Abstract
Background:
Neonatal seizures are common, but the impact of neonatal seizures on long-term neurologic outcome remains unclear. We addressed this question by analyzing data from an early-phase controlled trial of bumetanide to treat neonatal seizures.
Methods:
Neonatal seizure burden was calculated from continuous video-EEG data. Neurologic outcome was determined by standardized developmental tests and post-neonatal seizure recurrence.
Results:
Of 111 enrolled neonates, 43 were randomized to treatment or control groups. There were no differences in neurologic outcome between treatment and control groups. A subgroup analysis was performed for 84 neonates with acute perinatal brain injury (57 HIE, 18 stroke, 9 ICH), most of whom (70%) had neonatal seizures. There was a significant negative correlation between seizure burden and developmental scores (p<0.01). Associations between seizure burden and developmental scores were stronger in HIE and stroke groups compared with ICH (p<0.05).
Conclusion:
Bumetanide showed no long-term beneficial or adverse effects, as expected based on treatment duration versus duration of neonatal seizures. For neonates with perinatal brain injury, higher neonatal seizure burden correlated significantly with worse developmental outcome, particularly for ischemic versus hemorrhagic brain injury. These data highlight the need for further investigation of the long-term effects of both neonatal seizure severity and etiology.
Keywords: Neonatal seizures, Bumetanide, Hypoxic ischemic encephalopathy, Stroke, Intracranial hemorrhage
Introduction:
Seizures are common in neonates with acute perinatal brain injury, including hypoxic-ischemic encephalopathy (HIE), ischemic stroke, and intracranial hemorrhage (ICH), and seizures in this population are often prolonged and refractory to treatment.1,2 Complicating clinical care, neonatal seizures can be difficult to detect, as clinical manifestations of the seizure may be subtle3,4 and many seizures are electrographic only, especially after initial anti-seizure medication (ASM) administration.5 Therefore, the American Clinical Neurophysiology Society (ACNS) guidelines recommend continuous video-EEG (cvEEG) monitoring for at-risk neonates to detect seizures.6
With increased prolonged cvEEG monitoring for critically ill neonates, an accumulating body of evidence, primarily in neonatal HIE, has shown a correlation between higher neonatal seizure burden and worse short-term1,7,8 and long-term9–15 outcome measures, including increased mortality and abnormal neurodevelopmental outcome. There is, however, a relative paucity of studies investigating the relationship between seizure burden and long-term outcome in neonates with other types of acute perinatal brain injury, including stroke and ICH. Work in animal models has demonstrated that neuronal injury secondary to seizure activity is significantly worse in the setting of hypoxia-ischemia,16,17 suggesting that the effect of neonatal seizure burden on long-term neurologic outcome may crucially depend on the seizure etiology, with worse outcome in patients with hypoxic-ischemic injury. This hypothesis has yet to be carefully studied in the clinical setting.
Here we report the developmental outcome and rate of post-neonatal seizure recurrence from a randomized, controlled, double-blind, early-phase trial of bumetanide as add-on therapy to phenobarbital to treat neonatal seizures.18 Neonates enrolled in this study had cvEEG during the entire period of acute neonatal seizures with quantification of seizure burden, as well as follow-up evaluation with standardized developmental psychological measures. Since the trial included subjects with stroke and ICH as well as HIE, we could evaluate the effect of seizure etiology on the relationship between seizure burden and long-term neurologic outcome, in addition to evaluating any effect of bumetanide on neurologic outcome.
Methods:
Study design and participants
The study population consisted of neonates enrolled between 2010 and 2017 in a randomized controlled double-blind trial of bumetanide as add-on therapy to phenobarbital to treat seizures.18 In this early-phase trial, one of three escalating dosages (0.1 – 0.3 mg / kg) was administered and the acute pharmacokinetics and EEG responses were studied. The single dose of bumetanide was not expected to have a sustained effect on seizure control over the entire period (days) of susceptibility to seizures.18 This multi-center trial was conducted in four neonatal intensive care units (NICUs) in Boston, MA. All neonates were born at post-menstrual age 34–44 weeks and either had clinically suspected and/or EEG-confirmed seizures or were deemed high risk for seizures due to a diagnosis of HIE, focal stroke, ICH, acute meningoencephalitis, brain malformation, or a suspected/known genetic disorder. Exclusion criteria included seizures secondary to transient metabolic abnormalities, diagnosis of inborn errors of metabolism, prior administration of bumetanide, furosemide, phenytoin, or ≥40 mg/kg phenobarbital, total bilirubin >15 mg/dL, treatment with ECMO, or risk of imminent death.
This study had IRB approval at all participating NICUs, and parents/guardians provided written informed consent. The trial was prospectively registered with clinicaltrials.gov (NCT00830531).
Measures
Continuous video-EEG (cvEEG) data were collected for all enrolled neonates, starting soon after or prior to enrollment, if cvEEG was initiated by the clinical team. The cvEEG was continued for at least 48 hours post-randomization or longer if seizures persisted. Neonatal seizure burden (in min/h) for each subject was defined as the sum of all minutes of seizure activity from onset of first suspected or confirmed seizure to end of last EEG-confirmed seizure, divided by the number of hours in the period from first to last seizure. Calculating seizure burden over an arbitrarily chosen standardized duration of recording (e.g., 48 hours) could under- or over-estimate seizure burden; therefore, seizure burden was measured over the entire period of seizure activity for each subject. Neonatal seizure burden was also calculated as total minutes of seizure activity recorded by cvEEG. Subjects with clinically suspected seizures but no seizures recorded by subsequent EEG were assigned a seizure burden of zero, since seizure diagnosis and seizure burden could not be determined accurately. Incidence of status epilepticus, defined as ≥ 30 minutes of EEG seizure activity in a 60-minute period, was determined from analysis of EEG data.
Seizure etiology was determined by review of the clinical data together with cvEEG, neuroradiology reports, and laboratory data. Seizure location and type by cvEEG was compared with location of any brain injury reported in neuroimaging studies to confirm etiology if neonates had multiple diagnoses (e.g., brain malformation and ICH). A neuroradiologist (PEG) reviewed cases of ICH to determine if there was imaging evidence of associated global hypoxic ischemic brain injury or focal arterial stroke in addition to hemorrhagic brain injury.
Between 17 and 31 months of age, subjects underwent developmental psychological evaluation. The primary outcome measure was the Bayley Scales of Infant and Toddler Development, 3e (Bayley-III),19 and the secondary outcome measure was the Vineland Adaptive Behavior Scales, 2e (Vineland-II).20 Each child was individually administered the Bayley-III, a comprehensive measure used to identify developmental progress during early childhood. Domains included in our analysis were Cognitive, Language, and Motor. Each family completed the interview version of the Vineland-II, which is designed to measure adaptive behavior of individuals from birth to the elderly years. Domains included in our analysis were Adaptive Behavior, Communication, and Motor Skills. Bayley-III and Vineland-II composite scores are reported as standard scores (mean = 100, standard deviation = 15 in a healthy, normal population).
Post-neonatal seizure recurrence was determined by review of notes available in the medical record from appointments with neurology, developmental medicine, or comprehensive well child visits with the primary care physician. Any relevant notes through January 2021 were included in the chart review. Subjects with one or more afebrile seizures were considered to meet criteria for post-neonatal seizure recurrence.
Subjects were divided into groups with normal or abnormal neurologic outcome based on criteria revised from a study of outcome in neonatal HIE.13 Subjects were defined as having an abnormal neurologic outcome if they had one or more of the following: a diagnosis of epilepsy, Gross Motor Function Classification System (GMFCS) level of three to five,21 sensorineural hearing loss (SNHL) requiring cochlear implant, and/or Bayley-III composite scores less than 85 in all three domains (Cognitive, Language, Motor), or less than 70 in any individual domain. In subjects without Bayley-III composite scores, Vineland-II composite scores were used as a proxy. Subjects not meeting these criteria were defined as having a normal neurologic outcome.
