Abstract
Moloney murine leukemia virus (M-MuLV) is a replication-competent, simple retrovirus that induces T-cell lymphoma with a mean latency of 3 to 4 months. During the preleukemic period (4 to 10 weeks postinoculation) a marked decrease in thymic size is apparent for M-MuLV-inoculated mice in comparison to age-matched uninoculated mice. We were interested in studying whether the thymic regression was due to an increased rate of thymocyte apoptosis in the thymi of M-MuLV-inoculated mice. Neonatal NIH/Swiss mice were inoculated subcutaneously (s.c.) with wild-type M-MuLV (approximately 105 XC PFU). Mice were sacrificed at 4 to 11 weeks postinoculation. Thymic single-cell suspensions were prepared and tested for apoptosis by two-parameter flow cytometry. Indications of apoptosis included changes in cell size and staining with 7-aminoactinomycin D or annexin V. The levels of thymocyte apoptosis were significantly higher in M-MuLV-inoculated mice than in uninoculated control animals, and the levels of apoptosis were correlated with thymic atrophy. To test the relevance of enhanced thymocyte apoptosis to leukemogenesis, mice were inoculated with the Mo+PyF101 enhancer variant of M-MuLV. When inoculated intraperitoneally, a route that results in wild-type M-MuLV leukemogenesis, mice displayed levels of enhanced thymocyte apoptosis comparable to those seen with wild-type M-MuLV. However, in mice inoculated s.c., a route that results in attenuated leukemogenesis, significantly lower levels of apoptosis were observed. This supported a role for higher levels of thymocyte apoptosis in M-MuLV leukemogenesis. To examine the possible role of mink cell focus-forming (MCF) recombinant virus in raising levels of thymocyte apoptosis, MCF-specific focal immunofluorescence assays were performed on thymocytes from preleukemic mice inoculated with M-MuLV and Mo+PyF101 M-MuLV. The results indicated that infection of thymocytes by MCF virus recombinants is not required for the increased level of apoptosis and thymic atrophy.
Moloney murine leukemia virus (M-MuLV) is a replication-competent, simple retrovirus. When inoculated into neonatal mice, it induces T lymphomas with a mean latency period of 3 to 4 months in 100% of the animals (13). Phenotypically, tumor cells can be classified as immature T lymphocytes. Because of its predictable time course for disease induction, M-MuLV provides a model system in which the changes that occur before the onset of leukemia can be examined.
Generally, M-MuLV leukemogenesis involves both early and late events. Late events include long terminal repeat activation of proto-oncogenes (8, 13, 14). Overexpression of these genes presumably leads to uncontrolled proliferation of the cells and subsequent transformation. Although these insertional activation events are essential for tumor formation, other, earlier events establish a preleukemic state within the animal that is required for efficient disease induction (11). These early changes include defects in bone marrow hematopoiesis, splenomegaly, and thymic atrophy.
A valuable tool in the study of M-MuLV pathogenesis is an enhancer variant of M-MuLV, Mo+PyF101 M-MuLV (10). Mo+PyF101 M-MuLV is similar to wild-type M-MuLV, except that it contains the enhancer elements of the F101 strain of murine polyomavirus inserted into the U3 region of the long terminal repeat, just downstream of the wild-type M-MuLV enhancer sequences. Both viruses, however, encode exactly the same viral proteins. When inoculated subcutaneously (s.c.) into neonatal mice, Mo+PyF101 M-MuLV displays attenuated pathogenicity (4, 10). Thus, comparisons between mice inoculated s.c. with wild-type M-MuLV and those inoculated with Mo+PyF101 M-MuLV have provided insights into the viral and physiological events leading to leukemogenesis. It was later observed that if the route of inoculation of Mo+PyF101 M-MuLV was switched to the intraperitoneal (i.p.) route, animals developed disease with wild-type-like kinetics (2). Moreover, i.p. inoculation with Mo+PyF101 M-MuLV induces the preleukemic states seen in wild-type M-MuLV-inoculated mice, whereas s.c. inoculation of Mo+PyF101 M-MuLV generally does not induce these changes.
One of the characteristics of an M-MuLV infection is the generation of mink cell focus-forming (MCF) virus recombinants (18). During the course of an M-MuLV infection, the input ecotropic virus undergoes an in vivo recombinational event with endogenous polytropic proviral sequences. The resulting MCF virus differs from the input virus in its envelope gene sequences. Several lines of evidence suggest important roles for MCF viruses in both early and late steps of M-MuLV-induced leukemogenesis (reviewed in reference 13). Most importantly for this investigation, MCF virus recombinants have been detected in mice inoculated by the i.p. route with Mo+PyF101 M-MuLV (wild-type-like pathogenicity) (3), whereas MCF virus recombinants have not been detected in mice inoculated s.c. with Mo+PyF101 M-MuLV (attenuated pathogenicity) (4).
The focus of this study was to examine the role of thymic atrophy as a preleukemic event in M-MuLV-induced leukemogenesis. Thymic size in preleukemic M-MuLV-inoculated mice is typically reduced in comparison to that of uninoculated, age-matched control mice (12). The thymus is an organ that supports T-lymphocyte maturation and differentiation through interactions with stromal elements (21). As thymocytes undergo differentiation, they receive signals from the stromal cells (i.e., cortical and medullary epithelial cells, bone marrow-derived macrophages, and dendritic cells) that result in positive and negative selection (23). The result of either a lack of a positive selection signal or the presence of a negative one is the elimination of the thymocyte by the apoptotic death pathway (29).
Due to the dynamic nature of the thymus, it seemed possible that M-MuLV infection was interfering with positive and/or negative selection processes, resulting in aberrant apoptosis of thymocytes and accounting for the size differentials seen between M-MuLV-inoculated and uninoculated mice. In order to test this hypothesis and the relevance of thymic atrophy to disease, we examined the levels of thymocyte apoptosis in preleukemic mice inoculated with M-MuLV and Mo+PyF101 M-MuLV (s.c. versus i.p.). The role of MCF virus recombinants in thymic atrophy was also investigated. The results of those studies are reported here.
