Abstract
Rationale
Cigarette smoking contributes to the risk of death through different mechanisms.
Objectives
To determine how causes of and clinical features associated with death vary in tobacco cigarette users by lung function impairment.
Methods
We stratified current and former tobacco cigarette users enrolled in Genetic Epidemiology of Chronic Obstructive Pulmonary Disease (COPDGene) into normal spirometry, PRISm (Preserved Ratio Impaired Spirometry), Global Initiative for Chronic Obstructive Lung Disease (GOLD) 1–2 COPD, and GOLD 3–4 COPD. Deaths were identified via longitudinal follow-up and Social Security Death Index search. Causes of death were adjudicated after a review of death certificates, medical records, and next-of-kin interviews. We tested associations between baseline clinical variables and all-cause mortality using multivariable Cox proportional hazards models.
Measurements and Main Results
Over a 10.1-year median follow-up, 2,200 deaths occurred among 10,132 participants (age 59.5 ± 9.0 yr; 46.6% women). Death from cardiovascular disease was most frequent in PRISm (31% of deaths). Lung cancer deaths were most frequent in GOLD 1–2 (18% of deaths vs. 9–11% in other groups). Respiratory deaths outpaced competing causes of death in GOLD 3–4, particularly when BODE index ⩾7. St. George’s Respiratory Questionnaire score ⩾25 was associated with higher mortality in all groups: Hazard ratio (HR), 1.48 (1.20–1.84) normal spirometry; HR, 1.40 (1.05–1.87) PRISm; HR, 1.80 (1.49–2.17) GOLD 1–2; HR, 1.65 (1.26–2.17) GOLD 3–4. History of respiratory exacerbations was associated with higher mortality in GOLD 1–2 and GOLD 3–4, quantitative emphysema in GOLD 1–2, and airway wall thickness in PRISm and GOLD 3–4.
Conclusions
Leading causes of death vary by lung function impairment in tobacco cigarette users. Worse respiratory-related quality of life is associated with all-cause mortality regardless of lung function.
Keywords: mortality, smokers, spirometry, exacerbations, respiratory-related quality of life
At a Glance Commentary
Scientific Knowledge on the Subject
Mortality adjudication analyses of clinical trials that enrolled participants with smoking-related chronic obstructive pulmonary disease (COPD) suggest the proportion of respiratory deaths is higher when lung function is lower. However, outside of clinical trials, the distribution of causes of death in current and former tobacco cigarette users with different severities of lung function impairment has not been fully characterized.
What This Study Adds to the Field
In this analysis of the COPDGene cohort, death from cardiovascular disease was most frequent in PRISm (preserved ratio impaired spirometry), lung cancer deaths were most frequent in GOLD (Global Initiative for Chronic Obstructive Lung Disease) 1–2 COPD, and respiratory deaths outpaced competing causes of death in GOLD 3–4 COPD, especially when the BODE (body mass index, airflow obstruction, dyspnea, exercise capacity) index was ⩾7. Impaired baseline respiratory-related quality of life was independently associated with higher 10-year all-cause mortality regardless of lung function.
Cigarette smoking is responsible for one of every five deaths in the United States, most commonly because of respiratory disease, cardiovascular disease, and malignancy (1, 2). The primary cause of death in individuals with a smoking history depends on a host of genetic, clinical, and behavioral determinants, including susceptibility to lung injury. Mortality adjudication analyses of clinical trials that enrolled participants with smoking-related chronic obstructive pulmonary disease (COPD) suggest the proportion of respiratory deaths is higher when the FEV1 is lower. For example, in the European Respiratory Society Study on Chronic Obstructive Pulmonary Disease (mean FEV1 77% predicted), 11% of deaths were attributed to respiratory causes (3). In contrast, the proportions of respiratory deaths in the Toward a Revolution in COPD Health (mean FEV1 44% predicted) and Understanding Potential Long-Term Impacts on Function with Tiotropium (mean FEV1 48% predicted) clinical trials were 35% and 38%, respectively (4–7). Outside of mortality data from COPD clinical trials, the distribution of causes of death in clinically diverse tobacco cigarette users with and without airflow obstruction has not been fully characterized.
Beyond FEV1, clinical parameters such as the burden of respiratory symptoms and the frequency of respiratory exacerbations help capture the heterogeneity of smoking-related lung disease (8–11). In addition, chest imaging features such as the extent of emphysema and airway wall thickness on computed tomography (CT) further inform the nature and severity of such disease (10–12). These features have become increasingly available in clinical practice as chest CTs are routinely obtained for a number of indications ranging from screening for lung cancer to evaluation for pulmonary embolism. However, the impact of these clinical and imaging characteristics on long-term all-cause and respiratory-related mortality has not been comprehensively examined in tobacco cigarette users with different severities of lung function impairment.
We hypothesized that leading causes of death and the association of common clinical and imaging features with death vary by the type and severity of lung function impairment in individuals with a smoking history. We tested this hypothesis using data from the Genetic Epidemiology of Chronic Obstructive Pulmonary Disease (COPDGene) study that enrolled current and former tobacco cigarette users with an appreciable range of lung disease severity. Some of the results of this analysis have been previously reported in the form of an abstract (13).
Methods
Study Population and Measurements
COPDGene is a multicenter longitudinal cohort study in the United States that enrolled individuals between the ages of 45 and 80 years with current or former tobacco use (⩾10 pack-years) (14). Exclusion criteria included a history of lung disease other than asthma, active cancer under treatment, a lung mass suspicious of malignancy, a recent myocardial infarction, and a history of radiation therapy to the chest.
Data on demographics, smoking history, comorbidities, and number of respiratory exacerbations in the year before enrollment were collected from participants at the baseline visit. Exacerbations were defined as acute worsening of respiratory symptoms requiring treatment with antibiotics and/or systemic corticosteroids. Respiratory-related quality of life was assessed through the administration of the SGRQ (St. George’s Respiratory Questionnaire), with a total score of ⩾25 denoting significant impairment (range, 0–100) (15). Spirometry was performed using an Easy-One spirometer (ndd Medical Technologies) at the baseline visit before and after administration of 180 μg of albuterol. The BODE (Body mass index, airflow Obstruction, Dyspnea, Exercise capacity) index was calculated using the body mass index (BMI), postbronchodilator FEV1% predicted, mMRC (modified Medical Research Council) dyspnea severity score, and the distance walked on a 6-minute-walk test (range, 0–10, with higher scores portending a worse prognosis) (16).
Participants underwent a volumetric chest CT at the baseline visit, as previously described (14). Emphysema was quantified as the percent of lung volume with attenuation <−950 Hounsfield Units at full inspiration. Airway wall thickness was assessed using Pi10, a standardized measure of the square root of the wall area for a hypothetical airway with an internal perimeter of 10 mm (VIDA Diagnostics) (17).
The study protocol was approved by the institutional review boards of participating centers, and all participants gave their written informed consent.
Identification and Adjudication of Deaths
Participants were enrolled from January 2008 to June 2011 and followed through August 2020 for vital status determination. Deaths were identified via the Longitudinal Follow-up Program of COPDGene and a search of the Social Security Death Index (18). The COPDGene Death Adjudication Committee then reviewed all available sources of information, including death certificates, medical records, and next-of-kin interviews, to determine the cause of death according to the principles of mortality adjudication adopted by the Clinical Endpoints Committee of the Toward a Revolution in COPD Health study (see the Appendix in the online supplement) (6). Causes of death that could not be definitively adjudicated after a review of all available information were classified as inconclusive. Deaths for which adequate sources of information could not be obtained had their cause classified as missing.
