Abstract
Astaxanthin has high utilization value in functional food because of its strong antioxidant capacity. However, the astaxanthin content of Phaffia rhodozyma is relatively low. Adaptive laboratory evolution is an excellent method to obtain high-yield strains. TiO2 is a good inducer of oxidative stress. In this study, different concentrations of TiO2 were used to domesticate P. rhodozyma, and at a concentration of 1000 mg/L of TiO2 for 105 days, the optimal strain JMU-ALE105 for astaxanthin production was obtained. After fermentation, the astaxanthin content reached 6.50 mg/g, which was 41.61% higher than that of the original strain. The ALE105 strain was fermented by batch and fed-batch, and the astaxanthin content reached 6.81 mg/g. Transcriptomics analysis showed that the astaxanthin synthesis pathway, and fatty acid, pyruvate, and nitrogen metabolism pathway of the ALE105 strain were significantly upregulated. Based on the nitrogen metabolism pathway, the nitrogen source was adjusted by ammonium sulphate fed-batch fermentation, which increased the astaxanthin content, reaching 8.36 mg/g. This study provides a technical basis and theoretical research for promoting industrialization of astaxanthin production of P. rhodozyma.
One-Sentence Summary
A high-yield astaxanthin strain (ALE105) was obtained through TiO2 domestication, and its metabolic mechanism was analysed by transcriptomics, which combined with nitrogen source regulation to further improve astaxanthin yield.
Keywords: Astaxanthin, Phaffia rhodozyma, TiO2 domestication, Fermentation, Transcriptomics
Graphical Abstract
Graphical Abstract.

Introduction
Astaxanthin is a carotenoid (Cao et al., 2021) with strong antioxidant capacity, which is widely used in functional foods (Stachowiak & Szulc, 2021), aquaculture (Lu & Lu, 2022), cosmetics (Cheng et al., 2019), and other fields. At present, the research of astaxanthin in the field of functional food mainly focuses on antihypertension (Mokhtari et al., 2021), enhancing immunity (Park et al., 2010), protecting retina (Otsuka et al., 2013), preventing low-density lipoprotein oxidative damage (Visioli & Artaria, 2017), etc. Phaffia rhodozyma/Xanthophyllomyces dendrorhous is one of the important sources of natural astaxanthin. It has rapid heterotrophic metabolism and high cell density in the fermenter. Moreover, it is one of the potential astaxanthin production strains (Miao et al., 2021). However, the astaxanthin yield of naturally isolated P. rhodozyma is low, while the different sources of strains lead to large differences in astaxanthin yield. At present, the astaxanthin content of high-yield strains reported is about 6–10 mg/g (Gassel et al., 2013; Jiang et al., 2017; Pan et al., 2020; Torres-Haro et al., 2021; Yang et al., 2023). The methods to improve the astaxanthin content of P. rhodozyma primarily include genetic engineering, random mutagenesis, and optimization of culture conditions (Rodríguez-Sáiz et al., 2010). Given the difficulty and high cost of genetic engineering methods (Liu et al., 2014), the positive mutation rate obtained by random mutation is low (Jung & Till, 2021), and the improvement of astaxanthin content is very limited. In recent years, it has been found that phytohormones have certain potential in promoting astaxanthin synthesis of P. rhodozyma, but the research is relatively small (Nutakor et al., 2022). Thus, at present, the astaxanthin content of P. rhodozyma remains difficult to reach the level of industrial production (Ye & Bhatia, 2012).
Adaptive laboratory evolution (ALE) uses environmental factors to change strain characteristics of strains to obtain stable and favourable mutations, thus obtaining high-yield strains (Lee & Kim, 2020). ALE is easier to perform in microbiological systems, with its easy operation, simple experimental set-up, and tight control of experimental variables (Dragosits & Mattanovich, 2013). At present, ALE is effective in regulating the metabolic process of strains, which can overcome some shortcomings of existing metabolic engineering, such as the lack of genomic insights and the limitations of genetic tools (Dasgupta et al., 2020; Shi & Zhao, 2017). Godara & Kao (2021) domesticated Saccharomyces cerevisiae by H2O2 to achieve a β-caryophyllene yield of 104.7 ± 6.2 mg/L; Yi et al. (2015) domesticated Phaeodactylum tricornutum using partial removal of diatom medium components, thereby resulting in a significant increase in the yield of rock algae xanthophylls in their cells; and Sun et al. (2018) domesticated Schizochytrium with high salt to achieve a lipid yield of 80.14 g/L, an increase of 53.31% compared to the original strain.
TiO2 is a natural oxide of elemental titanium with low biological toxicity (Grande & Tucci, 2016) and is a good inducer of oxidative stress reactions (Dong et al., 2015). TiO2 can induce redox reactions in cells to form a large number of reactive oxygen species (Tachikawa et al., 2007). Previous studies have shown that under TiO2 stress, P. rhodozyma can accelerate glucose conversion and increase the yield of astaxanthin and astaxanthin precursors (Zhang et al., 2021). Guan et al. (2022) added 500 mg/L of TiO2 to the culture medium, thereby making the astaxanthin content of P. rhodozyma reach 4.7 mg/g, which is significantly higher than that of the original strain. However, the direct addition of TiO2 during fermentation has significant limitations, facing problems such as TiO2 separation and unstable yields, while the yield of P. rhodozyma astaxanthin is low when no TiO2 is added. Stable and genetically evolved high-yielding strains obtained through TiO2 domestication screening can still significantly increase astaxanthin production without TiO2 in the fermentation process. Meanwhile, transcriptomics is an important method to study the regulation and phenotype of gene expression in living organisms at the RNA level. At the same time, the application of transcriptomics in P. rhodozyma has gradually increased (Luna-Flores et al., 2023; Shi et al., 2022). Transcriptomics can be used to analyse the metabolic mechanism of the evolution of domesticated high-yield strains.
Therefore, in this study, different concentrations of TiO2 were added to the seed medium for 154 days of subculture and domestication, and the changes of astaxanthin, carotenoid, biomass, and other parameters were monitored during the domestication process to obtain a strain of P. rhodozyma with high astaxanthin content. In addition, the mechanism of the domesticated strain was also studied by transcriptomics. This study provides a theoretical basis for the synthesis of astaxanthin by domesticated P. rhodozyma and a certain technical basis for promoting the industrial application of astaxanthin.
Materials and Methods
Materials and Reagents
The strain JMU-MVP14 of P. rhodozyma (Li et al., 2022; Xiao et al., 2015; Yang et al., 2023) was obtained from the strain bank of the Fujian Key Laboratory of Food Microbiology and Enzyme Engineering. Astaxanthin standard products were purchased from Yuanye Biotechnology Co., Ltd (SHH, CHN). 3,5-Dinitrosalicylic acid reagent (DNS) was purchased from Fei Jing Biotechnology Co., Ltd (FJ, CHN). Methanol (chromatographic grade) was purchased from Sigma–Aldrich (CA, USA). Moreover, dimethyl sulphoxide, anhydrous ethanol, glucose, magnesium sulphate heptahydrate, calcium chloride dihydrate, sodium chloride, potassium dihydrogen phosphate, ammonium sulphate, and so on were all analytically pure and purchased from Sinopharm Chemical Reagent Co (SHH, CHN). Yeast extract powder and tryptone were purchased from Huankai Microbial Technology Co., Ltd (GD, CHN). TiO2 was analytical grade, purchased from Xilong Science Co., Ltd (GD, CHN).
Strains and Culture
The seed medium was formulated as follows: yeast extract powder 10 g/L, tryptone 20 g/L, glucose 20 g/L, and pH was 6 (FE28, Mettler Toledo, Zurich, CH). Fermentation medium was formulated as follows: glucose 20 g/L, KH2PO4 1 g/L, NaCl 0.1 g/L, MgSO4·7H2O 0.5 g/L, CaCl2·2H2O 0.1 g/L, yeast extract powder 0.2 g/L, and pH was 6. Phaffia rhodozyma stored at −80°C was inoculated onto agar seed solid medium and incubated at 22°C for 5–7 days. A single colony was selected and inoculated into 30 mL of liquid seed medium and incubated at 22°C and 200 rpm (ZWYR-2102, Zhicheng, SHH, CHN) for 5–7 days. After passing two generations in a seed medium, the seed culture (3% v/v) was transferred to a fresh seed medium and incubated at 22°C for 5–7 days at 200 rpm.
Domestication Process
The above secondary seeds were inoculated with 3% inoculum into 30 mL seed medium with TiO2 concentrations of 250, 500, 750, and 1000 mg/L, respectively, and cultured at 22°C and 200 rpm for 5–7 days. Then, the above operation was repeated for continuous domestication and cultivation. Every 35 days of cultivation, 3% inoculum was added to the seed medium without TiO2 for 5 days, and then 9% inoculum was added to the 30 mL fermentation medium, and fermentation was conducted at 200 rpm at 22°C for 5 days. Finally, cell dry weight, astaxanthin, and carotenoid contents were measured at the end of fermentation.
