Abstract

Interfacial strain in heteroepitaxial oxide thin films is a powerful tool for discovering properties and recognizing the potential of materials performance. Particularly, facilitating ion conduction by interfacial strain in oxide multilayer thin films has always been seen to be a highly promising route to this goal. However, the effect of interfacial strain on ion transport properties is still controversial due to the difficulty in deconvoluting the strain contribution from other interfacial phenomena, such as space charge effects. Here, we show that interfacial strain can effectively tune the ionic conductivity by successfully growing multilayer thin films composed of an ionic conductor Gd-doped CeO2 (GDC) and an insulator RE2O3 (RE = Y and Sm). In contrast to compressively strained GDC-Y2O3 multilayer films, tensile strained GDC-Sm2O3 multilayer films demonstrate the enhanced ionic conductivity of GDC, which is attributed to the increased concentration of oxygen vacancies. In addition, we demonstrate that increasing the number of interfaces has no impact on the further enhancement of the ionic conductivity in GDC-Sm2O3 multilayer films. Our findings demonstrate the unambiguous role of interfacial strain on ion conduction of oxides and provide insights into the rational design of fast ion conductors through interface engineering.
Keywords: interfacial strain, pulsed laser deposition, epitaxial strain-induced ionic conductivity, oxide multilayer thin films, fluorite structure, bixbyite oxide structure
1. Introduction
Achieving fast ion transport at reduced temperatures is a key requirement to develop advanced oxide-based energy applications, including solid oxide fuel cells,1,2 batteries,3,4 oxygen permeable membranes,5 and catalysts.6 In recent years, with the development of thin film technologies, such as pulsed laser deposition (PLD), growing attention has been directed toward investigating ion conduction in oxide thin film heterostructures, where tuning interfacial strain is known as a control knob for altering the migration of oxygen ions7−10 and the formation of oxygen vacancies11−15 at heterointerfaces.
In oxide thin film heterostructures, strain can be introduced into heterointerfaces via lattice mismatch.14 However, by increasing the lattice mismatch and film thickness, the interface can change from coherent to incoherent as dislocations are introduced to relieve strain.10 Appropriate lattice mismatch and film thickness (a few nanometers thick) are thus essential for obtaining suitable strain levels (∼±3%) to elucidate the interfacial strain effect on ion conduction. However, ultrathin films exhibit extremely large resistance, which makes the choice of substrate critical to avoid the substrate shunt current from dominating the transport. To accurately and reliably evaluate the oxygen ion conductivity, it is necessary to use insulating substrates, such as Al2O3, MgO, and NdGaO3 (NGO).16−18 Despite their insulating properties, such substrates generally cause a large lattice mismatch with oxide thin films, resulting in poor crystallinity of the films, which, in turn, hampers the identification of the strain effect on ion conduction.
Furthermore, depending on the combination of materials, it is difficult to deconvolute the strain contribution from multiple factors, such as space charge layers (SCLs), to the ionic conductivity in multilayer thin films. For example, in the case of undoped ionic conductors with a low intrinsic defect concentration, such as CeO2, creating a heterointerface with insulating oxides enabled the enhancement of the ionic conductivity of CeO2 due to the formation of SCLs.19 Heterointerfaces between two different doped ion conductors, such as a lateral multilayer composed of Sm-doped CeO2 (SDC) and Y-doped ZrO2 (YSZ),20 also enhanced the ionic conductivity without the formation of SCLs, which were likely negligible anyway due to the large intrinsic defect concentrations.10 However, due to the simultaneous contribution of two ionic conductors to the total conductivity, deconvoluting the strain contribution to the total conductivity was impossible.
As a result of such difficulties noted in these material systems and limited appropriate substrates, the effect of strain on enhancing the ionic conductivity is still quite controversial.10,20−23 What is needed to explore the effect of strain on ion conduction is a precise synthesis of high-quality multilayer thin films on insulating substrates and the careful selection of material systems. In particular, an ionic conductor with abundant oxygen vacancies and an insulator are needed to exclude the SCLs and the contribution to the ionic conductivity, respectively. In addition, the lattice mismatch between these materials should be in the range of ∼±2% to form a coherent interface. To meet these requirements, a combination of fluorite Gd-doped CeO2 (GDC) and bixbyite RE2O3 (RE = Y and Sm) is a promising material combination.
In this work, we present the effect of strain on ionic conductivity by successfully creating heterointerfaces composed of an ionic conductor GDC and an insulator RE2O3 (RE = Y and Sm) grown on insulating Al2O3 substrates. Considering the lattice mismatch between fluorite GDC and bixbyite RE2O3 (RE = Y and Sm), Sm2O3 (a = 10.935 Å),24 and Y2O3 (a = 10.604 Å)25 introduce tensile (∼1.1%) and compressive (∼−2%) strain, respectively, into GDC (a = 5.410 Å).26 The interfacial strain is modulated by controlling the thickness of each layer in epitaxial GDC-RE2O3 multilayer thin films on Al2O3. In contrast to compressive strain, tensile strain results in an increase in the oxygen vacancy concentration, which, in turn, leads to the enhanced ionic conductivity of GDC. This work demonstrates that controlling interfacial strain in multilayer thin films is a simple and effective means to tune the ionic conductivity of oxides.