Statistical Analyses
Analyses included all participants and were also stratified by the following lung function categories on the basis of postbronchodilator spirometry: Normal spirometry (FEV1/FVC ⩾ 0.7 and FEV1 ⩾ 80% predicted), preserved ratio impaired spirometry (PRISm: FEV1/FVC ⩾ 0.7 and FEV1 < 80% predicted) (19), GOLD 1–2 (FEV1/FVC < 0.7 and FEV1 ⩾ 50% predicted), and GOLD 3–4 (FEV1/FVC < 0.7 and FEV1 < 50% predicted) (20). We compared the proportions of each cause of death between any two lung function categories using the chi-square test. For each lung function category, we estimated survival probabilities stratified by the number of exacerbations in the year before enrollment (⩾2 vs. <2) and by baseline SGRQ score (⩾25 vs. <25) using the Kaplan-Meier product limit estimator.
We constructed multivariable Cox proportional hazards models with all-cause mortality as the outcome and the following predictors of interest: ⩾2 exacerbations in the year before enrollment, SGRQ ⩾ 25, mMRC ⩾ 2, and chronic bronchitis (defined as chronic cough and sputum production for at least 3 months per year for two consecutive years) in separate clinical models, and 1% absolute increase in emphysema and a one–standard-deviation increase in Pi10 in separate imaging models. Clinical models were adjusted for age, sex, race, BMI, smoking status, smoking pack-years, highest level of school completed, postbronchodilator FEV1% predicted, and study site. Imaging models were adjusted for the same variables as clinical models except for scanner make instead of the study site.
To account for dependent censoring from competing causes of death, we applied inverse probability weighting to Kaplan-Meier curves to model probabilities of death from respiratory disease, cardiovascular disease, and lung cancer in each lung function category. We also applied inverse probability weighting to multivariable Cox proportional hazards models with respiratory mortality as the outcome. These models were weighted for demographic and clinical variables that could affect risk and cause of mortality in tobacco cigarette users, including age, sex, race, BMI, smoking status, smoking pack-years, time since smoking cessation, postbronchodilator FEV1% predicted, the highest level of school completed, personal and family history of cancer, and self-reported history of coronary artery disease, congestive heart failure, and diabetes mellitus.
All analyses were performed in R software version 3.4.0. A P value less than 0.05 was considered statistically significant.
Results
Baseline Characteristics
The baseline characteristics of the 10,132 participants (4,387 normal spirometry, 1,262 PRISm, 2,713 GOLD 1–2, and 1,770 GOLD 3–4) are summarized in Table 1. The mean age was 59.5 years. Women and Black individuals accounted for 46.6% and 33.2% of the cohort, respectively. The proportion of participants with at least two respiratory exacerbations in the year before enrollment increased with the severity of lung function impairment: 2.7% in normal spirometry, 7.4% in PRISm, 10.7% in GOLD 1–2, and 23.3% in GOLD 3–4. The proportion of participants with SGRQ ⩾ 25 was lowest in normal spirometry (26.0%), highest in GOLD 3–4 (89.7%), and intermediate in PRISm (51.0%) and GOLD 1–2 (50.1%). Median percent emphysema on CT was 2.2%, lowest in PRISm (0.7%) and highest in GOLD 3–4 (18.8%). Mean Pi10 was 3.68 mm, lowest in normal spirometry (3.65 mm), and highest in PRISm and GOLD 3–4 (3.73 and 3.75 mm, respectively).
Table 1.
Baseline Characteristics of Individuals with a Smoking History
| All (N = 10,132) |
Normal Spirometry (n = 4,387) |
PRISm (n = 1,262) |
GOLD 1–2 (n = 2,713) |
GOLD 3–4 (n = 1,770) |
|
|---|---|---|---|---|---|
| Age, yr | 59.5 ± 9.0 | 56.6 ± 8.4 | 57.2 ± 8.2 | 62.3 ± 8.9 | 64.2 ± 8.1 |
| Female, % | 4,723 (46.6) | 2,067 (47.1) | 679 (53.8) | 1,230 (45.3) | 747 (42.2) |
| Black, % | 3,366 (33.2) | 1,807 (41.2) | 541 (42.9) | 660 (24.3) | 358 (20.2) |
| Body mass index, kg/m2 | 28.8 ± 6.3 | 28.9 ± 5.8 | 31.9 ± 7.3 | 28.3 ± 5.9 | 27.3 ± 6.3 |
| Currently smoking, % | 5,364 (52.9) | 2,617 (59.7) | 804 (63.7) | 1,394 (51.4) | 549 (31.0) |
| Smoking pack-years | 44.2 ± 25.0 | 37.2 ± 20.2 | 42.6 ± 24.2 | 49.0 ± 26.3 | 55.4 ± 28.1 |
| Postbronchodilator FEV1, L | 2.2 ± 0.9 | 2.9 ± 0.7 | 2.1 ± 0.5 | 2.1 ± 0.7 | 1.0 ± 0.4 |
| Postbronchodilator FEV1, % predicted | 76.3 ± 25.5 | 97.4 ± 11.5 | 70.2 ± 8.4 | 72.5 ± 14.5 | 34.2 ± 10.0 |
| Postbronchodilator FEV1/FVC, % | 66.7 ± 16.2 | 78.7 ± 5.2 | 76.6 ± 4.9 | 60.3 ± 7.7 | 39.8 ± 10.4 |
| BODE index | 1 [0–3] | 0 [0–1] | 1 [0–3] | 1 [0–3] | 5 [4–7] |
| ⩾2 respiratory exacerbations in yr before study enrollment, n (%)* | 916 (9.0) | 119 (2.7) | 94 (7.4) | 290 (10.7) | 413 (23.3) |
| SGRQ total score ⩾ 25, n (%) | 4,731 (46.7) | 1,142 (26.0) | 643 (51.0) | 1,359 (50.1) | 1,587 (89.7) |
| mMRC score ⩾ 2, n (%) | 4,239 (41.8) | 1,027 (23.4) | 589 (46.7) | 1,140 (42.0) | 1,483 (83.8) |
| Chronic bronchitis, n (%) | 1,940 (19.1) | 551 (12.6) | 226 (17.9) | 647 (23.8) | 516 (29.2) |
| Self-reported coronary artery disease, n (%) | 654 (6.5) | 170 (3.9) | 87 (6.9) | 234 (8.6) | 163 (9.2) |
| Self-reported congestive heart failure, n (%) | 322 (3.2) | 58 (1.3) | 59 (4.7) | 84 (3.1) | 121 (6.8) |
| Self-reported diabetes mellitus, n (%) | 1,328 (13.1) | 506 (11.5) | 273 (21.6) | 312 (11.5) | 237 (13.4) |
| Personal history of cancer (before enrollment), n (%) | 494 (4.9) | 158 (3.6) | 43 (3.4) | 159 (5.9) | 134 (7.6) |
| Family history of cancer, n (%) | 3,766 (37.2) | 1,518 (34.6) | 525 (41.6) | 1,017 (37.5) | 706 (39.9) |
| Emphysema on chest CT, % of total lung volume† | 2.2 [0.7–7.3] | 1.1 [0.5–2.8] | 0.7 [0.3–1.8] | 4.1 [1.5–9.5] | 18.8 [7.6–30.2] |
| Pi10, mm‡ | 3.68 ± 0.13 | 3.65 ± 0.11 | 3.73 ± 0.13 | 3.67 ± 0.13 | 3.75 ± 0.14 |
Definition of abbreviations: BODE = Body mass index, airflow Obstruction, Dyspnea severity, Exercise capacity index; CT = computed tomography; GOLD = Global Initiative for Chronic Obstructive Lung Disease spirometry grade; mMRC = modified Medical Research Council dyspnea score; PRISm = preserved ratio impaired spirometry; SGRQ = St. George’s Respiratory Questionnaire.
