Abstract
PURPOSE
For survivors of childhood cancer treated with doxorubicin, dexrazoxane is cardioprotective for at least 5 years. However, longer-term data are lacking.
METHODS
Within the Children's Oncology Group and the Dana Farber Cancer Institute's Childhood Acute Lymphoblastic Leukemia Consortium, we evaluated four randomized trials of children with acute lymphoblastic leukemia or Hodgkin lymphoma, who received doxorubicin with or without dexrazoxane, and a nonrandomized trial of patients with osteosarcoma who all received doxorubicin with dexrazoxane. Cumulative doxorubicin doses ranged from 100 to 600 mg/m2 across these five trials, and dexrazoxane was administered uniformly (10:1 mg/m2 ratio) as an intravenous bolus before doxorubicin. Cardiac function was prospectively assessed in survivors from these trials, plus a matched group of survivors of osteosarcoma treated with doxorubicin without dexrazoxane. Two-dimensional echocardiograms and blood biomarkers were analyzed centrally in blinded fashion. Multivariate analyses adjusted for demographic characteristics, cumulative doxorubicin dose, and chest radiotherapy determined the differences and associations by dexrazoxane status.
RESULTS
From 49 participating institutions, 195 participants were assessed at 18.1 ± 2.7 years since cancer diagnosis (51% dexrazoxane-exposed; cumulative doxorubicin dose 297 ± 91 mg/m2). Dexrazoxane administration was associated with superior left ventricular fractional shortening (absolute difference, +1.4% [95% CI, 0.3 to 2.5]) and ejection fraction (absolute difference, +1.6% [95% CI, 0.0 to 3.2]), and lower myocardial stress per B-type natriuretic peptide (–6.7 pg/mL [95% CI, –10.6 to –2.8]). Dexrazoxane was associated with a reduced risk of having lower left ventricular function (fractional shortening < 30% or ejection fraction < 50%; odds ratio, 0.24 [95% CI, 0.07 to 0.81]). This protective association was primarily seen in those treated with cumulative doxorubicin doses ≥ 250 mg/m2.
CONCLUSION
Among young adult-aged survivors of childhood cancer, dexrazoxane was associated with a cardioprotective effect nearly 20 years after initial anthracycline exposure.
INTRODUCTION
Improvements in the treatment of childhood cancer have been one of the major medical advances of the past 50 years, with 5-year survival increasing from < 60% to more than 85% today.1 These advances are attributed to refinements in surgery, radiotherapy, and combination chemotherapy, including anthracyclines. Although a newer generation of molecularly targeted agents is receiving much attention, contemporary therapy for most pediatric cancers still relies on anthracyclines and other cytotoxic chemotherapeutics.2 Anthracyclines are routinely used to treat children and adolescents with newly diagnosed acute lymphoblastic and myeloid leukemias, most lymphomas, neuroblastoma, sarcomas, and advanced-stage liver and kidney tumors.
CONTEXT
Key Objective
This multi-institutional cooperative group study sought to determine the long-term efficacy of dexrazoxane in mitigating anthracycline-related cardiotoxicity in survivors of childhood cancer treated on dexrazoxane-containing clinical trials.
Knowledge Generated
We found that among survivors of childhood cancer assessed on average 18 years after cancer diagnosis, those assigned to doxorubicin with dexrazoxane were significantly more likely to have preserved left ventricular function (measured by echocardiography) and lower myocardial stress (measured by serum natriuretic peptide levels) compared with the group assigned to doxorubicin alone. The cardioprotective effect of dexrazoxane was primarily observed in survivors treated with greater cumulative doses of doxorubicin (250 mg/m2 or greater).
Relevance (J.W. Friedberg)
Dexrazoxane should be considered as a standard cardioprotectant in children with cancer receiving a cumulative dose of at least 250 mg/m2 of doxorubicin.*
*Relevance section written by JCO Editor-in-Chief Jonathan W. Friedberg, MD.
Cardiomyopathy is the major late toxicity of anthracycline therapy. The cumulative incidence of clinical heart failure in long-term survivors of childhood cancer approaches 25% by age 40 years for some groups.3 Cumulative anthracycline dose is one of the most important predictors for cardiomyopathy; additional predictors include radiation to the heart, younger age at exposure, female sex, hypertension, and other potentially modifiable risk factors.4 Although overall late mortality and morbidity have improved in children treated in the 1990s compared with the 1970s, late cardiovascular morbidity has not declined appreciably over this time period.5,6
For these reasons, implementation of an effective cardioprotective strategy for newly diagnosed children and adolescents receiving anthracyclines is important. Dexrazoxane is a bisdioxopiperazine that has been tested and used as a cardioprotectant.7 Randomized trials of dexrazoxane in anthracycline-exposed adult patients with breast cancer have demonstrated its efficacy in minimizing the risk of both clinical and subclinical heart failure.8 As a result, dexrazoxane is approved by the US Food and Drug Administration for patients with breast cancer who are receiving ≥ 300 mg/m2 of doxorubicin-based therapy. ASCO and the European Society for Medical Oncology both recommend consideration of dexrazoxane use for any adult patient with cancer expected to receive high-dose anthracycline therapy exceeding 250-300 mg/m2 (total doxorubicin equivalent dose).9,10 However, long-term data on the cardioprotective efficacy of dexrazoxane in survivors of childhood cancer are lacking, although short- and medium-term data indicate its potential benefit.11-13 We addressed this gap in knowledge by conducting a prospective multi-institutional follow-up study (Children's Oncology Group [COG] ALTE11C2; ClinicalTrials.gov identifier: NCT01790152) of childhood cancer survivors previously treated on dexrazoxane-containing clinical trials.
