Abstract
BACKGROUND
Early-life severe respiratory syncytial virus (RSV) bronchiolitis is a risk factor for childhood asthma. Because azithromycin may attenuate airway inflammation during RSV bronchiolitis, we evaluated whether it would reduce the occurrence of post-RSV recurrent wheeze.
METHODS
We prospectively enrolled 200 otherwise healthy 1- to 18-month-old children hospitalized with RSV bronchiolitis in this single-center, double-blind, placebo-controlled study and randomly assigned them to receive oral azithromycin (10 mg/kg daily for 7 days, followed by 5 mg/kg daily for 7 days) or placebo. Randomization was stratified by recent open-label antibiotic use. The primary outcome was the occurrence of recurrent wheeze, defined as a third episode of post-RSV wheeze over the following 2 to 4 years.
RESULTS
As an indication of the biologic activity of azithromycin, nasal wash interleukin-8 levels, at day 14 after randomization, were lower among azithromycin-treated participants (P<0.01). Despite evidence of biologic activity, azithromycin did not reduce the risk of post-RSV recurrent wheeze (47% in the azithromycin group vs. 36% in the placebo group; adjusted hazard ratio, 1.45; 95% confidence interval [CI], 0.92 to 2.29; P=0.11). Azithromycin also did not modify the risk of recurrent wheeze among participants already receiving other antibiotic treatment at the time of enrollment (hazard ratio, 0.94; 95% CI, 0.43 to 2.07). There was a potential signal among antibiotic-naïve participants who received azithromycin to have an increased risk of recurrent wheeze (hazard ratio, 1.79; 95% CI, 1.03 to 3.1).
CONCLUSIONS
Azithromycin therapy for 14 days during acute severe RSV bronchiolitis did not reduce recurrent wheeze occurrence over the following 2 to 4 years. Our data suggest no benefit of azithromycin administration with the goal of preventing recurrent wheeze in later life. (Funded by the National Heart, Lung, and Blood Institute; ClinicalTrials.gov number, NCT02911935.)
Introduction
Asthma, the most common chronic disease in childhood, has significant effects on children, their families, and the health care system,1 making asthma prevention a high priority. Early-life respiratory syncytial virus (RSV) bronchiolitis, especially when severe,2,3 is a well-known risk factor for the development of childhood asthma.4–10 We reported previously that by 7 years of age, 48% of infants hospitalized for RSV bronchiolitis had a physician diagnosis of asthma, and that 75% had experienced at least three subsequent wheezing episodes.8
Previous attempts to prevent postbronchiolitis wheezing using medications typically prescribed for asthma, such as inhaled glucocorticoids,11–13 systemic glucocorticoids,14–16 and montelukast,17,18 have been largely unsuccessful, likely related to their minimal effect on noneosinophilic (i.e., neutrophilic) airway inflammation,19 the dominant pattern seen during viral bronchiolitis.20,21 Macrolides provide clinical benefits in inflammatory airway diseases with a dominant neutrophilic airway inflammation component, such as cystic fibrosis, potentially through antiinflammatory activities.22–24 Therefore, we hypothesized that targeting neutrophilic inflammation may attenuate post-RSV wheezing. We reported previously on a pilot trial of 40 infants hospitalized with RSV bronchiolitis, in which treatment with azithromycin at the time of RSV bronchiolitis reduced the likelihood of recurrent wheeze over the following year.25 These findings provided the clinical equipoise for this larger, potentially confirmatory, randomized controlled clinical trial.
The potential benefit of an antimicrobial for the prevention of asthma must be considered in the context of the risks of asthma development that have been associated with antibiotic use,26–31 including during acute bronchiolitis.32 However, the studies showing these risks were retrospective observational studies and were complicated by indication bias and reverse causation. We performed the current controlled clinical trial, which we termed Azithromycin to Prevent Wheezing following Severe RSV Bronchiolitis–II (APW-RSV II), on the basis of the hypothesis that azithromycin therapy, during severe RSV bronchiolitis, would reduce the occurrence of recurrent wheeze during the preschool years.
Methods
APW-RSV II was a double-blind, placebo-controlled, parallel-group, single-center randomized trial that was conducted between November 2016 and May 2021. A comprehensive description of the study methodology and rationale is described elsewhere.33 The study protocol is provided with the full text of this article.
TRIAL PARTICIPANTS
We prospectively enrolled otherwise healthy children, 1 to 18 months of age, who were admitted to St. Louis Children’s Hospital with severe RSV bronchiolitis. RSV infection was confirmed by positive nasopharyngeal swab results by polymerase chain reaction and/or direct antigen detection. Severe bronchiolitis was defined by the presence of at least two of the following: respiratory rate greater than 40 breaths per minute; cough; wheezing; the presence of rales, crackles, and/or rhonchi heard on chest auscultation; or paradoxical chest movements (retractions).18 These clinical parameters were recorded by the clinical team and were obtained from the medical records by the trial coordinators. Inclusion and exclusion criteria are listed in Table S1 in the Supplementary Appendix. Concomitant clinically indicated treatment with a nonmacrolide antibiotic was allowed at enrollment or during the study. To treat during the period of active airway inflammation, children were randomly assigned to treatment within 7 days from symptom onset.
TRIAL PROTOCOLS
The study design is described in Figure 1A. The study included a randomized active treatment phase of 2 weeks and an observational phase of up to 48 months. Participants were enrolled during three consecutive RSV seasons (each starting in the autumn of 2016, 2017, or 2018 and ending in the respective spring). Parents or legal guardians provided written informed consent. Eligible children were randomly assigned to receive azithromycin or placebo in a 1:1 fashion based on a blocked allocation sequence. Random assignment was conducted during hospitalization and was stratified by the presence or absence of open-label nonmacrolide antibiotic use during the preceding 2 weeks. The random allocation sequence and block size were concealed from all participants and study personnel except for the statisticians and pharmacists. Azithromycin was administered orally as 10 mg/kg once daily for 7 days, followed by 5 mg/kg once daily for 7 days (rationale described elsewhere33). The matched placebo was manufactured by the St. Louis Children’s Hospital investigational pharmacy.33
Figure 1.
