Abstract
Background:
Gestational phthalate and phenol exposure disrupts adipogenesis, contributing to obesity in mice. Whether gestational phthalate or phenol exposure is associated with infant body composition has not been investigated in humans.
Objective:
We examined associations between biomarkers of phthalate and phenol exposure in midpregnancy and infant size and body composition at birth and at 5 months of age.
Methods:
Analyses were conducted among 438 infants from the Healthy Start prospective pregnancy cohort. Sixteen phthalate and phenol biomarkers were quantified in spot urine samples collected at 24–28 wk of gestation. Infant outcomes measured at birth and at 5 months of age included size [weight (in grams)] and body composition [fat and lean masses (in grams); percentage fat mass]. Single- (linear) and multipollutant (quantile g-computation) models were used to estimate associations of phthalate and phenol biomarkers with infant outcomes at birth and at 5 months of age. Models were adjusted for sociodemographics, sample collection timing, and lifestyle factors and used to examine for effect modification by infant sex.
Results:
In single-pollutant models, mono-benzyl phthalate and di--butyl phthalate were inversely associated with percentage fat mass [: (95% CI: , ) and (95% CI: , 0.01), respectively] in male but not female infants at birth. Similar, but less precise, associations were observed at 5 months of age. In multipollutant models, a 1-quartile increase in the phthalate and phenol biomarker mixture was inversely associated with percentage fat mass at birth [ (95% CI: , 0.1)] and at 5 months of age [ (95% CI: , )] among males, but associations were null among females [0.48 (95% CI: , 1.75) and (95% CI: , 1.41), respectively]. Similar associations were observed with infant weight.
Conclusion:
In this U.S.-based prospective cohort, gestational phthalate and phenol biomarkers were inversely associated with infant weight and fat mass, particularly in males. https://doi.org/10.1289/EHP12500
Introduction
Regular use of phthalates and phenols in consumer products has led to frequent and chronic exposure to these chemicals,1 including among pregnant women. Gestational exposure to phthalates and phenols has been linked to adverse fetal growth outcomes, including reductions in ultrasound measures of fetal weight2,3 and birthweight.4–6 However, although these observations are supported by in vivo animal models, much of the epidemiologic literature remains inconsistent with respect to the direction and magnitude of the effect.7
This heterogeneity in the epidemiologic literature may be partially explained by a focus on birthweight. Weight is a composite measure of fat mass (i.e., adipose) and lean mass (i.e., skeletal weight, muscle tissue, and other organs) and—although fat mass only accounts for of birthweight—almost half of the variance in birthweight can be explained by fat mass.8 Several phenols have been shown to influence the fate of stem cells toward the adipocyte lineage and away from osteoblasts,9,10 and several phthalates, including di(2-ethylhexyl) phthalate (DEHP), have been shown to promote adipogenesis and alter the expression of key adipogenic pathways, such as peroxisome proliferator activated receptor gamma.11–13 Therefore, more specific measures of body composition may help to clarify effects of gestational exposure to phthalates and phenols on fetal growth outcomes.
To our knowledge, no human studies have explicitly examined the influence of gestational exposure to phthalates and phenols on neonatal or infant body composition. Moreover, there is limited literature on the joint effects of these chemicals on infant size and growth outcomes, despite moderate-to-high correlation between these exposures. To address this literature gap, we examined single- and multipollutant associations between biomarkers of phthalate and phenol exposure in midpregnancy with infant size and body composition at birth and at 5 months of age in the Healthy Start cohort.
Methods
Study Participants and Design
The Healthy Start study is a prospective cohort study that recruited 1,410 pregnant individuals years of age with singleton pregnancies, enrolled before 24 wk of gestation from obstetrics clinics at the University of Colorado Hospital within the Anschutz Medical Campus of the University of Colorado-Denver between 2009 and 2014. Participants were excluded if they had a history of stillbirth or extremely preterm birth, had diabetes, asthma treated with steroids, cancer, or medication-dependent psychiatric illness. Of the original 1,410 individuals enrolled, 19 experienced fetal demise and an additional 9 withdrew prior to delivery.
Study procedures included four research visits: two visits during pregnancy at a median [interquartile range (IQR)] of 17 (15–20) and 27 (25–29) wk of gestation, one visit at delivery, and one visit at a median (IQR) of 5 (4–6) months postnatally. Research visits included questionnaires, sample collection, and anthropometric and body composition measures conducted by study staff. The present analysis was conducted among a convenience sample of 446 pregnant individuals who provided spot urine samples during the second study visit. We additionally restricted our sample based on having infant body composition assessed at delivery () or postnatal () visits. The final analytic sample comprised 438 infants. A subset of 24 participants agreed to provide three spot urine samples every 2 wk after the second study visit at a median (IQR) of 30 (26–31), 32 (29–33), and 34 (31–35) wk of gestation.14
Ethics approval was obtained from the Colorado Multiple Institutional Review Board and all participants provided written informed consent prior to the first study visit. The involvement of the Centers for Disease Control and Prevention (CDC) laboratory was determined not to constitute engagement in human subjects research.
Urinary Biomarkers of Phthalates and Phenols
Spot urine samples were collected in sterile collection cups and stored at until ready for shipment. Urinary concentrations of phthalate metabolites and phenols were measured at the CDC laboratory according to previously published guidelines.15,16 The phthalate metabolites included in analyses were mono-ethyl phthalate (MEP), mono-benzyl phthalate (MBzP), mono-3-carboxypropyl phthalate (MCPP), mono carboxyisooctyl phthalate (MCOP), and mono carboxyisononyl phthalate (MCNP), mono--butyl phthalate (MnBP), mono-hydroxybutyl phthalate (MHBP), mono-isobutyl phthalate (MiBP), mono-hydroxyisobutyl phthalate (MHiBP), mono-2-ethylhexyl phthalate (MEHP), mono-2-ethyl-5-hydroxyhexyl phthalate (MEHHP), mono-2-ethyl-5-oxohexyl phthalate (MEOHP), and mono-2-ethyl-5-carboxypentyl phthalate (MECPP). We also calculated the molar sum of the di--butyl phthalate metabolites ( from MnBP and MHBP), the di-isobutyl phthalate metabolites ( from MiBP and MHiBP), and the DEHP metabolites ( from MEHP, MEHHP, MEOHP, and MECPP). Molar concentrations were converted to nanograms per milliliter by multiplying DBP, DiBP, and DEHP by the molecular weights of MnBP, MiBP, and MECPP, respectively.17 The phenols included in analyses were 2,4 dichlorophenol, 2,5 dichlorophenol, bisphenol A (BPA), bisphenol S (BPS), benzophenone-3, methyl paraben, propyl paraben, and triclosan. Two other phthalate metabolites (monomethyl phthalate and mono-isononyl phthalate) and two phenols (ethyl paraben and butyl paraben) were measured by the lab but excluded from analyses owing to the large numbers () of values below the limit of detection (LOD).18 For concentrations below the LOD, we obtained instrument values when possible or substituted one-half the minimum reported value for that biomarker, as has been done previously in this cohort.14
Urine creatinine concentrations were measured at the CDC using a Roche/Hitachi Cobas 6000 Analyzer (Roche Diagnostics). The O’Brien method19 was used to correct for urinary creatinine. For this, we identified age and prepregnancy body mass index (BMI) category of the gestational parent, and gestational week at sample collection as significant () predictors of urinary creatinine. Models were fit to predict urinary creatinine based on these variables, and we standardized exposure biomarkers for each participant using the following formula: , where is the creatinine-standardized exposure biomarker concentration, is the observed exposure biomarker concentration, is the predicted creatinine concentration from our model, and is the observed creatinine concentration.
Infant Outcomes
Primary infant outcomes were infant size and body composition, including total body mass (i.e., weight in grams), fat mass (in grams; percentage), and lean mass (in grams) measured using whole body air displacement plethysmography (PEA POD; COSMED, Inc.) within 72 h of delivery and again at months of age. The PEA POD is a validated instrument that relies on a two-compartment model for estimating body mass in infants.20,21 PEA POD measures were conducted in duplicate and, if measures deviated by , in triplicate; the average of the closest two measures was used for analyses. Fat mass percentage was calculated as fat mass/().
Secondary infant outcomes included sex-specific -scores for weight-for-age and weight-for-length based on the World Health Organization’s Growth Standards,22 fat mass and lean mass indexes, and rapid infant growth. Length measures were conducted by study staff in duplicate or—if measures deviated by —triplicate; the average of the closest two measures was used for analyses. Fat and lean mass indexes accounted for infant size by dividing by squared infant length [fat mass index: fat mass (in kilograms)/length ; lean mass index: lean mass (in kilograms)/length ].23 Rapid infant growth was calculated for a change in weight-for-age and weight-for-length -scores between delivery and 5 months of age of .24,25
Covariates
Model covariates were determined a priori from a directed acyclic graph (Figure S1).26 A study questionnaire ascertained characteristics of the gestational parent including age, race and ethnicity (categorized as Hispanic or Latina, non-Hispanic White or Caucasian, Non-Hispanic Black or African American, all others), prepregnancy BMI (categorized as , 25.0–29.9, ), highest education level completed [ grade, high school degree or General Education Development (GED), some college or associate’s degree, 4-y college degree, graduate degree], previous pregnancies (categorized as any, none), and any smoking during pregnancy (yes, no). Participants selected the race and ethnicity category they identified as from the following categories: Hispanic or Latina, White or Caucasian, Black or African American, Asian or Pacific Islander, American Indian or Alaskan Native, or other. Diet during pregnancy [usual intake Healthy Eating Index (HEI-2010) score and daily calories]27 was derived from 24-h diet recalls. Physical activity during pregnancy [metabolic equivalent for task (MET)-hours/week]28 was derived from the Pregnancy Physical Activity Questionnaire. Variables derived from medical records included infant sex assigned at birth (male, female) and total gestational weight gain.29,30 Final models were adjusted for age, race and ethnicity, prepregnancy BMI category, highest education level completed, any previous pregnancies, smoking during pregnancy, gestational age at biological sample collection, infant sex, diet during pregnancy, physical activity during pregnancy, and gestational weight gain. In models for infant outcomes at the 5-month follow-up, we additionally adjusted for infant age.