To evaluate the relationship between seizure burden and neurologic diagnosis with neurologic outcome and seizure recurrence, we analyzed neonatal seizure burden, developmental psychological scores, and seizure recurrence data for all enrolled neonates with acute perinatal brain injury and available follow-up data. This analysis included only neonates with HIE, stroke, or ICH; neonates with brain malformation, confirmed genetic disorder, or acute meningoencephalitis were excluded because of the known association of these disorders with long-term epilepsy and adverse developmental outcome independent of neonatal seizure burden.
Statistical analysis
Fisher’s exact tests (for categorical variables) and rank-based Kruskal-Wallis tests (for continuous variables) were used to assess differences in demographic and medical characteristics, seizure burden and recurrence, and developmental psychological scores by group (randomized vs. nonrandomized, bumetanide vs. control, and by neurologic diagnosis). Spearman rank (robust to outlying observations) and linear regression were used to assess the relationship between neonatal seizure burden and developmental psychological scores, including exploratory assessment of interactions with seizure etiology. These findings were confirmed via two robust regression methods: M-estimation with iterated reweighted least squares (giving lower weight to observations with higher residuals) and generalized estimating equations with sandwich variance estimators (robust to assumptions of normality and homoscedasticity of residuals). Logistic regression and receiver operating characteristic (ROC) curves were used to assess the relationship between neonatal seizure burden and dichotomous seizure recurrence and abnormal neurologic outcome. R version 3.6.0 (R Foundation for Statistical Computing, Vienna, Austria) was used for all analyses.
Results:
Neonatal characteristics of enrolled trial subjects
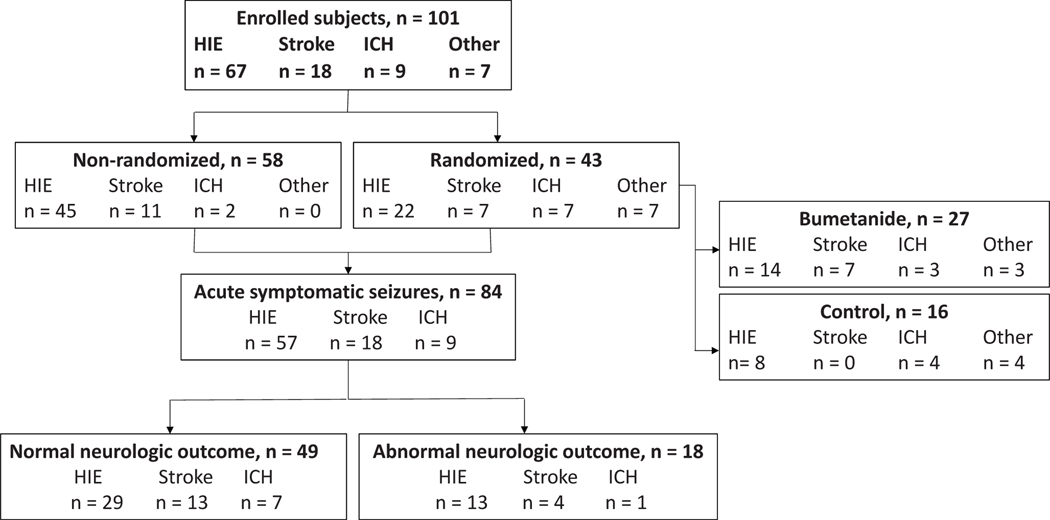
Of the 111 subjects enrolled in the study, ten met exclusion criteria after enrollment. Forty-three of the remaining 101 enrolled subjects were randomized to treatment (bumetanide, n=27) or control (standard therapy, n=16) groups (Table 1, Figure 1). Fifty-eight subjects were enrolled but did not meet criteria for randomization (non-randomized group), either because of a lack of persistent seizures after a loading dose of phenobarbital (n=54) or missed EEG seizures (n=4).
Table 1.
Neonatal characteristics and neurologic outcome in Boston Bumetanide Trial subjects.
| Randomized | Non-randomized | ||
|---|---|---|---|
|
|
|
||
| Neonatal characteristics | Bumetanide (n=27) | Control (n=16) | (n=58) |
| Male, n (%) | 14 (52) | 7 (44) | 32 (55) |
| Gestational age, w, median (IQR) | 39 (39, 40) | 40 (39, 41) | 39 (38, 40) |
| Birth weight, kg, median (IQR) | 3.4 (3.0, 3.7) | 3.3 (3.0, 3.5) | 3.2 (3.0, 3.6) |
| Race, n (%) | |||
| White | 23 (96) | 12 (92) | 33 (77) |
| Black/African American | 0 | 0 | 8 (19) |
| Other | 1 (4) | 1 (8) | 2 (5) |
| Unreported | 3 | 3 | 15 |
| Ethnicity, Hispanic or Latino, n (%) | 2 (7) | 4 (25) | 11 (19) |
| Neurologic diagnosis, n (%) | |||
| HIE | 14 (52) | 8 (50) | 45 (78) |
| Stroke | 7 (26) | 0 | 11 (19) |
| ICH | 3 (11) | 4 (25) | 2 (3) |
| Othera | 3 (11) | 4 (25) | 0 |
| Received therapeutic hypothermia, n (%) | 10 (37) | 5 (31) | 42 (72) |
| Clinical or EEG-confirmed neonatal seizures, n (%) | 27 (100) | 16 (100) | 29 (50) |
| Neonatal seizure burden, min/hr, median (IQR) | 3.1 (1.3, 4.9) | 1.2 (0.3, 2.7) | 0.0 (0.0, 0.9) |
| Neonatal status epilepticus, n (%) | 11 (41) | 1 (6) | 5 (9) |
| EEG duration, hr, median (IQR) | 88.4 (71.2, 99.9) | 81.9 (69.6, 95.6) | 64.5 (47.9, 83.0) |
| Neurodevelopmental testing | (n=21) | (n=12) | (n=39) |
| Age at testing, m, median (IQR) | 19 (18, 20) | 19 (18, 22) | 19 (18, 22) |
| Bayley-III Composite Scores, mean ± SD | |||
| Cognitive | 88.2 ± 16.9 | 90.6 ± 16.7 | 95.0 ± 17.3 |
| Language | 86.5 ± 17.6 | 87.9 ± 20.0 | 90.0 ± 17.6 |
| Motor | 84.4 ± 20.3 | 92.3 ± 21.1 | 93.5 ± 16.6 |
| Vineland-II Composite Scores, mean ± SD | |||
| Adaptive Behavior | 89.7 ± 12.0 | 78.8 ± 23.5 | 92.3 ± 16.0 |
| Communication | 88.4 ± 12.2 | 81.2 ± 22.9 | 90.2 ± 17.5 |
| Motor Skills | 88.8 ± 19.0 | 77.6 ± 31.3 | 96.4 ± 20.1 |
| Post-neonatal outcome, n/total (%) | |||
| Post-neonatal seizure(s) | 9/26 (35) | 4/13 (31) | 4/51 (8) |
| Confirmed SNHL | 2/26 (8) | 0/13 (0) | 1/54 (2) |
| Suspected or confirmed CVI | 0/24 (0) | 3/13 (23) | 1/46 (2) |
Abbreviations: IQR, interquartile range; HIE, hypoxic-ischemic encephalopathy; ICH, intracranial hemorrhage; SD, standard deviation; SNHL, sensorineural hearing loss; CVI, cerebral visual impairment
Other neurologic diagnoses included brain malformation, confirmed genetic disorder, acute meningoencephalitis.