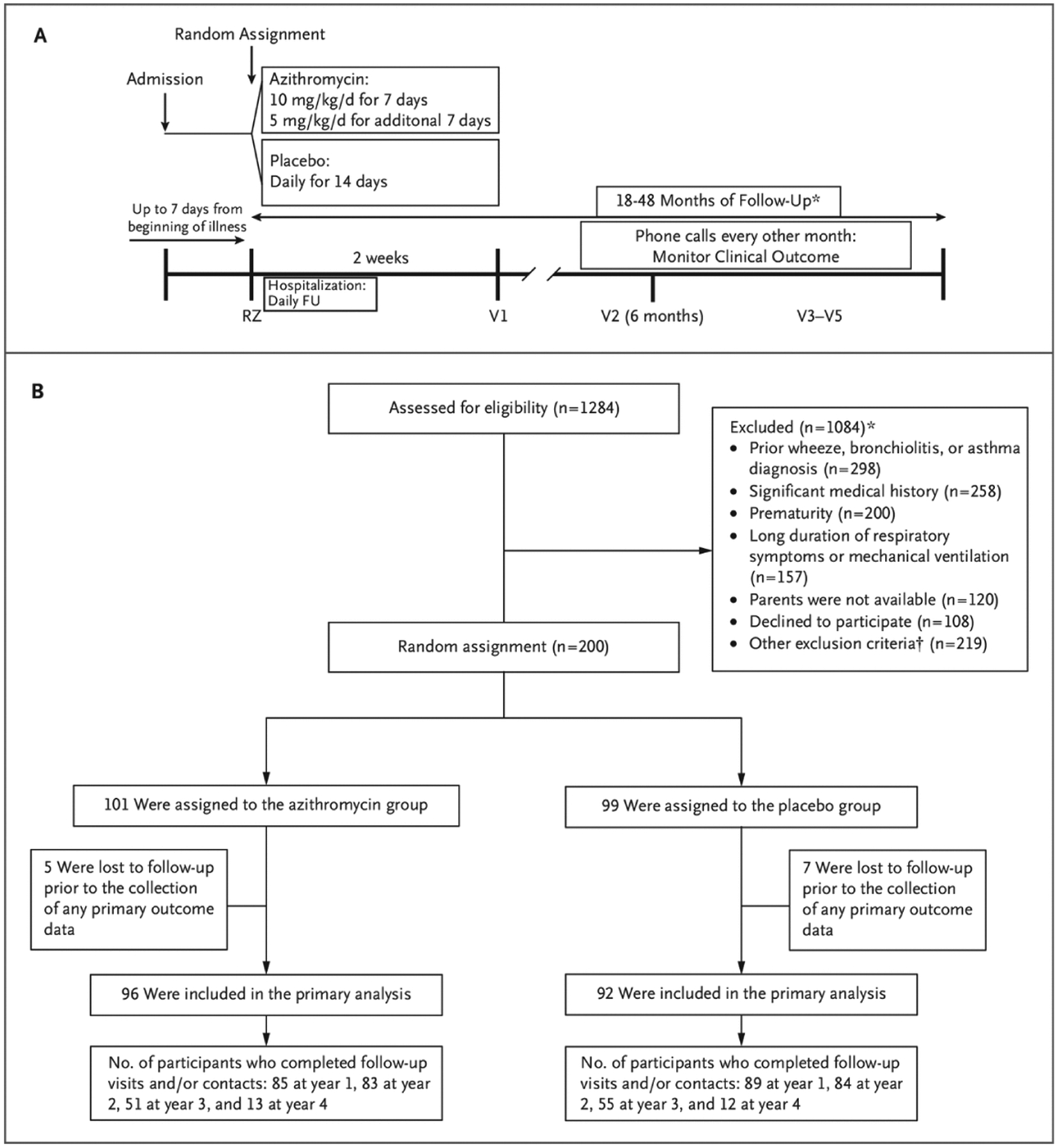
Study Design and Patient Enrollment, Random Assignment, Group Assignments, and Analysis.
A, Study design. B, Enrollment, random assignment, group assignments, and analysis. Participants enrolled at year 1 could have up to 4 years of follow-up, participants enrolled at year 2 could have up to 3 years of follow-up, and participants enrolled at year 3 could have up to 2 years of follow-up. FU denotes follow-up, RZ Randomization visit, V1 visit #1, V2 visit #2, and V3–V5 visits #3–5.
* Some children had more than one reason for exclusion. See Table S2 for a comprehensive listing of exclusions.
Inpatient bronchiolitis clinical care was directed by the participant’s attending physician according to a standardized care path based on the American Academy of Pediatrics guidelines,34 which emphasize supportive care and strongly discourage the use of systemic glucocorticoids and antibiotics. See section V.K. in the study protocol provided with the full text of this article for assessment of medication adherence.
Study outcomes were assessed during clinic visits conducted at 6, 18, 30, and 42 months following random assignment and by telephone interviews every other month (Fig. 1A). To maximize follow-up, the duration of follow-up was variable on the basis of year of enrollment: participants enrolled at year 1 could have up to 4 years of follow-up, participants enrolled at year 2 could have up to 3 years of follow-up, and participants enrolled at year 3 could have up to 2 years of follow-up. Beginning in January 2020, phone interviews were conducted instead of clinic visits as a result of the Covid-19 pandemic.
OUTCOME MEASURES
The primary outcome of recurrent wheeze was defined as the occurrence of a third episode of parent-reported wheezing after the index episode of severe RSV bronchiolitis. Recurrent wheeze is an intermediate end point associated with future asthma development that has been utilized effectively in previous studies.8,35,36
Episodes of wheezing were captured at each of the study telephone interviews and clinic visits using the question, “Has your child’s chest sounded wheezy or whistling (with and without a cold)?” adapted from the International Study of Asthma and Allergies in Childhood37 and utilized in our previous RSV studies.8,25 A new wheezing episode was defined if at least 7 days without wheezing had been reported since the previous episode. The timeframe for post-RSV wheezing started at the end of the study treatment period. Wheezing reported during the initial RSV bronchiolitis episode was not included in the count of post-RSV wheezing episodes.
Secondary and exploratory outcomes are listed in Table S2.
