IDELA, including patients who completed the primary study but decided not to enroll in the open extension study, as well as patients who received IDELA/R, with or without PD, who transitioned to the extension study. OS was analyzed using the ITT set, including data from both primary and extension studies for all randomly assigned patients (IDELA/R and placebo/R) without adjustment for the crossover.
Time-to-event end points were summarized using Kaplan-Meier methods. For Kaplan-Meier estimates, 95% CIs were calculated based on variances estimated using the Greenwood formula. Hazard ratios (HR) and 95% CIs were calculated, when appropriate, using the Cox proportional hazard model, and P values were calculated from the log-rank test. ORR were summarized using frequencies and percentages. Odds ratios and the corresponding 95% CI were presented for the between-arm comparisons.
Multivariable analyses were performed for PFS, ORR, and OS in the IDELA/R-to-IDELA group to explore the influences of several baseline variables. Stepwise regression method was used, and P was .2 for the entry level and .1 for the exit level.
RESULTS
Baseline Characteristics, Demographics, and Disposition of the ITT Population
As reported previously for the primary study, 7 patient demographic and baseline characteristics were balanced across treatment arms. Baseline patient demographics and characteristics, along with the most common prior therapies, stratified by whether the patients enrolled in the extension study, are in the Data Supplement. Overall, 65% of patients were men, and 90% were white; the median age was 71 years (range, 47 to 92 years). Patients received a median of three prior lines of therapy and had median time from diagnosis to study entry of approximately 8.5 years (range, 0.6 to 26.6 years). Either del(17p) or TP53 gene mutation was present at screening in 43.2% of patients and 83.6% of patients had unmutated IGHV. The most common prior regimens included bendamustine with rituximab, fludarabine with cyclophosphamide and rituximab, and rituximab alone.
A total of 220 patients were randomly assigned to IDELA/R or placebo/R (n = 110 in each group; Fig 1B) from May 2012 to August 2013. 7 In the IDELA/R arm, 14 patients met the primary end point of PD or death; four of these patients transitioned to the blinded part of the extension study to receive IDELA 300 mg twice daily. Twenty-five patients discontinued the primary study, mostly because of AEs (n = 9) or withdrawal by patient (n = 12). The remaining 71 patients without PD completed the primary study and enrolled in the open-label part of the extension study (Figs 1A and 1B).
Overall, 161 patients transitioned from the primary study to the extension study (n = 75 patients from the IDELA/R arm and n = 86 from the placebo/R arm; Fig 1B). Among the four patients with PD from the IDELA/R arm who received IDELA 300 mg twice daily, two patients died (pneumonia, underlying CLL disease) and two discontinued the study (diarrhea, other reason). Of 71 patients without PD who continued IDELA in the open-label extension study, 10 patients died; 20 experienced PD; and 41 discontinued the study, primarily as a result of AEs (n = 22; Fig 1B). As of August 16, 2018, no patients remained in the extension study.
Final Efficacy Results From the Primary Study
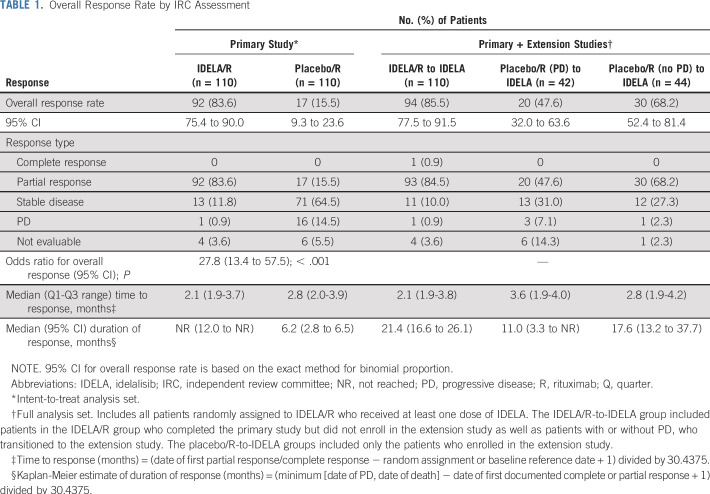
Primary study results from the final data cutoff of October 15, 2014, are presented in Table 1 and Figure 2A. During the primary study, one patient in the IDELA/R group experienced Richter transformation, which led to study discontinuation.
TABLE 1.
Overall Response Rate by IRC Assessment
FIG 2.
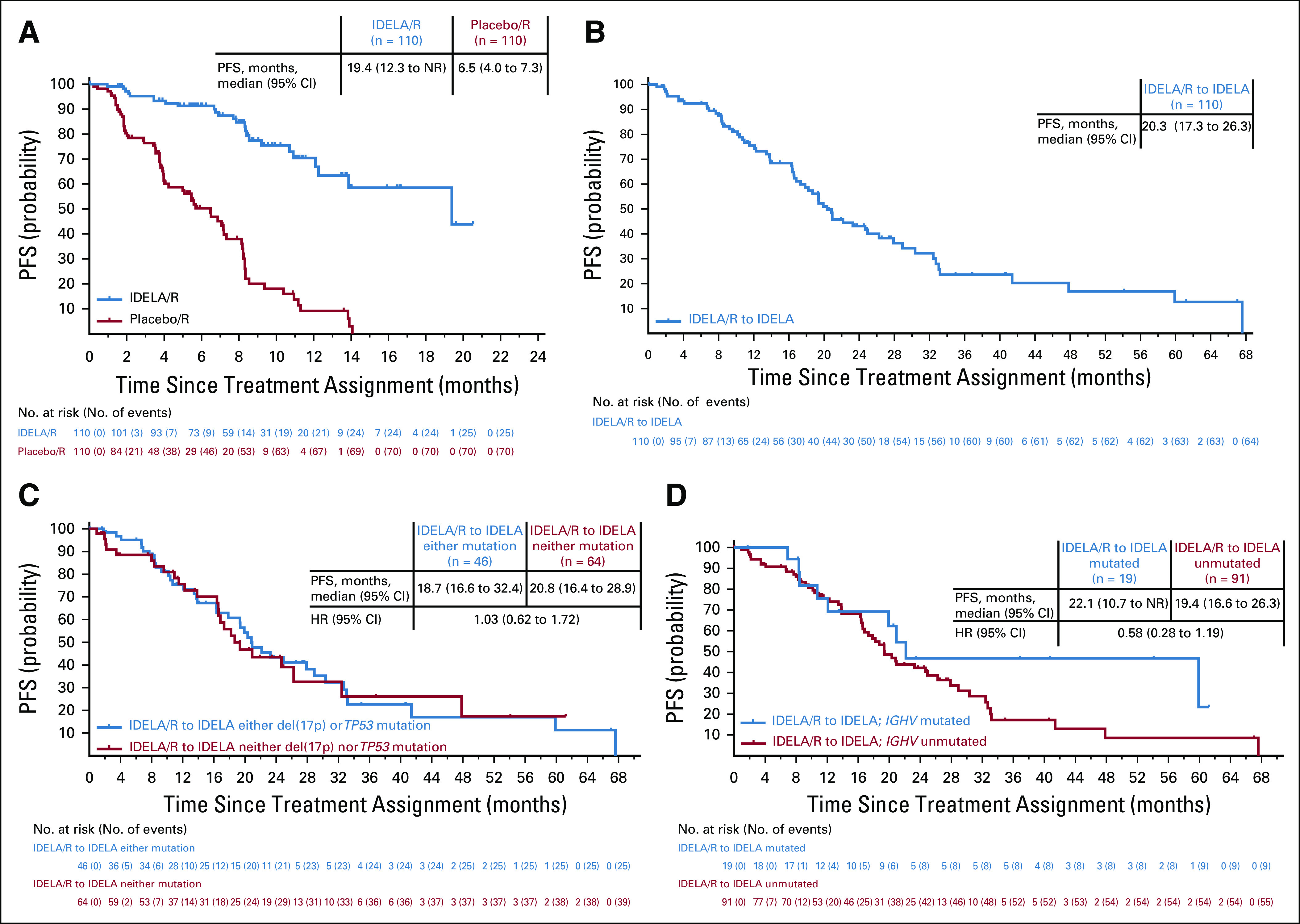