Figure 1.
Study subject groupings. Algorithm demonstrating distribution of seizure etiologies among trial subjects
There were no significant differences in neonatal characteristics between the randomized bumetanide and control groups (Table 1), apart from a lower neonatal seizure burden in the control group (p=0.007), which occurred by chance (pre-treatment seizure burden was similarly lower in control subjects).18 Of note, the randomized (R) and non-randomized (NR) groups differed in neurologic diagnosis (R: 51% HIE, 16% Stroke, 16% ICH, 16% Other; NR: 78% HIE, 19% Stroke, 3% ICH, 0% Other; p<0.001), and the non-randomized group had a higher rate of therapeutic hypothermia compared with the randomized group (R: 35%, NR: 72%; p<0.001) due to a larger proportion of subjects with HIE. As expected, presence of neonatal seizures (R: 100%, NR: 50%; p<0.001), neonatal seizure burden (R: median 2.2 min/hr, interquartile range [IQR] 1.0, 3.8; NR: 0 min/hr, IQR 0, 0.9; p<0.001), and duration of EEG monitoring (R: median 88.1 hr, IQR 70.9, 99.9; NR: 64.5 hr, IQR 47.9, 83.0; p<0.001) were lower in the non-randomized vs. the randomized group.
Post-neonatal outcome in enrolled trial subjects
Among the 101 enrolled subjects, eight (7%) died prior to follow-up. Developmental psychological scores (Bayley-III and/or Vineland-II) were available for 72 subjects (21 treatment, 12 control, 39 non-randomized); 21 subjects did not attend developmental psychological testing (5 treatment, 1 control, 15 non-randomized). Most subjects (n=61) had composite scores for both the Bayley-III and Vineland-II; three had only Bayley-III testing and eight had only Vineland-II testing. Seizure recurrence data was available for 90 subjects (26 treatment, 13 control, 51 non-randomized).
Developmental psychological testing was performed at a median age of 19 months (IQR 18, 20) (Table 1). Bayley-III and Vineland-II scores were not significantly different between the randomized (n=33) and non-randomized groups (n=39) or between the bumetanide (n=21) and control groups (n=12). Within the control group, mean Bayley-III scores were higher than mean Vineland-II scores due to three subjects who had low Vineland scores but were unable to come to clinic for Bayley-III testing. For all subjects, developmental outcome and functioning indices were lower than expected for age (one-sample t-test; Bayley-III: Cognitive, 92.3 ± 17.1 (mean ± SD), p<0.001; Language, 88.7 ± 17.7, p<0.001; Motor, 90.7 ± 18.6, p<0.001; Vineland-II: Adaptive Behavior, 89.2 ± 16.8, p<0.001; Communication, 88.1 ± 17.3, p<0.001; Motor Skills, 91.1 ± 22.7, p=0.002).
There was no difference in the rate of post-neonatal seizures between the bumetanide (9/26, 35%) and control (4/13, 31%) groups. Compared with the randomized group, the non-randomized group had a significantly lower rate of post-neonatal seizures (R: 13/39, 33%; NR: 4/51, 8%; p=0.003). Since all randomized neonates had neonatal seizures but only 50% of the non-randomized neonates had neonatal seizures, we repeated this analysis including only subjects with neonatal seizures. In this latter analysis, the difference in post-neonatal seizure recurrence was not statistically significant (randomized 13/39, 33%, non-randomized 4/26, 15%, p=0.15).
The relationship between neonatal seizure burden and post-neonatal outcome in all study subjects
To evaluate the relationship between neonatal seizure burden, neurologic diagnosis, and post-neonatal outcome, we analyzed data for the 84 subjects diagnosed with acute perinatal brain injury (HIE, stroke, or ICH, Figure 1) who had developmental psychological testing and/or seizure recurrence data. This included all subjects, including both randomized and non-randomized groups. The majority had HIE (57, 68%), followed by stroke (18, 21%) and ICH (9, 11%) (Table 2). Only subjects with HIE received therapeutic hypothermia (49/57, 86%). Subjects with ICH had no evidence of global hypoxic ischemic brain injury or focal arterial stroke by review of neuroimaging and clinical data. Most subjects (70%) had neonatal seizures, and there was a statistically significant difference in neonatal seizure burden across etiology groups; subjects with HIE had the lowest seizure burden (p=0.03). Seven subjects (8%) had only clinically suspected seizures prior to EEG lead placement, with no seizures captured on subsequent EEG monitoring, and were therefore classified as having a seizure burden of zero. Five of the seven subjects were treated with ASM after their clinically suspected seizure(s); two subjects had a single clinically suspected seizure with no ASM treatment. Time between first clinically suspected seizure and start of EEG monitoring was not significantly different between patients with clinically suspected seizures only (median 7.2 hr, IQR 4.8, 10.6) versus those with EEG-confirmed seizures (5.3 hr, IQR −1.0, 12.2). For all subjects included in the analysis, median duration of EEG monitoring was 73.4 hours (IQR 50.5, 91.0), with no differences among the HIE, stroke, and ICH groups.
Table 2.
Neonatal characteristics and neurologic outcome of subjects with perinatal brain injury
| HIE | Stroke | ICH | |
|---|---|---|---|
|
|
|
|
|
| Neonatal characteristics | (n = 57) | (n = 18) | (n = 9) |
| Male, n (%) | 30 (53) | 9 (50) | 6 (67) |
| Gestational age, w, median (IQR) | 39 (38, 40) | 38 (38, 40) | 39 (39, 40) |
| Birth weight, kg, median (IQR) | 3.2 (3.0, 3.6) | 3.3 (3.1, 3.4) | 3.5 (2.9, 3.6) |
| Race, n (%) | |||
| White | 38 (83) | 12 (86) | 8 (100) |
| Black/African American | 5 (11) | 2 (14) | 0 |
| Other | 3 (7) | 0 | 0 |
| Unreported | 11 | 4 | 1 |
| Ethnicity, Hispanic or Latino, n (%) | 11 (19) | 3 (17) | 0 |
| Received therapeutic hypothermia, n (%) | 49 (86) | 0 | 0 |
| Clinical or EEG-confirmed neonatal seizures, n (%) | 33 (58) | 18 (100) | 8 (89) |
| Neonatal seizure burden, min/hr, median (IQR) | 0.0 (0.0, 3.1) | 1.5 (0.8, 3.6) | 3.4 (0.1, 5.5) |
| Neonatal status epilepticus, n (%) | 9 (16) | 4 (22) | 2 (22) |
| EEG duration, hr, median (IQR) | 74.5 (52.1, 92.0) | 57.7 (48.1, 80.5) | 83.4 (71.3, 105.5) |
| Neurodevelopmental testing | (n = 46) | (n = 16) | (n = 7) |
| Age at testing, m, median (IQR) | 20 (18, 22) | 18 (18, 20) | 19 (18, 22) |
| Bayley-III Composite Scores, mean ± SD | |||
| Cognitive | 93.2 ± 17.9 | 97.7 ± 8.6 | 96.4 ± 9.9 |
| Language | 90.4 ± 18.5 | 92.5 ± 12.3 | 92.1 ± 10.1 |
| Motor | 92.5 ± 18.6 | 93.3 ± 12.3 | 97.4 ± 7.8 |
| Vineland-II Composite Scores, mean ± SD | |||
| Adaptive Behavior | 90.0 ± 17.6 | 93.7 ± 10.1 | 93.0 ± 4.5 |
| Communication | 88.7 ± 17.7 | 93.3 ± 12.3 | 91.3 ± 6.5 |
| Motor Skills | 92.6 ± 24.1 | 95.3 ± 11.9 | 97.2 ± 7.3 |
| Post-neonatal outcome, n/total (%) | |||
| Post-neonatal seizure(s) | 8/56 (14) | 4/18 (22) | 1/9 (11) |
| Confirmed SNHL | 3/57 (5) | 0/18 (0) | 0/9 (0) |
| Suspected or confirmed CVI | 3/50 (6) | 0/18 (0) | 0/8 (0) |
| Abnormal neurologic outcome | 13/42 (31) | 4/17 (24) | 1/8 (13) |
Abbreviations: HIE, hypoxic-ischemic encephalopathy; ICH, intracranial hemorrhage; IQR, interquartile range; SD, standard deviation; SNHL, sensorineural hearing loss; CVI, cerebral visual impairment
Developmental psychological testing was obtained in 69 (82%) of the 84 subjects with acute perinatal brain injury included in the outcome analysis (Table 2). For these subjects, developmental outcome and functioning indices were also lower than expected for age (one-sample t-test; Bayley-III: Cognitive, 94.8 ± 15.2, p=0.01; Language, 91.1 ± 16.1, p<0.001; Motor, 93.3 ± 16.0, p=0.003; Vineland-II: Adaptive Behavior, 91.1 ± 15.5, p<0.001; Communication, 90.0 ± 15.7, p<0.001; Motor Skills, 93.6 ± 20.9, p=0.02). Notably, there was a statistically significant correlation between neonatal seizure burden and scores across Bayley-III Cognitive (Spearman r= −0.45, p<0.001), Language (r= −0.33, p=0.01), and Motor domains (r= −0.31, p=0.02), as well as Vineland-II Adaptive Behavior (r= −0.37, p=0.005), Communication (r= −0.37, p=0.003), and Motor Skills domains (r= −0.34, p=0.01). In addition to the composite scores used in the above analyses, Bayley-III and Vineland-II subscales were examined for outliers, and no individual subcomponent altered the overall results significantly; these subscales were not individually included in the analysis to help prevent multiple comparison statistical errors.