Self-identified race and ethnicity were included in models owing to culturally driven patterns of personal care product use,31 diet,32 and unmeasured social factors (e.g., stress, discrimination) impacting exposure and outcome status.33,34 We did not adjust for gestational age at birth or pregnancy complications because they could be mediators of our association of interest.35
Statistical Analysis
Statistical analyses were performed using SAS (version 9.4; SAS Institute, Inc.) and R (version 4.0.4; R Development Core Team). Analyses were performed on a subset of participants with complete data for urinary biomarker concentrations and either delivery or 5-month outcomes (). Variable distributions and descriptive statistics for covariates and outcomes [ (SDs) and (%)] as well as exposures (quartiles) were examined. Covariate and outcome distributions were examined and compared for the enrolled, analytic, and subset samples, as well as for included (analytic sample) and excluded participants. Exposure and outcome correlations were determined using Spearman rank correlation and visualized using a heatmap. For each exposure, the intraclass correlation coefficients (ICCs) were calculated from a subset of participants who provided urine samples at three 2-wk intervals in mid-to-late pregnancy, as previously reported.14 A linear mixed effects model was fit to each log-transformed creatinine-standardized biomarker to determine ICCs in this subset. For comparison, we calculated the mean ICC in studies from a 2022 review on variability in urine biomarkers.36 These mean ICCs were calculated only for studies of pregnancy and were preferentially limited to creatinine- or specific-gravity standardized ICCs if multiple ICCs were provided within a single study. Exposure concentrations were log-transformed for analyses.
In our analytic sample, missing covariate data included usual intake HEI-2010 score (), usual intake daily calories (), and gestational age at biological sample collection (). Fifteen participants were missing outcomes at birth, and 81 were missing outcomes at the 5-month follow-up. Missing covariate and outcome data in the analytic sample was imputed using 20 multiple chained equations and included all variables from our main models in addition to gestational age at delivery, age at postnatal visit, infant length and length-for-age -scores, HEI-2010 score and daily calories from the 24-h dietary recall closest to the second study visit, energy expenditure and rate of gestational weight gain for the first and second trimesters,30 conditions complicating pregnancy (hypertension, hypertensive disorders of pregnancy, asthma, diabetes, thyroid disease, psychiatric disorders), mode of delivery, and any birth complications.
Prior to running analyses, we assessed the linearity of the associations by testing quadratic terms in multipollutant models. These initial data checks did not suggest strong deviations from linearity. Furthermore, based on prior literature,7,37 we sought to explore sex-specific associations through examination of effect modification by infant sex. These models required the assumption of linearity. We therefore proceeded with single- and multipollutant analyses under the assumption of linearity as described below.
Single-Pollutant analysis.
Multivariable linear regression models were used to estimate the and 95% confidence interval (CI) for the difference in each continuous infant outcome per log-unit increase in creatinine-standardized phthalate or phenol biomarker concentration. Models for outcomes at birth and at 5 months of age were run separately. Multivariable Poisson models with robust standard errors estimated the relative risk (RR) and 95% CI of experiencing rapid infant growth in weight-for-age or weight-for-length per log-unit increase in creatinine-standardized phthalate or phenol biomarker concentration. In a secondary analysis, we examined effect modification by infant sex.
Multipollutant analysis.
In multipollutant analyses, quantile g-computation were used to estimate the effect of simultaneously increasing all exposure biomarkers within the mixture by 1 quartile on each infant outcome.38 Parametric generalized linear regression models estimated the and 95% CI for each continuous infant outcome. Models for outcomes at birth and at 5 months of age were run separately. Parametric generalized binomial regression models estimated the marginal RR and 95% CI for rapid infant growth in weight-for-age or weight-for-length. Models were run separately for each exposure biomarker mixture: phthalates (Phthalate mixture), phenols (Phenol mixture), and phthalates and phenols (Overall mixture). The Phthalate mixture model was adjusted for phenols, and the Phenol mixture model adjusted for phthalates. In secondary analyses, we examined effect modification by infant sex using the qgcompint package.39
Sensitivity analyses.
We imputed covariate and outcome data in our primary analysis given that multiple imputation of missing data is generally always preferred to ignoring missingness.40 However, to ensure that our imputation approach did not bias results, we repeated our primary analyses for a complete case data set ().
Given that prior studies involving phthalates frequently lack adjustment for diet and other lifestyle confounders,7 we created unadjusted and minimally adjusted models and reran the primary analyses for better comparability to the literature. Minimally adjusted models included age, race and ethnicity, prepregnancy BMI, highest education level completed, any previous pregnancies, smoking during pregnancy, gestational age at biological sample collection, and infant sex assigned at birth. Models at 5 months of age additionally adjusted for infant age.
To ensure covariates could not be along the causal pathway (i.e., to ensure temporality), we created models with alternate adjustments and reran the primary analyses. These alternate adjustments included age, race and ethnicity, prepregnancy BMI, highest education level completed, any previous pregnancies, smoking during pregnancy, gestational age at biological sample collection, HEI-2010 score and daily calories from the 24-h dietary recall closest to the second study visit, and energy expenditure and rate of gestational weight gain for the first and second trimesters. Models at 5 months additionally adjusted for infant age.
Urinary concentrations of phthalate and phenol biomarkers have short half-lives,41 resulting in exposure misclassification and attenuation bias.42 Posteriori disattenuation has been shown to reduce bias resulting from the use of one urine sample to assess exposure.42,43 Thus, in a sensitivity analysis, we performed posteriori disattenuation of our single-pollutant linear regression models43 by dividing estimates and standard errors by their ICCs from the subset.
Results
Descriptive Analysis
Of the 1,410 pregnant individuals enrolled in the Healthy Start cohort, our analytic sample comprised 438 infants with complete exposure and PEA POD assessments at either birth or the 5-month follow-up. Characteristics in the analytic sample appeared generally similar to the enrolled sample, but there were differences in sample characteristics between the enrolled, analytic, and subset samples (Table S1). Notably, more participants in the subset sample identified as non-Hispanic White or Caucasian. Some differences were also observed between included (analytic) and excluded samples (Table S2). For example, included (analytic) participants had a lower energy expenditure, higher gestational weight gain, and smaller infants. Pregnant participants in our analytic sample were generally highly educated (47.9% with at least a 4-y degree), multiparous (62.3%), and nonsmokers (92.7%), with a BMI of (53.7%) (Table 1). A quarter identified as Hispanic or Latina, 12.1% identified as Non-Hispanic Black or African American, 57.3% identified as non-Hispanic White or Caucasian, with the rest (6.4%) identifying as Asian or Pacific Islander, American Indian or Alaskan Native, or other. Participants typically provided their urine sample in the morning (94.8%). Infants were delivered at a mean of wk of gestation, with a mean weight of g.
Table 1.
Participant characteristics for the analytical sample (), Healthy Start cohort, 2009–2014.
| Characteristics | or (%) |
|---|---|
| Maternal age (y) | |
| Self-identified race and ethnicity | |
| Hispanic or Latina | 106 (24.2) |
| Non-Hispanic White or Caucasian | 251 (57.3) |
| Non-Hispanic Black or African American | 53 (12.1) |
| All othersa | 28 (6.4) |
| Prepregnancy BMI () | |
| 235 (53.7) | |
| 25.0–29.9 | 123 (28.1) |
| 80 (18.3) | |
| Highest education level completed | |
| grade | 59 (13.5) |
| High school degree or GED | 72 (16.4) |
| Some college or associate’s degree | 97 (22.2) |
| 4-y college degree | 96 (21.9) |
| Graduate degree | 114 (26.0) |
| Any previous pregnancies | |
| Yes | 273 (62.3) |
| No | 165 (37.7) |
| Smoking during pregnancy | |
| Yes | 32 (7.3) |
| No | 406 (92.7) |
| Diet (usual intake) | |
| HEI-2010 score | |
| Daily calories | |
| Physical activity (MET-h/wk) | |
| Energy expenditure for entire pregnancy | |
| Gestational weight gain (kg) | |
| Gestational age at biological sample collection (wk) | |
| Infant sex assigned at birth | |
| Male | 234 (53.4) |
| Female | 204 (46.5) |
Note: Missing data includes usual intake HEI-2010 score (), usual intake daily calories (), gestational age at biological sample collection (). GED, General Education Development; MET, metabolic equivalent for task; SD, standard deviation.
All others included self-identified Asian or Pacific Islander, American Indian or Alaskan Native, or other.
