Abstract
The development of new high-performing battery materials is critical for meeting the energy storage requirements of portable electronics and electrified transportation applications. Owing to their exceptionally high rate capabilities, high volumetric capacities, and long cycle lives, Wadsley–Roth compounds are promising anode materials for fast-charging and high-power lithium-ion batteries. Here, we present a study of the Wadsley–Roth-derived NaNb13O33 phase and examine its structure and lithium insertion behavior. Structural insights from combined neutron and synchrotron diffraction as well as solid-state nuclear magnetic resonance (NMR) are presented. Solid-state NMR, in conjunction with neutron diffraction, reveals the presence of sodium ions in perovskite A-site-like block interior sites as well as square-planar block corner sites. Through combined experimental and computational studies, the high rate performance of this anode material is demonstrated and rationalized. A gravimetric capacity of 225 mA h g–1, indicating multielectron redox of Nb, is accessible at slow cycling rates. At a high rate, 100 mA h g–1 of capacity is accessible in 3 min for micrometer-scale particles. Bond-valence mapping suggests that this high-rate performance stems from fast multichannel lithium diffusion involving octahedral block interior sites. Differential capacity analysis is used to identify optimal cycling rates for long-term performance, and an 80% capacity retention is achieved over 600 cycles with 30 min charging and discharging intervals. These initial results place NaNb13O33 within the ranks of promising new high-rate lithium-ion battery anode materials that warrant further research.
Introduction
As we transition from fossil fuels to low-carbon energy sources, faster charging, higher energy, and higher power lithium-ion batteries (LIBs) are required to support widespread electrification. LIBs typically use graphitic carbon as the anode material given its high lithium storage capacity, low cost, and low voltage vs Li+/Li, which leads to a high energy density when paired with a 4-V-class cathode material such as Li(Ni,Mn,Co,Al)O2.1,2 However, the low voltage of graphite lithiation relative to the lithium metal deposition potential can lead to electrolytic side reactions and lithium metal plating, the results of which are increased impedance, shortened battery lifetimes, and thermal runaway should lithium dendrites traverse the separator.3−5 These issues, which are especially problematic at high current densities, have motivated the search for alternative anode materials that operate between 1.0 and 2.0 V vs Li+/Li.6,7
Wadsley–Roth compounds are a family of (mostly) transition metal oxides that have garnered interest as fast-charging LIB anodes. Many of these compounds were first reported in the 1950s–1960s8−13 but were not considered as electrode materials until the seminal work of Cava et al.14−16 Research into Wadsley–Roth electrodes declined until two 2011 papers by Han, Goodenough, and colleagues demonstrated excellent rate performance, respectable capacities, and stable cycling with the Wadsley–Roth phase TiNb2O7 as a lithium intercalation anode.6,7 Wadsley–Roth compounds typically operate above 0.8 V vs Li+/Li and are thus not subject to lithium plating or deleterious side reactions with the electrolyte. They also tend to insert more than one lithium per transition metal with a minor volume expansion. This allows for large and reversible lithium storage capacities and good long-term electrochemomechanical stability. Finally, Wadsley–Roth anodes have demonstrated excellent high-rate performance.6,7,17−21
Wadsley–Roth compounds are characterized by m × n blocks of corner-sharing MO6 octahedra joined by edge-sharing octahedra in “shear” planes, as first described by Roth in 1965.9,10 Unlike one-dimensional shear structures and layered vacancy-ordered Wadsley–Roth derivatives that exhibit relatively poor capacity retention upon electrochemical cycling,22−24 Wadsley–Roth phases exhibit good stability upon repeated lithium (de)intercalation because of the structural rigidity and minimal volume expansion imparted by the shear planes.18,25−28 Moreover, a combination of experimental and computational studies on Wadsley–Roth phases have provided evidence for rapid, quasi-one-dimensional lithium diffusion down parallel octahedral block channels paired with facile interchannel hopping, which helps to redistribute lithium and prevent blockages.26,29,30 This combination of long-range diffusion and short-range redistribution accounts for the extremely high lithium diffusion coefficients reported for Wadsley–Roth electrodes, which are on par with those of lithium-conducting solid electrolytes.18,19,31 Additional studies have shown good electronic conductivity in Wadsley–Roth phases once they are partially reduced by either oxygen loss or lithium insertion.14,29,32−34 This rapid intercalation behavior has been observed in micrometer-scale particles, indicating that Wadsley–Roth phases do not require nanostructuring for high-rate performance.
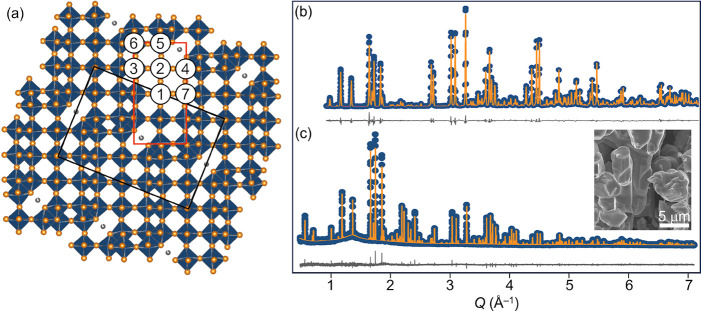
The present study comprises an in-depth structural and initial electrochemical investigation of the Wadsley–Roth-like phase NaNb13O33. The structure of this composition within the Nb2O5–Na2O solid solution system was first reported in 1965.35 Its block-defect structure and sodium conductivity were investigated in 1984,36 and its thermodynamic stability has been the subject of several reports.37−39 This compound deviates from the typical Wadsley–Roth structure in that transition-metal-centered octahedra are missing from the corners of the blocks, and instead, sodium ions occupy square planar sites. This is a relatively rare Na+ coordination that results in large rectangular tunnels connecting the Na sites. Following the (m × n)∞ Wadsley–Roth nomenclature where m and n are the block dimensions in units of octahedra and the subscript denotes the connectivity of the blocks in the block plane, we propose that the deviation in NaNb13O33 should be denoted (5×3–2)∞ (Figure 1a).
Figure 1.
(a) Schematic of the NaNb13O33 crystal structure highlighting the (5×3–2)∞ blocks of NbO6 octahedra and each unique Nb site. (b) Neutron powder diffraction of 1-NNO obtained at 300 K, λc = 1.5 Å. (c) Synchrotron X-ray powder diffraction of 1-NNO at 300 K, λc = 0.45788 Å. The diffuse background feature is due to the Kapton capillary sample holder. The inset shows an SEM image of pristine 1-NNO particles
We report structural insights from combined neutron and synchrotron diffraction of NaNb13O33 along with 23Na solid-state nuclear magnetic resonance spectroscopy. A new picture of the atomic-scale defect structure emerges with sodium cations occupying not only the square-planar sites but also perovskite-like sites within the block interiors. Density functional theory calculations of the electronic structure and bond-valence sum mapping of the electrostatic landscape suggest good electronic and ionic conductivity. This is confirmed by the electrochemical behavior of this material in Li half-cell batteries, which reveal excellent rate capability and long-term cyclability with micrometer-sized NaNb13O33 particles. Finally, galvanostatic cycling suggests Nb multielectron redox, which enables large amounts of lithium to be stored in this promising new anode material.
Methods
Solid-State Synthesis of NaNb13O33
NaNb13O33 was prepared by multiple independent solid-state methods. In the first preparation method, resulting in the sample hereafter termed 1-NNO, Nb2O5 (CBMM, 99.8%, white powder, 1:1 T-phase:H-phase) and Na2CO3 (Sigma, 99.95–100.05%, anhydrous, white powder, dried at 250 °C for 8 h) were hand-mixed and ground in an agate mortar and pestle for 10 min before being pressed into two 5 g, 25.4 mm diameter pellets at 300 MPa. The pellets were placed in a Pt crucible and fired in a three-stage heating program, all with 5 °C min–1 heating rates, at 400 °C for 6 h, 820 °C for 12 h, and 1150 °C for 18 h before being cooled in the furnace under zero power. Note that the sample prepared for synchrotron diffraction used a single heating step at 1150 °C for 24 h.
In the second solid-state preparation method, which resulted in the sample termed 2-NNO, NaNbO3 was first prepared by hand-grinding a 1:1 molar ratio of Nb2O5 (Materion, ≥99%) and Na2CO3 (Sigma, ≥99%) in an agate mortar and pestle for 20 min. The resulting mixture was pressed into 700 mg, 13 mm pellets under 3.5 t of pressure. Pellets were placed on a bed of sacrificial powder within an alumina crucible, heated to 1100 °C at a rate of 10 °C min–1, sintered for 12 h, and then air-quenched. The same grinding, pelletizing, heating, and quenching procedure was then repeated to produce NaNb13O33 from a hand-ground mixture of NaNbO3 and Nb2O5 in a 1:6 molar ratio.