Values represent counts (proportions) for categorical variables and means ± standard deviations or medians [interquartile intervals] for continuous variables.
Respiratory exacerbations were defined as those requiring treatment with antibiotics and/or systemic corticosteroids.
Emphysema is defined as the extent of low attenuation area (voxels <−950 Hounsfield Units at total lung capacity) as a percent of total lung volume (available for 9,419 participants).
Pi10 is a standardized measure of airway wall thickness on CT, defined as the square root of the wall area of a theoretical airway with an internal perimeter of 10 mm (available for 9,358 participants).
Causes of Death
Over a median follow-up of 10.1 years, 2,200 deaths occurred: 436 in normal spirometry, 228 in PRISm, 600 in GOLD 1–2, and 936 in GOLD 3–4. Of the 2,200 recorded deaths, 1,757 had their causes reviewed to date. Of these, 409 (23.3%) had their cause classified as missing because of an inability to obtain adequate sources of information. The proportions of deaths with a missing cause were similar between lung function categories (Table E1 in the online supplement). Other than lower smoking pack-years (51.2 ± 25.2 vs. 54.6 ± 30.7) and higher FEV1% predicted (59.1 ± 26.1 vs. 55.5 ± 28.1), participants with a missing cause of death had similar demographic and clinical characteristics to those with adjudicated causes of death (Table E2).
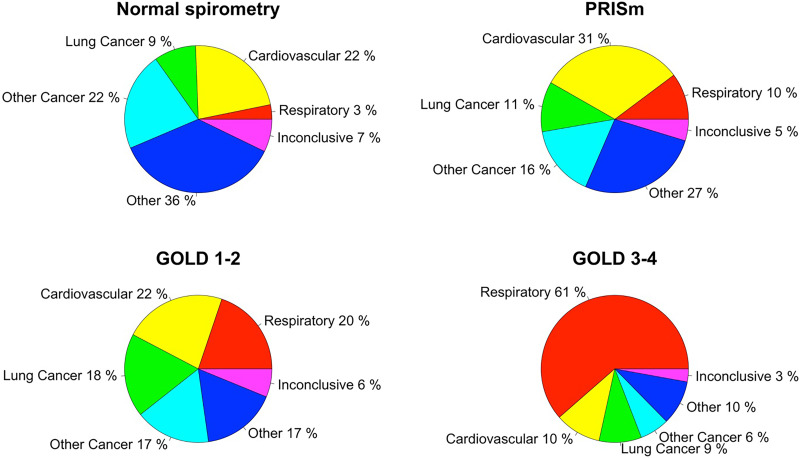
Leading causes of death in participants with normal spirometry included cardiovascular disease (22%) and malignancies other than lung cancer (22%) (Figure 1 and Table E1). The leading cause of death in PRISm was cardiovascular disease (31%). In GOLD 1–2 participants, deaths from cardiovascular disease (22%), respiratory disease (20%), lung cancer (18%), and malignancies other than lung cancer (17%) occurred with nearly similar incidence. By contrast, respiratory events accounted for most deaths (61%) in GOLD 3–4 participants. The proportion of respiratory-related deaths was significantly higher with increasing severity of lung function impairment (Table E3). Cardiovascular deaths were proportionately less frequent in GOLD 3–4, and lung cancer deaths were proportionately more frequent in GOLD 1–2 relative to other lung function categories.
Figure 1.
Distribution of causes of death by baseline lung function category. GOLD = Global Initiative for Chronic Obstructive Lung Disease; PRISm = preserved ratio impaired spirometry.
We then examined causes of death by spirometry group further stratified by sex, race, and smoking status (Table E4 and Figures E1–E3). Female participants with PRISm had a higher incidence of lung cancer deaths compared with male participants with PRISm (16.9% vs. 5.9%; P = 0.047). Black participants with normal spirometry had a higher incidence of cardiovascular deaths compared with White participants with normal spirometry (28.6% vs. 17.4%; P = 0.03). Participants who formerly smoked were more likely to die from respiratory causes than those still smoking, particularly within the normal spirometry (7.5% vs. 1.6%; P = 0.02) and GOLD 1–2 (26.8% vs. 13.2%; P = 0.002) groups.
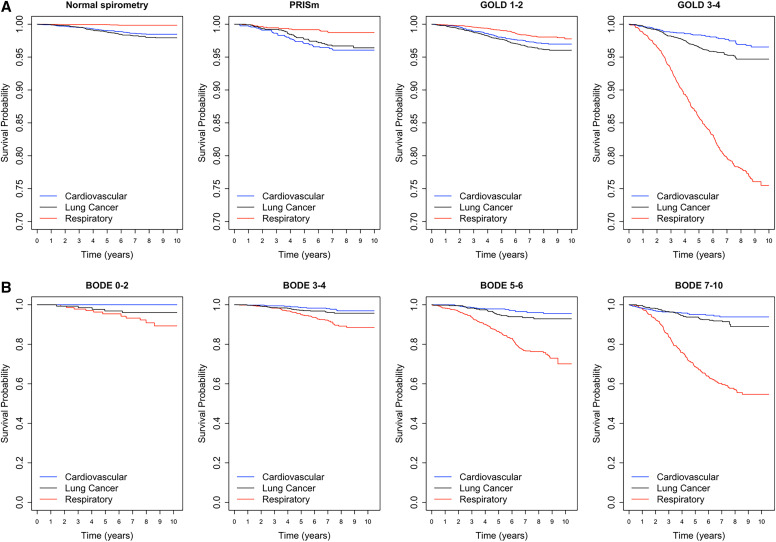
In inverse-weighted probability cause-specific mortality analyses examining respiratory, cardiovascular, and lung cancer deaths by lung function category, current and former tobacco cigarette users with GOLD 3–4 had a substantially higher risk of respiratory deaths (Figure 2A). Among these GOLD 3–4 participants, the risk of respiratory deaths was highest relative to lung cancer and cardiovascular deaths among those with a BODE index ⩾7 (Figure 2B).
Figure 2.
Kaplan-Meier plots of inverse-weighted survival probabilities of respiratory, cardiovascular, and lung cancer deaths in A) all participants by lung function category and B) GOLD 3–4 participants by BODE (Body mass index, airflow Obstruction, Dyspnea, and Exercise capacity) index category. GOLD = Global Initiative for Chronic Obstructive Lung Disease; PRISm = preserved ratio impaired spirometry.