METHODS
Participants
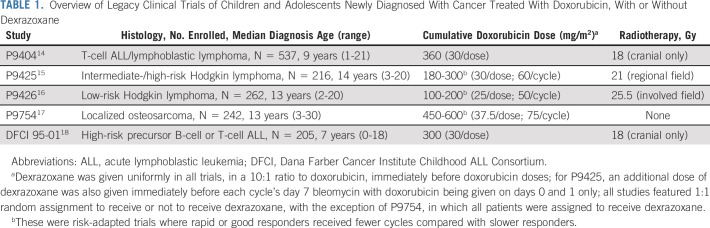
Eligible ALTE11C2 participants included those enrolled on legacy COG protocols P9404, P9425, P9426, and P9754, and Dana Farber Cancer Institute (DFCI) Childhood Acute Lymphoblastic Leukemia Consortium protocol 95-01, all of which assigned patients to doxorubicin doses ranging from 100 to 600 mg/m2 (Table 1).14-18 No other anthracyclines were used in these protocols. P9404, P9425, P9426, and DFCI 95-01 were all phase III clinical trials of acute lymphoblastic leukemia/lymphoma or Hodgkin lymphoma that featured upfront random assignment to treatment with or without dexrazoxane. P9754 was a trial for localized osteosarcoma in which all patients received doxorubicin with dexrazoxane. Therefore, for ALTE11C2, a comparison group (non–dexrazoxane-exposed localized osteosarcoma patients treated with ≥ 450 mg/m2 doxorubicin contemporaneously and who otherwise matched P9754's eligibility criteria) also was recruited from participating sites. Eligible ALTE11C2 participants had to be in remission from their primary cancer and could not have had any subsequent malignant neoplasm treated with a cardiotoxic drug. Primary cancer treatment information, including doxorubicin doses and radiation exposures, was obtained from COG and DFCI. Dexrazoxane was administered uniformly as one intravenous bolus in a 10:1 mg/m2 dose ratio to doxorubicin in all five trials.
TABLE 1.
Overview of Legacy Clinical Trials of Children and Adolescents Newly Diagnosed With Cancer Treated With Doxorubicin, With or Without Dexrazoxane
Measurements
Participants were recruited by their treating institution in the United States and Canada for a one-time assessment, which included anthropometric measurements and a ≥ 10-hour fasting blood draw. Serum samples were analyzed for cardiomyocyte injury (high-sensitivity cardiac troponin T) and myocardial stress (B-type natriuretic peptides [BNP] and N-terminal pro-BNP). Other markers of inflammation and heart failure (high-sensitivity C-reactive protein, galectin-3, growth differentiation factor-15, soluble urokinase plasminogen activator receptor, and ST2) were collected for exploratory analyses. Samples were shipped to a Clinical Laboratory Improvement Amendments–certified laboratory and analyzed, with evaluators blinded to dexrazoxane status. Participants' medical histories were updated by research staff, including documenting the presence of cardiovascular and related diseases (hypertension, dyslipidemia, and diabetes).
Participants underwent a standardized two-dimensional, M-mode and Doppler echocardiogram following the ALTE11C2 protocol.19 Digitized copies of the study echocardiograms were centrally reviewed and remeasured by researchers blinded to dexrazoxane and clinical status (S.A. and N.S.). The primary protocol-specified echocardiographic outcome was end-diastolic wall thickness-to-dimension ratio as a measure of left ventricular (LV) pathologic remodeling.20 Prespecified secondary outcomes included indices of LV systolic (fractional shortening [FS] and biplane Simpson's ejection fraction [EF]) and diastolic function. In addition to these usual indices of myocardial mechanics, we also measured longitudinal, circumferential, and radial strain with post hoc speckle tracking (by S.A.; TomTec Imaging Systems, Munich, Germany). All study procedures were approved by the Central Institutional Review Board of the US National Institutes of Health and review boards at participating sites. All participants or their legal guardians provided informed consent.
Analyses