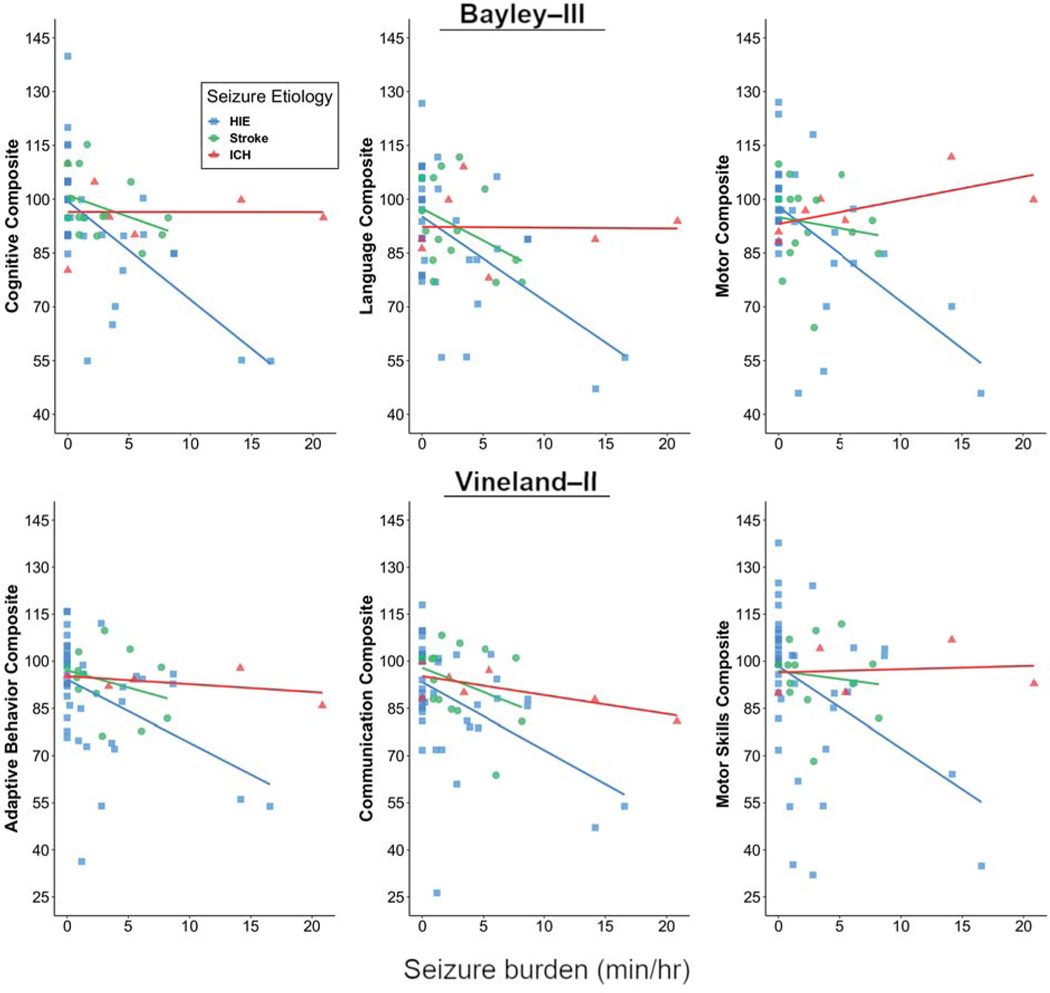
Although higher neonatal seizure burden correlated with worse outcome, in exploratory analyses the neurologic diagnosis (HIE, stroke, or ICH) modified the relationship between neonatal seizure burden and the Bayley-III Cognitive and Motor composite scores (Cognitive interaction p=0.01, Language interaction p=0.09, Motor interaction p=0.006, Figure 2). These interactions were not statistically significant for the Vineland-II composite scores. We then compared the correlation between neonatal seizure burden and developmental scores in neonates with ischemic injury (HIE, stroke) to those with hemorrhagic injury (ICH). Comparison of these two groups demonstrated a stronger association between seizure burden and developmental psychological scores in the HIE/stroke group than in the ICH group for all Bayley-III domains (Cognitive interaction p= 0.01, Language interaction p=0.04, Motor interaction p=0.008). These results are confirmed by comparing Spearman rank correlations between seizure burden and Bayley-III scores (r= −0.54, p<0.001 for Cognitive; r= −0.39, p=0.005 for Language; r= −0.47 for Motor for the HIE/stroke group vs. r= −0.13, p=0.79 for Cognitive; r= 0.12, p=0.80 for Language; r= 0.80, p=0.03 for Motor for the ICH group). In these exploratory models, the statistically significant interaction effects of seizure burden on developmental psychological scores by neurologic diagnosis were confirmed in robust regression analyses based on M-estimation or generalized estimating equations.
Figure 2.
Developmental psychological composite scores (Bayley-III and Vineland-II) and neonatal seizure burden for subjects with acute perinatal brain injury (HIE, stroke, or ICH).
Post-neonatal seizure data were available for 83 subjects (Table 2), with post-neonatal seizures occurring in 13, all of whom had a history of neonatal seizures. This represented a seizure recurrence rate of 22% (13/58 subjects with neonatal seizures). Neonatal status epilepticus was not significantly associated with post-neonatal seizure recurrence, as we found seizure recurrence in 4 of 15 (27%) of subjects with neonatal status epilepticus, compared with 9 of 68 (13%) subjects without status epilepticus (p=0.24). Median age of seizure recurrence was 34 months (IQR 12, 60). All subjects with seizure recurrence had afebrile focal seizures (n=13). Some subjects had additional seizure types, including complex febrile seizures (n=4), afebrile generalized seizures (n=4), and infantile spasms (n=2). Twelve of the 13 subjects remained on ASM treatment at last follow-up, with a median of one ASM (IQR 1, 2). There were no statistically significant differences in the post-neonatal seizure recurrence across neurologic diagnosis, and there was no association between neonatal seizure burden and post-neonatal seizures.
