Abstract
Periodontitis is a prevalent human disease of inflammation-induced bone destruction. Through studies in patient lesions of rare and common forms of periodontitis and animal model experimentation, Th17/IL-17 related immune pathways have emerged as mediators of disease pathology. In this focused review, we examine mechanisms of induction, amplification and pathogenicity of Th17 cells in periodontitis.
Keywords: Osteoimmunology, Periodontitis, Th17 cells, oral microbiome, bone loss
Introduction
The term osteoimmunology was coined more than two decades ago [1] to describe the interdisciplinary research field that deals with the cross-regulation between bone cells and the immune system. Indeed, the osseous and immune systems are intimately interconnected. During development, physical proximity brings together bone with the immune system, as the bone marrow provides the intimate microenvironment where hematopoiesis primarily occurs giving rise to blood and immune cell types [2]. With increased appreciation of the primary role of the stromal/osseous microenvironment in regulating hematopoiesis under both physiologic and pathologic conditions [3,4], it has become evident that there is constant communication between the osseous and immune system during development. Additionally, one of the key cell types of the osseous system, the osteoclast is of myeloid origin [5]. However, the first appreciation regarding the direct communication of the immune and osseous systems came from the setting of inflammation in the context of periodontitis, when “osteoclast activating factor” was discovered as an immune cytokine stimulating osteoclastogenesis [6]. This factor was later renamed IL-1β. In fact, the context of inflammatory-bone diseases became the setting where most of the biological mechanisms related to immune triggering of bone destruction/erosion have been discovered. Through this work it became evident that shared immune mediators and cell types have been implicated in the pathology of various inflammatory bone diseases such as rheumatoid arthritis (RA), osteoarthritis (OA) and periodontitis. As such, the myeloid derived cytokines IL-1β, Tumor necrosis factor (TNF) ɑ and IL-6 have emerged as common mediators and druggable targets for a variety of inflammatory bone diseases [7–12]. Beyond myeloid cells, T cells and associated factors have been well established as mediators of inflammatory bone loss [13]. In particular, the T cell subset Th17 and its associated cytokines (IL-23/IL-17) have emerged as pathogenic drivers for various forms of inflammatory bone loss diseases with successful therapeutic targeting of these pathways, particularly related to specific forms of inflammatory arthritis (psoriatic arthritis (PsA) and subtype of RA patients) [14].
In this focused review, we will present basic concepts related to the oral inflammatory bone disease periodontitis and discuss unique and shared mechanisms to the induction and pathogenic role of Th17-related immune responses in periodontitis as compared with other inflammatory bone diseases.
1. Periodontitis is a prototypical disease of inflammation-induced bone loss
Periodontitis is one of the most prevalent human inflammatory diseases which affects oral mucosa (gingiva) and structural tissues which support the dentition, including connective tissue, tooth-associated cementum and alveolar bone. In its severe forms, periodontitis affects approximately 8% of the general population in the United States [15] and has been epidemiologically associated with the co-occurrence of various comorbidities including diabetes, cardiovascular disease, and RA [16–19].
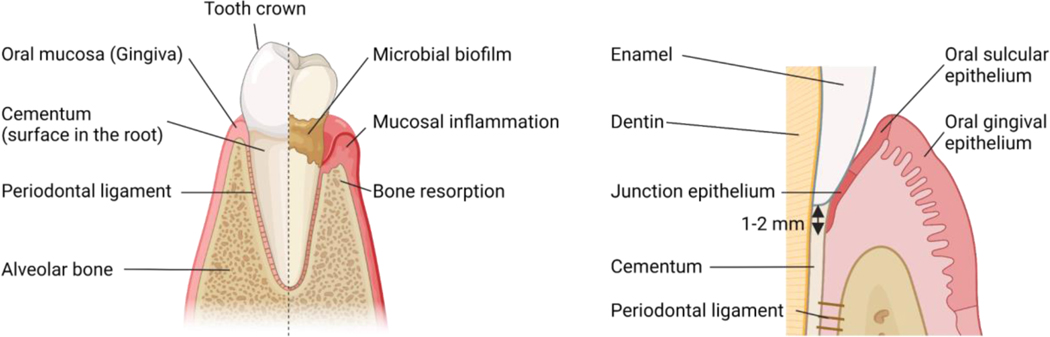
Disease is characterized by the accumulation of a “dysbiotic” microbiome on the root surface of the tooth and mucosal inflammation which becomes pathogenic leading to tissue destruction with loss of tooth supporting structures including connective tissue, tooth cementum and bone and ultimately loss of teeth in severe cases. In health, teeth “sit” inside the alveolar (jaw) bone, which provides support. The alveolar bone is connected to the tooth root surface (a hard tissue structure named cementum) through connective tissue fibers (namely, periodontal ligament). The tooth is typically supported by bone through most of its root, and this support ends 1–2 mm below the clinical crown (the part of a tooth above gum) of the tooth. This 1–2 mm is the area where the oral mucosa connects with the tooth through a very few layers of epithelium (namely Junctional epithelium (JE)) which is connected to the tooth via hemidesmosomes [20,21]. (Fig.1) With inflammation, JE becomes quickly ulcerated and disconnected from the tooth, allowing for microbial translocation. When inflammation is limited to the gingival mucosal tissues, the disease is termed gingivitis and is typically reversible with removal of the dysbiotic microbiome. However, in susceptible individuals’ inflammation becomes more destructive leading to further periodontal tissue destruction: destruction of the connection between tooth and bone, destruction of bone and migration of the epithelium with formation of a periodontal pocket. (Fig. 1)
Figure 1.
Anatomy of tooth and periodontal tissue.
Tooth supporting structures include the gingiva (oral mucosa), the tooth root structure cementum, the alveolar bone and the periodontal ligament which connects the cementum to bone. Oral mucosa has three distinct epithelial regions, Junctional epithelium (JE): the area where the oral mucosa connects to the tooth through a very thin epithelium layer, the Oral gingival epithelium (OGE): Epithelial layer which is exposed to the outside of the tooth and the Oral sulcular (crevicular) epithelium (OSE): In periodontitis, the dysbiotic microbiome on the root surface triggers mucosal inflammation in the gingiva and subsequent loss of connective tissue attachment and bone loss.
2. Dysbiotic microbiome as a disease trigger in periodontitis
The surface of the tooth root (subgingival area) is colonized by a complex microbial biofilm, even in the setting of health. In fact, the subgingival microbiome is one of the most complex microbial communities in the human body [22,23]. However, in the setting of periodontitis, the subgingival microbiome undergoes significant changes, which have been well characterized by multiple research groups [17,24,25]. This disease-associated dysbiotic microbiome is characterized by a significant increase in total microbial biomass and an increase in microbial diversity with over-representation of particular periodontitis-associated species, including Porphyromonas gingivalis, Tannerella forsythia, Treponema denticola: a triad of bacteria known as the “red complex”, and Filifactor alocis [24,26–28]. This dysbiotic microbiome is thought to have increased virulence potentially associated with its ability to trigger excessive inflammatory responses [29], but also associated with the adaptation into an anaerobic and inflammatory environment. In turn, local inflammation is thought to provide nutrients to the microbial community and further perpetuate microbial dysbiosis, creating a vicious self-reinforcing cycle [17,24].
Importantly, microbial dysbiosis is not sufficient to precipitate periodontitis. Therefore, bacterial plaque due to lack of oral and dental care does not necessarily lead to periodontitis, but is thought to trigger disease in individuals with host susceptibility or in cooperation with additional risk factors, such as additional disease comorbidities and/or environmental factors such as smoking [30]. However, because microbial triggering is a key event for the initiation and perpetuation of periodontal disease pathogenesis, current standard of care treatments relies on the removal of microbial biofilm [31].
Current treatment for periodontitis is largely nonspecific, broadly aimed at removing the dysbiotic microbiome and when necessary intervening surgically. Removal of microbial biofilm is typically accomplished with mechanical instrumentation and in some cases with use of adjunct antibiotic treatment, with further surgical interventions in severe cases [32]. However, there are no targeted treatments approved to date for inhibiting specific mechanisms involved in inflammatory bone loss of periodontitis. Furthermore, whether distinct biological mechanisms should be targeted in the treatment of particular subgroups of periodontitis patients also remains unclear. Currently, we lack compelling evidence to subclassify periodontitis patients based on diverse mechanisms implicated. Further evidence is needed to identify valuable targets and potentially identify patient groups where select modulation may be applicable [33]. Further understanding of disease’s triggers, susceptibility and pathogenesis may inform therapeutic intervention but also progress the field of immune-osseous interaction in inflammatory disease.