Scanning Electron Microscopy (SEM)
To characterize the morphology and size of 1- and 2-NNO particles, NaNb13O33 pellets were hand-ground for approximately 10 min in an agate mortar and pestle. The powdered samples were then pressed onto double-sided carbon tape and measured with a Hitachi S4800 (1-NNO) or an Apreo C FEG (ThermoFisher) (2-NNO) microscope. SEM images were collected using secondary electron (SE) detection with 10 kV accelerating voltage.
Diffraction
Synchrotron X-ray powder diffraction (SXRPD) data were collected on both 1- and 2-NNO at 300 K on the 11-BM beamline at the Advanced Photon Source at Argonne National Laboratory with a wavelength λ = 0.45788 Å. Time-of-flight neutron powder diffraction (TOF-NPD) data of 1-NNO were obtained on the POWGEN diffractometer at the Spallation Neutron Source (SNS) located at Oak Ridge National Laboratory.40 Approximately 10 g of NaNb13O33 was loaded into an 8 mm diameter cylindrical vanadium can. Data were collected on POWGEN detector bank 2 with a center wavelength of 1.5 Å at 20 and 300 K with scan times of 75 and 95 min, respectively, and detector bank 3 with a center wavelength of 2.665 Å at 300 K for 5 min. Combined Rietveld analysis on SXRPD and TOF-NPD data was performed using GSAS-II.41 Fitting information and refined patterns of both samples are included in the Supporting Information (S1–S2).
Nuclear Magnetic Resonance (NMR)
To better understand the local structure of as-prepared NaNb13O33, magic-angle spinning (MAS) solid-state 23Na NMR was performed on 1- and 2-NNO compounds at room temperature.
Solid-state 23Na MAS NMR spectra were acquired on 1-NNO using a Bruker AVANCE III 400 MHz (9.4 T) spectrometer and a 1.6 mm Phoenix HX MAS probe or a 4 mm Bruker HX MAS probe. The 1-NNO sample was packed into zirconia rotors in air and rotated about the magic angle at 12.5 kHz to obtain high signal-to-noise spectra using a 4 mm rotor and at 38 kHz for the variable-temperature study using a 1.6 mm rotor. One-pulse spectra were collected using an rf pulse of either 0.8 μs (1.6 mm probe) or 1.3 μs (4 mm probe), corresponding to half the length of a liquid-state 23Na π/2 pulse to account for the doubling of the nutation frequency of this spin-3/2 nucleus in the solid state. T1 values were measured with a saturation–recovery pulse sequence, and recycle delays were subsequently set to 5 × T1. For comparison, spectra of NaNbO3 and Na13Nb35O94 were obtained using similar data acquisition parameters. 23Na shifts of 1-NNO, NaNbO3, and Na13Nb35O94 were referenced to aqueous 1.0 M NaCl at 0 ppm. Actual sample temperatures during variable-temperature MAS NMR data acquisition differ from the value measured by the thermocouple due to frictional heating of the sample. The sample temperature was externally calibrated by leveraging the temperature dependence of the 207Pb shift of a lead nitrate reference sample.42,43 A solid-state 23Na MAS NMR spectrum of the 2-NNO material was also acquired, as detailed in the Supporting Information.
Electronic Structure and Bond Valence Sum Calculations
To understand the electronic properties of the host structure, density functional theory (DFT) calculations were performed using the Vienna Ab initio Simulation Project (VASP)44−46 code, projector augmented-wave (PAW) pseudopotentials,47,48 and the general-gradient-approximation Perdew–Burke–Ernzerhof (PBE)49 functional. Structural models were obtained from a joint refinement of 1-NNO neutron and synchrotron XRD diffraction patterns obtained at 20 K. Lattice parameters and atomic positions were optimized by using a plane-wave energy cutoff of 500 eV and a k-point mesh with a length-density parameter of Rk = 25 Å, corresponding to a 7 × 2 × 2 Γ-centered mesh. Structural relaxation was followed by static calculations of the projected density of states (DOS) and electronic band structure, which was postprocessed using the Sumo package.50
To estimate the pathways for ion intercalation in the three-dimensional NaNb13O33 host structure, bond valence sum mapping was performed with either Li+ or Na+ as the mobile ion using softBV-GUI ver. 1.2.7.51−53 Energy barriers for ion hopping within the NaNb13O33 unit cell were visualized in VESTA54 using an isosurface energy cutoff of 0.1 valence units.
Finally, DFT, as implemented in plane wave code CASTEP v19.11, was used to compute the quadrupolar tensor of NaNb13O33. The calculations were performed using “on-the-fly” ultrasoft pseudopotentials and the PBE exchange–correlation functional.49,55−57 The plane-wave basis set was truncated at an energy cutoff of 700 eV, and integration over reciprocal space was performed using a 2 × 7 × 2 Monkhorst–Pack grid.58 Structures were geometry optimized prior to NMR calculations.59−61 Spectral simulations of the calculated quadrupolar tensor were performed with the Solid Lineshape Analysis (SOLA) tool in TopSpin v4.0.9.
Electrochemical Characterization
Electrodes composed of 2-NNO, Super P carbon black, and poly(vinylidene difluoride) (PVDF) were fabricated in a weight ratio of 80:10:10. Pristine 2-NNO was blended with carbon black (MTI Super P) in a mortar and pestle and mixed into a solution of PVDF (MTI) in N-methyl-2-pyrrolidone (NMP, Sigma-Aldrich, 40 g L–1) to produce a slurry. The slurry was cast onto copper foil using a doctor blade, giving a coating thickness of approximately 40 μm. The resulting film was vacuum-dried at 80 °C overnight and punched into 14 mm diameter discs, resulting in approximate active material mass loadings of 2.0–5.5 mg cm–2. The electrodes were incorporated into CR2032 coin cells from MTI in an argon-filled glovebox, with polished lithium metal (MTI) as a counter electrode and glass fiber separators (Whatman GF/C). An electrolyte of 1.0 M lithium bis(trifluoromethanesulfonyl)imide (LiTFSI, Sigma-Aldrich) dissolved in 1:1 v/v ethylene carbonate (EC, Sigma-Aldrich) and dimethyl carbonate (DMC, Sigma-Aldrich) was added to the cell in a ratio of 25 μL of electrolyte per milligram of active material. Half-cells of 2-NNO electrodes vs sodium metal were fabricated in a similar manner, as detailed in the Supporting Information.
On the basis of reduction of Nb5+ to Nb4+, 13 Li or Na ions inserted into NaNb13O33 would yield a theoretical capacity of 198 mA h g–1 based on the molecular mass of the pristine compound as is standard for anode materials. Cycling rate is defined as C/n, where n is the number of hours to complete a single charge or discharge. Therefore, a C/10 rate corresponds to the current density needed to fully charge or discharge to the theoretical capacity in 10 h or a current density of 19.8 mA g–1. All electrochemical experiments were performed using Biologic potentiostats.
Results and Discussion
Characterization of the NaNb13O33 Host Structure
Scanning electron microscopy (SEM) images of pristine 1-NNO show particles on the order of 5–50 μm, while SEM images of 2-NNO show particles on the order of 1–10 μm (Figure 1 inset and Figure S4). Synchrotron powder X-ray diffraction (SXPRD) and neutron powder diffraction (NPD) patterns were collected and jointly refined to examine phase purity, octahedral distortions, and sodium position(s) and occupancy(ies) in NaNb13O33. X-ray diffraction confirms the phase purity of the NaNb13O33 samples prepared using the two synthesis methods (Figures 1c and S2). Neutron diffraction gives atomic displacement parameters (ADPs) for each Nb and Na site within the structure at 20 and 300 K (Figure 1b). Square-planar Na shows a relatively large atomic displacement parameter (ADP) at room temperature, indicating greater motion of the Na+ ions around their nominal positions in the crystal structure. ADPs and second-order Jahn–Teller distortion parameters for the NbO6 octahedra are listed in Table S1.
