Abstract
PURPOSE
To improve skin cancer screening among survivors of childhood cancer treated with radiotherapy where skin cancers make up 58% of all subsequent neoplasms. Less than 30% of survivors currently complete recommended skin cancer screening.
PATIENTS AND METHODS
This randomized controlled comparative effectiveness trial evaluated patient and provider activation (PAE + MD) and patient and provider activation with teledermoscopy (PAE + MD + TD) compared with patient activation alone (PAE), which included print materials, text messaging, and a website on skin cancer risk factors and screening behaviors. Seven hundred twenty-eight participants from the Childhood Cancer Survivor Study (median age at baseline 44 years), age > 18 years, treated with radiotherapy as children, and without previous history of skin cancer were randomly assigned (1:1:1). Primary outcomes included receiving a physician skin examination at 12 months and conducting a skin self-examination at 18 months after intervention.
RESULTS
Rates of physician skin examinations increased significantly from baseline to 12 months in all three intervention groups: PAE, 24%-39%, relative risk [RR], 1.65, 95% CI, 1.32 to 2.08; PAE + MD, 24% to 39%, RR, 1.56, 95% CI, 1.25 to 1.97; PAE + MD + TD, 24% to 46%, RR, 1.89, 95% CI, 1.51 to 2.37. The increase in rates did not differ between groups (P = .49). Similarly, rates of skin self-examinations increased significantly from baseline to 18 months in all three groups: PAE, 29% to 50%, RR, 1.75, 95% CI, 1.42 to 2.16; PAE + MD, 31% to 58%, RR, 1.85, 95% CI, 1.52 to 2.26; PAE + MD + TD, 29% to 58%, RR, 1.95, 95% CI, 1.59 to 2.40, but the increase in rates did not differ between groups (P = .43).
CONCLUSION
Although skin cancer screening rates increased more than 1.5-fold in each of the intervention groups, there were no differences between groups. Any of these interventions, if implemented, could improve skin cancer prevention behaviors among childhood cancer survivors.
INTRODUCTION
Currently, in the United States, 85% of children treated for cancer will achieve 5-year survival.1 In addition, there are an estimated 500,000 adult survivors of childhood cancer,2 more than 60% of whom were treated with radiation therapy.3 The Childhood Cancer Survivor Study (CCSS) has previously demonstrated that among survivors treated with radiation, skin cancers are the most common subsequent neoplasms, making up 58% of all subsequent neoplasms.3 An estimated 20 years after treatment exposure, these survivors are at 30-fold increased risk of developing basal cell carcinoma,4 and 2.5- to 5-fold increased risk of melanoma5,6 compared with the general population, with many survivors developing multiple basal cell carcinomas.
CONTEXT
Key Objective
To our knowledge, this was the first randomized trial to test various methods for improving the early detection of skin cancer among childhood cancer survivors. Participants were randomly assigned to patient activation alone (PAE) versus patient and provider activation (PAE + MD) and patient and provider activation with teledermoscopy (PAE + MD + TD).
Knowledge Generated
Rates of physician examinations and skin self-examinations improved more than 1.5-fold in all three intervention groups. However, the increase in rates did not differ between groups.
Relevance (S. Bhatia)
Low-cost patient activation strategies need to be tested in the setting of implementation trials to promote early detection of skin cancer in childhood cancer survivors treated with radiation.*
*Relevance section written by JCO Associate Editor Smita Bhatia, MD, MPH, FASCO.
Among the general population, early detection of skin cancer is associated with improved survival rates and reduced individual and health care costs.7-9 On the basis of this premise, current US practice guidelines by the National Cancer Institute for detection of early-onset skin cancer among childhood cancer survivors who received radiotherapy (PDQ, evidence-based data summary) recommend an annual dermatologic examination.10 Additionally, the Children's Oncology Group Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers recommends an annual dermatologic skin examination focusing on skin lesions and pigmented nevi in the radiation field as well as monthly skin self-examinations (SSEs).11
Adherence to screening recommendations is low among radiation exposed survivors, with only 27% of patients reporting that they received physician screening for skin cancer by skin examination of irradiated areas12 and adherence rates to Children's Oncology Guidelines for skin cancer screening being 22%.13 Thus, a key challenge among this high-risk population is to improve adherence to skin cancer screening. We conducted a randomized, controlled comparative effectiveness trial to determine whether patient and physician activation and patient and physician activation with dermoscopy would improve skin cancer surveillance compared with patient activation alone.
PATIENTS AND METHODS
Population
Participants were recruited from CCSS, which includes 25,665 5-year survivors of childhood cancer from 31 institutions diagnosed before age 21 years and between 1970 and 1999.14,15 Eligibility criteria for recruitment to the Advancing Survivors' Knowledge of skin cancer (ASK) study included (1) age 18 years or older; (2) previously treated with radiation therapy; (3) visited a primary care physician or oncologist in the previous 2 years or planning a visit in the next year; (4) no personal history of skin cancer; (5) ability to receive text messages; and (6) ability to use the DermLite teledermoscopy device (3Gen, San Juan Capistrano, CA). Consent was obtained verbally via the telephone, online, or was ascertained by receipt of completed paper surveys. Participants were not excluded if diagnosed and treated as an adult with cancer, as they remain at high risk for nonmelanoma skin cancer from their childhood radiation exposure. The CCSS does not collect treatment exposure information for adult-onset malignancies, nor any treatment provided beyond the 5-year time point of survival from the primary malignancy. Detailed recruitment procedures have been previously reported.16
Ethical Statement
The study Protocol (online only; ClinicalTrials.gov identifier: NCT02046811) was reviewed and approved by the institutional review boards of Harvard T.H. Chan School of Public Health and St Jude Children's Research Hospital. All the participants provided consent before study enrollment.
Study Design and Random Assignment
Using the Patient Activation Model framework, the objective was to compare the additive effect of education through patient and provider activation (PAE + MD) and patient and provider activation with teledermoscopy (PAE + MD + TD) compared with education through patient activation alone (PAE) to improve skin cancer screening rates. The development of the interventions was previously described in detail.16,17 We hypothesized that PAE + MD and PAE + MD + TD would lead to higher rates of physician skin examinations at 12 months and higher rates of SSEs at 12 and 18 months after intervention compared with PAE. Participants were enrolled and randomly assigned on the basis of a three-arm parallel (1:1:1) design conducted using a uniform number generator and stratified by sex and education. The study Protocol (ClinicalTrials.gov identifier: NCT02046811) was reviewed and approved by the institutional review boards of Harvard T.H. Chan School of Public Health and St Jude Children's Research Hospital.