3. A primer on Th17 biology
In 2006, different labs separately reported on a newly identified helper T cell subset, the IL-17-producing CD4+ helper T cells termed Th17 cells [34–36]. The transcription factor RAR-related orphan receptor gamma (RORγt) was first defined as the signature transcriptional regulator for Th17, yet additional transcription factors have been shown to be involved in the differentiation, commitment and pathogenicity of Th17 including the nuclear receptor RORɑ, the basic leucine zipper transcriptional factor ATF-like (BATF) and interferon-regulatory factor 4 (IRF4), aryl hydrocarbon receptor (Ahr) [37]. Differentiation of Th17 cell was originally shown to depend on cooperative signaling from the cytokines transforming growth factor beta (TGF-β) and IL-6, and there after additional cytokine molecules have been demonstrated to trigger Th17 differentiation in various contexts including the interleukins, IL-1β, IL-21 and IL-23 [38].
Physiologically, Th17 cells are encountered at barrier surfaces such as the gastrointestinal tract and skin and mediate critical immuno-protective functions associated with barrier defense [39]. Th17 cells and their signature cytokine IL-17A have been clearly demonstrated to induce epithelial barrier defenses, to promote barrier integrity and to play a central role in the recruitment of neutrophils during injury and infection [39]. Through these critical functions, Th17 cells have been shown to promote antibacterial and antifungal barrier defenses. In fact, humans with genetic defects in molecules involved in Th17 development and IL-17 signaling present a dominant phenotype of oral and mucocutaneous fungal infections (Candidiasis) and cutaneous and lung bacterial infections [40]. Yet, beyond physiologic immuno-protective roles, Th17 cells have been implicated in chronic inflammatory and autoimmune diseases. Experimental works in animal models have demonstrated a role for Th17 and related cytokines in experimental autoimmune encephalomyelitis (EAE), collagen-induced arthritis (CIA), models of inflammatory bowel disease (IBD), ankylosing spondylitis (AS), psoriasis, experimental uveitis and periodontitis [41]. In humans, inhibition of the cytokine IL-17 has been very successful in the treatment of psoriasis and PsA, while inhibition of the upstream mediator IL-23 has been implemented for the treatment of both psoriasis as well as Crohn’s disease, demonstrating a critical role of these pathways in human autoimmunity and inflammation [41]. (Table1)
4. Th17 cells are pathogenic drivers of inflammatory bone loss in periodontitis
Th17 cells and associated pathways have been associated with immune-osseous interactions implicated in the pathogenesis of diseases associated with inflammatory bone loss such as RA, AS, OA and periodontitis [2].
Upregulation of Th17 and associated responses have been documented in the majority of arthritis mouse models, with the exception of the TNF overproducing models [42,43]. Furthermore, antibody inhibition of IL-17A or IL-17 receptor A (IL-17RA) has resulted in decreased clinical scores and bone loss in a variety of arthritis animal models including the CIA mouse model, the formalin-fixed Borrelia burgdorferi immunize mouse model and rat adjuvant-induced model [44–46]. Additionally, antigen-specific (collagen type 2-specific) Th17 have been found in opposition to osteoclasts in the subchondral area in inflamed joints of the CIA model [47]; and administration of IL-17 into a normal mouse joint induced cartilage degradation [48]. Th17 cells and IL-17A have been documented to be enriched in RA patient joint synovium. The experiment of human bone explant cultures has demonstrated that blockade of the bone-derived endogenous IL-17 by specific inhibitors resulted in a protective effect: inhibition of bone destruction. Those reports strongly suggested Th17/IL-17 as therapeutic targets in RA [49,50]. Yet, inhibition of IL-17A in patients with RA has shown modest results of efficacy, particularly in specific subsets of patients non-responsive to TNF inhibitors [49]. Additionally, targeting of IL-17 related pathways has shown efficacy particularly for the treatment of PsA and promise for spondyloarthritis (SpA), suggesting a role in particular disease subsets of inflammatory bone loss [2,49].
Similar to other inflammatory bone diseases, the Th17/IL-17 axis has been implicated in the pathogenesis of periodontitis. Work from many laboratories has clearly demonstrated the upregulation of cytokines related to the Th17 response, including IL-17A, IL-17F, IL-23(p19), IL-21 in tissue lesions of patients with both chronic and aggressive forms of periodontal disease [51]. In fact, levels of the cytokines IL-17A, IL-23 and IL-21 within periodontal mucosal tissues have been shown to correlate with the severity of bone destruction [52–55]. Furthermore, the expansion of Th17 cells has been documented both in common [55–57] and rare genetic forms of periodontitis [58].
In experimental models of periodontal disease, Th17 cells accumulate after disease induction [55,59,60] and play a pathogenic role as drivers of inflammation and bone destruction in these models. Indeed, inhibition of either the differentiation of Th17 cells through genetic models (Signal transducer and activator of transcription 3: Stat3, CD4CreStat3fl/fl mice and LckCreRorcfl/f mice) or the signature cytokines IL-17A and IL-17F significantly suppressed gingival inflammation and bone destruction in various models of periodontitis [55,59].
In humans, whether Th17 cells and related cytokines are pathogenic drivers of periodontal bone loss has not been conclusively established. However, patients with a deficiency in Th17 differentiation due to a loss of function mutation in the STAT3 gene, have been shown to have very low Th17 cells in the circulation and in gingival tissues with reduced gingival inflammation and periodontal bone loss compared to the general populations [55]. Furthermore, inhibition of p40 (antibody: ustekinumab), the common chain of IL-23 and IL-12 has led to significant reduction of periodontal inflammation in a genetic form of periodontitis, Leukocyte adhesion deficiency (LAD) [61] and has led to the initiation of a clinical trial for the treatment of LAD-associated immune pathologies with ustekinumab [62]. (Table 1)
Table 1.
TH17cells in different inflammatory/autoimmune diseases.

|
5. Disease-associated microbiota trigger Th17 accumulation in periodontitis
A unique aspect of periodontitis compared to other inflammatory bone diseases, such as RA and OA, is that it is initiated by a dysbiotic microbiome [26,59]. While in settings of autoimmune inflammatory bone loss Th17 are thought to be specific to autoantigen triggers, in periodontitis models Th17 cells expand in oral mucosal gingival tissues, in response to the accumulation of a dysbiotic microbiome [55,59]. Indeed, broad spectrum antibiotic treatment inhibited expansion of Th17 cells in experimental periodontitis and associated bone loss [55,59–61,63,64].
Dependence of Th17 on microbes for their induction at barrier sites was first demonstrated in the gastrointestinal tract and skin, with germ-free mice having significantly lower frequency of Th17 cells in the lower intestine [65] and skin [66]. In the gingival mucosa, Th17 physiologically accumulates with age, even in the absence of live commensal microbiota with germ free (GF) mice having comparable proportions and numbers of Th17 cells to specific pathogen-free (SPF) counterparts [67]. In the healthy gingiva, local damage from mastication becomes a tissue-specific trigger for the induction of homeostatic Th17 cells [67]. However, in disease, further amplification of Th17 depends on the accumulation of dysbiotic microbiomes. While specific microbiota capable of triggering Th17 immunity have not been defined to date, experimental inhibition of anaerobes using the narrow spectrum antibiotic Metronidazole inhibited Th17 induction without corresponding reduction of total microbial load. The result indicates that the specific class of microbiota are implicated in periodontitis and Th17 induction [55]. Supporting the role of anaerobes in triggering pathogenic inflammatory responses, Metronidazole is the antibiotic of choice of treatment of aggressive forms of periodontitis [68]. Induction of Th17 cells in the gingiva is shown to depend on cognate antigen recognition in health [67] and T cell receptor (TCR) engagement is also evident during disease expansion of Th17 in periodontitis [55], yet specific antigen recognition and dependence is not detailed to date.
