Chimeric antigen receptor T cell (CART) therapy has shown outstanding results in hematological malignancies.1–13 To date, 6 engineered CART products have been approved by US Food and Drug Administration and European Medicines Agency - European Union for B cell non-Hodgkin lymphoma (NHL), B cell acute lymphoblastic leukemia (B-ALL), and multiple myeloma.2–13 Despite the high rate of responses, disease refractoriness or relapse are common, and therefore, there is a growing interest in understanding the determinants of CART immunotherapy outcomes.14–17 Several mechanisms of CART resistance have been identified, including product-related factors (ie, reduced CAR T cell persistence and T cell exhaustion) and tumor-intrinsic factors (ie, antigen-negative escape, overexpression of immunomodulatory molecules, impairment of apoptotic pathways, and the complex anti-inflammatory tumor microenvironment composition).17 Various strategies have been proposed to address these issues,18,19 but a deeper knowledge of additional and concomitant causes of failure is direly needed. In fact, for many patients, it is still not possible to precisely pinpoint the cause of disease relapse or progression.
In the last 2 decades, the role of gut microbiota has been deeply investigated in several human diseases, including autoimmunity, neurodegeneration, metabolic and cardiovascular diseases, cancer, and others.20–23 In particular, the gut microbiota has been recognized as a critical modulator of immune response after immune checkpoint blockade (ICB)24–27 and in allogeneic hematopoietic stem cell transplantation (allo-HCT).28,29 Initial clinical evidence suggests that the gut microbiota may also influence the antitumor effects of CAR T cell therapy.30–33 In this perspective article, we review the currently available evidence of the role of the microbiota in CART, including preclinical and clinical published data. Despite the limited current clinical evidence, we attempt to speculate on the potential therapeutic approaches that could be developed to modulate the microbiota and enhance CART efficacy and reduce toxicity.
The human gut microbiota is a complex microcosm of commensal, symbiotic, or pathogenic microorganisms, including bacteria, viruses, fungi, and archaea, that modulate inflammatory, metabolic, immunological, and kinetic processes in the host. While the gut microbiota remains the most commonly studied, the microbiota of the skin, oral cavity, respiratory system, vagina, and tumors are increasingly recognized for their associations with human diseases and therapeutic outcomes.34 Since the gut microbiota was recognized as an essential regulator of the immune system, several groups have investigated its role in cancer, highlighting a close relationship between gut microbiota disruption and tumorigenesis, therapeutic effects, tumor escape, and modulation of immune responses.35 Early evidence of its role in immunotherapy came from studies on ICB, such as anti-CTLA-424,36,37 and anti-PD-1 therapies.25–27 Bifidobacterium and Akkermansia muciniphila were associated with therapeutic efficacy, as they enhanced dendritic cell activity and CD8+ T cell response in the tumor microenvironment.26,27,38,39 These bacteria have been implicated in antitumor responses, and autoimmune diseases and tolerance, revealing the extensive involvement of the gut microbiota in systemic immune activity.40
Besides ICB, the role of the gut microbiota has been clinically investigated in the setting of chemotherapy, radiation therapy, and allogeneic stem cell transplant. Yoon et al have recently reported that gut dysbiosis is already present at diagnosis in a cohort of treatment-naïve, newly diagnosed patients with diffuse large B-cell lymphoma, and a high abundance of Enterobacteriaceae during R-CHOP chemo-immunotherapy (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone) correlates with febrile neutropenia and poor survival.41 In comparison, Lachnospiraceae and Enterococcaceae have been linked to postradiation restoration of hematopoiesis and gastrointestinal repair, revealing a possible protective role against gastrointestinal dysfunction in leukemia patients undergoing radiotherapy.42 Moreover, the effects of high-dose chemotherapy on fecal diversity have been studied in a cohort of autologous-HCT recipients, in which a low gut microbiota diversity has been associated with decreased progression-free survival.43 In the setting of allo-HCT, myeloablative conditioning regimens with chemo and high-dose radiation have been shown to dramatically reduce the alpha diversity of the gut microbiota,44 a measure of the unique bacteria present and their relative frequencies, which is significantly correlated with post-allo-HCT survival and disease relapse.28,29,45 Analyses of clinical data have demonstrated a strong relationship between the abundance of Blautia46 and Clostridia47 and acute graft-versus-host disease (GVHD). Furthermore, a mechanistic link between Enterococcus domination and GVHD has been established.48 Conversely, the presence of Lachnospiraceae has been associated with a reduced occurrence of chronic GVHD.49 In addition, the obligate anaerobes Faecalibacterium, Ruminococcus, and Akkermansia were strongly associated with immune reconstitution after allo-HCT, whereas Staphylococcus abundance affected the lymphopoiesis.50,51 The gut microbiota exerts significant control over the expression of major histocompatibility complex (MHC) class II on intestinal epithelial cells, thereby influencing the promotion of GVHD.52 Notably, the administration of oral vancomycin peritransplantation has been shown to reduce bacterial MHC II inducers, leading to a decrease in CD4+ T cell-mediated GVHD. This result has been recently corroborated in a large clinical allo-HCT cohort.53
Overall, these data demonstrate that the microbiota is fundamental for immune regulation and influences the outcomes of anticancer therapies.