T-test, Wilcoxon rank-sum, chi-square, and Fisher's exact tests were used to compare continuous and categorical patient characteristics and outcomes by dexrazoxane status as appropriate. Prespecified subanalyses included comparing outcomes for dexrazoxane- versus non–dexrazoxane-assigned subjects within sex-specific stratum and cumulative doxorubicin dose stratum (< 250 and ≥ 250 mg/m2 per international consensus recommendations).21 For continuous outcomes, multivariable linear regression was used to examine differences by dexrazoxane status, adjusting for sex, age at cancer diagnosis, current age, doxorubicin dose, radiation to the heart (yes/no), and history of cardiomyopathy requiring medications or heart transplant. The distribution of outcomes was assessed by residual and Q-Q plots, and log-transformed if there were potential departures from normality. Multivariable logistic regression adjusting for the same covariates assessed the association (odds ratio [OR]) of having lower LV systolic function with dexrazoxane assignment, which was defined as FS < 30% or EF < 50%. Individuals with a history of cardiomyopathy were coded as having lower LV systolic function regardless of current FS or EF. All analyses were performed using Stata (by D.R.D., version 17, StataCorp, College Station, TX).
RESULTS
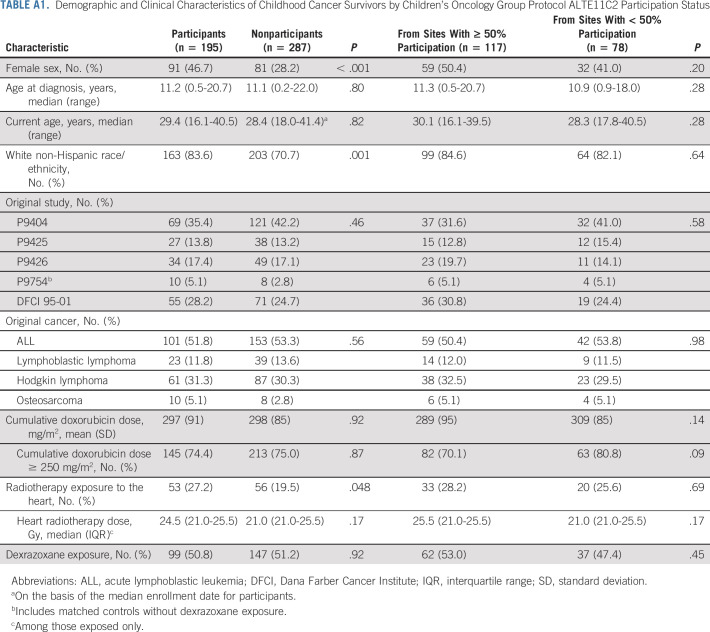
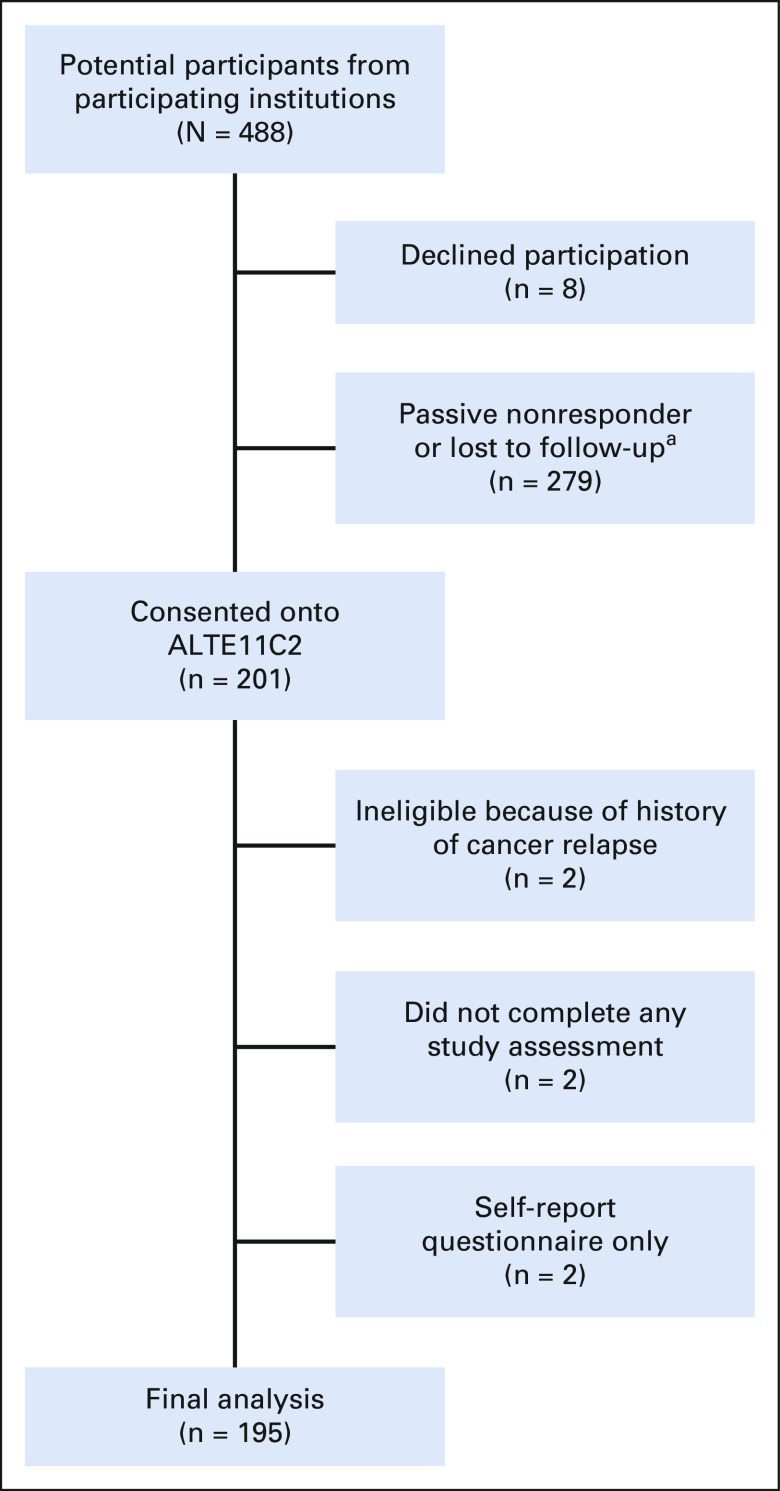
Of 488 potentially eligible participants from 49 participating institutions, 201 (41.2%) were enrolled between May 2014 and March 2021. Among nonparticipants, eight declined participation and the remainder were either passive nonresponders or assumed to be lost to follow-up (Appendix Fig A1, online only). Among enrolled participants, two were found to be ineligible because of history of relapse, two consented but did not complete any study assessments, and two consented to self-report questionnaire submission only, resulting in 195 participants in the final analysis (50.8% assigned to receive dexrazoxane). When characteristics between nonparticipants and participants were compared, nonparticipants were more likely to be male and to belong to a racial or ethnic minority, and less likely to have had heart radiation exposure (Appendix Table A1, online only). However, characteristics were similar among participants enrolled from institutions that recruited a majority of their participants versus those recruited from sites with < 50% participation.
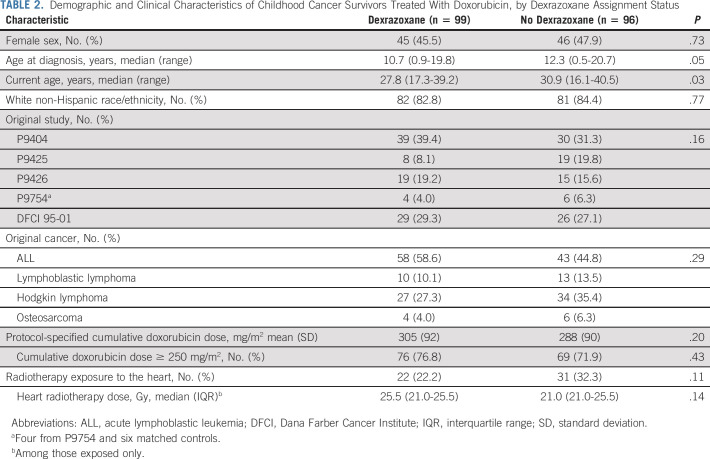
Participants had a mean (±standard deviation) attained age of 28.8 ± 5.3 years at study participation and were 18.1 ± 2.7 years since cancer diagnosis. Characteristics between those treated with and without dexrazoxane were similar except that dexrazoxane-assigned participants were slightly younger at time of cancer diagnosis and at study assessment (Table 2). Otherwise, the two groups had similar mean protocol-specified doxorubicin doses (305 ± 92 mg/m2 v 288 ± 90 mg/m2; P = .20) and proportions with heart radiation exposure (22.2% v 32.3.%; P = .11).
TABLE 2.
Demographic and Clinical Characteristics of Childhood Cancer Survivors Treated With Doxorubicin, by Dexrazoxane Assignment Status
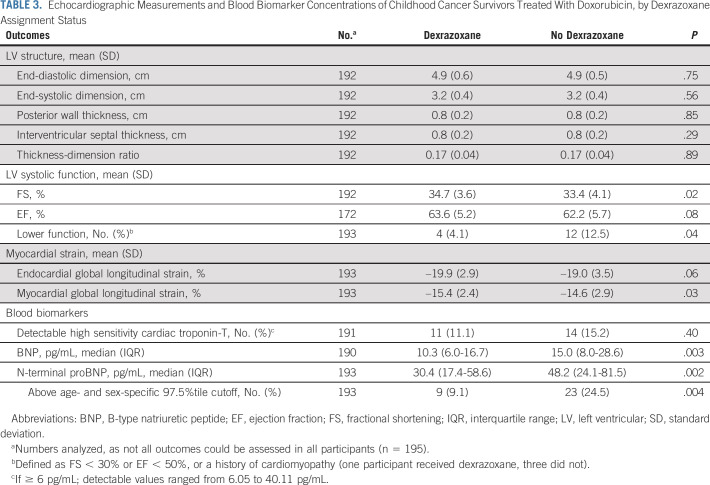
Univariate analyses did not reveal significant differences in the LV structure including the wall thickness-dimension ratio (Table 3). However, the dexrazoxane group had better LV systolic function compared with the no dexrazoxane group, with greater FS (34.7 ± 3.6% v 33.4 ± 4.1%; P = .02), EF (63.6 ± 5.2% v 62.2 ± 5.7%; P = .08), and fewer participants with lower LV function (4.1% v 12.5%; P = .04). Measurements of global longitudinal strain also favored the dexrazoxane group (P = .03-.06). The dexrazoxane group also had significantly lower BNP and NT-proBNP levels, indicating reduced myocardial stress (P < .01); this included a significantly smaller proportion of participants with NT-proBNP levels above an age- and sex-specific 97.5th percentile cutoff (P = .004).22
TABLE 3.