TRIAL OVERSIGHT
The trial was funded by the National Heart, Lung, and Blood Institute (NHLBI) and was approved by the Washington University Human Research Protection Office and by the Data and Safety Monitoring Board convened by the NHLBI to monitor the study. The Food and Drug Administration (FDA) approved an Investigational New Drug application for azithromycin use (exempt status, PIND 112359).
All authors vouch for the accuracy and completeness of the data, the accuracy of the analyses, the fidelity of the trial to the protocol, and the decision to submit the manuscript for publication.
STATISTICAL ANALYSIS AND POWER CALCULATION
A comprehensive description of the statistical analysis plan is available in the study methods article.33 In brief, the primary analysis tested the statistical null hypothesis of equal recurrent wheeze rates between the azithromycin and placebo groups. We used a modified intention-to-treat analysis (N = 188), because there were no follow-up data for 12 participants. Initially, we used a Kaplan-Meier survival analysis and a log-rank test to test for recurrent wheeze rate differences between treatment groups. We then proceeded to a multivariable Cox regression analysis where fixed-time covariates that indicated some evidence of a difference between the treatment groups (P<0.10; assessed using chi-square, t-, or Wilcoxon rank-sum test, as appropriate) and time-varying covariates were considered for inclusion in a stepwise multivariable model. Race and a parental history of asthma were forced into the multivariable model on the basis of their strong association with asthma inception.38
For the recurrent wheeze outcome, we tested for interactions between group assignment and prespecified baseline characteristics (Table S5) to evaluate the consistency of treatment effects across covariates. Furthermore, because random assignment was stratified by the presence or absence of open-label nonmacrolide antibiotics, and because we hypothesized that the effect of azithromycin may differ between children enrolled while receiving nonstudy antibiotics and those who were antibiotic naïve, we performed a post hoc exploratory analysis to investigate the effect of the intervention in each of these randomization strata including tests of interactions. For secondary and exploratory rate outcomes, we used a negative binomial regression analysis with an offset term to adjust for variable duration of follow-up. Additional analyses of secondary and exploratory outcomes included the Kaplan-Meier survival analysis, analysis of covariance (ANCOVA), Cox regression analysis, chi-square analysis, and Wilcoxon rank-sum tests. Analyses were conducted using SAS version 9.4 (SAS Institute Inc, Cary, NC), and P values <0.05 were considered significant. No multiplicity adjustments for the secondary and exploratory end points were defined. Therefore, only point estimates and 95% confidence intervals (CIs) are provided. The CIs have not been adjusted for multiple comparisons and should not be used to infer definitive treatment effects.
Power computations were based on two-sided tests at the 0.05 level of significance. We assumed that the survival curves generated in this study would be similar to the curves generated in our previous pilot trial25 and the longitudinal cohort study8 that involved similar patients. On the basis of conservative assumptions regarding hazard rates over the course of follow-up (see the statistical analysis plan in the Supplementary Appendix), we assumed that the hazard rate ratio comparing the azithromycin group with the placebo group was 0.77. These computations indicated that 188 participants (94 per group) yielded a power of 0.9 to detect this hazard rate ratio between the two treatment arms, assuming a dropout rate of 20%. Twelve additional participants were enrolled to ensure adequate power (N5200). Further details regarding the analyses can be found in the statistical analysis plan within the protocol.
Results
PATIENTS
We identified 1284 children hospitalized with RSV bronchiolitis. A total of 200 children met the eligibility criteria and were randomly assigned to treatment (Fig. 1B). Reasons for exclusion are listed in Table S3. The baseline characteristics (Table 1) were generally similar between treatment groups, with the exceptions of pet exposure and tobacco smoke exposure. Twelve participants withdrew before the first follow-up visit (five in the azithromycin group and seven in the placebo group). A total of 188 participants (96 in the azithromycin group and 92 in the placebo group) had at least one follow-up visit, with an overall median follow-up duration of 35.7 months (interquartile range [IQR], 23.9 to 36). The group was broadly representative of the population of children who experience severe RSV bronchiolitis (see Table S13). Retention data are presented in Table S4. Good adherence to the study medication, defined as having taken more than 80% of the doses, was noted for 92% and 93% of participants in the azithromycin and placebo groups, respectively.
Table 1.
Baseline Characteristics of the Study Population.*
| Characteristic | Azithromycin (n=101) | Placebo (n=99) |
|---|---|---|
| Median age at enrollment — mo | 3.9 (2.3, 7.2) | 2.8 (1.7, 6.6) |
| Male sex | 54 (53.5) | 55 (55.6) |
| Race and ethnicity (n = 199) | ||
| African American | 19 (18.8) | 21 (21.4) |
| Caucasian | 72 (71.3) | 72 (73.5) |
| More than one race | 10 (9.9) | 5 (5.1) |
| Birth weight — kg | 3.3±0.5 | 3.4±0.5 |
| Birth by cesarean section | 29 (28.7) | 28 (28.3) |
| Gestational age at birth — wk | 38.9±1.2 | 38.8±1.2 |
| Maternal smoking during pregnancy | 19 (18.8) | 13 (13.1) |
| History of breast feeding | 75 (74.3) | 80 (80.8) |
| History of eczema | 16 (15.8) | 13 (13.1) |
| Food allergy diagnosis | 3 (3) | 5 (5.1) |
| Parental history of asthma | 33 (32.7) | 39 (39.4) |
| Parental history of other atopic diseases | 60 (59.4) | 69 (69.7) |
| Pet exposure | 59 (58.4) | 71 (71.7) |
| Tobacco smoke exposure | 40 (39.6) | 26 (26.3) |
| Median duration of hospital stay — hr (n = 198) | 51 (33, 89) | 56 (39, 77) |
| Median duration of oxygen requirement, if required — hr (n = 114)† | 46 (26, 70) | 48 (18, 62) |
| Lowest oxygen saturation on room air — % (n = 197) | 90.6±4.4 | 90.7±5.2 |
| Need for BiPAP ventilation (n = 198)‡ | 8 (7.9) | 5 (5.2) |
| Bronchiolitis severity scoreg§ | 5.3±1.8 | 5.2±1.8 |
| Other nonstudy antibiotic (nonmacrolide) use | 30 (29.7) | 28 (28.3) |
Values are presented as the median (interquartile range), n (%), or mean (±SD). The total sample comprised 200 participants unless otherwise specified.