Strains were screened after 70 days of domestication. Subsequently, 5–10 optimal single colonies were selected for fermentation treatment based on the colour of P. rhodozyma at each concentration by culturing the domesticated P. rhodozyma at different TiO2 concentrations in a solid medium. Based on the fermentation data, the highest-yielding strain was selected for further domestication. At the same time, if the carotenoid or astaxanthin content of the domesticated strain decreases but is still higher than that of the original strain, the concentration of TiO2 will be increased to further stimulate P. rhodozyma; if the yield of domesticated strains was lower than that of the original strains at different TiO2 concentrations, the domestication was stopped. In this experiment, TiO2 concentrations were increased to 500, 750, 1000, and 1250 mg/L on day 70 of domestication. Domestication was stopped after collecting samples by analysis on day 154.
Batch and Fed-Batch Fermentation
Batch fermentation was primarily carried out by introducing the original strain and the optimal strain after domestication into a 5 L fermenter containing 2 L fermentation broth (glucose concentration is 40 g/L) with 10% inoculum, and cultivated for 5 days at 300 rpm, 22°C, pH = 4 and dissolved oxygen content of 40% (BIOTECH-5BG-3, bXBio, SHH, CHN). Herein, the fermentation broth was taken every 8 hr to determine its carotenoid, OD600 value, biomass, glucose concentration, and other parameters. Fed-batch fermentation was based on batch fermentation by adding 600 g/L of glucose solution to maintain its glucose concentration at approximately 20–25 g/L.
Nitrogen source control was achieved by adding 1 g/L ammonium sulphate at the initial stage of batch and fed-batch fermentation.
Biomass and Glucose Determination
In addition, 1 mL of fermentation broth was taken in a 2 mL centrifuge tube, centrifuged at13201 × g (5417R, Eppendorf, HAM, GER) for 5 min and the supernatant was discarded. The centrifuge tube was dried in an oven (LS-0610.150L, Thermo, MA, USA) at 60°C to a constant weight, and its dry weight of cells was weighed by an analytical balance.
A certain amount of glucose was oven dried, and 50 mg of glucose was dissolved in 45 mL of distilled water and then fixed to 50 mL to obtain a high-concentration glucose solution of 1 mg/mL. Then, 20, 40, 60, 80, 100, 140, 180, and 220 μL of glucose solution was mixed with 480, 460, 440, 420, 400, 360, 320, and 280 μL of distilled water, respectively. Afterwards, 500 μL of DNS reagent was added, followed by the reaction in a boiling water bath for 10 min. The absorbance at 540 nm was measured (Epoch2T, BioTek, VT, USA). The glucose standard curve was obtained by plotting glucose concentration (mg/mL) as abscissa and OD540 as ordinate. Subsequently, 500 μL of yeast suspension was collected in a 1.5 mL centrifuge tube, centrifuged at 13201 × g for 3 min, and the supernatant was diluted 100-fold (5 μL of supernatant was placed in 495 μL of distilled water). Then, 500 μL of DNS reagent was added and mixed well, and the reaction was performed in a boiling water bath for 10 min. Following cooling, 200 μL was transferred to a 96-well plate and its absorbance at 540 nm was measured and substituted into a glucose standard curve to calculate the glucose concentration.
Carotenoids Determination
Herein, 1.00 mg of astaxanthin standard was weighed, dissolved in anhydrous ethanol, and fixed to 100 mL to obtain 10 mg/L of astaxanthin standard. Astaxanthin standards 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, and 10 mL were measured and fixed to 10 mL with anhydrous ethanol to obtain standard solutions with concentrations of 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, and 10 mg/L, respectively. Then, the absorbance of each concentration of astaxanthin standard solution at 478 nm was measured. The carotenoid standard curve was obtained by taking OD478 as the vertical coordinate and astaxanthin concentration (mg/L) as the horizontal coordinate.
Furthermore, 500 μL of fermentation broth was pipetted into a 2 mL centrifuge tube, centrifuged at 13201 × g for 5 min, and the supernatant was discarded. Then, 500 μL of dimethyl sulphoxide preheated at 60°C was added to the thallus after washing twice with distilled water. After sufficient shaking, the mixture was placed in a water bath at 50°C and heated to break the wall for 5 min. Subsequently, 1 mL of absolute ethanol was added to the centrifuge tube, and the extract was allowed to stand for 15 min in the dark, and centrifuged at 13201 × g for 5 min. The supernatant was transferred into a 10 mL centrifuge tube, and the above extraction steps were repeated until the precipitate was white. Then, the supernatant of the extract was transferred to the same centrifuge tube, fixed to 5 mL with anhydrous ethanol, and the absorbance was measured at 478 nm. Finally, the carotenoid content in the fermentation broth was calculated by substituting it into the carotenoid standard curve.
Astaxanthin Determination
The above-obtained astaxanthin standard solution of each concentration was filtered through a 0.22 μm organic filter membrane and then analysed by liquid chromatography. The astaxanthin peak area was used as the vertical coordinate and the concentration of astaxanthin (mg/L) was used as the horizontal coordinate to make the astaxanthin standard curve. The condition of high performance liquid chromatography (HPLC): 150 mm × 3.9 mm liquid chromatographic column (Nova-Pak C18, Waters, MA, USA); mobile phase: (A) water and (B) methanol; detection wavelength: 478 nm; injection volume: 10 μL; flow rate: 0.8 mL/min; and column temperature: room temperature. The elution gradient was as follows: 85% B at 0 min, 100% B at 40 min, and 85% B at 45 min.
The above treatment sample was filtered using a 0.22 μm organic filter membrane and then subjected to liquid chromatography (1260 Infinity, Agilent, CA, USA) analysis. In addition, the concentration of astaxanthin in the fermentation broth was calculated by substituting the above standard curve. Liquid chromatograms of the original and domesticated optimal strains of astaxanthin were shown in Fig. S1.
Observation of Cellular Structure
A volume of 1 mL of fermentation broth of the original strain and the optimal domesticated strain were centrifuged at 13201 × g for 5 min and the supernatant was discarded. The yeast was washed three times with distilled water, then 1 mL of 2.5% glutaraldehyde was added and fixed at 4°C for 12 hr. The yeast was then rinsed with 0.1 mol/L phosphate buffer, and, finally the organisms were dried in a vacuum freeze dryer and then their cell structure was observed by scanning electron microscopy.
Nitrogen Source Screening and Optimization
ALE105 strain was inoculated with 3% inoculum to 30 mL fermentation medium supplemented with 0.5 mM ammonium sulphate, ammonium chloride, sodium nitrate, and potassium nitrate, respectively, and cultured at 22°C for 5 days at 200 rpm. The biomass and astaxanthin yield were measured, and the optimal nitrogen source was selected according to the astaxanthin content.
ALE105 strain was inoculated in 30 mL fermentation medium with the optimal nitrogen source of 0.5, 1, and 1.5 g/L at 3% inoculation amount, cultured at 22°C at 200 rpm for 5 days, measured the biomass and astaxanthin yield, and screened the optimal concentration according to the astaxanthin content.
ALE105 strain was inoculated in 30 mL fermentation medium with 3% inoculation amount, and the optimal nitrogen source was added at the optimal concentration on the 0, 1, 2, 3, and 4 days, respectively, and cultured at 22°C at 200 rpm for 5 days. The biomass and astaxanthin yield were measured, and the optimal addition time was selected according to the astaxanthin content.
RNA Sequencing, Assembly, and Annotation
The original strain and the most ideal domesticated strain were fermented for 5 days, and RNA was extracted for quality inspection. Eukaryotic mRNAs were enriched with magnetic beads with Oligo (dT). Subsequently, a fragmentation buffer was added to randomly interrupt the mRNA. First-strand cDNA was synthesized using mRNA as a template with a six-base random primer followed by buffer, dNTPs and DNA polymerase I to synthesise the second-strand cDNA, and double-stranded cDNA was subsequently purified using AMPure XP beads. Then, purified double-stranded cDNA was end-repaired, A-tailed, and ligated with sequencing adapters, followed by fragment size selection with AMPure XP beads, and, finally polymerase chain reaction (PCR) enrichment to obtain the final cDNA library.
The raw data obtained by sequencing were screened and statistically analysed. Genomic alignment analysis was performed using the HISAT2 software. The expression level of each gene in each sample was calculated using feature Counts software. DESeq2 was used for differential expression analysis of genes. Differential genes were annotated and enriched using the Gene Ontology (GO) and Kyoto Encylopaedia of Genes and Genomes (KEGG). The transcriptome study of ALE105 strain and original strain was performed by Zhongke New Life Biotechnology Co., Ltd (SHH, CHN). The raw data have been uploaded to NCBI (PRJNA981295).