Echocardiographic Measurements and Blood Biomarker Concentrations of Childhood Cancer Survivors Treated With Doxorubicin, by Dexrazoxane Assignment Status
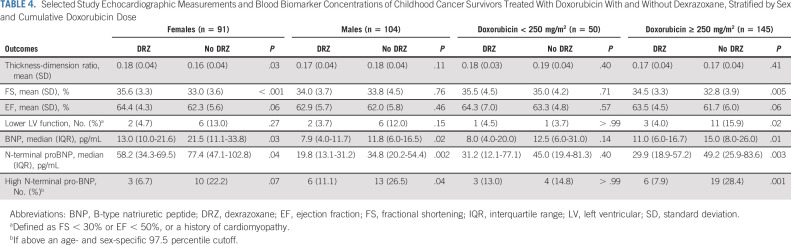
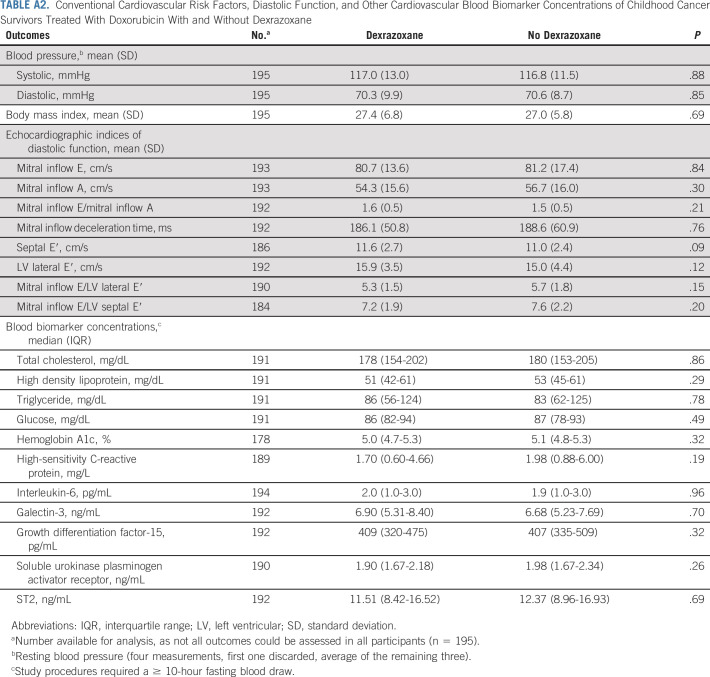
In subanalyses, differences in LV systolic function favoring dexrazoxane appear to be more evident in female versus male participants, and among those treated with doxorubicin doses ≥ 250 mg/m2 versus < 250 mg/m2 (Table 4). In sensitivity analyses, measurements of LV systolic function did not substantially differ among participants enrolled from sites with ≥ 50% versus < 50% participation (data not shown). Dexrazoxane status also was not associated with differences in conventional cardiovascular risk factors, diastolic function, or other blood biomarkers (Appendix Table A2, online only).
TABLE 4.
Selected Study Echocardiographic Measurements and Blood Biomarker Concentrations of Childhood Cancer Survivors Treated With Doxorubicin With and Without Dexrazoxane, Stratified by Sex and Cumulative Doxorubicin Dose
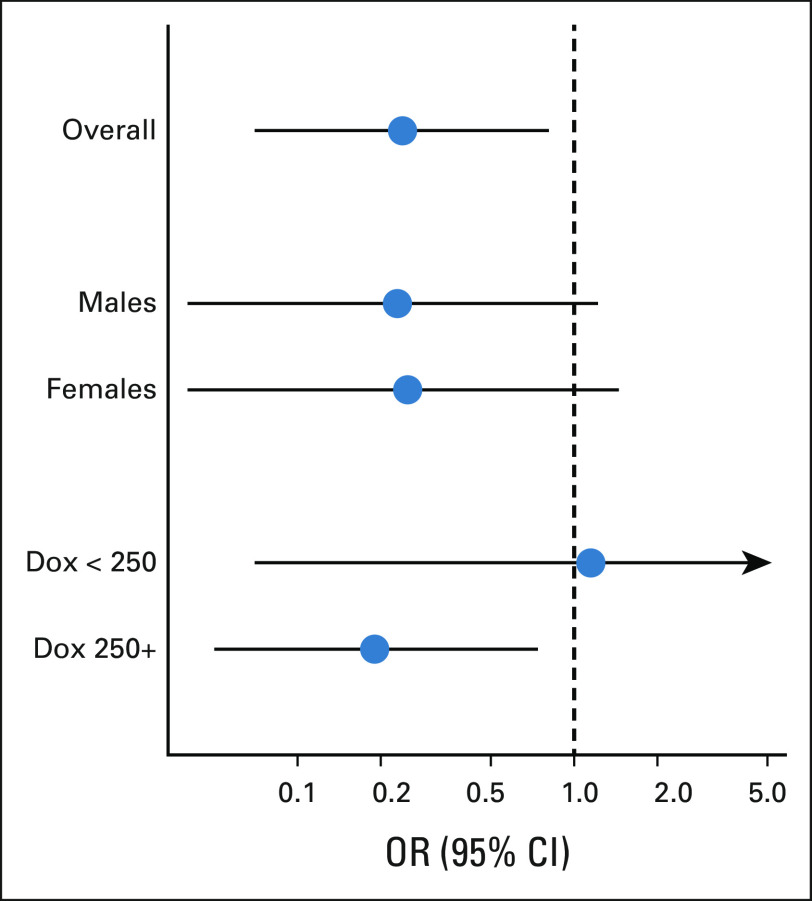
Multivariable analyses confirmed these results, with dexrazoxane assignment associated with better LV systolic function (FS absolute difference: +1.4% [95% CI, 0.3 to 2.5]; EF absolute difference: +1.6% [95% CI, 0.0 to 3.2]), and blood biomarkers consistent with lower myocardial stress (BNP, –6.7 pg/mL [95% CI, –10.6 to –2.8]; NT pro-BNP, –22.1 pg/mL [95% CI, –34.3 to –9.8]; Table 5). The absolute differences in global longitudinal strain, although not statistically significant, also favored dexrazoxane (endocardial, –0.8% [95% CI, –1.7 to 0.1]; myocardial, –0.8% [95% CI, –1.5 to 0.0]). Finally, the risk of reduced LV function was lower in the dexrazoxane group (OR, 0.24 [95% CI, 0.07 to 0.81; Fig 1). Dexrazoxane's protective association was specific to participants treated with ≥ 250 mg/m2 of doxorubicin (OR, 0.19 [95% CI, 0.05 to 0.76]) and not seen in those receiving < 250 mg/m2 (OR, 1.17 [95% CI, 0.07 to 20.35]). If a more stringent definition for LV systolic dysfunction was adopted (FS < 28%, EF < 50%), the association with dexrazoxane was similar but less precise—among participants treated with doxorubicin ≥ 250 mg/m2, OR, 0.29 (95% CI, 0.05 to 1.50).