Of the 84 subjects with perinatal brain injury included in the outcome analysis, 49 (58%) were classified as having a normal neurologic outcome, 18 (21%) had an abnormal neurologic outcome, and 17 (20%) had insufficient data for classification (Table 2). Neonatal characteristics were similar between abnormal and normal outcome groups. Thirteen of 42 (31%) subjects with HIE, four of 17 (24%) subjects with stroke, and one of 8 (13%) subjects with ICH had abnormal neurologic outcome, with no significant difference across neurologic diagnosis. For subjects with HIE, treatment with therapeutic hypothermia was almost significantly different between the normal (27/30, 90%) and abnormal (9/13, 69%) groups (p=0.06).
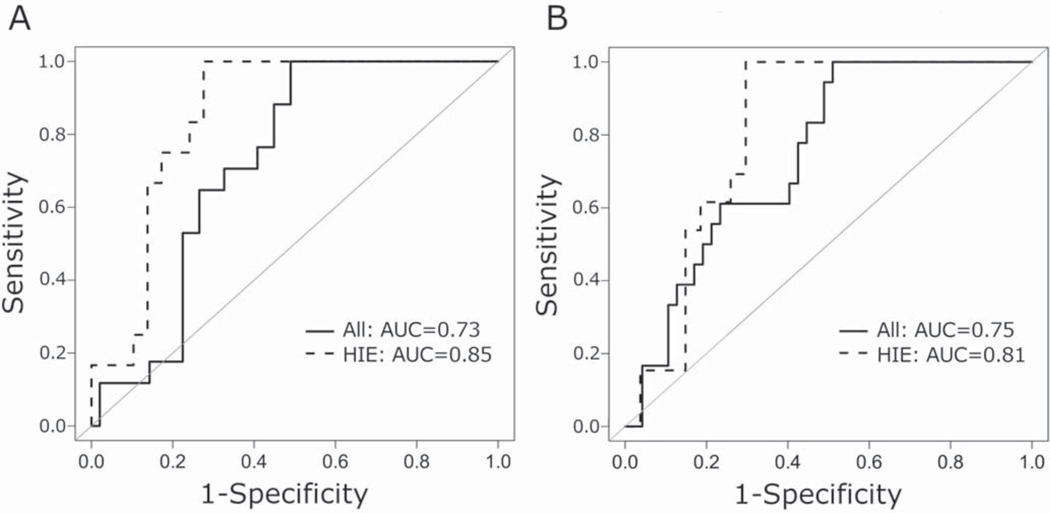
While neonatal seizures were more common in the abnormal outcome group (18/18, 100%) compared with the normal outcome group (32/49, 65%, p=0.003), neonatal status epilepticus was not significantly associated with abnormal outcome. Abnormal outcome was found in 6 of 15 (40%) subjects with neonatal status epilepticus versus 12 of 52 (23%) subjects without neonatal status epilepticus (p=0.21). In contrast, neonatal seizure burden (min/hr) was significantly higher in the abnormal outcome group (median 3.6; IQR 1.2, 4.5) compared with the normal outcome group (0; IQR 0, 3.1; p=0.005). The area under the receiver operating characteristic (ROC) curve for normal versus abnormal outcome and neonatal seizure burden (min/hr) was 0.73 for the entire cohort and 0.85 for HIE only (Figure 3A). Similar results were obtained when seizure burden was analyzed as total seizure minutes recorded, with a higher seizure burden in the abnormal (median 102.0; IQR 28.2, 188.3) versus the normal outcome group (3.9; IQR 0, 73.6; p=0.002) and comparable areas under the ROC curve (entire cohort = 0.75, HIE only = 0.81; Figure 3B).
Figure 3.
Receiver operating characteristic curves for normal versus abnormal outcome and (A) seizure burden in min/hr and (B) seizure burden in total seizure minutes. Sensitivity = true positive rate. (1-specificity) = false positive rate.
Discussion:
Here we present long-term neurologic outcome data from a randomized, controlled, double-blind trial of bumetanide as add-on therapy to phenobarbital to treat neonatal seizures,18 and find no significant differences in developmental outcome or post-neonatal seizure recurrence between treatment and control groups. The trial was an early phase, dose-finding trial designed to test the safety, pharmacokinetics, and pharmacodynamics of a single dose of bumetanide. As such, the trial was not designed to evaluate the efficacy of bumetanide to reduce total neonatal seizure burden or improve long-term neurologic outcome. These outcome data demonstrated no long-term benefits or adverse effects of a single dose of bumetanide.
In this study, we evaluated the contribution of neonatal seizures and seizure burden to long-term neurologic outcome in neonates with acute perinatal brain injury. The effect of bumetanide on long-term outcome is expected to be secondary to its effects on neonatal seizure burden, and, as noted above, there were no differences between the treatment and control groups in neurologic outcome after a single dose of bumetanide. Furthermore, all subjects received multiple doses of standard ASMs for neonatal seizures, which would be expected to outweigh by far the effects of a single dose of bumetanide on seizure burden. Although the median half-life (elimination = pharmacokinetics) of bumetanide in neonates was 16 hours, the duration of antiseizure action (bumetanide effect = pharmacodynamics) is expected to be considerably shorter than that, particularly compared with the duration of antiseizure action of the other ASMs administered and the period of susceptibility to seizures (days). Therefore, we used neonatal EEG and outcome data from all enrolled subjects, regardless of randomization status. While neonatal seizure burden did not correlate with post-neonatal seizure recurrence, there was a significant and consistent correlation between the presence of neonatal seizures and higher neonatal seizure burden with worse developmental outcome. Interestingly, this relationship between neonatal seizure burden and later neurodevelopmental scores was modified by seizure etiology, with stronger correlation in subjects with HIE/stroke compared with ICH. This effect was significant despite the small number of subjects with ICH in our study. To our knowledge, this effect of seizure etiology modulating the effect of seizure burden has not been examined previously. Prior studies have focused on neonates with HIE, and our data highlight a need for larger prospective studies of neonatal seizure burden and neurologic outcome in neonates with other seizure etiologies.
The neurologic outcome in all trial subjects is similar to previously published studies in neonates at risk for seizures. Developmental scores from both the Bayley-III and Vineland-II were lower than expected for age, in agreement with previous studies reporting abnormal developmental outcome in neonates with acute perinatal brain injury, particularly those with neonatal seizures.9–15,22 The 22% incidence of post-neonatal seizures is also consistent with previously reported rates of seizure recurrence, which range from 5 to up to 56% of surviving patients with a history of neonatal seizures.23–31 Sensorineural hearing loss was only observed among the subjects with HIE, with three affected subjects (two in the bumetanide group and one in the non-randomized group). This represented 5% of enrolled neonates with HIE, which is lower than what has been previously reported (10%) in neonates with HIE, likely related to inclusion of subjects with mild HIE (and no seizures) in the non-randomized group.32 Cerebral visual impairment was also seen exclusively in the HIE group, at a rate (5%) in line with prior data (3–4%).33
Our analysis of subjects with acute perinatal brain injury (HIE, stroke, or ICH) demonstrated a strong correlation between continuous measures of neonatal seizure burden and developmental outcome, adding to the growing evidence that higher seizure burden is correlated with worse neurodevelopmental outcome.12–15 Prior papers examining the relationship between seizure burden and short- and long-term outcome measures have used a variety of measures to quantify seizure burden, including categorization of seizure burden based on seizure number9–11,14,31 or aEEG data,15 quantification of total seizure duration in seconds,12 minutes,13 hours,9 or days,31 and quantification of maximum hourly seizure burden.13 Because of the variability of EEG monitoring duration in our study, we measured seizure burden as minutes of seizure activity divided by the number of hours in the period from first to last seizure (min/hr). Additionally, to allow for direct comparison of our results to a similar study by Kharoshankaya et al.13 conducted in neonates with HIE, we calculated seizure burden as total minutes of seizure activity and used a binary normal/abnormal neurologic outcome scale. Here again, our data show that the group with abnormal outcome were more likely to have had neonatal seizures and a significantly higher neonatal seizure burden, with median seizure burdens and ROC areas under the curve similar to those presented by Kharoshankaya et al.13 In contrast to that study, however, we did not find a clear threshold for seizure burden to predict abnormal outcome. Indeed, our continuous analysis suggests that with increasing neonatal seizure burden, there is increasing risk for an abnormal outcome.