Correlations between measures of size (weight, weight-for-age, weight-for-age -score) and adiposity (fat mass percentage, grams, index) ranged from 0.37 to 0.78, with stronger correlations observed between these measures at 5 months of age than at birth (Figure S2). We observed moderate () positive correlations within, but not between, phthalates and phenols (Figure S3). Given the high () positive correlations and shared parent compounds, , , and were used in subsequent analyses. Most phthalate metabolites and phenols were highly detected in urine (average percentage detection ) and ICCs for three repeated measures at 2-wk intervals in a subset of 24 participants ranged from 0.00 for to 0.86 for benzophenone-3 (Table 2). As expected owing to the short time frame between repeated samples in midpregnancy, ICC values in the Healthy Start cohort were generally higher than those observed in prior pregnancy studies, as summarized in a recent review (Tables S3–S4).36
Table 2.
Description of biomarkers ( creatinine) in gestational urine samples.
| Biomarker | Abbreviation | Healthy Start analytic sample ()a | Healthy Start subset ()b | Roggeman et al.36,c | ||||
|---|---|---|---|---|---|---|---|---|
| LOD () | Percentage detected (%) | Percentile | ICC | ICC | ||||
| 25th | 50th | 75th | ||||||
| Phthalates | ||||||||
| Mono-ethyl phthalate | MEP | 0.6 | 99.8 | 16.0 | 34.6 | 99.9 | 0.60 | 0.45 |
| Mono-benzyl phthalate | MBzP | 0.3 | 97.3 | 2.3 | 5.6 | 12.6 | 0.48 | 0.48 |
| Mono-3-carboxypropyl phthalate | MCPP | 0.2 | 96.3 | 1.1 | 2.1 | 4.2 | 0.23 | 0.21 |
| Mono carboxyisooctyl phthalate | MCOP | 0.2 | 100.0 | 8.5 | 18.6 | 48.0 | 0.25 | 0.26 |
| Mono carboxyisononyl phthalate | MCNP | 0.2 | 99.1 | 1.9 | 3.1 | 5.6 | 0.18 | 0.08 |
| Mono--butyl phthalate | MnBP | 0.4 | 96.6 | 5.1 | 11.2 | 18.3 | 0.04 | 0.43 |
| Mono-hydroxybutyl phthalate | MHBP | 0.4 | 72.1 | 0.6 | 1.2 | 2.0 | 0.52 | — |
| phthalate metabolitesd | — | — | 5.6 | 12.3 | 20.1 | 0.00 | — | |
| Mono-isobutyl phthalate | MiBP | 0.2 | 98.9 | 3.9 | 8.2 | 14.4 | 0.59 | 0.42 |
| Mono-hydroxyisobutyl phthalate | MHiBP | 0.4 | 95.4 | 2.2 | 3.8 | 6.3 | 0.57 | — |
| phthalate metabolitese | — | — | 6.3 | 12.1 | 20.2 | 0.61 | — | |
| Mono-2-ethylhexyl phthalate | MEHP | 0.5 | 72.8 | 0.5 | 1.2 | 2.5 | 0.58 | 0.28 |
| Mono-2-ethyl-5-hydroxyhexyl phthalate | MEHHP | 0.2 | 99.3 | 2.5 | 5.0 | 10.2 | 0.28 | 0.21 |
| Mono-2-ethyl-5-oxohexyl phthalate | MEOHP | 0.2 | 99.1 | 2.6 | 4.7 | 8.9 | 0.31 | 0.24 |
| Mono-2-ethyl-5-carboxypentyl phthalate | MECPP | 0.2 | 100.0 | 6.8 | 11.2 | 20.4 | 0.39 | 0.28 |
| phthalate metabolitesf | — | — | 13.6 | 24.1 | 43.3 | 0.36 | — | |
| Phenols | ||||||||
| 2,4 dichlorophenol | — | 0.1 | 96.1 | 0.4 | 0.6 | 1.2 | 0.74 | — |
| 2,5 dichlorophenol | — | 0.1 | 95.0 | 0.8 | 1.8 | 5.7 | 0.84 | — |
| Bisphenol A | BPA | 0.1 | 97.7 | 0.6 | 1.1 | 1.9 | 0.39 | 0.20 |
| Bisphenol S | BPS | 0.1 | 87.0 | 0.2 | 0.3 | 0.6 | 0.25 | 0.20 |
| Benzophenone-3 | — | 0.2 | 99.5 | 36.3 | 110.3 | 438.9 | 0.86 | — |
| Methyl paraben | — | 1.0 | 100.0 | 47.8 | 142.5 | 343.4 | 0.82 | 0.47 |
| Propyl paraben | — | 0.1 | 100.0 | 5.2 | 27.6 | 95.0 | 0.79 | 0.49 |
| Triclosan | — | 1.0 | 92.7 | 4.6 | 15.3 | 63.4 | 0.83 | — |
Note: Biomarkers are creatinine-standardized based on the O’Brien method.19 ICC, intraclass correlation coefficient; LOD, limit of detection.
Analytic sample of the Healthy Start cohort conducted during 2009–2014 enrolled participants who provided a urine sample midpregnancy and had at least one time point with outcome assessment.
Subset within Healthy Start cohort conducted during 2009–2014 enrolled participants who provided at least three urine samples in mid-to-late pregnancy.
Mean value calculated from the 2022 review by Roggeman et al.36 on variability in urine biomarkers of nonpersistent chemical exposures. Mean values calculated only for studies of pregnancy, and preferentially limited to creatinine or specific-gravity standardized ICCs when multiple ICCs were provided in one study. Missing ICCs are for biomarkers without values provided in the review. See Tables S3 and S4 for more details.
Sum of di--butyl phthalate metabolites: MnBP and MHBP.
Sum of di-isobutyl phthalate metabolites: MiBP and MHiBP.
Sum of di(2-ethylhexyl) phthalate metabolites: MEHP, MEOHP, MEHHP, and MECPP.
Single-Pollutant Analysis
We identified several notable associations between individual phthalate and phenol biomarkers and infant outcomes (Figure 1; Tables S5–S6) based on the magnitude of the effect estimate, width of CIs, and consistency of associations across primary and secondary outcomes. MBzP was inversely associated with infant weight [: (95% CI: , 2.6) g], fat mass [ (95% CI: , 2.9) g; (95% CI: , 0.14) %], and lean mass [ (95% CI: , 5.6) g] at birth; associations were attenuated at 5 months of age. MEP was inversely associated with infant weight [ (95% CI: , 1.2) g] and fat mass [ (95% CI: , ) g; (95% CI: , 0.1) %], but not with lean mass [ (95% CI: , 20) g] at 5 months of age. No biomarkers were individually associated with risk of rapid infant growth (Table S7).
Figure 1.
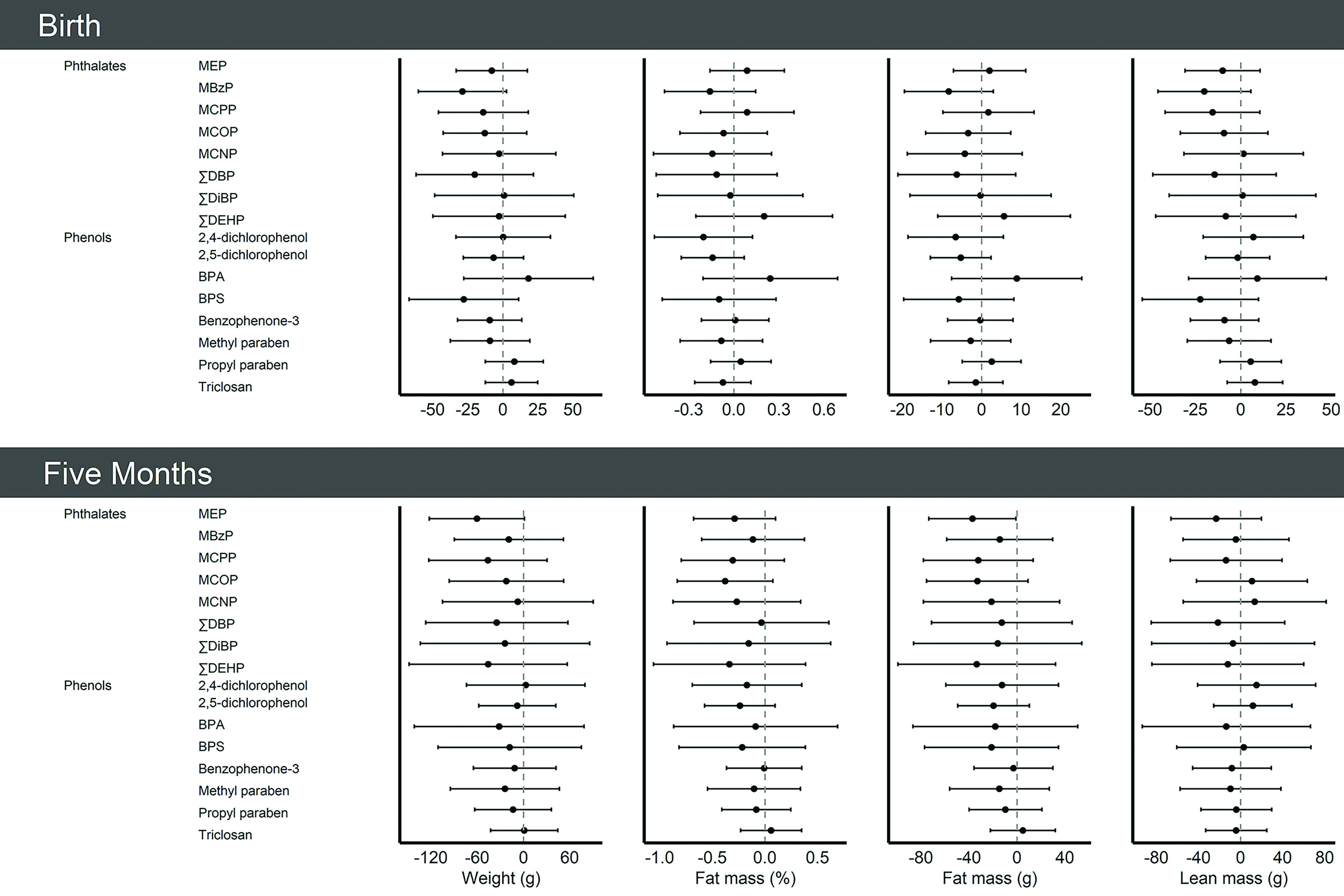
Single-pollutant model results [ (95% CI)] per log-unit increase in creatinine-standardized midpregnancy phthalate or phenol biomarker concentration with infant size and body composition at birth and at 5 months of age in the analytic sample (), Healthy Start cohort, 2009–2014. Models were adjusted for age, race and ethnicity, prepregnancy body mass index category, highest education level completed, any previous pregnancies, smoking during pregnancy, gestational age at biological sample collection, infant sex, diet during pregnancy, physical activity during pregnancy, and gestational weight gain. In models for infant outcomes at the 5-month follow-up, we additionally adjusted for infant age. See Tables S5 and S6 for more details. Note: BPA, bisphenol A; BPS, bisphenol S; CI, confidence interval; MBzP, mono-benzyl phthalate; MCNP, mono carboxyisononyl phthalate; MCOP, mono carboxyisooctyl phthalate; MCPP, mono-3-carboxypropyl phthalate; MEP, mono-ethyl phthalate; , sum of the of di--butyl phthalate metabolites; , sum of di(2-ethylhexyl) phthalate metabolites; , sum of di-isobutyl phthalate metabolites.