TABLE 5.
Multivariable Differences in Selected Echocardiographic Measurements and Blood Biomarker Concentrations of Childhood Cancer Survivors Treated With Doxorubicin With Dexrazoxane Versus Doxorubicin Without Dexrazoxane (referent group)
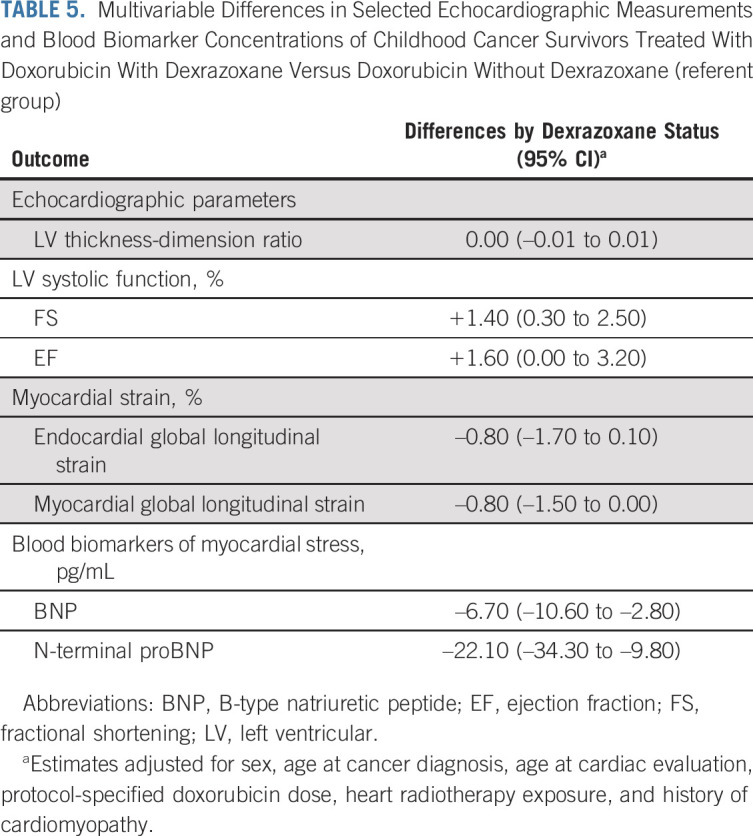
FIG 1.
Odds of having lower left ventricular function (fractional shortening < 30% or ejection fraction < 50%) among childhood cancer survivors treated with Dox and dexrazoxane versus Dox without dexrazoxane (referent group). Results overall, by sex, and by lower and greater cumulative Dox doses (< 250 or ≥ 250 mg/m2). Dox, doxorubicin; OR, odds ratio.
DISCUSSION
In this prospective multi-institutional study, we found that long-term survivors of childhood cancer treated with dexrazoxane tended to have better LV systolic function and lower blood biomarker levels of myocardial stress compared with those not treated with dexrazoxane. These benefits were accentuated in survivors receiving higher anthracycline (doxorubicin) doses. Most participants had been treated on clinical trials in which they were randomly assigned to dexrazoxane, and all long-term study outcomes were interpreted by assessors blinded to dexrazoxane status.