2. Experimental Details
2.1. Synthesis of GDC-RE2O3 (RE = Y and Sm) Multilayer Films
Epitaxial GDC-RE2O3 (RE = Y and Sm) multilayer films with different thicknesses of GDC (∼5, ∼10, ∼30, and ∼50 nm) were grown by PLD on single-crystal (0001) Al2O3 substrates. To achieve the desired strain in the GDC films, the thickness of the RE2O3 layers was consistently set at 100 nm on Al2O3 substrates to ensure their relaxation state. The substrates were attached to the PLD substrate holder using a small amount of silver paint for thermal contact. PLD was performed using a KrF excimer laser at λ = 248 nm, 10 Hz pulse rate, and ∼1 J/cm2 fluence under an oxygen partial pressure, p(O2), of 1.3 × 10–4 atm (100 mTorr) at 700 °C. After completing the deposition, the samples were cooled to room temperature in the PLD chamber for 1 h under a p(O2) of 1.3 × 10–4 atm (100 mTorr).
2.2. Characterization of Physical and Chemical Properties
Oxide phase purity and crystallography of the films were investigated via high-resolution X-ray diffraction (HRXRD) using a four-circle diffractometer. Measurements were performed using in-plane and out-of-plane configurations. The thicknesses of the films were characterized by X-ray reflectivity (XRR) measurements. In situ HRXRD was performed on a four-circle diffractometer in a p(O2) of 1 atm and a controlled temperature stage (DHS 900, Anton Paar). Silver paste was used to adhere the thin film sample to the heating plate. The heating rate was ∼10 °C min–1, and the temperature was held for 20 min at each temperature (25 and 700 °C) before XRD data were collected. Sample realignment was conducted at each temperature to maximize the XRD intensities. A full range θ–2θ normal scan was collected, and then high-resolution θ–2θ normal scans of GDC (111) and Al2O3 (0002) were collected. As the thermocouple for this experiment was placed inside the heating stage, a small difference between the set and actual temperatures on the sample surface cannot be excluded. Raman scattering measurements were performed with an Xplora plus Raman spectrometer. A 50× magnification long working distance (8 mm) objective was used with a laser excitation wavelength of 638 nm. The Raman spectra were collected using the LabSpec 6 software every 1 min.
2.3. Evaluation of Ionic Conductivity
To measure the ionic conductivity of the GDC single layer and GDC-RE2O3 (RE = Y and Sm) multilayer films, in-plane dc measurements were performed with a source meter (Keithley 2450) in a two-electrode configuration geometry using silver paste electrodes painted onto the film surface. The dc measurements were carried out at a temperature of 450 to 700 °C. The dc measurements were also performed while varying the oxygen partial pressure p(O2) from 10–3 to 1 atm.
3. Results and Discussion
3.1. Crystallinity of the Epitaxial GDC-RE2O3 (RE = Y and Sm) Multilayer Films
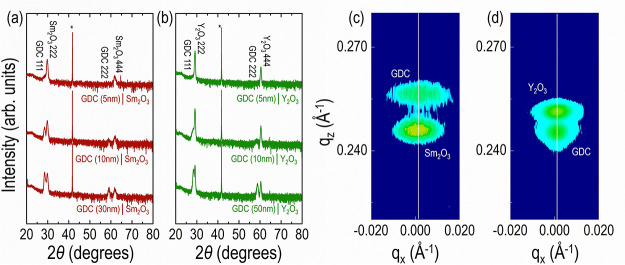
Prior to the synthesis of multilayer thin films, the growth of single layer films was required to optimize the growth conditions for each layer. XRD θ–2θ scans shown in Figure 1 confirmed that GDC and RE2O3 (RE = Sm and Y) single layer films were grown epitaxially. The films showed only (hhh) peaks, indicating (111)-oriented films on (0001) Al2O3. The ϕ scans of the GDC and RE2O3 (RE = Sm and Y) single layer films showed that (200) GDC, (400) RE2O3 (RE = Sm and Y), and (113) Al2O3 have strong peaks with 6-fold symmetry (Figure 1b), which reveals the in-plane crystallographic relationships between GDC, RE2O3 (RE = Sm and Y) and Al2O3 (in-plane 30° rotation). According to the 6-fold symmetry of the (0001) Al2O3 and 3-fold symmetry of the (111) GDC and RE2O3 (RE = Sm and Y),27 single layers of GDC and RE2O3 (RE = Sm and Y) could be deposited on (0001) Al2O3 with the following epitaxy relationship: (111) GDC or (111) RE2O3||(0001) Al2O3 and [1̅10] GDC or [1̅10] RE2O3||[1̅10] Al2O3, [11̅0] GDC or [11̅0] RE2O3||[1̅10] Al2O3 (Figure 1c). The deposition of GDC and RE2O3 (RE = Sm and Y) with a 3-fold symmetry can be achieved in two different directions on (0001) Al2O3, resulting in the 6-fold symmetry observed in the ϕ scans (Figure 1b). It is worth noting that epitaxial Sm2O3 thin films were successfully grown on Al2O3. In fact, despite the advantages of using Sm2O3 as a counterpart material in oxide multilayer films, the growth of epitaxial Sm2O3 films is difficult compared to that of other rare-earth oxides,28,29 which, in turn, leads to the limited range of strain that can be induced by the lattice mismatch with other oxides.
Figure 1.
(a) XRD θ–2θ patterns of the epitaxial single layer GDC, Sm2O3, and Y2O3 films grown on Al2O3 substrates. (b) XRD ϕ scans of the 113 reflections of Al2O3 substrate, 200 reflections of GDC film, and 400 reflections of RE2O3 (RE = Y and Sm). (c) Schematic illustration of in-plane lattice matching based on XRD θ–2θ patterns and ϕ scans (black circles represent oxygens of Al2O3).