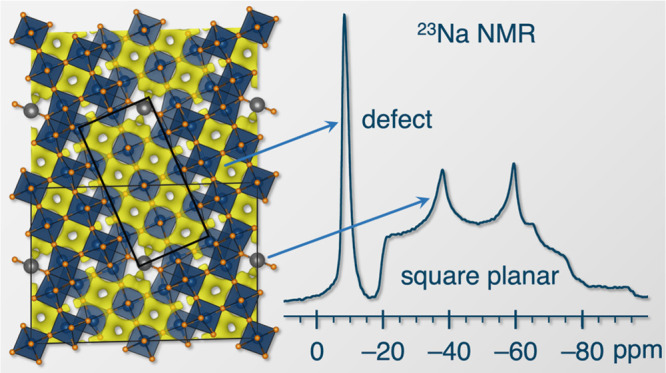
23Na solid-state MAS NMR of NaNb13O33 revealed two environments (Figure 2, black curve): a sharp resonance at −8 ppm and a broad resonance centered at −50 ppm, with respective relative integrated intensities of 16:84. The T1 relaxation times of the high- and low-frequency components are 3.1 and 0.91 s, respectively. The relaxation difference is sensible given that the low-frequency resonance has a larger quadrupolar interaction, and quadrupolar relaxation is expected to be the dominant relaxation mechanism for 23Na in diamagnetic NaNb13O33. This low-frequency resonance, with an isotropic shift of −15 ppm, a large quadrupolar coupling constant of CQ = 3.74 MHz, and asymmetry ηQ = 0.42 (Figure 2, blue curve), corresponds to a spherically asymmetric Na site. It is therefore assigned to the square-planar coordinated Na site in the NaNb13O33 crystal structure. The quadrupolar parameters measured for this Na site, and particularly the magnitude of the quadrupolar coupling, are in good agreement with results from CASTEP NMR calculations (CQ = 3.96 MHz; ηQ = 0.13) on the Na-ordered parent structure.
Figure 2.
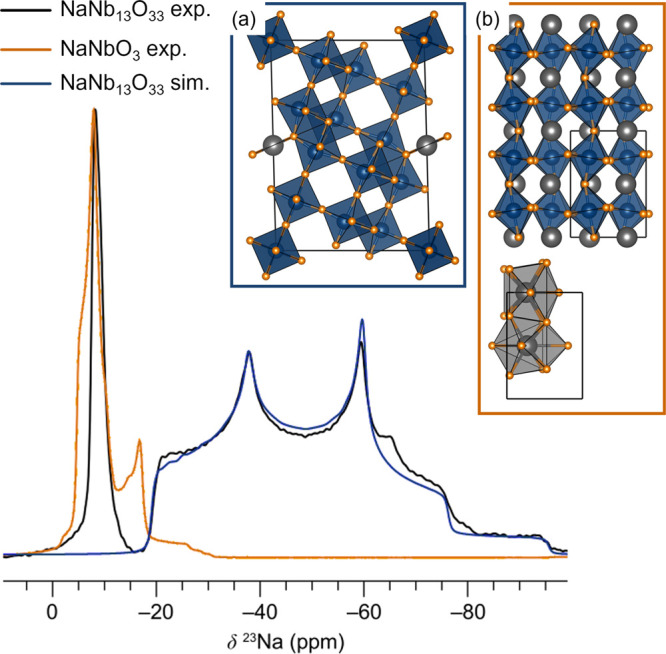
23Na MAS NMR of 1-NNO featuring two distinct resonances (black curve): a fit of the quadrupolar environment with a center of mass at approximately −50 ppm (blue curve) and an overlaid experimental spectrum of NaNbO3 (orange curve). Spectra were acquired at room temperature at 9.4 T and 12.5 kHz MAS. Box (a) shows the NaNb13O33 structure with its square-planar Na environment, which is assigned to the strongly quadrupolar line shape, while box (b) shows the Na environments in NaNbO3. Grey spheres and polyhedra correspond to sodium, blue octahedra are NbO6.
The sharp Na environment, however, cannot be explained by the Na-ordered pristine NaNb13O33 structure with its single square-planar Na coordination. Curiously, it is consistent with the presence of Na in the 12-coordinate site within the octahedral block channels (i.e., the perovskite A-site) of this Wadsley–Roth-derived phase. A Na atom occupying this perovskite-like site within NaNb13O33 would have a mean distance from the 12 nearest atoms of 2.82 Å, which is quite similar to that of NaNbO3, and indeed, the chemical shift is virtually identical with that of Na in perovskite NaNbO3 (Figure 2, orange curve). However, no secondary phase of NaNbO3 was observed in either high-resolution SXRPD or NPD, and the line shape of the sharp resonance in the NaNb13O33 sample is narrower than that observed in any of the polymorphs of NaNbO3.62,63 The narrower resonance could be the result of (i) fast local Na dynamics or (ii) a less distorted Na local environment in NaNb13O33—or perhaps a combination of both.
In NaNbO3, the Na sites are highly distorted (Figure 2b).64 With a Goldschmidt tolerance factor less than one, NbO6 octahedra in NaNbO3 may tilt and rotate around the Na sites, while second-order Jahn–Teller distortions associated with Nb5+ add to the asymmetry. Conversely, the presence of significant edge-sharing in NaNb13O33 prevents octahedral rotation and tilting, and the occupancy of sodium on the perovskite A-site position in NaNb13O33 is far less than unity (vide infra), which will affect electrostatic repulsion. Hence, Na ions in the perovskite-like sites of NaNb13O33 are expected to be in more symmetrical environments than those in NaNbO3.
Overall, there is evidence to suggest that the additional NMR resonance should be attributed to a fraction of Na in the interstitial, perovskite A-site-like positions of NaNb13O33 rather than an impurity phase such as NaNbO3. This idea is supported by further evidence from diffraction. First, in the joint refinement, the occupancy of Na in the square-planar site freely refines to 0.85(4) at 20 K and 0.89(2) at 300 K, which are within error and similar to the 84% integrated NMR signal intensity obtained for the low-frequency component of 1-NNO (87% for 2-NNO (Figure S3)). We note that there are challenges to the precise quantification of NMR signal intensities of sites with significantly different quadrupolar coupling interaction magnitudes.65 In the case of a spin-3/2 nucleus such as 23Na, 60% of the total intensity is contained in the satellite transitions66 that are expected to be distributed over the spinning sidebands for the highly quadrupolar square-planar site but would be expected to be partially or fully folded into the sharp centerband of the perovskite-like site. Thus, a reasonable bound on the relative sodium occupancies from these data sets would be 84%–93% for the broad low-frequency signal and 7%–16% for the narrow high-frequency signal.
Second, Fourier difference maps of the neutron diffraction data at 20 K reveal nuclear density near the center of the perovskite-like sites in NaNb13O33 (Figure S9). The data are too noisy to yield accurate refined Na occupations or ADPs within each perovskite-like site, which is not surprising given that there is only one Na per NaNb13O33 formula unit, and there are six such sites that are expected to sum to 7%–16% of the Na, so the occupancies may only be around 1%–2% per site. Neutron diffraction was helpful here because the relative scattering of Na vs Nb is roughly 1:2 with neutrons, compared to 1:4 with X-rays, and because nuclear density is less diffuse than electron density.
The third and final piece of evidence for this assignment of the NMR data, and for the presence of point defects in NaNb13O33, comes from phase boundary mapping.67,68 With this technique, the target compound NaNb13O33 is purposefully made off-stoichiometric on the Na-poor side and the Na-rich side of the phase diagram until a secondary phase appears. In the case of NaNb13O33 prepared under the 1-NNO synthesis conditions, the adjacent Na-poor phase is H–Nb2O5 and the adjacent Na-rich phase is the bronze-like phase Na13Nb35O94. With excess Nb2O5 added to the synthesis, reflections from H–Nb2O5 are clearly visible in the XRD pattern, and there is nearly no change in the 23Na NMR spectrum (Figures S6 and S7), suggesting that the Na-containing phase is unaffected. With excess Na2CO3 added to the synthesis, small Bragg reflections from the bronze-like phase become visible in XRD, and significant 23Na NMR signal intensity appears around −22 ppm, along with broadening around the base of the signal at −8 ppm (Figure S6). A spectrum of pure Na13Nb35O94 reveals a strong resonance at −22 ppm and a weaker resonance at −8 ppm that is four times broader than the sharp signal at −8 ppm in NaNb13O33 (Figure S6). The crystal structure of Na13Nb35O94 contains four unique sodium sites, with two in four-sided tunnels, much like the perovskite-like site in NaNb13O33, and two in pentagonal tunnels that are common in bronze-like compounds. Although it goes beyond the scope of this work, we propose that the resonance at −8 ppm in Na13Nb35O94 originates from the perovskite A-cation-like site, which would be consistent with those of NaNb13O33 and NaNbO3.
Taken together, the structural data show that NaNb13O33 contains approximately 90% Na on the square-planar site with 10% distributed onto the perovskite A-site-like environments within the (5×3–2)∞ blocks of NbO6 octahedra.