Three clinical studies on the role of the microbiota in CART have been published so far. Despite the retrospective nature of the analyses, these preliminary data suggest that the gut microbiota influences the response and toxicity to CART immunotherapy (results summarized in Table 1). In the first of these studies, conducted by Smith et al, a large cohort of patients with relapsed or refractory B-ALL and large B-cell lymphoma received anti-CD19 CART at 2 institutions, the Memorial Sloan Kettering Cancer Center and the University of Pennsylvania, using both CD28 and 4-1BB costimulated CART products.30 As expected, in 228 patients with NHL and B-ALL, exposure to antibiotics in general and in particular to broad-spectrum anaerobe-targeting ones (piperacillin-tazobactam, imipenem-cilastatin, and/or meropenem [PIM]) within 4 weeks before CART administration corresponded with decreased alpha diversity. More importantly, preinfusion PIM exposure was associated with reduced progression-free survival and overall survival in a multivariable regression model after adjusting for potential confounders. In addition, within the lymphoma subcohort, individuals exposed to antibiotics before CART infusion exhibited a higher incidence of immune effector cell-associated neurotoxicity syndrome. Conversely, in a separate prospective cohort of 48 patients, high abundance of obligate anaerobes such as Ruminococcus, Bacteroides, Faecalibacterium, and Akkermansia was associated with complete response at day 100.30 These results were consistent with the observations derived from previous studies of ICB, where a favorable gut microbiota (high diversity and abundance of Ruminococcaceae and Faecalibacterium) promoted antigen presentation and improved effector T cell function in patients treated with anti-PD-1 therapy.25–27 More recently, Stein-Thoeringer et al confirmed these data in a cohort of American and German patients affected by NHL who received axi-cel, tisa-cel, or liso-cel infusion but were not exposed to antibiotics in the 3 weeks before CART infusion.33 Bifidobacterium longum abundance and peptidoglycan biosynthesis strongly correlated with long-term survival and response to CART, independently of demographic or clinical variables. Furthermore, Lachnospira pectinoschiza and Akkermansia muciniphila significantly correlated with CD3+ and CD4+ T cell counts at the time of T cell apheresis, while Bacteroides, Blautia, and Faecalibacterium prausnitzii negatively impacted on CD3+ and CD8+ T cell levels.33 Finally, the authors highlighted Akkermansia as potentially involved in better quality and performance of the manufactured T cell product. In line with the above-mentioned studies, Hu et al described a different pattern of intestinal microbes in multiple myeloma patients who achieved complete remission (CR) after anti-BCMA CART treatment31 (Table 1). Noteworthy, they found different amino acid metabolism pathways enriched in responders and nonresponders and observed that Bifidobacterium was enriched in CR patients and associated with cytokine release syndrome (CRS). In contrast, they were unable to analyze the microbiome in relation to neurotoxicity incidence and severity due to the small number of events in the cohort. Whether the microbiota influences CRS remains an open question, because neither Smith et al nor Stein-Thoeringer et al reported a correlation between gut microbiota composition and CRS. In particular, it is important to determine which antibiotics are more toxic to the gut microbiota and consequently reduce CART efficacy and increase toxicity. For example, in the retrospective cohort by Smith et al, the authors compared 2 commonly used antibiotics for the treatment of neutropenic fever: piperacillin-tazobactam and cefepime. Interestingly, piperacillin-tazobactam, a known anaerobe-targeting antibiotic that importantly affects the microbiota composition, was associated with reduced progression-free survival and overall survival as compared with cefepime. While a randomized trial would be needed to confirm a differential effect of the 2 antibiotics, it is becoming clear that the use of specific antibiotics might impair CART function. Lastly, it is fundamental to study the impact of antibiotics also in the days and weeks after CART infusion, when their administration is tightly linked to CRS and frequently requires broad-spectrum antibiotic therapy due to the challenging differential diagnosis with sepsis. Pathogen-directed treatments, time-reduced antibiotic exposures, or even only observation in nonneutropenic patients could minimize the dysbiosis and the perturbation of the immune system. In addition, the incidence of CRS is directly related to high tumor burden,1,4,10,12 and therefore its detrimental effect may be reduced by tailoring therapies to achieve better response before CART infusion.
Table 1.