Dynamic Parameters
Using the following equation:
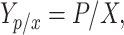 |
(1) |
where Yp/x = Specific yield (mg/g), P = product concentration (mg/L), and X = cell concentration (g/L).
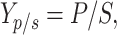 |
(2) |
where Yp/s = product yield (mg/g), P = product concentration (mg/L), and S = glucose concentration (g/L).
 |
(3) |
where Yx/s = growth yield (mg/g), S = glucose concentration (g/L), and X = the cell concentration (g/L).
 |
(4) |
where Qx = cell growth rate (g/L h), X = the cell concentration (g/L), and T = time (hr).
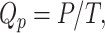 |
(5) |
where Qp = product formation rate (mg/L h), P = product concentration (mg/L), and T = time (hr).
 |
(6) |
where μ = specific growth rate (h−), X = cell concentration (g/L), and T = time (hr).
Statistical Analysis
Each biological replicate was counted triplicate. Excel, Origin2022, and SPSS were used for data processing and analysis to make relevant icons. Significance was determined by p and p < 0.05 was considered significant.
Results and Discussion
Biomass, Glucose, Carotenoid, and Astaxanthin Analysis at Each Stage of TiO2 Domestication at Different Concentrations
The domestication process has a great impact on the growth of microorganisms, and its growing status is an important indicator of the domestication effect (Fu et al., 2012). At different stages of domestication, this study inoculated P. rhodozyma into an unadded TiO2 fermentation medium for fermentation, and the biomass results were shown in Fig. 1A. The dry weight of P. rhodozyma cells slightly increased in the middle of the pre-domestication period (day 70) with a maximum value of 6 g/L at a TiO2 concentration of 250 mg/L, and the lowest dry weight of 3.1 g/L at a TiO2 concentration of 750 mg/L. Meanwhile, in the middle and late stages of domestication (day 105), when the TiO2 concentration was increased from 500 and 750 mg/L to 750 and 1000 mg/L, respectively, the growth of P. rhodozyma was no longer inhibited and the dry weight of cells was slightly increased compared to day 70. A decreasing trend in the dry weight of P. rhodozyma domesticated by different TiO2 concentrations was observed at the end of domestication (day 154). It has been shown that the addition of 500 and 1000 mg/L of TiO2 to the fermentation medium resulted in a small increase in P. rhodozyma biomass; the addition of 1500 mg/L TiO2 resulted in a decrease in biomass (Zhang et al., 2019). Zhang et al. (2020) found that the number of P. rhodozyma cells would be slightly higher than the blank control under the exogenous addition of 500 mg/L TiO2. Combined with the results of this experimental study, TiO2 as a low toxic compound, P. rhodozyma had a strong ability to adapt and had less influence on its growth condition. The domestication of P. rhodozyma with an appropriate concentration of TiO2 would promote its growth and reproduction. In the domestication stage, P. rhodozyma was subcultured for 22 times. Long-time continuous subculture led to the degeneration and aging of P. rhodozyma and the reduction of its tolerance to TiO2, which led to the reduction of biomass in the later stage of domestication (day 154). It has been shown that yeast that has been passed on for more than 25 generations deteriorates significantly, leading to a reduction in yeast quality and fermentation performance. (Gibson et al., 2008; Longo et al., 2012). Almeida et al. also showed a decrease in biomass in the later stages of domestication of Laurentii UFV-1 strains (2023). The studies reported above were consistent with the results of this experiment.
Fig. 1.
(A) Cell dry weight; (B) carotenoid concentration; (C) carotenoid content; (D) astaxanthin concentration; and (E) astaxanthin content of P. rhodozyma domesticated by TiO2 at different stages. Note: Different lowercase letters indicate significant differences (p < 0.05).
After the P. rhodozyma was domesticated to different concentrations of TiO2, the domesticated strain was inoculated into a fermentation medium without TiO2 for fermentation culture. Figure 1B showed the carotenoid concentration. At the concentration of 1000 mg/L TiO2 on the 105th day of domestication, the carotenoid concentration of P. rhodozyma reached the maximum value of 79.27 mg/L, which was 38.46% higher than that of the original strain. The carotenoid concentration decreased at the end of domestication (154th day). The carotenoid content was shown in Fig. 1C. The carotenoid content of the domesticated strain at the concentration of 1000 mg/L TiO2 on the 105th day reached the maximum value of 15.06 mg/g, which was 40.35% higher than that of the original strain.
The astaxanthin concentration was shown in Fig. 1D. In the middle stage of domestication (the first 70 days), P. rhodozyma had the best domestication effect at the concentration of 500 mg/L TiO2, particularly on the 35th day, its yield reached 28.65 mg/L, which was 16.99% higher than the original strain. Moreover, at the concentration of 1000 mg/L TiO2 after domestication for 105 days, the astaxanthin concentration of the P. rhodozyma strain reached the maximum of 34.66 mg/L. The astaxanthin content was shown in Fig. 1E. Furthermore, at the concentration of 500 mg/L TiO2 on the 35th day of domestication, the astaxanthin content reached 5.30 mg/g, which was 15.47% higher than that of the original strain. At the concentration of 1000 mg/L TiO2 for 105 days after domestication, the astaxanthin content of P. rhodozyma reached the maximum of 6.50 mg/L, which was 41.61% higher than that of the original strain. Phaffia rhodozyma was stimulated to increase its astaxanthin content by adding 500 mg/L TiO2 to the fermentation (Zhang et al., 2020). Thus, in this study, P. rhodozyma was domesticated at 500 mg/L TiO2 for 35 days, and the astaxanthin content significantly increased, while the yield significantly decreased after 35 days, thereby indicating a better stimulation effect at this concentration in the short term of addition. Fu et al. (2013) domesticated Salvia dulcis by light conditions and their carotenoid yield increased substantially. Godara et al. (2019) used H2O2 to domesticate S. cerevisiae and found that the increased tolerance to oxidative stress was accompanied by increased carotenoid yield in most strains. In the process of adaptive domestication, the tolerance of microorganisms to the environment will significantly decrease with the increase of domestication time, thereby resulting in degradation. Through the domestication of S. cerevisiae, Kutyna et al. (2012) found that its glycerol yield greatly increased in the early and middle stages of domestication and showed significant degeneration in the later stages. The composition and proportion of metabolites in the body of microorganisms would also change after domestication. Yi et al. (2015) used oxidative stress-driven domestication to increase carotenoid yield in Trichoderma sp. The increase was primarily in lithophanes, while chlorophyll and β-carotene yield showed a decrease in the later stages after an increase in the first and middle stages.
This study successfully screened the P. rhodozyma with the highest astaxanthin content at day 105 and 1000 mg/L TiO2 concentration through 154 days of domestication, and the strain was fermented in the fermentation medium without TiO2. Moreover, the carotenoid and astaxanthin yield were significantly higher than the original strain. The strain could still maintain the characteristics of high astaxanthin yield without exogenous TiO2 stimulation, which indicated that the adaptive domestication had undergone heritable evolution, and the resistance to oxidative stress had been steadily improved. However, with the reduction of the tolerance of P. rhodozyma to TiO2, degradation occurred in the later stage of domestication.
Biomass, Glucose, Carotenoid, and Astaxanthin Analysis of Shake Flask Fermentation of ALE105 Strain
Based on the above research results, the strain with the highest astaxanthin yield in the process of TiO2 domestication was the strain with 1000 mg/L TiO2 domesticated for 105 days, which was named JMU-ALE105. Exogenous TiO2 could accelerate the uptake and utilization of glucose by P. rhodozyma (Zhang et al., 2021). Thus, the growth and utilization of glucose of the ALE105 strain, ALE105 and original strains were inoculated into the fermentation medium without TiO2 for shake flask fermentation and glucose concentration was measured daily to better study the heritability evolution. As shown in Fig. 2A, the ALE105 strain absorbed glucose faster and the utilization rate of glucose was also 17.60% higher than the original strain. The high utilization of glucose by the ALE105 strain made its biomass increase rapidly and entered a stable period earlier. Hu et al. (2021) domesticated Vibrio schizoicus through low temperatures, and the glucose consumption rate of the domesticated strain was significantly increased.
Fig. 2.
Original strain and ALE105 shake flask fermentation: (A) glucose residue and cell dry weight curves; (B) carotenoid content; and (C) astaxanthin content.