Features Associated with All-Cause Mortality
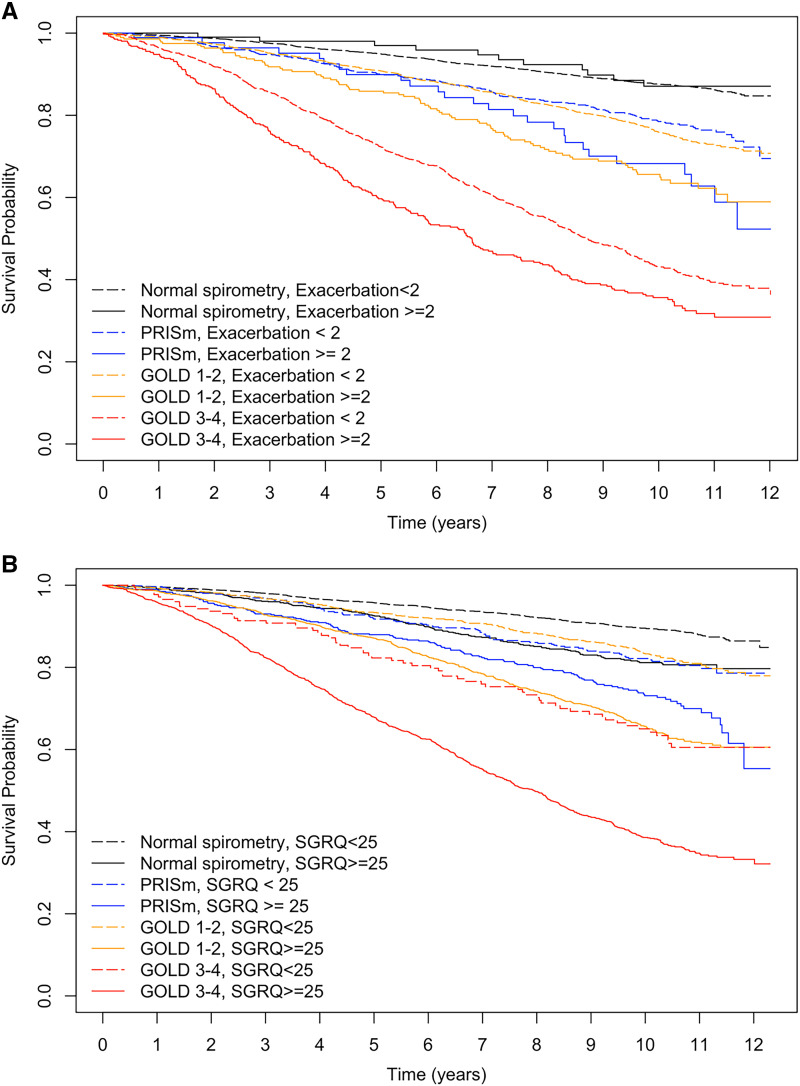
Participants with at least two respiratory exacerbations in the year preceding enrollment had worse survival probabilities compared with those with fewer than two exacerbations within the PRISm, GOLD 1–2, and GOLD 3–4 groups (all pairwise log-rank test P < 0.05) (Figure 3A). Participants with SGRQ ⩾ 25 had worse survival probabilities compared with those with SGRQ < 25 within all lung function categories (all pairwise log-rank test P < 0.05) (Figure 3B). In particular, the survival probability estimate was similar among normal spirometry participants with SGRQ ⩾ 25 and GOLD 1–2 participants with SGRQ less than 25 (log-rank test P = 0.4). Likewise, the survival probability estimate was similar among GOLD 1–2 participants with SGRQ ⩾ 25 and GOLD 3–4 participants with SGRQ less than 25 (log-rank test P = 0.7).
Figure 3.
Kaplan-Meier plots of survival probabilities by lung function category, stratified by A) the number of respiratory exacerbations in the year before enrollment; and B) the baseline score of St. George’s Respiratory Questionnaire. GOLD = Global Initiative for Chronic Obstructive Lung Disease; PRISm = preserved ratio impaired spirometry.
In multivariable Cox proportional hazards models including all participants, a history of at least two respiratory exacerbations in the year before enrollment (HR, 1.44; 95% CI, 1.28–1.62) and SGRQ ⩾ 25 (HR 1.60; 95% CI, 1.43–1.79) were associated with higher all-cause mortality (Table 2). In subgroup analyses, the exacerbation history association with mortality was observed in the GOLD 1–2 and GOLD 3–4 groups, whereas SGRQ ⩾ 25 was associated with mortality in all lung function groups. Compared with at least two exacerbations, a history of at least one exacerbation in the year before enrollment remained associated with all-cause mortality but with a lower magnitude of effect in GOLD 1–2 (HR, 1.13; 95% CI, 1.06–1.22) and GOLD 3–4 (HR, 1.10; 95% CI, 1.05–1.15). More severe dyspnea (mMRC ⩾ 2) was associated with all-cause mortality (HR, 1.57; 95% CI, 1.41–1.74), including in all lung function groups except for normal spirometry. Chronic bronchitis was also associated with all-cause mortality (HR, 1.21; 95% CI, 1.09–1.34) and had a similar effect size in all lung function groups, with statistical significance in GOLD 1–2 and GOLD 3–4. Furthermore, disease awareness was an important prognostic factor as baseline self-reported diagnoses of COPD and/or emphysema (HR, 1.29; 95% CI, 1.15–1.45) and of coronary artery disease and/or congestive heart failure (HR, 1.34; 95% CI, 1.18–1.51) were independently associated with all-cause mortality.
Table 2.
Association of Clinical and Imaging Variables with All-Cause Mortality in Individuals with a Smoking History
| All HR (95% CI) P value | Normal Spirometry HR (95% CI) P value | PRISm HR (95% CI) P value | GOLD 1–2 HR (95% CI) P value | GOLD 3–4 HR (95% CI) P value | |
|---|---|---|---|---|---|
| Clinical variables* | |||||
| ⩾2 respiratory exacerbations in yr before enrollment | 1.44 (1.28–1.62) <0.001 |
0.94 (0.51–1.71) 0.83 |
1.45 (0.95–2.22) 0.09 |
1.45 (1.15–1.84) 0.002 |
1.43 (1.23–1.66) <0.001 |
| SGRQ total score ⩾ 25 | 1.60 (1.43–1.79) <0.001 |
1.48 (1.20–1.84) <0.001 |
1.40 (1.05–1.87) 0.02 |
1.80 (1.49–2.17) <0.001 |
1.65 (1.26–2.17) <0.001 |
| mMRC ⩾ 2 | 1.57 (1.41–1.74) <0.001 |
1.21 (0.96–1.52) 0.10 |
1.57 (1.18–2.09) 0.002 |
1.84 (1.53–2.20) <0.001 |
1.53 (1.24–1.88) <0.001 |
| Chronic bronchitis | 1.21 (1.09–1.34) <0.001 |
1.26 (0.97–1.64) 0.08 |
1.29 (0.93–1.81) 0.13 |
1.21 (1.01–1.46) 0.04 |
1.25 (1.08–1.45) 0.003 |
| Imaging variables† | |||||
| % Emphysema (per 1% increase) | 1.01 (1.01–1.02) <0.001 |
1.01 (0.97–1.06) 0.53 |
0.99 (0.93–1.05) 0.71 |
1.02 (1.01–1.03) <0.001 |
1.00 (1.00–1.01) 0.21 |
| Pi10 (per 1 SD = 0.13 mm increase) | 1.09 (1.04–1.14) <0.001 |
1.13 (1.00–1.28) 0.06 |
1.25 (1.07–1.46) 0.004 |
1.05 (0.96–1.15) 0.32 |
1.10 (1.03–1.17) 0.005 |
Definition of abbreviations: CI = confidence interval; GOLD = Global Initiative for Chronic Obstructive Lung Disease spirometry grade; HR = hazard ratio; mMRC = modified Medical Research Council dyspnea scale; PRISm = Preserved ratio impaired spirometry; SD = standard deviation; SGRQ = St. George’s Respiratory Questionnaire.
Separate clinical models, each adjusted for age, sex, race, body mass index, smoking status, smoking pack-years, the highest level of school completed, postbronchodilator FEV1% predicted, and study site.
Separate imaging models, each adjusted for the same variables as clinical models with the exception of scanner make instead of the study site.