MATERIALS AND METHODS
Viruses and inoculation of mice.
Viral stocks were cell culture supernatants derived from NIH 3T3 cells confluently infected with either M-MuLV or Mo+PyF101 M-MuLV (10). Viral titers were determined by the UV/XC plaque assay (24). Neonatal NIH/Swiss mice were inoculated s.c. with 0.2 ml of wild-type M-MuLV viral stock (approximately 105 XC PFU) or either s.c. or i.p. with 0.2 ml of Mo+PyF101 M-MuLV viral stock (approximately 105 XC PFU). Mice were sacrificed by cervical dislocation at 4 to 12 weeks postinoculation. Thymi were dissected, removed, rinsed briefly in ice-cold phosphate-buffered saline (PBS), and immediately placed on ice. Thymic single-cell suspensions were prepared by gently teasing the organ apart in PBS and passing it through a 94-μm (pore-size) wire mesh (Bellco Glass), allowing thymocytes to flow through, with stromal components remaining on the mesh. Thymocytes were then washed twice in ice-cold PBS and counted with a hemacytometer.
Flow cytometric analyses for apoptosis.
Approximately 2 × 106 thymocytes were incubated with 1 μg of 7-aminoactinomycin D (7-AAD) per ml in ice-cold PBS for 20 min on ice, in the dark. Thymocytes treated with 7-AAD were then analyzed simultaneously by flow cytometry (FACSCalibur; Becton Dickinson) for forward angle scatter (FSC), as a measure of cell size, and 7-AAD fluorescence. Alternatively, thymocyte single-cell suspensions were incubated with annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI) (with an apoptosis detection kit; R & D Systems) per the manufacturer’s protocol and were subjected to flow cytometric analysis. In all cases, cell debris was gated out on the basis of forward scatter and side scatter analysis.
In our first attempts at examining the levels of thymocyte apoptosis in these mice, we used a higher concentration of 7-AAD (20 μg/ml) (25). However, this concentration did not effectively discriminate among live, early-stage apoptotic, and late-stage apoptotic murine thymocytes. Other studies using the higher concentration of 7-AAD focused on human thymocytes (25), which seem to possess somewhat different flow cytometric properties than murine thymocytes (9, 15, 20). Apoptotic murine thymocytes generally show a distinct downshift in forward scatter characteristics (15, 30, 32), indicative of the early shrinkage in cell size which occurs before later-stage DNA cleavage and membrane alterations (26, 30, 32). In two-dimensional flow cytometry for FSC and 7-AAD staining, apoptotic cells were defined as those falling within two gates. The R4 gate identified cells that were smaller by FSC with no increases in 7-AAD staining; they were considered early-stage apoptotic cells. The R3 gate identified smaller cells that also showed increases in 7-AAD staining; they were considered intermediate and late-stage apoptotic.
Focal immunofluorescence assay (FIA).
The monoclonal antibody specific for the MCF virus envelope glycoprotein used was MAb 514 (5). To assay for thymocytes that express infectious MCF virus (28), serial dilutions of thymocytes from inoculated and uninoculated mice were prepared and overlaid onto NIH 3T3 cells (8 × 104 cells/5-cm-diameter dish seeded the previous day). Cells were cocultured overnight in the presence of 8 μg of Polybrene per ml. Thymocytes were washed off twice with cold PBS, and the NIH 3T3 cells were refed and allowed to grow to confluency (approximately 4 to 5 days). Upon reaching confluency, the NIH 3T3 cultures were incubated with MAb 514, rinsed, and subsequently incubated with an FITC-conjugated goat anti-mouse immunoglobulin G (IgG), IgA, and IgM secondary antibody diluted 1:200. Foci of infected cells were visualized by fluorescence microscopy and counted.
RESULTS
M-MuLV-induced thymic atrophy correlates with enhanced thymocyte apoptosis.
In order to test whether the decreases in thymic size observed in preleukemic M-MuLV-inoculated mice were due to enhanced levels of thymocyte apoptosis, M-MuLV-inoculated mice and uninoculated age-matched controls were analyzed by flow cytometry for two hallmarks of apoptotic death—shrinkage in cell size and the cleavage of genomic DNA at internucleosomal sites (6, 27). FSC, as a measure of cell size (30), and 7-AAD fluorescence were therefore used as indicators of apoptosis (15, 25, 27). 7-AAD is a DNA intercalator that will fluoresce when stimulated by 488-nm-wavelength light. In normal live cells, 7-AAD is unable to cross the cell membrane and is therefore unable to intercalate into the DNA. However, in cells that are in the late stages of apoptosis, when cell membrane permeability is compromised and the DNA has been extensively cleaved, 7-AAD can enter the cell and intercalate into the DNA much more efficiently. Since apoptotic thymocytes decrease in size relatively early in the apoptotic death pathway, two-dimensional analysis for cell size and 7-AAD staining provides an excellent discrimination of cells in early stages of apoptosis from those in later stages of apoptosis. Apoptotic thymocytes will have one of two characteristics: (i) small size with low 7-AAD fluorescence, indicative of early-stage apoptosis, and (ii) small size with high 7-AAD fluorescence, indicative of late-stage apoptosis.