Infants were supplemented with oxygen if they had oxygen saturation of less than 90% on room air.
No child required invasive ventilation. BiPAP denotes bilevel positive airway pressure.
Scores range from 0 to 12 with higher numbers indicating worse disease.
PRIMARY OUTCOME — EFFECT OF AZITHROMYCIN ON POST-RSV RECURRENT WHEEZE
Forty-five patients (47%) in the azithromycin group developed recurrent wheeze compared with 33 (36%) in the placebo group. There was no significant difference in recurrent wheeze rates between participants in the azithromycin and placebo groups (Fig. 2A), as indicated by both the unadjusted hazard ratio (1.49; 95% CI, 0.95 to 2.34; P=0.08) and the adjusted hazard ratio (1.45; 95% CI, 0.92 to 2.29; P=0.11). None of the prespecified tests for interactions between baseline covariates and treatment assignment were significant (Table S5).
Figure 2.
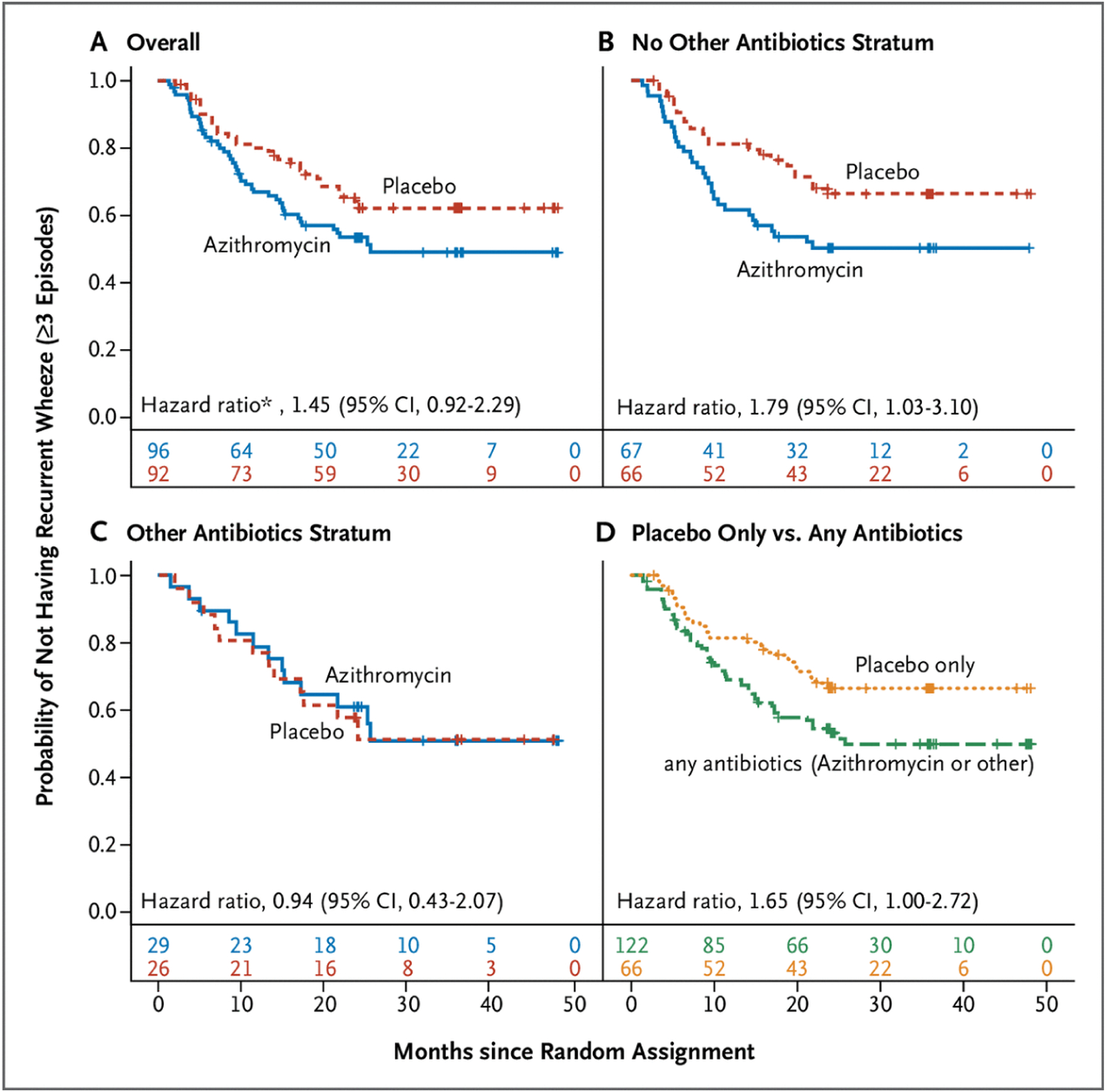
Time to Post–Respiratory Syncytial Virus Third Episode of Wheezing (Recurrent Wheeze).
A, Among the whole cohort (the primary outcome). B, Among participants who had not received open-label nonmacrolide antibiotics (“other antibiotics”) at or within 14 days before random assignment. C, Among participants who had received open-label nonmacrolide antibiotics (“other antibiotics”) at or within 14 days before random assignment. D, A post hoc analysis of the time to recurrent wheeze among participants who received placebo and had not received other antibiotics (i.e., had not received any antibiotic) versus participants who received any antibiotics (azithromycin and/or open-label nonmacrolide antibiotic). CI denotes confidence interval. Numbers below the x-axis in each graph indicate the number at risk at each time point.
* Hazard ratio adjusted for parental history of asthma, race, tobacco smoke exposure, and pet exposure.