In the early stage of fermentation, P. rhodozyma primarily used glucose for growth and reproduction and synthesis of intermediate metabolites and rapidly synthesized astaxanthin and carotenoid after entering the stable stage (Schmidt et al., 2011). The astaxanthin content of the original P. rhodozyma JMU-MVP14 strain was 4.9 mg/g (Ni et al., 2011). In this study, the original and ALE105 strains were further studied in a shake flask fermentation for 5 days to observe their fermentation conditions. As shown in Fig. 2B and C, the original strain primarily synthesized carotenoid and astaxanthin in the first 3 days, while the ALE105 strain still synthesized carotenoid and astaxanthin in large quantities on the 4th day. Astaxanthin synthesis was not detected in the original strain on the 1st day. The ALE105 strain entered the stable phase to synthesise astaxanthin on the 1st day. The carotenoid and astaxanthin synthesis rates of the ALE105 strain on the 5th day of fermentation were 0.588 and 0.296 mg/(L h), respectively, which were significantly higher than the reports of the original strain (Xiao et al., 2015). The ALE105 strain obtained in this study entered the astaxanthin synthesis stage earlier than the original strain, and the synthesis time of carotenoids and astaxanthin was longer. On the 5th day of fermentation, the carotenoid and astaxanthin content of the ALE105 strain increased by 15.10 and 37.91%, respectively as compared with the original strain, thereby indicating that more intermediate pigments and metabolites of the ALE105 strain transferred to the astaxanthin synthesis pathway during fermentation, thus resulting in a significant increase in astaxanthin yield.
Biomass, Glucose, Carotenoid, and Astaxanthin Analysis of ALE105 Strain in Batch and Fed-Batch Fermentation
The glucose consumption curve of P. rhodozyma was shown in Fig. 3A. During the batch fermentation process, the ALE105 strain consumed more glucose than the original strain, and the consumption of glucose increased by 6.87%. During fed-batch fermentation, the original strain showed higher consumption of glucose, whereas the ALE105 strain primarily showed rapid utilization of glucose in the first 96 hr. Furthermore, based on the results in Fig. 3B, the changes in the cell dry weight of the original and ALE105 strains were similar during the batch fermentation process. The cell dry weight increased rapidly after 8–32 hr of fermentation, and slowly after 32 hr, and finally reached 5.73 and 5.50 g/L, respectively. In the process of fed-batch fermentation, the cell dry weight of the original strain slightly changed from 8–40 to 48–120 hr, and finally reached 21.23 g/L, significantly higher than 5.73 g/L of the ALE105 strain.
Fig. 3.
Batch and fed-batch fermentation of original strain and ALE 105: (A) growth curve; (B) cell dry weight; (C) carotenoid concentration; (D) carotenoid content; (E) astaxanthin concentration; and (F) astaxanthin content.
Harith et al. (2020) achieved a biomass of 18 g/L by continuous fermentation with glucose supplementation. Ho et al. (1999) used a batch replenishment fermentation with constant dissolved oxygen to cultivate P. rhodozyma and found that the organisms reached a maximum biomass of 17.4 g/L. Therefore, the biomass of the original strain was significantly increased through fed-batch fermentation, thereby indicating that glucose was used for cell growth and reproduction. However, fed-batch fermentation had no significant effect on the biomass of the ALE105 strain, thereby indicating that this consumed glucose did not promote the growth and reproduction of its cells, which might be used in the respiratory metabolic pathway.
The carotenoid yield of the original strain and ALE105 strain were shown in Fig. 3C and D. The carotenoid concentration of the original strain and ALE105 strain through batch fermentation finally reached 59.22 and 92.31 mg/L, respectively. Finally, the carotene concentration of the original strain and ALE105 strain reached 80.37 and 165.90 mg/L after fed-batch fermentation. The carotenoid content of the ALE105 strain by batch and fed-batch fermentation reached 16.8 and 14.03 mg/g, respectively, which was 62.38% and 80.80% higher than that of the original strain.
The astaxanthin yield of the original and ALE105 strains were shown in Fig. 3E and F. Through batch fermentation, the astaxanthin concentration of the ALE105 strain was 35.95 mg/L, which was 34.6% higher than that of the original strain. Meanwhile, through fed-batch fermentation, the astaxanthin concentration of the original strain was 45.05 mg/L, slightly higher than the 39.05 mg/L of the ALE105 strain. It was also found that the original strain detected astaxanthin on the 3rd day by fed-batch fermentation method, thereby indicating that feeding would affect the astaxanthin synthesis time of the original strain. Based on the analysis of astaxanthin content, the astaxanthin content of the ALE105 strain was 6.53 mg/g in the batch fermentation, which was 41.04% higher than that of the original strain. In fed-batch fermentation, the astaxanthin content of the ALE105 strain was 6.83 mg/g, which was 222.17% higher than that of the original strain.
Through scanning electron microscopy analysis of the cell morphology of ALE105 strain on the 5th day of fermentation, it was found that its cell volume was significantly smaller than the original strain (Fig. S2). Bommasamudram et al. (2022) found that the cell morphology of high-quality Lactobacilli obtained through domestication also occurs. The change of cell morphology may be more conducive to the synthesis of astaxanthin in P. rhodozyma.
Villegas-Mendez et al. (2021) showed that the biomass concentration of fed-batch fermentation of P. rhodozyma yeast increased; however, the microorganism decreased the carotenoid production per unit biomass production. Moriel et al. (2005) added glucose through fed-batch fermentation, and the biomass of P. rhodozyma yeast significantly increased. Combined with the growth curve analysis of P. rhodozyma, the original strain consumed more glucose for cell growth and reproduction through feeding, thereby resulting in a significant increase in its biomass. Therefore, although the carotenoid concentration significantly increased, the carotenoid content decreased. The ALE105 strain used more glucose for carotenoid and astaxanthin synthesis, thereby resulting in higher carotenoid and astaxanthin yield without significant changes in biomass, and thus a substantial increase in content. Therefore, it was speculated that TiO2 domestication would change the distribution ratio of glucose in P. rhodozyma, which would make more sugar flow into the direction of carotenoid production rather than be used for cell growth and reproduction.
Analysis of Key Genes of ALE105 Strain by Transcriptomics
Transcriptome analysis was performed on the fermentation broth of the original and ALE105 strains to reveal the mechanism of TiO2 domestication. The results were showed in Fig. 4, there were 1 835 genes with different expressions, of which 1 073 and 762 genes were upregulated and downregulated, respectively. Multiple important metabolic pathways of the ALE105 strain changed and were found through GO and KEGG enrichment and pathway analyses.
Fig. 4.
Volcanic map of differential gene expression distribution between original strain and ALE105.
The relevant pathway of astaxanthin synthesis was selected in this experiment, such as glycolysis/gluconeogenesis, fatty acid biosynthesis, pyruvate metabolism, cell cycle, nitrogen metabolism, and so on (Fig. 5). The ALE105 strain up-regulated the expression of fructose kinase, 6-phosphate fructose kinase, phosphoenolpyruvate carboxyl kinase, and pyruvate dehydrogenase E2 components in the glycolysis/gluconeogenesis pathway compared with the original strain, which indicated that ALE105 strain had a stronger ability to utilise glucose, could promote the synthesis of acetyl-CoA and provide more precursors for subsequent astaxanthin synthesis (Martinez-Moya et al., 2015). In the pathway of pyruvate metabolism, the expression of the acetyl-CoA carboxylase gene was upregulated, thereby indicating that the ALE105 strain had a stronger ability to synthesise acetyl-CoA into fatty acids. The key genes of astaxanthin synthesis pathway, such as phytoene desaturase, phytoene synthase, and astaxanthin synthetase, were upregulated, indicating that ALE105 had stronger astaxanthin synthesis ability. Fatty acid synthase subunit β in the fatty acid biosynthetic pathway also exhibited upregulation, and this enzyme also promoted the synthesis of fatty acids in P. rhodozyma.
Fig. 5.
Cluster diagram of key genes for the difference between original strain and ALE105. Note: Gene upregulation (red); and gene downregulation (blue).
ACBC1 and ACBC10 genes in the ABC transport tool of the ALE105 strain were also upregulated. The ABC transport protein played an important role in the accumulation of secondary metabolites and transmembrane transport and had a great impact on the synthesis of astaxanthin by P. rhodozyma (Liu et al., 2022). In the nitrogen metabolism pathway, glutamate dehydrogenase, and glutamine synthetase were also upregulated compared with the original strain. These two enzymes played an important role in the consumption and utilization of nitrogen sources, which showed that the ALE105 strain had a higher utilization rate of nitrogen sources. Moreover, the expression of cell division control proteins and cell cycle regulatory proteins was upregulated in the cell cycle pathway in the ALE105 strain, and the effect of cell cycle proteins on the growth cycle of yeast may lead to increased astaxanthin yield (Dragosits & Mattanovich, 2013). HMG-CoA reductase in the biosynthesis pathway of the terpene skeleton was also upregulated. This enzyme could act on the mevalonate pathway and C5 isoprene biosynthesis, thereby promoting the synthesis of astaxanthin precursors (Miao et al., 2011).