Unlike most prior outcome studies, our study included detailed EEG data for neonates with stroke and ICH in addition to HIE, so we were also able to directly compare the effects of seizure burden across different seizure etiologies. It has long been observed that seizure etiology plays an important role in determining long-term outcome, with worse injury corresponding with worse outcome (e.g., global HIE with bilateral or diffuse injury having worse outcome than a unilateral focal stroke).34,35 Prior work has also shown that certain diagnoses, such as infection, brain malformations, and genetic disorders, are predictors of a worse prognosis, compared with HIE.36 Interestingly, evidence from animal models suggests that injury secondary to hypoxia-ischemia is exacerbated by the presence of seizure activity,16,17 suggesting that seizure burden may play a more important role in determining outcome in a brain with hypoxic-ischemic injury, versus a brain that did not suffer from lack of oxygen and blood flow. However, the differential effects of neonatal seizure burden with and without hypoxic-ischemic injury have not been carefully studied in the clinical setting. Our results suggest that seizure etiology does indeed affect the relationship between seizure burden and developmental outcome in perinatal brain injury. Specifically, subjects with ICH, as compared with those with HIE or stroke, had a weaker correlation between neonatal seizure burden and developmental psychological scores. Notably, as can be appreciated in Figure 2, there were subjects with ICH who had high developmental scores despite having had a high neonatal seizure burden. Although experimental models show that hemorrhage adjacent to or involving cortical neurons may cause acute seizures and later epilepsy,37 neonatal seizures without ischemic injury are associated with generally favorable neurobehavioral outcome.38 This preliminary evidence suggests that the injurious effects of seizure activity may be specific to global or local hypoxic-ischemic etiologies of acute seizures, and thus the prognostic importance of seizure burden is dependent on seizure etiology. However, as our study included relatively few subjects with ICH, it will be important to repeat these analyses in a larger cohort.
Another factor to consider is the degree to which seizure burden is a proxy for severity and/or extent of brain injury. Several studies have demonstrated that, in neonates with HIE, seizure burden correlates with HIE severity22 and/or the extent of brain injury seen on MRI.10,39–41 This has led to the argument that seizure burden is merely a proxy for the severity of brain injury, rather than an additional contributor to worse neurologic outcome,22 although one study still found a significant correlation between seizures and outcome after controlling for severity of injury seen on brain MRI.10 Additionally, in the studies that examined the relationship between seizure burden and brain MRI,10,39–41 MRIs were obtained at a mean or median of five to nine days of age, which is generally after the resolution of neonatal seizures secondary to acute injury. Therefore, it is possible that the injury seen on MRI was in part secondary to seizure activity. In contrast, seizures associated with hemorrhage adjacent to cortical neurons without a large area of associated ischemic injury may not exacerbate brain injury or worsen later cognitive outcome. Our data support the notion that seizure burden in neonates with hemorrhagic brain injury may be less related to extent of injury or may not exacerbate the brain injury compared with neonates with global or local hypoxia ischemia such as occurs with HIE and stroke, explaining in part why the correlation between seizure burden and outcome is weaker. Further research with larger populations and detailed neuroimaging analysis will be helpful in clarifying the relationship between injury severity, seizure burden, and long-term outcome.
In terms of post-neonatal seizures, we found, not surprisingly, that all subjects with post-neonatal seizures had a history of neonatal seizures. However, we did not find a statistically significant correlation between neonatal seizure burden or status epilepticus and post-neonatal seizure recurrence. This is in contrast to prior studies, which demonstrated a relationship between status epilepticus and later life epilepsy in neonates with neonatal encephalopathy,27 as well as presence of neonatal seizures and risk of later epilepsy in patients with a history of neonatal stroke.29 A recent study in infants with acute brain injury also demonstrated a relationship between days of neonatal seizures and increased risk of post-neonatal epilepsy.31 Several factors may explain why we did not find a significant relationship between seizure burden and post-neonatal seizure recurrence. First, as noted above, variable methods for quantifying neonatal seizure burden have been used. Second, the number of subjects with post-neonatal seizure recurrence was small overall (n=13), and a larger number might be required to detect an association with neonatal seizure burden. Indeed, the fact that 27% of subjects with vs. 13% without status epilepticus had seizure recurrence, suggests that with more subjects we may have been able to detect a statistically significant association between status epilepticus and seizure recurrence. Third, since post-neonatal seizure recurrence can be observed years after the neonatal period,27 it is likely that our follow-up period was too short to detect all subjects who will eventually develop post-neonatal seizures. Finally, risk factors that we were unable to measure in this study likely play a role in post-neonatal seizure recurrence, including extent and location of brain injury and genetic factors.
Limitations of our study include lack of a direct measure of brain injury severity. Also, although our study is one of the larger studies of neonates with acute perinatal brain injury to date, the number of subjects with etiologies such as stroke or ICH remains small. It will be important to conduct similar analyses with larger populations of patients to determine if the effects we find are robust. Finally, our follow-up data consisted of developmental testing during the preschool years, which likely do not capture the full spectrum of long-term neurologic impairments that can be seen in patients with a history of acute perinatal brain injury, as more subtle neuropsychological impairments manifest at an older age.42
Strengths of our study include the prolonged neonatal EEG recordings with quantification of neonatal seizure burden. Additionally, the availability of detailed and prospectively obtained developmental psychological testing by a blinded investigator allowed for rigorous and objective assessment of developmental outcome. Finally, this is one of the few studies to date to include neonates with stroke and ICH in a study of seizure burden and long-term neurologic outcome, and our finding of differences between seizure etiologies is a compelling argument for more studies of this kind. Indeed, heterogeneity in the relationship between seizure burden and outcome across different seizure etiologies could explain in part some of the conflicting findings on the impact of treating neonatal electrographic seizures on long-term outcome.43
Overall, our data demonstrate the lack of long-term beneficial or adverse effects of a single dose of bumetanide to treat neonatal seizures, when comparing treatment and standard therapy control groups. Our results add to the literature demonstrating a clear link between higher neonatal seizure burden and worse neurodevelopmental outcome, and we present the preliminary but novel finding that this relationship was stronger for neonates with global or focal arterial ischemic brain injury than intracranial hemorrhage. Thus, there is a need for further research, particularly with the goal of finding more effective treatments for neonatal seizures that might reduce adverse long-term sequelae of neonatal seizures.
Data Availability Statement:
The datasets generated during and/or analyzed during the current study are not publicly available due to protection of patient data but are available from the corresponding author on reasonable request.
Acknowledgments:
The authors are grateful for the contributions of many physician, nursing, pharmaceutical, laboratory and research staff at all four sites, and are especially grateful for the participation of the families. The trial was funded by NIH/NINDS grant 5R01 NS066929, and grants from the CURE foundation, Harvard Catalyst - Harvard Clinical and Translational Science Center, Tufts Clinical and Translational Science Institute (CTSI), the Charles H. Hood Foundation, the Translational Research Program at Boston Children’s Hospital, and the Mooney Family Initiative for Translation and Clinical Studies in Rare Diseases.