There was evidence of effect modification of the association between phthalates biomarkers and infant size and fat mass by infant sex, with results suggesting inverse associations with infant weight and fat mass among males but not females (Figure 2). At birth, and were inversely associated with infant weight in males and had null () or positive () associations in females ( for all comparisons) (Tables S8–S9). Also at birth, MBzP and were inversely associated with infant fat mass for males, whereas associations for females were null (MBzP) or positive () ( for all comparisons). Conversely, MCNP was inversely associated with fat mass in females, whereas associations were null in males ( for all comparisons). Sex-based disparities persisted at 5 months of age but were less precise (Tables S10–S11). Several biomarkers were generally positively associated with the RR of rapid infant growth in weight-for-age (MBzP, , ) or weight-for-length (propyl paraben) in males and inversely associated with the RR of rapid infant growth in females, although effect sizes were small ( for all comparisons) (Table S12). Results were consistent across primary and secondary study outcomes.
Figure 2.
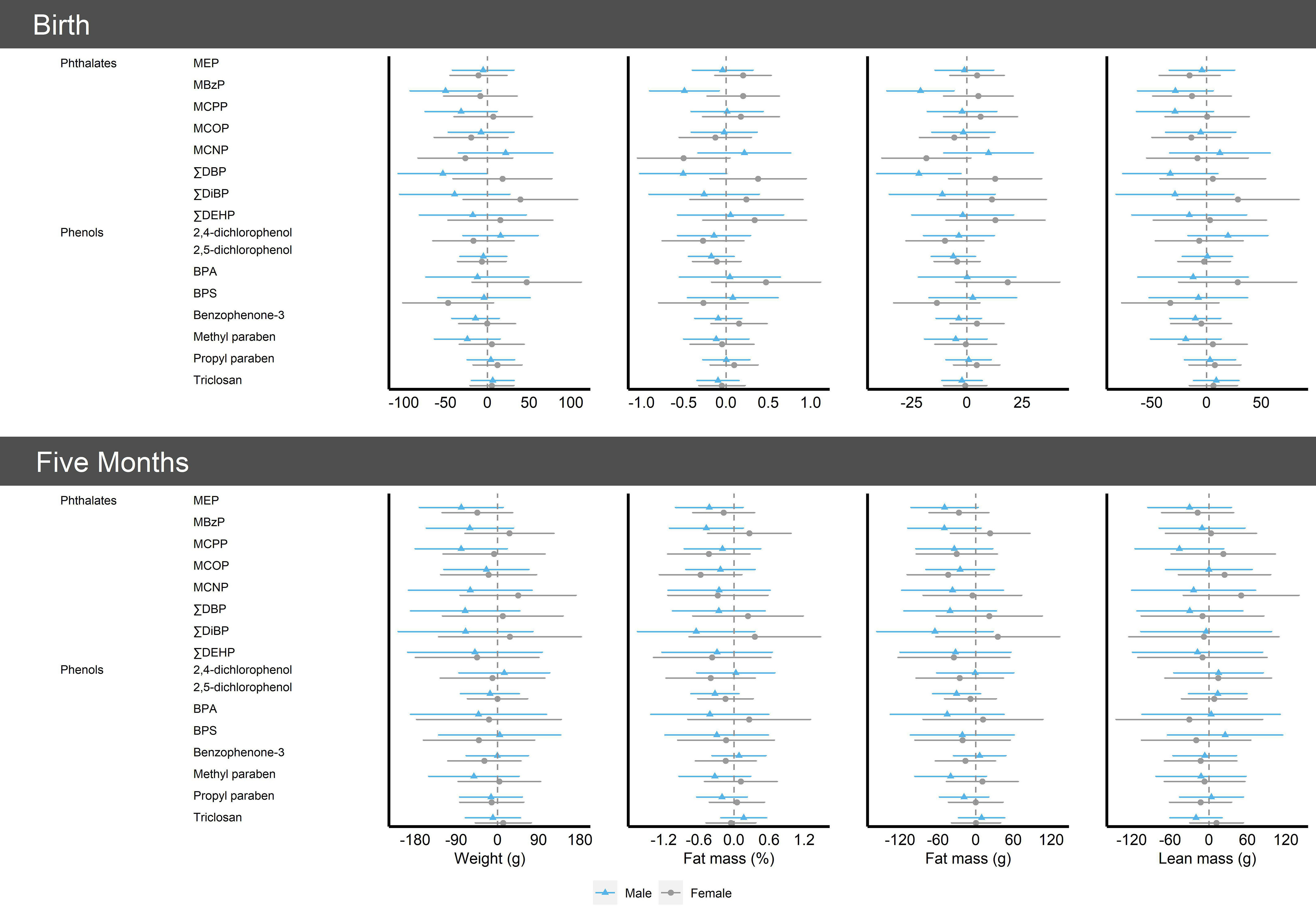
Single-pollutant model results [ (95% CI)] per log-unit increase in creatinine-standardized midpregnancy phthalate or phenol biomarker concentration with infant size and body composition at birth and at 5 months of age by infant sex in the analytic sample (), Healthy Start cohort, 2009–2014. Models were adjusted for age, race and ethnicity, prepregnancy body mass index category, highest education level completed, any previous pregnancies, smoking during pregnancy, gestational age at biological sample collection, infant sex, diet during pregnancy, physical activity during pregnancy, and gestational weight gain. In models for infant outcomes at the 5-month follow-up, we additionally adjusted for infant age. See Tables S8–S11 for more details. Note: BPA, bisphenol A; BPS, bisphenol S; CI, confidence interval; MBzP, mono-benzyl phthalate; MCNP, mono carboxyisononyl phthalate; MCOP, mono carboxyisooctyl phthalate; MCPP, mono-3-carboxypropyl phthalate; MEP, mono-ethyl phthalate; , sum of the of di--butyl phthalate metabolites; , sum of di(2-ethylhexyl) phthalate metabolites; , sum of di-isobutyl phthalate metabolites.
Multipollutant Analysis
Based on the magnitude of the effect estimate, width of CIs, and consistency of associations across primary and secondary outcomes, we determined phthalate and phenol biomarkers mixtures were associated with infant outcomes (Figure 3; Table S13). Associations were stronger at 5 months of age than at birth and were strongest for the Overall mixture of phthalate and phenol biomarkers than for the Phthalate and Phenol mixtures analyzed individually. For example, a 1-quartile increase in the Phthalate mixture was inversely associated with fat mass percentage at birth and at 5 months of age [ (95% CI: , 0.50) and (, 0.05), respectively]. This association for the Phenol mixture was (95% CI: , 0.46) and (95% CI: , 0.47), respectively. For the Overall mixture, this association was (95% CI: , 0.51) and (95% CI: , ), respectively.
Figure 3.