This study also found that the downregulation of acid phosphatase corresponding to thiamine metabolism, riboflavin metabolism, and yeast meiosis of ALE105 strain could make phytic acid accumulate in yeast, and the oxidation resistance of phytic acid could be used to cope with the oxidative stress induced by titanium dioxide (Zhang et al., 2021). The down-regulated genes of the ALE105 strain were primarily concentrated in oxidative phosphorylation and ribosomal pathway, and down-regulated malate dehydrogenase and NADH dehydrogenase were also detected. However, the downregulation of malate dehydrogenase in the tricarboxylic acid cycle decreases NADH production and inhibited the oxidative phosphorylation pathway, thereby resulting in less ATP in the respiratory chain, which in turn affects ribosome function (Wang et al., 2019).
The main way to increase the production of astaxanthin of P. rhodozyma is to improve the synthetic pathway of astaxanthin and inhibit the competitive pathway. Li et al. (2022) adjusted the metabolic node by directly adding additives, and increased astaxanthin production by strengthening the carotenoid synthesis pathway and weakening the competitive pathway. Luna‐Flores et al. (2023) found that the enhancement of the electron transport chain of the mutant strain is conducive to inhibiting the competitive pathway, thereby promoting the synthesis of astaxanthin. Shi et al. (2022) studied a mutant strain and found that its high production of astaxanthin was also mainly related to the inhibition of amino acid metabolism pathway. The transcriptomic results of this study were consistent with those reported above. Under TiO2 domestication, strain ALE105 enhanced astaxanthin production by enhancing glycolysis, pyruvate metabolism, fatty acid metabolism, carotenoid synthesis, and other pathways associated with astaxanthin synthesis, while inhibiting the tricarboxylic acid cycle, amino acid metabolism, respiratory chain, and other competing pathways for astaxanthin synthesis (Fig. 6).
Fig. 6.
Main metabolic pathway of the ALE105 strain. Note: Gene upregulation (red); gene downregulation (blue); HK = hexokinase; GCDH = glucose dehydrogenase; PDH = pyruvate dehydrogenase; HMG-CoAR = 3-hydroxy-3-methyl glutaryl coenzyme A reductase; MVK = mevalonate kinase; IPP isomerase = isopentenyl pyrophosphate isomerase; crtE = geranylgeranyl pyrophosphate synthase; crtYB = phytoene synthase; crtI = phytoene desaturase; crtS = astaxanthin synthase; ACC = acetyl-CoA carboxylase; FAS = fatty acid synthetase; MDH = malate dehydrogenase; GDH = glutamate dehydrogenase; and GS = glutamine synthetase.
Analysis of Fermentation Kinetic Parameters of the Fermenter
Batch fermentation and fed-batch fermentation data of the original and ALE105 strains were compared and the results were shown in Table 1. The growth yield YX/S of ALE105 strain by batch fermentation and fed-batch fermentation was 0.19 and 0.16 g/g, which was lower than 0.21 and 0.55 g/g of the original strain, respectively. This result reflected the utilization of glucose by P. rhodozyma in growth, thereby indicating that the original strain used more glucose for growth and reproduction than the ALE105 strain. In batch fermentation, the yield coefficient YP/S of glucose by astaxanthin corresponding to the ALE105 strain was 1.25 mg/g higher than that of the original strain, thereby indicating that the ALE105 strain used more glucose for the biosynthesis of astaxanthin as compared with the original strain. By comparing the two fermentation methods of the ALE105 strain, the astaxanthin yield of glucose by fed-batch fermentation was lower than that by batch fermentation, thereby indicating that the effect of promoting astaxanthin synthesis by fed-batch fermentation was not evident. For the ALE105 strain, the supplemented glucose was not available for astaxanthin synthesis, probably because of its low biomass (Fig. 3B), thereby limiting the synthesis of astaxanthin. Therefore, increasing its biomass may also be a key factor in further improving astaxanthin yield. Controlled nitrogen sources can effectively increase the biomass of P. rhodozyma (Flores-Cotera et al., 2001) Thus, herein, the nitrogen source of the ALE105 strain would be controlled by this method to further obtain high biomass and astaxanthin production.
Table 1.
Comparison of Kinetic Parameters between Batch Fermentation and Fed-Batch Fermentation in Fermenter
| Parameter | Batch fermentation (control) | Batch fermentation (ALE105) | Fed-batch fermentation (control) | Fed-batch fermentation (ALE105) |
|---|---|---|---|---|
| Total sugar concentration S (g/L) | 40.00 ± 0.00c | 40.00 ± 0.00c | 63.32 ± 0.47a | 59.46 ± 0.72b |
| Residual sugar concentration S (g/L) | 13.10 ± 0.04c | 11.26 ± 0.06d | 24.42 ± 0.13a | 24.01 ± 0.09b |
| Fermentation time T (h) | 120.00a | 120.00a | 120.00a | 120.00a |
| Maximum cell dry weight X (g/L) | 5.73 ± 0.25b | 5.50 ± 0.10b | 21.23 ± 0.15a | 5.73 ± 0.15b |
| Specific yield of carotenoid (carotenoid content) Yp/x (mg/g) | 10.34 ± 0.36c | 16.79 ± 0.57a | 7.76 ± 0.11d | 14.03 ± 0.49b |
| Specific yield of astaxanthin (astaxanthin content) Yp/x (mg/g) | 4.63 ± 0.23b | 6.53 ± 0.18a | 2.12 ± 0.08c | 6.81 ± 0.41a |
| Growth yield Yx/s (g/g) | 0.21 ± 0.002b | 0.19 ± 0.009c | 0.55 ± 0.005a | 0.16 ± 0.014d |
| Carotenoid yield Yp/s (mg/g) | 2.20 ± 0.13c | 3.21 ± 0.07b | 4.26 ± 0.24a | 2.26 ± 0.18c |
| Astaxanthin yield Yp/s (mg/g) | 0.98 ± 0.02c | 1.25 ± 0.11a | 1.16 ± 0.15b | 1.10 ± 0.04b |
| Cell-specific production rate Qx (g/(L h)) | 0.05 ± 0.002b | 0.05 ± 0.001b | 0.18 ± 0.001a | 0.05 ± 0.001b |
| Carotenoid specific production rate Qp (mg/(L h)) | 0.49 ± 0.007d | 0.77 ± 0.013b | 1.40 ± 0.019a | 0.67 ± 0.007c |
| Astaxanthin-specific production rate Qp (mg/(L h))) | 0.22 ± 0.002d | 0.30 ± 0.005c | 0.37 ± 0.016a | 0.33 ± 0.012b |
| Specific growth rate μ (h−) | 0.023 ± 0.007b | 0.022 ± 0.002b | 0.038 ± 0.006a | 0.023 ± 0.005b |
| Proportion of astaxanthin in carotenoids (%) | 44.74 ± 2.31b | 38.94 ± 0.97c | 27.15 ± 1.01d | 48.58 ± 2.10a |
Note: Different lowercase letters indicate significant differences (p < 0.05).
Nitrogen Source Regulation ALE105 Strain Batch and Fed-Batch Fermentation
Luna-Flores et al. (2022) found that low concentrations of ammonium sulphate enhanced P. rhodozyma biomass and astaxanthin content. Wang et al. (2019) also believed that ammonium sulphate was an effective nitrogen source to optimize the culture medium and increase the yield of P. rhodozyma.
This study screened four nitrogen sources and optimized the optimal nitrogen source. The results are shown in Figs. S3–S5. The results showed that the addition of 1 g/L ammonium sulphate at the initial moment of fermentation was most effective in promoting astaxanthin synthesis in strain ALE105. Further fermentation was carried out with the addition of 1 g/L ammonium sulphate in batch and fed-batch fermentation, the results of which are shown in Table 2 and Fig. S6. During the batch fermentation, the cell dry weight of the ALE105 strain finally reached 8.40 g/L by initially adding 1 g/L of ammonium sulphate, which was 52.73% higher than that of the non-added group. During batch fermentation, the initial addition of ammonium sulphate also had a great impact on the astaxanthin yield of the ALE105 strain. Its astaxanthin concentration and astaxanthin content reached 68.91 mg/L and 8.21 mg/g, respectively, which were 91.68% and 25.53% higher than those of the non-added group. During fed-batch fermentation, the difference between the ammonium sulphate-added group and the non-added group was significant. The cell dry weight in the added group, carotenoid concentration, astaxanthin concentration, and astaxanthin content reached 21.93 g/L, 252.85 mg/L, 183.06 mg/L, and 8.36 mg/g, which was increased by 282.72%, 214.61%, 368.78%, and 22.58%, respectively, as compared with the non-added group.
Table 2.