Progression-free survival (PFS) for (A) placebo/R and IDELA/R (intent-to-treat [ITT] population, primary study), and patients were censored at the time of transition to open-label IDELA; (B) IDELA/R-to-IDELA (full analysis set (FAS) population, primary and extension studies); (C) the IDELA/R-to-IDELA arm stratified by presence or absence of del(17p)/TP53 mutation (either/or and neither/nor; FAS); (D) the IDELA/R-to-IDELA arm stratified by presence or absence of IGHV mutated versus unmutated status (FAS). IDELA/R-to-IDELA included all patients who received IDELA/R in the primary randomized study, including patients who did not enroll in open extension, as well as patients who experienced progressive disease and transitioned to the double-blind extension study and patients without PD who completed the primary study and enrolled in the open-label extension study. The ITT population included all randomly assigned patients with treatment assignments designated according to random assignment in the primary study. The FAS population included all patients in the ITT set who received at least one dose of idelalisib, with treatment assignments designated according to random assignment in the primary study. HR, hazard ratio; IDELA, idelalisib; NR, not reached; R, rituximab.
Long-Term Efficacy of IDELA
The cumulative median follow-up for PFS in the IDELA/R-to-IDELA group was 18 months (range, 0.3 to 67.6 months), and the median Kaplan-Meier–determined PFS was 20.3 months (95% CI, 17.3 to 26.3 months; Fig 2B), as of the August 16, 2018, data cutoff. PFS rates for patients in the IDELA/R-to-IDELA arm were 92.4% and 76.7% at 24 and 48 weeks, respectively. The median PFS was 20.8 months (95% CI, 16.4 to 28.9 months) in patients with neither del(17p) nor TP53 mutation and was 18.7 months (95% CI, 16.6 to 32.4 months) in patients with either del(17p) or TP53 mutation (unadjusted HR, 1.03; 95% CI, 0.62 to 1.72; P = .9012 per unstratified log-rank test; Fig 2C). The median PFS was 22.1 months versus 19.4 months in patients with IGHV mutated versus unmutated statuses (unadjusted HR, 0.58; 95% CI, 0.28 to 1.19; P = .1327 per unstratified log-rank test; Fig 2D). For comparison, this analysis performed in patients treated with rituximab alone in the primary study revealed that the presence of del(17p) and TP53 mutation was associated with adverse outcomes, including a median PFS of 4.0 months (95% CI, 3.7 to 5.7 months) versus a median PFS of 8.1 months (95% CI, 5.1 to 8.2 months) for patients with neither genetic aberration (Data Supplement). In the same patient group, the median PFS was longer in patients with mutated IGHV (8.5 months) than in patients with unmutated IGHV (5.6 months). No patients in the IDELA/R-to-IDELA group experienced documented, confirmed Richter transformation during the extension study.
The ORR for patients in the IDELA/R-to-IDELA arm was 85.5% (95% CI, 77.5% to 91.5%) and there was one complete response (Table 1). The median duration of response was 21.4 months (95% CI, 16.6 to 26.1 months).
The median OS were 40.6 (95% CI, 28.5 to 57.3 months) and 34.6 months (95% CI, 16.0 months to not reached) for patients randomly assigned to the IDELA/R and placebo/R groups, respectively. The adjusted HR for OS was 0.8 (95% CI, 0.5 to 1.1) and favored IDELA/R treatment compared with placebo/R (P = .1343 per stratified log-rank test; Fig 3A). The survival rates at 12 and 24 months were 89.3% and 69.8% in the IDELA/R arm, respectively, compared with 68.1% and 51.5% in the placebo/R arm, respectively. Treatment with IDELA/R significantly prolonged survival in patients with either del(17p) or TP53 mutations compared with those treated with placebo/R (HR, 0.59; 95% CI, 0.35 to 1.01; P = .0504; Figs 3B and 3C). As of August 16, 2018, according to the OS analysis, 106 patients died; 50 (45.5%) were in the IDELA/R arm, and 56 (50.9%) were in the placebo/R arm. The main causes of death were largely consistent with advanced CLL and the underlying frailty, age, and poor prognosis of the population and AEs.
FIG 3.