Based on the optimized growth conditions of single layers, epitaxial GDC-RE2O3 (RE = Sm and Y) multilayer films were deposited on Al2O3 (Figure 2). The films clearly show the presence of only (hhh) peaks of GDC and RE2O3 (RE = Sm and Y), indicating that the films grew epitaxially. To precisely control the interfacial strain, the thickness of GDC was carefully modulated in the nanometer range, which was a challenge in this study. One concern could be the cation diffusion between the GDC and RE2O3 during film deposition and ionic conductivity measurements. However, previous studies already reported that there was no cation interdiffusion between fluorite CeO2 and bixbyite RE2O3 due to the extremely low diffusion coefficients of rare-earth cations in CeO2 at 650 °C and below.19,30,31 XRD reciprocal space maps (RSMs) around the 103 Bragg reflections of the Al2O3 indicate that Sm2O3 and Y2O3 introduced in-plane tensile and compressive strain, respectively, into GDC (Figure 2c,d).
Figure 2.
XRD θ–2θ patterns of the epitaxial (a) GDC-Sm2O3 and (b) GDC-Y2O3 multilayer films grown on Al2O3 substrates. X-ray reciprocal space maps (RSMs) of the (c) tensile strained GDC-Sm2O3 and (d) compressively strained GDC-Y2O3 multilayer films.
3.2. Strain in the Epitaxial GDC-RE2O3 (RE = Y and Sm) Multilayer Films
The systematic change in the c-axis lattice constant of GDC was observed as the thickness of GDC decreased in the GDC-RE2O3 (RE = Sm and Y) multilayer films, leading to the change in the in-plane lattice strain of GDC due to the Poisson’s ratio (Figure 3a). The in-plane lattice strain of GDC in the GDC-RE2O3 (RE = Sm and Y) multilayer films is shown in Figure 3b. The in-plane strain of GDC increased as the GDC thickness decreased. The GDC film with a thickness of ∼5 nm on Sm2O3 was found to have a strain of ∼1.2%, whereas the same thickness of GDC on Y2O3 resulted in a strain of −1.9%. For GDC thicknesses less than 50 nm, a negligible change in compressive strain was observed compared to the tensile GDC-Sm2O3 case. This trend implies that the compressive strain induced at a GDC thickness of ∼50 nm has already reached the theoretical compressive strain calculated from the bulk lattice parameters of GDC and Y2O3.25,26 These strain calculations based on changes in the in-plane lattice parameter of GDC are in good agreement with the RSMs discussed in Figure 2.
Figure 3.
Changes of (a) c-axis lattice parameter and (b) in-plane strain of GDC extracted from GDC-RE2O3 (RE = Y and Sm) multilayer films as a function of GDC thickness.
3.3. Ionic Conductivity of the Epitaxial GDC-RE2O3 (RE = Y and Sm) Multilayer Films
As discussed earlier, careful attention should be paid to the selection of substrates and counterpart materials to access the ionic conductivity of oxide multilayer thin films. If the counterpart materials or substrates have comparable resistance with the materials under study, then the measured ionic conductivity can be attributed to all components. Therefore, the resistance from the substrate and RE2O3 (RE = Sm and Y) without the GDC thin film layer should be confirmed to be at least 1 order of magnitude lower than that of the GDC thin film for meaningful measurements.32,33 In this study, we confirmed that the ionic conductivities of the substrates and RE2O3 (RE = Sm and Y) were at least 3 orders of magnitude lower than that of bulk GDC (Figure S1).34 In addition, the resistances of the substrates and RE2O3 were more than 1 order of magnitude larger than that of the GDC thin films.
Figure 4a shows the Arrhenius plots of the conductivity measured in the temperature range of 450 to 750 °C in air for the GDC-RE2O3 (RE = Sm and Y) multilayer films, as well as the strain-relaxed GDC single layer as a reference deposited epitaxially on Al2O3. The ionic conductivity of the GDC-RE2O3 (RE = Sm and Y) multilayer films did not change with oxygen partial pressure. This result indicates that the conductivity of the GDC-RE2O3 (RE = Sm and Y) multilayer films was attributed to the ionic conductivity within our test range (Figure S2).35 An increase in the ionic conductivity of GDC by tensile strain in the GDC-Sm2O3 multilayer films was observed. By decreasing the thickness of GDC in the GDC-Sm2O3 multilayer films, the tensile strain increased, resulting in an approximate twofold enhancement in the ionic conductivity of GDC compared to a GDC single layer. While the strain-enhanced ionic conductivity was not significant, the enhancement in the in-plane ionic conductivity of doped ionic conductors by lattice strain is generally less than 1 order of magnitude due to the structural limitations for coherency strain.10,20,23,36−41 Interestingly, decreasing the GDC thickness resulted in a slight increase in the activation energy from 0.7 to 0.8 eV, which is mainly attributed to the distortion of the strained lattice.33,42,43 The GDC-Y2O3 multilayer films with compressive strain exhibited a reduction in the ionic conductivity, resulting in an approximate twofold decrement compared to a GDC single layer. However, for GDC-Y2O3 multilayer films with a GDC thickness below ∼50 nm, the compressive strain did not significantly affect ionic conductivity and activation energy, which is consistent with the observation of negligible changes in compressive strain with varying GDC thickness as discussed in Figure 3b.
Figure 4.
Temperature dependence of the ionic conductivity of (a) GDC-RE2O3 (RE = Sm and Y) multilayer films. The ionic conductivity of GDC single layer (relaxed) is also plotted for comparison. (b) Ionic conductivity comparison between (111)- and (001)-oriented GDC films.