Comparison of Batch Fermentation and Fed-Batch Fermentation of ALE105 Strain Supplemented Ammonium Sulphate Fermenter
| Parameter | Batch fermentation | Batch fermentation [(NH4)2SO4] |
Fed-batch fermentation | Fed-batch fermentation [(NH4)2SO4] |
|---|---|---|---|---|
| Cell dry weight (mg/L) | 5.50 ± 0.10c | 8.40 ± 0.53b | 5.73 ± 0.15c | 21.93 ± 0.81a |
| Carotenoid concentration (mg/L) | 92.31 ± 1.55b | 71.09 ± 2.23d | 80.37 ± 0.85c | 252.85 ± 0.47a |
| Carotenoid content (mg/g) | 16.79 ± 0.57a | 8.48 ± 0.38d | 14.03 ± 0.49b | 11.53 ± 0.25c |
| Astaxanthin concentration (mg/L) | 35.95 ± 0.67c | 68.91 ± 2.96b | 39.05 ± 1.44c | 183.06 ± 6.48a |
| Astaxanthin content (mg/g) | 6.54 ± 0.18b | 8.21 ± 0.71a | 6.82 ± 0.41b | 8.36 ± 0.60a |
| Proportion of astaxanthin in carotenoids (%) | 38.94 ± 0.97d | 96.93 ± 1.22a | 48.58 ± 2.10c | 72.39 ± 0.60b |
Note: Different lowercase letters indicate significant differences (p < 0.05).
Combined with the data in Table 1, the astaxanthin concentration and astaxanthin content were increased by 306.35% and 294.34%, respectively, in the ALE105 fed-batch fermentation supplemented with ammonium sulphate group compared with the original strain. Furthermore, the proportion of astaxanthin in carotenoids of the ALE105 strain added with ammonium sulphate significantly increased, thereby reaching 96.93% at the highest, 148.72% and 115.56% higher than that of the non-added group and the original strain, respectively. This result indicated that the addition of moderate amounts of ammonium sulphate could promote astaxanthin synthesis while increasing the biomass of the ALE105 strain. The promotion of astaxanthin synthesis was accomplished more by weakening the accumulation of other pigments in the carotenoid pathway and enhancing astaxanthin synthesis, thereby resulting in a significant increase in the proportion of astaxanthin in carotenoids.
Conclusion
The effect of domestication on the growth and astaxanthin yield of P. rhodozyma was investigated in this study using TiO2 to treat the ALE of P. rhodozyma. The domestication results showed that the ALE105 strain with the highest astaxanthin yield was obtained at the concentration of 1000 mg/L TiO2 on the 105th day of domestication after 154 days of domestication. The strain obtained stable heritable dominant mutation, its utilization rate of glucose was 17.60% higher than that of the original strain, and the yield of carotenoid and astaxanthin was also significantly increased. Among these, the astaxanthin content reached 6.50 mg/g, which was 41.61% higher than that of the original strain. The astaxanthin content of the ALE105 strain reached 6.81 mg/g by fed-batch fermentation. The transcriptome data showed that the genes related to fatty acid metabolism, pyruvate metabolism, fatty acid biosynthesis, cell cycle, nitrogen metabolism, and other pathways of the ALE105 strain were significantly upregulated, whereas the respiratory chain and ribosomal pathways were downregulated, thereby making more carbon sources flow into the astaxanthin synthesis pathway, thus increasing the yield of astaxanthin. The nitrogen source was regulated by ammonium sulphate fed-batch fermentation, and finally, the astaxanthin content reached 8.36 mg/g, 294.33% higher than that of the original strain. The ALE is one of the effective methods to develop astaxanthin-producing strains for industrial application.
Supplementary Material
Acknowledgements
We appreciate the transcriptome assay undertaken by Zhongke New Life Biotechnology Co., Ltd.
Contributor Information
Liang Yang, College of Ocean Food and Biological Engineering, Jimei University, Xiamen 361021, China; Fujian Provincial Key Laboratory of Food Microbiology and Engineering, Xiamen 361021, China.
Hao-Yi Yang, College of Ocean Food and Biological Engineering, Jimei University, Xiamen 361021, China; Fujian Provincial Key Laboratory of Food Microbiology and Engineering, Xiamen 361021, China.
Li You, College of Ocean Food and Biological Engineering, Jimei University, Xiamen 361021, China; Fujian Provincial Key Laboratory of Food Microbiology and Engineering, Xiamen 361021, China.
Hui Ni, College of Ocean Food and Biological Engineering, Jimei University, Xiamen 361021, China; Fujian Provincial Key Laboratory of Food Microbiology and Engineering, Xiamen 361021, China; Research Center of Food Biotechnology of Xiamen City, Xiamen 361021, China.
Ze-Dong Jiang, College of Ocean Food and Biological Engineering, Jimei University, Xiamen 361021, China; Fujian Provincial Key Laboratory of Food Microbiology and Engineering, Xiamen 361021, China; Research Center of Food Biotechnology of Xiamen City, Xiamen 361021, China.
Xi-Ping Du, College of Ocean Food and Biological Engineering, Jimei University, Xiamen 361021, China; Fujian Provincial Key Laboratory of Food Microbiology and Engineering, Xiamen 361021, China; Research Center of Food Biotechnology of Xiamen City, Xiamen 361021, China.
Yan-Bing Zhu, College of Ocean Food and Biological Engineering, Jimei University, Xiamen 361021, China; Fujian Provincial Key Laboratory of Food Microbiology and Engineering, Xiamen 361021, China; Research Center of Food Biotechnology of Xiamen City, Xiamen 361021, China.
Ming-Jing Zheng, College of Ocean Food and Biological Engineering, Jimei University, Xiamen 361021, China; Fujian Provincial Key Laboratory of Food Microbiology and Engineering, Xiamen 361021, China; Research Center of Food Biotechnology of Xiamen City, Xiamen 361021, China.
Li-Jun Li, College of Ocean Food and Biological Engineering, Jimei University, Xiamen 361021, China; Fujian Provincial Key Laboratory of Food Microbiology and Engineering, Xiamen 361021, China; Research Center of Food Biotechnology of Xiamen City, Xiamen 361021, China.
Rui Lin, College of Ocean and Earth Sciences, and Research and Development Center for Ocean Observation Technologies, Xiamen University, Xiamen 361008, China.
Zhi-Peng Li, College of Ocean Food and Biological Engineering, Jimei University, Xiamen 361021, China; Fujian Provincial Key Laboratory of Food Microbiology and Engineering, Xiamen 361021, China; Research Center of Food Biotechnology of Xiamen City, Xiamen 361021, China.
Qing-Biao Li, College of Ocean Food and Biological Engineering, Jimei University, Xiamen 361021, China; Fujian Provincial Key Laboratory of Food Microbiology and Engineering, Xiamen 361021, China; Research Center of Food Biotechnology of Xiamen City, Xiamen 361021, China.
Author Contributions
Liang Yang: design of the study and methodology, data curation, and writing—original draft. Hao-Yi Yang and Li You: data curation and writing—original draft preparation. Hui Ni, Ze-Dong Jiang, Xi-Ping Du, Yan-Bing Zhu, Ming-Jing Zheng, and Li-Jun Li: design of the study and methodology. Rui Lin: provide study guidance. Zhi-Peng Li: design of the study and methodology, writing—reviewing and editing, supervision, and project administration. Qing-Biao Li: writing—reviewing and editing, supervision, and project administration.
Funding
This work was supported by the National Natural Science Foundation (32001672; 22038012); General Program of Fujian Natural Science Foundation (2020J01678); General Project of Fujian Provincial Department of Education (JAT190339), and Major Special Topic on Science and Technology in Fujian Province (2020NZ012015).