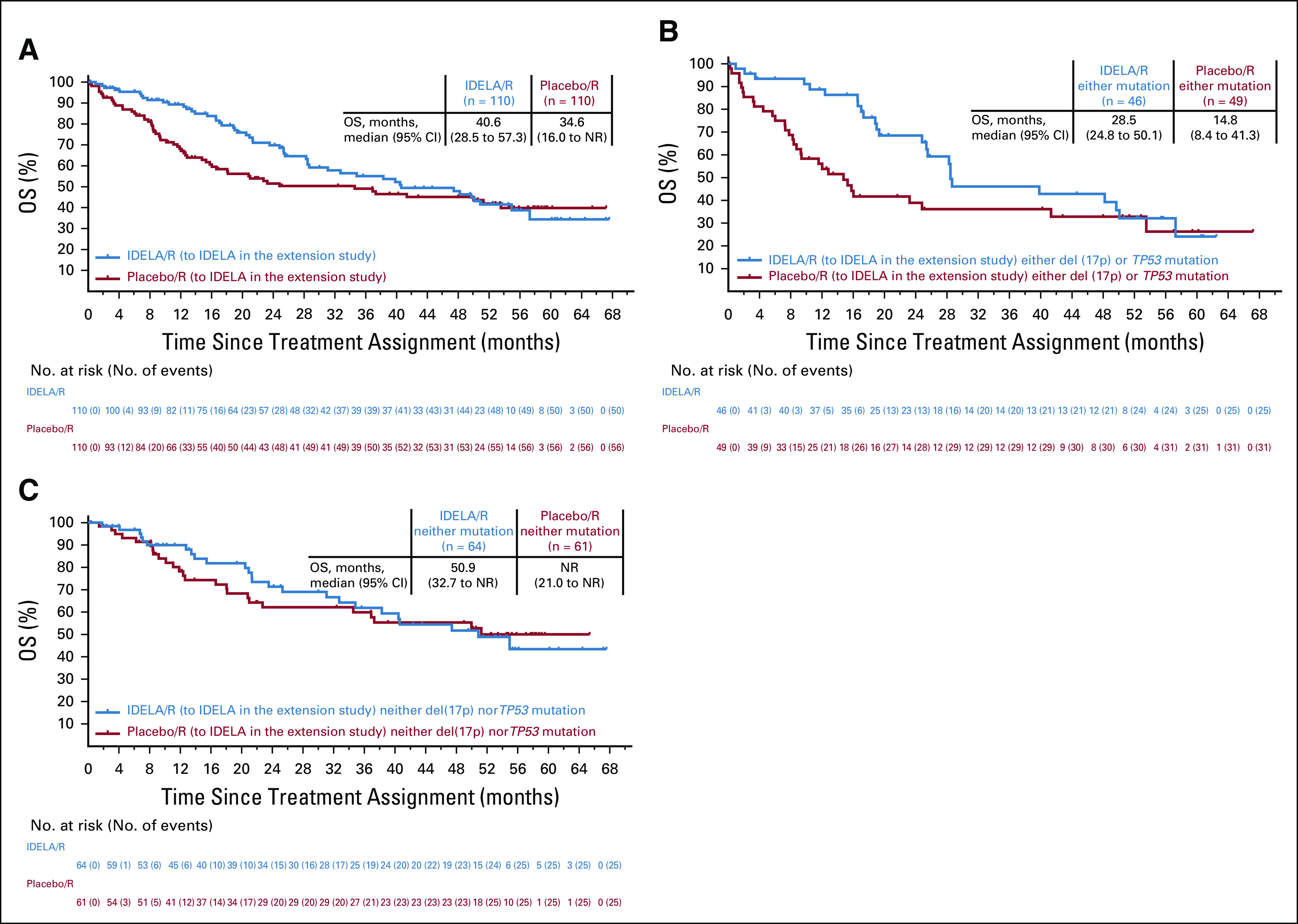
Overall survival (OS) in (A) the intent-to-treat population with treatment assignments according to initial random assignment; (B) patients with either del(17p) or TP53 mutation; (C) patients with neither del(17p) nor TP53 mutation. Insets show median (95% Cl) OS for each treatment group. IDELA, idelalisib; NR, not reached; R, rituximab.
Multivariable analyses revealed statistically significant improvement in clinical outcomes for patients in the IDELA/R-to-IDELA group who were age 70 years or older (ORR), had earlier tumor stages per Binet staging (PFS and OS), were men, had mutated IGHV, or had received less than three prior therapies (OS; Data Supplement).
Efficacy of IDELA Monotherapy in Patients Who Initially Received Placebo/R
For patients randomly assigned to placebo/R in the primary study who transitioned to IDELA monotherapy in the extension study, the median PFS during IDELA treatment for those with (n = 42) and without PD (n = 44) was 6.9 months (95% CI, 4.1 to 10.7 months) and 16.2 months (95% CI, 8.8 to 26.2 months), respectively (Data Supplement). The ORR during the extension study was 47.6% (95% CI, 32.0% to 63.6%) for those with PD and was 68.2% (95% CI, 52.4% to 81.5%) for those without PD; all were partial responses (Table 1). For the 42 patients who experienced PD during the primary study, the median PFS during the primary study (while receiving placebo/R) was 3.8 months (95% CI, 2.9 to 5.7 months), and the ORR was 7.1% (95% CI, 1.5% to 19.5%). One patient randomly assigned to placebo/R developed Richter transformation after the transition to the extension study (IDELA 150 mg treatment). The OS data for patients in the placebo/R arm who did or did not enroll in the extension study are shown in the Data Supplement.
Safety
The safety analyses have been compared between short-term exposure to IDELA in the primary study (median duration of exposure, 8.1 months; quarter 1 to quarter 3 range, 5.6 to 11.1 months) and prolonged exposure to IDELA across the primary and extension studies (median duration of response, 16.2 months; quarter 1 to quarter 3 range, 8.2 to 25.8 months). The results have not been adjusted for the length of observation. The numbers of patients still receiving IDELA after 1 and 2 years from the start of the randomized study were 64 (58.2%) and 32 (29.1%), respectively.