We detected modest echocardiographic differences in LV systolic function (< 2% absolute difference in either FS or EF). However, the mean age of our participants was only 29 years, and clinical heart failure following treatment for childhood cancer has a long latency period. For example, internationally validated prediction models show that even for survivors at high risk for cardiomyopathy, the cumulative incidence of clinical cardiomyopathy by age 30 years is < 5%, but that it increases to > 10% by age 40 years.3 As such, the differences we found in this study may become more prominent as survivors age and that any benefit from dexrazoxane could become more evident with further follow-up.23,24 Mild depression of LV function has been associated with subsequent increased all-cause mortality in other pediatric populations such as HIV-infected children.25 Finally, it is important to acknowledge that the absolute differences in LV function reported in this study can be within the intraobserver and interobserver variability of measurements at an individual level.20,26 However, we sought to enhance the rigor of our results by centrally remeasuring all study echocardiograms in blinded fashion. Central remeasurement has been shown to improve the reliability of echocardiographic measurements in cardiac clinical trials, which focus on mean group differences.27
Aside from minimizing anthracycline exposure, dexrazoxane is the only other primary cardioprotective strategy available to children and adolescents with cancer. Studies examining potential cardioprotection with other agents (eg, coenzyme-Q10, L-carnitine, and N-acetylcysteine), liposomal formulations of anthracyclines, or prolonged anthracycline infusions have not found those strategies to benefit children and adolescents.2,8,28,29 Within COG, new liposomal anthracycline formulations (eg, ClinicalTrials.gov identifier: NCT04293562) are being tested, but efficacy data are not yet available. However, although dexrazoxane appears to offer some cardioprotection, this protection is not complete.30 Periodic echocardiographic surveillance is recommended for all survivors treated with anthracyclines, and if the heart was exposed to radiotherapy.21 Although our study identified significant differences in serum natriuretic peptide levels, there is no currently accepted role for using this biomarker for post cancer-therapy cardiomyopathy surveillance.31
Our findings are consistent with earlier reports from randomized trials of dexrazoxane. Dexrazoxane was associated with significantly reduced serum cardiac troponin concentrations immediately after doxorubicin exposure in the DFCI 95-01 study.11 Five years after therapy, the dexrazoxane group had less pathologic LV remodeling (a greater wall thickness-dimension ratio) and better LV systolic function; these differences were particularly pronounced in females.12 In patients treated on protocol P9404, with 3 years of follow-up, similar associations were found between dexrazoxane treatment and acute serum troponin concentrations, LV pathologic remodeling, and systolic function.13 Nonrandomized pediatric data from patients with acute myeloid leukemia treated with daunorubicin and mitoxantrone also suggest that dexrazoxane minimized LV systolic dysfunction during therapy and for several years into follow-up.32 Finally, the magnitude of the potential risk reduction we observed for lower LV systolic function is nearly identical to those reported in meta-analyses of randomized clinical trials (primarily of older adult patients with breast cancer), where the aggregated risk ratio for clinical heart failure was 0.18 (95% CI, 0.10 to 0.32).8 In these meta-analyses, when subclinical heart failure also was assessed, the effect associated with dexrazoxane remained highly protective (risk ratio, 0.29 [95% CI, 0.20 to 0.41]). Meta-analyses of nonrandomized studies in children found similar results at a median follow-up < 10 years.33
Historically, dexrazoxane use has been limited in children.34,35 In part, its use was limited because evidence of long-term benefits was lacking, as well as concern about acute toxicities, possible effects on cancer recurrence rates, and induced second cancers. Although concurrent administration of dexrazoxane appeared to be associated with greater hematologic toxicity and infections in children with Hodgkin lymphoma,15,16 this association was not observed in children with lymphoblastic leukemia and lymphoma, or in pooled summaries of trials in adults.8,13 In our other long-term follow-up studies of patients in these randomized trials, dexrazoxane was not associated with a difference in overall survival, risk of relapse, or second cancers after nearly 20 years of follow-up.36 Similarly, dexrazoxane has not been associated with differential relapse rates in randomized trials of adults with cancer.8
There are some additional limitations to our results. Although we assessed the impact of dexrazoxane on patients who received a broad range of doxorubicin doses (100-600 mg/m2), the trials of interest did not use other anthracyclines (eg, daunorubicin and idarubicin) or mitoxantrone (an anthraquinone). As such, our results may not fully apply to patients treated with these other agents.37 However, nonrandomized data from children with myeloid leukemia treated with daunorubicin and mitoxantrone suggest that dexrazoxane mitigates short-term LV systolic dysfunction.32 We also were able to prospectively assess only 41% of potential participants. Given the fragmentary nature of health care in the United States (and to a lesser degree, Canada), most nonparticipants were no longer actively seen by the institutions that provided their pediatric cancer treatment, and were likely lost to follow-up.
Although response bias may have influenced our results, the direction of any bias is not obvious. The observation that about half the enrolled participants had received dexrazoxane was reassuring. Nonparticipants were more likely to be male and belong to a racial or ethnic minority, but these characteristics did not differ by dexrazoxane status. As such, although our participation rates could limit generalizability, we do not believe it affects the internal validity of our results. Nevertheless, to address potential response bias, in a recent publication, we linked patients enrolled in these five clinical trials to multiple administrative data sets: Medicaid, the US National Death Index, the Organ Procurement and Transplantation Network (US registry of solid organ transplantation), and the Pediatric Health Information System (administrative and billing data from 50 children's hospitals).36 Only one participant (not treated with dexrazoxane) was found to have received a heart transplant.36 Analysis of these administrative data also indicated that serious cardiovascular outcomes occurred less commonly in the dexrazoxane group compared with the no dexrazoxane group (4% v 18%), including cardiomyopathy (4% v 8%), findings that are consistent with the direction of our current study.36
We believe this study provides evidence that dexrazoxane offers measurable long-term cardioprotection for children and adolescents with cancer receiving anthracycline-based chemotherapy. Per a recently published international consensus guideline, oncology providers should consider administering dexrazoxane to children and adolescents expected to receive ≥ 250 mg/m2 of cumulative doxorubicin, or an equivalent amount of other anthracyclines.38 Use of dexrazoxane could be a patient-centered decision in instances where patients are expected to receive lower cumulative anthracycline doses, accounting for other risk factors, including the risk of slow early response or cancer relapse, and whether intensification strategies in these situations may require additional cardiotoxic treatments. However, given the very long time frame for cardiomyopathy development in survivors of childhood cancer, combined with preclinical data that show cardiomyocyte injury following even low doses of doxorubicin exposure, the risk benefits of dexrazoxane use in this situation should be carefully weighed.39
APPENDIX
TABLE A1.
Demographic and Clinical Characteristics of Childhood Cancer Survivors by Children's Oncology Group Protocol ALTE11C2 Participation Status
TABLE A2.
Conventional Cardiovascular Risk Factors, Diastolic Function, and Other Cardiovascular Blood Biomarker Concentrations of Childhood Cancer Survivors Treated With Doxorubicin With and Without Dexrazoxane
FIG A1.
Children's Oncology Group ALTE11C2 study participation flow chart. aInstitutions did not differentiate between participants who were lost to follow-up versus participants who were felt to be reached but did not respond to study approaches.
Eric J. Chow
Research Funding: Abbott
Richard Aplenc
Expert Testimony: Vorys, Sater, Seymour, and Pease LLP
Smita Bhatia
This author is an Associate Editor for Journal of Clinical Oncology. Journal policy recused the author from having any role in the peer review of this manuscript.
Steven D. Colan
Employment: Rocket Pharma
Research Funding: Rocket Pharma
Louis S. Constine
Honoraria: UpToDate, Springer, Lippincott
Lisa M. Kopp
Employment: Arcus Biosciences, Covance
Stock and Other Ownership Interests: Arcus Biosciences
Wendy M. Leisenring
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
No other potential conflicts of interest were reported.
DISCLAIMER
Funders were not involved with the data collection, analysis, or interpretation of the study. The content is solely the responsibility of the authors and does not necessarily represent the official views of the US National Institutes of Health.
PRIOR PRESENTATION
Presented at the 2016 American Society of Hematology annual meeting, San Diego, CA, December 5, 2016; and at the 2020 ASCO annual meeting, virtual, May 29, 2020.