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Almeida E. L. M., Ventorim R. Z., Ferreira M. A. M., Costa M. D., Mantovani H. C., Silveira W. B. (2023). New Papiliotrema laurentii UFV-1 strains with improved acetic acid tolerance selected by adaptive laboratory evolution. Fungal Genetics and Biology, 164, 103765. 10.1016/j.fgb.2022.103765 [DOI] [PubMed] [Google Scholar]
- Bommasamudram J., Kumar P., Kapur S., Sharma D., Devappa S. (2022). Development of thermotolerant Lactobacilli cultures with improved probiotic properties using adaptive laboratory evolution method. Probiotics and Antimicrobial Proteins, 0123456789. 10.1007/s12602-021-09892-3 [DOI] [PubMed] [Google Scholar]
- Cao Y., Yang L., Qiao X., Xue C., Xu J. (2023). Dietary astaxanthin: An excellent carotenoid with multiple health benefits. Critical Reviews in Food Science and Nutrition, 63(18), 3019–3045. 10.1080/10408398.2021.1983766 [DOI] [PubMed] [Google Scholar]
- Cheng X. Y., Xiong Y. J., Yang M. M., Zhu M. J. (2019). Preparation of astaxanthin mask from Phaffia rhodozyma and its evaluation. Process Biochemistry, 79, 195–202. 10.1016/j.procbio.2018.12.027 [DOI] [Google Scholar]
- Dasgupta A., Chowdhury N., De R. K. (2020). Metabolic pathway engineering: Perspectives and applications. Computer Methods and Programs in Biomedicine, 192, 105436. 10.1016/j.cmpb.2020.105436 [DOI] [PubMed] [Google Scholar]
- Dong H., Zeng G., Tang L., Fan C., Zhang C., He X., He Y. (2015). An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Research, 79, 128–146. 10.1016/j.watres.2015.04.038 [DOI] [PubMed] [Google Scholar]
- Dragosits M., Mattanovich D. (2013). Adaptive laboratory evolution—Principles and applications for biotechnology. Microbial Cell Factories, 12(1), 1–17. 10.1186/1475-2859-12-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Cotera L. B., Martín R., Sánchez S. (2001). Citrate, a possible precursor of astaxanthin in Phaffia rhodozyma: Influence of varying levels of ammonium, phosphate and citrate in a chemically defined medium. Applied Microbiology and Biotechnology, 55(3), 341–347. 10.1007/s002530000498 [DOI] [PubMed] [Google Scholar]
- Fu W. Q., Gudmundsson O., Feist A. M., Herjolfsson G., Brynjolfsson S., Palsson B. (2012). Maximizing biomass productivity and cell density of Chlorella vulgaris by using light-emitting diode-based photobioreactor. Journal of Biotechnology, 161(3), 242–249. 10.1016/j.jbiotec.2012.07.004 [DOI] [PubMed] [Google Scholar]
- Fu W., Gudmundsson O., Paglia G., Herjolfsson G., Andresson O. S., Palsson B. O., Brynjolfsson S. (2013). Enhancement of carotenoid biosynthesis in the green microalga Dunaliella salina with light-emitting diodes and adaptive laboratory evolution. Applied Microbiology and Biotechnology, 97(6), 2395–2403. 10.1007/s00253-012-4502-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassel S., Schewe H., Schmidt I., Schrader J., Sandmann G. (2013). Multiple improvement of astaxanthin biosynthesis in Xanthophyllomyces dendrorhous by a combination of conventional mutagenesis and metabolic pathway engineering. Biotechnology Letters, 35(4), 565–569. 10.1007/s10529-012-1103-4 [DOI] [PubMed] [Google Scholar]
- Gibson B. R., Lawrence S. J., Boulton C. A., Box W. G., Graham N. S., Linforth R. S. T., Smart K. A. (2008). The oxidative stress response of a lager brewing yeast strain during industrial propagation and fermentation. FEMS Yeast Research, 8(4), 574–585. 10.1111/j.1567-1364.2008.00371.x [DOI] [PubMed] [Google Scholar]
- Godara A., Kao K. C. (2021). Adaptive laboratory evolution of β-caryophyllene producing Saccharomyces cerevisiae. Microbial Cell Factories, 20(1), 1–13. 10.1186/s12934-021-01598-z[ 10.1186/s12934-021-01598-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godara A., Rodriguez M. A. G., Weatherston J. D., Peabody G. L., Wu H. J., Kao K. C. (2019). Beneficial mutations for carotenoid production identified from laboratory-evolved Saccharomyces cerevisiae. Journal of Industrial Microbiology and Biotechnology, 46(12), 1793–1804. 10.1007/s10295-019-02241-y [DOI] [PubMed] [Google Scholar]
- Grande F., Tucci P. (2016). Titanium dioxide nanoparticles: A risk for human health?, Mini-reviews in Medicinal Chemistry, 16(9), 762–769. 10.2174/1389557516666160321114341 [DOI] [PubMed] [Google Scholar]
- Guan X., Zhang J., Xu N., Cai C., Lu Y., Liu Y., Dai W., Wang X., Nan B., Li X., Wang Y. (2023). Optimization of culture medium and scale-up production of astaxanthin using corn steep liquor as substrate by response surface methodology. Preparative Biochemistry & Biotechnology.53(4),443–453. 10.1080/10826068.2022.2098324 [DOI] [PubMed] [Google Scholar]
- Harith Z. T., Lima M. D., Charalampopoulos D., Chatzifragkou A. (2020). Optimised production and extraction of astaxanthin from the yeast Xanthophyllomyces dendrorhous. Microorganisms, 8(3), 430. 10.3390/microorganisms8030430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho K. P., Tam C. Y., Zhou B. (1999). Growth and carotenoid production of Phaffia Rhodozyma in fed-batch cultures with different feeding methods. Biotechnology Letters, 21(2), 175–178. 10.1023/A:1005487709974 [DOI] [Google Scholar]
- Hu X., Tang X., Bi Z., Zhao Q., Ren L. (2021). Adaptive evolution of microalgae Schizochytrium sp. under high temperature for efficient production of docosahexaeonic acid. Algal Research, 54(30), 102212. 10.1016/j.algal.2021.102212 [DOI] [Google Scholar]
- Jiang G. L., Zhou L. Y., Wang Y. T., Zhu M. J. (2017). Astaxanthin from Jerusalem artichoke: Production by fed-batch fermentation using Phaffia rhodozyma and application in cosmetics. Process Biochemistry, 63, 16–25. 10.1016/j.procbio.2017.08.013 [DOI] [Google Scholar]
- Jung C., Till B. (2021). Mutagenesis and genome editing in crop improvement: Perspectives for the global regulatory landscape. Trends in Plant Science, 26(12), 1258–1269. 10.1016/j.tplants.2021.08.002 [DOI] [PubMed] [Google Scholar]
- Kutyna D. R., Varela C., Stanley G. A., Borneman A. R., Henschke P. A., Chambers P. J. (2012). Adaptive evolution of Saccharomyces cerevisiae to generate strains with enhanced glycerol production. Applied Microbiology and Biotechnology, 93(3), 1175–1184. 10.1007/s00253-011-3622-7 [DOI] [PubMed] [Google Scholar]
- Lee S. R., Kim P. (2020). Current status and applications of adaptive laboratory evolution in industrial microorganisms. Journal of Microbiology and Biotechnology, 30(6), 793–803. 10.4014/jmb.2003.03072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z. P., Chen L., Yang H., Li T., Du X., He N., Jiang Z., Lijun L., Ni H. (2022). The role of key genes in astaxanthin biosynthesis in Phaffia rhodozyma by transcript level and gene knockout. Frontiers in Bioengineering and Biotechnology, 9, 158–166. 10.3389/fbioe.2021.812309. [DOI] [Google Scholar]
- Liu J., Sun Z., Gerken H., Liu Z., Jiang Y., Chen F. (2014). Chlorella zofingiensis as an alternative microalgal producer of astaxanthin: Biology and industrial potential. Marine Drugs, 12(6), 3487–3515. 10.3390/md12063487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Yi H., Zhan H., Wang L., Wang J., Li Y., Liu B. (2022). Gibberellic acid-induced fatty acid metabolism and ABC transporters promote astaxanthin production in Phaffia rhodozyma. Journal of Applied Microbiology, 132(1), 390–400. 10.1111/jam.15187 [DOI] [PubMed] [Google Scholar]
- Longo V. D., Shadel G. S., Kaeberlein M., Kennedy B. (2012). Replicative and chronological aging in Saccharomyces cerevisiae. Cell Metabolism, 16(1), 18–31. 10.1016/j.cmet.2012.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q., Lu Y. J. (2022). Microalga- and yeast-based astaxanthin production via nutrient recovery from wastewater for aquaculture practice: An emerging technology for sustainable development. Journal of Chemical Technology & Biotechnology, 97(11), 3035–3048. 10.1002/jctb.7164 [DOI] [Google Scholar]
- Luna-Flores C. H., Wang A., Cui Z., von Hellens J., Speight R. E. (2023). An enhanced electron transport chain improved astaxanthin production in Phaffia rhodozyma. Biotechnology and Bioengineering, 120,5, 1382–1398. 10.1002/bit.28332 [DOI] [PubMed] [Google Scholar]