3.4. Effect of Crystallographic Orientations on Ionic Conductivity
In addition to tuning epitaxial strain, controlling crystallographic orientations of oxides can be another promising approach to enhance ionic conductivity since oxygen transport and defect formation energies can be altered depending on the crystallographic orientations.44,45 The orientation of epitaxial films is mainly dependent upon that of the substrates. In contrast to single-crystal (0001) Al2O3, which resulted in strained (111)-oriented epitaxial GDC films, strain-relaxed, (001)-oriented epitaxial GDC films were grown on single-crystal (110) NGO substrates (Figure S3). Details about the XRD analysis of (001)-oriented epitaxial GDC films can be found in the Supporting Information. We confirmed that the conductivity of the (001)-oriented GDC film was dominated by an ionic contribution within our tests (Figure S4). Interestingly, as shown in Figure 4b, the ionic conductivity of the (001)-oriented GDC film was higher than that of the (111)-oriented GDC film, most likely due to the different migration paths for oxygen vacancies that vary with the crystallographic plane.33,44,46 This result is further supported by the fact that the activation energy required for the migration of oxygen vacancies is lower in the ⟨001⟩ direction than in the ⟨111⟩ direction.44 Consequently, controlling the in-plane strain in (001)-oriented GDC films is expected to result in enhanced ionic conductivity compared to the (111)-oriented GDC films,46 but further studies are needed to confirm this hypothesis.
3.5. Influence of the Number of Strained Interfaces on Ionic Conductivity
As discussed earlier, the structural limitations for coherency strain constrain the enhancement of the in-plane ionic conductivity of GDC. To overcome such limitations, increasing the number of strained interfaces by synthesizing oxide superlattices can be an approach.47,48 While oxide superlattices have shown great promise for creating unique materials properties,49−54 the effect of the increased number of interfaces on ionic conductivity is still under debate.20,22,47,48,55 To systematically investigate the effect of the number of interfaces on ionic conductivity, superlattices of [(GDC)1|(Sm2O3)1]n, where n is the number of GDC-Sm2O3 bilayers, were successfully synthesized with n equal to 1, 3, 6, and 20. The presence of distinct satellite peaks in the XRD θ–2θ pattern for n = 20 indicates the characteristic features of a superlattice structure (Figure S5), signifying the presence of well-defined and high-quality interfaces between the two constituent oxides.20,22 To keep the same amount of tensile strain on the GDC film, we only changed the number of layers and fixed the GDC thickness at 5 nm. Interestingly, no significant difference in the ionic conductivity was shown by increasing the number of interfaces from 1 to 20 (Figure 5). This observation confirms that the number of interfaces does not play a crucial role in determining the ionic conductivity of [(GDC)1|(Sm2O3)1]n superlattices, which is consistent with other studies.22,55 A plausible explanation for ionic conductivity that is independent of the number of interfaces may be made by considering the geometric constraints of planar thin films. Specifically, as the total thickness of the superlattice increases, the interfaces can be degraded due to the formation of misfit dislocations. Ultimately, this degradation can result in incoherent interface structures, which adversely affect the ionic conductivity of the system.55,56 This hypothesis can be supported by a slight decrease in the ionic conductivity when n = 20 in the [(GDC)1|(Sm2O3)1]n superlattices. Furthermore, the activation energies of the superlattices, ranging from 0.7 to 0.8 eV, were found to be randomly distributed without exhibiting a distinct trend. Further studies are needed to see if controlling the thickness of each layer to retain coherent interface structures can further enhance the ionic conductivity of [(GDC)1|(Sm2O3)1]n superlattices.
Figure 5.

Temperature dependence of the ionic conductivity of [(GDC)1|(Sm2O3)1]n superlattices. The ionic conductivity of the GDC single layer (relaxed) is also plotted for comparison.
3.6. Relation between Oxygen Vacancy Concentration and Strain
Tensile strain is generally known to decrease the energy required for the formation of oxygen vacancies, increasing the concentration of oxygen vacancies.7,15 This increase in oxygen vacancies is a major contributor to increased ionic conductivity in oxides.57,58 In order to examine the change of oxygen vacancies depending on strain, in situ HRXRD measurements were performed. Here, we calculated the unit cell volumes of GDC-RE2O3 (RE = Sm and Y) multilayer films because the unit cell volume of GDC changes with varying concentrations of oxygen vacancies.45,59 Based on the results of the temperature-dependent unit cell volume (Figure 6a), we calculated the thermal expansion coefficient (TEC) of the GDC single layer and GDC-RE2O3 (RE = Sm and Y) multilayer films (2.4 × 10–5–3.1 × 10–5 °C–1), which were found to be comparable with the previously reported GDC bulk TEC60 (1.25 × 10–5 °C–1). The unit cell volume of the GDC-Sm2O3 multilayer film under tensile strain was observed to be larger than that of the GDC single layer. Conversely, the unit cell volume of the GDC-Y2O3 multilayer film under compressive strain was found to be smaller. This observation strongly indicates that tensile strain promotes an increase in the concentration of oxygen vacancies, while compressive strain has the opposite effect, reducing their concentration. The increased oxygen vacancies by tensile strain are further supported by the results of Raman spectroscopy measurements (Figure 6b). The peak of the tensile strained GDC-Sm2O3 multilayer film shifted to lower energy and became broader compared to relaxed GDC single layer and compressively strained GDC-Y2O3 multilayer film. Previous studies demonstrated a similar trend in the peak shift to lower Raman energy and broadening with increased oxygen vacancy concentration in GDC.61,62 Thus, our in situ HRXRD and Raman spectroscopy measurements confirmed that the increased concentration of oxygen vacancies resulting from tensile strain in GDC-RE2O3 (RE = Sm and Y) multilayer films can enable enhanced ionic conductivity.
Figure 6.