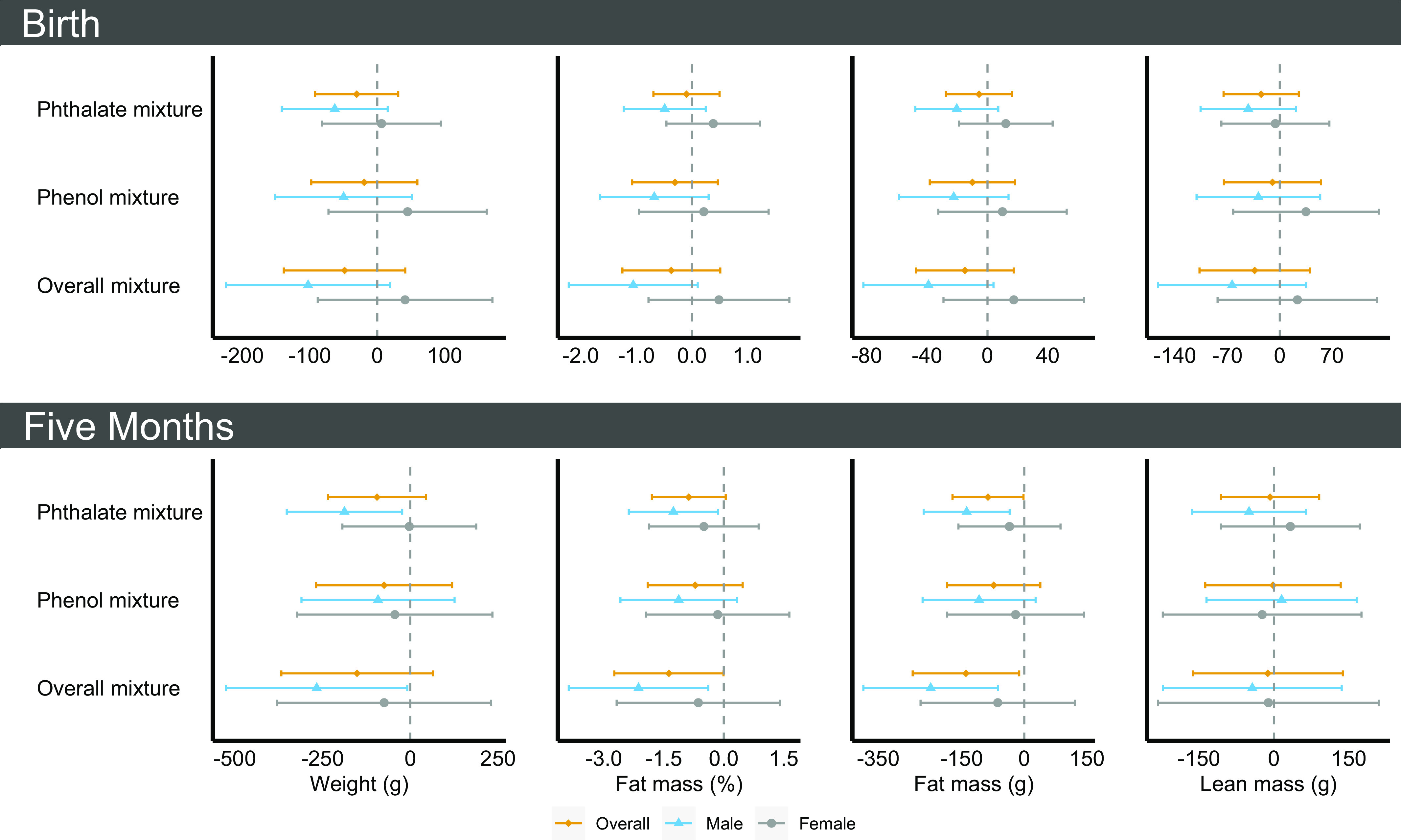
Multipollutant model results [ (95% CI)] per 1-quartile increase in creatinine-standardized midpregnancy phthalate or phenol biomarker concentration with infant size and body composition at birth and at 5 months of age overall and by infant sex in the analytic sample (), Healthy Start cohort, 2009–2014. Models were adjusted for age, race and ethnicity, prepregnancy body mass index category, highest education level completed, any previous pregnancies, smoking during pregnancy, gestational age at biological sample collection, infant sex, diet during pregnancy, physical activity during pregnancy, and gestational weight gain. In models for infant outcomes at the 5-month follow-up, we additionally adjusted for infant age. See Tables S13 and S14 for more details. Note: CI, confidence interval.
Similar to single-pollutant analysis, male infants were disproportionately smaller for increased phthalate and phenol exposure biomarkers (Table S14). Of note, male infants had lower fat mass at birth [ (95% CI: , 7.1) g and (95% CI: , 0.25) %] and at 5 months of age [ (95% CI: , ) g and (95% CI: , ) %] for a 1-quartile increase in the Phthalate mixture; associations were null in female infants ( for all comparisons). Similar associations were observed for the Overall mixture. For example, male infants had lower fat mass percentage at birth [ (95% CI: , 0.1)] for a 1-quartile increase in the Overall mixture; female infants had no notable differences in fat mass percentage [0.48 (95% CI: , 1.75)] for the same time frame (). For a 1-quartile increase in the Phthalate, Phenol, and Overall mixtures, male infants had a generally heightened risk of rapid growth and female infants had a lower risk of rapid growth, although CIs were imprecise and included the null. Results were consistent across primary and secondary study outcomes.
Sensitivity Analyses
In the complete case analysis, birthweight was inversely associated with MBzP [ (95% CI: , )] and [ (95% CI: , )] (Table S15). Further, fat mass at birth was inversely associated with 2,4 dichlorophenol [ (95% CI: , )] and 2,5 dichlorophenol [ (95% CI: , )] (Table S16). Findings from single-pollutant models for rapid infant growth (Table S17) and multipollutant models were similar to our primary analysis (Table S18).
Associations for unadjusted (Tables S19–S22), minimally adjusted (Tables S23–S26), and alternate (Tables S27–S30) models were similar to those from our primary analysis, although the magnitude of associations was stronger in fully adjusted models from our primary analysis. For example, a 1-quartile increase in the Overall mixture was inversely associated with infant percentage fat mass at 5 months of age in unadjusted [ (95% CI: , 0.54)], minimally [ (95% CI: , 0.04)], alternate [ (95% CI: , 0.04)], and fully [ (95% CI: , )] adjusted models.
Single-pollutant linear regression estimates were generally strengthened but less precise with posteriori disattenuation (Tables S31–32). For example, MBzP was associated with a (, 5.2)-g difference in weight at birth using corrected estimates; this difference was (, ) g in our primary analysis.
Discussion
Biomarkers of nonpersistent consumer product chemicals including phthalates and phenols were highly detected among pregnant participants in the Healthy Start study. Exposure to many contemporary phthalates and phenols (e.g., DEHP, BPA) has been decreasing as exposure to replacement chemicals (e.g., BPS) have increased.44 However, the high detection in this study population is in line with several U.S. studies occurring during 2010–201645,46 Concentrations of phenol biomarkers measured midpregnancy were imprecisely inversely associated with infant size (weight) and adiposity at birth and at 5 months of age. Prior observational studies have reported inconsistent and insignificant findings between gestational exposure to phenols and fetal growth outcomes, as summarized in a 2019 review.6 We found that concentrations of phthalate biomarkers measured midpregnancy—in particular MBzP and —were inversely associated with infant size and fat mass at birth and at 5 months of age. This is in line with prior observational studies that have reported more consistent but overall modest associations between gestational exposure to phthalates and fetal growth outcomes, as summarized in a 2019 review.7 Combined exposure to phthalates and phenols midpregnancy was inversely associated with infant size and fat mass at birth and at 5 months of age. In stratified models, these associations were observed only among male rather than female infants; the latter exhibited either null or positive associations between exposures and fat mass, although CIs were imprecise. To our knowledge, this is the first study to examine infant body composition in relation to gestational phthalate and phenol exposures. Given evidence suggesting gestational phthalate and phenol exposure may be associated with reduced fetal growth7 and laboratory studies linking phthalates and phenols to impaired adipogenesis,9–12 further studies to clarify the association between these chemical exposures during pregnancy and body composition and growth outcomes are needed.
The in utero environment has a profound effect on fetal development. Exposure to nonpersistent chemicals including phthalates and phenols during pregnancy has been inversely associated with fetal growth in animal and epidemiologic studies.7 However, evidence from the epidemiologic studies has been equivocal, potentially due to inconsistencies and limitations in outcome assessment. Ultrasound measures of fetal growth are error-prone47,48 and constrained in their depiction of fetal anatomy,49 and birthweight is a proxy measure of fetal growth composed of multiple compartments, including fat and lean mass. Reliance on these measures has provided incomplete insight into associations and potentially obscured the clinical implications of fetal exposure to phthalates and phenols. Birthweight has a U-shaped association with obesity, particularly among males,50 and rapid infant growth is a recognized risk factor for obesity.51 However, anthropometric measures likely reflect lean mass more than fat mass.52 Recent studies from Healthy Start and an Ethiopian cohort have reported that neonatal fat mass exhibits a U-shaped association with later adiposity,53 with the smallest infants experiencing rapid fat accretion and subsequently an adverse metabolic profile.52 In the present study, we observed poor-to-moderate correlations between measures of infant size and adiposity, suggesting body weight is not a strong marker of adiposity. Precise assessments of infant fat and lean mass using noninvasive methods, such as air displacement plethysmography, could therefore provide further clarification of the effects of gestational phthalate and phenol exposures on adiposity development and metabolic health.