In the gastrointestinal (GI) tract and skin, particular microbiota have been shown to trigger Th17 induction. Segmented filamentous bacteria (SFB) in the gut, and Staphylococcus epidermidis in the skin were the first commensal microbes shown to specifically induce Th17 cell differentiation in their respective barriers in the setting of health [66,69]. In the GI tract, SFB-specific induction of Th17 cells in health and during inflammation depended on the cooperative action of the acute phase protein serum amyloid A (SAA) and cytokine IL-23 both in health and disease states [70–73]. While in the skin, S. epidermidis induction of Th17 depended on the cytokine IL-1β. Antigen specificity of Th17 to be commensal specific has also been shown in the gut [74]. Commensal-induction of Th17 has also been demonstrated in the setting of disease. SFB has been shown to trigger Th17 in the gut not only during heath but also in the setting of inflammation. Actinobacterium Eggerthella lenta, a human microbe, has been associated with induction of Th17 in mice and humans in the gut in the setting of IBD [75]. Additionally, Actinobacterium Bifidobacterium adolescentis has been shown to trigger induction of Th17 cells in an antigen-specific manner and to contribute to severity of arthritis in mice [75].
While amplification of Th17 during disease has been clearly shown to be microbe-dependent in experimental models of periodontitis, to date specific constituents of the microbiome that may be implicated in disease-associated Th17 induction have not been identified in mice or humans. Furthermore, it remains unclear whether Th17 in periodontitis are antigen-specific towards particular periodontitis related pathobionts. Induction of Th17 cells in the gingiva is shown to depend on cognate antigen recognition in health [67] and T cell receptor (TCR) engagement is also evident during disease expansion of Th17 in periodontitis [55], yet specific antigen recognition and dependence is not detailed to date.
However, cytokine dependence for gingival Th17 induction in health and disease has been explored. Th17 cells depend on the cytokine IL-6 for their induction in health and disease [55,59]. IL-6 KO mice had almost complete abrogation of gingival Th17 in periodontitis, although both IL-6 and IL-23 are necessary in disease for Th17 induction [55]. Of interest in the tongue and in the setting of candida infection, Th17 cells are shown to depend on IL-1 indicating tissue and disease specificity for the induction of IL-17 immunity even within the oral mucosal niches [76]. However, while the cellular source of IL-6 in mice has been identified to be the oral epithelium, the cellular source of IL-23 and mechanisms related to IL-23 induction in disease both in mice and humans are not fully elucidated. IL-23 has been linked to pathogenicity of Th17 in other settings [77] consistent with its role in other settings; it appears to be a switch between health and disease associated with Th17 in experimental periodontitis. Indeed, IL-23 is currently explored as a disease target for a Mendelian form of periodontitis in LAD-1.
IL-23 has been previously associated with pathogenicity in inflammatory bone diseases including periodontitis, RA and SpA. Increased levels of IL-23 have been documented in lesions from common and rare forms of periodontitis [51]. In RA, increased levels of IL-23 have been documented in serum and synovial fluid, suggesting IL-23 as a disease biomarker [78]. Furthermore, polymorphisms of the IL-23 receptor (IL-23R) are a risk factor for AS and PsA, which indicates that IL-23 is also involved in the pathogenesis of SpA [79]. IL-23R is expressed by pathogenic Th17 in experimental arthritis [80]. Additionally, IL-23 synergizes with other cytokines such as IL-17 and TNFα to mediate inflammatory bone loss. Finally, IL-23 inhibition has been shown to attenuate paw swelling and joint destruction in CIA rats [81], but has also been clinically effective in reducing clinical manifestations of PsA and SpA. (Table 1)
6. Th17-mediated bone destruction in periodontitis
To date, most of the existing evidence points to a pathogenic role for Th17 cells in experimental models of periodontitis, primarily through its signature cytokine IL-17. Indeed, inhibition of cytokine IL-17A or IL-17A/F has led to significant protection from inflammatory bone loss in the ligature model of periodontitis [55,59]. IL-17 inhibition has also led to protection from natural age-dependent periodontal bone loss [67] and in the context of diabetes-associated periodontitis [82], Developmental endothelial locus-1 (DEL-1) deficiency associated periodontitis [83] and Leukocyte adhesion deficiency type1 (LAD-1) associated periodontitis in mice [58].
One mechanism by which the IL-17 cytokine appears to mediate inflammatory bone destruction is through excessive recruitment of neutrophils and neutrophil mediated immunopathology. Indeed, in the ligature experimental periodontitis (LIP) model and DEL-1 KO mice with spontaneous periodontitis, inhibition of IL-17 led to inhibition of neutrophil granulopoiesis factors and signature chemokines: granulocyte-colony stimulating factor (G-CSF), chemokine (C-X-C motif) ligand (CXCL) 1, CXCL2), leading to reduced neutrophil recruitment to tissues [63,84]. Furthermore, while abrogation of neutrophil recruitment [85] [58] is linked to periodontitis, reduction in neutrophil numbers (using monoclonal antibodies) has led to protection from periodontal bone loss, demonstrating a role for neutrophil-mediated destruction in periodontal models [63]. Neutrophil accumulation is a well-documented feature not only in experimental periodontitis but importantly in human disease. Furthermore, neutrophil activation and neutrophil-mediated pathways including production of reactive oxygen species (ROS), neutrophil proteases and neutrophil extracellular traps (NETs) have been suspected as inflammatory triggers in periodontitis [56,86–88] Indeed, neutrophil activation and NETosis has been implicated in a variety of inflammatory pathologies, including inflammatory bone loss in RA [56,89]. Recently, our lab has documented that neutrophil activation through Fibrin-CD11b binding mediates inflammatory bone loss, partially through activation of NETosis [90].
However, it is clear that IL-17 has pleiotropic roles, beyond neutrophil recruitment, even in the context of periodontitis. A clear example of this is IL-17 mediating inflammatory bone loss in models of defective neutrophil transmigration into tissues. In Lymphocyte Function-associated Antigen 1 (LFA-1) KO mice, neutrophils are unable to transmigrate into tissues. However, IL-17A is a pathogenic driver of inflammatory bone loss in the absence of tissue neutrophils. In this context, inhibition of IL-17 is associated with overall reduction in inflammatory molecules but also reduced expression of the classic osteoclastogenic factor: receptor activator of nuclear factor kappa-Β ligand (RANKL). Yet, the cellular targets for IL-17 signaling in this context are not clear. While it has been clearly shown that osteoblast and periodontal ligament cell expression of RANKL is critical for periodontal bone loss [59], it is still not clear whether IL-17 directly or indirectly signals to these cell subsets to mediate bone resorption. (Fig.2)
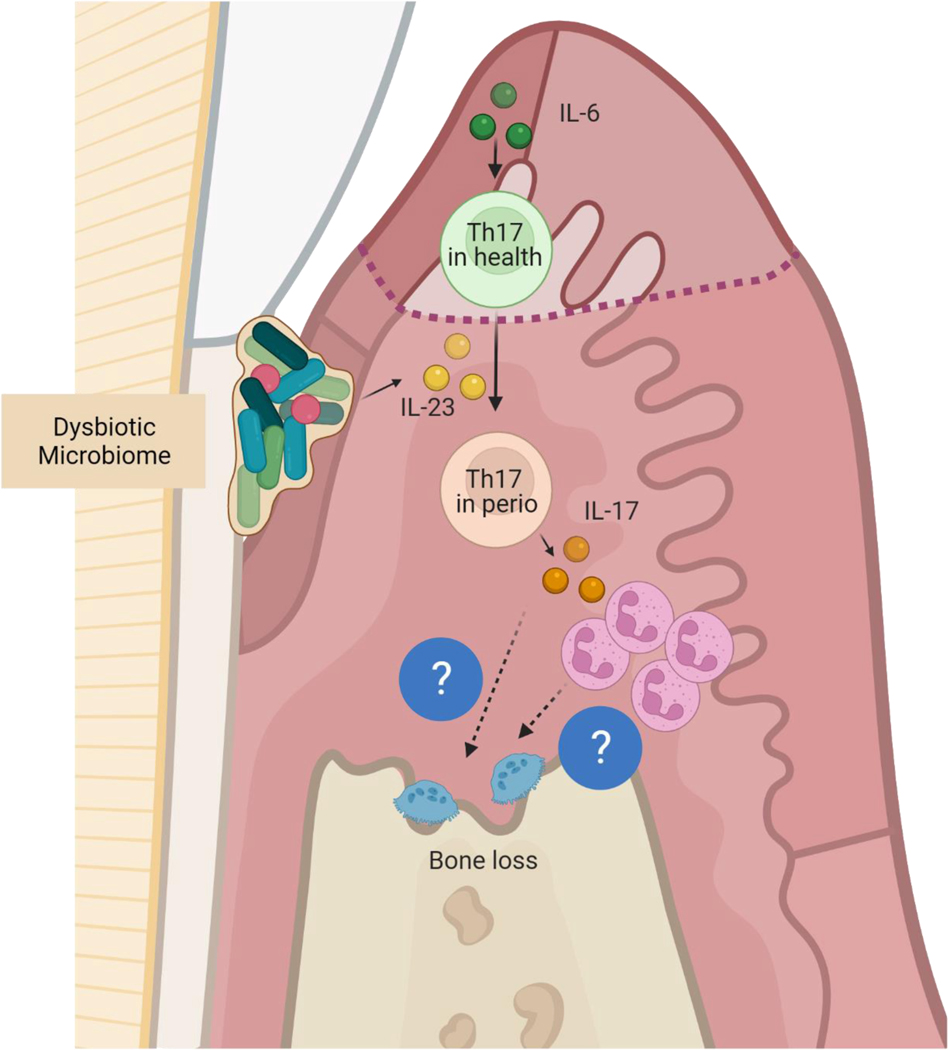
Figure 2.