Gut Microbiota Composition and Outcomes in CART-treated Patients
| Publication | Disease | Patients, n (%) | CART, n (%) | AB Exposure, n (%) | Response | Toxicity | |||
|---|---|---|---|---|---|---|---|---|---|
| CR at 3 mo, n (%) | PFS (non-AB vs AB) |
OS (non-AB vs AB) |
CRS (non-AB vs AB) | ICANS (non-AB vs AB) | |||||
| Exposure to AB pre-CART infusion (Smith et al., 2022) | NHL ALL |
137 (60.1) 91 (39.9) |
Anti-CD19, 228 (100): Axi-cel, 72 (31.6) Tisa-cel, 101 (44.3) 19–28z, 55 (24.1) |
No AB, 83 (36.4) Any AB, 145 (63.6) |
Yes, 117 (51.3) No, 111 (48.7) |
NHL-ALL: HR 1.71 (1.12–2.59; P = 0.011) | |||
| NHL: HR 1.29 (0.82–2.01; P = 0.256) | NHL: HR 2.54 (1.41–4.56; P = 0.001) | P = 0.179 | P = 0.013 | ||||||
| P-I-M, 47 (20.6) | NHL-ALL: HR 2.58 (1.68–3.98; P < 0.001) | P = 0.058 | P = 0.023 | ||||||
| NHL: HR 1.83 (1.03–3.27; P = 0.038) | NHL: HR 3.37 (1.77–6.44; P < 0.001) | P = 0.154 | P = 0.002 | ||||||
| ALL: HR 1.96 (1.15–3.35; P = 0.012) | ALL: HR 2.12 (1.2–3.76; P = 0.008) | P = 0.525 | P = 0.254 | ||||||
| Microbiota analysis: NHL ALL |
48 (100) 46 (95.8) 2 (4.2) |
19–28z, 2 (4.2) Axi-cel, 21 (43.8) Tisa-cel, 23 (47.9) Brexu-cel, 2 (4.2) |
Reduced alpha diversity in CART-treated patients (P = 0.0023). Reduced beta diversity in CART-treated patients (P < 0.001). |
Yes, 23 (47.9) No, 25 (52.1) |
High abundance of Ruminococcus, Bacteroides, Faecalibacterium in CR; Akkermansia the top enriched dominant taxon in responders; High abundance of Veillonellales, family Veillonellaceae in NR. |
Blautia, Ruminococcus, Bacteroides, Faecalibacterium associated with the absence of toxicities. | |||
| Microbiota modifications pre- and post-CART in MM (Hu et al., 2022) | MM | 99 (100) | ORR 91 (95)a CR 53 (55.8)a |
||||||
| Microbiota analysis: MM |
81 (81.8) | Anti-BCMA, 81 (100) | Higher alpha diversity in CR vs PR/NR patients; Bifidobacterium, Prevotella, Colllinsella, Sutterella enriched in patients in CR pre- and post-CART; Sutterella associated with prolonged PFS post-CART. |
High abundance of Bifidobacterium and Leuconostoc in patients with CRS. | |||||
| Exposure to ABT pre-CART infusion (Stein-Thoeringer et al., 2023) |
NHL | 172 (100) | Anti-CD19: Axi-cel, 122 (70.9) Tisa-cel, 49 (28.5) Liso-cel, 1 (0.6) |
No AB, 110 (64) Any AB, 62 (36) |
Yes, 82 (47.7) | HR 2.04 (1.38–3.00; P = 0.0009) | HR 2.39 (1.46–3.91; P = 0.0027) | ||
| High-risk ABb, 36 (20.9) | No, 90 (52.3) | HR 3.05 (1.96–4.75; P < 0.0001) | HR 2.99 (1.69–5.29; P = 0.0003) | P = 0.085 | 43.4% vs 63.8% (P = 0.039) | ||||
| Microbiota analysis: NHL | 116 (67.4) | Reduced alpha diversity in high-risk AB-treated patients; High abundance of Prevotella, Veillonella, Enterococcus spp. in high-risk AB-treated patients. |
Pre-CART high abundance of Bacteroides, Ruminococcus, Eubacterium and Akkermansia in CR patients; Bifidobacterium longum associated with response at 6 mo post-CART; High Bacteroides stercoris in NR patients. |
||||||
aResponse at 3 months was assessed in the efficacy analysis cohort of 95 patients.
bHigh-risk AB: piperacillin/tazobactam, meropenem, cefepime, ceftazidime.
AB = antibiotics; ALL = B-cell acute lymphoblastic leukemia; Axi-cel = axicabtagene ciloleucel; Brexu-cel = brexucabtagene autoleucel; CART = chimeric antigen receptor T cell therapy; CR = complete response; CRS = cytokine releasing syndrome; HR = hazard ratio; ICANS = Immune effector cell-associated neurotoxicity syndrome; NHL = non-Hodgkin lymphoma; NR = no response; ORR = overall response rate; OS = overall survival; PFS = progression-free survival; P-I-M = piperacillin/tazobactam, imipenem, meropenem; PR = partial response; Tisa-cel = tisagenlecleucel.
Antibiotic-induced dysbiosis and its effects on immunotherapy outcomes have also been studied in preclinical models. The first observation on CART came from Kuczma et al, who reported that the administration of broad-spectrum antibiotics did not dampen the tumor killing of anti-CD19 CART in murine models of A20 B-cell lymphoma receiving pre-CART lymphodepletion with cyclophosphamide (CTX). Of note, the alteration of the gut microbiome was significantly associated with prolonged persistence of CART and duration of B-cell aplasia in these mice.54 Interestingly, the antitumor effect of CTX was reduced by antibiotics in the CTX-only treated mice, confirming the dependence of CTX efficacy on its immunomodulatory effects.55 In contrast, Uribe-Herranz et al recently demonstrated that immunocompetent mice receiving oral vancomycin—a nonabsorbed Gram-positive bacterium-specific antibiotic—after murine anti-CD19 CART administration experienced better control of A20 lymphoma and B16 melanoma tumors as compared with mice treated with anti-Gram-negative antimicrobials or antibiotic-naïve.32 Mechanistically, the authors found that tumors of vancomycin-treated mice were enriched with endogenous CD8+ T cells and CD11b+ CD103+ dendritic cells. Additionally, markers of T cell activation and cytotoxicity, such as granzyme B, perforin 1, and interferon γ (IFNγ), were increased, along with upregulation of factors involved in the antigen presentation pathway. These data suggested that endogenous CD8+ T cells and dendritic cells might potentiate CART activity via the cross-presentation of tumor-associated antigens, and was further confirmed by the fact that the administration of an anti-MHC-I blocking antibody abrogated these results. Borrowing from the experience of ICB-treated preclinical models,56,57 mice depleted of their own gut microbiota underwent fecal transplantation from healthy human donors, then injected with lymphoma cells and treated with CART and oral vancomycin. Greater tumor shrinkage, higher levels of intratumor dendritic cells, and markedly increased cytotoxic markers were found in mice exposed to CART and vancomycin compared with the controls. Moreover, Ruminococcaceae and Lachnospiraceae were decreased along with tryptophan, biliary acids, and short-chain fatty acids (SCFAs), which have been shown to impair antigen presentation and CD8+ T cell activation.58 However, other studies have reported different SCFAs effects, whose activity depends on host conditions and immunological environment.59–62 In a retrospective analysis, Uribe-Herranz et al also showed that B-ALL patients receiving oral vancomycin (likely for Clostridium difficile infection) had higher CART cell expansion than patients not exposed to this antibiotic.32 Despite the small population size, these preliminary data demonstrate that the gut microbiota modulates the immune responses by boosting CART expansion and directing the endogenous immune reactions toward antitumor effects.