- Luna-Flores C. H., Wang A., von Hellens J., Speight R. E. (2022). Towards commercial levels of astaxanthin production in Phaffia rhodozyma. Journal of Biotechnology, 350, 42–54. 10.1016/j.jbiotec.2022.04.001 [DOI] [PubMed] [Google Scholar]
- Martinez-Moya P., Niehaus K., Alcaino J., Baeza M., Cifuentes V. (2015). Proteomic and metabolomic analysis of the carotenogenic yeast Xanthophyllomyces dendrorhous using different carbon sources. BMC Genomics [Electronic Resource], 16(1), 1–18. 10.1186/s12864-015-1484-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao L. L., Chi S., Hou T. T., Liu Z. P., Li Y. (2021). The damage and tolerance mechanisms of Phaffia rhodozyma mutant strain MK19 grown at 28°C. Microbial Cell Factories, 20(1), 1–13. 10.1186/s12934-020-01479-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao L. L., Chi S., Tang Y., Su Z., Yin T., Guan G., Li Y. (2011). Astaxanthin biosynthesis is enhanced by high carotenogenic gene expression and decrease of fatty acids and ergosterol in a Phaffia rhodozyma mutant strain. FEMS Yeast Research, 11(2), 192–201. 10.1111/j.1567-1364.2010.00705.x [DOI] [PubMed] [Google Scholar]
- Mokhtari E., Rafiei S., Shokri-Mashhadi N., Saneei P. (2021). Impact of astaxanthin supplementation on blood pressure: A systematic review and meta-analysis of randomized controlled trials. Journal of Functional Foods, 87, 104860. 10.1016/j.jff.2021.104860 [DOI] [Google Scholar]
- Moriel D. G., Chociai M. B., Machado I. M. P., Fontana J. D., Bonfim T. M. B. (2005). Effect of feeding methods on the astaxanthin production by Phaffia rhodozyma in fed-batch process. Brazilian Archives of Biology and Technology, 48(3), 397–401. 10.1590/S1516-89132005000300010 [DOI] [Google Scholar]
- Ni H., Hong Q. L., Xiao A. F., Li L. J., Cai H. N., Su W. J. (2011). Characterization and evaluation of an astaxanthin over-producing Phaffia rhodozyma. Chinese Journal of Biotechnology, 27, 1065–1075. [PubMed] [Google Scholar]
- Nutakor C., Kanwugu O. N., Kovaleva E. G., Glukhareva T. V. (2022). Enhancing astaxanthin yield in Phaffia rhodozyma: Current trends and potential of phytohormones. Applied Microbiology and Biotechnology, 106(9–10), 3531–3538. 10.1007/s00253-022-11972-5 [DOI] [PubMed] [Google Scholar]
- Otsuka T., Shimazawa M., Nakanishi T., Ohno Y., Inoue Y., Tsuruma K., Ishibashi T., Hara H. (2013). The protective effects of a dietary carotenoid, astaxanthin, against light-induced retinal damage. Journal of Pharmacological Sciences, 123(3), 209–218. 10.1254/jphs.13066FP [DOI] [PubMed] [Google Scholar]
- Pan X. S., Wang B. B., Duan R., Jia J., Li J., Xiong W. D., Ling X. P., Chen C. X., Huang X. H., Zhang G. L., Lu Y. H. (2020). Enhancing astaxanthin accumulation in Xanthophyllomyces dendrorhous by a phytohormone: Metabolomic and gene expression profiles. Microbial Biotechnology, 13(5), 1446–1460. 10.1111/1751-7915.13567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. S., Chyun J. H., Kim Y. K., Line L. L., Chew B. P. (2010). Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutrition & Metabolism, 7,1, 1–10. 10.1186/1743-7075-7-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Sáiz M., De La Fuente J. L., Barredo J. L. (2010). Xanthophyllomyces dendrorhous for the industrial production of astaxanthin. Applied Microbiology and Biotechnology, 88(3), 645–658. 10.1007/s00253-010-2814-x [DOI] [PubMed] [Google Scholar]
- Schmidt I., Schewe H., Gassel S., Jin C., Buckingham J., Hümbelin M., Sandmann G., Schrader J. (2011). Biotechnological production of astaxanthin with Phaffia rhodozyma/Xanthophyllomyces dendrorhous. Applied Microbiology and Biotechnology, 89(3), 555–571. 10.1007/s00253-010-2976-6 [DOI] [PubMed] [Google Scholar]
- Shi S., Zhao H. (2017). Metabolic engineering of oleaginous yeasts for production of fuels and chemicals. Frontiers in Microbiology, 8, 1–16. 10.3389/fmicb.2017.02185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z. H., He X. X., Zhang H. L., Guo X. N., Cheng Y. F., Liu X. L., Wang Z. Y., He X. P. (2022). Whole genome sequencing and RNA-seq-driven discovery of new targets that affect carotenoid synthesis in Phaffia rhodozyma. Frontiers in Microbiology, 13, 1–11. 10.3389/fmicb.2022.837894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachowiak B., Szulc P. (2021). Astaxanthin for the food industry. Molecules (Basel, Switzerland), 26(9), 1–18. 10.3390/molecules26092666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X. M., Ren L. J., Bi Z. Q., Ji X. J., Zhao Q. Y., Huang H. (2018). Adaptive evolution of microalgae Schizochytrium sp. under high salinity stress to alleviate oxidative damage and improve lipid biosynthesis. Bioresource Technology, 267(30), 438–444. 10.1016/j.biortech.2018.07.079 [DOI] [PubMed] [Google Scholar]
- Tachikawa T., Fujitsuka M., Majima T. (2007). Mechanistic insight into the TiO2 photocatalytic reactions: Design of new photocatalysts. The Journal of Physical Chemistry C, 111(14), 5259–5275. 10.1021/jp069005u [DOI] [Google Scholar]
- Torres-Haro A., Verdín J., Kirchmayr M. R., Arellano-Plaza M. (2021). Metabolic engineering for high yield synthesis of astaxanthin in Xanthophyllomyces dendrorhous. Microbial Cell Factories, 20(1), 1–17. 10.1186/s12934-021-01664-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villegas-Méndez M. Á., Papadaki A., Pateraki C., Balagurusamy N., Montañez J., Koutinas A. A., Morales-Oyervides L. (2021). Fed-batch bioprocess development for astaxanthin production by Xanthophyllomyces dendrorhous based on the utilization of Prosopis sp. pods extract. Biochemical Engineering Journal, 166, 107844. 10.1016/j.bej.2020.107844 [DOI] [Google Scholar]
- Visioli F., Artaria C. (2017). Astaxanthin in cardiovascular health and disease: Mechanisms of action, therapeutic merits, and knowledge gaps. Food & Function, 8(1), 39–63. 10.1039/c6fo01721e [DOI] [PubMed] [Google Scholar]
- Wang B., Pan X., Jia J., Xiong W., Manirafasha E., Ling X., Yinghua L. (2019). Strategy and regulatory mechanisms of glutamate feeding to enhance astaxanthin yield in Xanthophyllomyces dendrorhous. Enzyme and Microbial Technology, 125, 45–52. 10.1016/j.enzmictec.2019.02.010 [DOI] [PubMed] [Google Scholar]
- Xiao A. F., Jiang X. L., Ni H., Yang Q. M., Cai H. N. (2015). Study on the relationship between intracellular metabolites and astaxanthin accumulation during Phaffia rhodozyma fermentation. Electronic Journal of Biotechnology, 18(3), 148–153. 10.1016/j.ejbt.2015.02.002 [DOI] [Google Scholar]
- Yang H. Y., Yang L., Du X. P., He N., Jiang Z. D., Zhu Y. B., Li L. J., Ni H., Li Q. B., Li Z. P. (2023). Metabolomics of astaxanthin biosynthesis and corresponding regulation strategies in Phaffia rhodozyma. Yeast. 10.1002/yea.3854 [DOI] [PubMed] [Google Scholar]
- Ye V. M., Bhatia S. K. (2012). Pathway engineering strategies for production of beneficial carotenoids in microbial hosts. Biotechnology Letters, 34(8), 1405–1414. 10.1007/s10529-012-0921-8 [DOI] [PubMed] [Google Scholar]
- Yi Z., Xu M., Magnusdottir M., Zhang Y., Brynjolfsson S., Fu W. Q. (2015). Photo-oxidative stress-driven mutagenesis and adaptive evolution on the marine diatom Phaeodactylum tricornutum for enhanced carotenoid accumulation. Marine Drugs, 13(10), 6138–6151. 10.3390/md13106138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Li Q. R., Liu J., Lu Y., Wang Y., Wang Y. (2020). Astaxanthin overproduction and proteomic analysis of Phaffia rhodozyma under the oxidative stress induced by TiO2. Bioresource Technology, 311, 123525. 10.1016/j.biortech.2020.123525 [DOI] [PubMed] [Google Scholar]
- Zhang J., Li Q. R., Lu Y., Guan X., Liu J., Xu N., Cai C., Li X., Nan B., Liu J., Wang Y. (2021). Astaxanthin overproduction of Phaffia rhodozyma PR106 under titanium dioxide stress by transcriptomics and metabolic regulation analysis. Bioresource Technology, 342, 125957. 10.1016/j.biortech.2021.125957 [DOI] [PubMed] [Google Scholar]
- Zhang J., Li Q. R., Zhang M. H., You Y., Wang Y., Wang Y. H. (2019). Enhancement of carotenoid biosynthesis in Phaffia rhodozyma PR106 under stress conditions. Bioscience, Biotechnology, and Biochemistry, 83(12), 2375–2385. 10.1080/09168451.2019.1650633 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.