SECONDARY OUTCOMES
The direction of effect for prespecified secondary outcomes was similar to that of the primary outcome, and none differed between the treatment groups. There were no differences between the azithromycin group and the placebo group in the annualized number of days with any respiratory symptoms (26.2 vs. 22.2 days per year; rate ratio, 1.18; 95% CI, 0.86 to 1.62), days with albuterol use (4 vs. 3.3 days per year; rate ratio, 1.22; 95% CI, 0.60 to 2.48), number of oral glucocorticoid courses (0.23 vs. 0.17 courses per year; rate ratio, 1.34; 95% CI, 0.70 to 2.57), and number of antibiotic courses (1.2 vs. 1.3 courses per year; rate ratio, 0.92; 95% CI, 0.70 to 1.22) (Table S6). In the azithromycin group, 15.6% of the participants had been given an asthma diagnosis by the end of trial data collection compared with 8.7% of patients in the control group. The hazard ratio for having an asthma diagnosis by the end of data collection was 1.95 (95% CI, 0.83 to 4.61).
PRESPECIFIED EXPLORATORY OUTCOMES
Annual rates of days of respiratory-related daycare absence (2.2 vs. 1.1 days per year; rate ratio, 1.94; 95% CI, 1.14 to 3.30) and days with parental absence from work (1.7 vs. 0.74 days per year; rate ratio, 2.23; 95% CI, 1.29 to 3.86) were higher in the azithromycin group.
In the azithromycin group, 36.5% of the participants had a fourth wheezing episode compared with 31.5% in the placebo group. The time to fourth wheezing episode did not differ between the groups (hazard ratio, 1.28; 95% CI, 0.78 to 2.09).
None of the other exploratory outcomes differed between treatment groups (Fig. 3; Table S7).
Figure 3.

Forest Plot of Annualized Rate Ratios from a Negative Binomial Regression Analysis of Prespecified Exploratory Outcomes.
The rate ratio corresponds to the azithromycin (AZM) group compared with the placebo group. Estimates greater than 1 indicate that the AZM group did worse compared with the placebo group, and values less than 1 indicate that the placebo group did worse compared with the AZM group. ER denotes emergency room.
To evaluate the potential effect of the COVID-19 pandemic, which began less than 1 year after the last patient was randomly assigned in year 3, we assessed wheezing rate by calendar year of follow-up in exploratory analyses and found a lower rate of wheezing beginning in 2020 (Table S8).
EXPLORATORY ANALYSIS OF THE EFFECT OF OTHER, NONSTUDY ANTIBIOTIC USE ON RECURRENT WHEEZE
Participants were eligible to enroll if they had received open-label nonmacrolide antibiotics at or within the 14 days before random assignment. Twenty-nine participants (30%) in the azithromycin group and 26 (28%) in the placebo group received a nonstudy antibiotic, most commonly amoxicillin (Table S9).
Given that nonstudy antibiotic use was a study stratification variable, we conducted post hoc exploratory subgroup Kaplan-Meier analyses on the basis of nonstudy antibiotic use, which revealed markedly different recurrent wheeze rates (Fig. 2B to 2D) Among participants receiving an open-label antibiotic (n=55), the addition of azithromycin therapy was not associated with the risk of recurrent wheeze relative to placebo (44.8% vs. 46.2%; hazard ratio, 0.94; 95% CI, 0.43 to 2.07). In contrast, among antibiotic-naïve participants (n=133), azithromycin therapy resulted in a risk of subsequent recurrent wheeze relative to placebo of 47.8% vs. 31.8%; hazard ratio 1.79; 95% CI, 1.03 to 3.10. The interaction term between study medication and nonstudy antibiotic status was not significant (P=0.19). A comparison of participants who received any antibiotic (azithromycin as study medication and/or any other antibiotic at random assignment) with those without antibiotic exposure (placebo and no other antibiotics) showed that any antibiotic exposure was associated with a risk of recurrent wheeze of 46.7% versus 31.8% for those without antibiotic exposure (hazard ratio, 1.65; 95% CI, 1.00 to 2.72).
EFFECT OF AZITHROMYCIN ON NASAL INTERLEUKIN-8 LEVELS
To assess the effect of azithromycin on neutrophilic airway inflammation, interleukin (IL)-8 levels were measured in nasal wash samples obtained at baseline, 2 weeks, and 6 months after random assignment. IL-8 levels were similar for the azithromycin and placebo groups at random assignment (median 406.3 pg/ml [IQR, 94.8 to 1351.6] vs. 359.4 pg/ml [IQR, 124.8 to 993.7]) and 6 months (median 356.7 pg/ml [IQR, 49.2 to 1075.4] vs. 250.4 pg/ml [IQR, 52 to 839.3]), but IL-8 levels were significantly lower in the azithromycin group compared with the placebo group at 2 weeks (median 64.9 pg/ml [IQR, 1.8 to 328.1] vs. 225.1 pg/ml [IQR, 47.5 to 641.2], P<0.01) (Fig. 4). In addition to the prespecified ANCOVA, we compared the groups at 2 weeks using the Wilcoxon rank-sum test as a sensitivity analysis because the data were strongly skewed, and results from this analysis similarly indicated a significant difference (P=0.01).
Figure 4.