Adipogenesis initiates at wk of gestation, adipocytes form at wk, fat lobules form at wk, and adipose tissue accrual accelerates in the third trimester with lipid accretion rates peaking close to delivery (36–40 wk of gestation).54 The number and size of adipocytes are programmed in utero in response to environmental and metabolic cues, through processes under endocrine regulation and with sex-based differences.13,55 Sex differences in adiposity emerge as early as birth, and individuals assigned female sex at birth tend to have more fat mass than those assigned male alongside greater gains in adiposity in infancy.56 Males and females both express estrogen and androgen receptors in adipose tissue, but females tend to have higher expression of estrogen receptors than males.57 This sexual dimorphism helps drive differential adipocyte metabolism and expansion, with estrogens enhancing preadipocyte proliferation and differentiation into insulin-sensitive adipocytes and lipolysis and androgens performing opposing functions.57 In rat models, phthalates decrease the number of adipocytes at birth but increase the size and lipid storage of adipocytes, resulting in an adaptive catch-up adipogenesis and subsequent adipose tissue dysfunction and glucose intolerance, particularly in male rats.13 In mice models, phenols increase the number of adipocytes at birth by changing the fate of stem cells toward the adipocyte lineage and away from osteoblasts.9,10 Several phthalates and phenols are endocrine disrupters, and they can mimic or alter sex steroid levels and thereby differentially affect adipose tissue development in males and females.7,57,58 However, although laboratory studies and biologic plausibility support a link between gestational phthalate and phenol exposures with obesity, epidemiologic evidence of these effects is scarce.
Our study is the first to focus on associations with infant body composition, but several observational studies have investigated associations with childhood body composition.59–68 These cohorts have reported positive,61,62,66,67 inverse,60,63–65 and no associations59,68 between gestational phthalate or phenol exposures with childhood adiposity. Although associations were commonly sexually dimorphic, there is little agreement in prior literature regarding whether males or females are disproportionately affected by exposure. Ferguson et al. recently reported gestational phthalate exposures [MEP (median concentration in ), MBzP (4.5), MCPP (2.0), MBP (9.0), MiBP (6.0), MCOP (15.7), ()] inversely associated with adiposity -scores at birth (weight-for-length) and positively associated with adiposity -scores (BMI) at 3 and 4 years of age, with slightly higher magnitude of associations in males, in a prospective pregnancy cohort conducted at four U.S. sites with recruitment during 2010–2012.69 Placing our results into the context of these prior laboratory and epidemiologic studies, midpregnancy phthalate and, to a lesser extent, phenol exposure appears to be influencing adipogenesis in a sex-dependent manner to result in initial decreases in infant fat mass among males. By 5 months of age, we continued to note lower fat mass with increased gestational phthalate and phenol exposure, although these associations were less precise. We also noted a heightened risk of rapid infant growth (i.e., catch-up growth) in exposed males. Differences in associations at birth and at 5 months of age might suggest some findings could be spurious, particularly those observed in single-pollutant models. On the other hand, it might also suggest a pattern of development whereby gestational phthalate and phenol exposure results in initial decreases in adiposity followed by rapid fat accretion from birth through infancy and into childhood. At 5 months of age, we may have been capturing a window of time when the difference in size and fat mass between exposed and unexposed infants narrows as fat accretion accelerates in exposed infants. Follow-up beyond 5 months of age should enable a better understanding of the potential long-term effects of gestational phthalate and phenol exposures on body composition and growth.
Our results should be considered in light of study limitations. Although a relatively large sample () was analyzed, this convenience sample represents approximately one-third of the full Healthy Start study and may not be generalizable. We note some differences in risk of rapid infant growth, whereby our analytic sample had higher infant fat mass and risk of rapid growth in weight-for-length by the postnatal visit than the excluded sample. Participants in our analytic sample had lower gestational energy expenditure and higher gestational weight gain, which may explain these differences in postnatal infant growth and fat outcomes. Encouragingly, these differences were not present at the neonatal visit, appeared generally small in magnitude (e.g., fat mass was 25% vs. 24% in the included and excluded samples, respectively), and did not occur for risk of rapid growth in weight-for-age. Further, multiple imputation of covariates and outcomes should have provided unbiased estimates for associations.40,70 A complete case sensitivity analysis provided comparable findings to our primary analysis. Other sources of bias may be due to the use of only one time point for exposure assessment, which results in attenuation bias (i.e., bias toward the null).43 To correct for this bias, it is recommended that posteriori disattenuation be applied using internal rather than external ICCs.42 In single-pollutant analyses, we applied a posteriori disattenuation sensitivity analysis for continuous outcomes to correct for this bias. Unfortunately, in single-pollutant analyses for noncontinuous outcomes and all multipollutant analyses, we were unable to apply bias correction methods owing to the categorization of the outcomes and exposures. We also noted that the associations were usually greater in magnitude with adjustment for confounders, suggesting negative confounding. Thus, associations in our study, which were generally modest and imprecise, may actually have been greater than observed. Our ICCs were generally higher than those observed in prior pregnancy studies, likely owing to the 2-wk intervals for exposure assessment occurring within one window. This allowed us to characterize midpregnancy exposure, but even after correction, nonclassical error may arise in case we did not target the relevant exposure window when assessing exposure. Adipogenesis initiates after the first trimester and continues until delivery. We therefore hypothesize that midpregnancy is an exposure window with high relevance for fetal adipose development.
Our study had several strengths. Study results were robust to numerous modeling approaches, adjustments, outcome definitions, and in sensitivity analyses. A strength of our multipollutant modeling approach is that quantile g-computation allowed us to quantify the total mixture effect on our outcomes.38 Mixture methods have been underused in the phthalate and phenol literature,71 but are crucial for the appropriate specification and quantification of the human exposure experience. Quantile g-computation is advantageous for its interpretability, computational ease, and minimal bias, as well as its ability to incorporate nonlinearity and nonadditivity of mixture components.38 Other criticisms of prior epidemiologic studies include inadequate adjustment for potential confounders. Phthalates and phenols are commonly found in food packaging and may be more common among those with obesogenic dietary patterns,7,72 making adjustment for diet of critical importance. Reassuringly for other studies of these associations, additional adjustment for diet quality, total dietary intake, physical activity, and gestational weight gain did not substantially influence model results. Finally, we examined numerous primary and secondary outcomes, finding consistent results across outcomes. Notably, study conclusions remained the same for weight and weight-for-age -score outcomes, and across all means of assessing fat mass (percentage, grams, and fat mass index).
Conclusions
In this U.S.-based prospective cohort, midpregnancy phthalate and phenol exposure was modestly inversely associated with infant weight and adiposity before 5 months of age, particularly in males. Our findings suggest midpregnancy phthalate and phenol exposure may have sexually dimorphic impacts on adipogenesis, resulting in initial decreases in fat mass, which may be followed by rapid growth among male infants. Additional follow-up is needed to understand the potential clinical implications of observed associations.
Supplementary Material
Acknowledgments
This work was supported by extramural grants from the National Institute of Diabetes, Digestive and Kidney Diseases (R01DK076648, to D.D.), the National Institute of Environmental Health Sciences (R01ES022934), the National Institutes of Health Office of the Director (UH3OD023248, to D.D.) and the Intramural Research Program of the National Institute of Environmental Health Sciences. Funding sources were not involved in the study design, collection, analysis, and interpretation of the data or in the writing and decision to submit the manuscript for publication.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC). Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the U.S. Department of Health and Human Services.
Data access may be provided upon reasonable request and a proposal submitted to the Healthy Start cohort study team.