Study Interventions
Participants randomly assigned to the PAE only intervention group received a single set of mailed print materials, monthly text messages, and 12-month access to the ASK website, which included a video guide on how to conduct a SSE. The intervention targeted improving survivors' awareness of their heightened risk of skin cancer while encouraging them to (1) carefully examine their skin using pictorial diagrams of how to conduct a skin self-examination, photographs of abnormal lesions, and prominent body sites to examine (particularly those in the radiation field); (2) request provider skin examinations with the use of a printable checklist for their care provider; and (3) develop a collaborative care plan with their provider that addresses common responsibility for monitoring and timely follow-up on new and changing moles and lesions. Thirteen text messages were sent during the 12-month intervention period, which included reminders of these goals.
Participants assigned to the PAE + MD arm received the PAE intervention. Additionally, their physicians were mailed physician activation print materials, including information about survivors' increased risk for skin cancer education to provide a full-body skin examination at the next appointment, access to the patient and provider sections of the study website, and additional written resources (eg, efficacy data on skin examinations).
Participants assigned to the PAE + MD + TD arm received the PAE + MD intervention. In addition, they received a dermatoscope to attach to their smartphone for acquisition of high-resolution dermoscopic images, and a customized instructional video on how to use the device. Participants were instructed to send photographs of suspicious moles or lesions to the study dermatologist by a secure portal. The results were sent to the participant's physician, encouraging referral to a dermatologist for a clinical examination if needed, and emphasizing the importance of monthly self-examinations.
Primary Outcomes
The two primary outcomes were participant-reported physician skin examination within 12 months after intervention and participant-reported SSE in the 2 months before assessment at 18 months. Completion of a physician skin examination was assessed at baseline and 12 months as most participants were making annual and not midyear routine health care visits. SSE reporting has been previously validated18,19 and participants were asked, “How many times in the past two months have you carefully checked your whole body (including the skin on your back and back of your legs) for any sign of skin cancer?” Answer choices included never, once, and two or more times. For analyses, this variable was scored as binary: never versus at least once. Because SSE is a personal practice and one that high-risk patients should be performing routinely, we measured this outcome at 12 and 18 months (the primary end point).
Secondary Outcome
Participants were also asked if they carefully examined each of nine areas of the body (the front of your body from the waist up, the front of your thighs and legs, the bottom of your feet, your calves, the backs of your thighs, your buttocks, the lower parts of your back, your upper back, and your scalp).18,19 Participant-reported body parts self-examined was scored as the mean of the total number of body parts examined and assessed at baseline, 12 months, and 18 months (primary end point).
Additional Measures
We collected self-reported sociodemographic information including current age, education, and skin type at baseline evaluation. Sex, race/ethnicity, age at diagnosis, childhood cancer diagnosis, chemotherapy exposure, and maximum radiation therapy location and dose were previously collected by CCSS.14,15 To evaluate adherence, we asked participants whether they read all, some, or none of the print materials and the 13 text messages, and whether they used the study website. We assessed participant use of teledermoscopy on the basis of the number of individuals who submitted dermoscopic images to the study website. We used the 13-item short version of the Patient Activation Measure to assess patient knowledge, skills, beliefs, motivations, and behaviors needed to become activated or actively engaged in their health care. Scoring resulted in four activation stages: (1) importance of taking an active role as a patient; (2) confidence and knowledge to take action; (3) action toward health maintenance and improvement; and (4) staying the course even under stress.20,21
Statistical Analysis
Descriptive analyses characterized distributions of patient characteristics (counts and percentages) overall and by intervention group. For the primary analyses, we fit a series of repeated-measures marginal models via generalized estimating equations.22 In each model, we included separate indicators for intervention group, time period (i.e. baseline, 12 months, and 18 months, as appropriate), and their interaction terms. The interaction terms were the primary coefficients of interest since they characterize whether and how changes over time in the outcome vary across intervention arms. A log-link function facilitated interpretation of contrasts in terms of relative risks (RRs; for physician skin examination and SSE) or relative rates (for body parts examined). Standard errors were estimated using the robust sandwich error after having adopted working independence in the estimation procedure. For each outcome, analyses were performed with and without covariate adjustment for sex, current age (years, categorized as < 35, 35-39, 40-44, 45-49, 50-54, and 55+), education (≤ high school, some college, college graduate, and postgraduate), and skin type (very fair, fair, olive, and dark/very dark).
Approximately 4% of participants were excluded because of missing data in age and skin type at baseline, as well as in the primary outcomes at baseline and during follow-up. To investigate the potential impact of missing data, we performed a series of complete-case and available data inverse probability-weighted analyses.23 All analyses were performed in R v4.03.24
Sample Size Estimation
Sample size calculations were based on previously reported physician-led and self-screening rates for skin cancer. Using a main effects model, with at least 80% power to detect a 15% difference across any of the arms and an alpha of .025, and an estimated 25% attrition rate by month 18, we proposed to recruit 801 subjects to be randomly assigned into three arms. Further details are included in the published Protocol.16
Sensitivity Analysis
Post hoc, we reanalyzed the main efficacy analyses restricted to those participants who were nonadherent at baseline with respect to the primary and secondary outcomes.