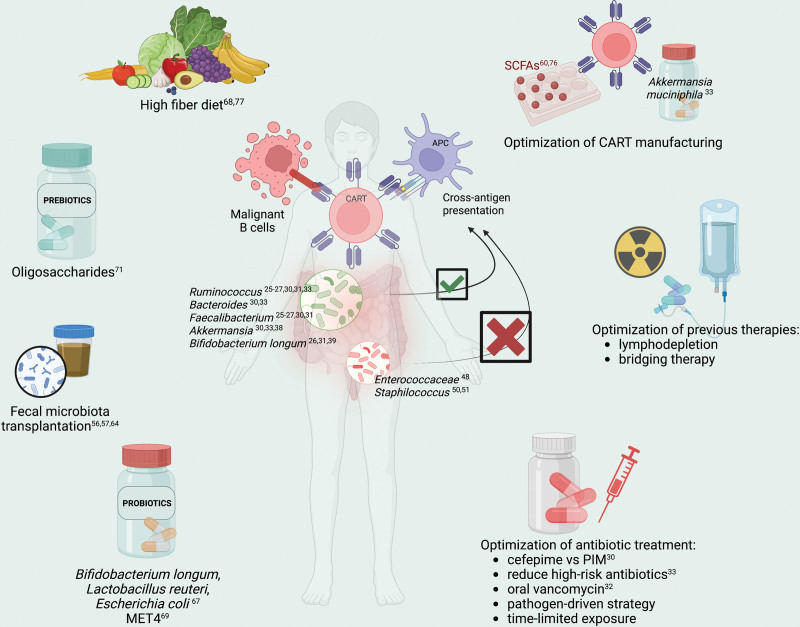
Many therapeutic strategies have been developed to modulate the microbiota activity and bolster the antitumor effects of immunotherapies, such as fecal microbiota transplantation (FMT), probiotics, prebiotics, diet, and adjustments in antimicrobial therapy (Figure 1). The encouraging results achieved in patients treated with ICB and allo-HCT suggest a plausible role of these interventions in CART as well. However, results of clinical trials in CART-treated patients are still awaited. FMT consists of the transfer of a complex and heterogeneous community of microorganisms from a healthy donor, screened for the absence of pathogens, to a recipient. The rationale behind its application is that the re-establishment of gut microbial homeostasis leads to the restoration of beneficial functions of the microbiome, including providing colonization resistance, producing beneficial metabolites, and restoring crosstalk with the mucosal immune system.63 FMT has been successfully implemented for the treatment of recurrent Clostridioides difficile infections and has recently shown promising results both in ICB-treated patients56,57 and those with acute GVHD after allogeneic allo-HCT.64 Multiple clinical studies are ongoing, investigating its role as an adjuvant of ICB, in GVHD treatment, inflammatory bowel disease, and infections both in adults and children (NCT05273255, NCT04729322; NCT04935684, NCT03819803, NCT03812705; NCT04729322, NCT03806803, NCT05739825; NCT05361785, NCT03613545, and NCT03378167). Despite the encouraging results, safety concerns have recently emerged due to the potential transmission of unknown organisms and some caveats, such as the lack of process control and reproducibility at large scale.65
Figure 1.
Possible strategies to enhance CART immunotherapy by modulating the gut microbiota. CART = chimeric antigen receptor T cell therapy; MET4 = Microbial Ecosystem Therapeutics; PIM = piperacillin-tazobactam, imipenem, meropenem; SCFAs = short-chain fatty acids.