SUPPORT
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: Eric J. Chow, Sanjeev Aggarwal, Richard Aplenc, Saro H. Armenian, K. Scott Baker, Smita Bhatia, Louis S. Constine, Caroline Laverdière, Wendy M. Leisenring, Barbara L. Asselin, Cindy L. Schwartz, Steven E. Lipshultz
Financial support: Eric J. Chow, Steven E. Lipshultz
Administrative support: Eric J. Chow, Steven E. Lipshultz
Provision of study materials or patients: Eric J. Chow, Richard Aplenc, Saro H. Armenian, Caroline Laverdière, Barbara L. Asselin, Cindy L. Schwartz
Collection and assembly of data: Eric J. Chow, Sanjeev Aggarwal, Richard Aplenc, Nancy Blythe, Louis S. Constine, Caroline Laverdière, Nao Sasaki, Lynda M. Vrooman, Barbara L. Asselin, Cindy L. Schwartz, Steven E. Lipshultz
Data analysis and interpretation: Eric J. Chow, Sanjeev Aggarwal, David R. Doody, Richard Aplenc, Saro H. Armenian, K. Scott Baker, Smita Bhatia, Steven D. Colan, Louis S. Constine, David R. Freyer, Lisa M. Kopp, Caroline Laverdière, Wendy M. Leisenring, Lynda M. Vrooman, Barbara L. Asselin, Cindy L. Schwartz, Steven E. Lipshultz
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Dexrazoxane and Long-Term Heart Function in Survivors of Childhood Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Eric J. Chow
Research Funding: Abbott
Richard Aplenc
Expert Testimony: Vorys, Sater, Seymour, and Pease LLP
Smita Bhatia
This author is an Associate Editor for Journal of Clinical Oncology. Journal policy recused the author from having any role in the peer review of this manuscript.
Steven D. Colan
Employment: Rocket Pharma
Research Funding: Rocket Pharma
Louis S. Constine
Honoraria: UpToDate, Springer, Lippincott
Lisa M. Kopp
Employment: Arcus Biosciences, Covance
Stock and Other Ownership Interests: Arcus Biosciences
Wendy M. Leisenring
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
No other potential conflicts of interest were reported.
REFERENCES
- 1.Howlader N, Noone AM, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975-2018. Bethesda, MD: National Cancer Institute; 2021. [Google Scholar]
- 2. Armenian SH, Armstrong GT, Aune G, et al. Cardiovascular disease in survivors of childhood cancer: Insights into epidemiology, pathophysiology, and prevention. J Clin Oncol. 2018;36:2135–2144. doi: 10.1200/JCO.2017.76.3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chow EJ, Chen Y, Kremer LC, et al. Individual prediction of heart failure among childhood cancer survivors. J Clin Oncol. 2015;33:394–402. doi: 10.1200/JCO.2014.56.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lipshultz SE, Adams MJ, Colan SD, et al. Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: Pathophysiology, course, monitoring, management, prevention, and research directions: A scientific statement from the American Heart Association. Circulation. 2013;128:1927–1995. doi: 10.1161/CIR.0b013e3182a88099. [DOI] [PubMed] [Google Scholar]
- 5. Armstrong GT, Chen Y, Yasui Y, et al. Reduction in late mortality among 5-year survivors of childhood cancer. N Engl J Med. 2016;374:833–842. doi: 10.1056/NEJMoa1510795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gibson TM, Mostoufi-Moab S, Stratton KL, et al. Temporal patterns in the risk of chronic health conditions in survivors of childhood cancer diagnosed 1970-99: A report from the Childhood Cancer Survivor Study cohort. Lancet Oncol. 2018;19:1590–1601. doi: 10.1016/S1470-2045(18)30537-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Herman EH, Ferrans VJ, Jordan W, et al. Reduction of chronic daunorubicin cardiotoxicity by ICRF-187 in rabbits. Res Commun Chem Pathol Pharmacol. 1981;31:85–97. [PubMed] [Google Scholar]
- 8. van Dalen EC, Caron HN, Dickinson HO, et al. Cardioprotective interventions for cancer patients receiving anthracyclines. Cochrane Database Syst Rev. 2011:CD003917. doi: 10.1002/14651858.CD003917.pub3. [DOI] [PubMed] [Google Scholar]
- 9. Armenian SH, Lacchetti C, Barac A, et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2017;35:893–911. doi: 10.1200/JCO.2016.70.5400. [DOI] [PubMed] [Google Scholar]
- 10. Curigliano G, Lenihan D, Fradley M, et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann Oncol. 2020;31:171–190. doi: 10.1016/j.annonc.2019.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lipshultz SE, Rifai N, Dalton VM, et al. The effect of dexrazoxane on myocardial injury in doxorubicin-treated children with acute lymphoblastic leukemia. N Engl J Med. 2004;351:145–153. doi: 10.1056/NEJMoa035153. [DOI] [PubMed] [Google Scholar]
- 12. Lipshultz SE, Scully RE, Lipsitz SR, et al. Assessment of dexrazoxane as a cardioprotectant in doxorubicin-treated children with high-risk acute lymphoblastic leukaemia: Long-term follow-up of a prospective, randomised, multicentre trial. Lancet Oncol. 2010;11:950–961. doi: 10.1016/S1470-2045(10)70204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Asselin BL, Devidas M, Chen L, et al. Cardioprotection and safety of dexrazoxane in patients treated for newly diagnosed T-cell acute lymphoblastic leukemia or advanced-stage lymphoblastic non-Hodgkin lymphoma: A report of the Children's Oncology Group randomized trial Pediatric Oncology Group 9404. J Clin Oncol. 2016;34:854–862. doi: 10.1200/JCO.2015.60.8851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Asselin BL, Devidas M, Wang C, et al. Effectiveness of high dose methotrexate in T-cell lymphoblastic leukemia and advanced stage lymphoblastic lymphoma: A randomized study by the Children's Oncology Group (POG 9404) Blood. 2011;118:874–883. doi: 10.1182/blood-2010-06-292615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schwartz CL, Constine LS, Villaluna D, et al. A risk-adapted, response-based approach using ABVE-PC for children and adolescents with intermediate- and high-risk Hodgkin lymphoma: The results of P9425. Blood. 2009;114:2051–2059. doi: 10.1182/blood-2008-10-184143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tebbi CK, Mendenhall NP, London WB, et al. Response-dependent and reduced treatment in lower risk Hodgkin lymphoma in children and adolescents, results of P9426: A report from the Children's Oncology Group. Pediatr Blood Cancer. 2012;59:1259–1265. doi: 10.1002/pbc.24279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schwartz CL, Wexler LH, Krailo MD, et al. Intensified chemotherapy with dexrazoxane cardioprotection in newly diagnosed nonmetastatic osteosarcoma: A report from the Children's Oncology Group. Pediatr Blood Cancer. 2016;63:54–61. doi: 10.1002/pbc.25753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moghrabi A, Levy DE, Asselin B, et al. Results of the Dana-Farber Cancer Institute ALL Consortium Protocol 95-01 for children with acute lymphoblastic leukemia. Blood. 2007;109:896–904. doi: 10.1182/blood-2006-06-027714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cheitlin MD, Armstrong WF, Aurigemma GP, et al. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography—Summary article: A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (ACC/AHA/ASE Committee to update the 1997 guidelines for the clinical application of echocardiography) J Am Coll Cardiol. 2003;42:954–970. doi: 10.1016/s0735-1097(03)01065-9. [DOI] [PubMed] [Google Scholar]