RESULTS
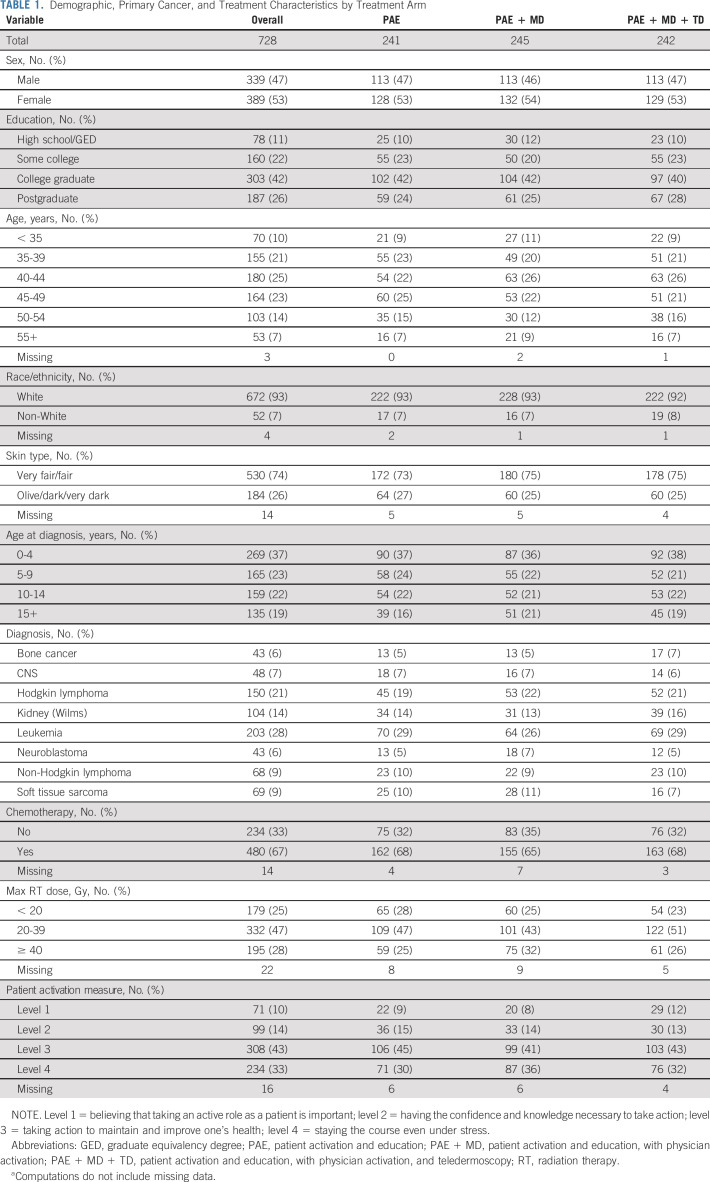
Overall, 1,353 CCSS participants were assessed for eligibility and 728 were randomly assigned, as shown in Figure 1. Among participants randomly assigned, 53% were female and 74% reported fair skin type (Table 1). The median age at baseline was 43.9 years (range, 29.9-64.7 years) and the median age at cancer diagnosis was 6 years (range, 0-20 years). All participants were treated with radiation therapy and 67% also received chemotherapy. Participant study retention was 89% at 12 months and 90% at 18 months (Appendix Fig A1, online only).
FIG 1.
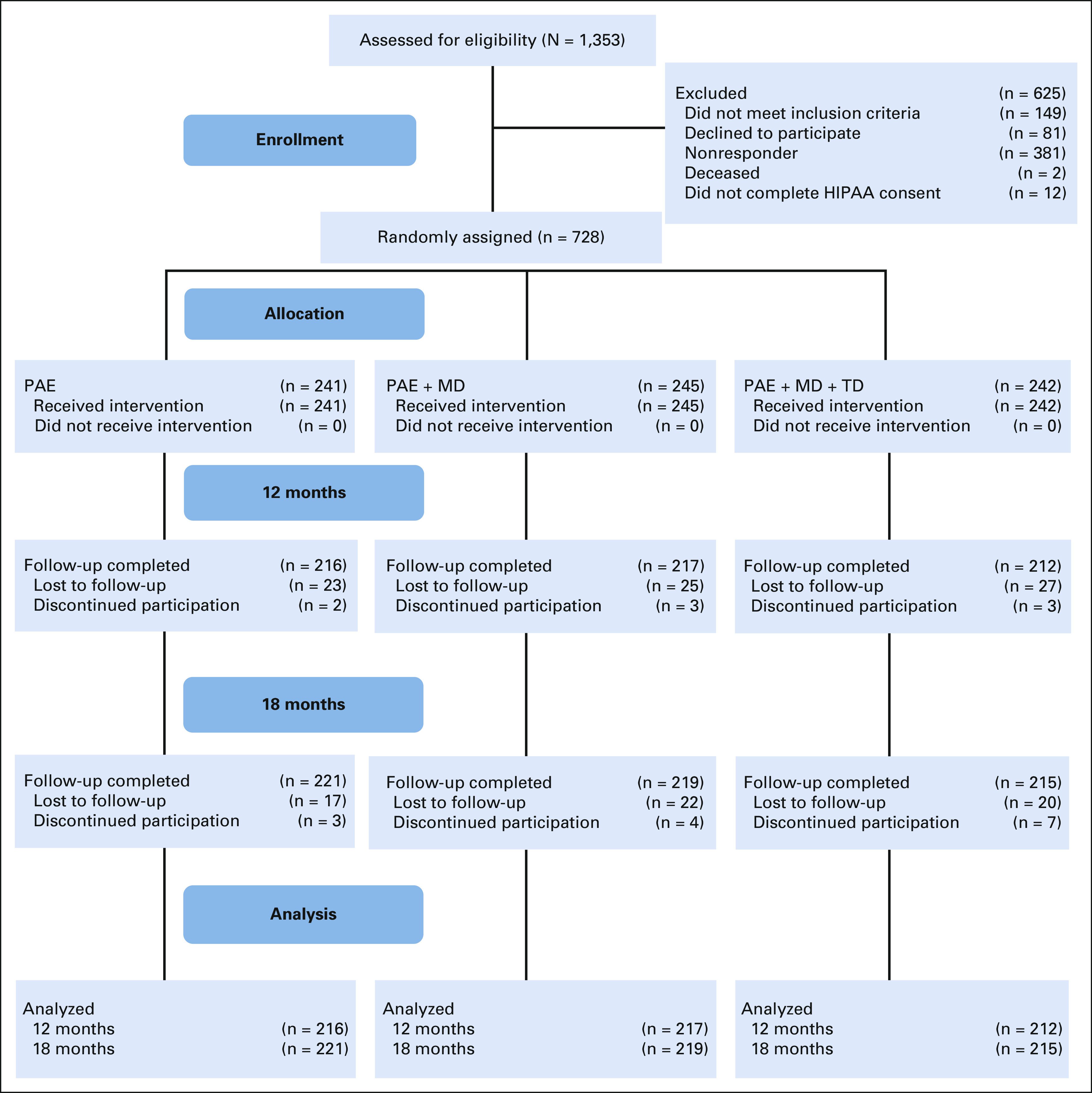
CONSORT diagram. All 728 participants were contacted for the 18-month follow-up, even if they did not complete the 12-month follow-up, hence the slightly increased follow-up rate at 18 months. HIPPA, Health Insurance Portability and Accountability Act; PAE, patient activation and education; PAE + MD, patient activation and education, with physician activation; PAE + MD + TD, patient activation and education, with physician activation, and teledermoscopy.
TABLE 1.