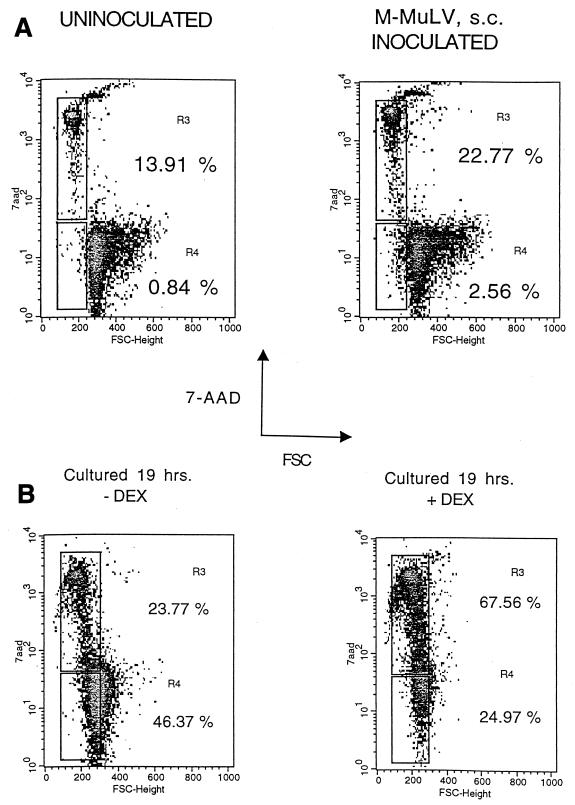
Thymocyte single-cell suspensions were prepared from mice and subjected to flow cytometric analyses. Typical results from this type of two-parameter analysis are shown in Fig. 1A. Thymocytes from an uninoculated animal showed 0.84% of cells in the R4 region (early apoptotic, i.e., small size with low 7-AAD fluorescence) and 13.91% of cells in the R3 region (intermediate and late apoptotic, i.e., small size with elevated 7-AAD fluorescence). In contrast, an M-MuLV-inoculated animal showed 2.56% of cells in the R4 range, more than twice as many as the uninoculated animal, and 22.77% of cells in the R3 region. Thus, the increase in apoptotic cells in M-MuLV-inoculated mice involved both early and late stages of apoptosis. Increases in the percentages of cells in the R3 and R4 regions in M-MuLV-inoculated mice generally correlated with thymic atrophy (see below).
FIG. 1.
Detection of apoptosis in thymocytes. (A) Thymocytes from 6-week-old uninoculated and M-MuLV-inoculated mice were subjected to two-parameter flow cytometric analysis for FSC and 7-AAD fluorescence. 7-AAD fluorescence is plotted on a logarithmic scale. Regions R3 and R4 represent thymocytes that are in the intermediate-to-late and early stages of apoptosis, respectively. Percentages of cells in both these regions are shown. (B) Dexamethasone-induced apoptosis. Primary thymocytes from an uninoculated mouse were cultured in vitro for 19 h in the presence (+DEX) or absence (−DEX) of 0.1 mM dexamethasone. The cells were then incubated with 7-AAD and subjected to two-parameter flow cytometric analysis to determine the percentages of cells in the R3 and R4 regions of late-to-intermediate- and early-stage apoptosis. Percentages of cells in both these regions are shown.
As a further test that cells in the R3 and R4 regions were apoptotic, primary thymocytes from an uninoculated mouse were cultured in vitro in the presence of the glucocorticoid dexamethasone to induce apoptosis (22, 25). The primary thymocytes were cultured for 19 h in the presence or absence of 0.1 mM dexamethasone, incubated with 7-AAD, and then subjected to two-parameter flow cytometric analysis. The data in Fig. 1B show that dexamethasone treatment led to higher percentages of cells in the R3 and R4 regions, consistent with these regions representing apoptotic cells. It should be noted that the profile of the control thymocytes incubated in the absence of dexamethasone for 19 h showed substantially higher levels of apoptosis than the thymocytes analyzed directly after removal from the animal (Fig. 1). This was likely due to the fact that thymocytes are extremely sensitive to their environment and will spontaneously undergo apoptosis when placed into culture (7), presumably stemming from loss of contact with thymic stromal elements that provide survival signals. It was necessary to slightly adjust the R3 and R4 gates for the in vitro-incubated thymocytes so that early- and late-stage apoptotic cells fell into these regions.
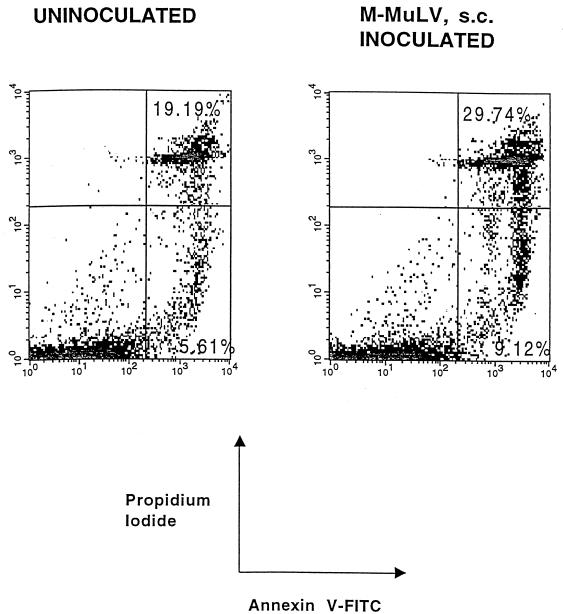
As an independent test for apoptosis, cells were stained with annexin V-FITC and PI. Annexin V is a calcium and phospholipid binding protein that selectively binds to negatively charged phospholipids (16). Under specific salt and calcium concentrations, annexin V preferentially binds phosphatidylserine (PS) (1). Very early in the apoptotic pathway, cells rearrange their membrane asymmetry with respect to PS by translocating PS from the inner leaflet of the cell membrane to the outer side. Therefore, cells that are undergoing apoptosis can bind annexin V. This property can be used to detect apoptotic cells by flow cytometry (31). Figure 2 shows the results of a two-parameter flow cytometric analysis, of the same 6-week-old uninoculated and M-MuLV-inoculated mice studied to produce the data in Fig. 1A, for staining by annexin V-FITC and PI (which binds DNA). The comparison of the percentages of annexin V-positive cells (upper and lower right quadrants) for the uninoculated animal (19.19 and 5.61%, respectively) with those for the M-MuLV-inoculated animal (29.74 and 9.12%, respectively) also indicated that there was an enhanced level of apoptosis in the inoculated animal. Moreover, it was possible to distinguish early-stage apoptotic cells from late-stage apoptotic cells: the early ones were annexin V positive and PI negative (lower right quadrant), and the late ones were positive for both annexin V and PI (upper right quadrant). Thus, by this independent analysis, the thymocytes from M-MuLV-inoculated mice showed higher levels of apoptosis than those from uninoculated mice. It should be noted that the annexin V-FITC and PI staining gave somewhat higher percentages of apoptotic cells than procedures described in Fig. 1. This may reflect the ability of annexin V to detect cells at an earlier stage of apoptosis than is evident from a change in cell size, as well as a somewhat greater overall ability to detect either early- or late-stage apoptotic cells.