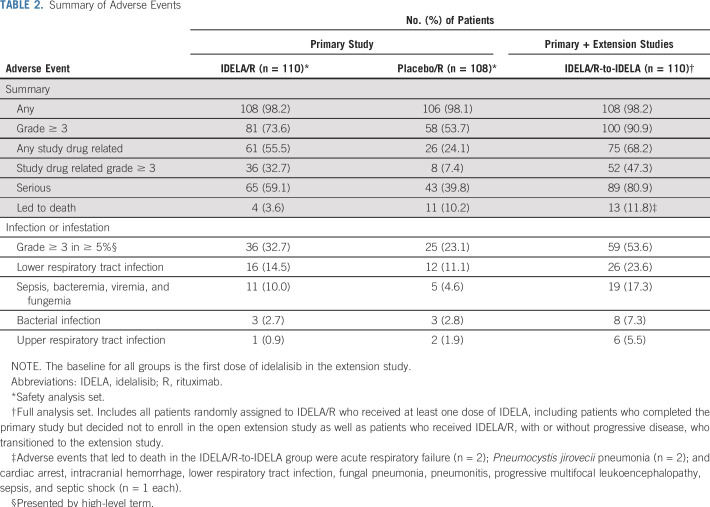
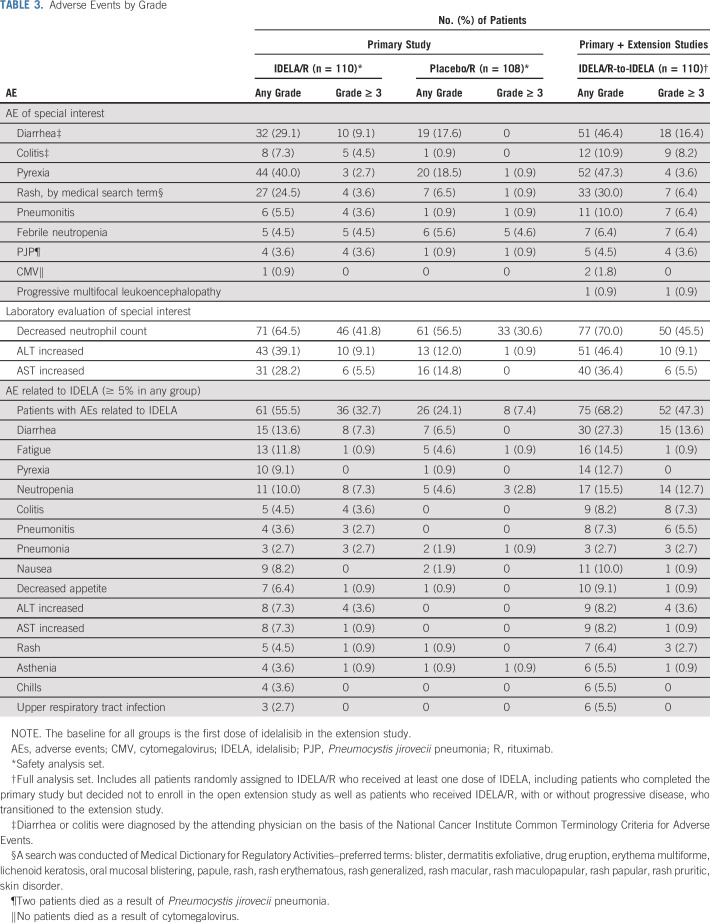
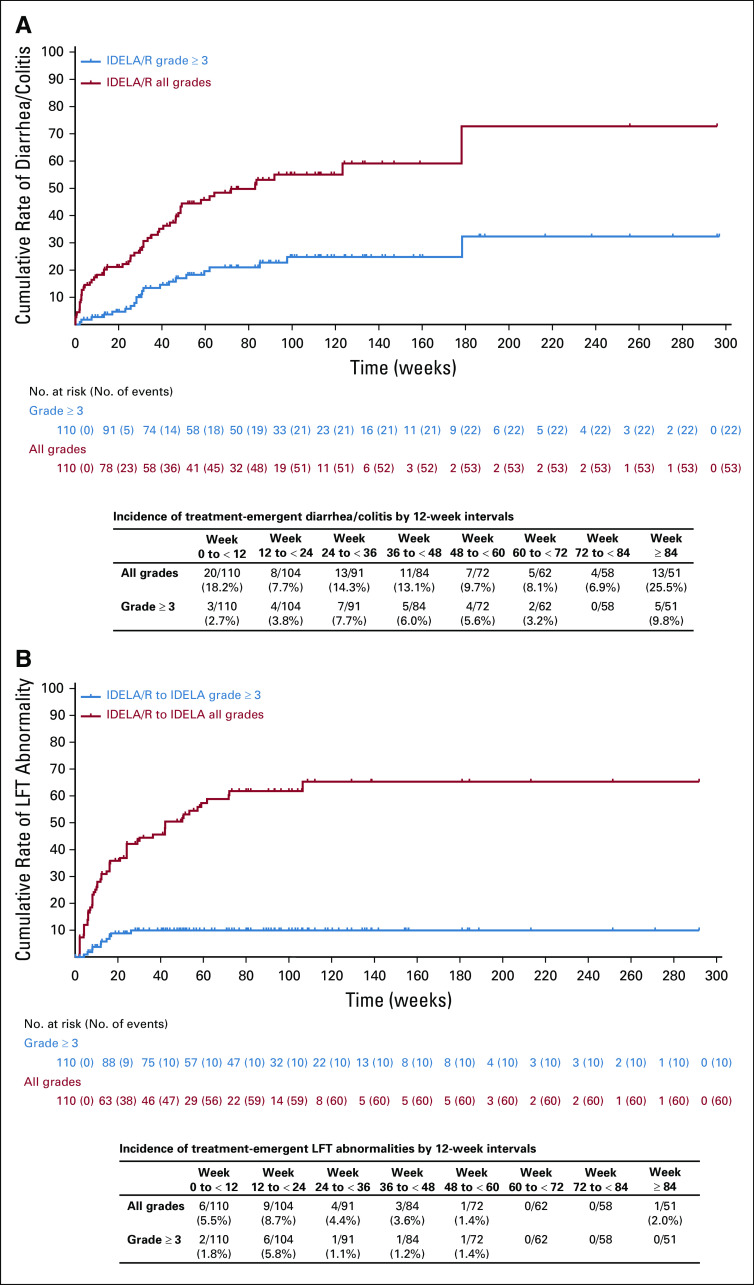
An overall summary of AEs that occurred during the primary and extension studies is shown in Table 2. All of the most common all-grade and grade 3 or greater AEs that occurred in at least 15% of patients in the IDELA/R, placebo/R and IDELA/R-to-IDELA groups are summarized in the Data Supplement. The most frequently reported AEs in the IDELA/R group in the primary study were pyrexia (40.0%), fatigue (30.9%), and diarrhea (29.1%; Table 3). Among patients who experienced diarrhea, 9.1% had grade 3 or greater diarrhea, and 8.2% had grade 2 diarrhea. The median times to onset and resolution of grade 3 or greater diarrhea/colitis were 24.4 and 2.1 weeks, respectively. With longer exposure to IDELA, the frequency of diarrhea increased to 46.4%, which included 17.3% of patients with grade 2 diarrhea and 16.4% of patients with grade 3 or greater diarrhea (33.6% had grade 2 or greater diarrhea; Fig 4A). The median times to onset and resolution of grade 2 or greater diarrhea in the IDELA/R-to-IDELA arm were 31.4 weeks and 2.1 weeks, respectively. Among 12 (10.9%) of 110 patients in the IDELA/R-to-IDELA arm who had colitis, nine (8.2%) had grade 3 or greater colitis. Of these nine patients, eight also had grade 2 or greater diarrhea, and seven of these received treatment for colitis. In total, 11 patients underwent colon and/or rectum biopsies to assist the diagnosis of diarrhea/colitis. More detailed characterization of some of these biopsy results have been published elsewhere. 12 Of the nine patients who discontinued IDELA treatment because of immune-related AEs, seven were rechallenged, and three of these had no AE recurrence (Data Supplement).