In imaging models including all participants, percent emphysema (HR, 1.01; 95% CI, 1.01–1.02 per 1% absolute increase) and Pi10 (HR, 1.09; 95% CI, 1.04–1.14 per one standard deviation increase) were associated with higher all-cause mortality (Table 2). In subgroup analyses, the emphysema association was significant in GOLD 1–2 only, whereas the Pi10 association was significant in PRISm (HR, 1.25; 95% CI, 1.07–1.46) and GOLD 3–4 (HR, 1.10; 95% CI, 1.03–1.17).
Because information on our clinical and imaging predictors of interest may be concurrently available in clinical practice, we ran additional models simultaneously containing all of them. Compared with the models in Table 2, all associations were maintained except for SGRQ ⩾ 25 in PRISm and exacerbation history in GOLD 1–2 (Table E5).
Features Associated with Respiratory Mortality
In multivariable models including all participants, a history of at least two respiratory exacerbations in the year before enrollment (HR, 1.74; 95% CI, 1.43–2.11), SGRQ ⩾ 25 (HR, 2.27; 95% CI, 1.59–3.22), mMRC ⩾ 2 (HR, 1.80; 95% CI, 1.37–2.38), chronic bronchitis (HR, 1.53, 95% CI, 1.26–1.85), the extent of emphysema (HR, 1.02; 95% CI, 1.01–1.03 per 1% absolute increase) and Pi10 (HR, 1.12; 95% CI, 1.02–1.22 per one standard deviation increase) were all associated with increased risk of respiratory mortality (Table E6). In subgroup analyses, all six features were associated with an increased risk of respiratory mortality in participants with normal spirometry, GOLD 1–2 and GOLD 3–4, except for mMRC ⩾ 2 in normal spirometry and Pi10 in GOLD 3–4. Among participants with PRISm, only Pi10 was significant. Results of multivariable models simultaneously including all clinical and imaging parameters of interest for respiratory mortality are shown in Table E7. Some of the effect estimates in the normal spirometry and PRISm groups were unstable because of the low number of respiratory deaths in these groups.
Discussion
This COPDGene analysis, one of the largest, most rigorous (in terms of death adjudication methods), and most inclusive (in terms of the range of lung disease severity) COPD mortality analyses, informs causes of death and provides insight into the association of common clinical and imaging features with risk of death among current and former tobacco cigarette users with different severities of lung function impairment.
In addition to the crucial step of smoking cessation, the different distributions of causes of death by category of lung function impairment have important implications for the clinical care of current and former tobacco cigarette users. Our data suggest that those with normal spirometry, PRISm, and GOLD 1–2 COPD would benefit from timely screening for lung cancer and targeted assessments for cardiovascular diseases such as coronary artery disease, arrhythmias, and systolic and diastolic heart failure. Similar to several population-based cohorts, we found that participants with PRISm were at a significantly increased risk of cardiovascular mortality, likely because of a high prevalence of cardiac comorbidities and the metabolic syndrome (21–23).
We also show lung cancer is a leading cause of death in GOLD 1–2, which makes these patients particularly important targets for screening. Despite evidence that annual lung cancer screening with low-dose chest CT decreases mortality from lung cancer (24, 25), this resource remains significantly underused in the United States (26). In patients with advanced COPD, especially those with GOLD 3–4 spirometry and a BODE index of at least seven, our analysis suggests the risk of respiratory death significantly outweighs competing risks from other causes of death, including lung cancer. As with all patients, the decision to screen these patients for lung cancer should be on the basis of shared decision-making between patients and their providers, considering each patient’s values, clinical history, and life expectancy. Our findings, combined with previous reports of lack of mortality benefit when screening patients with GOLD 3–4 COPD, may help inform this decision (27). Regardless, these patients should be evaluated for therapies with a demonstrated survival benefit in COPD, including long-term oxygen therapy in those with severe hypoxia (28, 29), lung volume reduction surgery in those with upper lobe-predominant emphysema and low baseline exercise capacity (30), home noninvasive positive pressure ventilation in those with chronic hypercapnia (31, 32), and arguably inhaled corticosteroids in those at risk for COPD exacerbations (33).
In additional subgroup analyses of causes of death, we found that female participants with PRISm were more likely to die of lung cancer than male participants with PRISm. The reasons are unclear but may lie at the intersection of hormonal (both endogenous and exogenous), metabolic, and genetic differences (34). It remains to be determined whether this differential rate of lung cancer deaths between males and females with PRISm will be observed in other cohorts and whether it will decrease with the new lung cancer screening guidelines that now include younger individuals with a lesser smoking history (35).
An analysis of SPIROMICS (SubPopulations and InteRmediate Outcome Measures in COPD Study) showed that symptomatic (defined as having a CAT [COPD assessment test] score of at least 10) current or former tobacco cigarette users with preserved lung function had a higher rate of respiratory exacerbations compared with asymptomatic counterparts with mild to moderate COPD (9). Others have demonstrated that respiratory symptoms in this group of individuals are associated with a higher risk of all-cause and respiratory mortality (36, 37). In our analysis, using SGRQ ⩾ 25 (equivalent to CAT ⩾ 10) (38), impaired respiratory-related quality of life was associated with increased all-cause mortality in all spirometry groups, including normal spirometry and PRISm. To our knowledge, this relationship has not been previously investigated in PRISm. The exact biological underpinnings and the optimal clinical management of respiratory disease in current and former tobacco cigarette users without spirometric airflow obstruction remain to be determined. Data from SPIROMICS showed that symptomatic individuals with a smoking history and normal spirometry had thicker airway walls and higher total airway mucin concentrations than their asymptomatic counterparts, thereby suggesting one possible pathologic basis for this phenotype (39). We also found greater airway wall thickness was independently associated with higher all-cause and respiratory mortality in participants with PRISm, which further supports this hypothesis. However, in the recently published Redefining Therapy in Early COPD clinical trial, inhaled dual bronchodilator therapy did not decrease the burden of respiratory symptoms in tobacco cigarette users without airflow obstruction on spirometry (40). Collectively, these data call for continued research on this symptomatic population with a smoking history and preserved lung function, which consists of millions of individuals in the United States, to improve their clinical outcomes (10).
Among participants with airflow obstruction, both exacerbation history and SGRQ score of at least 25 were associated with higher all-cause and respiratory mortality. Several available maintenance inhalers and oral medications improve symptom burden and reduce exacerbation frequency in patients with COPD. However, no definitive data exist regarding their benefits on long-term survival, which highlights the sore need for additional therapies for patients with COPD. Regarding imaging characteristics, the extent of emphysema on chest CT was independently associated with increased all-cause and respiratory mortality in participants with GOLD 1–2 COPD. This could be because these individuals are at the highest risk for accelerated emphysema progression because of local inflammatory and biomechanical effects in the emphysematous regions of their lungs (41).
We acknowledge the limitations of our study. First, our analysis did not capture the ongoing medical care of participants, such as their daily medications, supplemental oxygen use, influenza and pneumonia vaccination status, participation in pulmonary rehabilitation, and referral to annual lung cancer screening, all of which could impact their risk and cause of death. Second, our model for respiratory exacerbations was limited to self-reported events occurring in the year preceding enrollment, but year-to-year variation in the frequency of exacerbations has been described (42). Third, the spirometric categorization of participants may not fully reflect the severity of smoking-related lung disease. Although postbronchodilator FEV1/FVC less than 0.70 is a common population-based standard for defining airflow obstruction, individuals with a smoking history not meeting this definition can still experience significant respiratory morbidity (11). Fourth, individuals with active cancer under treatment were not enrolled. Fifth, the causes and risk factors of death may be different in individuals with a lesser smoking history than those in our study. Along the same lines, our cohort, which was recruited on the basis of smoking history, includes participants who have already had a lifetime of risk that cannot be accounted for, although we mitigated this effect by using inverse weight probability methods. Sixth, the cause of death in 23.3% of participants was missing because of the inability to obtain adequate records for adjudication. Seventh, we acknowledge that the interpretation of predictor of interest effect estimates in multivariable models can be complicated because of several factors, including the potential heterogeneity of such effect estimates across levels of other covariates (43). In addition, a better understanding of how mortality risk factors vary in women and minorities is essential, given the known associations of sex and race with COPD risk, manifestations, and outcomes (44, 45).