Near-ambient Na dynamics were investigated with variable-temperature 23Na NMR (Figure S8). The spectra collected from −13 to 72 °C are nearly temperature-independent. A small drift in the resonance frequency is observed, but no appreciable line-broadening occurs across the temperatures measured. At low temperatures, no additional environments appear, indicating that the Na+ ions have not been “frozen out”, and at elevated temperatures, the line widths do not narrow or merge, indicating no appreciable chemical exchange between Na sites. Bovin’s study of NaNb13O33 reported Na+-ion conductivities from 6 × 10–2 to 6 × 10–4 mS cm–1 for temperatures from 450 to 275 °C.36 It is therefore unsurprising that significant Na dynamics are not observed over the much lower temperature range investigated here, which is also the relevant temperature range for most Li-ion battery applications.
Characterization of NaNb13O33 as a Battery Electrode Material
Bond-valence sum energy (BVSE) mapping was performed on NaNb13O33 to approximate its Li- and Na-ion diffusion pathways and thus its performance as a host material for Li- and Na-ion battery applications. BVSE is a simple electrostatic method that uses the bond valence site energy approach to estimate the energy barriers relevant to ion diffusion within a crystal structure.69 In this method, a mobile cation is chosen (here, Li+ or Na+), and the interaction energy between this ion and the entire structure, except for other ions of the same type, is calculated over a grid of locations throughout the structure. The resulting array of BVSE energies provides an estimation of relative hopping energy barriers, a representation of the energy landscape for the mobile ion, and a picture of conduction pathways.21,51−53,70,71 The energy isosurface for Δv = 0.1 valence units is displayed for NaNb13O33 with Li+ and Na+ as mobile ions in Figure 3, where Δv is the half-width (in valence units) of the volume accessible to the mobile ion.69 From the extensive Li+ conduction pathways visible at this cutoff, BVSE suggests low-energy barriers for intrablock hopping in the ac plane as well as low-energy-barrier pathways down the b axis within the blocks (Figure 3a,c). This combination of facile 1D diffusion down block channels and intrablock Li redistribution is consistent with previous studies of Wadsley–Roth Li diffusion mechanisms, and it has been credited for their Li diffusion coefficients on par with those of solid electrolyte materials.18,19,29−31 Conversely, BVSE does not predict facile Na+ hopping within octahedral blocks or down channels (Figure 3b,d). The only predicted low-energy-barrier Na+ motion is localized within each NbO6 cage and existing square-planar sites, suggesting that NaNb13O33 would make a poor Na-ion conductor.
Figure 3.
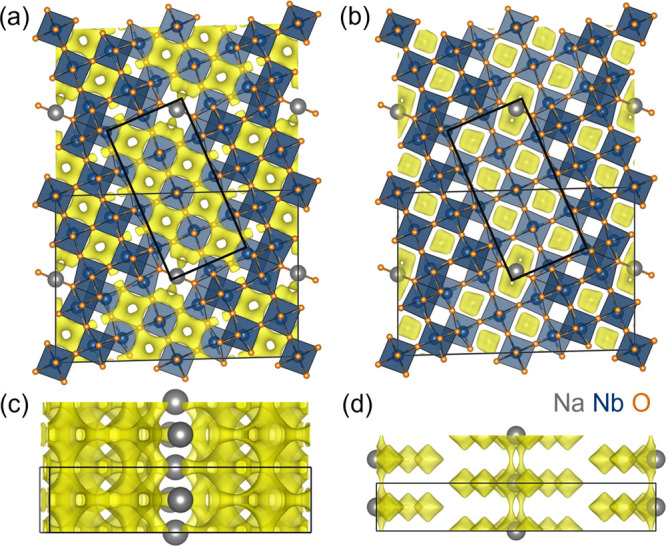
Bond-valence sum energy map of Li+ (a, c) and Na+ (b, d) as mobile ions in ordered NaNb13O33 viewed in the ac plane (top) and the ab plane (bottom). Low-energy-barrier Li-ion conduction pathways (Δv = 0.1 valence units) are shown in yellow.
Electronic structure calculations of the relaxed structure, starting from the model refined from 20 K neutron diffraction data, show a band diagram consisting of both flat and dispersive energy bands (Figure 4). Previous computational studies of similar Wadsley–Roth compounds have shown each n × m block capable of holding one localized electron in Nb d-orbitals dispersed over several octahedra.26,72 These orbitals form a relatively flat band in the band diagram because the corner-sharing octahedra are too far apart to allow for extensive electron delocalization. Conversely, overlapping orbitals between edge-sharing octahedra create dispersive conduction bands. When the number of electrons per block exceeds unity, these latter states allow electron delocalization along the b-axis via the shear planes.20,21,73 In the band structure of NaNb13O33, a relatively flat band is visible at the bottom of the conduction band (1.7–2.2 eV), indicating that electrons doping the structure from initial lithiation occupy such localized states as was seen with previous Wadsley–Roth compounds. With electron doping, the more dispersive bands beginning from 1.9 eV become accessible, suggesting electron delocalization along overlapping Nb d-orbitals and O p-orbitals in the shear planes. Experimental studies have demonstrated metallic conductivity along the shear planes when the one-electron-per-block threshold is exceeded.18,29,74 Thus, n-doping Wadsley–Roth phases with lithium insertion transform them from wide-band-gap insulators to good conductors. This insulator-to-metal transition as a function of lithiation has been observed at low lithium contents and obviates the need for nanostructuring or electron doping to achieve sufficient electronic conduction. From this band structure, we can therefore expect an insulator-to-metal transition and therefore good electronic conductivity as an electrode from NaNb13O33.
Figure 4.
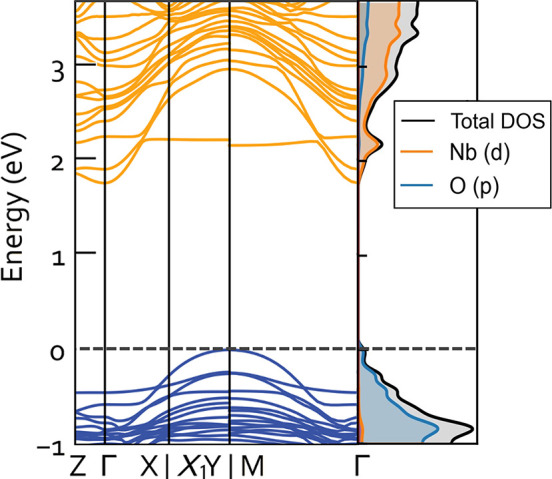
Band structure and density of states for NaNb13O33 calculated from the structure model from refinement of 1-NNO NPD obtained at 20 K. The Fermi level (dashed line) sits on top of the valence band.
Electrochemical Performance of NaNb13O33 in Li Cells
Galvanostatic cycling of 2-NNO after three formation cycles at C/15 is shown in Figure 5a. Both the lithiation and delithiation profiles exhibit at least ten different regions (labeled with Roman numerals), indicating a series of transitions between solid-solution and two-phase regions. To better identify these continuous and discontinuous phase transitions, we turned to differential capacity analysis (dQ/dV vs V). Figure 5b shows at least five peaks and four valleys. Each peak in the dQ/dV vs V plot suggests a phase transition that the active material undergoes during each lithiation and delithiation, with peaks in NaNb13O33 at 1.66 1.64, 1.58, 1.38, and 1.14 V vs Li+/Li. The overpotential and capacity offset between lithiation and delithiation are both small, indicating high energy efficiency and high Coulombic reversibility, respectively (Figure 5a).
Figure 5.
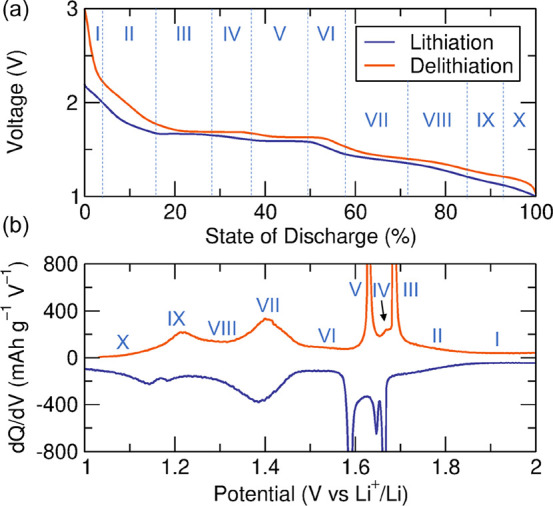
Galvanostatic charge/discharge (GCD) profiles and differential capacity analysis of a LixNaNb13O33 vs Li cell after three C/15 formation cycles. (a) Galvanostatic profiles during lithiation and delithiation between 1 and 3 V vs Li+/Li at a rate of C/15. (b) Corresponding dQ/dV vs V plot for the GCD curves in (a). Regions in (a) and the corresponding features in (b) are labeled with Roman numerals. The cell had an areal active material mass loading of approximately 2 mg cm–2.