TABLE 2.
Summary of Adverse Events
TABLE 3.
Adverse Events by Grade
FIG 4.
Cumulative rates of all-grade and grade 3 or greater (A) diarrhea/colitis and (B) abnormal LFTs in the IDELA/R-to-IDELA group. Inset tables provide incidence rates by 12-week intervals. IDELA, idelalisib; LFT, liver function tests; R, rituximab.
Of the four patients treated with IDELA 300 mg twice daily, grade 3 or greater IDELA-related AEs occurred; these were neutropenia (n = 2) and diarrhea (n = 1). The fourth patient had no IDELA-related AEs.
During the primary study, more patients in the IDELA/R group than in the placebo/R group experienced elevated ALT levels (39.1% v 12.0%, respectively) and AST levels (28.2% v 14.8%, respectively). Grade 3 or greater elevated ALT and AST occurred in 9.1% and 5.5% of patients in the IDELA/R group, respectively, and in 0.9% and no patients in the placebo/R group, respectively. The median time to onset and resolution of grade 3 or greater ALT and AST elevation were 12.1 and 4.1 weeks. In the IDELA/R-to-IDELA arm, there were no additional occurrences of elevated grade 3 or greater ALT and AST despite longer exposure to IDELA (Table 3; Fig 4B).
Other AEs of special interest included Pneumocystis jirovecii pneumonia (PJP) infections that occurred in four patients (3.6%) in the IDELA/R group and in one patient (0.9%) in the placebo/R group; all were grade 3 or greater, and two were fatal. In the IDELA/R-to-IDELA group, one additional grade 2 PJP infection occurred (Table 3). In the IDELA/R arm, 62 of 110 patients received prophylaxis. Of the five patients who experienced PJP across both primary and extension studies, none received anti-PJP prophylaxis. In addition to PJP infections, there were 22 occurrences of fungal infections in the IDELA/R-to-IDELA group: five occurrences of oral candidiasis; three occurrences each of esophageal candidiasis, candida infections (location not specified), and fungal infection (location not specified); two occurrences of fungal pneumonia; and one occurrence each of hepatic candidiasis, respiratory moniliasis, bronchopulmonary aspergillosis, anal fungal infection, fungal skin infection, and vulvovaginal mycotic infection. Two patients in the IDELA/R-to-IDELA group had grade 1 and 2 nonfatal cytomegalovirus infections. Across the primary and extension studies, rates of grade 3 or greater infections in the IDELA/R-to-IDELA group were 19.1%, 7.7%, 11.0%, 7.1%, 8.3%, 9.7%, and 5.2% in each 12-week interval up to 84 weeks, and the rate was 35.3% after 84 weeks. Mean IgG levels fluctuated slightly with time and exhibited no notable trends or changes from baseline; greater variability could be observed at the patient level (data not shown).
Two patients experienced atrial fibrillation in the primary study—one in each of the treatment arms. In the IDELA/R-to-IDELA group, atrial fibrillation occurred in four patients (3.6%), and grade 3 or greater treatment-emergent bleeding events were noted in seven patients (6.4%). Four of these patients were taking anticoagulants. No serious AEs of hypertension were reported.
Across both the primary and extension studies, AEs that resulted in death in the IDELA/R-to-IDELA group occurred in 13 patients (11.8%; Table 2). AEs that resulted in the death of at least one patient included acute respiratory failure (n = 2) and PJP (n = 2).
DISCUSSION
Elderly patients with CLL who have medical comorbidities generally are poor candidates for chemotherapy and have limited treatment options. 13,14 At the time of this study, there were few defined treatment options that offered more than palliative or supportive care. Rituximab monotherapy was commonly used in the therapy of such patients, but it had limited efficacy. In this pivotal phase III study, treatment of this patient population with the IDELA/R combination resulted in significantly better clinical outcomes compared with those seen with rituximab alone (ORR, 83.6% v 15.5%; PFS, 19.4 v 6.5 months). These clinical outcomes were then confirmed with longer follow -up (ORR, 85.5%; PFS, 20.3 months). In addition, although the sample size was small, data from the placebo/R arm suggested that those who experienced PD during treatment with rituximab alone still obtained clinical benefit from subsequent monotherapy with IDELA.
We observed improved OS for patients treated with IDELA/R compared with placebo/R; this survival benefit of IDELA/R versus placebo/R was greatest among patients with del(17p) or TP53 mutations. Among patients treated with IDELA/R, multivariable analysis demonstrated that the presence of del(17p) or TP53 mutations did not negatively affect clinical outcomes compared with those seen in patients who lack these adverse markers, which is a unique finding among novel agents in CLL. After approximately 4 years of follow-up, the OS curves converged. This may be attributed to patient crossover to the extension study and/or to subsequent therapies received after disease progression, neither of which were prospectively recorded in this study. Ibrutinib was approved in 2013 and became commercially available during the conduct of this study, which likely affected subsequent therapy options for some patients. 15
Only two patients with Richter transformation were identified. However, biopsies at progression were not specified in the protocol, and some occurrences may have been missed.
The long-term safety of IDELA did not reveal any previously unreported toxicity. The incidence of grade 3 or greater elevation of liver function tests plateaued after approximately 20 weeks of treatment and did not increase with long-term exposure to IDELA. Although reports have linked IDELA to increased rates of PJP infections, 16,17 these events were seen only in patients without PJP prophylaxis, which reinforces the recommendation for PJP prophylaxis for patients treated with IDELA.