Gingival Th17/IL-17 axis in health and periodontal disease.
In health IL-6, primarily derived by oral epithelial cells in response to mechanical forces of mastication, induces Th17. In periodontitis, homeostatic IL-6 and IL-23 induced by the dysbiotic microbiota leads to pathogenic expansion of Th17 which drives immune pathology through their cytokine IL17A via neutrophil dependent and independent mechanisms. Ultimately osteoclast activation mediates alveolar bone loss.
7. Potential features of pathogenicity of periodontitis - associated Th17 cells
While the pathogenic role for IL-17 has been well documented in animal models of periodontitis, it is unclear whether Th17 cells have additional, cell intrinsic pathogenic features that can contribute to periodontal immunopathology. In other inflammatory settings, Th17 cells have been documented to acquire a pathogenic gene signature with additional factors (beyond IL-17 secretion) implicated in their pathogenicity. Early studies defined unique transcriptional signatures of proinflammatory Th17 cells in models of EAE [77] and determined the ability of “pathogenic” Th17 cells to drive disease through, not only IL-17 secretion but also secretion of additional mediators such as Interferon gamma (IFNɣ) and Granulocyte-macrophage colony-stimulating factor (GM-CSF) [91]. Additionally, while “pathogenic” signatures may have unique features depending on tissue and disease, a shared feature has been their dependence on IL-23 and their expression of STAT3. In fact, IL23 dependance for pathogenic Th17 has justified targeting IL23 versus IL17 in certain diseases. [92].
In the gingiva, specific “pathogenic” transcriptional signatures have not been documented that would distinguish health vs disease specific Th17 populations. Furthermore, Th17 subpopulations capable of inducing disease upon transfer have also not been defined. However, Th17 in periodontitis do share features previously associated with pathogenicity in other disease settings. In human disease, Th17 cells have been shown to be majority tissue resident, effector memory T cells (Trem) [93] which secrete predominantly IL-17A, with smaller proportions of cells secreting GM-CSF, IFNɣ and IL-22 [55]. Transcriptional profiles of Th17 in human periodontitis also reveal expression of IL17F, CCR6 and STAT3 [56]. In experimental models, while health-associated Th17 cells depend on IL-6, expansion of Th17 in LIP necessitates IL-23, similar to IL-23 dependence of Th17 in most pathologies [55]. Furthermore, depletion of Stat3 in CD4 cells resulted in protection from periodontitis, indicating a role for Stat3 in disease-associated Th17 cells [55]. However, periodontitis-associated Th17 cells are not shown to co-secrete IFNγ, a pathogenic Th17 cytokine in other settings [55,59,60].
Another, potential pathogenic feature of periodontitis-associated Th17 is the fact that they were converted from Foxp3+ cells in the setting of inflammation. In RA models CD25lowFoxp3+CD4+ T cells have been shown to lose Foxp3 expression (called exFoxp3 cells) and undergo transdifferentiation into Th17 cells. Fate mapping analysis demonstrated that IL-17-expressing exFoxp3 Th17 cells accumulated in inflamed joints and were more potent osteoclastogenic T cells than were naȉve CD4+ T cells-derived Th17 cells. Notably, exFoxp3 Th17 cells were characterized by the expression of SRY-Box transcription factor 4 (Sox4), chemokine (C-C motif) receptor 6 (CCR6), chemokine ligand 20 (CCL20), IL-23R and receptor activator of NF-κB ligand RANKL [80]. In periodontitis, exFoxp3 Th17 cells express more Th17 signature cytokines (Il17a and Il17f), transcription factor Rort and RANKL [59] than Th17 in health. Increased IL-17A/F production can contribute to pathogenicity. RANKL expression on T cells also contributes to inflammatory bone loss, albeit not as the major source of RANKL-mediated osteoclastogenesis [59]. In arthritis models the major source of RANKL contributing to osteoclastogenesis are synovial fibroblasts, not T cells [94,95]. Although Th17 cells have been shown to secrete RANKL, RANKL on Th17 cells alone is not sufficient for the induction of osteoclast differentiation [96].
8. Th17 pathways to osteoclastogenesis
Similar to other inflammatory bone loss diseases, osteoclast activation is the final step towards induction of bone destruction in periodontitis. Indeed, upregulation of the classical osteoclastogenic factor RANKL and accumulation of osteoclasts on the bone surface are features for both human and experimental periodontitis [97,98]. RANKL and its decoy receptor Osteoprotegerin (OPG) play very important roles in osteoclast differentiation and activation [99]. RANKL, which can be membrane bound or secreted, binds to RANK expressed on osteoclasts to activate them into bone resorbing phagocytes. Alternatively, the decoy receptor OPG which is expressed on a variety of cell types, inhibits and regulates their functions [100,101]. Upregulation of RANKL and accumulation of osteoclasts on the alveolar bone surface is indeed a classical finding in all types of periodontitis models [102,103]. Furthermore, an increased RANKL/OPG ratio has been reported in gingival tissues from periodontitis patients compared to healthy volunteers in multiple clinical studies [104]. In animal models, RANKL inhibition through osteoprotegerin has been shown to inhibit bone loss in experimental periodontitis [97]. Furthermore, mice deficient in OPG have been shown to develop severe alveolar bone loss with increased presence of osteoclasts [97,105].
While RANKL is expressed by multiple cell types, it appears that RANKL from osteoblasts and periodontal ligament cells most significantly contribute to osteoclastogenesis and bone loss, with T cell expressed RANKL contributing modestly to osteoclastogenesis, in the ligature periodontitis model [59].
It is not well understood whether Th17 and the IL-17 cytokines contribute directly or indirectly to RANKL upregulation and osteoclastogenesis in periodontitis. Nuclear localization of NF-kB signaling in osteoblasts has been shown to be a key for osteoclastogenesis in the oral cavity [106], however whether IL-17 (alone and/or in coordination with other proinflammatory cytokines) contributes to this process is not well determined in periodontitis. Consistent with a role for IL17 in directly mediating RANKL expression, in RA experimental models, IL-17 has been shown to upregulate RANKL in synovial fibroblasts to mediate inflammatory bone loss [95]. (Fig.2)
9. Shared mechanisms in Periodontitis and RA
Th17 and IL-17 related pathways emerge as shared culprits involved in inflammatory bone loss for both periodontitis and RA. Importantly, a strong epidemiological connection between periodontitis and RA has been well defined over the years. Indeed, patients with RA present with a higher incidence and severity of periodontal disease compared to the general population [19]. Significant periodontitis has also been documented in cohorts of new onset RA patients (NORA) [107], instigating the idea that periodontitis may even be an initiating factor for the development of RA [19]. Periodontitis- associated microbiota have been implicated as triggers of early events in RA [108], but also systemic inflammation caused by periodontitis, has been shown to induce epigenetic rewiring of the bone marrow niche, leading to sustained systemic inflammation and increased susceptibility to RA [109]. Whether periodontitis associated microbial and/or host factors contribute to the emergence of Th17-related immunopathology in both diseases, or if shared genetic susceptibilities underlie the emergence of Th17 immunopathology in a subset of RA and periodontitis patients is yet to be defined. Moreover, given the evident co-occurrence of the two disease entities, and the shared clinical feature of inflammation-induced bone destruction further understanding of mechanisms underlying shared susceptibility and pathogenesis will be very valuable.