A highly pursued approach is the use of probiotics, that is, direct administration of specific bacterial taxa or pool of them to modulate the microbiota. Despite the results achieved in controlling infectious disease recurrence66 and metabolic disorders,40 the beneficial effects of probiotics in combination with immunotherapy for cancer are still debated. On the one hand, Bender et al have reported antitumor effects of Lactobacillus reuteri, Bifidobacterium longum, and Escherichia coli in mice treated with ICB, due to the expansion of IFNγ-producing T cells in the tumor microenvironment.67 On the other hand, Spencer et al did not observe better survival and response rates in melanoma patients treated with ICB and exposed to probiotics. Moreover, they showed that mice administered anti–PD-L1 and probiotics—namely Bifidobacterium longum and Lactobacillus rhamnosus—had greater tumor size than the probiotics-untreated mice and a significantly reduced frequency of IFNγ-positive CD8+ T cells in tumor microenviroment.68 To overcome the complexity of FMT and the potential detrimental effect of selected probiotics, the first in-human trial of a cultivated microbial consortium (Microbial Ecosystem Therapeutics [MET4]) administered as a cotherapy for ICB has been published recently, achieving its primary safety and tolerability endpoints. However, the association between microbial changes and clinical response could not be assessed in this early-phase trial.69 These controversial findings highlight the need for further investigation, and clinical trials are underway to evaluate the potential enhancement of immunotherapy with selected probiotics (NCT04995653, NCT05220124, and NCT05462496) and MET4 (NCT03686202).
Another possible approach to modulate the gut microbiota is the administration of prebiotics, nondigestible dietary substances that stimulate the growth and metabolism of beneficial bacteria in the gut,70 such as inulin, fructo-oligosaccharides, and galacto-oligosaccharides. The clinical administration of an oligosaccharide mixture and resistant starch to allo-HCT recipients, from the pretransplant to day 28, has led to lower mucosa injury and acute GVHD occurrence, mainly by preserving butyrate-producing bacterial populations.71 Depending on the host conditions and the immunological milieu, SCFAs promote the polarization toward a regulatory signature and IL-10-mediated immune tolerance or enhance the cytotoxic behavior of CD8+ T cells.59,61,62 In a GVHD preclinical model, butyrate directly influenced the T cells through the GRP109A receptor. Immunosuppressed mice injected with GRP109A knock-out T cells showed lower levels of markers of IFNγ, TNF, IL-2, increased abundance of the taxa Clostridium and Blautia, and experienced less GVHD than the wild-type, as the result of induced apoptosis of the T cells.72 Therefore, butyrate stimulates the survival and alloreactivity of T cells, and conversely, hinders antigen presentation in vitro and interferes with the cross-priming activity in vivo, abrogating the advantageous effects of vancomycin combined with radiotherapy73 and CART immunotherapy.32 Further data showed that tryptophan, a gut microbial-derived metabolite, signaling through aryl hydrocarbon receptors (AhR), activated the tumor-associated macrophages (TAM) and induced T cell exhaustion in cancer models.74,75 On the contrary, the removal of dietary tryptophan reduced TAM AhR activity and promoted intratumoral accumulation of TNFα+IFNγ+CD8+ T cells, resulting in increased tumor shrinkage. Due to their ability to modulate T cell differentiation and the accessibility of chromatin via the HDAC activity, SCFAs such as butyrate and propionate have also been investigated as supplements during the T cell expansion, showing a more stable transgene expression in SCFAs cocultured cells.60,76 Recently, Spencer et al demonstrated that patients with adequate fiber intake had longer progression-free survival and higher response rates over those with insufficient fiber intake, which correlated with greater microbial alpha diversity and Ruminococcaceae and Faecalibacterium abundances.68 Furthermore, Simpson et al reported that patients on higher fiber and omega-3 consumption achieved greater antitumor immune responses and reduced toxicities after ICB therapy.77 Concurrently, the ketogenic diet and its principal ketone body 3-hydroxybutyrate have been shown to stimulate the production of SCFAs and Bifidobacterium in a mouse model.78 According to previous reports,26,39 a high abundance of Bifidobacterium enhanced the antitumor activity of the ICB through the activation of the endogenous dendritic cells and CD8+ cytotoxic lymphocytes. Overall, SCFAs and diet may favorably impact immunotherapies and enhance the antitumor T cell response in vivo or could be implemented in the in vitro manufacturing CART. Since no clinical data are available, the potential role of dietary approaches in CART-treated patients should be investigated in clinical trials.
Antibiotics profoundly affect the microbiota composition and are commonly used in hematological patients, both as infection prophylaxis and treatment. Broad-spectrum antibiotics cause severe depletion of obligate anaerobes and gut microbe metabolites, compromising the efficacy of immunotherapy or being associated with toxicities. Indeed, they have been associated with a high mortality rate after allogeneic transplantation,28,79 reduced antitumor efficacy of ICB,80 and, more recently, reduced survival in CART-treated patients.30,33 A pathogen-directed treatment instead of the empiric broad-spectrum one could perhaps minimize the dysbiosis and favor the immune system response against tumor, but the specificity of antibiotics is poor. Interestingly, to examine the effects of different antibiotics on the microbiota composition, a phase II clinical trial is ongoing in allo-HCT recipients comparing the effects of piperacillin-tazobactam versus cefepime, meant as a microbiota-sparing strategy (NCT03078010).