Demographic, Primary Cancer, and Treatment Characteristics by Treatment Arm
Primary and Secondary Outcomes
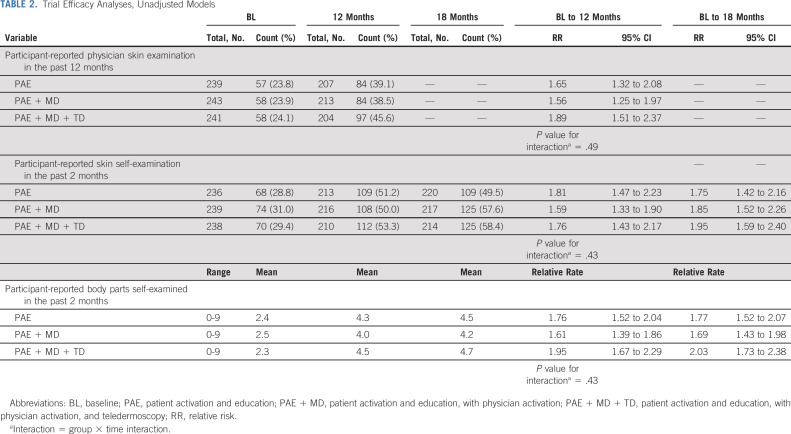
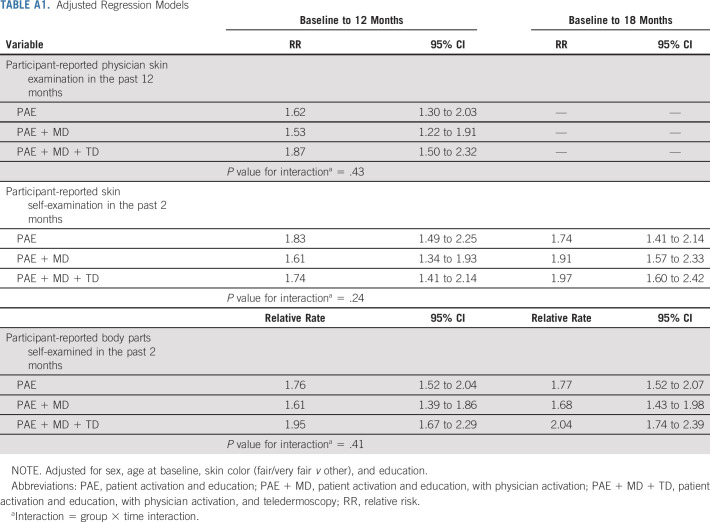
Compared with baseline, rates of completion of physician skin examination, SSE, and self-examination of an increased number of body parts within 12 and 18 months after intervention were higher in all three intervention groups (Table 2, Fig 2; adjusted analyses provided in Appendix Table A1, online only). Among survivors randomly assigned to the PAE only group, participant-reported physician skin examination within 12 months increased from 23.8% at baseline to 39.1% (RR, 1.65; 95% CI, 1.32 to 2.08). For survivors randomly assigned to the PAE + MD group, physician skin examination increased from 23.9% to 38.5% (RR, 1.56; 95% CI, 1.25 to 1.97) and for those in the PAE + MD + TD group, there was an increase from 24.1% to 45.6% (RR, 1.89; 95% CI, 1.51 to 2.37; Table 2). There was no statistically significant difference in the rate of increase across the three intervention groups (P = .49).
TABLE 2.
Trial Efficacy Analyses, Unadjusted Models
FIG 2.
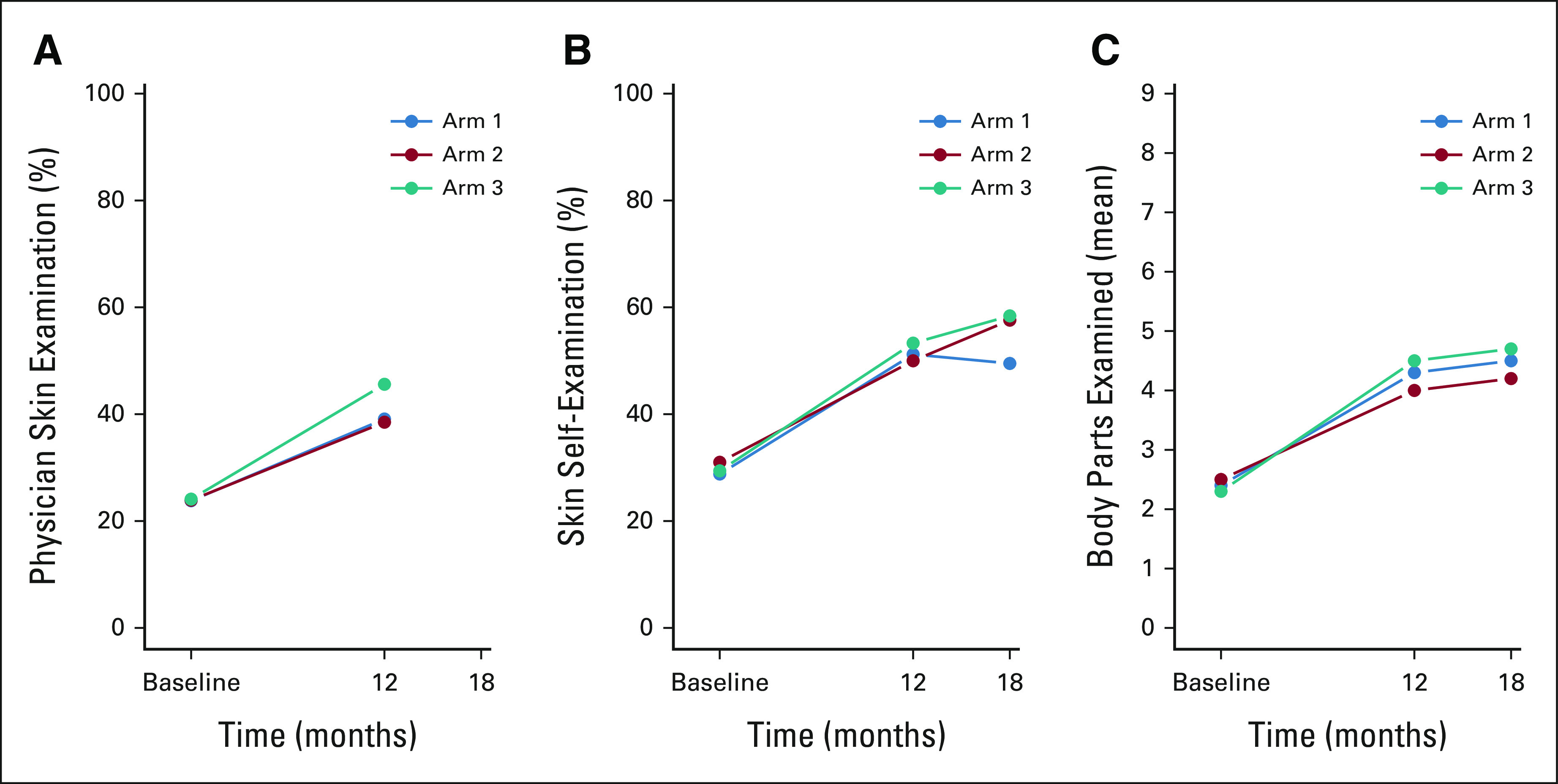
Visual representation of within-arm changes throughout the study: (A) physician skin examination in the past 12 months, (B) skin self-examination in the past 2 months, and (C) body parts self-examined in the past 2 months. Arm 1 = PAE, arm 2 = PAE + MD, and arm 3 = PAE + MD + TD. PAE, patient activation and education; PAE + MD, patient activation and education, with physician activation; PAE + MD + TD, patient activation and education, with physician activation, and teledermoscopy.