- 20. Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 21. Armenian SH, Hudson MM, Mulder RL, et al. Recommendations for cardiomyopathy surveillance for survivors of childhood cancer: A report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2015;16:e123–e136. doi: 10.1016/S1470-2045(14)70409-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fradley MG, Larson MG, Cheng S, et al. Reference limits for N-terminal-pro-B-type natriuretic peptide in healthy individuals (from the Framingham Heart Study) Am J Cardiol. 2011;108:1341–1345. doi: 10.1016/j.amjcard.2011.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lipshultz SE, Lipsitz SR, Sallan SE, et al. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J Clin Oncol. 2005;23:2629–2636. doi: 10.1200/JCO.2005.12.121. [DOI] [PubMed] [Google Scholar]
- 24. Border WL, Sachdeva R, Stratton KL, et al. Longitudinal changes in echocardiographic parameters of cardiac function in pediatric cancer survivors. JACC CardioOncol. 2020;2:26–37. doi: 10.1016/j.jaccao.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fisher SD, Easley KA, Orav EJ, et al. Mild dilated cardiomyopathy and increased left ventricular mass predict mortality: The prospective P2C2 HIV Multicenter Study. Am Heart J. 2005;150:439–447. doi: 10.1016/j.ahj.2005.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Greupner J, Zimmermann E, Grohmann A, et al. Head-to-head comparison of left ventricular function assessment with 64-row computed tomography, biplane left cineventriculography, and both 2- and 3-dimensional transthoracic echocardiography: Comparison with magnetic resonance imaging as the reference standard. J Am Coll Cardiol. 2012;59:1897–1907. doi: 10.1016/j.jacc.2012.01.046. [DOI] [PubMed] [Google Scholar]
- 27. Lipshultz SE, Easley KA, Orav EJ, et al. Reliability of multicenter pediatric echocardiographic measurements of left ventricular structure and function: The prospective P(2)C(2) HIV study. Circulation. 2001;104:310–316. doi: 10.1161/01.cir.104.3.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van Dalen EC, van der Pal HJ, Caron HN, et al. Different dosage schedules for reducing cardiotoxicity in cancer patients receiving anthracycline chemotherapy. Cochrane Database Syst Rev. 2009:CD005008. doi: 10.1002/14651858.CD005008.pub2. [DOI] [PubMed] [Google Scholar]
- 29. van Dalen EC, Michiels EM, Caron HN, et al. Different anthracycline derivates for reducing cardiotoxicity in cancer patients. Cochrane Database Syst Rev. 2010:CD005006. doi: 10.1002/14651858.CD005006.pub2. [DOI] [PubMed] [Google Scholar]
- 30. Kopp LM, Womer RB, Schwartz CL, et al. Effects of dexrazoxane on doxorubicin-related cardiotoxicity and second malignant neoplasms in children with osteosarcoma: A report from the Children's Oncology Group. Cardiooncology. 2019;5:15. doi: 10.1186/s40959-019-0050-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Leerink JM, Verkleij SJ, Feijen EAM, et al. Biomarkers to diagnose ventricular dysfunction in childhood cancer survivors: A systematic review. Heart. 2019;105:210–216. doi: 10.1136/heartjnl-2018-313634. [DOI] [PubMed] [Google Scholar]
- 32. Getz KD, Sung L, Alonzo TA, et al. Effect of dexrazoxane on left ventricular systolic function and treatment outcomes in patients with acute myeloid leukemia: A report from the Children's Oncology Group. J Clin Oncol. 2020;38:2398–2406. doi: 10.1200/JCO.19.02856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shaikh F, Dupuis LL, Alexander S, et al. Cardioprotection and second malignant neoplasms associated with dexrazoxane in children receiving anthracycline chemotherapy: A systematic review and meta-analysis. J Natl Cancer Inst. 2016;108:djv357. doi: 10.1093/jnci/djv357. [DOI] [PubMed] [Google Scholar]
- 34. Walker DM, Fisher BT, Seif AE, et al. Dexrazoxane use in pediatric patients with acute lymphoblastic or myeloid leukemia from 1999 and 2009: Analysis of a national cohort of patients in the Pediatric Health Information Systems database. Pediatr Blood Cancer. 2013;60:616–620. doi: 10.1002/pbc.24270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Seif AE, Walker DM, Li Y, et al. Dexrazoxane exposure and risk of secondary acute myeloid leukemia in pediatric oncology patients. Pediatr Blood Cancer. 2015;62:704–709. doi: 10.1002/pbc.25043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chow EJ, Aplenc R, Vrooman LM, et al. Late health outcomes after dexrazoxane treatment: A report from the Children's Oncology Group. Cancer. 2022;128:788–796. doi: 10.1002/cncr.33974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Feijen EAM, Leisenring WM, Stratton KL, et al. Derivation of anthracycline and anthraquinone equivalence ratios to doxorubicin for late-onset cardiotoxicity. JAMA Oncol. 2019;5:864–871. doi: 10.1001/jamaoncol.2018.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. de Baat EC, van Dalen EC, Mulder RL, et al. Primary cardioprotection with dexrazoxane in patients with childhood cancer who are expected to receive anthracyclines: Recommendations from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Child Adolesc Health. 2022;6:885–894. doi: 10.1016/S2352-4642(22)00239-5. [DOI] [PubMed] [Google Scholar]
- 39. Lipshultz SE, Rifai N, Sallan SE, et al. Predictive value of cardiac troponin T in pediatric patients at risk for myocardial injury. Circulation. 1997;96:2641–2648. doi: 10.1161/01.cir.96.8.2641. [DOI] [PubMed] [Google Scholar]