FIG. 2.
Annexin V-PI staining. Thymocytes from the same 6-week-old animals for which results are shown in Fig. 1 were incubated with FITC-conjugated annexin V and PI, and flow cytometric analysis was performed. Percentages of late-stage apoptotic cells (upper right quadrant) and early-stage apoptotic cells (lower right quadrant) are shown. Both FITC-conjugated annexin V and PI fluorescence are plotted on logarithmic scales.
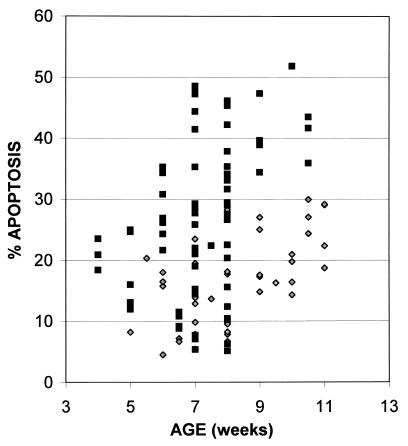
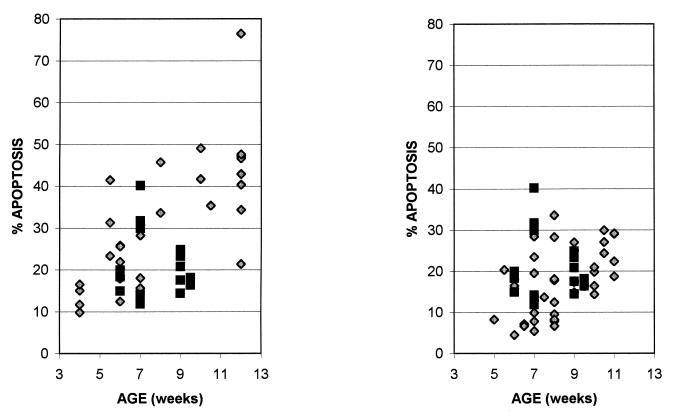
To test the generality of the increased apoptosis in thymi from M-MuLV-inoculated mice, multiple animals were examined over a time course of 4 to 11 weeks postinoculation by the sort of analysis shown in Fig. 1. Figure 3 shows the cumulative data for all animals, as a function of age, with the sum of the percentages of cells in the R3 and R4 regions as the measure of thymocyte apoptosis. Each data point represents one animal. The level of thymocyte apoptosis in uninoculated mice remained relatively constant between 5 and 10 weeks of age. In contrast, M-MuLV-inoculated mice showed higher levels of thymocyte apoptosis, particularly from 6 to 10.5 weeks of age. Statistical analysis of the data with a two-sample t test resulted in a calculated t score (tcalc) of 4.19 (t < 0.001, one-tailed test), demonstrating a statistically significant difference between the two groups.
FIG. 3.
Thymocyte apoptosis in M-MuLV-inoculated and uninoculated mice. Thymocytes from uninoculated (diamonds) and M-MuLV-inoculated (squares) mice were analyzed for apoptosis, calculated as the sum of the percentages of cells in the R3 and R4 regions of intermediate-to-late- and early-stage apoptosis (see Fig. 1A). Mice were sacrificed between 4 and 11 weeks postinoculation. All data points represent individual animals.
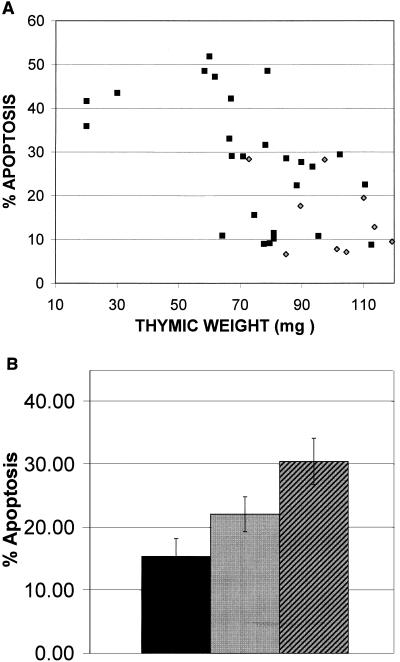
The initial hypothesis for these experiments was that the accelerated thymic atrophy in M-MuLV-inoculated mice was due to enhanced levels of thymocyte apoptosis. To address this, the relationship between apoptotic value and visible thymic atrophy was analyzed, as shown in Fig. 4. Inoculated animals were scored according to the extent of thymic atrophy at the time of necropsy, determined by thymic weight, prior to flow cytometry. Only animals of 7 to 10.5 weeks of age were used for this analysis, as normal thymic regression became evident in uninoculated animals after 11 weeks. As shown in Fig. 4A, uninoculated mice with a mean thymic weight of 97.47 mg had low apoptotic values (<30%). For M-MuLV-inoculated mice, some animals exhibited normal thymic weight while others showed reduced weight (i.e., accelerated thymic regression). For the M-MuLV-inoculated mice there was a general negative correlation between thymic weight and apoptotic value. In Fig. 4B, the M-MuLV-inoculated animals were divided into two groups: those with no atrophy (weights within 1 standard deviation of the mean thymic weight of the control group) and those with atrophy (weights below 1 standard deviation). Uninoculated mice showed an average of 15.4% apoptosis and an average thymic weight of 97.47 mg. M-MuLV-inoculated mice showing no atrophy had an average of 22.1% apoptosis, while those displaying signs of atrophy had an average of 30.47% apoptosis. Thus, the degree of thymic atrophy in the M-MuLV-inoculated mice was generally consistent with the levels of thymocyte apoptosis. These results support the hypothesis that M-MuLV-induced thymic atrophy is related to enhanced levels of thymocyte apoptosis.