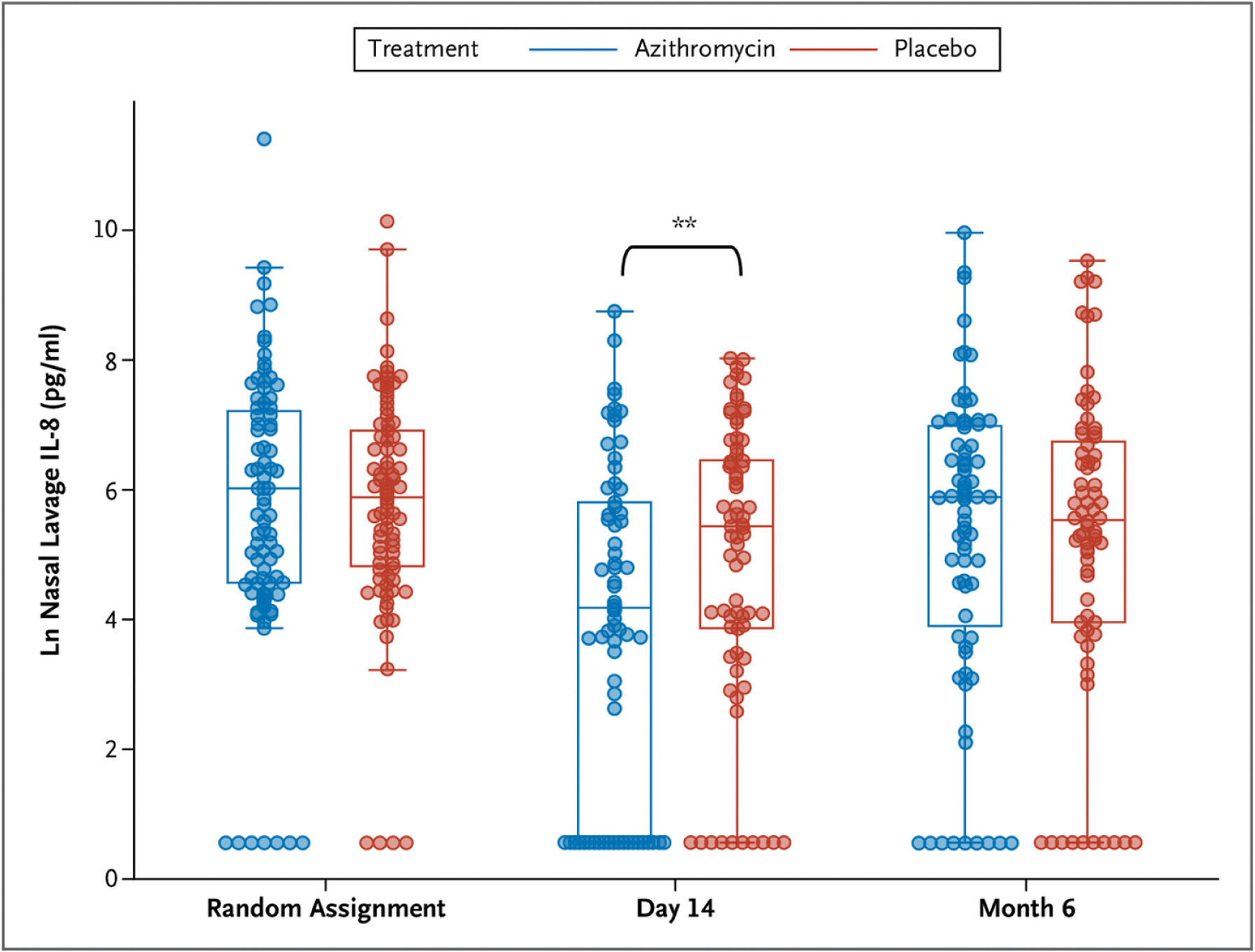
Nasal Lavage Interleukin-8 Levels at Randomization, End of Study Medication Treatments (Day 14), and 6 Months after Randomization.
** = P<0.01.
ANTIBIOTIC RESISTANCE STUDIES
The treatment groups did not differ in rates of detection of azithromycin-resistant microorganisms in nasal samples obtained at the end of study treatment (2 weeks) or 6 months later (Table S10).
SERIOUS ADVERSE EVENTS AND ADVERSE EVENTS
Serious adverse events were reported in 15 participants (15%) and 16 participants (16%) in the azithromycin and placebo groups, respectively (Tables S11 and S12). None of the serious adverse events were determined to be acutely related to the study medication. The total number of participants with adverse events or gastrointestinal adverse events was similar between groups (Table S11).
Discussion
In this trial, we found that despite acutely reducing upper airway IL-8 levels, azithromycin did not modify the risk of recurrent wheeze or other indicators of respiratory morbidity in young children with acute severe RSV bronchiolitis. Our randomized, masked, placebo-controlled, prospective trial failed to demonstrate a protective effect of azithromycin on recurrent wheeze. These clinical outcome data are distinct from our prior open-label pilot trial25 and highlight the need for high-quality evidence to use in decision-making in this setting.
The lack of a beneficial effect on recurrent wheeze is supported by the secondary and prespecified exploratory outcomes all indicating that azithromycin has no benefit and potentially may be harmful. On the basis of this directionality, and because random assignment was stratified by other nonmacrolide antibiotic therapy, we performed exploratory subgroup analyses that suggested a higher recurrent wheeze risk among antibiotic-naïve participants who were treated with azithromycin and a higher recurrent wheeze risk among participants who were treated with any antibiotic (azithromycin or nonstudy antibiotic).
An augmented risk of recurrent wheeze and asthma following early-life antibiotic use, including azithromycin,32 has been described in retrospective and observational studies.26–32,39 However, causality cannot be established from these previous studies because of their limitations of reverse causation, recall bias, and confounding by indication such as antibiotic use as a marker for early-life respiratory infections, a known risk factor for asthma. Our study largely avoids these limitations through its prospective and randomized, placebo-controlled design because unlike in prior observational studies, our participants shared the same respiratory viral infection, azithromycin exposure was randomly assigned, and study encounters occurred every 2 months, minimizing recall bias. We cannot draw firm conclusions from our secondary and exploratory outcomes about enhanced risk with antibiotic treatment, because we did not control for multiple comparisons. However, the alignment of our data with the above-noted observational data indicates that antibiotic exposure during severe RSV bronchiolitis (e.g., azithromycin and/or a nonmacrolide antibiotic) may actually augment the risk of recurrent wheeze during the preschool years. This finding is of high relevance because 30% to 90% of infants hospitalized with bronchiolitis receive antibiotics40 despite guidelines advocating against this practice.34
The mechanisms though which azithromycin (or other antimicrobials) alter recurrent wheeze outcomes following RSV bronchiolitis remain uncertain, but modification of the airway and/or gut microbiomes is likely. Azithromycin may acutely reduce neutrophilic airway inflammation, and hence airway IL-8 levels, by eliminating specific bacteria, such as Moraxella,41 that have been associated with asthma inception.42,43 However, early-life azithromycin may have long-term negative effects on the development of the gut microbiome predisposing to asthma, consistent with findings that germ-free44 or antibiotic-treated mice45,46 are more prone to develop allergic inflammation and asthma. Azithromycin’s effects on the gut microbiome may be age dependent because uninterrupted maturation of the human gut microbiome, in the first year of life, was shown to be protective against asthma development.47 Finally, the dose and the type of antibiotic may also be important factors in determining future asthma risk.31
This study has several important strengths, including targeting hospitalized patients with RSV bronchiolitis, the subgroup at the greatest risk for post-RSV wheezing episodes.2,3 The study was well powered to detect potential benefit of the intervention, and it achieved excellent retention. Our trial also has limitations. The analysis based on nonstudy antibiotic use was not prespecified, and hence is considered exploratory and requires further assessment. Because children with previous use of macrolide antibiotics were excluded from the trial, we do not know whether prior azithromycin use would have the same effect as prior nonmacrolide antibiotic use. Although our ultimate goal was to examine an asthma prevention strategy, the primary outcome was defined as recurrent wheeze during 2 to 4 years of follow-up, as was the case in previous studies in this field.35,36 The study primary outcome measure was wheeze assessed by the parents, which has a relatively low correlation with wheeze assessed by physicians.48 However, a systematic bias is unlikely because both groups were evaluated by the same method. Finally, the COVID-19 pandemic affected the final year of observation. Fewer wheezing episodes were noted in both treatment arms during the pandemic, likely owing to social distancing, lower prevalence of most viral infections, and lower levels of air pollution. However, these factors should have affected both study groups equally. During the pandemic, we performed study phone calls instead of in-person visits. However, our original study design included five phone calls and only one in-person visit each year; this minor deviation is unlikely to have affected the study results.