Diarrhea increased with longer exposure: observed rates of all-grade, grade 2, and grade 3 or greater diarrhea were 46.4%, 17.3%, and 16.4%, respectively. Of the 18 total cases of grade 3 or greater diarrhea, 10 (55.5%) occurred within the first 52 weeks of therapy. According to protocol, colon biopsies were not required, nor were central pathologic analyses performed. In prior reports, typical findings have included the presence of cytomegalovirus infection, glandular changes, apoptosis, ischemia, and inflammation. 12,18,19 Steroid therapy was recommended for management of grade 3 or greater diarrhea. The median time to symptom resolution was approximately 2 weeks; however, there were insufficient numbers of patients to determine if steroid therapy affected the duration of symptoms. In both the primary and the extension studies, treatment discontinuations because of AEs or patient withdrawal exceeded discontinuation because of disease progression or death.
The longer-term data presented here confirm the previously reported efficacy of targeting PI3K with idelalisib in patients with relapsed/refractory CLL and support the use of IDELA/R in this patient population with careful management of potential AEs.
ACKNOWLEDGMENT
We thank Lyndah Dreiling, Yeonhee Kim, and Jie Gao from Gilead Sciences, Foster City, CA, for their assistance with early drafts of the manuscript and with data analyses. We thank Ewa Wandzioch and Meryl Gersh of AlphaBioCom, King of Prussia, PA, for medical writing assistance funded by Gilead Sciences.
Footnotes
Presented at the Annual Meetings of the American Society of Hematology, New Orleans, LA, December 7-10, 2013; and San Francisco, CA, December 6-9, 2014.
Supported by Gilead Sciences.
Preprint version available on bioRxiv.
AUTHOR CONTRIBUTIONS
Conception and design: Jeff P. Sharman, Steven E. Coutre, Richard R. Furman, John M. Pagel, Peter Hillmen, Andrew D. Zelenetz, Thomas J. Kipps, Paula Cramer, Michael Hallek, Susan O’Brien, Stephan Stilgenbauer
Collection and assembly of data: Jeff P. Sharman, Steven E. Coutre, Richard R. Furman, John M. Pagel, Peter Hillmen, Jacqueline C. Barrientos, Andrew D. Zelenetz, Thomas J. Kipps, Ian W. Flinn, Herbert Eradat, Thomas Ervin, Andrew R. Pettitt, Shuo Ma, Eugen Tausch, Paula Cramer, Susan M. O’Brien, Stephan Stilgenbauer
Provision of study material or patients: Jeff P. Sharman, Steven E. Coutre, Richard R. Furman, Bruce D. Cheson, John M. Pagel, Peter Hillmen, Jacqueline C. Barrientos, Andrew D. Zelenetz, Thomas J. Kipps, Ian W. Flinn, Paolo Ghia, Herbert Eradat, Thomas Ervin, Bertrand Coiffier, Andrew R. Pettitt, Shuo Ma, Eugen Tausch, Paula Cramer, Michael Hallek, Stephan Stilgenbauer
Data analysis and interpretation: Jeff P. Sharman, Steven E. Coutre, Richard R. Furman, Bruce D. Cheson, John M. Pagel, Peter Hillmen, Jacqueline C. Barrientos, Andrew D. Zelenetz, Thomas J. Kipps, Ian W. Flinn, Paolo Ghia, Herbert Eradat, Nicole Lamanna, Bertrand Coiffier, Andrew R. Pettitt, Shuo Ma, Paula Cramer, Julie Huang, Siddhartha Mitra, Michael Hallek, Stephan Stilgenbauer
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work : All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Final Results of a Randomized, Phase III Study of Rituximab With or Without Idelalisib Followed by Open-Label Idelalisib in Patients With Relapsed Chronic Lymphocytic Leukemia
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Jeff P. Sharman
Leadership: US Oncology
Consulting or Advisory Role: Pharmacyclics, Celgene, TG Therapeutics, Genentech, Abbvie, Acerta Pharma, AstraZeneca
Research Funding: Pharmacyclics (Inst), Genentech (Inst), Celgene (Inst), Acerta Pharma (Inst), Gilead Sciences (Inst), Seattle Genetics (Inst), TG Therapeutics (Inst), Merck (Inst), Takeda (Inst)
Expert Testimony: Gilead Sciences
Steven E. Coutre
Stock and Other Ownership Interests: Pharmacyclics (I)
Consulting or Advisory Role: Genentech, Roche, Celgene, Pharmacyclics, Gilead Sciences, Abbvie, Novartis, Janssen Oncology, BeiGene
Research Funding: Celgene (Inst), Pharmacyclics (Inst), Gilead Sciences (Inst), Abbvie (Inst), Janssen Oncology (Inst), Acerta Pharma (Inst), AstraZeneca (Inst), Millenium Pharmaceuticals (Inst)
Travel, Accommodations, Expenses: Celgene, Gilead Sciences, Novartis, Abbvie, Pharmacyclics, Janssen Oncology, BeiGene
Richard R. Furman
Honoraria: Janssen, Pharmacyclics, Genentech, Roche
Consulting or Advisory Role: Pharmacyclics, Janssen Biotech, Gilead Sciences, Genentech, Roche, Abbvie, Sunesis Pharmaceuticals, Loxo, TG Therapeutics, Verastem, Acerta Pharma, AstraZeneca
Research Funding: Acerta Pharma, TG Therapeutics
Expert Testimony: Abbvie
Other Relationship: Incyte, Janssen Biotech, Abbvie
Bruce D. Cheson
Consulting or Advisory Role: Acerta Pharma, TG Therapeutics, Bayer, Roche, Genentech, Abbvie, Pharmacyclics, Janssen, Morphosys, Sunesis Pharmaceuticals, Celgene
Research Funding: Roche (Inst), Genentech (Inst), Acerta Pharma (Inst), Gilead Sciences (Inst), Abbvie (Inst), TG Therapeutics (Inst)
John M. Pagel
Consulting or Advisory Role: Pharmacyclics, Gilead Sciences
Peter Hillmen
Honoraria: Janssen, Abbvie, Roche
Research Funding: Janssen (Inst), Pharmacyclics (Inst), Roche (Inst), Gilead Sciences (Inst)
Travel, Accommodations, Expenses: Janssen, Abbvie