FIG. 4.
Thymic atrophy correlates with elevated levels of thymocyte apoptosis. At the time of sacrifice, thymi were dissected, removed, and weighed, as a measure of thymic atrophy. (A) A comparison of the levels of thymocyte apoptosis (calculated as the sum of the percentages of cells in regions R3 and R4) with thymic weights (in milligrams) in M-MuLV-inoculated (squares) and uninoculated (diamonds) mice is shown. All data points represent individual animals. (B) Thymi from M-MuLV-inoculated mice were scored as having either no thymic atrophy (thymic weight was within 1 standard deviation of the mean thymic weight of uninoculated mice) or thymic atrophy (thymic weight was more than 1 standard deviation below the mean thymic weight of uninoculated mice). A comparison of the mean levels of thymocyte apoptosis observed in these mice and uninoculated mice is shown. Black bar, uninoculated mice (mean weight, 97.47 mg); gray bar, no atrophy (mean weight, 96.36 mg); hatched bar, atrophy (mean weight, 63.74 mg). Average thymic weights of all three categories are displayed, and error bars are based on standard error values.
Relevance of enhanced levels of thymocyte apoptosis to M-MuLV-induced leukemogenesis.
We tested whether the higher levels of thymocyte apoptosis in preleukemic M-MuLV-inoculated mice were related to disease induction. To do so, we employed the Mo+PyF101 M-MuLV enhancer variant. As described in the introduction, Mo+PyF101 M-MuLV has greatly attenuated leukemogenicity when inoculated s.c. On the other hand, when inoculated i.p., the virus shows the same leukemogenicity as wild-type M-MuLV. The rate of leukemogenesis for wild-type M-MuLV is the same for either route of inoculation (mean latency of 3 to 4 months in 100% of the animals).
We therefore measured the levels of thymocyte apoptosis in Mo+PyF101 M-MuLV-inoculated mice. Thymic single-cell suspensions from mice inoculated with Mo+PyF101 M-MuLV by the i.p. route were assayed for apoptosis by flow cytometry, as described above. The left panel of Fig. 5 shows the collective data for mice inoculated i.p. with Mo+PyF101 M-MuLV versus the data for uninoculated animals. The animals inoculated i.p. with Mo+PyF101 M-MuLV showed elevated levels of apoptosis at 6 to 12 weeks. Statistical analysis using a two-sample t test resulted in a tcalc score of 5.2 (t < 0.001, one-tailed test), demonstrating a statistically significant difference between the levels of thymocyte apoptosis in uninoculated mice and those in Mo+PyF101 M-MuLV-inoculated (i.p.) mice.
FIG. 5.
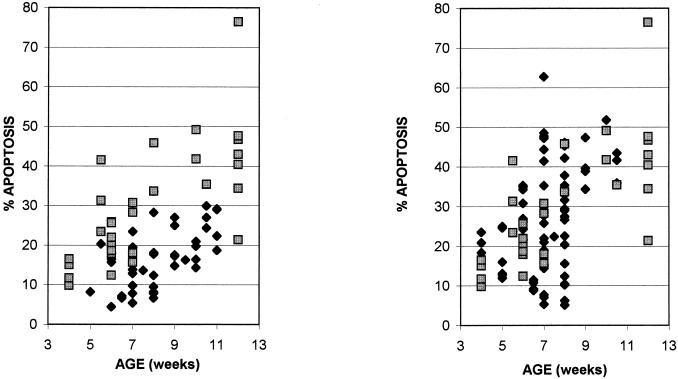
Thymocyte apoptosis in mice inoculated i.p. with Mo+PyF101 M-MuLV. (Left panel) Thymocytes from uninoculated mice (diamonds) and mice inoculated i.p. with Mo+PyF101 M-MuLV (squares) were analyzed for apoptosis, determined as the sum of the percentages of cells in the R3 and R4 regions of intermediate-to-late- and early-stage apoptosis. Mice were sacrificed between 4 and 12 weeks postinoculation. All data points represent individual animals. (Right panel) A comparison of the levels of thymocyte apoptosis in mice inoculated s.c. with wild-type M-MuLV (diamonds) and mice inoculated i.p. with Mo+PyF101 M-MuLV (squares) is shown.
The increased level of apoptosis for the animals inoculated i.p. with Mo+PyF101 resembled the level of apoptosis in mice inoculated s.c. with wild-type M-MuLV (Fig. 5, right panel). Thus, when Mo+PyF101 M-MuLV was inoculated into mice under conditions where it showed efficient leukemogenicity, an increased level of apoptosis was observed in preleukemic thymocytes.
The level of thymocyte apoptosis was also measured for mice inoculated with Mo+PyF101 M-MuLV by the s.c. route (attenuated leukemogenicity). As shown in Fig. 6 (left panel), mice inoculated s.c. with Mo+PyF101 M-MuLV showed lower levels of thymocyte apoptosis than those inoculated i.p. Statistical analysis using a two-sample t test resulted in a tcalc score of 2.67 (t < 0.005, one-tailed test), demonstrating a statistically significant difference between the levels of thymocyte apoptosis in mice inoculated i.p. with Mo+PyF101 M-MuLV and those in mice inoculated s.c. Hence, this virus induced higher levels of apoptosis when it was inoculated into mice under conditions where it efficiently induces disease. On the other hand, the levels of apoptosis were similar for mice inoculated s.c. with Mo+PyF101 M-MuLV and uninoculated mice (Fig. 6, right panel). The lower level of apoptosis in mice inoculated s.c. with Mo+PyF101 M-MuLV correlated with the attenuated pathogenicity of Mo+PyF101 M-MuLV when inoculated s.c. Thus, studies with Mo+PyF101 M-MuLV supported the hypothesis that enhanced levels of thymocyte apoptosis are important for efficient leukemogenesis by M-MuLV.
FIG. 6.