In the PAE group, SSE increased from 28.8% at baseline to 51.2% at 12-month follow-up (RR, 1.81; 95% CI, 1.47 to 2.23) and to 49.5% at 18 months (RR, 1.75; 95% CI, 1.42 to 2.16). In the PAE + MD group, SSE increased from 31% at baseline to 50% at 12 months (RR, 1.59; CI, 1.33 to 1.90) and to 57.6% at 18 months (RR, 1.85; CI, 1.52 to 2.26). In the PAE + MD + TD group SSE increased from 29.4% at baseline to 53.3% at 12 months (RR, 1.76; CI, 1.43 to 2.17) and to 58.4% at 18 months (RR, 1.95; CI, 1.59 to 2.40). There was no statistically significant difference in the rate of increase across the three groups (P = .43).
Among survivors assigned to PAE, the mean number of body parts self-examined increased from 2.4 at baseline to 4.3 at 12 months (relative rate, 1.76; 95% CI, 1.52 to 2.04) and to 4.5 at 18 months (relative rate, 1.77; 95% CI, 1.52 to 2.07). For PAE + MD, the mean increased from 2.5 at baseline to 4.0 at 12 months (relative rate, 1.61; CI, 1.39 to 1.86) and to 4.2 at 18 months (relative rate, 1.69; CI, 1.43 to 1.98). Survivors in the PAE + MD + TD intervention group reported an increase in the mean number of body parts self-examined from 2.3 at baseline to 4.5 at 12 months (relative rate, 1.95; CI, 1.67 to 2.29) and to 4.7 at 18 months (relative rate, 2.03; CI, 1.73 to 2.38). There was no statistically significant difference in the rate of increase across the three intervention groups (P = .43). The results were unchanged when accounting for data missingness.
Sensitivity Analysis
At baseline, there were 536 nonadherers with respect to PSE, 490 with respect to SSE and 334 with respect to BSE (ie, indicated zero number of body parts examined). There was a statistically significant increase from baseline to the 12-month follow-up within each of the three arms, but no statistically significant difference in the rate of the increase between the arms (P values for interactions are .387, .062, and .576, respectively).
Exploratory Analyses With Cancer Outcomes
At 12 months, 103 participants in the PAE arm had undergone at least one clinical skin examination, with 21 undergoing at least one biopsy and seven self-reporting a diagnosis of cancer (basal cell, squamous cell, melanoma in situ, or melanoma). In the PAE + MD and PAE + MD + TD arms, 110 and 123 participants, respectively, underwent at least one clinical skin examination. Among these, 28 and 35 patients underwent at least one biopsy, with 12 and seven self-reported cancer diagnoses, respectively.
Adherence to Protocol Assessment and Patient Activation Levels
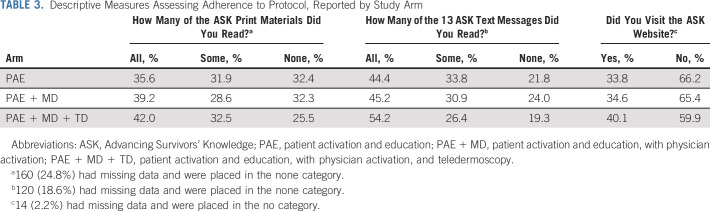
Adherence to the intervention was similar across the three intervention groups (Table 3). Sixty-eight percent of the PAE and PAE + MD groups reported having read some or all of the study print materials, as did 75% of the PAE + MD + TD group. Text messages were read by 78%, 76%, and 81% of groups, respectively, and the ASK website was reviewed by 34%, 35%, and 40%, respectively. Among survivors randomly assigned to PAE + MD + TD, 17% of participants sent dermoscopic images to the study website. For descriptive purposes, participants with missing adherence data were treated as nonadherent and included under the none category.
TABLE 3.
Descriptive Measures Assessing Adherence to Protocol, Reported by Study Arm
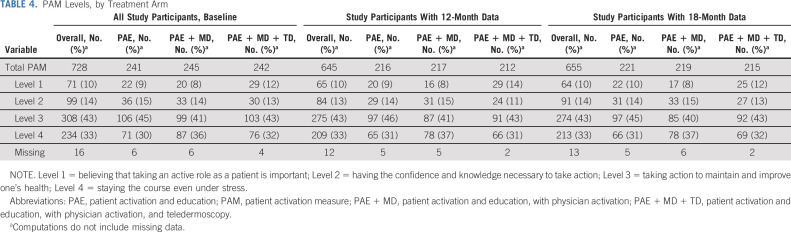
Patient activation level, which assessed overall engagement in one's health care rather than skin cancer–specific activities, was also similar across the study groups (Table 4). At baseline, activation levels including taking an active role and staying the course even under stress were reported by 75%, 76%, and 75% within the PAE, PAE + MD, and PAE + MD + TD groups, respectively. These rates were maintained at 12 and 18 months after intervention.
TABLE 4.
PAM Levels, by Treatment Arm
DISCUSSION
In this randomized controlled comparative effectiveness trial among survivors of childhood cancer at high risk for skin cancer after radiotherapy exposure, there was no statistically significant evidence of a difference in increased surveillance for skin cancer across the three intervention groups, suggesting that the use of additional provider activation and teledermoscopy did not increase rates of screening over patient activation alone. However, with an observed consistently modest increase in screening for both physician skin examination and SSE in all three groups, it appears that patient activation in the form of print materials in combination with mHealth strategies (text messages and use of the ASK website) significantly improves screening rates.