In conclusion, azithromycin therapy during early-life acute severe RSV bronchiolitis did not reduce recurrent wheeze occurrence over the following 2 to 4 years. Our findings are consistent with national bronchiolitis guidelines,34 which recommend against the use of antibiotics during acute bronchiolitis because of a lack of effect on the acute illness, and they extend this recommendation by demonstrating the lack of a salutary effect on recurrent wheeze.
Supplementary Material
Acknowledgments
Funding for the APW-RSV II clinical trial was provided by the National Heart, Lung, and Blood Institute (grant R01HL130876). This trial is registered at Clinicaltrials.gov (NCT02911935).
Footnotes
Disclosures
Author disclosures and other supplementary materials are available at evidence.nejm.org.
References
- 1.Akinbami LJ, Moorman JE, Bailey C, et al. Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010. NCHS Data Brief 2012;94:1–8. [PubMed] [Google Scholar]
- 2.Carroll KN, Wu P, Gebretsadik T, et al. The severity-dependent relationship of infant bronchiolitis on the risk and morbidity of early childhood asthma. J Allergy Clin Immunol 2009;123:1055–1061, 1061.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Escobar GJ, Masaquel AS, Li SX, Walsh EM, Kipnis P. Persistent recurring wheezing in the fifth year of life after laboratory-confirmed, medically attended respiratory syncytial virus infection in infancy. BMC Pediatr 2013;13:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B, Bjorkstén B. Asthma and immunoglobulin E antibodies after respiratory syncytial virus bronchiolitis: a prospective cohort study with matched controls. Pediatrics 1995;95:500–505. [PubMed] [Google Scholar]
- 5.Sigurs N. Epidemiologic and clinical evidence of a respiratory syncytial virus-reactive airway disease link. Am J Respir Crit Care Med 2001;163:S2–S6. [DOI] [PubMed] [Google Scholar]
- 6.Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med 2000; 161:1501–1507. [DOI] [PubMed] [Google Scholar]
- 7.Sigurs N, Gustafsson PM, Bjarnason R, et al. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med 2005;171:137–141. [DOI] [PubMed] [Google Scholar]
- 8.Bacharier LB, Cohen R, Schweiger T, et al. Determinants of asthma after severe respiratory syncytial virus bronchiolitis. J Allergy Clin Immunol 2012;130:91–100.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simões EAF, Chirikov V, Botteman M, Kwon Y, Kuznik A. Long-term assessment of healthcare utilization 5 years after respiratory syncytial virus infection in US infants. J Infect Dis 2020;221:1256–270. [DOI] [PubMed] [Google Scholar]
- 10.Fauroux B, Simões EAF, Checchia PA, et al. The burden and long-term respiratory morbidity associated with respiratory syncytial virus infection in early childhood. Infect Dis Ther 2017;6:173–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blom D, Ermers M, Bont L, van Aalderen WM, van Woensel JB. Inhaled corticosteroids during acute bronchiolitis in the prevention of post-bronchiolitic wheezing. Cochrane Database Syst Rev 2007;1: CD004881. [DOI] [PubMed] [Google Scholar]
- 12.Ermers MJ, Rovers MM, van Woensel JB, Kimpen JL, Bont LJ. The effect of high dose inhaled corticosteroids on wheeze in infants after respiratory syncytial virus infection: randomised double blind placebo controlled trial. BMJ 2009;338:b897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zomer-Kooijker K, van der Ent CK, Ermers MJ, Rovers MM, Bont LJ. Lack of long-term effects of high-dose inhaled beclomethasone for respiratory syncytial virus bronchiolitis: a randomized placebo-controlled trial. Pediatr Infect Dis J 2014;33:19–23. [DOI] [PubMed] [Google Scholar]
- 14.Bülow SM, Nir M, Levin E, et al. Prednisolone treatment of respiratory syncytial virus infection: a randomized controlled trial of 147 infants. Pediatrics 1999;104:e77. [DOI] [PubMed] [Google Scholar]
- 15.Jartti T, Lehtinen P, Vanto T, et al. Evaluation of the efficacy of prednisolone in early wheezing induced by rhinovirus or respiratory syncytial virus. Pediatr Infect Dis J 2006;25:482–488. [DOI] [PubMed] [Google Scholar]
- 16.Simoes EA. Treatment and prevention of respiratory syncytial virus lower respiratory tract infection. Long-term effects on respiratory outcomes. Am J Respir Crit Care Med 2001;163:S14–S17. [DOI] [PubMed] [Google Scholar]
- 17.Peng WS, Chen X, Yang XY, Liu EM. Systematic review of montelukast’s efficacy for preventing post-bronchiolitis wheezing. Pediatr Allergy Immunol 2014;25:143–50. [DOI] [PubMed] [Google Scholar]
- 18.Bisgaard H, Flores-Nunez A, Goh A, et al. Study of montelukast for the treatment of respiratory symptoms of post-respiratory syncytial virus bronchiolitis in children. Am J Respir Crit Care Med 2008; 178:854–860. [DOI] [PubMed] [Google Scholar]
- 19.Berry M, Morgan A, Shaw DE, et al. Pathological features and inhaled corticosteroid response of eosinophilic and non-eosinophilic asthma. Thorax 2007;62:1043–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Everard ML, Swarbrick A, Wrightham M, et al. Analysis of cells obtained by bronchial lavage of infants with respiratory syncytial virus infection. Arch Dis Child 1994;71:428–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marguet C, Bocquel N, Benichou J, et al. Neutrophil but not eosinophil inflammation is related to the severity of a first acute epidemic bronchiolitis in young infants. Pediatr Allergy Immunol 2008;19: 157–165. [DOI] [PubMed] [Google Scholar]
- 22.Friedlander AL, Albert RK. Chronic macrolide therapy in inflammatory airways diseases. Chest 2010;138:1202–1212. [DOI] [PubMed] [Google Scholar]