Symmetric rate capability was also characterized in NaNb13O33 half-cells (Figure 6) after three formation cycles at C/15. At 6C and 20C current densities, NaNb13O33 delivered 108 and 80 mAh g–1 in approximately 5.4 and 1.2 min, respectively, indicating fast Li+ insertion and extraction despite primary particles on the order of 1–10 μm (Figure S4 inset). Furthermore, at higher active material mass loadings (5.5 mg cm–2), NaNb13O33 still exhibited a specific capacity of 95 mAh g–1 at 10 C (Figure S10). We note that the clear plateaus in the galvanostatic profiles and peaks in the dQ/dV curves are suppressed or merge at rates of 6C and above (Figure S11). The application of higher currents can lead to a nonequilibrium distribution of lithium through the electrode and/or individual particles, resulting in the coexistence of phases as indicated by the increasing width and decreasing height and area of the dQ/dV peaks (Figure S12). Increasing polarization with increasing current density is captured in the shifting dQ/dV peak locations. As a result, charge and discharge cutoff voltages are prematurely reached without achieving full removal or insertion of lithium (Figure S12a,e). These shifts in polarization are symmetric across lithiation and delithiation, indicating that ohmic and faradaic losses are similar during charge and discharge in this material.
Figure 6.
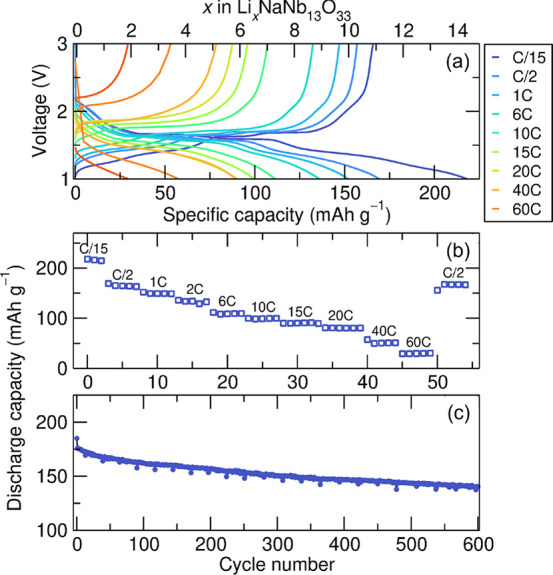
Rate performance of a LixNaNb13O33//Li cell with an areal active material mass loading of 2 mg cm–2. (a) Representative galvanostatic charge–discharge curves at various C-rates after three formation cycles at C/15. (b) Specific capacities from (a) as a function of cycle number with increments in C-rate. (c) Specific capacity as a function of cycle number. Long-term cycling between 1 and 3 V was performed at a rate of 2C following two formation cycles at a rate of C/10.
Based on the above analysis, long-term cycling of lithium in NaNb13O33 half-cells from 1 to 3 V vs Li+/Li was conducted at a charge/discharge rate of 2C after two C/10 formation cycles. After 600 cycles, the material shows 80% capacity retention (Figure 6c). As the number of cycles increases, the electrochemical features are not as pronounced compared to those of the first few cycles (Figures S13 and S14). This suggests a material transformation such as decreased long-range order or increased heterogeneity upon cycling. We note that the cycled cells experienced periodic temperature fluctuations, which accounts for the outliers in discharge capacity. We also note that no attempts were made to optimize this electrochemical performance, although it could likely be further improved through, e.g., calendering, conductive carbon network optimization, or electrolyte optimization.
The state-of-the-art high-rate lithium-ion battery anode material is lithium titanate spinel Li4Ti5O12 (LTO). Based on a three-electron reduction to Li7Ti5O12, the gravimetric capacity of lithium titanate is 175.1 mAh g–1.75 However, bulk Li4Ti5O12 does not function well as an anode material and must be carbon-coated and nanoscaled to achieve high-rate (de)intercalation.75,76 These synthetic modifications add cost and complexity to the manufacturing process, while nanoparticles show decreased packing density and enhanced side reactions between the electrode particle surfaces and the electrolyte.77 Additionally, LTO contains a large quantity of inactive lithium, which is disadvantageous in terms of resource utilization and because the price of lithium has seen a multifold increase in recent years. Wadsley–Roth phases have become well-known for their ability to rapidly store large quantities of lithium (above 200 mAh g–1, beyond one Li per transition metal) even in micrometer-scale particles and in the absence of carbon coating.18−20 Generally, only 3/5 of the Ti4+ is reduced in Li4Ti5O12 while Wadsley–Roth phases see full one-electron or multielectron redox, leading to higher gravimetric and volumetric capacities in the latter phases. The initial results on bulk NaNb13O33 show that it is capable of lithium storage in excess of 200 mA h g–1 and retains about 50% capacity in a 5 min discharge relative to its 15 h discharge. Nonetheless, the maximum low-rate capacity and the high-rate capacity retention are lower than those observed in Wadsley–Roth phases such as TiNb2O7 and Nb16W5O55. Additional electrochemical testing under optimized and standardized conditions would be warranted to consider the commercial applicability of NaNb13O33 as a high-rate oxide anode. Synthetic modifications such as doping and/or carbon coating could be pursued to improve the electronic conductivity and enhance the rate performance by minimizing ohmic losses observed at high current densities (Figure 6a).
Electrochemical sodiation of 2-NNO electrodes was also attempted. NaNb13O33 half-cells vs Na metal were cycled at rates from C/10 to C/100. As predicted by variable-temperature NMR and BVSE mappings, little to no sodium intercalation was observed (Figure S15). The calculated sodium hopping barriers in related Wadsley–Roth phases are extremely high.29 It appears that the channel sizes and inflexibility of the framework are well-suited for rapid and reversible lithium intercalation but effectively hinder sodium conduction.
Conclusion
Like many Wadsley–Roth phases, NaNb13O33 displays excellent lithium storage capacity that is largely maintained at high rates and over long-term cycling, even with micrometer-scale particles. Combined X-ray and neutron diffraction and 23Na solid-state NMR spectroscopy reveal that this Wadsley–Roth-like structure contains vacancies on the square-planar sodium site and that some sodium is present in the perovskite-like sites inside the 5 × 3 octahedral blocks of NaNb13O33. Reduction of Nb from the 5+ to the 4+ and partially to the 3+ oxidation state enables large lithium capacities, which do not seem inhibited by the presence of Na point defects in the block interiors, although the precise effects of this Na disorder on Li conduction were not investigated. Future studies might attempt to minimize the extent of this disorder, perhaps through lower-temperature synthesis techniques, and this should be easily quantifiable with 23Na solid-state NMR. Bond valence mapping suggests fast, quasi-1D Li diffusion down parallel block channels and facile hopping between neighboring channels, which explains the excellent rate performance observed (e.g., 100 mA h g–1 at 20 C) despite the presence of tunnel blockages and large particle sizes. DFT electronic structure calculations are consistent with previous studies and suggest that there will be good electronic conductivity primarily along overlapping niobium d-orbitals and oxygen p-orbitals within shear planes once NaNb13O33 is n-doped by Li insertion. Intercalation of Na was also tested, but high Na hopping barriers prevented any significant electrochemical energy storage, in agreement with BVSE calculations. Altogether, this work provides structural and electrochemical insights into a promising new high-rate lithium-ion battery anode material and expands the use of Wadsley–Roth battery materials into new compositional and structural phase space.
Acknowledgments
The work done at Northwestern was supported as part of the Joint Center for Energy Storage Research, an Energy Innovation Hub funded by the U.S. Department of Energy, Office of Science, Basic Energy Sciences. The work done at UC Santa Barbara and University of Southern California was supported part of the Center for Synthetic Control Across Length-scales for Advancing Rechargeables (SCALAR), an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Basic Energy Sciences under DE-SC0019381. This work made use of the IMSERC X-ray and NMR facilities at Northwestern University, which have received support from the Soft and Hybrid Nanotechnology Experimental (SHyNE) Resource (NSF ECCS-2025633), International Institute of Nanotechnology, and Northwestern University. The research reported here also made use of shared facilities of the National Science Foundation (NSF) Materials Research Science and Engineering Center (MRSEC) at UC Santa Barbara, NSF DMR 1720256, a member of the Materials Research Facilities Network (www.mrfn.org). A.R.P. thanks Raynald Giovine and Farnaz Kaboudvand at UC Santa Barbara for their help with NMR acquisition and electronic structure calculations, respectively. K.J.G. thanks Alicia Manjon Sanz for support collecting neutron diffraction data during the COVID-19 pandemic. Computational work was supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences, Materials Sciences and Engineering Division under Contract DE-AC02-05-CH11231 (Materials Project Program KC23MP). This research used resources of the Advanced Photon Source (GUP70176), a US Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract DE-AC02-06CH11357 as well as resources at the Spallation Neutron Source, a DOE Office of Science User Facility operated by the Oak Ridge National Laboratory. A.R.P. acknowledges the NSF for a Graduate Research Fellowship, Grant 2139319.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.chemmater.3c01066.