In summary, although preclinical and clinical studies have demonstrated that the gut microbiota plays a key role in regulating the response to immunotherapy, defining the underlying mechanisms is still a challenge. Furthermore, due to the high rate of resistance, improving the efficacy of CART is a priority. Based on Stein-Thoeringer et al’s data,33 Lachnospira pectinoschiza and Akkermansia muciniphila might be administered preapheresis to ameliorate lymphocyte collection, whereas SCFAs could be directed to activate T cells and stimulate their survival, both during the in vitro manufacture and the in vivo expansion. In addition, modifying food intake toward a high-fiber or ketogenic diet would be significant in modulating cellular metabolism and maintaining gut microbiota diversity, being a feasible and cost-effective approach. Antibiotics play a crucial role in altering the gut microbiota and their impact on post-CART survival make a pathogen-driven treatment strategy highly recommended to preserve the microbial alpha diversity and harness its beneficial effect on immunotherapy. Further studies are needed to confirm the beneficial effects of oral vancomycin in CART expansion. In conjunction, a patient-tailored lymphodepleting regimen could enhance the cross-antigen presentation by endogenous antigen-presenting cells and stimulate CART survival in vivo. Given the results reached in allo-HCT and ICB-treated patients, FMT may be a matter of interest in CART-treated patients, perhaps in those most exposed to antibiotics. Noteworthy, the impact of antibiotics and dysbiosis on CART efficiency postinfusion needs to be analyzed.
In conclusion, the gut microbiota has demonstrated a profound influence on cancer therapy and immune responses. Preliminary data have suggested its potential role in modulating the effects of CAR T cell therapies, but the underlying mechanisms are not yet fully understood. In this perspective, we have highlighted several potential therapeutic strategies to enhance the efficacy of engineered T cells and optimize the management of CART-treated patients by harnessing the potential of the gut microbiota. Clinical trials are needed to explore the efficacy of these treatments and achieve more robust results.
AUTHOR CONTRIBUTIONS
GG provided substantial contribution to the content and wrote the article. RS and GG contributed substantially to discussion of the content and reviewed the article. MvdB reviewed the article before submission. MR contributed to all the aspects of the article: discussion of the content, edited, and reviewed the article before submission.
DISCLOSURES
The authors have no conflicts of interest to disclose.
SOURCES OF FUNDING
The authors declare no sources of funding.
REFERENCES
- 1.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378:439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2018;380:45–56. [DOI] [PubMed] [Google Scholar]
- 4.Fowler NH, Dickinson M, Dreyling M, et al. Tisagenlecleucel in adult relapsed or refractory follicular lymphoma: the phase 2 ELARA trial. Nat Med. 2022;28:325–332. [DOI] [PubMed] [Google Scholar]
- 5.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377:2531–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Locke FL, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med. 2021;386:640–654. [DOI] [PubMed] [Google Scholar]
- 7.Jacobson CA, Chavez JC, Sehgal AR, et al. Axicabtagene ciloleucel in relapsed or refractory indolent non-Hodgkin lymphoma (ZUMA-5): a single-arm, multicentre, phase 2 trial. Lancet Oncol. 2022;23:91–103. [DOI] [PubMed] [Google Scholar]
- 8.Wang M, Munoz J, Goy A, et al. KTE-X19 CAR t-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2020;382:1331–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah BD, Ghobadi A, Oluwole OO, et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet. 2021;398:491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396:839–852. [DOI] [PubMed] [Google Scholar]
- 11.Abramson JS, Solomon SR, Arnason J, et al. Lisocabtagene maraleucel as second-line therapy for large B-cell lymphoma: primary analysis of the phase 3 TRANSFORM study. Blood. 2023;141:1675–1684. [DOI] [PubMed] [Google Scholar]
- 12.Berdeja JG, Madduri D, Usmani SZ, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet. 2021;398:314–324. [DOI] [PubMed] [Google Scholar]
- 13.Munshi NC, Anderson LD, Shah N, et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med. 2021;384:705–716. [DOI] [PubMed] [Google Scholar]
- 14.Laetsch TW, Maude SL, Rives S, et al. Three-year update of tisagenlecleucel in pediatric and young adult patients with relapsed/refractory acute lymphoblastic leukemia in the ELIANA trial. J Clin Oncol. 2023;41:1664–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol. 2019;20:31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chong EA, Ruella M, Schuster SJ, Lymphoma Program Investigators at the University of Pennsylvania. Five-year outcomes for refractory B-cell lymphomas with CAR T-cell therapy. N Engl J Med. 2021;384:673–674. [DOI] [PubMed] [Google Scholar]
- 17.Ghilardi G, Braendstrup P, Chong EA, et al. CAR-T TREK through the lymphoma universe, to boldly go where no other therapy has gone before. Br J Haematol. 2021;193:449–465. [DOI] [PubMed] [Google Scholar]
- 18.Lemoine J, Ruella M, Houot R. Born to survive: how cancer cells resist CAR T cell therapy. J Hematol Oncol. 2021;14:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee YG, Guruprasad P, Ghilardi G, et al. Modulation of BCL-2 in both T cells and tumor cells to enhance chimeric antigen receptor T-cell Immunotherapy against cancer. Cancer Discov. 2022;12:2372–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cullin N, Azevedo Antunes C, Straussman R, et al. Microbiome and cancer. Cancer Cell. 2021;39:1317–1341. [DOI] [PubMed] [Google Scholar]