Conclusions
The causes of and clinical factors associated with death among individuals with a smoking history vary depending on lung function. Impaired respiratory-related quality of life and airway wall thickness on chest CT are important prognostic factors in tobacco cigarette users without airflow obstruction, including those with PRISm. In addition to smoking cessation, more research is needed to identify effective management strategies in this understudied patient population. Among patients with advanced COPD, particularly those with BODE ⩾ 7, our data suggest respiratory deaths significantly outweigh the risks from competing causes of death and can help inform discussions regarding lung cancer screening and advanced COPD treatment options.
Acknowledgments
COPDGene Investigators – Core Units
Administrative Center: James D. Crapo, M.D. (PI); Edwin K. Silverman, M.D., Ph.D. (PI); Barry J. Make, M.D.; Elizabeth A. Regan, M.D., Ph.D. Genetic Analysis Center: Terri H. Beaty, Ph.D.; Peter J. Castaldi, M.D., M.Sc.; Michael H. Cho, M.D., M.P.H.; Dawn L. DeMeo, M.D., M.P.H.; Adel El Boueiz, M.D., M.M.Sc.; Marilyn G. Foreman, M.D., M.S.; Auyon Ghosh, M.D.; Lystra P. Hayden, M.D., M.M.Sc.; Craig P. Hersh, M.D., M.P.H.; Jacqueline Hetmanski, M.S.; Brian D. Hobbs, M.D., M.M.Sc.; John E. Hokanson, M.P.H., Ph.D.; Wonji Kim, Ph.D.; Nan Laird, Ph.D.; Christoph Lange, Ph.D.; Sharon M. Lutz, Ph.D.; Merry-Lynn McDonald, Ph.D.; Dmitry Prokopenko, Ph.D.; Matthew Moll, M.D., M.P.H.; Jarrett Morrow, Ph.D.; Dandi Qiao, Ph.D.; Elizabeth A. Regan, M.D., Ph.D.; Aabida Saferali, Ph.D.; Phuwanat Sakornsakolpat, M.D.; Edwin K. Silverman, M.D., Ph.D.; Emily S. Wan, M.D.; Jeong Yun, M.D., M.P.H. Imaging Center: Juan Pablo Centeno; Jean-Paul Charbonnier, Ph.D.; Harvey O. Coxson, Ph.D.; Craig J. Galban, Ph.D.; MeiLan K. Han, M.D., M.S.; Eric A. Hoffman, Stephen Humphries, Ph.D.; Francine L. Jacobson, M.D., M.P.H.; Philip F. Judy, Ph.D.; Ella A. Kazerooni, M.D.; Alex Kluiber; David A. Lynch, M.B.; Pietro Nardelli, Ph.D.; John D. Newell, Jr., M.D.; Aleena Notary; Andrea Oh, M.D.; Elizabeth A. Regan, M.D., Ph.D.; James C. Ross, Ph.D.; Raul San Jose Estepar, Ph.D.; Joyce Schroeder, M.D.; Jered Sieren; Berend C. Stoel, Ph.D.; Juerg Tschirren, Ph.D.; Edwin Van Beek, M.D., Ph.D.; Bram van Ginneken, Ph.D.; Eva van Rikxoort, Ph.D.; Gonzalo Vegas Sanchez-Ferrero, Ph.D.; Lucas Veitel; George R. Washko, M.D.; Carla G. Wilson, M.S. PFT QA Center, Salt Lake City, UT: Robert Jensen, Ph.D. Data Coordinating Center and Biostatistics, National Jewish Health, Denver, CO: Douglas Everett, Ph.D.; Jim Crooks, Ph.D.; Katherine Pratte, Ph.D.; Matt Strand, Ph.D.; Carla G. Wilson, M.S. Epidemiology Core, University of Colorado Anschutz Medical Campus, Aurora, CO: John E. Hokanson, M.P.H., Ph.D.; Erin Austin, Ph.D.; Gregory Kinney, M.P.H., Ph.D.; Sharon M. Lutz, Ph.D.; Kendra A. Young, Ph.D. Mortality Adjudication Core: Surya P. Bhatt, M.D.; Jessica Bon, M.D.; Alejandro A. Diaz, M.D., M.P.H.; MeiLan K. Han, M.D., M.S.; Barry Make, M.D.; Susan Murray, Sc.D.; Elizabeth Regan, M.D.; Xavier Soler, M.D.; Carla G. Wilson, M.S. Biomarker Core: Russell P. Bowler, M.D., Ph.D.; Katerina Kechris, Ph.D.; Farnoush Banaei-Kashani, Ph.D.
COPDGene Investigators – Clinical Centers
Ann Arbor VA: Jeffrey L. Curtis, M.D.; Perry G. Pernicano, M.D. Baylor College of Medicine, Houston, TX: Nicola Hanania, M.D., M.S.; Mustafa Atik, M.D.; Aladin Boriek, Ph.D.; Kalpatha Guntupalli, M.D.; Elizabeth Guy, M.D.; Amit Parulekar, M.D. Brigham and Women’s Hospital, Boston, MA: Dawn L. DeMeo, M.D., M.P.H.; Craig Hersh, M.D., M.P.H.; Francine L. Jacobson, M.D., M.P.H.; George Washko, M.D. Columbia University, New York, NY: R. Graham Barr, M.D., Dr.P.H.; John Austin, M.D.; Belinda D’Souza, M.D.; Byron Thomashow, M.D. Duke University Medical Center, Durham, NC: Neil MacIntyre, Jr., M.D.; H. Page McAdams, M.D.; Lacey Washington, M.D. HealthPartners Research Institute, Minneapolis, MN: Charlene McEvoy, M.D., M.P.H.; Joseph Tashjian, M.D. Johns Hopkins University, Baltimore, MD: Robert Wise, M.D.; Robert Brown, M.D.; Nadia N. Hansel, M.D., M.P.H.; Karen Horton, M.D.; Allison Lambert, M.D., M.H.S.; Nirupama Putcha, M.D., M.H.S. Lundquist Institute for Biomedical Innovation at Harbor UCLA Medical Center, Torrance, CA: Richard Casaburi, Ph.D., M.D.; Alessandra Adami, Ph.D.; Matthew Budoff, M.D.; Hans Fischer, M.D.; Janos Porszasz, M.D., Ph.D.; Harry Rossiter, Ph.D.; William Stringer, M.D. Michael E. DeBakey VAMC, Houston, TX: Amir Sharafkhaneh, M.D., Ph.D.; Charlie Lan, D.O. Minneapolis VA: Christine Wendt, M.D.; Brian Bell, M.D.; Ken M. Kunisaki, M.D., M.S. Morehouse School of Medicine, Atlanta, GA: Eric L. Flenaugh, M.D.; Hirut Gebrekristos, Ph.D.; Mario Ponce, M.D.; Silanath Terpenning, M.D.; Gloria Westney, M.D., M.S. National Jewish Health, Denver, CO: Russell Bowler, M.D., Ph.D.; David A. Lynch, M.B. Reliant Medical Group, Worcester, MA: Richard Rosiello, M.D.; David Pace, M.D. Temple University, Philadelphia, PA: Gerard Criner, M.D.; David Ciccolella, M.D.; Francis Cordova, M.D.; Chandra Dass, M.D.; Gilbert D’Alonzo, D.O.; Parag Desai, M.D.; Michael Jacobs, Pharm.D.; Steven Kelsen, M.D., Ph.D.; Victor Kim, M.D.; A. James Mamary, M.D.; Nathaniel Marchetti, D.O.; Aditi Satti, M.D.; Kartik Shenoy, M.D.; Robert M. Steiner, M.D.; Alex Swift, M.D.; Irene Swift, M.D.; Maria Elena Vega-Sanchez, M.D. University of Alabama, Birmingham, AL: Mark Dransfield, M.D.; William Bailey, M.D.; Surya P. Bhatt, M.D.; Anand Iyer, M.D.; Hrudaya Nath, M.D.; J. Michael Wells, M.D. University of California, San Diego, CA: Douglas Conrad, M.D.; Xavier Soler, M.D., Ph.D.; Andrew Yen, M.D. University of Iowa, Iowa City, IA: Alejandro P. Comellas, M.D.; Karin F. Hoth, Ph.D.; John Newell, Jr., M.D.; Brad Thompson, M.D. University of Michigan, Ann Arbor, MI: MeiLan K. Han, M.D. M.S.; Ella Kazerooni, M.D., M.S.; Wassim Labaki, M.D., M.S.; Craig Galban, Ph.D.; Dharshan Vummidi, M.D. University of Minnesota, Minneapolis, MN: Joanne Billings, M.D.; Abbie Begnaud, M.D.; Tadashi Allen, M.D. University of Pittsburgh, Pittsburgh, PA: Frank Sciurba, M.D.; Jessica Bon, M.D.; Divay Chandra, M.D., M.Sc.; Joel Weissfeld, M.D., M.P.H. University of Texas Health, San Antonio, San Antonio, TX: Antonio Anzueto, M.D.; Sandra Adams, M.D.; Diego Maselli-Caceres, M.D.; Mario E. Ruiz, M.D.; Harjinder Singh.