Jacqueline C. Barrientos
Honoraria: Janssen
Consulting or Advisory Role: Pharmacyclics, Abbvie, Gilead Sciences, Genentech, Bayer
Research Funding: Pharmacyclics (Inst)
Travel, Accommodations, Expenses: Janssen
Andrew D. Zelenetz
Stock and Other Ownership Interests: Adaptive Biotechnologies
Honoraria: National Comprehensive Cancer Network, Clinical Care Options, Physician Education Resource, Oncology Information Group
Consulting or Advisory Role: Gilead Sciences, Amgen, Genentech, Roche, Celgene, Medscape, AstraZeneca, DAVA Oncology, MorphoSys, BeiGene, Cancer Support Community, Janssen, MEI Pharma, Novartis, Pfizer, Prime Education, TRM Oncology, Vaniam Group, Verastem, Pharmacyclics, Karyopharm Therapeutics
Research Funding: Genentech, Roche, Gilead Sciences, MEI Pharma, BeiGene
Bertrand Coiffier
Honoraria: Celgene, Mundipharma, Gilead Sciences, AstraZeneca, Pfizer, Celltrion, Novartis
Consulting or Advisory Role: Celgene, Mundipharma, Gilead Sciences, AstraZeneca, Pfizer,Celltrion, Novartis
Thomas J. Kipps
Employment: University of California, San Diego, Health Moores Cancer Center
Honoraria: Pharmacyclics, AbbVie, Janssen, Celgene, Genentech, Roche, Gilead, DAVA Oncology
Consulting or Advisory Role: AbbVie, Pharmacyclics, Celgene, Genentech, Roche, Janssen, DAVA Oncology, Gilead Sciences
Speakers' Bureau: Verastem, Pharmacyclics
Ian W. Flinn
Consulting or Advisory Role: Abbvie (Inst), Seattle Genetics (Inst), TG Therapeutics (Inst), Verastem (Inst)
Research Funding : Acerta Pharma (Inst), Agios (Inst), Calithera Biosciences (Inst), Celgene (Inst), Constellation Pharmaceuticals (Inst), Genentech (Inst), Gilead Sciences (Inst), Incyte (Inst), Infinity Pharmaceuticals (Inst), Janssen (Inst), Karyopharm Therapeutics (Inst), Kite Pharma (Inst), Novartis (Inst), Pharmacyclics (Inst), Portola Pharmaceuticals (Inst), Roche (Inst), TG Therapeutics (Inst), Trillium Therapeutics (Inst), Abbvie (Inst), ArQule (Inst), BeiGene (Inst), Curis (Inst), FORMA Therapeutics (Inst), Forty Seven (Inst), Merck (Inst), Pfizer (Inst), Takeda (Inst), Teva (Inst), Verastem (Inst), Gilead Sciences (Inst), AstraZeneca (Inst), Juno Therapeutics (Inst), Unum Therapeutics (Inst), MorphoSys (Inst)
Paolo Ghia
Honoraria: Abbvie, BeiGene, Janssen Oncology, Gilead Sciences, Juno Therapeutics, Sunesis Pharmaceuticals, Roche
Consulting or Advisory Role: Abbvie, BeiGene, Janssen, Gilead Sciences, Sunesis Pharmaceuticals, Juno Therapeutics
Speakers' Bureau: Gilead Sciences
Research Funding: Abbvie, Janssen Oncology, Gilead Sciences, Sunesis Pharmaceuticals, Novartis
Herbert Eradat
Honoraria: Genentech, Roche, Abbvie, Gilead Sciences
Consulting or Advisory Role: Genentech, Roche, Abbvie, Gilead Sciences, Pharmacyclics
Speakers' Bureau: Genentech, Roche, Abbvie, Gilead Sciences, Pharmacyclics
Research Funding: Abbvie, Genentech, Roche, Gilead Sciences, Seattle Genetics, Celgene, miRagen, Pharmacyclics
Travel, Accommodations, Expenses: Genentech, Roche, Abbvie
Nicole Lamanna
Consulting or Advisory Role: Celgene, Genentech, Abbvie, ProNAi, Pharmacyclics, Juno Therapeutics, Roche, Janssen, AstraZeneca, Gilead Sciences
Research Funding: Genentech (Inst), Abbvie (Inst), Infinity Pharmaceuticals (Inst), Gilead Sciences (Inst), ProNAi (Inst), BeiGene (Inst), AstraZeneca (Inst), Verastem (Inst), Juno Therapeutics (Inst), TG Therapeutics (Inst), Acerta Pharma (Inst), Loxo (Inst)
Bertrand Coiffier
Honoraria: Celgene, Mundipharma, Gilead Sciences, AstraZeneca, Pfizer, Celltrion, Novartis
Consulting or Advisory Role: Celgene, Mundipharma, Gilead Sciences, AstraZeneca, Pfizer, Celltrion, Novartis
Andrew R. Pettitt
Research Funding: Celgene (Inst), Gilead Sciences (Inst), Roche (Inst), NAPP Pharmaceuticals (Inst), GlaxoSmithKline (Inst), Novartis (Inst), Verastem (Inst)
Travel, Accommodations, Expenses: Gilead, Kite, Celgene
Shuo Ma
Consulting or Advisory Role: Genentech, Roche, Gilead Sciences, Abbvie, Novartis, Pharmacyclics, Janssen Oncology, AstraZeneca, Bioverativ, Omniprex
Speakers' Bureau: Pharmacyclics, Genentech, Roche, Janssen Oncology, AstraZeneca
Research Funding: Pharmacyclics (Inst), Gilead Sciences (Inst), Acerta Pharma (Inst), Abbvie (Inst), Novartis (Inst), Incyte (Inst)
Eugen Tausch
Consulting or Advisory Role: Roche
Speakers' Bureau: Roche, Abbvie
Travel, Accommodations, Expenses: Abbvie
Paula Cramer
Honoraria: Abbvie, Acerta Pharma, AstraZeneca, Janssen-Cilag
Research Funding: Acerta Pharma (Inst), Gilead Sciences (Inst), Janssen-Cilag (Inst), Roche (Inst), Novartis (Inst)
Travel, Accommodations, Expenses: Abbvie, Roche, Janssen-Cilag
Julie Huang
Employment: Gilead Sciences
Stock and Other Ownership Interests: Gilead Sciences
Siddhartha Mitra
Employment: Five Prime Therapeutics, Gilead Sciences
Stock and Other Ownership Interests: Gilead Sciences, Five Prime Therapeutics
Michael Hallek
Honoraria: Roche, Janssen, Abbvie, Gilead Sciences
Consulting or Advisory Role: Janssen, Abbvie, Gilead Sciences, Genentech, Roche
Speakers' Bureau: Janssen, Abbvie, Gilead Sciences, Roche, Genentech
Research Funding: Roche, Abbvie, Janssen, Gilead Sciences
Travel, Accommodations, Expenses: Roche, Janssen
Susan M. O’Brien
Employment: University of California, Irvine