Mice inoculated s.c. with Mo+PyF101 M-MuLV. (Left panel) Thymocytes from mice inoculated s.c. with Mo+PyF101 M-MuLV were analyzed for apoptosis, determined as the sum of the percentages of cells in the R3 and R4 regions of intermediate-to-late- and early-stage apoptosis. A comparison of the levels of thymocyte apoptosis in mice inoculated i.p. with Mo+PyF101 M-MuLV (diamonds) and those for mice inoculated s.c. (squares) is shown. (Right panel) A comparison of the levels of thymocyte apoptosis for uninoculated mice (diamonds) and those observed for mice inoculated s.c. with Mo+PyF101 M-MuLV (squares) is shown.
Role of MCF recombinant virus in M-MuLV-induced thymocyte apoptosis.
MCF virus recombinants can be found in almost all M-MuLV-induced tumors, suggesting that they are advantageous for tumorigenesis, although their exact role is not completely understood (see the introduction). It has been previously suggested that MCF viruses play a role in early preleukemic events (4). This was based on the fact that s.c. inoculation with Mo+PyF101 M-MuLV does not result in preleukemic physical changes, such as accelerated thymic atrophy (11, 13), and that at the same time mice inoculated s.c. with this virus do not show development of MCF viruses (4). Moreover, when the same virus is inoculated i.p., the mice concomitantly show thymic atrophy and generate MCF virus recombinants (3).
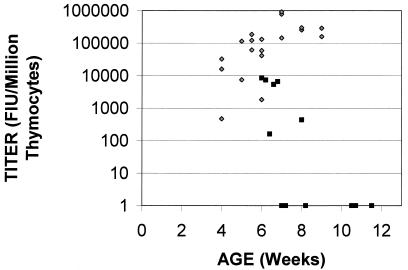
In order to address the role of MCF viruses in M-MuLV-induced enhanced thymocyte apoptosis, we assayed thymocytes for the production of infectious MCF virus. To detect cells expressing MCF virus, an FIA was used (see Materials and Methods) (28). This assay quantifies MCF virus-infected cells by employing a monoclonal antibody (MAb 514) that is specific for the MCF virus envelope glycoprotein (5). In this method, an initial MCF virus-infected cell acts as an infectious center to create a focus of infected cells that can be visualized by immunofluorescent staining for the polytropic envelope glycoprotein. Figure 7 shows the rate of appearance of MCF virus recombinants in mice inoculated s.c. with wild-type M-MuLV. MCF virus recombinants could be detected at high levels in thymocytes from inoculated mice beginning at 4 weeks. The appearance of MCF virus infection in these inoculated animals accompanied (or preceded) accelerated thymic atrophy and enhanced levels of apoptosis. This was consistent with the hypothesis that MCF virus recombinants are involved in establishing M-MuLV-induced preleukemic events.
FIG. 7.
MCF recombinant virus infection of thymocytes in preleukemic mice. Thymocytes from mice inoculated s.c. with M-MuLV (diamonds) and from mice inoculated i.p. with Mo+PyF101 M-MuLV (squares) were tested for productive MCF virus infection by FIA. Titers are shown on a logarithmic scale (FIU per 106 thymocytes).
In contrast, when mice inoculated i.p. with Mo+PyF101 M-MuLV were examined, 6 of 12 mice showed no detectable MCF virus recombinants by 11 weeks of age, and the remaining six had very low levels (<104 focal infectivity units [FIU] per 106 thymocytes), even though the great majority of them (9 of 12) showed thymic atrophy and enhanced levels of apoptosis. Thus, despite the positive correlation of MCF virus appearance and enhanced levels of thymocyte apoptosis observed in wild-type M-MuLV-inoculated mice, the results for the mice inoculated i.p. with Mo+PyF101 M-MuLV indicated that MCF virus infection of thymocytes does not play an obligate role in enhancing the level of thymocyte apoptosis. For the latter mice, high-level MCF virus infection of thymocytes did not precede or accompany the onset of thymic atrophy and enhanced levels of thymocyte apoptosis.
DISCUSSION
In this paper, preleukemic changes in the thymi of M-MuLV-inoculated mice were studied. It was found that enhanced levels of thymocyte apoptosis were evident beginning at 6 weeks postinoculation. The thymocyte apoptosis was assessed by several different flow cytometric criteria, including decreased cell size and enhanced staining with 7-AAD or annexin V. The enhanced levels of thymocyte apoptosis were also correlated with accelerated thymic atrophy in M-MuLV-inoculated mice. This was plausible since the thymus is a very dynamic organ, with a continual turnover of thymocytes undergoing positive and negative selection. Both of these selection processes involve apoptosis. Thus, enhanced rates of thymocyte apoptosis without compensating increases in thymocyte division would result in decreased numbers of thymocytes in an M-MuLV-infected thymus.
Once increased preleukemic thymocyte apoptosis had been documented, we investigated whether it was likely to be important for leukemogenesis by M-MuLV. Experiments with the Mo+PyF101 M-MuLV variant were supportive of a role for enhanced levels of thymocyte apoptosis in leukemogenesis. When Mo+PyF101 M-MuLV was inoculated by the i.p. route (whereby it efficiently induces leukemia), enhanced levels of thymocyte apoptosis and thymic atrophy equivalent to those observed in wild-type M-MuLV-inoculated animals were observed. On the other hand, when the same virus was inoculated s.c. (by which leukemogenicity is attenuated), substantially less thymocyte apoptosis and thymic atrophy were observed. Hence, Mo+PyF101 M-MuLV-induced thymic atrophy and enhanced levels of thymocyte apoptosis were positively correlated with efficient leukemogenesis.
The exact mechanism by which M-MuLV-induced thymic atrophy and enhanced levels of thymocyte apoptosis putatively facilitate leukemogenesis remains to be determined. In AKR mice, potentially leukemic cells (PLC) have been detected by transplantation experiments (17). These PLC are initially found in the bone marrow and spleen but not in the thymus; at later times they can be detected in the thymus as well. Phenotypic characterization of the PLC indicated that they have the properties of prothymocytes. By analogy, M-MuLV-enhanced levels of apoptosis in the thymus of an inoculated mouse might result in enhanced recruitment of preleukemic prothymocytes from the bone marrow or spleen into the thymus, where development of the tumor ultimately occurs.