- 21.Nishida A, Inoue R, Inatomi O, et al. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin J Gastroenterol. 2018;11:1–10. [DOI] [PubMed] [Google Scholar]
- 22.Quigley EMM. Microbiota-brain-gut axis and neurodegenerative diseases. Curr Neurol Neurosci Rep. 2017;17:94. [DOI] [PubMed] [Google Scholar]
- 23.Zhang S, Cai Y, Meng C, et al. The role of the microbiome in diabetes mellitus. Diabetes Res Clin Pract. 2021;172:108645. [DOI] [PubMed] [Google Scholar]
- 24.Dubin K, Callahan MK, Ren B, et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat Commun. 2016;7:10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gopalakrishnan V, Spencer CN, Nezi L, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matson V, Fessler J, Bao R, et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018;359:104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359:91–97. [DOI] [PubMed] [Google Scholar]
- 28.Peled JU, Gomes ALC, Devlin SM, et al. Microbiota as predictor of mortality in allogeneic hematopoietic-cell transplantation. N Engl J Med. 2020;382:822–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taur Y, Jenq RR, Perales MA, et al. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood. 2014;124:1174–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith M, Dai A, Ghilardi G, et al. Gut microbiome correlates of response and toxicity following anti-CD19 CAR T cell therapy. Nat Med. 2022;28:713–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu Y, Li J, Ni F, et al. CAR-T cell therapy-related cytokine release syndrome and therapeutic response is modulated by the gut microbiome in hematologic malignancies. Nat Commun. 2022;13:5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uribe-Herranz M, Beghi S, Ruella M, et al. Modulation of the gut microbiota engages antigen cross-presentation to enhance antitumor effects of CAR T cell immunotherapy. Mol Ther. 2023;31:686–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stein-Thoeringer CK, Saini NY, Zamir E, et al. A non-antibiotic-disrupted gut microbiome is associated with clinical responses to CD19-CAR-T cell cancer immunotherapy. Nat Med. 2023;29:906–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12:31–46. [DOI] [PubMed] [Google Scholar]
- 35.Zitvogel L, Ayyoub M, Routy B, et al. Microbiome and anticancer immunosurveillance. Cell. 2016;165:276–287. [DOI] [PubMed] [Google Scholar]
- 36.Chaput N, Lepage P, Coutzac C, et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol. 2017;28:1368–1379. [DOI] [PubMed] [Google Scholar]
- 37.Vétizou M, Pitt JM, Daillère R, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350:1079–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Derosa L, Routy B, Thomas AM, et al. Intestinal Akkermansia muciniphila predicts clinical response to PD-1 blockade in patients with advanced non-small-cell lung cancer. Nat Med. 2022;28:315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sivan A, Corrales L, Hubert N, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350:1084–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cani PD, Depommier C, Derrien M, et al. Akkermansia muciniphila: paradigm for next-generation beneficial microorganisms. Nat Rev Gastroenterol Hepatol. 2022;19:625–637. [DOI] [PubMed] [Google Scholar]
- 41.Yoon SE, Kang W, Choi S, et al. The influence of microbial dysbiosis on immunochemotherapy-related efficacy and safety in diffuse large B-cell lymphoma. Blood. 2023;141:2224–2238. [DOI] [PubMed] [Google Scholar]
- 42.Guo H, Chou W-C, Lai Y, et al. Multi-omics analyses of radiation survivors identify radioprotective microbes and metabolites. Science. 2020;370:eaay9097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khan N, Lindner S, Gomes ALC, et al. Fecal microbiota diversity disruption and clinical outcomes after auto-HCT: a multicenter observational study. Blood. 2021;137:1527–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shouval R, Waters NR, Gomes ALC, et al. Conditioning regimens are associated with distinct patterns of microbiota injury in allogeneic hematopoietic cell transplantation. Clin Cancer Res. 2023;29:165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peled JU, Devlin SM, Staffas A, et al. Intestinal microbiota and relapse after hematopoietic-cell transplantation. J Clin Oncol. 2017;35:1650–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jenq RR, Taur Y, Devlin SM, et al. Intestinal blautia is associated with reduced death from graft-versus-host disease. Biol Blood Marrow Transplant. 2015;21:1373–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burgos da Silva M, Ponce DM, Dai A, et al. Preservation of the fecal microbiome is associated with reduced severity of graft-versus-host disease. Blood. 2022;140:2385–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stein-Thoeringer CK, Nichols KB, Lazrak A, et al. Lactose drives enterococcus expansion to promote graft-versus-host disease. Science. 2019;366:1143–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Markey KA, Schluter J, Gomes ALC, et al. The microbe-derived short-chain fatty acids butyrate and propionate are associated with protection from chronic GVHD. Blood. 2020;136:130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miltiadous O, Waters NR, Andrlová H, et al. Early intestinal microbial features are associated with CD4 T-cell recovery after allogeneic hematopoietic transplant. Blood. 2022;139:2758–2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schluter J, Peled JU, Taylor BP, et al. The gut microbiota is associated with immune cell dynamics in humans. Nature. 2020;588:303–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koyama M, Mukhopadhyay P, Schuster IS, et al. MHC class II antigen presentation by the intestinal epithelium initiates graft-versus-host disease and is influenced by the microbiota. Immunity. 2019;51:885–898.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koyama M, Hippe DS, Srinivasan S, et al. Intestinal microbiota controls graft-versus-host disease independent of donor-host genetic disparity. Immunity. 2023. Aug 8. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kuczma MP, Ding Z-C, Li T, et al. The impact of antibiotic usage on the efficacy of chemoimmunotherapy is contingent on the source of tumor-reactive T cells. Oncotarget. 2017;8:111931–111942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Viaud S, Saccheri F, Mignot G, et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science. 2013;342:971–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baruch EN, Youngster I, Ben-Betzalel G, et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science. 2021;371:602–609. [DOI] [PubMed] [Google Scholar]