Funding:
The trial was funded by NIH/NINDS grant 5R01 NS066929, and grants from the CURE foundation, Harvard Catalyst - Harvard Clinical and Translational Science Center, Tufts Clinical and Translational Science Institute (CTSI), the Charles H. Hood Foundation, the Translational Research Program at Boston Children’s Hospital, and the Mooney Family Initiative for Translation and Clinical Studies in Rare Diseases.
APPENDIX
Appendix
Boston Bumetanide Trial Group Members:
| Member | Institution |
|---|---|
| Sarah Barnett, MD | Boston Children’s Hospital |
| Gerard Berry, MD | Boston Children’s Hospital |
| Joseph H. Chou, MD | Massachusetts General Hospital |
| Helen A. Christou, MD | Brigham and Women’s Hospital |
| Jonathan M. Davis, MDCM | Floating Hospital for Children/Tufts Medical Center |
| Min Dong, PhD | Cincinnati Children’s Hospital Medical Center |
| Carmen Rosa Fortuno, MD | Boston Children’s Hospital |
| John N. Gaitanis, MD | Floating Hospital for Children/Tufts Medical Center |
| David A. Griesemer, MD | Floating Hospital for Children/Tufts Medical Center |
| Breda Hayes, MD | Boston Children’s Hospital |
| Xiaoping Huang, MD | Boston Children’s Hospital |
| Robert M. Insoft, MD, FAAP | Brigham and Women’s Hospital |
| Frances E. Jensen, MD | Boston Children’s Hospital |
| Fengxin Lu, MD | Boston Children’s Hospital |
| Prajakta Mangeshkar, MS | Boston Children’s Hospital |
| Deirdre O’Reilly, MD, MPH | Boston Children’s Hospital |
| Danielle B. Pier, MD | Boston Children’s Hospital |
| Christine Powell, MA | Boston Children’s Hospital |
| Arnold J. Sansevere, MD | Boston Children’s Hospital |
| Adam Simmons, MPH, CCRC | Boston Children’s Hospital |
| Avantika Singh, MD | Boston Children’s Hospital |
| Christian Stopp, MS | Boston Children’s Hospital |
| Ju Tang, MD, PhD | Floating Hospital for Children/Tufts Medical Center |
| Alexander A. Vinks, PhD, PharmD | Cincinnati Children’s Hospital Medical Center |
| Linh N. Vu, MA | Boston Children’s Hospital |
Footnotes
Ethics approval statement: IRB approval was obtained from all study sites prior to subject enrollment.
Patient consent statement: Parent(s)/guardian(s) of the patients provided written informed consent.
Clinical trial registration: The trial was prospectively registered with clinicaltrials.gov (NCT00830531).
Social media information Dr Soul’s twitter handle is @DrJanetSoul
Lay language synopsis: Seizures in newborns are commonly caused by brain injury from asphyxia (decreased brain blood flow and oxygen), strokes, and bleeds. This study adds to the growing evidence that frequent seizures in the newborn period are linked to worse cognitive and language development at an older age. This study also provides new preliminary evidence that the impact of seizures on development may depend in part on the underlying cause of the brain injury, with a stronger link between high seizure activity and later development in injuries due to lack of oxygen/blood flow (e.g., asphyxia, strokes) compared with bleeding in the brain.
Draft Tweet: Trowbridge et al. provide preliminary evidence that the impact of neonatal seizure burden on developmental outcomes may depend on seizure etiology, with a stronger link between seizure burden and outcome in ischemic (HIE, stroke) versus hemorrhagic injuries.
Conflict of interest disclosure: The authors report no conflicts of interest.
Potential conflicts of interest: The authors have no conflicts of interest to report.
References
- 1.Glass HC, Shellhaas RA, Wusthoff CJ, Chang T, Abend NS, Chu CJ, et al. Contemporary Profile of Seizures in Neonates: A Prospective Cohort Study. J Pediatr. 2016;174:98–103.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rennie JM, de Vries LS, Blennow M, Foran A, Shah DK, Livingstone V, et al. Characterisation of neonatal seizures and their treatment using continuous EEG monitoring: a multicentre experience. Arch Dis Child Fetal Neonatal Ed. 2019;104:F493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malone A, Ryan CA, Fitzgerald A, Burgoyne L, Connolly S, Boylan GB. Interobserver agreement in neonatal seizure identification. Epilepsia. 2009;50:2097–101. [DOI] [PubMed] [Google Scholar]
- 4.Glass HC, Wusthoff CJ, Shellhaas RA, Tsuchida TN, Bonifacio SL, Cordeiro M, et al. Risk factors for EEG seizures in neonates treated with hypothermia. Neurology. 2014;82:1239–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scher MS, Alvin J, Gaus L, Minnigh B, Painter MJ. Uncoupling of EEG-clinical neonatal seizures after antiepileptic drug use. Pediatr Neurol. 2003;28:277–80. [DOI] [PubMed] [Google Scholar]
- 6.Shellhaas RA, Chang T, Tsuchida T, Scher MS, Riviello JJ, Abend NS, et al. The American Clinical Neurophysiology Society’s Guideline on Continuous Electroencephalography Monitoring in Neonates. J Clin Neurophysiol Off Publ Am Electroencephalogr Soc. 2011;28:611–7. [DOI] [PubMed] [Google Scholar]
- 7.van Rooij LGM, Toet MC, van Huffelen AC, Groenendaal F, Laan W, Zecic A, et al. Effect of treatment of subclinical neonatal seizures detected with aEEG: randomized, controlled trial. Pediatrics. 2010;125:e358–366. [DOI] [PubMed] [Google Scholar]
- 8.Lemmon ME, Bonifacio SL, Shellhaas RA, Wusthoff CJ, Greenberg RG, Soul JS, et al. Characterization of Death in Infants With Neonatal Seizures. Pediatr Neurol. 2020;113:21–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McBride MC, Laroia N, Guillet R. Electrographic seizures in neonates correlate with poor neurodevelopmental outcome. Neurology. 2000;55:506–13. [DOI] [PubMed] [Google Scholar]
- 10.Glass HC, Glidden D, Jeremy RJ, Barkovich AJ, Ferriero DM, Miller SP. Clinical Neonatal Seizures are Independently Associated with Outcome in Infants at Risk for Hypoxic-Ischemic Brain Injury. J Pediatr. 2009;155:318–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murray DM, Boylan GB, Ryan CA, Connolly S. Early EEG findings in hypoxic-ischemic encephalopathy predict outcomes at 2 years. Pediatrics. 2009;124:e459–467. [DOI] [PubMed] [Google Scholar]
- 12.Srinivasakumar P, Zempel J, Trivedi S, Wallendorf M, Rao R, Smith B, et al. Treating EEG Seizures in Hypoxic Ischemic Encephalopathy: A Randomized Controlled Trial. Pediatrics. 2015;136:e1302–1309. [DOI] [PubMed] [Google Scholar]
- 13.Kharoshankaya L, Stevenson NJ, Livingstone V, Murray DM, Murphy BP, Ahearne CE, et al. Seizure burden and neurodevelopmental outcome in neonates with hypoxic-ischemic encephalopathy. Dev Med Child Neurol. 2016;58:1242–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fitzgerald MP, Massey SL, Fung FW, Kessler SK, Abend NS. High electroencephalographic seizure exposure is associated with unfavorable outcomes in neonates with hypoxic-ischemic encephalopathy. Seizure. 2018;61:221–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basti C, Maranella E, Cimini N, Catalucci A, Ciccarelli S, Del Torto M, et al. Seizure burden and neurodevelopmental outcome in newborns with hypoxic-ischemic encephalopathy treated with therapeutic hypothermia: A single center observational study. Seizure. 2020;83:154–9. [DOI] [PubMed] [Google Scholar]