(a) Unit cell volume change as a function of temperature of GDC-RE2O3 (RE = Sm and Y) multilayer films and GDC single layer obtained from in situ HRXRD measured from 25 to 700 °C in air. (b) Raman peak shift of GDC-RE2O3 (RE = Sm and Y) multilayer films and GDC single layer obtained from Raman spectroscopy measurements.
4. Conclusions
In summary, we successfully synthesized multilayer thin films composed of ionic conducting GDC and insulating rare-earth oxides RE2O3 (RE = Y and Sm), demonstrating the unambiguous effect of strain on ionic conductivity. Compared to compressively strained GDC-Y2O3 multilayer films, tensile strained GDC-Sm2O3 multilayer films exhibited improved ionic conductivity. The creation of more oxygen vacancies induced by tensile strain, as confirmed by in situ HRXRD and Raman spectroscopy results, was responsible for the enhancement of ionic conductivity. However, there was no significant difference in the ionic conductivity by increasing the number of interfaces in GDC-Sm2O3 superlattices. We also showed that (001)-oriented GDC films were beneficial for enhancing ionic conductivity compared to (111)-oriented GDC films. Our results highlight the importance of properly designing oxide multilayers to identify the interfacial strain effect on ionic conductivity and the potential of forming coherent interfacial strain to tune the ionic conductivity.
Acknowledgments
Film synthesis and in situ HRXRD measurements were conducted as part of a user project at the Center for Nanophase Materials Sciences (CNMS), which is a US Department of Energy, Office of Science User Facility at Oak Ridge National Laboratory.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsaelm.3c00724.
Ionic conductivity measurements and high-resolution X-ray diffraction (HRXRD) (PDF)
Author Contributions
G.Y.: Methodology, investigation, formal analysis, visualization, writing—original draft; M.E.L.: Methodology, investigation, writing—review and editing; H.R.C.: Investigation, writing—review and editing; J.K.: Investigation, writing—review and editing; J.K.K.: Visualization, writing—review and editing; C.M.R.: Synthesis environment, visualization, writing—review and editing; D.L.: Supervision, conceptualization, methodology, writing—review and editing.
This research was funded by the U.S. Department of Energy (DOE), Office of Science (OS), Basic Energy Sciences (BES), grant number DE-SC0021363, and National Science Foundation (grant number 2110033).
The authors declare no competing financial interest.
Supplementary Material
References
- Shao Z. P.; Haile S. M. A High-performance Cathode for the Next Generation of Solid-oxide Fuel Cells. Nature 2004, 431, 170–173. 10.1038/nature02863. [DOI] [PubMed] [Google Scholar]
- Wachsman E. D.; Lee K. T. Lowering the Temperature of Solid Oxide Fuel Cells. Science 2011, 334, 935–939. 10.1126/science.1204090. [DOI] [PubMed] [Google Scholar]
- Dunn B.; Kamath H.; Tarascon J. M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928–935. 10.1126/science.1212741. [DOI] [PubMed] [Google Scholar]
- Tarascon J. M.; Armand M. Issues and Challenges Facing Rechargeable Lithium Batteries. Nature 2001, 414, 359–367. 10.1038/35104644. [DOI] [PubMed] [Google Scholar]
- Hendriksen P. V.; Larsen P. H.; Mogensen M.; Poulsen F. W.; Wiik K. Prospects and Problems of Dense Oxygen Permeable Membranes. Catal. Today 2000, 56, 283–295. 10.1016/S0920-5861(99)00286-2. [DOI] [Google Scholar]
- Voskresenskaya E. N.; Roguleva V. G.; Anshits A. G. Oxidant Activation Over Structural Defects of Oxide Catalysts in Oxidative Methane Coupling. Catal. Rev. 1995, 37, 101–143. 10.1080/01614949508007092. [DOI] [Google Scholar]
- De Souza R. A.; Ramadan A.; Hörner S. Modifying the Barriers for Oxygen-vacancy Migration in Fluorite-structured CeO2 Electrolytes Through Strain: A Computer Simulation Study. Energy Environ. Sci. 2012, 5, 5445–5453. 10.1039/C2EE02508F. [DOI] [Google Scholar]
- Lee D.; Lee H. Controlling Oxygen Mobility in Ruddlesden–Popper Oxides. Materials 2017, 10, 368 10.3390/ma10040368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp J. L. M. Ionic Diffusion as a Matter of Lattice-strain for Electroceramic Thin Films. Solid State Ionics 2012, 207, 1–13. 10.1016/j.ssi.2011.09.009. [DOI] [Google Scholar]