X-ray and neutron diffraction Rietveld fits, nuclear magnetic resonance spectra and scanning electron micrographs of pristine NaNb13O33 samples, X-ray diffraction of NaNbO3 used for NMR analysis, NMR and X-ray diffraction supporting phase mapping, variable-temperature 23Na NMR spectra, Fourier difference map of NaNb13O33, and additional galvanostatic cycling data, including representative dQ/dV curves and analysis and sodium half-cell cycling (PDF)
Crystallographic information file of NaxNb13O33 at 300 K (NB: sodium occupancies were refined freely to test the model stability and thus do not sum precisely to NaNb13O33; see main text for details) (CIF)
Crystallographic information file of NaxNb13O33 at 20 K (NB: sodium occupancies were refined freely to test the model stability and thus do not sum precisely to NaNb13O33; see main text for details) (CIF)
Author Present Address
Department of Chemistry and Biochemistry, University of California, San Diego, La Jolla, CA, 92093
The authors declare the following competing financial interest(s): K.J.G. is a stakeholder in a start-up company commercializing niobium-based anode materials.
Author Status
S.R.N.: Deceased May 24, 2023.
Notes
Note: Several days before the submission of this manuscript, a paper appeared that focused on the electrochemical performance of NaNb13O33.78 In this work, the room-temperature and low-temperature performance of NaNb13O33 are studied in detail. The applied focus of that work differs from the fundamental crystal chemical questions addressed through combined high-resolution diffraction and solid-state NMR in the present study.
Supplementary Material
References
- Belharouak I.; Sun Y.-K.; Liu J.; Amine K. Li(Ni1/3Co1/3Mn1/3)O2 As a Suitable Cathode for High power Applications. J. Power Sources 2003, 123, 247–252. 10.1016/S0378-7753(03)00529-9. [DOI] [Google Scholar]
- Itou Y.; Ukyo Y. Performance of LiNiCoO2 Materials for Advanced Lithium-Ion Batteries. J. Power Sources 2005, 146, 39–44. 10.1016/j.jpowsour.2005.03.091. [DOI] [Google Scholar]
- Vetter J.; Novák P.; Wagner M. R.; Veit C.; Möller K.-C.; Besenhard J.; Winter M.; Wohlfahrt-Mehrens M.; Vogler C.; Hammouche A. Ageing Mechanisms in Lithium-Ion Batteries. J. Power Sources 2005, 147, 269–281. 10.1016/j.jpowsour.2005.01.006. [DOI] [Google Scholar]
- Aurbach D.; Zinigrad E.; Cohen Y.; Teller H. A Short Review of Failure Mechanisms of Lithium Metal and Lithiated Graphite Anodes in Liquid Electrolyte Solutions. Solid State Ion. 2002, 148, 405–416. 10.1016/S0167-2738(02)00080-2. [DOI] [Google Scholar]
- Abraham K. Directions in Secondary Lithium Battery Research and Development. Electrochim. Acta 1993, 38, 1233–1248. 10.1016/0013-4686(93)80054-4. [DOI] [Google Scholar]
- Han J.-T.; Huang Y.-H.; Goodenough J. B. New Anode Framework for Rechargeable Lithium Batteries. Chem. Mater. 2011, 23, 2027–2029. 10.1021/cm200441h. [DOI] [Google Scholar]
- Han J.-T.; Goodenough J. B. 3-V Full Cell Performance of Anode Framework TiNb2O7/Spinel LiNi0.5Mn1.5O4. Chem. Mater. 2011, 23, 3404–3407. 10.1021/cm201515g. [DOI] [Google Scholar]
- Magnéli A. Structures of the ReO3-Type with Recurrent Dislocations of Atoms: ‘Homologous Series’ of Molybdenum and Tungsten Oxides. Acta Crystallogr. 1953, 6, 495–500. 10.1107/S0365110X53001381. [DOI] [Google Scholar]
- Roth R.; Wadsley A. Mixed Oxides of Titanium and Niobium: the Crystal Structure of TiNb24O62 (TiO2·12Nb2O5). Acta Crystallogr. 1965, 18, 724–730. 10.1107/S0365110X65001664. [DOI] [Google Scholar]
- Roth R. S.; Wadsley A. D. Multiple Phase Formation in the Binary System Nb2O5–WO3. I. Preparation and Identification of Phases. Acta Crystallogr. 1965, 19, 26–32. 10.1107/S0365110X65002712. [DOI] [Google Scholar]
- Roth R. S.; Wadsley A. D. Multiple Phase Formation in the Binary System Nb2O5–WO3. II. The Structure of the Monoclinic Phases WNb12O33 and W5Nb16O55. Acta Crystallogr. 1965, 19, 32–38. 10.1107/S0365110X65002724. [DOI] [Google Scholar]
- Roth R. S.; Wadsley A. D. Multiple Phase Formation in the Binary System Nb2O5–WO3. III. The Structures of the Tetragonal Phases W3Nb14O44 and W8Nb18O69. Acta Crystallogr. 1965, 19, 38–42. 10.1107/S0365110X65002736. [DOI] [Google Scholar]
- Roth R. S.; Wadsley A. D. Multiple Phase Formation in the Binary System Nb2O5–WO3. IV. The Block Principle. Acta Crystallogr. 1965, 19, 42–47. 10.1107/S0365110X65002748. [DOI] [Google Scholar]
- Cava R. J.; Murphy D. W.; Zahurak S. M. Lithium Insertion in Wadsley–Roth Phases Based on Niobium Oxide. J. Electrochem. Soc. 1983, 130, 2345. 10.1149/1.2119583. [DOI] [Google Scholar]
- Cava R. J.; Murphy D. W.; Zahurak S. M. Secondary Lithium Cells Employing Vanadium Tungsten Oxide Positive Electrodes. J. Electrochem. Soc. 1983, 130, 243–245. 10.1149/1.2119672. [DOI] [Google Scholar]
- Cava R.; Murphy D.; Rietman E.; Zahurak S.; Barz H. Lithium Insertion, Electrical Conductivity, and Chemical Substitution in Various Crystallographic Shear Structures. Solid State Ion. 1983, 9, 407–411. 10.1016/0167-2738(83)90267-9. [DOI] [Google Scholar]
- Griffith K. J.; Senyshyn A.; Grey C. P. Structural Stability from Crystallographic Shear in TiO2–Nb2O5 Phases: Cation Ordering and Lithiation Behavior of TiNb24O62. Inorg. Chem. 2017, 56, 4002–4010. 10.1021/acs.inorgchem.6b03154. [DOI] [PubMed] [Google Scholar]
- Griffith K. J.; Wiaderek K. M.; Cibin G.; Marbella L. E.; Grey C. P. Niobium Tungsten Oxides for High-Rate Lithium-Ion Energy Storage. Nature 2018, 559, 556–563. 10.1038/s41586-018-0347-0. [DOI] [PubMed] [Google Scholar]
- Griffith K. J.; Grey C. P. Superionic Lithium Intercalation through 2 × 2 nm2 Columns in the Crystallographic Shear Phase Nb18W8O69. Chem. Mater. 2020, 32, 3860–3868. 10.1021/acs.chemmater.9b05403. [DOI] [Google Scholar]
- Preefer M. B.; Saber M.; Wei Q.; Bashian N. H.; Bocarsly J. D.; Zhang W.; Lee G.; Milam-Guerrero J.; Howard E. S.; Vincent R. C.; Melot B. C.; Van der Ven A.; Seshadri R.; Dunn B. S. Multielectron Redox and Insulator-to-Metal Transition upon Lithium Insertion in the Fast-Charging, Wadsley-Roth Phase PNb9O25. Chem. Mater. 2020, 32, 4553–4563. 10.1021/acs.chemmater.0c00560. [DOI] [Google Scholar]
- Wyckoff K. E.; Robertson D. D.; Preefer M. B.; Teicher S. M. L.; Bienz J.; Kautzsch L.; Mates T. E.; Cooley J. A.; Tolbert S. H.; Seshadri R. High-Capacity Li+ Storage through Multielectron Redox in the Fast-Charging Wadsley-Roth Phase (W0.2V0.8)3O7. Chem. Mater. 2020, 32, 9415–9424. 10.1021/acs.chemmater.0c03496. [DOI] [Google Scholar]