References
- 1.Calafat AM, Valentin-Blasini L, Ye X. 2015. Trends in exposure to chemicals in personal care and consumer products. Curr Environ Health Rep 2(4):348–355, PMID: , 10.1007/s40572-015-0065-9. [DOI] [PubMed] [Google Scholar]
- 2.Ferguson KK, Meeker JD, Cantonwine DE, Chen YH, Mukherjee B, McElrath TF. 2016. Urinary phthalate metabolite and bisphenol A associations with ultrasound and delivery indices of fetal growth. Environ Int 94:531–537, PMID: , 10.1016/j.envint.2016.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferguson KK, Meeker JD, Cantonwine DE, Mukherjee B, Pace GG, Weller D, et al. 2018. Environmental phenol associations with ultrasound and delivery measures of fetal growth. Environ Int 112:243–250, PMID: , 10.1016/j.envint.2017.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Golestanzadeh M, Riahi R, Kelishadi R. 2019. Association of exposure to phthalates with cardiometabolic risk factors in children and adolescents: a systematic review and meta-analysis. Environ Sci Pollut Res Int 26(35):35670–35686, PMID: , 10.1007/s11356-019-06589-7. [DOI] [PubMed] [Google Scholar]
- 5.Ghazipura M, McGowan R, Arslan A, Hossain T. 2017. Exposure to benzophenone-3 and reproductive toxicity: a systematic review of human and animal studies. Reprod Toxicol 73:175–183, PMID: , 10.1016/j.reprotox.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 6.Zhong Q, Peng M, He J, Yang W, Huang F. 2020. Association of prenatal exposure to phenols and parabens with birth size: a systematic review and meta-analysis. Sci Total Environ 703:134720, PMID: , 10.1016/j.scitotenv.2019.134720. [DOI] [PubMed] [Google Scholar]
- 7.Kamai EM, McElrath TF, Ferguson KK. 2019. Fetal growth in environmental epidemiology: mechanisms, limitations, and a review of associations with biomarkers of non-persistent chemical exposures during pregnancy. Environ Health 18(1):43, PMID: , 10.1186/s12940-019-0480-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Catalano PM, Tyzbir ED, Allen SR, McBean JH, McAuliffe TL. 1992. Evaluation of fetal growth by estimation of neonatal body composition. Obstet Gynecol 79(1):46–50, PMID: . [PubMed] [Google Scholar]
- 9.Hu P, Chen X, Whitener RJ, Boder ET, Jones JO, Porollo A, et al. 2013. Effects of parabens on adipocyte differentiation. Toxicol Sci 131(1):56–70, PMID: , 10.1093/toxsci/kfs262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu P, Overby H, Heal E, Wang S, Chen J, Shen CL, et al. 2017. Methylparaben and butylparaben alter multipotent mesenchymal stem cell fates towards adipocyte lineage. Toxicol Appl Pharmacol 329:48–57, PMID: , 10.1016/j.taap.2017.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiu CY, Sun SC, Chiang CK, Wang CC, Chan DC, Chen HJ, et al. 2018. Plasticizer di(2-ethylhexyl)phthalate interferes with osteoblastogenesis and adipogenesis in a mouse model. J Orthop Res 36(4):1124–1134, PMID: , 10.1002/jor.23740. [DOI] [PubMed] [Google Scholar]
- 12.Pomatto V, Cottone E, Cocci P, Mozzicafreddo M, Mosconi G, Nelson ER, et al. 2018. Plasticizers used in food-contact materials affect adipogenesis in 3T3-L1 cells. J Steroid Biochem Mol Biol 178:322–332, PMID: , 10.1016/j.jsbmb.2018.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strakovsky RS, Lezmi S, Shkoda I, Flaws JA, Helferich WG, Pan YX. 2015. In utero growth restriction and catch-up adipogenesis after developmental di (2-ethylhexyl) phthalate exposure cause glucose intolerance in adult male rats following a high-fat dietary challenge. J Nutr Biochem 26(11):1208–1220, PMID: , 10.1016/j.jnutbio.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polinski KJ, Dabelea D, Hamman RF, Adgate JL, Calafat AM, Ye X, et al. 2018. Distribution and predictors of urinary concentrations of phthalate metabolites and phenols among pregnant women in the Healthy Start study. Environ Res 162:308–317, PMID: , 10.1016/j.envres.2018.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silva MJ, Samandar E, Preau JL Jr, Reidy JA, Needham LL, Calafat AM. 2007. Quantification of 22 phthalate metabolites in human urine. J Chromatogr B Analyt Technol Biomed Life Sci 860(1):106–112, PMID: , 10.1016/j.jchromb.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 16.Ye X, Kuklenyik Z, Needham LL, Calafat AM. 2005. Automated on-line column-switching HPLC-MS/MS method with peak focusing for the determination of nine environmental phenols in urine. Anal Chem 77(16):5407–5413, PMID: , 10.1021/ac050390d. [DOI] [PubMed] [Google Scholar]
- 17.Pacyga DC, Haggerty DK, Nicol M, Henning M, Calafat AM, Braun JM, et al. 2022. Identification of profiles and determinants of maternal pregnancy urinary biomarkers of phthalates and replacements in the Illinois Kids Development Study. Environ Int 162:107150, PMID: , 10.1016/j.envint.2022.107150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lubin JH, Colt JS, Camann D, Davis S, Cerhan JR, Severson RK, et al. 2004. Epidemiologic evaluation of measurement data in the presence of detection limits. Environ Health Perspect 112(17):1691–1696, PMID: , 10.1289/ehp.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Brien KM, Upson K, Cook NR, Weinberg CR. 2016. Environmental chemicals in urine and blood: improving methods for creatinine and lipid adjustment. Environ Health Perspect 124(2):220–227, PMID: , 10.1289/ehp.1509693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Butte NF, Hopkinson JM, Wong WW, Smith EO, Ellis KJ. 2000. Body composition during the first 2 years of life: an updated reference. Pediatr Res 47(5):578–585, PMID: , 10.1203/00006450-200005000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Ma G, Yao M, Liu Y, Lin A, Zou H, Urlando A, et al. 2004. Validation of a new pediatric air-displacement plethysmograph for assessing body composition in infants. Am J Clin Nutr 79(4):653–660, PMID: , 10.1093/ajcn/79.4.653. [DOI] [PubMed] [Google Scholar]
- 22.Schumacher D, Borghi E, Polonsky J. 2023. Package ‘anthro’. Computation of the WHO Child Growth Standards. https://github.com/dirkschumacher/anthro: CRAN; [accessed 5 June 2021]. [Google Scholar]
- 23.Beunders VAA, Roelants JA, Hulst JM, Rizopoulos D, Hokken-Koelega ACS, Neelis EG, et al. 2021. Early weight gain trajectories and body composition in infancy in infants born very preterm. Pediatr Obes 16(6):e12752, PMID: , 10.1111/ijpo.12752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Fluiter KS, van Beijsterveldt I, Breij LM, Acton D, Hokken-Koelega ACS. 2020. Association between fat mass in early life and later fat mass trajectories. JAMA Pediatr 174(12):1141–1148, PMID: , 10.1001/jamapediatrics.2020.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monteiro POA, Victora CG. 2005. Rapid growth in infancy and childhood and obesity in later life—a systematic review. Obes Rev 6(2):143–154, PMID: , 10.1111/j.1467-789X.2005.00183.x. [DOI] [PubMed] [Google Scholar]
- 26.Textor J, van der Zander B, Gilthorpe MS, Liskiewicz M, Ellison GT. 2016. Robust causal inference using directed acyclic graphs: the R package ‘dagitty.’ Int J Epidemiol 45(6):1887–1894, PMID: , 10.1093/ije/dyw341. [DOI] [PubMed] [Google Scholar]
- 27.Shapiro ALB, Kaar JL, Crume TL, Starling AP, Siega-Riz AM, Ringham BM, et al. 2016. Maternal diet quality in pregnancy and neonatal adiposity: the Healthy Start study. Int J Obes (Lond) 40(7):1056–1062, PMID: , 10.1038/ijo.2016.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harrod CS, Chasan-Taber L, Reynolds RM, Fingerlin TE, Glueck DH, Brinton JT, et al. 2014. Physical activity in pregnancy and neonatal body composition: the Healthy Start study. Obstet Gynecol 124(2 pt 1):257–264, PMID: , 10.1097/AOG.0000000000000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lemas DJ, Brinton JT, Shapiro AL, Glueck DH, Friedman JE, Dabelea D. 2015. Associations of maternal weight status prior and during pregnancy with neonatal cardiometabolic markers at birth: the Healthy Start study. Int J Obes (Lond) 39(10):1437–1442, PMID: , 10.1038/ijo.2015.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Starling AP, Brinton JT, Glueck DH, Shapiro AL, Harrod CS, Lynch AM, et al. 2015. Associations of maternal BMI and gestational weight gain with neonatal adiposity in the Healthy Start study. Am J Clin Nutr 101(2):302–309, PMID: , 10.3945/ajcn.114.094946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chan M, Mita C, Bellavia A, Parker M, James-Todd T. 2021. Racial/ethnic disparities in pregnancy and prenatal exposure to endocrine-disrupting chemicals commonly used in personal care products. Curr Environ Health Rep 8(2):98–112, PMID: , 10.1007/s40572-021-00317-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pacyga DC, Sathyanarayana S, Strakovsky RS. 2019. Dietary predictors of phthalate and bisphenol exposures in pregnant women. Adv Nutr 10(5):803–815, PMID: , 10.1093/advances/nmz029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dominguez TP, Dunkel-Schetter C, Glynn LM, Hobel C, Sandman CA. 2008. Racial differences in birth outcomes: the role of general, pregnancy, and racism stress. Health Psychol 27(2):194–203, PMID: , 10.1037/0278-6133.27.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larrabee Sonderlund A, Schoenthaler A, Thilsing T. 2021. The association between maternal experiences of interpersonal discrimination and adverse birth outcomes: a systematic review of the evidence. Int J Environ Res Public Health 18(4):1465, PMID: , 10.3390/ijerph18041465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilcox AJ, Weinberg CR, Basso O. 2011. On the pitfalls of adjusting for gestational age at birth. Am J Epidemiol 174(9):1062–1068, PMID: , 10.1093/aje/kwr230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roggeman M, Gys C, Klimowska A, Bastiaensen M, Wielgomas B, Ait Bamai Y, et al. 2022. Reviewing the variability in urinary concentrations of non-persistent organic chemicals: evaluation across classes, sampling strategies and dilution corrections. Environ Res 215(pt 2):114332, PMID: , 10.1016/j.envres.2022.114332. [DOI] [PubMed] [Google Scholar]
- 37.Sood S, Shekhar S, Santosh W. 2017. Dimorphic placental stress: a repercussion of interaction between endocrine disrupting chemicals (EDCs) and fetal sex. Med Hypotheses 99:73–75, PMID: , 10.1016/j.mehy.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 38.Keil AP, Buckley JP, O’Brien KM, Ferguson KK, Zhao S, White AJ. 2020. A quantile-based g-computation approach to addressing the effects of exposure mixtures. Environ Health Perspect 128(4):047004, PMID: , 10.1289/EHP5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keil A. 2021. qgcompint: quantile G-Computation Extensions for Effect Measure Modification. Version R package version 0.6.6.