- 16.Wirrell EC, Armstrong EA, Osman LD, Yager JY. Prolonged seizures exacerbate perinatal hypoxic-ischemic brain damage. Pediatr Res. 2001;50:445–54. [DOI] [PubMed] [Google Scholar]
- 17.Yager JY, Armstrong EA, Miyashita H, Wirrell EC. Prolonged neonatal seizures exacerbate hypoxic-ischemic brain damage: correlation with cerebral energy metabolism and excitatory amino acid release. Dev Neurosci. 2002;24:367–81. [DOI] [PubMed] [Google Scholar]
- 18.Soul JS, Bergin AM, Stopp C, Hayes B, Singh A, Fortuno CR, et al. A Pilot Randomized, Controlled, Double-Blind Trial of Bumetanide to Treat Neonatal Seizures. Ann Neurol. 2021;89:327–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bayley N. Bayley scales of infant and toddler development. 3rd ed. San Antonio, TX: PsychCorp; 2006. [Google Scholar]
- 20.Sparrow SS, Cicchetti V, Balla A. Vineland adaptive behavior scales. 2nd ed. Circle Pines, MN: American Guidance Service; 2005. [Google Scholar]
- 21.Palisano RJ, Rosenbaum P, Bartlett D, Livingston MH. Content validity of the expanded and revised Gross Motor Function Classification System. Dev Med Child Neurol. 2008;50:744–50. [DOI] [PubMed] [Google Scholar]
- 22.Kwon JM, Guillet R, Shankaran S, Laptook AR, McDonald SA, Ehrenkranz RA, et al. Clinical seizures in neonatal hypoxic-ischemic encephalopathy have no independent impact on neurodevelopmental outcome: secondary analyses of data from the neonatal research network hypothermia trial. J Child Neurol. 2011;26:322–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee J, Croen LA, Lindan C, Nash KB, Yoshida CK, Ferriero DM, et al. Predictors of outcome in perinatal arterial stroke: a population-based study. Ann Neurol. 2005;58:303–8. [DOI] [PubMed] [Google Scholar]
- 24.Guillet R, Kwon J. Seizure recurrence and developmental disabilities after neonatal seizures: outcomes are unrelated to use of phenobarbital prophylaxis. J Child Neurol. 2007;22:389–95. [DOI] [PubMed] [Google Scholar]
- 25.Ronen GM, Buckley D, Penney S, Streiner DL. Long-term prognosis in children with neonatal seizures: a population-based study. Neurology. 2007;69:1816–22. [DOI] [PubMed] [Google Scholar]
- 26.Pisani F, Orsini M, Braibanti S, Copioli C, Sisti L, Turco EC. Development of epilepsy in newborns with moderate hypoxic-ischemic encephalopathy and neonatal seizures. Brain Dev. 2009;31:64–8. [DOI] [PubMed] [Google Scholar]
- 27.Glass HC, Hong KJ, Rogers EE, Jeremy RJ, Bonifacio SL, Sullivan JE, et al. Risk Factors For Epilepsy In Children With Neonatal Encephalopathy. Pediatr Res. 2011;70:535–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wusthoff CJ, Kessler SK, Vossough A, Ichord R, Zelonis S, Halperin A, et al. Risk of later seizure after perinatal arterial ischemic stroke: a prospective cohort study. Pediatrics. 2011;127:e1550–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fox CK, Glass HC, Sidney S, Smith SE, Fullerton HJ. Neonatal seizures triple the risk of a remote seizure after perinatal ischemic stroke. Neurology. 2016;86:2179–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clancy RR, Legido A. Postnatal epilepsy after EEG-confirmed neonatal seizures. Epilepsia. 1991;32:69–76. [DOI] [PubMed] [Google Scholar]
- 31.Shellhaas RA, Wusthoff CJ, Numis AL, Chu CJ, Massey SL, Abend NS, et al. Early-life epilepsy after acute symptomatic neonatal seizures: A prospective multicenter study. Epilepsia. 2021;62:1871–82. [DOI] [PubMed] [Google Scholar]
- 32.Smit E, Liu X, Gill H, Sabir H, Jary S, Thoresen M. Factors Associated with Permanent Hearing Impairment in Infants Treated with Therapeutic Hypothermia. J Pediatr. 2013;163:995–1000. [DOI] [PubMed] [Google Scholar]
- 33.Laptook AR, Shankaran S, Tyson JE, Munoz B, Bell EF, Goldberg RN, et al. Effect of Therapeutic Hypothermia Initiated After 6 Hours of Age on Death or Disability Among Newborns With Hypoxic-Ischemic Encephalopathy. JAMA. 2017;318:1550–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mizrahi EM, Clancy RR. Neonatal seizures: early-onset seizure syndromes and their consequences for development. Ment Retard Dev Disabil Res Rev. 2000;6:229–41. [DOI] [PubMed] [Google Scholar]
- 35.Tekgul H, Gauvreau K, Soul J, Murphy L, Robertson R, Stewart J, et al. The current etiologic profile and neurodevelopmental outcome of seizures in term newborn infants. Pediatrics. 2006;117:1270–80. [DOI] [PubMed] [Google Scholar]
- 36.Garfinkle J, Shevell MI. Prognostic factors and development of a scoring system for outcome of neonatal seizures in term infants. Eur J Paediatr Neurol EJPN Off J Eur Paediatr Neurol Soc. 2011;15:222–9. [DOI] [PubMed] [Google Scholar]
- 37.Hammond EJ, Ramsay RE, Villarreal HJ, Wilder BJ. Effects of intracortical injection of blood and blood components on the electrocorticogram. Epilepsia. 1980;21:3–14. [DOI] [PubMed] [Google Scholar]
- 38.Huang L, Cilio MR, Silveira DC, McCabe BK, Sogawa Y, Stafstrom CE, et al. Long-term effects of neonatal seizures: a behavioral, electrophysiological, and histological study. Brain Res Dev Brain Res. 1999;118:99–107. [DOI] [PubMed] [Google Scholar]
- 39.Glass HC, Nash KB, Bonifacio SL, Barkovich AJ, Ferriero DM, Sullivan JE, et al. Seizures and magnetic resonance imaging-detected brain injury in newborns cooled for hypoxic-ischemic encephalopathy. J Pediatr. 2011;159:731–735.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nash KB, Bonifacio SL, Glass HC, Sullivan JE, Barkovich AJ, Ferriero DM, et al. Video-EEG monitoring in newborns with hypoxic-ischemic encephalopathy treated with hypothermia. Neurology. 2011;76:556–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shah DK, Wusthoff CJ, Clarke P, Wyatt JS, Ramaiah SM, Dias RJ, et al. Electrographic seizures are associated with brain injury in newborns undergoing therapeutic hypothermia. Arch Dis Child - Fetal Neonatal Ed. 2014;99:F219–24. [DOI] [PubMed] [Google Scholar]
- 42.van Handel M, Swaab H, de Vries LS, Jongmans MJ. Long-term cognitive and behavioral consequences of neonatal encephalopathy following perinatal asphyxia: a review. Eur J Pediatr. 2007;166:645–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hunt RW, Liley HG, Wagh D, Schembri R, Lee KJ, Shearman AD, et al. Effect of Treatment of Clinical Seizures vs Electrographic Seizures in Full-Term and Near-Term Neonates: A Randomized Clinical Trial. JAMA Netw Open. 2021;4:e2139604. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available due to protection of patient data but are available from the corresponding author on reasonable request.