- Korte C.; Peters A.; Janek J.; Hesse D.; Zakharov N. Ionic Conductivity and Activation Energy for Oxygen Ion Transport in Superlattices-The Semicoherent Multilayer System YSZ (ZrO2 + 9.5 mol% Y2O3)/Y2O3. Phys. Chem. Chem. Phys. 2008, 10, 4623–4635. 10.1039/b801675e. [DOI] [PubMed] [Google Scholar]
- Guo X. X.; Maier J. Ionically Conducting Two-Dimensional Heterostructures. Adv. Mater. 2009, 21, 2619–2631. 10.1002/adma.200900412. [DOI] [PubMed] [Google Scholar]
- Morgan D.; Mayeshiba T. (Invited) Ab Initio Studies of Strain Effects on Perovskite Oxygen Vacancy Formation and Migration Energetics. ECS Meet. Abstr. 2016, MA2016-01, 1489 10.1149/MA2016-01/30/1489. [DOI] [Google Scholar]
- Lee D.; Jacobs R.; Jee Y.; Seo A.; Sohn C.; Ievlev A. V.; Ovchinnikova O. S.; Huang K.; Morgan D.; Lee H. N. Stretching Epitaxial La0.6Sr0.4CoO3−δ for Fast Oxygen Reduction. J. Phys. Chem. C 2017, 121, 25651–25658. 10.1021/acs.jpcc.7b06374. [DOI] [Google Scholar]
- Herklotz A.; Lee D.; Guo E. J.; Meyer T. L.; Petrie J. R.; Lee H. N. Strain Coupling of Oxygen Non-stoichiometry in Perovskite Thin Films. J. Phys.: Condens. Matter 2017, 29, 493001 10.1088/1361-648X/aa949b. [DOI] [PubMed] [Google Scholar]
- Aidhy D. S.; Liu B.; Zhang Y. W.; Weber W. J. Strain-Induced Phase and Oxygen-Vacancy Stability in Ionic Interfaces from First-Principles Calculations. J. Phys. Chem. C 2014, 118, 30139–30144. 10.1021/jp507876m. [DOI] [Google Scholar]
- Zhang Y. W.; Jin S.; Yang Y.; Li G. B.; Tian S. J.; Jia J. T.; Liao C. S.; Yan C. H. Electrical Conductivity Enhancement in Nanocrystalline (RE2O3)0.08(ZrO2)0.92 (RE = Sc, Y) Thin Films. Appl. Phys. Lett. 2000, 77, 3409–3411. 10.1063/1.1328099. [DOI] [Google Scholar]
- Kim S. M.; Son J. W.; Lee K. R.; Kim H.; Kim H. R.; Lee H. W.; Lee J. H. Substrate Effect on the Electrical Properties of Sputtered YSZ Thin Films for Co-planar SOFC Applications. J. Electroceram. 2010, 24, 153–160. 10.1007/s10832-008-9550-y. [DOI] [Google Scholar]
- Chen L.; Chen C.; Huang D.; Lin Y.; Chen X.; Jacobson A. High Temperature Electrical Conductivity of Epitaxial Gd-doped CeO2 Thin Films. Solid State Ionics 2004, 175, 103–106. 10.1016/j.ssi.2004.09.034. [DOI] [Google Scholar]
- Shen W.; Jiang J.; Ni C.; Voras Z.; Beebe T. P.; Hertz J. L. Two-dimensional Vacancy Trapping in Yttria Doped Ceria. Solid State Ionics 2014, 255, 13–20. 10.1016/j.ssi.2013.11.012. [DOI] [Google Scholar]
- Sanna S.; Esposito V.; Tebano A.; Licoccia S.; Traversa E.; Balestrino G. Enhancement of Ionic Conductivity in Sm-doped Ceria/Yttria-stabilized Zirconia Heteroepitaxial Structures. Small 2010, 6, 1863–1867. 10.1002/smll.200902348. [DOI] [PubMed] [Google Scholar]
- Garcia-Barriocanal J.; Rivera-Calzada A.; Varela M.; Sefrioui Z.; Iborra E.; Leon C.; Pennycook S. J.; Santamaria J. Colossal Ionic Conductivity at Interfaces of Epitaxial ZrO2: Y2O3/SrTiO3 Heterostructures. Science 2008, 321, 676–680. 10.1126/science.1156393. [DOI] [PubMed] [Google Scholar]
- Pergolesi D.; Fabbri E.; Cook S. N.; Roddatis V.; Traversa E.; Kilner J. A. Tensile Lattice Distortion Does Not Affect Oxygen Transport in Yttria-Stabilized Zirconia–CeO2 Heterointerfaces. ACS Nano 2012, 6, 10524–10534. 10.1021/nn302812m. [DOI] [PubMed] [Google Scholar]
- Aydin H.; Korte C.; Rohnke M.; Janek J. Oxygen Tracer Diffusion Along Interfaces of Strained Y2O3/YSZ Multilayers. Phys. Chem. Chem. Phys. 2013, 15, 1944–1955. 10.1039/C2CP43231E. [DOI] [PubMed] [Google Scholar]
- Lee S.; Zhang W.; Khatkhatay F.; Jia Q.; Wang H.; MacManus-Driscoll J. L. Strain Tuning and Strong Enhancement of Ionic Conductivity in SrZrO3–RE2O3 (RE = Sm, Eu, Gd, Dy, and Er) Nanocomposite Films. Adv. Funct. Mater. 2015, 25, 4328–4333. 10.1002/adfm.201404420. [DOI] [Google Scholar]
- Gaboriaud R. J.; Pailloux F.; Guerin P.; Paumier F. Yttrium Oxide Thin Films, Y2O3, Grown by Ion Beam Sputtering on Si. J. Phys. D: Appl. Phys. 2000, 33, 2884–2889. 10.1088/0022-3727/33/22/304. [DOI] [Google Scholar]
- Wang B.; Lewis R. J.; Cormack A. N. Computer Simulations of Large-scale Defect Clustering and Nanodomain Structure in Gadolinia-doped Ceria. Acta Mater. 2011, 59, 2035–2045. 10.1016/j.actamat.2010.12.003. [DOI] [Google Scholar]
- Schichtel N.; Korte C.; Hesse D.; Janek J. Elastic Strain at Interfaces and its Influence on Ionic Conductivity in Nanoscaled Solid Electrolyte Thin Films—Theoretical Considerations and Experimental Studies. Phys. Chem. Chem. Phys. 2009, 11, 3043–3048. 10.1039/b900148d. [DOI] [PubMed] [Google Scholar]