By comparison, prior prevention studies among other populations at increased risk for melanoma showed inconsistent results. For example, several clinical trials conducted among individuals at high risk for melanoma (patients and first-degree relatives of patients with melanoma, men age > 50 years) using a 12-month follow-up or longer found increased rates of SSE in the intervention groups compared with control groups,25-28 while others did not.29,30 Characteristics of successful interventions included modality of delivery (website or video, with reminders), intervention content tailored to personal characteristics (eg, screening history and personal motivation), and intervention duration (12 months).25-28 A 2018 systematic review on mHealth interventions for skin cancer31 found that among trials in the general population, text messages and personalized e-mails with reminders were effective in increasing rates of sun protection32,33 and skin surveillance behaviors,34 but phone applications were less effective.35,36 For interventions delivered via text messaging and personalized e-mails, reported mediators of effect included self-efficacy and motivation to perform sun-protective behaviors33; moderators of effect included age (< 32 years), skin phenotype (fair and very fair), and planning at baseline to perform skin examinations.34 In the single trial examining the feasibility of using a dermoscope at home, 94% people reported the tool was easy to use and 86% reported it motivated them to check their skin, 18% had difficulty photographing hard-to-reach areas, and 35% needed assistance forwarding the images.37
It appears likely that among survivors of childhood cancer treated with radiation, who are at high risk for skin cancer, patient activation print materials used in combination with mHealth strategies (ie, text messaging and ASK website) are at least as effective at promoting skin cancer screening behaviors as other more resource-intensive strategies. These findings provide clear direction for future implementation and dissemination as the intervention with the lowest resource requirements, PAE, should have greater potential for successful uptake and effectiveness as more involved interventions targeting physician engagement or dermoscopy.
In the current trial, approximately two thirds of participants read the print materials and text messages, while only one third accessed the ASK website, providing guidance for future tailoring. Our rate of adherence to website interventions is slightly higher than those found in prior studies of website utilization for skin cancer prevention trials that are generally low (eg, 19% at 3-week follow-up and 23% at 12-week follow-up).38 Furthermore, in the PAE + MD + TD arm, only a minority of patients (< 20%) sent dermoscopic images for further evaluation. This could be attributable to a low incidence of problematic moles or lesions, low self-efficacy for identifying problematic lesions, confidentiality concerns about sharing personal data, or mistrust of dermoscopy as a viable mHealth strategy to facilitate self-detection of skin cancer. Any one of these factors could have contributed to the lack of differential effect across groups.
A number of limitations need to be considered. Participants in this trial were members of the CCSS cohort, which may not be representative of all survivors of childhood cancers. Participating survivors are largely similar in their distribution of key demographic characteristics to the population-based SEER database but might not be representative in terms of routine/ongoing screening and follow-up care, and level of compliance.39 Second, this trial used patient-reported outcomes, which may be influenced by social desirability bias and recall bias, and the items used to assess the behavioral outcomes were not specifically referring to the area of the skin exposed to radiation. Third, in this comparative effectiveness study, we used two active arms and one active comparator, but did not include a true, nonactive control arm. Although it is impossible to evaluate the exact impact of this design choice, we believe a true control would be unlikely to have measurably changed the trial results, given the low rate (23%-24%) of physician skin examination and SSE at baseline.
In conclusion, although there were no differences found between the intervention groups, which were designed to incrementally include more resources, the rates of physician and SSE increased more than 1.5-fold in all three groups. On the basis of our findings, for survivors of childhood cancer treated with radiation, we recommend that future implementation trials of early detection of skin cancer using self- and provider-administered skin examinations be centered on low-cost patient activation strategies including a combination of print and mHealth strategies (texting and website) similar to those found to be effective in the current study.
ACKNOWLEDGMENT
The authors thank Dana-Farber Cancer Institute's Health Communication Core for developing the project materials.
APPENDIX
TABLE A1.
Adjusted Regression Models
FIG A1.
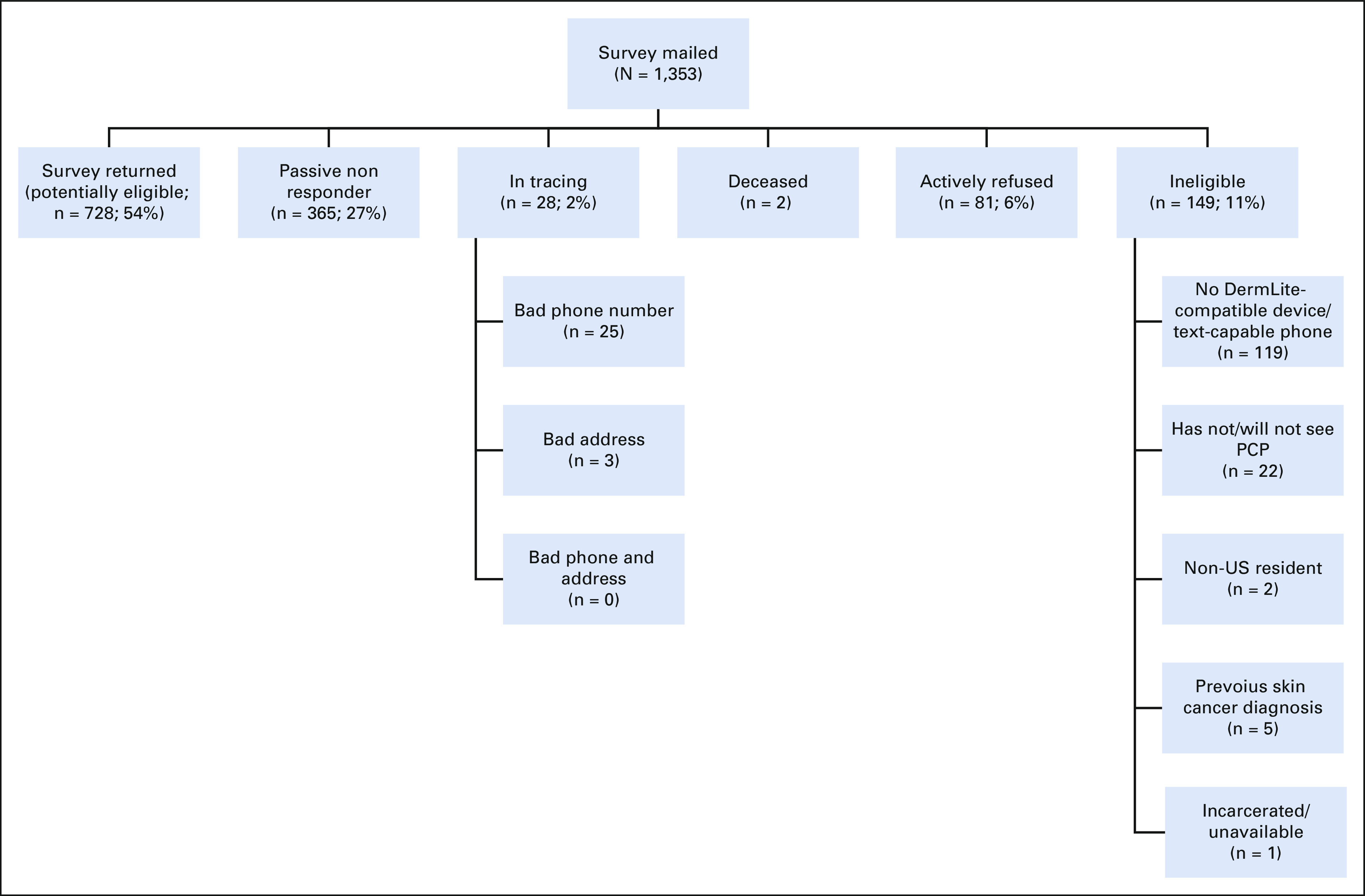
Final recruitment status for the ASK intervention trial. PCP, primary care physician.