Conclusions
Periodontitis shares features with distal inflammatory and autoimmune conditions such as inflammation-driven pathology of psoriasis in the skin, colitis in the GI tract and inflammatory bone loss in arthritis. A common feature of all these conditions both in humans and experimental models is the amplification of Th17/IL-17 mediated immune responses. While distinct local triggers appear to mediate Th17 induction in the various disease entities, some mechanisms of triggering and pathogenicity appear to be shared throughout the disease process. Comparing and contrasting features of Th17 immunity in various contexts will not only help advance fundamental understanding of relevant immune mechanisms but may aid in targeting common mechanisms in various disease settings.
Highlights.
Periodontitis is a very prevalent disease of inflammation-induced bone destruction
Th17/IL-17 -related pathways are upregulated in tissues from periodontitis patients
Th17 cells are pathogenic drivers of inflammatory bone loss in experimental periodontitis
A dysbiotic microbiome triggers Th17 amplification in periodontitis
Th17- mediated bone loss in periodontitis largely depends on IL-17
Acknowledgements
The authors thank Teresa G. Wild in Moutsopoulos lab for proofreading, Biorender.com for figure/table illustrations.
Founding sources
This work was funded in part by the intramural programs of NIH/NIDCR.
Footnotes
Declaration of competing interest
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Arron JR, Choi Y, Bone versus immune system, Nature. 408 (2000) 535–536. [DOI] [PubMed] [Google Scholar]
- [2].Tsukasaki M, Takayanagi H, Osteoimmunology: evolving concepts in bone-immune interactions in health and disease, Nat. Rev. Immunol 19 (2019) 626–642. [DOI] [PubMed] [Google Scholar]
- [3].Tikhonova AN, Dolgalev I, Hu H, Sivaraj KK, Hoxha E, Cuesta-Domínguez Á, Pinho S, Akhmetzyanova I, Gao J, Witkowski M, Guillamot M, Gutkin MC, Zhang Y, Marier C, Diefenbach C, Kousteni S, Heguy A, Zhong H, Fooksman DR, Butler JM, Economides A, Frenette PS, Adams RH, Satija R, Tsirigos A, Aifantis I, The bone marrow microenvironment at single-cell resolution, Nature. 569 (2019) 222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Baccin C, Al-Sabah J, Velten L, Helbling PM, Grünschläger F, Hernández-Malmierca P, Nombela-Arrieta C, Steinmetz LM, Trumpp A, Haas S, Combined single-cell and spatial transcriptomics reveal the molecular, cellular and spatial bone marrow niche organization, Nat. Cell Biol 22 (2020) 38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Charles JF, Aliprantis AO, Osteoclasts: more than “bone eaters,” Trends Mol. Med 20 (2014) 449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Horton JE, Raisz LG, Simmons HA, Oppenheim JJ, Mergenhagen SE, Bone resorbing activity in supernatant fluid from cultured human peripheral blood leukocytes, Science. 177 (1972) 793–795. [DOI] [PubMed] [Google Scholar]
- [7].Bresnihan B, Alvaro-Gracia JM, Cobby M, Doherty M, Domljan Z, Emery P, Nuki G, Pavelka K, Rau R, Rozman B, Watt I, Williams B, Aitchison R, McCabe D, Musikic P, Treatment of rheumatoid arthritis with recombinant human interleukin-1 receptor antagonist, Arthritis & Rheumatism. 41 (1998) 2196–2204. . [DOI] [PubMed] [Google Scholar]
- [8].Cohen S, Hurd E, Cush J, Schiff M, Weinblatt ME, Moreland LW, Kremer J, Bear MB, Rich WJ, McCabe D, Treatment of rheumatoid arthritis with anakinra, a recombinant human interleukin-1 receptor antagonist, in combination with methotrexate: results of a twenty-four-week, multicenter, randomized, double-blind, placebo-controlled trial, Arthritis Rheum. 46 (2002) 614–624. [DOI] [PubMed] [Google Scholar]
- [9].Feldmann M, Maini RN, Anti-TNFα Therapy of Rheumatoid Arthritis: What Have We Learned?, Annual Review of Immunology. 19 (2001) 163–196. 10.1146/annurev.immunol.19.1.163. [DOI] [PubMed] [Google Scholar]
- [10].Charles P, Elliott MJ, Davis D, Potter A, Kalden JR, Antoni C, Breedveld FC, Smolen JS, Eberl G, deWoody K, Feldmann M, Maini RN, Regulation of Cytokines, Cytokine Inhibitors, and Acute-Phase Proteins Following Anti-TNF-α Therapy in Rheumatoid Arthritis, The Journal of Immunology. 163 (1999) 1521–1528. [PubMed] [Google Scholar]
- [11].Nakahara H, Song J, Sugimoto M, Hagihara K, Kishimoto T, Yoshizaki K, Nishimoto N, Anti-interleukin-6 receptor antibody therapy reduces vascular endothelial growth factor production in rheumatoid arthritis, Arthritis Rheum. 48 (2003) 1521–1529. [DOI] [PubMed] [Google Scholar]
- [12].Emery P, Keystone E, Tony HP, Cantagrel A, van Vollenhoven R, Sanchez A, Alecock E, Lee J, Kremer J, IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial, Ann. Rheum. Dis 67 (2008) 1516–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Takayanagi H, Ogasawara K, Hida S, Chiba T, Murata S, Sato K, Takaoka A, Yokochi T, Oda H, Tanaka K, Nakamura K, Taniguchi T, T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-gamma, Nature. 408 (2000) 600–605. [DOI] [PubMed] [Google Scholar]
- [14].McGeachy MJ, Cua DJ, Gaffen SL, The IL-17 Family of Cytokines in Health and Disease, Immunity. 50 (2019) 892–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ, CDC Periodontal Disease Surveillance workgroup: James Beck (University of North Carolina, Chapel Hill, USA), Gordon Douglass (Past President, American Academy of Periodontology), Roy Page (University of Washin, Prevalence of periodontitis in adults in the United States: 2009 and 2010, J. Dent. Res 91 (2012) 914–920. [DOI] [PubMed] [Google Scholar]
- [16].Pihlstrom BL, Michalowicz BS, Johnson NW, Periodontal diseases, Lancet. 366 (2005) 1809–1820. [DOI] [PubMed] [Google Scholar]
- [17].Hajishengallis G, Periodontitis: from microbial immune subversion to systemic inflammation, Nat. Rev. Immunol 15 (2015) 30–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Tonetti MS, D’Aiuto F, Nibali L, Donald A, Storry C, Parkar M, Suvan J, Hingorani AD, Vallance P, Deanfield J, Treatment of periodontitis and endothelial function, N. Engl. J. Med 356 (2007) 911–920. [DOI] [PubMed] [Google Scholar]
- [19].Hajishengallis G, Chavakis T, Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities, Nat. Rev. Immunol 21 (2021) 426–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Nakamura M, Histological and immunological characteristics of the junctional epithelium, Jpn. Dent. Sci. Rev 54 (2018) 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jiang N, Guo W, Chen M, Zheng Y, Zhou J, Kim SG, Embree MC, Song KS, Marao HF, Mao JJ, Periodontal Ligament and Alveolar Bone in Health and Adaptation: Tooth Movement, in: Tooth Movement, Karger Publishers, 2016: pp. 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Deo PN, Deshmukh R, Oral microbiome: Unveiling the fundamentals, J. Oral Maxillofac. Pathol 23 (2019) 122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sedghi L, DiMassa V, Harrington A, Lynch SV, Kapila YL, The oral microbiome: Role of key organisms and complex networks in oral health and disease, Periodontol. 2000. 87 (2021) 107–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Abusleme L, Hoare A, Hong B-Y, Diaz PI, Microbial signatures of health, gingivitis, and periodontitis, Periodontol. 2000. 86 (2021) 57–78. [DOI] [PubMed] [Google Scholar]