- 40.Cole SR, Zivich PN, Edwards JK, Ross RK, Shook-Sa BE, Price JT, et al. 2023. Missing outcome data in epidemiologic studies. Am J Epidemiol 192(1):6–10, PMID: , 10.1093/aje/kwac179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sathyanarayana S. 2008. Phthalates and children’s health. Curr Probl Pediatr Adolesc Health Care 38(2):34–49, PMID: , 10.1016/j.cppeds.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 42.Vernet C, Philippat C, Agier L, Calafat AM, Ye X, Lyon-Caen S, et al. 2019. An empirical validation of the within-subject biospecimens pooling approach to minimize exposure misclassification in biomarker-based studies. Epidemiology 30(5):756–767, PMID: , 10.1097/EDE.0000000000001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perrier F, Giorgis-Allemand L, Slama R, Philippat C. 2016. Within-subject pooling of biological samples to reduce exposure misclassification in biomarker-based studies. Epidemiology 27(3):378–388, PMID: , 10.1097/EDE.0000000000000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buckley JP, Kuiper JR, Bennett DH, Barrett ES, Bastain T, Breton CV, et al. 2022. Exposure to contemporary and emerging chemicals in commerce among pregnant women in the United States: the Environmental Influences on Child Health Outcome (ECHO) Program. Environ Sci Technol 56(10):6560–6573, PMID: , 10.1021/acs.est.1c08942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shin HM, Dhar U, Calafat AM, Nguyen V, Schmidt RJ, Hertz-Picciotto I. 2020. Temporal trends of exposure to phthalates and phthalate alternatives in California pregnant women during 2007–2013: comparison with other populations. Environ Sci Technol 54(20):13157–13166, PMID: , 10.1021/acs.est.0c03857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim K, Shin HM, Busgang SA, Barr DB, Panuwet P, Schmidt RJ, et al. 2021. Temporal trends of phenol, paraben, and triclocarban exposure in California pregnant women during 2007–2014. Environ Sci Technol 55(16):11155–11165, PMID: , 10.1021/acs.est.1c01564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mayer C, Joseph KS. 2013. Fetal growth: a review of terms, concepts and issues relevant to obstetrics. Ultrasound Obstet Gynecol 41(2):136–145, PMID: , 10.1002/uog.11204. [DOI] [PubMed] [Google Scholar]
- 48.Cetin I, Boito S, Radaelli T. 2008. Evaluation of fetal growth and fetal well-being. Semin Ultrasound CT MR 29(2):136–146, PMID: , 10.1053/j.sult.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 49.Sepulveda W, Wong AE, Sepulveda F, Martinez-Ten P, Ximenes R. 2012. Fetal magnetic resonance imaging and three-dimensional ultrasound in clinical practice: general aspects. Best Pract Res Clin Obstet Gynaecol 26(5):575–591, PMID: , 10.1016/j.bpobgyn.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 50.Sun D, Wang T, Heianza Y, Huang T, Shang X, Lv J, et al. 2018. Birthweight and cardiometabolic risk patterns in multiracial children. Int J Obes (Lond) 42(1):20–27, PMID: , 10.1038/ijo.2017.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Singhal A. 2017. Long-term adverse effects of early growth acceleration or catch-up growth. Ann Nutr Metab 70(3):236–240, PMID: , 10.1159/000464302. [DOI] [PubMed] [Google Scholar]
- 52.Sauder KA, Perng W, Palumbo MP, Bloemsma LD, Carey J, Glueck DH, et al. 2021. Fat mass accretion from birth to 5 years and metabolic homeostasis in childhood: the Healthy Start study. J Clin Endocrinol Metab 106(6):1684–1691, PMID: , 10.1210/clinem/dgab115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Andersen GS, Wibaek R, Kaestel P, Girma T, Admassu B, Abera M, et al. 2018. Body composition growth patterns in early infancy: a latent class trajectory analysis of the Ethiopian iABC birth cohort. Obesity (Silver Spring) 26(7):1225–1233, PMID: , 10.1002/oby.22197. [DOI] [PubMed] [Google Scholar]
- 54.Orsso CE, Colin-Ramirez E, Field CJ, Madsen KL, Prado CM, Haqq AM. 2020. Adipose tissue development and expansion from the womb to adolescence: an overview. Nutrients 12(9):2735, PMID: , 10.3390/nu12092735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Desoye G, Herrera E. 2021. Adipose tissue development and lipid metabolism in the human fetus: the 2020 perspective focusing on maternal diabetes and obesity. Prog Lipid Res 81:101082, PMID: , 10.1016/j.plipres.2020.101082. [DOI] [PubMed] [Google Scholar]
- 56.Davis SM, Kaar JL, Ringham BM, Hockett CW, Glueck DH, Dabelea D. 2019. Sex differences in infant body composition emerge in the first 5 months of life. J Pediatr Endocrinol Metab 32(11):1235–1239, PMID: , 10.1515/jpem-2019-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Le Magueresse-Battistoni B. 2020. Adipose tissue and endocrine-disrupting chemicals: does sex matter? Int J Environ Res Public Health 17(24):9403, PMID: , 10.3390/ijerph17249403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rubin BS, Paranjpe M, DaFonte T, Schaeberle C, Soto AM, Obin M, et al. 2017. Perinatal BPA exposure alters body weight and composition in a dose specific and sex specific manner: the addition of peripubertal exposure exacerbates adverse effects in female mice. Reprod Toxicol 68:130–144, PMID: , 10.1016/j.reprotox.2016.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Montes JOA, Villarreal AB, Romieu I, Barr DB, Martínez KC, Cadena LH. 2022. Modification of the association by sex between the prenatal exposure to di(2-ethylhexyl) phthalate and fat percentage in a cohort of Mexicans schoolchildren. Int J Obes (Lond) 46(1):121–128, PMID: , 10.1038/s41366-021-00952-w. [DOI] [PubMed] [Google Scholar]
- 60.Buckley JP, Engel SM, Mendez MA, Richardson DB, Daniels JL, Calafat AM, et al. 2016. Prenatal phthalate exposures and childhood fat mass in a New York City cohort. Environ Health Perspect 124(4):507–513, PMID: , 10.1289/ehp.1509788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Berman YE, Doherty DA, Main KM, Frederiksen H, Hickey M, Keelan JA, et al. 2021. Associations between prenatal exposure to phthalates and timing of menarche and growth and adiposity into adulthood: a twenty-years birth cohort study. Int J Environ Res Public Health 18(9):4725, PMID: , 10.3390/ijerph18094725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sol CM, Santos S, Duijts L, Asimakopoulos AG, Martinez-Moral MP, Kannan K, et al. 2020. Fetal exposure to phthalates and bisphenols and childhood general and organ fat. A population-based prospective cohort study. Int J Obes (Lond) 44(11):2225–2235, PMID: , 10.1038/s41366-020-00672-7. [DOI] [PubMed] [Google Scholar]
- 63.Shoaff J, Papandonatos GD, Calafat AM, Ye X, Chen A, Lanphear BP, et al. 2017. Early-life phthalate exposure and adiposity at 8 years of age. Environ Health Perspect 125(9):097008, PMID: , 10.1289/EHP1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Buckley JP, Herring AH, Wolff MS, Calafat AM, Engel SM. 2016. Prenatal exposure to environmental phenols and childhood fat mass in the Mount Sinai Children’s Environmental Health Study. Environ Int 91:350–356, PMID: , 10.1016/j.envint.2016.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Harley KG, Aguilar Schall R, Chevrier J, Tyler K, Aguirre H, Bradman A, et al. 2013. Prenatal and postnatal bisphenol A exposure and body mass index in childhood in the CHAMACOS cohort. Environ Health Perspect 121(4):514–520, PMID: , 10.1289/ehp.1205548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hoepner LA, Whyatt RM, Widen EM, Hassoun A, Oberfield SE, Mueller NT, et al. 2016. Bisphenol A and adiposity in an inner-city birth cohort. Environ Health Perspect 124(10):1644–1650, PMID: , 10.1289/EHP205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Højsager FD, Kyhl HB, Frederiksen H, Juul A, Andersson AM, Andersen MS, et al. 2021. Prenatal exposure to butyl paraben is associated with fat percentage in 7-year-old boys. J Clin Endocrinol Metab 106(7):e2633–e2638, PMID: , 10.1210/clinem/dgab167. [DOI] [PubMed] [Google Scholar]
- 68.Kalloo G, Calafat AM, Chen A, Yolton K, Lanphear BP, Braun JM. 2018. Early life triclosan exposure and child adiposity at 8 years of age: a prospective cohort study. Environ Health 17(1):24, PMID: , 10.1186/s12940-018-0366-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ferguson KK, Bommarito PA, Arogbokun O, Rosen EM, Keil AP, Zhao S, et al. 2022. Prenatal phthalate exposure and child weight and adiposity from in utero to 6 years of age. Environ Health Perspect 130(4):047006, PMID: , 10.1289/EHP10077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Perkins NJ, Cole SR, Harel O, Tchetgen Tchetgen EJ, Sun B, Mitchell EM, et al. 2018. Principled approaches to missing data in epidemiologic studies. Am J Epidemiol 187(3):568–575, PMID: , 10.1093/aje/kwx348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chiu YH, Bellavia A, James-Todd T, Correia KF, Valeri L, Messerlian C, et al. 2018. Evaluating effects of prenatal exposure to phthalate mixtures on birth weight: a comparison of three statistical approaches. Environ Int 113:231–239, PMID: , 10.1016/j.envint.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Filardi T, Panimolle F, Lenzi A, Morano S. 2020. Bisphenol A and phthalates in diet: an emerging link with pregnancy complications. Nutrients 12(2):525, PMID: , 10.3390/nu12020525. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