- Jhang J. H.; Schaefer A.; Cartas W.; Epuri S.; Bäumer M.; Weaver J. F. Growth and Partial Reduction of Sm2O3(111) Thin Films on Pt(111): Evidence for the Formation of SmO(100). J. Phys. Chem. C 2013, 117, 21396–21406. 10.1021/jp4074416. [DOI] [Google Scholar]
- Guo Q.; Zhao Y.; Jiang C.; Mao W. L.; Wang Z. Phase Transformation in Sm2O3 at High Pressure: In situ Synchrotron X-ray Diffraction Study and ab initio DFT Calculation. Solid State Commun. 2008, 145, 250–254. 10.1016/j.ssc.2007.11.019. [DOI] [Google Scholar]
- Shen W.; Jiang J.; Hertz J. L. Beneficial Lattice Strain in Heterogeneously Doped Ceria. J. Phys. Chem. C 2014, 118, 22904–22912. 10.1021/jp506554z. [DOI] [Google Scholar]
- Jiang J.; Shen W.; Hertz J. L. Fabrication of Epitaxial Zirconia and Ceria Thin Films with Arbitrary Dopant and Host Atom Composition. Thin Solid Films 2012, 522, 66–70. 10.1016/j.tsf.2012.09.013. [DOI] [Google Scholar]
- Kim H. R.; Kim J. C.; Lee K. R.; Ji H. I.; Lee H. W.; Lee J. H.; Son J. W. ‘Illusional’ Nano-size Effect Due To Artifacts of In-plane Conductivity Measurements of Ultra-Thin Films. Phys. Chem. Chem. Phys. 2011, 13, 6133–6137. 10.1039/c0cp02673e. [DOI] [PubMed] [Google Scholar]
- Lee K. R.; Ahn K.; Chung Y. C.; Lee J. H.; Yoo H. I. Lattice Distortion Effect on Electrical Properties of GDC Thin Films: Experimental Evidence and Computational Simulation. Solid State Ionics 2012, 229, 45–53. 10.1016/j.ssi.2012.10.007. [DOI] [Google Scholar]
- Steele B. C. H. Appraisal of Ce1–yGdyO2–y/2 Electrolytes for IT-SOFC Operation at 500 °C. Solid State Ionics 2000, 129, 95–110. 10.1016/S0167-2738(99)00319-7. [DOI] [Google Scholar]
- Mogensen M.; Sammes N. M.; Tompsett G. A. Physical, Chemical and Electrochemical Properties of Pure and Doped Ceria. Solid State Ionics 2000, 129, 63–94. 10.1016/S0167-2738(99)00318-5. [DOI] [Google Scholar]
- Korte C.; Keppner J.; Peters A.; Schichtel N.; Aydin H.; Janek J. Coherency Strain and its Effect on Ionic Conductivity and Diffusion in Solid Electrolytes – An Improved Model for Nanocrystalline Thin Films and a Review of Experimental Data. Phys. Chem. Chem. Phys. 2014, 16, 24575–24591. 10.1039/C4CP03055A. [DOI] [PubMed] [Google Scholar]
- Schichtel N.; Korte C.; Hesse D.; Zakharov N.; Butz B.; Gerthsen D.; Janek J. On the Influence of Strain on Ion Transport: Microstructure and Ionic Conductivity of Nanoscale YSZ| Sc2O3 Multilayers. Phys. Chem. Chem. Phys. 2010, 12, 14596–14608. 10.1039/c0cp01018a. [DOI] [PubMed] [Google Scholar]
- Li B.; Zhang J.; Kaspar T.; Shutthanandan V.; Ewing R. C.; Lian J. Multilayered YSZ/GZO Gilms with Greatly Enhanced Ionic Conduction for Low Temperature Solid Oxide Fuel Cells. Phys. Chem. Chem. Phys. 2013, 15, 1296–1301. 10.1039/C2CP42964K. [DOI] [PubMed] [Google Scholar]
- Aydin H.; Korte C.; Janek J. 18O-tracer Diffusion Along Nanoscaled Sc2O3/Yttria Stabilized Zirconia (YSZ) Multilayers: On the Influence of Strain. Sci. Technol. Adv. Mater. 2013, 14, 035007 10.1088/1468-6996/14/3/035007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte C.; Schichtel N.; Hesse D.; Janek J. Influence of Interface Structure on Mass Transport in Phase Boundaries between Different Ionic Materials. Monatsh. Chem. Chem. Mon. 2009, 140, 1069–1080. 10.1007/s00706-009-0125-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karthikeyan A.; Ramanathan S. Temperature-dependent Interfacial Carrier Transport in Low-dimensional Oxides Using Ionic Conductor-insulator (YDZ-SiO2) Superlattices. J. Appl. Phys. 2008, 104, 124314 10.1063/1.3031220. [DOI] [Google Scholar]
- Zhang J.; Liang E. J.; Sun Q.; Jia Y. Oxygen Vacancy Formation and Migration in Sr-and Mg-Doped LaGaO3: A Density Functional Theory Study. Chin. Phys. B 2012, 21, 047201 10.1088/1674-1056/21/4/047201. [DOI] [Google Scholar]
- Omar S.; Wachsman E. D.; Jones J. L.; Nino J. C. Crystal Structure–Ionic Conductivity Relationships in Doped Ceria Systems. J. Am. Ceram. Soc. 2009, 92, 2674–2681. 10.1111/j.1551-2916.2009.03273.x. [DOI] [Google Scholar]
- Dholabhai P. P.; Adams J. B.; Crozier P.; Sharma R. A Density Functional Study of Defect Migration in Gadolinium Doped Ceria. Phys. Chem. Chem. Phys. 2010, 12, 7904–7910. 10.1039/b924534k. [DOI] [PubMed] [Google Scholar]