- 23.Cameron EJ, McSharry C, Chaudhuri R, Farrow S, Thomson NC. Long-term macrolide treatment of chronic inflammatory airway diseases: risks, benefits and future developments. Clin Exp Allergy 2012;42:1302–1312. [DOI] [PubMed] [Google Scholar]
- 24.Welte T Azithromycin: the holy grail to prevent exacerbations in chronic respiratory disease? Am J Respir Crit Care Med 2019;200: 269–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beigelman A, Isaacson-Schmid M, Sajol G, et al. Randomized trial to evaluate azithromycin’s effects on serum and upper airway IL-8 levels and recurrent wheezing in infants with respiratory syncytial virus bronchiolitis. J Allergy Clin Immunol 2015;135: 1171–8.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patrick DM, Sbihi H, Dai DLY, et al. Decreasing antibiotic use, the gut microbiota, and asthma incidence in children: evidence from population-based and prospective cohort studies. Lancet Respir Med 2020;8:1094–1105. [DOI] [PubMed] [Google Scholar]
- 27.Ni J, Friedman H, Boyd BC, et al. Early antibiotic exposure and development of asthma and allergic rhinitis in childhood. BMC Pediatr 2019;19:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toivonen L, Schuez-Havupalo L, Karppinen S, et al. Antibiotic treatments during infancy, changes in nasal microbiota, and asthma development: population-based cohort study. Clin Infect Dis 2021;72:1546–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fishman E, Crawford G, DeVries A, et al. Association between early-childhood antibiotic exposure and subsequent asthma in the US Medicaid population. Ann Allergy Asthma Immunol 2019;123: 186–192.e9. [DOI] [PubMed] [Google Scholar]
- 30.Aversa Z, Atkinson EJ, Schafer MJ, et al. Association of infant antibiotic exposure with childhood health outcomes. Mayo Clin Proc 2021;96:66–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Donovan BM, Abreo A, Ding T, et al. Dose, timing, and type of infant antibiotic use and the risk of childhood asthma. Clin Infect Dis 2020;70:1658–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen IL, Huang HC, Chang YH, et al. Effect of antibiotic use for acute bronchiolitis on new-onset asthma in children. Sci Rep 2018; 8:6090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Srinivasan M, Bacharier LB, Goss CW, et al. The Azithromycin to Prevent Wheezing following Severe RSV Bronchiolitis-II clinical trial: rationale, study design, methods, and characteristics of study population. Contemp Clin Trials Commun 2021;22:100798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ralston SL, Lieberthal AS, Meissner HC, et al. ; American Academy of Pediatrics. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics 2014;134:e1474–e1502. [DOI] [PubMed] [Google Scholar]
- 35.Litonjua AA, Carey VJ, Laranjo N, et al. Effect of prenatal supplementation with vitamin D on asthma or recurrent wheezing in offspring by age 3 years: the VDAART randomized clinical trial. JAMA 2016;315:362–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lynch SV, Wood RA, Boushey H, et al. Effects of early-life exposure to allergens and bacteria on recurrent wheeze and atopy in urban children. J Allergy Clin Immunol 2014;134:593–601.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Asher MI, Keil U, Anderson HR, et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J 1995;8:483–491. [DOI] [PubMed] [Google Scholar]
- 38.Biagini Myers JM, Schauberger E, He H, et al. A pediatric asthma risk score to better predict asthma development in young children. J Allergy Clin Immunol 2019;143:1803–1810.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abreo A, Gebretsadik T, Stone CA, Hartert TV. The impact of modifiable risk factor reduction on childhood asthma development. Clin Transl Med 2018;7:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Farley R, Spurling GK, Eriksson L, Del Mar CB. Antibiotics for bronchiolitis in children under two years of age. Cochrane Database Syst Rev 2014;10:CD005189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou Y, Bacharier LB, Isaacson-Schmid M, et al. Azithromycin therapy during respiratory syncytial virus bronchiolitis: upper airway microbiome alterations and subsequent recurrent wheeze. J Allergy Clin Immunol 2016;138:1215–1219.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Teo SM, Mok D, Pham K, et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe 2015;17:704–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Teo SM, Tang HHF, Mok D, et al. Airway microbiota dynamics uncover a critical window for interplay of pathogenic bacteria and allergy in childhood respiratory disease. Cell Host Microbe 2018; 24:341–352.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cahenzli J, Köller Y, Wyss M, Geuking MB, McCoy KD. Intestinal microbial diversity during early-life colonization shapes long-term IgE levels. Cell Host Microbe 2013;14:559–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Russell SL, Gold MJ, Hartmann M, et al. Early life antibiotic-driven changes in microbiota enhance susceptibility to allergic asthma. EMBO Rep 2012;13:440–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oyama N, Sudo N, Sogawa H, Kubo C. Antibiotic use during infancy promotes a shift in the T(H)1/T(H)2 balance toward T(H)2-dominant immunity in mice. J Allergy Clin Immunol 2001;107:153–159. [DOI] [PubMed] [Google Scholar]
- 47.Depner M, Taft DH, Kirjavainen PV, et al. Maturation of the gut microbiome during the first year of life contributes to the protective farm effect on childhood asthma. Nat Med 2020;26: 1766–1775. [DOI] [PubMed] [Google Scholar]
- 48.Levy ML, Godfrey S, Irving CS, et al. Wheeze detection: recordings vs. assessment of physician and parent. J Asthma 2004;41:845–853. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