Footnotes
Supported by the NHLBI (U01HL089897, U01HL089856, R01HL122438, K24HL138188, and K23HL151751) and the COPD Foundation (through contributions made to an Industry Advisory Board that has included AstraZeneca, Bayer Pharmaceuticals, Boehringer-Ingelheim, Genentech, GlaxoSmithKline, Novartis, Pfizer, and Sunovion).
Author Contributions: W.W.L., B.J.M., E.A.R., and M.K.H. made substantial contributions to the conception and design of the work. W.W.L., T.G., S.M., and M.K.H. made substantial contributions to data analysis and interpretation. W.W.L., S.M., and M.K.H. wrote the first draft of the manuscript. All authors revised the manuscript for important intellectual content.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202210-1887OC on May 9, 2023
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Collaborators: James D. Crapo, Edwin K. Silverman, Barry J. Make, Elizabeth A. Regan, Terri H. Beaty, Peter J. Castaldi, Michael H. Cho, Dawn L. DeMeo, Adel El Boueiz, Marilyn G. Foreman, Auyon Ghosh, Lystra P. Hayden, Craig P. Hersh, Jacqueline Hetmanski, Brian D. Hobbs, John E. Hokanson, Wonji Kim, Nan Laird, Christoph Lange, Sharon M. Lutz, Merry-Lynn McDonald, Dmitry Prokopenko, Matthew Moll, Jarrett Morrow, Dandi Qiao, Elizabeth A. Regan, Aabida Saferali, Phuwanat Sakornsakolpat, Edwin K. Silverman, Emily S. Wan, Jeong Yun, Juan Pablo Centeno, Jean-Paul Charbonnier, Harvey O. Coxson, Craig J. Galban, MeiLan K. Han, Eric A. Hoffman, Stephen Humphries, Francine L. Jacobson, Philip F. Judy, Ella A. Kazerooni, Alex Kluiber, David A. Lynch, Pietro Nardelli, John D. Newell, Jr., Aleena Notary, Andrea Oh, Elizabeth A. Regan, James C. Ross, Raul San Jose Estepar, Joyce Schroeder, Jered Sieren, Berend C. Stoel, Juerg Tschirren, Edwin Van Beek, Bram van Ginneken, Eva van Rikxoort, Gonzalo Vegas Sanchez-Ferrero, Lucas Veitel, George R. Washko, Carla G. Wilson, Robert Jensen, Douglas Everett, Jim Crooks, Katherine Pratte, Matt Strand, Carla G. Wilson, John E. Hokanson, Erin Austin, Gregory Kinney, Sharon M. Lutz, Kendra A. Young, Surya P. Bhatt, Jessica Bon, Alejandro A. Diaz, MeiLan K. Han, Barry Make, Susan Murray, Elizabeth Regan, Xavier Soler, Carla G. Wilson, Russell P. Bowler, Katerina Kechris, Farnoush Banaei-Kashani, Jeffrey L. Curtis, Perry G. Pernicano, Nicola Hanania, Mustafa Atik, Aladin Boriek, Kalpatha Guntupalli, Elizabeth Guy, Amit Parulekar, Dawn L. DeMeo, Craig Hersh, Francine L. Jacobson, George Washko, R. Graham Barr, John Austin, Belinda D’Souza, Byron Thomashow, Neil MacIntyre, Jr., H. Page McAdams, Lacey Washington, Charlene McEvoy, Joseph Tashjian, Robert Wise, Robert Brown, Nadia N. Hansel, Karen Horton, Allison Lambert, Nirupama Putcha, Richard Casaburi, Alessandra Adami, Matthew Budoff, Hans Fischer, Janos Porszasz, Harry Rossiter, William Stringer, Amir Sharafkhaneh, Charlie Lan, Christine Wendt, Brian Bell, Ken M. Kunisaki, Eric L. Flenaugh, Hirut Gebrekristos, Mario Ponce, Silanath Terpenning, Gloria Westney, Russell Bowler, David A. Lynch, Richard Rosiello, David Pace, Gerard Criner, David Ciccolella, Francis Cordova, Chandra Dass, Gilbert D’Alonzo, Parag Desai, Michael Jacobs, Steven Kelsen, Victor Kim, A. James Mamary, Nathaniel Marchetti, Aditi Satti, Kartik Shenoy, Robert M. Steiner, Alex Swift, Irene Swift, Maria Elena Vega-Sanchez, Mark Dransfield, William Bailey, Surya P. Bhatt, Anand Iyer, Hrudaya Nath, J. Michael Wells, Douglas Conrad, Xavier Soler, Andrew Yen, Alejandro P. Comellas, Karin F. Hoth, John Newell, Jr., Brad Thompson, MeiLan K. Han, Ella Kazerooni, Wassim Labaki, Craig Galban, Dharshan Vummidi, Joanne Billings, Abbie Begnaud, Tadashi Allen, Frank Sciurba, Jessica Bon, Divay Chandra, Joel Weissfeld, Antonio Anzueto, Sandra Adams, Diego Maselli-Caceres, Mario E. Ruiz, and Harjinder Singh
References
- 1.The health consequences of smoking–50 years of progress: a report of the surgeon general. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service, Office of the Surgeon General; 2014. [Google Scholar]
- 2. Carter BD, Abnet CC, Feskanich D, Freedman ND, Hartge P, Lewis CE, et al. Smoking and mortality—beyond established causes. N Engl J Med . 2015;372:631–640. doi: 10.1056/NEJMsa1407211. [DOI] [PubMed] [Google Scholar]
- 3. Pauwels RA, Löfdahl CG, Laitinen LA, Schouten JP, Postma DS, Pride NB, et al. European Respiratory Society Study on Chronic Obstructive Pulmonary Disease Long-term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking. N Engl J Med . 1999;340:1948–1953. doi: 10.1056/NEJM199906243402503. [DOI] [PubMed] [Google Scholar]
- 4. Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, et al. TORCH investigators Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med . 2007;356:775–789. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- 5. Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, et al. UPLIFT Study Investigators A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med . 2008;359:1543–1554. doi: 10.1056/NEJMoa0805800. [DOI] [PubMed] [Google Scholar]
- 6. McGarvey LP, John M, Anderson JA, Zvarich M, Wise RA, TORCH Clinical Endpoint Committee Ascertainment of cause-specific mortality in COPD: operations of the TORCH clinical endpoint committee. Thorax . 2007;62:411–415. doi: 10.1136/thx.2006.072348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Celli B, Decramer M, Kesten S, Liu D, Mehra S, Tashkin DP, UPLIFT Study Investigators Mortality in the 4-year trial of tiotropium (UPLIFT) in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med . 2009;180:948–955. doi: 10.1164/rccm.200906-0876OC. [DOI] [PubMed] [Google Scholar]