- [25].Duran-Pinedo AE, Chen T, Teles R, Starr JR, Wang X, Krishnan K, Frias-Lopez J, Community-wide transcriptome of the oral microbiome in subjects with and without periodontitis, ISME J. 8 (2014) 1659–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Abusleme L, Dupuy AK, Dutzan N, Silva N, Burleson JA, Strausbaugh LD, Gamonal J, Diaz PI, The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation, ISME J. 7 (2013) 1016–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL Jr, Microbial complexes in subgingival plaque, J. Clin. Periodontol 25 (1998) 134–144. [DOI] [PubMed] [Google Scholar]
- [28].Griffen AL, Beall CJ, Campbell JH, Firestone ND, Kumar PS, Yang ZK, Podar M, Leys EJ, Distinct and complex bacterial profiles in human periodontitis and health revealed by 16S pyrosequencing, ISME J. 6 (2012) 1176–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Solbiati J, Frias-Lopez J, Metatranscriptome of the Oral Microbiome in Health and Disease, J. Dent. Res 97 (2018) 492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Darveau RP, Periodontitis: a polymicrobial disruption of host homeostasis, Nat. Rev. Microbiol 8 (2010) 481–490. [DOI] [PubMed] [Google Scholar]
- [31].Kinane DF, Stathopoulou PG, Papapanou PN, Periodontal diseases, Nat Rev Dis Primers. 3 (2017) 17038. [DOI] [PubMed] [Google Scholar]
- [32].Sanz M, Herrera D, Kebschull M, Chapple I, Jepsen S, Beglundh T, Sculean A, Tonetti MS, EFP Workshop Participants and Methodological Consultants, Treatment of stage I-III periodontitis-The EFP S3 level clinical practice guideline, J. Clin. Periodontol 47 Suppl 22 (2020) 4–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Caton JG, Armitage G, Berglundh T, Chapple ILC, Jepsen S, Kornman KS, Mealey BL, Papapanou PN, Sanz M, Tonetti MS, A new classification scheme for periodontal and peri-implant diseases and conditions - Introduction and key changes from the 1999 classification, J. Clin. Periodontol. 45 Suppl 20 (2018) S1–S8. [DOI] [PubMed] [Google Scholar]
- [34].Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR, The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells, Cell. 126 (2006) 1121–1133. [DOI] [PubMed] [Google Scholar]
- [35].Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B, TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells, Immunity. 24 (2006) 179–189. [DOI] [PubMed] [Google Scholar]
- [36].Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT, Transforming growth factor-beta induces development of the T(H)17 lineage, Nature. 441 (2006) 231–234. [DOI] [PubMed] [Google Scholar]
- [37].Stockinger B, Omenetti S, The dichotomous nature of T helper 17 cells, Nat. Rev. Immunol 17 (2017) 535–544. [DOI] [PubMed] [Google Scholar]
- [38].Ahern PP, Izcue A, Maloy KJ, Powrie F, The interleukin-23 axis in intestinal inflammation, Immunol. Rev 226 (2008) 147–159. [DOI] [PubMed] [Google Scholar]
- [39].Abusleme L, Moutsopoulos NM, IL-17: overview and role in oral immunity and microbiome, Oral Dis. 23 (2017) 854–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Gaffen SL, Moutsopoulos NM, Regulation of host-microbe interactions at oral mucosal barriers by type 17 immunity, Sci Immunol. 5 (2020). 10.1126/sciimmunol.aau4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Gaffen SL, Jain R, Garg AV, Cua DJ, The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing, Nat. Rev. Immunol 14 (2014) 585–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zwerina K, Koenders M, Hueber A, Marijnissen RJ, Baum W, Heiland GR, Zaiss M, McLnnes I, Joosten L, van den Berg W, Zwerina J, Schett G, Anti IL-17A therapy inhibits bone loss in TNF-α-mediated murine arthritis by modulation of the T-cell balance, Eur. J. Immunol 42 (2012) 413–423. [DOI] [PubMed] [Google Scholar]
- [43].Hashimoto M, Th17 in Animal Models of Rheumatoid Arthritis, J. Clin. Med. Res 6 (2017). 10.3390/jcm6070073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Adamopoulos IE, Bowman EP, Immune regulation of bone loss by Th17 cells, Arthritis Res. Ther 10 (2008) 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Lubberts E, Koenders MI, Oppers-Walgreen B, van den Bersselaar L, Coenen-de Roo CJJ, Joosten LAB, van den Berg WB, Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of collagen-induced arthritis reduces joint inflammation, cartilage destruction, and bone erosion, Arthritis Rheum. 50 (2004) 650–659. [DOI] [PubMed] [Google Scholar]
- [46].Bush KA, Farmer KM, Walker JS, Kirkham BW, Reduction of joint inflammation and bone erosion in rat adjuvant arthritis by treatment with interleukin-17 receptor IgG1 Fc fusion protein, Arthritis Rheum. 46 (2002) 802–805. [DOI] [PubMed] [Google Scholar]
- [47].Pöllinger B, Junt T, Metzler B, Walker UA, Tyndall A, Allard C, Bay S, Keller R, Raulf F, Di Padova F, O’Reilly T, Horwood NJ, Patel DD, Littlewood-Evans A, Th17 cells, not IL-17+ γδ T cells, drive arthritic bone destruction in mice and humans, J. Immunol 186 (2011) 2602–2612. [DOI] [PubMed] [Google Scholar]
- [48].Lubberts E, van den Bersselaar L, Oppers-Walgreen B, Schwarzenberger P, Coenen-de Roo CJ, Kolls JK, Joosten LAB, van den Berg WB, IL-17 Promotes Bone Erosion in Murine Collagen-Induced Arthritis Through Loss of the Receptor Activator of NF-κB Ligand/Osteoprotegerin Balance, The Journal of Immunology. 170 (2003) 2655–2662. [DOI] [PubMed] [Google Scholar]
- [49].Taams LS, Interleukin-17 in rheumatoid arthritis: Trials and tribulations, J. Exp. Med 217 (2020). 10.1084/jem.20192048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].van den Berg WB, Miossec P, IL-17 as a future therapeutic target for rheumatoid arthritis, Nat. Rev. Rheumatol 5 (2009) 549–553. [DOI] [PubMed] [Google Scholar]
- [51].Zenobia C, Hajishengallis G, Basic biology and role of interleukin-17 in immunity and inflammation, Periodontol. 2000. 69 (2015) 142–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Lester SR, Bain JL, Johnson RB, Serio FG, Gingival concentrations of interleukin-23 and −17 at healthy sites and at sites of clinical attachment loss, J. Periodontol 78 (2007) 1545–1550. [DOI] [PubMed] [Google Scholar]
- [53].Takahashi K, Azuma T, Motohira H, Kinane DF, Kitetsu S, The potential role of interleukin-17 in the immunopathology of periodontal disease, J. Clin. Periodontol 32 (2005) 369–374. [DOI] [PubMed] [Google Scholar]
- [54].Dutzan N, Vernal R, Vaque JP, García-Sesnich J, Hernandez M, Abusleme L, Dezerega A, Gutkind JS, Gamonal J, Interleukin-21 expression and its association with proinflammatory cytokines in untreated chronic periodontitis patients, J. Periodontol 83 (2012) 948–954. [DOI] [PubMed] [Google Scholar]
- [55].Dutzan N, Kajikawa T, Abusleme L, Greenwell-Wild T, Zuazo CE, Ikeuchi T, Brenchley L, Abe T, Hurabielle C, Martin D, Morell RJ, Freeman AF, Lazarevic V, Trinchieri G, Diaz PI, Holland SM, Belkaid Y, Hajishengallis G, Moutsopoulos NM, A dysbiotic microbiome triggers TH17 cells to mediate oral mucosal immunopathology in mice and humans, Sci. Transl. Med 10 (2018). 10.1126/scitranslmed.aat0797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Williams DW, Greenwell-Wild T, Brenchley L, Dutzan N, Overmiller A, Sawaya AP, Webb S, Martin D, NIDCD/NIDCR Genomics and Computational Biology Core, Hajishengallis G , Divaris K, Morasso M, Haniffa M, Moutsopoulos NM, Human oral mucosa cell atlas reveals a stromal-neutrophil axis regulating tissue immunity, Cell. 184 (2021) 4090–4104.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Cardoso CR, Garlet GP, Crippa GE, Rosa AL, Júnior WM, Rossi MA, Silva JS, Evidence of the presence of T helper type 17 cells in chronic lesions of human periodontal disease, Oral Microbiol. Immunol 24 (2009) 1–6. [DOI] [PubMed] [Google Scholar]