Honoraria: Celgene, Janssen, Pharmacyclics, Gilead Sciences, Pfizer, Amgen, Astellas Pharma, GlaxoSmithKline, Aptose Biosciences, Vaniam Group, Abbvie, Sunesis Pharmaceuticals, Alexion Pharmaceuticals, Loxo, Eisai, TG Therapeutics
Consulting or Advisory Role: Amgen, Celgene, GlaxoSmithKline, Janssen Oncology, Aptose Biosciences, Vaniam Group, Abbvie, Genentech, Sunesis Pharmaceuticals, Alexion Pharmacewuticals, Astellas Pharma, Gilead Sciences, Pharmacyclics, TG Therapeutics, Pfizer
Research Funding: Acerta Pharma (Inst), Regeneron (Inst), Gilead Sciences (Inst), Pfizer (Inst), TG Therapeutics (Inst), Pharmacyclics (Inst), Kite Pharma (Inst), Sunesis Pharmaceuticals (Inst)
Travel, Accommodations, Expenses: Celgene, Janssen, Gilead Sciences, Regeneron
Stephan Stilgenbauer
Honoraria: AbbVie, AstraZeneca, Celgene, Gilead, GlaxoSmithKline, Hoffmann La-Roche, Janssen
Consulting or Advisory Role: AbbVie, AstraZeneca, Celgene, Gilead, GlaxoSmithKline, Hoffmann La-Roche, Janssen
Speakers' Bureau: AbbVie, AstraZeneca, Celgene, Gilead, GlaxoSmithKline, Hoffmann La-Roche, Janssen
Research Funding: AbbVie, AstraZeneca, Celgene, Gilead, GlaxoSmithKline, Hoffmann La-Roche, Janssen
Travel, Accommodations, Expenses: AbbVie, AstraZeneca, Celgene, Gilead, GlaxoSmithKline, Hoffmann La-Roche, Janssen
No other potential conflicts of interest were reported.
REFERENCES
- 1.SEER :Stat fact sheets: Chronic lymphocytic leukemia. https://seer.cancer.gov/statfacts/html/clyl.html [Google Scholar]
- 2.Shanafelt T:Treatment of older patients with chronic lymphocytic leukemia: Key questions and current answers.Hematology (Am Soc Hematol Educ Program) 2013:158-167,2013 [DOI] [PubMed] [Google Scholar]
- 3.Smolej L:Therapy of elderly/comorbid patients with chronic lymphocytic leukemia.Curr Pharm Des 18:3399-3405,2012 [DOI] [PubMed] [Google Scholar]
- 4.Sharman JP,Mato A,Kay NE,et al. : Demographics by age group and line of therapy in Chronic lymphocytic leukemia patients treated in US practices from the Connect CLL registry. Blood 124:3338, 2014. 25431474 [Google Scholar]
- 5.Lannutti BJ,Meadows SA,Herman SE,et al. :CAL-101, a p110delta selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability.Blood 117:591-594,2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoellenriegel J,Meadows SA,Sivina M,et al. :The phosphoinositide 3-kinase delta inhibitor, CAL-101, inhibits B-cell receptor signaling and chemokine networks in chronic lymphocytic leukemia.Blood 118:3603-3612,2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furman RR,Sharman JP,Coutre SE,et al. :Idelalisib and rituximab in relapsed chronic lymphocytic leukemia.N Engl J Med 370:997-1007,2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gilead Sciences: Zydelig (idelalisib) full prescribing information. https://www.gilead.com/~/media/Files/pdfs/medicines/oncology/zydelig/zydelig_pi.pdf.
- 9.European Medicines Agency : Zydelig summary of product characteristics. https://www.ema.europa.eu/en/documents/product-information/zydelig-epar-product-information_en.pdf
- 10.Hallek M,Cheson BD,Catovsky D,et al. :Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: A report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute Working Group 1996 guidelines.Blood 111:5446-5456,2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheson BD,Byrd JC,Rai KR,et al. :Novel targeted agents and the need to refine clinical end points in chronic lymphocytic leukemia.J Clin Oncol 30:2820-2822,2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeung CC,Hockenbery DM,Westerhoff M,et al. :Pathological assessment of gastrointestinal biopsies from patients with idelalisib-associated diarrhea and colitis.Future Oncol 14:2265-2277,2018 [DOI] [PubMed] [Google Scholar]
- 13.National Comprehensive Cancer Network :Clinical practice guidelines in oncology: Chronic lymphocytic leukemia/small lymphocytic lymphoma. https://www.nccn.org/professionals/physician_gls/default.aspx
- 14.Jain N,Thompson P,Ferrajoli A,et al. :Approaches to chronic lymphocytic leukemia therapy in the era of new agents: The conundrum of many options.Am Soc Clin Oncol Educ Book 23:580-591,2018 [DOI] [PubMed] [Google Scholar]
- 15.https://www.imbruvica.com/docs/librariesprovider7/default-document-library/prescribing-information.pdf Pharmacyclics: Imbruvica (ibrutinib) full prescribing information.
- 16.Jones JA,Robak T,Brown JR,et al. :Efficacy and safety of idelalisib in combination with ofatumumab for previously treated chronic lymphocytic leukaemia: An open-label, randomised phase 3 trial.Lancet Haematol 4:e114-e126,2017 [DOI] [PubMed] [Google Scholar]
- 17.Zelenetz AD,Barrientos JC,Brown JR,et al. :Idelalisib or placebo in combination with bendamustine and rituximab in patients with relapsed or refractory chronic lymphocytic leukaemia: Interim results from a phase 3, randomised, double-blind, placebo-controlled trial.Lancet Oncol 18:297-311,2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Louie CY,DiMaio MA,Matsukuma KE,et al. :Idelalisib-associated enterocolitis: Clinicopathologic features and distinction from other enterocolitides.Am J Surg Pathol 39:1653-1660,2015 [DOI] [PubMed] [Google Scholar]
- 19.Weidner AS,Panarelli NC,Geyer JT,et al. :Idelalisib-associated colitis: Histologic findings in 14 patients.Am J Surg Pathol 39:1661-1667,2015 [DOI] [PubMed] [Google Scholar]