Karen M. Emmons
Employment: Sleepwell Consultants
Consulting or Advisory Role: SleepCogni
Elena B. Elkin
Research Funding: Pfizer
Ashfaq Marghoob
Honoraria: DermLite, FotoFinder, Canfield, Heine
Patents, Royalties, Other Intellectual Property: Textbooks UpToDate
Uncompensated Relationships: Heine, Canfield, DermLite
Gregory T. Armstrong
Honoraria: Grail
No other potential conflicts of interest were reported.
SUPPORT
Supported by the National Cancer Institute (CA55727, GTA, principal investigator; 1 R01 CA175231, ACG, principal investigator). Support to St Jude Children's Research Hospital was also provided by the Cancer Center Support (CORE) grant (CA21765, C. Roberts, principal investigator) and the American Lebanese-Syrian Associated Charities.
CLINICAL TRIAL INFORMATION
Karen M. Emmons
Employment: Sleepwell Consultants
Consulting or Advisory Role: SleepCogni
Elena B. Elkin
Research Funding: Pfizer
Ashfaq Marghoob
Honoraria: DermLite, FotoFinder, Canfield, Heine
Patents, Royalties, Other Intellectual Property: Textbooks UpToDate
Uncompensated Relationships: Heine, Canfield, DermLite
Gregory T. Armstrong
Honoraria: Grail
No other potential conflicts of interest were reported.
A.C.G. and A.C. shared first authorship.
DATA SHARING STATEMENT
Data are available from the corresponding author upon reasonable request.
AUTHOR CONTRIBUTIONS
Conception and design: Alan C. Geller, Adina Coroiu, Robyn R. Keske, Sebastien Haneuse, Karen M. Emmons, Leslie L. Robison, Ann C. Mertens, Elena B. Elkin, Gregory T. Armstrong
Administrative support: Adina Coroiu, Robyn R. Keske, Jessica A. Davine, Leslie L. Robison, Gregory T. Armstrong
Collection and assembly of data: Adina Coroiu, Robyn R. Keske, Jessica A. Davine, Todd M. Gibson, Aaron J. McDonald, Leslie L. Robison, Gregory T. Armstrong
Data analysis and interpretation: Alan C. Geller, Adina Coroiu, Sebastien Haneuse, Karen M. Emmons, Casey L. Daniel, Todd M. Gibson, Aaron J. McDonald, Ann C. Mertens, Elena B. Elkin, Ashfaq Marghoob, Gregory T. Armstrong
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Advancing Survivors Knowledge (ASK Study) of Skin Cancer Surveillance After Childhood Cancer: A Randomized Controlled Trial in the Childhood Cancer Survivor Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
REFERENCES
- 1.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2018. National Cancer Institute; 2021. https://seer.cancer.gov/csr/1975_2018/ [Google Scholar]
- 2. Robison LL, Hudson MM. Survivors of childhood and adolescent cancer: Life-long risks and responsibilities. Nat Rev Cancer. 2014;14:61–70. doi: 10.1038/nrc3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Watt TC, Inskip PD, Stratton K, et al. Radiation-related risk of basal cell carcinoma: A report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2012;104:1240–1250. doi: 10.1093/jnci/djs298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Teepen JC, Kok JL, Kremer LC, et al. Long-term risk of skin cancer among childhood cancer survivors: A DCOG-LATER cohort study. J Natl Cancer Inst. 2019;111:845–853. doi: 10.1093/jnci/djy212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pappo AS, Armstrong GT, Liu W, et al. Melanoma as a subsequent neoplasm in adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer. 2013;60:461–466. doi: 10.1002/pbc.24266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Turcotte LM, Neglia JP, Reulen RC, et al. Risk, risk factors, and surveillance of subsequent malignant neoplasms in survivors of childhood cancer: A review. J Clin Oncol. 2018;36:2145–2152. doi: 10.1200/JCO.2017.76.7764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.SEER Statistics . SEER Cancer Stat Facts: Melanoma of the Skin. National Cancer Institute, 2022; https://seer.cancer.gov/statfacts/html/melan.html [Google Scholar]
- 8. Guy GP, Machlin SR, Ekwueme DU, Yabroff KR. Prevalence and costs of skin cancer treatment in the U.S., 2002–2006 and 2007–2011. Am J Prev Med. 2015;48:183–187. doi: 10.1016/j.amepre.2014.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gordon LG, Rowell D. Health system costs of skin cancer and cost-effectiveness of skin cancer prevention and screening: A systematic review. Eur J Cancer Prev. 2015;24:141–149. doi: 10.1097/CEJ.0000000000000056. [DOI] [PubMed] [Google Scholar]
- 10.PDQ Pediatric Treatment Editorial Board . PDQ Late Effects of Treatment for Childhood Cancer. National Cancer Institute; 2021. https://www.cancer.gov/types/childhood-cancers/late-effects-hp-pdq [Google Scholar]
- 11. Landier W, Bhatia S, Eshelman DA, et al. Development of risk-based guidelines for pediatric cancer survivors: The Children’s Oncology Group long-term follow-up Guidelines from the Children’s Oncology Group Late Effects Committee and Nursing Discipline. J Clin Oncol. 2004;22:4979–4990. doi: 10.1200/JCO.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 12. Nathan PC, Ness KK, Mahoney MC, et al. Screening and surveillance for second malignant neoplasms in adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Ann Intern Med. 2010;153:442–451. doi: 10.1059/0003-4819-153-7-201010050-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yan AP, Chen Y, Henderson TO, et al. Adherence to surveillance for second malignant neoplasms and cardiac dysfunction in childhood cancer survivors: A Childhood Cancer Survivor Study. J Clin Oncol. 2020;38:1711–1722. doi: 10.1200/JCO.19.01825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Leisenring WM, Mertens AC, Armstrong GT, et al. Pediatric cancer survivorship research: Experience of the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2319–2327. doi: 10.1200/JCO.2008.21.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: A National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27:2308–2318. doi: 10.1200/JCO.2009.22.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Daniel CL, Armstrong GT, Keske RR, et al. Advancing Survivors’ Knowledge (ASK) about skin cancer study: Study protocol for a randomized controlled trial. Trials. 2015;16:109. doi: 10.1186/s13063-015-0637-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Geller AC, Keske RR, Haneuse S, et al. Skin cancer early detection practices among adult survivors of childhood cancer treated with radiation. J Invest Dermatol. 2019;139:1898–1905.e2. doi: 10.1016/j.jid.2019.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weinstock MA, Martin RA, Risica PM, et al. Thorough skin examination for the early detection of melanoma. Am J Prev Med. 1999;17:169–175. doi: 10.1016/s0749-3797(99)00077-x. [DOI] [PubMed] [Google Scholar]