- [58].Moutsopoulos NM, Konkel J, Sarmadi M, Eskan MA, Wild T, Dutzan N, Abusleme L, Zenobia C, Hosur KB, Abe T, Uzel G, Chen W, Chavakis T, Holland SM, Hajishengallis G, Defective neutrophil recruitment in leukocyte adhesion deficiency type I disease causes local IL-17-driven inflammatory bone loss, Sci. Transl. Med 6 (2014) 229ra40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Tsukasaki M, Komatsu N, Nagashima K, Nitta T, Pluemsakunthai W, Shukunami C, Iwakura Y, Nakashima T, Okamoto K, Takayanagi H, Host defense against oral microbiota by bone-damaging T cells, Nat. Commun 9 (2018) 701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Kitamoto S, Nagao-Kitamoto H, Jiao Y, Gillilland MG 3rd, Hayashi A, Imai J, Sugihara K, Miyoshi M, Brazil JC, Kuffa P, Hill BD, Rizvi SM, Wen F, Bishu S, Inohara N, Eaton KA, Nusrat A, Lei YL, Giannobile WV, Kamada N, The Intermucosal Connection between the Mouth and Gut in Commensal Pathobiont-Driven Colitis, Cell. 182 (2020) 447–462.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Moutsopoulos NM, Zerbe CS, Wild T, Dutzan N, Brenchley L, DiPasquale G, Uzel G, Axelrod KC, Lisco A, Notarangelo LD, Hajishengallis G, Notarangelo LD, Holland SM, Interleukin-12 and Interleukin-23 Blockade in Leukocyte Adhesion Deficiency Type 1, N. Engl. J. Med 376 (2017) 1141–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Rezaei N, Translational Autoimmunity: Autoimmune Disease Associated with Different Clinical Features, Academic Press, 2022. [Google Scholar]
- [63].Moutsopoulos NM, Konkel JE, Tissue-Specific Immunity at the Oral Mucosal Barrier, Trends Immunol. 39 (2018) 276–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Huang N, Dong H, Luo Y, Shao B, Th17 Cells in Periodontitis and Its Regulation by A20, Front. Immunol 12 (2021) 742925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Ivanov II, de Frutos R, Manel N Yoshinaga K, Rifkin DB, Sartor RB, Finlay BB, Littman DR, Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine, Cell Host Microbe. 4 (2008) 337–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Naik S, Bouladoux N, Wilhelm C, Molloy MJ, Salcedo R, Kastenmuller W, Deming C, Quinones M, Koo L, Conlan S, Spencer S, Hall JA, Dzutsev A, Kong H, Campbell DJ, Trinchieri G, Segre JA, Belkaid Y, Compartmentalized control of skin immunity by resident commensals, Science. 337 (2012) 1115–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Dutzan N, Abusleme L, Bridgeman H, Greenwell-Wild T, Zangerle-Murray T, Fife ME, Bouladoux N, Linley H, Brenchley L, Wemyss K, Calderon G, Hong B-Y, Break TJ, Bowdish DME, Lionakis MS, Jones SA, Trinchieri G, Diaz PI, Belkaid Y, Konkel JE, Moutsopoulos NM, On-going Mechanical Damage from Mastication Drives Homeostatic Th17 Cell Responses at the Oral Barrier, Immunity. 46 (2017) 133–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Teughels W, Feres M, Oud V, Martín C, Matesanz P, Herrera D, Adjunctive effect of systemic antimicrobials in periodontitis therapy: A systematic review and meta-analysis, J. Clin. Periodontol 47 Suppl 22 (2020) 257–281. [DOI] [PubMed] [Google Scholar]
- [69].Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman DR, Induction of intestinal Th17 cells by segmented filamentous bacteria, Cell. 139 (2009) 485–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Sano T, Huang W, Hall JA, Yang Y, Chen A, Gavzy SJ, Lee J-Y, Ziel JW, Miraldi ER, Domingos AI, Bonneau R, Littman DR, An IL-23R/IL-22 Circuit Regulates Epithelial Serum Amyloid A to Promote Local Effector Th17 Responses, Cell. 163 (2015) 381–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Lee J-Y, Hall JA, Kroehling L, Wu L, Najar T, Nguyen HH, Lin W-Y, Yeung ST, Silva HM, Li D, Hine A, ‘ng Loke P, Hudesman D, Martin JC, Kenigsberg E, Merad M, Khanna KM, Littman DR, Serum Amyloid A Proteins Induce Pathogenic Th17 Cells and Promote Inflammatory Disease, Cell. 180 (2020) 79–91.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Wang Y, Yin Y, Chen X, Zhao Y, Wu Y, Li Y, Wang X, Chen H, Xiang C, Induction of Intestinal Th17 Cells by Flagellins From Segmented Filamentous Bacteria, Front. Immunol 0 (2019). 10.3389/fimmu.2019.02750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Wu W, Chen F, Liu Z, Cong Y, Microbiota-specific Th17 Cells: Yin and Yang in Regulation of Inflammatory Bowel Disease, Inflamm. Bowel Dis. 22 (2016) 1473–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Yang Y, Torchinsky MB, Gobert M, Xiong H, Xu M, Linehan JL, Alonzo F, Ng C, Chen A, Lin X, Sczesnak A, Liao J-J, Torres VJ, Jenkins MK, Lafaille JJ, Littman DR, Focused specificity of intestinal TH17 cells towards commensal bacterial antigens, Nature. 510 (2014) 152–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Alexander M, Ang QY, Nayak RR, Bustion AE, Sandy M, Zhang B, Upadhyay V, Pollard KS, Lynch SV, Turnbaugh PJ, Human gut bacterial metabolism drives Th17 activation and colitis, Cell Host Microbe. 30 (2022) 17–30.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Hernández-Santos N, Gaffen SL, Th17 cells in immunity to Candida albicans, Cell Host Microbe. 11 (2012) 425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Lee Y, Awasthi A, Yosef N, Quintana FJ, Xiao S, Peters A, Wu C, Kleinewietfeld M, Kunder S, Hafler DA, Sobel RA, Regev A, Kuchroo VK, Induction and molecular signature of pathogenic TH17 cells, Nat. Immunol 13 (2012) 991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Zaky DSE, El-Nahrery EMA, Role of interleukin-23 as a biomarker in rheumatoid arthritis patients and its correlation with disease activity, Int. Immunopharmacol 31 (2016) 105–108. [DOI] [PubMed] [Google Scholar]
- [79].Smith JA, Colbert RA, Review: The interleukin-23/interleukin-17 axis in spondyloarthritis pathogenesis: Th17 and beyond, Arthritis Rheumatol. 66 (2014) 231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Komatsu N, Okamoto K, Sawa S, Nakashima T, Oh-hora M, Kodama T, Tanaka S, Bluestone JA, Takayanagi H, Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis, Nat. Med 20 (2014) 62–68. [DOI] [PubMed] [Google Scholar]
- [81].Yago T, Nanke Y, Kawamoto M, Kobashigawa T, Yamanaka H, Kotake S, IL-23 and Th17 Disease in Inflammatory Arthritis, J. Clin. Med. Res 6 (2017). 10.3390/jcm6090081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Graves DT, Ding Z, Yang Y, The impact of diabetes on periodontal diseases, Periodontol. 2000. 82 (2020) 214–224. [DOI] [PubMed] [Google Scholar]
- [83].Kourtzelis I, Li X, Mitroulis I, Grosser D, Kajikawa T, Wang B, Grzybek M, von Renesse J, Czogalla A, Troullinaki M, Ferreira A, Doreth C, Ruppova K, Chen L-S, Hosur K, Lim J-H, Chung K-J, Grossklaus S, Tausche AK, Joosten LAB, Moutsopoulos NM, Wielockx B, Castrillo A, Korostoff JM, Coskun Ü, Hajishengallis G, Chavakis T, DEL-1 promotes macrophage efferocytosis and clearance of inflammation, Nat. Immunol 20 (2019) 40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Eskan MA, Jotwani R, Abe T, Chmelar J, Lim J-H, Liang S, Ciero PA, Krauss JL, Li F, Rauner M, Hofbauer LC, Choi EY, Chung K-J, Hashim A, Curtis MA, Chavakis T, Hajishengallis G, The leukocyte integrin antagonist Del-1 inhibits IL-17-mediated inflammatory bone loss, Nat. Immunol 13 (2012) 465–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Yu JJ, Ruddy MJ, Wong GC, Sfintescu C, Baker PJ, Smith JB, Evans RT, Gaffen SL, An essential role for IL-17 in preventing pathogen-initiated bone destruction: recruitment of neutrophils to inflamed bone requires IL-17 receptor-dependent signals, Blood. 109 (2007) 3794–3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Matthews JB, Wright HJ, Roberts A, Ling-Mountford N, Cooper PR, Chapple ILC, Neutrophil hyper-responsiveness in periodontitis, J. Dent. Res 86 (2007) 718–722. [DOI] [PubMed] [Google Scholar]