- Ahn K.; Chung Y. C.; Yoon K. J.; Son J. W.; Kim B. K.; Lee H. W.; Lee J. H. Lattice-Strain Effect on Oxygen Vacancy Formation in Gadolinium-Doped Ceria. J. Electroceram. 2014, 32, 72–77. 10.1007/s10832-013-9844-6. [DOI] [Google Scholar]
- Hinterberg J.; Zacherle T.; De Souza R. A. Activation Volume Tensor for Oxygen-Vacancy Migration in Strained CeO2 Electrolytes. Phys. Rev. Lett. 2013, 110, 205901 10.1103/PhysRevLett.110.205901. [DOI] [PubMed] [Google Scholar]
- Azad S.; Marina O. A.; Wang C. M.; Saraf L.; Shutthanandan V.; McCready D. E.; El-Azab A.; Jaffe J. E.; Engelhard M. H.; Peden C. H. F.; Thevuthasan S. Nanoscale Effects on Ion Conductance of Layer-by-Layer Structures of Gadolinia-Doped Ceria and Zirconia. Appl. Phys. Lett. 2005, 86, 131906 10.1063/1.1894615. [DOI] [Google Scholar]
- Peters A.; Korte C.; Hesse D.; Zakharov N.; Janek J. Ionic Conductivity and Activation Energy for Oxygen Ion Transport in Superlattices—The Multilayer System CSZ (ZrO2+ CaO)/Al2O3. Solid State Ionics 2007, 178, 67–76. 10.1016/j.ssi.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Ohtomo A.; Hwang H. Y. A High-Mobility Electron Gas at the LaAlO3/SrTiO3 Heterointerface. Nature 2004, 427, 423–426. 10.1038/nature02308. [DOI] [PubMed] [Google Scholar]
- Bousquet E.; Dawber M.; Stucki N.; Lichtensteiger C.; Hermet P.; Gariglio S.; Triscone J. M.; Ghosez P. Improper Ferroelectricity in Perovskite Oxide Artificial Superlattices. Nature 2008, 452, 732–736. 10.1038/nature06817. [DOI] [PubMed] [Google Scholar]
- Okamoto S.; Millis A. J. Electronic Reconstruction at an Interface between a Mott Insulator and a Band Insulator. Nature 2004, 428, 630–633. 10.1038/nature02450. [DOI] [PubMed] [Google Scholar]
- Mannhart J.; Schlom D. G. Oxide Interfaces—An Opportunity for Electronics. Science 2010, 327, 1607–1611. 10.1126/science.1181862. [DOI] [PubMed] [Google Scholar]
- Chakhalian J.; Freeland J. W.; Srajer G.; Strempfer J.; Khaliullin G.; Cezar J. C.; Charlton T.; Dalgliesh R.; Bernhard C.; Cristiani G.; Habermeier H.-U.; Keimer B. Magnetism at the Interface between Ferromagnetic and Superconducting Oxides. Nat. Phys. 2006, 2, 244–248. 10.1038/nphys272. [DOI] [Google Scholar]
- Tokura Y.; Nagaosa N. Orbital Physics in Transition-Metal Oxides. Science 2000, 288, 462–468. 10.1126/science.288.5465.462. [DOI] [PubMed] [Google Scholar]
- Shen W.; Hertz J. L. Ionic Conductivity of YSZ/CZO Multilayers with Variable Lattice Mismatch. J. Mater. Chem. A 2015, 3, 2378–2386. 10.1039/C4TA03892D. [DOI] [Google Scholar]
- Baiutti F.; Chiabrera F.; Acosta M.; Diercks D.; Parfitt D.; Santiso J.; Wang X.; Cavallaro A.; Morata A.; Wang H.; Chroneos A.; MacManus-Driscoll J.; Tarancon A. A High-Entropy Manganite in an Ordered Nanocomposite for Long-Term Application in Solid Oxide Cells. Nat. Commun. 2021, 12, 2660 10.1038/s41467-021-22916-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H.; Gür T. M.; Saito Y.; Prinz F. High Ionic Conductivity in Ultrathin Nanocrystalline Gadolinia-Doped Ceria Films. Appl. Phys. Lett. 2006, 89, 143107 10.1063/1.2358851. [DOI] [Google Scholar]
- Tsuchiya M.; Bojarczuk N. A.; Ramanathan S. Molecular Beam Synthesis and High Temperature Electrical Properties of Crystalline Ceria Thin Films. Appl. Phys. Lett. 2007, 91, 223101 10.1063/1.2818666. [DOI] [Google Scholar]
- Bishop S. R.; Duncan K. L.; Wachsman E. D. Surface and Bulk Oxygen Non-stoichiometry and Bulk Chemical Expansion in Gadolinium-doped Cerium Oxide. Acta Mater. 2009, 57, 3596–3605. 10.1016/j.actamat.2009.04.017. [DOI] [Google Scholar]
- Tietz F. Thermal Expansion of SOFC Materials. Ionics 1999, 5, 129–139. 10.1007/BF02375916. [DOI] [Google Scholar]
- Sediva E.; Bohdanov D.; Harrington G. F.; Rafalovskyi I.; Drahokoupil J.; Borodavka F.; Marton P.; Hlinka J. Anisotropic Strain in Rare-Earth Substituted Ceria Thin Films Probed by Polarized Raman Spectroscopy and First-Principles Calculations. ACS Appl. Mater. Interfaces 2020, 12, 56251–56259. 10.1021/acsami.0c14249. [DOI] [PubMed] [Google Scholar]
- Acharya S. A.; Gaikwad V. M.; D’Souza S. W.; Barman S. R. Gd/Sm Dopant-Modified Oxidation State and Defect Generation in Nano-Ceria. Solid State Ionics 2014, 260, 21–29. 10.1016/j.ssi.2014.03.008. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.