It also seems possible that M-MuLV-induced thymic atrophy and thymocyte apoptosis affect cellular immunity in inoculated mice. M-MuLV-inoculated mice do not generally seem to display substantial immunodeficiency. This might reflect the fact that thymic physiology appears fairly normal in M-MuLV-inoculated animals up to 6 weeks of age (see Results). Thus, the thymus would produce normal amounts of T lymphocytes up to that time. However, a premature decrease in thymocyte maturation might cause decreases in cellular immunity, particularly as animals age. One consequence could be that inoculated animals might not efficiently raise their cellular immune responses to neoantigens on developing tumors. However, it is unclear whether such a putative effect would play a role in M-MuLV leukemogenesis, since the tumors appear at approximately 4 months.
The mechanism by which M-MuLV infection leads to enhanced levels of thymocyte apoptosis is of considerable interest. Initially, our hypothesis was that formation of MCF virus recombinants played a role. Indeed, it has been shown previously that when Mo+PyF101 M-MuLV is inoculated into mice by the s.c. route, the slowly appearing tumors lack MCF viruses (4). On the other hand, i.p. inoculation with the same virus resulted in rapidly appearing tumors that contained MCF proviruses (3). In addition, other experiments done on in vitro bone marrow cultures from M-MuLV-inoculated mice indicated that bone marrow stroma showed a quantitative defect for growth that could be associated with MCF virus infection (19). The results for mice inoculated with wild-type M-MuLV supported the hypothesis, since high level MCF virus infection in thymocytes preceded thymic atrophy. However, in mice inoculated i.p. with Mo+PyF101 M-MuLV, little or no MCF virus was detected, even in animals that showed accelerated thymic atrophy. Thus, enhanced levels of thymocyte apoptosis could not be attributed to direct infection of these cells with MCF recombinant virus.
One possible explanation currently under investigation is that MCF recombinant virus infection in thymic stromal cells is important for enhancing levels of apoptosis. As mentioned above, we previously developed in vitro evidence for the role of MCF virus infection in bone marrow stroma defects; these defects are associated with decreased hematopoiesis (19). MCF virus infection of thymic stroma might interfere with the ability of the stroma to support proper thymocyte maturation (e.g., positive and negative selection), which could result in increased thymocyte apoptosis. It should be noted that the infectious center assays in Fig. 7 were carried out on nonadherent thymocytes, which are largely T lymphoid. Thus, MCF virus infection of stromal cells could have been missed. Experiments to assess the infection state of thymic stromal cells are currently in progress.
If the involvement of MCF virus recombinants is ruled out as a factor underlying increased thymocyte apoptosis, then infection of thymocytes or thymic stroma with M-MuLV itself might be considered as the cause. While this would be consistent with the fact that the preleukemic changes are not observed in uninoculated animals, it has been shown previously that the extent of thymocyte infection for animals inoculated with Mo+PyF101 M-MuLV by either the i.p. or s.c. route was the same (2). Any proposed mechanism for the increase of thymic apoptosis must take this result into account.
ACKNOWLEDGMENTS
This work was supported by NIH grant CA32455. C.B. was supported by NIH training grant 5T32-CA09054. The support of the Cancer Research Institute and the Optical Biology Core Facility of the Chao Family Comprehensive Cancer Center is acknowledged as well.
We thank Chris Hughes for help with flow cytometry.
REFERENCES
- 1.Andree H A, Reutelingsperger C P, Hauptmann R, Hemker H C, Hermens W T, Willems G M. Binding of vascular anticoagulant alpha (VAC alpha) to planar phospholipid bilayers. J Biol Chem. 1990;265:4923–4928. [PubMed] [Google Scholar]
- 2.Belli B, Fan H. The leukemogenic potential of an enhancer variant of Moloney murine leukemia virus varies with the route of inoculation. J Virol. 1994;68:6883–6889. doi: 10.1128/jvi.68.11.6883-6889.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belli B, Patel A, Fan H. Recombinant mink cell focus-inducing virus and long terminal repeat alterations accompany the increased leukemogenicity of the Mo+PyF101 variant of Moloney murine leukemia virus after intraperitoneal inoculation. J Virol. 1995;69:1037–1043. doi: 10.1128/jvi.69.2.1037-1043.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brightman B K, Rein A, Trepp D J, Fan H. An enhancer variant of Moloney murine leukemia virus defective in leukemogenesis does not generate detectable mink cell focus-inducing virus in vivo. Proc Natl Acad Sci USA. 1991;88:2264–2268. doi: 10.1073/pnas.88.6.2264. . (Erratum, 88:5066.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chesebro B, Britt W, Evans L, Wehrly K, Nishio J, Cloyd M. Characterization of monoclonal antibodies reactive with murine leukemia viruses: use in analysis of strains of Friend MCF and Friend ecotropic murine leukemia virus. Virology. 1983;127:134–148. doi: 10.1016/0042-6822(83)90378-1. [DOI] [PubMed] [Google Scholar]
- 6.Cohen J J. Apoptosis. Immunol Today. 1993;14:126–130. doi: 10.1016/0167-5699(93)90214-6. [DOI] [PubMed] [Google Scholar]
- 7.Cohen J J. Programmed cell death in the immune system. Adv Immunol. 1991;50:55–85. doi: 10.1016/s0065-2776(08)60822-6. [DOI] [PubMed] [Google Scholar]