- 19. Weinstock MA, Risica PM, Martin RA, et al. Reliability of assessment and circumstances of performance of thorough skin self-examination for the early detection of melanoma in the Check-It-Out Project. Prev Med. 2004;38:761–765. doi: 10.1016/j.ypmed.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 20. Hibbard JH, Stockard J, Mahoney ER, Tusler M. Development of the Patient Activation Measure (PAM): Conceptualizing and measuring activation in patients and consumers. Health Serv Res. 2004;39:1005–1026. doi: 10.1111/j.1475-6773.2004.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hibbard JH, Mahoney ER, Stockard J, Tusler M. Development and testing of a short form of the patient activation measure. Health Serv Res. 2005;40:1918–1930. doi: 10.1111/j.1475-6773.2005.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diggle P, Heagerty P, Liang KY, Zeger S. Analysis of Longitudinal Data. ed 2. Oxford, UK, Oxford University Press; 2002. [Google Scholar]
- 23. Robins JM, Rotnitzky A, Zhao LP. Analysis of semiparametric regression models for repeated outcomes in the presence of missing data. J Am Stat Assoc. 1995;90:106–121. [Google Scholar]
- 24.R Core Team . R: A Language and Environment for Statistical Computing. 2020. https://www.r-project.org/ [Google Scholar]
- 25. Bowen DJ, Burke W, Hay JL, et al. Effects of web-based intervention on risk reduction behaviors in melanoma survivors. J Cancer Surviv. 2015;9:279–286. doi: 10.1007/s11764-014-0412-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bowen DJ, Hay J, Meischke H, et al. Randomized trial of a web-based survivor intervention on melanoma prevention behaviors of first-degree relatives. Cancer Causes Control. 2019;30:225–233. doi: 10.1007/s10552-018-1096-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Janda M. Impact of a video-based intervention to improve the prevalence of skin self-examination in men 50 years or older: The randomized skin awareness trial. Arch Dermatol. 2011;147:799. doi: 10.1001/archdermatol.2011.48. [DOI] [PubMed] [Google Scholar]
- 28. Manne SL, Heckman CJ, Kashy DA, et al. Randomized controlled trial of the mySmartSkin web-based intervention to promote skin self-examination and sun protection among individuals diagnosed with melanoma. Transl Behav Med. 2021;11:1461–1472. doi: 10.1093/tbm/ibaa103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Geller AC, Emmons KM, Brooks DR, et al. A randomized trial to improve early detection and prevention practices among siblings of melanoma patients. Cancer. 2006;107:806–814. doi: 10.1002/cncr.22050. [DOI] [PubMed] [Google Scholar]
- 30. Robinson JK, Abou-el-Seoud D, Reavy R, et al. Persistence of partner-assisted skin self-examination supported by ‘being in this together’: A randomized trial. Br J Dermatol. 2020;183:571–573. doi: 10.1111/bjd.19048. [DOI] [PubMed] [Google Scholar]
- 31. Choi J, Cho Y, Woo H. mHealth approaches in managing skin cancer: systematic review of evidence-based research using integrative mapping. JMIR MHealth UHealth. 2018;6:e164. doi: 10.2196/mhealth.8554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Armstrong AW, Watson AJ, Makredes M, et al. Text-message reminders to improve sunscreen use: A randomized, controlled trial using electronic monitoring. Arch Dermatol. 2009;145:1230–1236. doi: 10.1001/archdermatol.2009.269. [DOI] [PubMed] [Google Scholar]
- 33. Szabó C, Ócsai H, Csabai M, Kemény L. A randomised trial to demonstrate the effectiveness of electronic messages on sun protection behaviours. J Photochem Photobiol B. 2015;149:257–264. doi: 10.1016/j.jphotobiol.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 34. Youl PH, Soyer HP, Baade PD, et al. Can skin cancer prevention and early detection be improved via mobile phone text messaging? A randomised, attention control trial. Prev Med. 2015;71:50–56. doi: 10.1016/j.ypmed.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 35. Buller DB, Berwick M, Lantz K, et al. Smartphone mobile application delivering personalized, real-time sun protection advice: A randomized clinical trial. JAMA Dermatol. 2015;151:497–504. doi: 10.1001/jamadermatol.2014.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Buller DB, Berwick M, Lantz K, et al. Evaluation of immediate and 12-week effects of a smartphone sun-safety mobile application: A randomized clinical trial. JAMA Dermatol. 2015;151:505–512. doi: 10.1001/jamadermatol.2014.3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Horsham C, Loescher Lj, Whiteman Dc, et al. Consumer acceptance of patient-performed mobile teledermoscopy for the early detection of melanoma. Br J Dermatol. 2016;175:1301–1310. doi: 10.1111/bjd.14630. [DOI] [PubMed] [Google Scholar]
- 38. Heckman CJ, Darlow SD, Ritterband LM, et al. Efficacy of an intervention to alter skin cancer risk behaviors in Young adults. Am J Prev Med. 2016;51:1–11. doi: 10.1016/j.amepre.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Phillips SM, Padgett LS, Leisenring WM, et al. Survivors of childhood cancer in the United States: Prevalence and burden of morbidity. Cancer Epidemiol Biomarkers Prev. 2015;24:653–663. doi: 10.1158/1055-9965.EPI-14-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the corresponding author upon reasonable request.