- McColl K.; Griffith K. J.; Dally R. L.; Li R.; Douglas J. E.; Poeppelmeier K. R.; Corà F.; Levin I.; Butala M. M. Energy Storage Mechanisms in Vacancy-Ordered Wadsley–Roth Layered Niobates. J. Mater. Chem. A 2021, 9, 20006–20023. 10.1039/D1TA02992D. [DOI] [Google Scholar]
- Bashian N. H.; Preefer M. B.; Milam-Guerrero J.; Zak J. J.; Sendi C.; Ahsan S. A.; Vincent R. C.; Haiges R.; See K. A.; Seshadri R.; Melot B. C. Understanding the Role of Crystallographic Shear on the Electrochemical Behavior of Niobium Oxyfluorides. J. Mater. Chem. A 2020, 8, 12623–12632. 10.1039/D0TA01406K. [DOI] [Google Scholar]
- McColl K.; Cora F. Fast Lithium-ion Conductivity in the ‘Empty-Perovskite’ n = 2 Ruddlesden–Popper-type Oxysulphide Y2Ti2S2O5. J. Mater. Chem. 2021, 9, 7068–7084. 10.1039/D0TA11358A. [DOI] [Google Scholar]
- Cava R. J.; Santoro A.; Murphy D.; Zahurak S.; Roth R. Structural Aspects of Lithium Insertion in Oxides: LixReO3 and Li2FeV3O8. Solid State Ion. 1981, 5, 323–326. 10.1016/0167-2738(81)90258-7. [DOI] [Google Scholar]
- Koçer C. P.; Griffith K. J.; Grey C. P.; Morris A. J. Cation Disorder and Lithium Insertion Mechanism of Wadsley–Roth Crystallographic Shear Phases from First Principles. J. Am. Chem. Soc. 2019, 141, 15121–15134. 10.1021/jacs.9b06316. [DOI] [PubMed] [Google Scholar]
- Vincent R. C.; Cheetham A. K.; Seshadri R. Structure and Lithium Insertion in Oxides of Molybdenum. APL. Mater. 2023, 11, 010902. 10.1063/5.0133518. [DOI] [Google Scholar]
- Parui K.; Lee A. D.; Gandhi S.; Butala M. M. R-Nb2O5 has an ‘Idealized’ V2O5 Structure and Wadsley–Roth-like Structural Stability during Li-ion Battery Cycling. J. Mater. Chem. 2023, 11, 5559–5567. 10.1039/D2TA08653K. [DOI] [Google Scholar]
- Griffith K. J.; Seymour I. D.; Hope M. A.; Butala M. M.; Lamontagne L. K.; Preefer M. B.; Kocer C. P.; Henkelman G.; Morris A. J.; Cliffe M. J.; Dutton S. E.; Grey C. P. Ionic and Electronic Conduction in TiNb2O7. J. Am. Chem. Soc. 2019, 141, 16706–16725. 10.1021/jacs.9b06669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koçer C. P.; Griffith K. J.; Grey C. P.; Morris A. J. Lithium Diffusion in Niobium Tungsten Oxide Shear Structures. Chem. Mater. 2020, 32, 3980–3989. 10.1021/acs.chemmater.0c00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z.; Xiang Y.; Wang D.; Fu R.; Yang Y.. NMR and MRI of Electrochemical Energy Storage Materials and Devices; Royal Society of Chemistry: 2021. [Google Scholar]
- Cava R.; Batlogg B.; Krajewski J.; Gammel P.; Poulsen H.; Peck W.; Rupp L. Antiferromagnetism and Metallic Conductivity in Nb12O29. Nature 1991, 350, 598–600. 10.1038/350598a0. [DOI] [Google Scholar]
- Cava R.; Batlogg B.; Krajewski J.; Poulsen H.; Gammel P.; Peck W. Jr.; Rupp L. Jr. Electrical and Magnetic Properties of Nb2O5–δ Crystallographic Shear Structures. Phys. Rev. B 1991, 44, 6973. 10.1103/PhysRevB.44.6973. [DOI] [PubMed] [Google Scholar]
- Ruscher C.; Salje E.; Hussain A. The Effect of High Polaron Concentration on the Polaron Transport in NbO2.5–x: Optical and Electrical Properties. J. Phys. C: Solid State Phys. 1988, 21, 3737. 10.1088/0022-3719/21/20/009. [DOI] [Google Scholar]
- Andersson S.; Selte K.; Kjekshus A.; Nielsen P. H.; Sjöberg B.; Larsen E. The Crystal Structure of NaNb13O33 and the Geometrical Principles of the Homologous Series NaM3n+1O8n+1. Acta Chem. Scand. 1965, 19, 557. 10.3891/acta.chem.scand.19-0557. [DOI] [Google Scholar]
- Bovin J. O. Ionic Conductivity and the Conductivity-Blocking Defect Structure of NaNb13O33. J. Solid State Chem. 1984, 54, 277–281. 10.1016/0022-4596(84)90156-7. [DOI] [Google Scholar]
- Irle E.; Blachnik R.; Gather B. The Phase Diagrams of Na2O and K2O with Nb2O5 and the Ternary System Nb2O5−Na2O–Yb2O3. Thermochim. Acta 1991, 179, 157–169. 10.1016/0040-6031(91)80344-I. [DOI] [Google Scholar]
- Popovič A.; Bencze L.; Koruza J.; Malič B.; Kosec M. Knudsen Effusion Mass Spectrometric Approach to the Thermodynamics of Na2O–Nb2O5 system. Int. J. Mass Spectrom. 2012, 309, 70–78. 10.1016/j.ijms.2011.08.028. [DOI] [Google Scholar]
- Appendino P. Contributo Allo Studio del Sistema Na2O-Nb2O5. Ann. Chim. Ital. 1973, 63, 277–281. [Google Scholar]
- Huq A.; Kirkham M.; Peterson P. F.; Hodges J. P.; Whitfield P. S.; Page K.; Hugle T.; Iverson E. B.; Parizzi A.; Rennich G. POWGEN: Rebuild of a Third-Generation Powder Diffractometer at the Spallation Neutron Source. J. Appl. Crystallogr. 2019, 52, 1189–1201. 10.1107/S160057671901121X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toby B. H.; Von Dreele R. B. GSAS-II: The Genesis of a Modern Open-Source all Purpose Crystallography Software PAckage. J. Appl. Crystallogr. 2013, 46, 544–549. 10.1107/S0021889813003531. [DOI] [Google Scholar]
- Bielecki A.; Burum D. P. Temperature Dependence of 207Pb MAS Spectra of Solid Lead Nitrate. An Accurate, Sensitive Thermometer for Variable-Temperature MAS. J. Magn. Reson. 1995, 116, 215–220. 10.1006/jmra.1995.0010. [DOI] [Google Scholar]
- Beckmann P. A.; Dybowski C. A Thermometer for Nonspinning Solid-State NMR Spectroscopy. J. Magn. Reson. 2000, 146, 379–380. 10.1006/jmre.2000.2171. [DOI] [PubMed] [Google Scholar]
- Kresse G.; Hafner J. Ab Initio Molecular Dynamics for Liquid metals. Phys. Rev. B 1993, 47, 558. 10.1103/PhysRevB.47.558. [DOI] [PubMed] [Google Scholar]
- Kresse G.; Furthmüller J. Efficiency of Ab-Initio Total Energy Calculations for Metals and Semiconductors Using a Plane-Wave Basis Set. Comput. Mater. Sci. 1996, 6, 15–50. 10.1016/0927-0256(96)00008-0. [DOI] [PubMed] [Google Scholar]
- Kresse G.; Furthmüller J. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B 1996, 54, 11169. 10.1103/PhysRevB.54.11169. [DOI] [PubMed] [Google Scholar]
- Kresse G.; Hafner J. Norm-Conserving and Ultrasoft Pseudopotentials for First-Row and Transition Elements. J. Condens. Matter Phys. 1994, 6, 8245. 10.1088/0953-8984/6/40/015. [DOI] [Google Scholar]
- Kresse G.; Joubert D. From Ultrasoft Pseudopotentials to the Projector Augmented-Wave Method. Phys. Rev. B 1999, 59, 1758. 10.1103/PhysRevB.59.1758. [DOI] [Google Scholar]
- Perdew J. P.; Burke K.; Ernzerhof M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865. 10.1103/PhysRevLett.77.3865. [DOI] [PubMed] [Google Scholar]
- Ganose A. M.; Jackson A. J.; Scanlon D. O. SUMO: Command-Line Tools for Plotting and Analysis of Periodic Ab Initio Calculations. J. Open Source Softw. 2018, 3, 717. 10.21105/joss.00717. [DOI] [Google Scholar]