- 57.Davar D, Dzutsev AK, McCulloch JA, et al. Fecal microbiota transplant overcomes resistance to anti–PD-1 therapy in melanoma patients. Science. 2021;371:595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nastasi C, Fredholm S, Willerslev-Olsen A, et al. Butyrate and propionate inhibit antigen-specific CD8+ T cell activation by suppressing IL-12 production by antigen-presenting cells. Sci Rep. 2017;7:14516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim CH. Control of lymphocyte functions by gut microbiota-derived short-chain fatty acids. Cell Mol Immunol. 2021;18:1161–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luu M, Riester Z, Baldrich A, et al. Microbial short-chain fatty acids modulate CD8+ T cell responses and improve adoptive immunotherapy for cancer. Nat Commun. 2021;12:4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luu M, Weigand K, Wedi F, et al. Regulation of the effector function of CD8+ T cells by gut microbiota-derived metabolite butyrate. Sci Rep. 2018;8:14430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rangan P, Mondino A. Microbial short-chain fatty acids: a strategy to tune adoptive T cell therapy. J ImmunoTher Cancer. 2022;10:e004147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sorbara MT, Pamer EG. Microbiome-based therapeutics. Nat Rev Microbiol. 2022;20:365–380. [DOI] [PubMed] [Google Scholar]
- 64.Qiao X, Biliński J, Wang L, et al. Safety and efficacy of fecal microbiota transplantation in the treatment of graft-versus-host disease. Bone Marrow Transplant. 2023;58:10–19. [DOI] [PubMed] [Google Scholar]
- 65.Araujo DV, Watson GA, Oliva M, et al. Bugs as drugs: the role of microbiome in cancer focusing on immunotherapeutics. Cancer Treat Rev. 2021;92:102125. [DOI] [PubMed] [Google Scholar]
- 66.Goldenberg JZ, Yap C, Lytvyn L, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;12):CD006095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bender MJ, McPherson AC, Phelps CM, et al. Dietary tryptophan metabolite released by intratumoral Lactobacillus reuteri facilitates immune checkpoint inhibitor treatment. Cell. 2023;186:1846–1862.e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Spencer CN, McQuade JL, Gopalakrishnan V, et al. Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science. 2021;374:1632–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Spreafico A, Heirali AA, Araujo DV, et al. First-in-class Microbial Ecosystem Therapeutic 4 (MET4) in combination with immune checkpoint inhibitors in patients with advanced solid tumors (MET4-IO trial). Ann Oncol. 2023;34:520–530. [DOI] [PubMed] [Google Scholar]
- 70.Gibson GR, Hutkins R, Sanders ME, et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14:491–502. [DOI] [PubMed] [Google Scholar]
- 71.Yoshifuji K, Inamoto K, Kiridoshi Y, et al. Prebiotics protect against acute graft-versus-host disease and preserve the gut microbiota in stem cell transplantation. Blood Adv. 2020;4:4607–4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Docampo MD, da Silva MB, Lazrak A, et al. Alloreactive T cells deficient of the short-chain fatty acid receptor GPR109A induce less graft-versus-host disease. Blood. 2022;139:2392–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Uribe-Herranz M, Rafail S, Beghi S, et al. Gut microbiota modulate dendritic cell antigen presentation and radiotherapy-induced antitumor immune response. J Clin Invest. 2020;130:466–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hezaveh K, Shinde RS, Klötgen A, et al. Tryptophan-derived microbial metabolites activate the aryl hydrocarbon receptor in tumor-associated macrophages to suppress anti-tumor immunity. Immunity. 2022;55:324–340.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu Y, Zhou N, Zhou L, et al. IL-2 regulates tumor-reactive CD8+ T cell exhaustion by activating the aryl hydrocarbon receptor. Nat Immunol. 2021;22:358–369. [DOI] [PubMed] [Google Scholar]
- 76.Moore TV, Scurti GM, DeJong M, et al. HDAC inhibition prevents transgene expression downregulation and loss-of-function in T-cell-receptor-transduced T cells. Mol Ther Oncolytics. 2021;20:352–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Simpson RC, Shanahan ER, Batten M, et al. Diet-driven microbial ecology underpins associations between cancer immunotherapy outcomes and the gut microbiome. Nat Med. 2022;28:2344–2352. [DOI] [PubMed] [Google Scholar]
- 78.Ferrere G, Tidjani Alou M, Liu P, et al. Ketogenic diet and ketone bodies enhance the anticancer effects of PD-1 blockade. JCI Insight. 2021;6:e145207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shono Y, Docampo MD, Peled JU, et al. Increased GVHD-related mortality with broad-spectrum antibiotic use after allogeneic hematopoietic stem cell transplantation in human patients and mice. Sci Transl Med. 2016;8:339ra71–339ra71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Khan U, Ho K, Hwang EK, et al. Impact of use of antibiotics on response to immune checkpoint inhibitors and tumor microenvironment. Am J Clin Oncol. 2021;44:247–253. [DOI] [PubMed] [Google Scholar]