- 8.Cuypers H T, Selten G, Quint W, Zijlstra M, Maandag E R, Boelens W, van Wezenbeek P, Melief C, Berns A. Murine leukemia virus-induced T-cell lymphomagenesis: integration of proviruses in a distinct chromosomal region. Cell. 1984;37:141–150. doi: 10.1016/0092-8674(84)90309-x. [DOI] [PubMed] [Google Scholar]
- 9.Darzynkiewicz Z, Juan G, Li X, Gorczyca W, Murakami T, Traganos F. Cytometry in cell necrobiology: analysis of apoptosis and accidental cell death (necrosis) Cytometry. 1997;27:1–20. [PubMed] [Google Scholar]
- 10.Davis B, Linney E, Fan H. Suppression of leukaemia virus pathogenicity by polyoma virus enhancers. Nature. 1985;314:550–553. doi: 10.1038/314550a0. [DOI] [PubMed] [Google Scholar]
- 11.Davis B R, Brightman B K, Chandy K G, Fan H. Characterization of a preleukemic state induced by Moloney murine leukemia virus: evidence for two infection events during leukemogenesis. Proc Natl Acad Sci USA. 1987;84:4875–4879. doi: 10.1073/pnas.84.14.4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis B R, Chandy K G, Brightman B K, Gupta S, Fan H. Effects of nonleukemogenic and wild-type Moloney murine leukemia virus on lymphoid cells in vivo: identification of a preleukemic shift in thymocyte subpopulations. J Virol. 1986;60:423–430. doi: 10.1128/jvi.60.2.423-430.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan H. Leukemogenesis by Moloney murine leukemia virus: a multistep process. Trends Microbiol. 1997;5:74–82. doi: 10.1016/S0966-842X(96)10076-7. [DOI] [PubMed] [Google Scholar]
- 14.Fan H, Chute H, Chao E, Pattengale P K. Leukemogenicity of Moloney murine leukemia viruses carrying polyoma enhancer sequences in the long terminal repeat is dependent on the nature of the inserted polyoma sequences. Virology. 1988;166:58–65. doi: 10.1016/0042-6822(88)90146-8. [DOI] [PubMed] [Google Scholar]
- 15.Fraker P J, King L E, Lill-Elghanian D, Telford W G. Quantification of apoptotic events in pure and heterogeneous populations of cells using the flow cytometer. Methods Cell Biol. 1995;46:57–76. doi: 10.1016/s0091-679x(08)61924-x. [DOI] [PubMed] [Google Scholar]
- 16.Grundmann U, Abel K J, Bohn H, Lobermann H, Lottspeich F, Kupper H. Characterization of cDNA encoding human placental anticoagulant protein (PP4): homology with the lipocortin family. Proc Natl Acad Sci USA. 1988;85:3708–3712. doi: 10.1073/pnas.85.11.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haran-Ghera N, Peled A, Leef F, Hoffman A D, Levy J A. Enhanced AKR leukemogenesis by the dual tropic viruses. I. The time and site of origin of potential leukemic cells. Leukemia. 1987;1:442–449. [PubMed] [Google Scholar]
- 18.Hartley J W, Wolford N K, Old L J, Rowe W P. A new class of murine leukemia virus associated with development of spontaneous lymphomas. Proc Natl Acad Sci USA. 1977;74:789–792. doi: 10.1073/pnas.74.2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Q X, Fan H. Combined infection by Moloney murine leukemia virus and a mink cell focus-forming virus recombinant induces cytopathic effects in fibroblasts or in long-term bone marrow cultures from preleukemic mice. J Virol. 1990;64:3701–3711. doi: 10.1128/jvi.64.8.3701-3711.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lyons A B, Samuel K, Sanderson A, Maddy A H. Simultaneous analysis of immunophenotype and apoptosis of murine thymocytes by single laser flow cytometry. Cytometry. 1992;13:809–821. doi: 10.1002/cyto.990130803. [DOI] [PubMed] [Google Scholar]
- 21.Mondino A, Khoruts A, Jenkins M K. The anatomy of T-cell activation and tolerance. Proc Natl Acad Sci USA. 1996;93:2245–2252. doi: 10.1073/pnas.93.6.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicoletti I, Migliorati G, Pagliacci M C, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139:271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 23.Robey E, Fowlkes B J. Selective events in T cell development. Annu Rev Immunol. 1994;12:675–705. doi: 10.1146/annurev.iy.12.040194.003331. [DOI] [PubMed] [Google Scholar]
- 24.Rowe W P, Pugh W E, Hartley J W. Plaque assay techniques for murine leukemia viruses. Virology. 1970;42:1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- 25.Schmid I, Uittenbogaart C H, Keld B, Giorgi J V. A rapid method for measuring apoptosis and dual-color immunofluorescence by single laser flow cytometry. J Immunol Methods. 1994;170:145–157. doi: 10.1016/0022-1759(94)90390-5. [DOI] [PubMed] [Google Scholar]
- 26.Sherwood S W, Schimke R T. Cell cycle analysis of apoptosis using flow cytometry. Methods Cell Biol. 1995;46:77–97. doi: 10.1016/s0091-679x(08)61925-1. [DOI] [PubMed] [Google Scholar]
- 27.Shi Y-B. Programmed cell death. New York, N.Y: Plenum; 1997. [Google Scholar]
- 28.Sitbon M, Nishio J, Wehrly K, Lodmell D, Chesebro B. Use of a focal immunofluorescence assay on live cells for quantitation of retroviruses: distinction of host range classes in virus mixtures and biological cloning of dual-tropic murine leukemia viruses. Virology. 1985;141:110–118. doi: 10.1016/0042-6822(85)90187-4. [DOI] [PubMed] [Google Scholar]
- 29.Surh C D, Sprent J. T-cell apoptosis detected in situ during positive and negative selection in the thymus. Nature. 1994;372:100–103. doi: 10.1038/372100a0. . (Comment, 372:44–45.) [DOI] [PubMed] [Google Scholar]
- 30.Telford W G, King L E, Fraker P J. Rapid quantitation of apoptosis in pure and heterogeneous cell populations using flow cytometry. J Immunol Methods. 1994;172:1–16. doi: 10.1016/0022-1759(94)90373-5. [DOI] [PubMed] [Google Scholar]
- 31.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 32.Watters D, Lavin M. Programmed cell death: the cellular and molecular biology of apoptosis. Chur, Switzerland: Harwood Academic Publishers; 1993. [Google Scholar]