- [87].Nicu EA, Rijkschroeff P, Wartewig E, Nazmi K, Loos BG, Characterization of oral polymorphonuclear neutrophils in periodontitis patients: a case-control study, BMC Oral Health. 18 (2018) 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].White PC, Chicca IJ, Cooper PR, Milward MR, Chapple ILC, Neutrophil Extracellular Traps in Periodontitis: A Web of Intrigue, J. Dent. Res 95 (2016) 26–34. [DOI] [PubMed] [Google Scholar]
- [89].Carmona-Rivera C, Carlucci PM, Moore E, Lingampalli N, Uchtenhagen H, James E, Liu Y, Bicker KL, Wahamaa H, Hoffmann V, Catrina AI, Thompson P, Buckner JH, Robinson WH, Fox DA, Kaplan MJ, Synovial fibroblast-neutrophil interactions promote pathogenic adaptive immunity in rheumatoid arthritis, Sci Immunol. 2 (2017). 10.1126/sciimmunol.aag3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Silva LM, Doyle AD, Greenwell-Wild T, Dutzan N, Tran CL, Abusleme L, Juang LJ, Leung J, Chun EM, Lum AG, Agler CS, Zuazo CE, Sibree M, Jani P, Kram V, Martin D, Moss K, Lionakis MS, Castellino FJ, Kastrup CJ, Flick MJ, Divaris K, Bugge TH, Moutsopoulos NM, Fibrin is a critical regulator of neutrophil effector function at the oral mucosal barrier, Science. 374 (2021) eabl5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Stem-like intestinal Th17 cells give rise to pathogenic effector T cells during autoimmunity, Cell. 184 (2021) 6281–6298.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Lee JS, Tato CM, Joyce-Shaikh B, Gulen MF, Cayatte C, Chen Y, Blumenschein WM, Judo M, Ayanoglu G, McClanahan TK, Li X, Cua DJ, Interleukin-23-Independent IL-17 Production Regulates Intestinal Epithelial Permeability, Immunity. 43 (2015) 727–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Dutzan N, Konkel JE, Greenwell-Wild T, Moutsopoulos NM, Characterization of the human immune cell network at the gingival barrier, Mucosal Immunol. 9 (2016) 1163–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Komatsu N, Takayanagi H, Immune-bone interplay in the structural damage in rheumatoid arthritis, Clin. Exp. Immunol 194 (2018) 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Danks L, Komatsu N, Guerrini MM, Sawa S, Armaka M, Kollias G, Nakashima T, Takayanagi H, RANKL expressed on synovial fibroblasts is primarily responsible for bone erosions during joint inflammation, Ann. Rheum. Dis 75 (2016) 1187–1195. [DOI] [PubMed] [Google Scholar]
- [96].Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, Tanaka S, Kodama T, Akira S, Iwakura Y, Cua DJ, Takayanagi H, Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction, J. Exp. Med 203 (2006) 2673–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Koide M, Kobayashi Y, Ninomiya T, Nakamura M, Yasuda H, Arai Y, Okahashi N, Yoshinari N, Takahashi N, Udagawa N, Osteoprotegerin-deficient male mice as a model for severe alveolar bone loss: comparison with RANKL-overexpressing transgenic male mice, Endocrinology. 154 (2013) 773–782. [DOI] [PubMed] [Google Scholar]
- [98].Lin P, Niimi H, Ohsugi Y, Tsuchiya Y, Shimohira T, Komatsu K, Liu A, Shiba T, Aoki A, Iwata T, Katagiri S, Application of Ligature-Induced Periodontitis in Mice to Explore the Molecular Mechanism of Periodontal Disease, Int. J. Mol. Sci 22 (2021). 10.3390/ijms22168900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, Van G, Itie A, Khoo W, Wakeham A, Dunstan CR, Lacey DL, Mak TW, Boyle WJ, Penninger JM, OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis, Nature. 397 (1999) 315–323. [DOI] [PubMed] [Google Scholar]
- [100].Li J, Sarosi I, Yan XQ, Morony S, Capparelli C, Tan HL, McCabe S, Elliott R, Scully S, Van G, Kaufman S, Juan SC, Sun Y, Tarpley J, Martin L, Christensen K, McCabe J, Kostenuik P, Hsu H, Fletcher F, Dunstan CR, Lacey DL, Boyle WJ, RANK is the intrinsic hematopoietic cell surface receptor that controls osteoclastogenesis and regulation of bone mass and calcium metabolism, Proc. Natl. Acad. Sci. U. S. A 97 (2000) 1566–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Kawai T, Matsuyama T, Hosokawa Y, Makihira S, Seki M, Karimbux NY, Goncalves RB, Valverde P, Dibart S, Li Y-P, Miranda LA, Ernst CWO, Izumi Y, Taubman MA, B and T lymphocytes are the primary sources of RANKL in the bone resorptive lesion of periodontal disease, Am. J. Pathol 169 (2006) 987–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Lin J, Bi L, Yu X, Kawai T, Taubman MA, Shen B, Han X, Porphyromonas gingivalis exacerbates ligature-induced, RANKL-dependent alveolar bone resorption via differential regulation of Toll-like receptor 2 (TLR2) and TLR4, Infect. Immun 82 (2014) 4127–4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Sojod B, Chateau D, Mueller CG, Babajko S, Berdal A, Lézot F, Castaneda B, RANK/RANKL/OPG Signalization Implication in Periodontitis: New Evidence from a RANK Transgenic Mouse Model, Front. Physiol 8 (2017) 338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Belibasakis GN, Bostanci N, The RANKL-OPG system in clinical periodontology, J. Clin. Periodontol 39 (2012) 239–248. [DOI] [PubMed] [Google Scholar]
- [105].Ozaki Y, Koide M, Furuya Y, Ninomiya T, Yasuda H, Nakamura M, Kobayashi Y, Takahashi N, Yoshinari N, Udagawa N, Treatment of OPG-deficient mice with WP9QY, a RANKL-binding peptide, recovers alveolar bone loss by suppressing osteoclastogenesis and enhancing osteoblastogenesis, PLoS One. 12 (2017) e0184904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Pacios S, Xiao W, Mattos M, Lim J, Tarapore RS, Alsadun S, Yu B, Wang C-Y, Graves DT, Osteoblast Lineage Cells Play an Essential Role in Periodontal Bone Loss Through Activation of Nuclear Factor-Kappa B, Sci. Rep 5 (2015) 16694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Scher JU, Sczesnak A, Longman RS, Segata N, Ubeda C, Bielski C, Rostron T, Cerundolo V, Pamer EG, Abramson SB, Huttenhower C, Littman DR, Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis, Elife. 2 (2013) e01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Konig MF, Abusleme L, Reinholdt J, Palmer RJ, Teles RP, Sampson K, Rosen A, Nigrovic PA, Sokolove J, Giles JT, Moutsopoulos NM, Andrade F, Aggregatibacter actinomycetemcomitans-induced hypercitrullination links periodontal infection to autoimmunity in rheumatoid arthritis, Sci. Transl. Med 8 (2016) 369ra176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Li X, Wang H, Yu X, Saha G, Kalafati L, Ioannidis C, Mitroulis I, Netea MG, Chavakis T, Hajishengallis G, Maladaptive innate immune training of myelopoiesis links inflammatory comorbidities, Cell. (2022). 10.1016/j.cell.2022.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Table 1
- [110].Schüler R, Brand A, Klebow S, Wild J, Veras FP, Ullmann E, Roohani S, Kolbinger F, Kossmann S, Wohn C, Daiber A, Münzel T, Wenzel P, Waisman A, Clausen BE, Karbach S, Antagonization of IL-17A Attenuates Skin Inflammation and Vascular Dysfunction in Mouse Models of Psoriasis, J. Invest. Dermatol 139 (2019) 638–647. [DOI] [PubMed] [Google Scholar]
- [111].Weaver CT, Elson CO, Fouser LA, Kolls JK, The Thl7 pathway and inflammatory diseases of the intestines, lungs, and skin, Annu. Rev. Pathol 8 (2013) 477–512. [DOI] [PMC free article] [PubMed] [Google Scholar]