- Chen H.; Wong L. L.; Adams S. SoftBV–A Software Tool for Screening the Materials Genome of Inorganic Fast Ion Conductors. Acta Crystallogr. B Struct. 2019, 75, 18–33. 10.1107/S2052520618015718. [DOI] [PubMed] [Google Scholar]
- Chen H.; Adams S. Bond Softness Sensitive Bond-Valence Parameters for Crystal Structure Plausibility Tests. IUCrJ. 2017, 4, 614–625. 10.1107/S2052252517010211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong L. L.; Phuah K. C.; Dai R.; Chen H.; Chew W. S.; Adams S. Bond Valence Pathway Analyzer—An Automatic Rapid Screening Tool for Fast Ion Conductors within softBV. Chem. Mater. 2021, 33, 625–641. 10.1021/acs.chemmater.0c03893. [DOI] [Google Scholar]
- Momma K.; Izumi F. VESTA 3 for Three-dimensional Visualization of Crystal, Volumetric and Morphology Data. J. Appl. Crystallogr. 2011, 44, 1272–1276. 10.1107/S0021889811038970. [DOI] [Google Scholar]
- Clark S. J.; Segall M. D.; Pickard C. J.; Hasnip P. J.; Probert M. I.; Refson K.; Payne M. C. First Principles Methods Using CASTEP. Z. Kristallogr. 2005, 220, 567–570. 10.1524/zkri.220.5.567.65075. [DOI] [Google Scholar]
- Vanderbilt D. Soft Self-Consistent Pseudopotentials in a Generalized Eigenvalue Formalism. Phys. Rev. B 1990, 41, 7892. 10.1103/PhysRevB.41.7892. [DOI] [PubMed] [Google Scholar]
- Perdew J. P.; Burke K.; Ernzerhof M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865. 10.1103/PhysRevLett.77.3865. [DOI] [PubMed] [Google Scholar]
- Monkhorst H. J.; Pack J. D. Special Points for Brillouin-zone Integrations. Phys. Rev. B 1976, 13, 5188. 10.1103/PhysRevB.13.5188. [DOI] [Google Scholar]
- Pickard C. J.; Mauri F. All-Electron Magnetic Response with Pseudopotentials: NMR Chemical Shifts. Phys. Rev. B 2001, 63, 245101. 10.1103/PhysRevB.63.245101. [DOI] [Google Scholar]
- Yates J. R.; Pickard C. J.; Mauri F. Calculation of NMR Chemical Shifts for Extended Systems Using Ultrasoft Pseudopotentials. Phys. Rev. B 2007, 76, 024401 10.1103/PhysRevB.76.024401. [DOI] [Google Scholar]
- Profeta M.; Mauri F.; Pickard C. J. Accurate First Principles Prediction of 17O NMR Parameters in SiO2: Assignment of the Zeolite Ferrierite Spectrum. J. Am. Chem. Soc. 2003, 125, 541–548. 10.1021/ja027124r. [DOI] [PubMed] [Google Scholar]
- Ashbrook S. E.; Le Polles L.; Gautier R.; Pickard C. J.; Walton R. I. 23Na Multiple-Quantum MAS NMR of the Perovskites NaNbO3 and NaTaO3. Phys. Chem. Chem. Phys. 2006, 8, 3423–3431. 10.1039/B604520K. [DOI] [PubMed] [Google Scholar]
- Johnston K. E.; Tang C. C.; Parker J. E.; Knight K. S.; Lightfoot P.; Ashbrook S. E. The Polar Phase of NaNbO3: A Combined Study by Powder Diffraction, Solid-State NMR, and First-Principles Calculations. J. Am. Chem. Soc. 2010, 132, 8732–8746. 10.1021/ja101860r. [DOI] [PubMed] [Google Scholar]
- Seidel P.; Hoffmann W. Verfeinerung der Kristallstruktur von NaNbO3 N. Bestimmung der Absoluten Konfiguration und des Zwillingsgesetzes. Z. Kristallogr. 1976, 143, 444–459. 10.1524/zkri.1976.143.jg.444. [DOI] [Google Scholar]
- Alemany L. B.; Massiot D.; Sherriff B. L.; Smith M. E.; Taulelle F. Observation and Accurate Quantification of 27Al MAS NMR Spectra of Some Al2SiO5 Polymorphs Containing Sites with Large Quadrupole Interactions. Chem. Phys. Lett. 1991, 177, 301–306 10.1016/0009-2614(91)85035-U. [DOI] [Google Scholar]
- Eden M.Annual Reports on NMR Spectroscopy; Elsevier: 2020; Vol. 101, pp 285–410. [Google Scholar]
- Ohno S.; Imasato K.; Anand S.; Tamaki H.; Kang S. D.; Gorai P.; Sato H. K.; Toberer E. S.; Kanno T.; Snyder G. J. Phase Boundary Mapping to Obtain n-type Mg3Sb2 -Based Thermoelectrics. Joule 2018, 2, 141–154. 10.1016/j.joule.2017.11.005. [DOI] [Google Scholar]
- Jood P.; Male J. P.; Anand S.; Matsushita Y.; Takagiwa Y.; Kanatzidis M. G.; Snyder G. J.; Ohta M. Na Doping in PbTe: Solubility, Band Convergence, Phase Boundary Mapping, and Thermoelectric Properties. J. Am. Chem. Soc. 2020, 142, 15464–15475. 10.1021/jacs.0c07067. [DOI] [PubMed] [Google Scholar]
- Brown I. D. Recent Developments in the Methods and Applications of the Bond Valence Model. Chem. Rev. 2009, 109, 6858–6919. 10.1021/cr900053k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avdeev M.; Sale M.; Adams S.; Rao R. P. Screening of the Alkali-metal Ion Containing Materials from the Inorganic Crystal Structure Database (ICSD) for High Ionic Conductivity Pathways using the Bond Valence Method. Solid State Ion. 2012, 225, 43–46. 10.1016/j.ssi.2012.02.014. [DOI] [Google Scholar]
- Xiao R.; Li H.; Chen L. High-throughput Design and Optimization of Fast Lithium Ion Conductors by the Combination of Bond-valence Method and Density Functional Theory. Sci. Rep. 2015, 5, 14227. 10.1038/srep14227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koçer C. P.; Griffith K. J.; Grey C. P.; Morris A. J. First-principles Study of Localized and Delocalized Electronic States in Crystallographic Shear Phases of Niobium Oxide. Phys. Rev. B 2019, 99, 075151. 10.1103/PhysRevB.99.075151. [DOI] [Google Scholar]
- Saber M.; Preefer M. B.; Kolli S. K.; Zhang W.; Laurita G.; Dunn B.; Seshadri R.; Van der Ven A. Role of Electronic Structure in Li Ordering and Chemical Strain in the Fast Charging Wadsley–Roth Phase PNb9O25. Chem. Mater. 2021, 33, 7755–7766. 10.1021/acs.chemmater.1c02059. [DOI] [Google Scholar]
- Kocer C.First-Principles Studies of Complex Oxide Materials. Ph.D. Thesis, University of Cambridge, 2021. [Google Scholar]
- Shen L.; Zhang X.; Uchaker E.; Yuan C.; Cao G. Li4Ti5O12 Nanoparticles Embedded in a Mesoporous Carbon Matrix as a Superior Anode Material for High Rate Lithium Ion Batteries. Adv. Energy Mater. 2012, 2, 691–698. 10.1002/aenm.201100720. [DOI] [Google Scholar]
- Prakash A.; Manikandan P.; Ramesha K.; Sathiya M.; Tarascon J.; Shukla A. Solution-combustion Synthesized Nanocrystalline Li4Ti5O12 as High-rate Performance Li-ion Battery Anode. Chem. Mater. 2010, 22, 2857–2863. 10.1021/cm100071z. [DOI] [Google Scholar]
- Yuan T.; Tan Z.; Ma C.; Yang J.; Ma Z.-F.; Zheng S. Challenges of Spinel Li4Ti5O12 for Lithium-ion Battery Industrial Applications. Adv. Energy Mater. 2017, 7, 1601625. 10.1002/aenm.201601625. [DOI] [Google Scholar]
- Gao J.; et al. Sodium Niobate with a Large Interlayer Spacing: A Fast-Charging, Long-Life, and Low-Temperature Friendly Lithium-Storage Material. Advanced Science 2023, 10, 2300583. 10.1002/advs.202300583. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.