- 8. Agusti A, Calverley PM, Celli B, Coxson HO, Edwards LD, Lomas DA, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) investigators Characterisation of COPD heterogeneity in the ECLIPSE cohort Respir Res 2010. 11 122 20831787 [Google Scholar]
- 9. Woodruff PG, Barr RG, Bleecker E, Christenson SA, Couper D, Curtis JL, et al. SPIROMICS Research Group Clinical significance of symptoms in smokers with preserved pulmonary function. N Engl J Med . 2016;374:1811–1821. doi: 10.1056/NEJMoa1505971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Regan EA, Lynch DA, Curran-Everett D, Curtis JL, Austin JH, Grenier PA, et al. Genetic Epidemiology of COPD (COPDGene) Investigators Clinical and radiologic disease in smokers with normal spirometry. JAMA Intern Med . 2015;175:1539–1549. doi: 10.1001/jamainternmed.2015.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lowe KE, Regan EA, Anzueto A, Austin E, Austin JHM, Beaty TH, et al. COPDGene 2019: redefining the diagnosis of chronic obstructive pulmonary disease Chronic Obstr Pulm Dis (Miami) 2019. 6 384 399 31710793 [Google Scholar]
- 12. Labaki WW, Martinez CH, Martinez FJ, Galbán CJ, Ross BD, Washko GR, et al. The role of chest computed tomography in the evaluation and management of the patient with chronic obstructive pulmonary disease. Am J Respir Crit Care Med . 2017;196:1372–1379. doi: 10.1164/rccm.201703-0451PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Labaki WW, Gu T, Murray S, Curtis JL, Soler X, Bhatt SP, et al. Causes and predictors of death in smokers with and without COPD: an analysis of the COPDGene cohort. Am J Respir Crit Care Med . 2019;199:A3995. [Google Scholar]
- 14. Regan EA, Hokanson JE, Murphy JR, Make B, Lynch DA, Beaty TH, et al. Genetic epidemiology of COPD (COPDGene) study design. COPD . 2010;7:32–43. doi: 10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George’s Respiratory Questionnaire. Am Rev Respir Dis . 1992;145:1321–1327. doi: 10.1164/ajrccm/145.6.1321. [DOI] [PubMed] [Google Scholar]
- 16. Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, Mendez RA, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med . 2004;350:1005–1012. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- 17. Nakano Y, Wong JC, de Jong PA, Buzatu L, Nagao T, Coxson HO, et al. The prediction of small airway dimensions using computed tomography. Am J Respir Crit Care Med . 2005;171:142–146. doi: 10.1164/rccm.200407-874OC. [DOI] [PubMed] [Google Scholar]
- 18. Stewart JI, Moyle S, Criner GJ, Wilson C, Tanner R, Bowler RP, et al. For The Copdgene Investigators Automated telecommunication to obtain longitudinal follow-up in a multicenter cross-sectional COPD study. COPD . 2012;9:466–472. doi: 10.3109/15412555.2012.690010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wan ES, Castaldi PJ, Cho MH, Hokanson JE, Regan EA, Make BJ, et al. COPDGene Investigators Epidemiology, genetics, and subtyping of preserved ratio impaired spirometry (PRISm) in COPDGene. Respir Res . 2014;15:89. doi: 10.1186/s12931-014-0089-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med . 2017;195:557–582. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 21. Wijnant SRA, De Roos E, Kavousi M, Stricker BH, Terzikhan N, Lahousse L, et al. Trajectory and mortality of preserved ratio impaired spirometry: the Rotterdam study. Eur Respir J . 2020;55:1901217. doi: 10.1183/13993003.01217-2019. [DOI] [PubMed] [Google Scholar]
- 22. Wan ES, Balte P, Schwartz JE, Bhatt SP, Cassano PA, Couper D, et al. Association between preserved ratio impaired spirometry and clinical outcomes in US adults. JAMA . 2021;326:2287–2298. doi: 10.1001/jama.2021.20939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Higbee DH, Granell R, Davey Smith G, Dodd JW. Prevalence, risk factors, and clinical implications of preserved ratio impaired spirometry: a UK Biobank cohort analysis. Lancet Respir Med . 2022;10:149–157. doi: 10.1016/S2213-2600(21)00369-6. [DOI] [PubMed] [Google Scholar]
- 24. Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, et al. National Lung Screening Trial Research Team Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med . 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. de Koning HJ, van der Aalst CM, de Jong PA, Scholten ET, Nackaerts K, Heuvelmans MA, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med . 2020;382:503–513. doi: 10.1056/NEJMoa1911793. [DOI] [PubMed] [Google Scholar]
- 26. Jemal A, Fedewa SA. Lung cancer screening with low-dose computed tomography in the United States-2010 to 2015. JAMA Oncol . 2017;3:1278–1281. doi: 10.1001/jamaoncol.2016.6416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Young RP, Hopkins RJ. Chronic obstructive pulmonary disease (COPD) and lung cancer screening. Transl Lung Cancer Res . 2018;7:347–360. doi: 10.21037/tlcr.2018.05.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nocturnal Oxygen Therapy Trial Group. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Ann Intern Med . 1980;93:391–398. doi: 10.7326/0003-4819-93-3-391. [DOI] [PubMed] [Google Scholar]
- 29. Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Report of the Medical Research Council Working Party. Lancet . 1981;1:681–686. [PubMed] [Google Scholar]
- 30. Fishman A, Martinez F, Naunheim K, Piantadosi S, Wise R, Ries A, et al. National Emphysema Treatment Trial Research Group A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med . 2003;348:2059–2073. doi: 10.1056/NEJMoa030287. [DOI] [PubMed] [Google Scholar]
- 31. Köhnlein T, Windisch W, Köhler D, Drabik A, Geiseler J, Hartl S, et al. Non-invasive positive pressure ventilation for the treatment of severe stable chronic obstructive pulmonary disease: a prospective, multicentre, randomised, controlled clinical trial. Lancet Respir Med . 2014;2:698–705. doi: 10.1016/S2213-2600(14)70153-5. [DOI] [PubMed] [Google Scholar]
- 32. Murphy PB, Rehal S, Arbane G, Bourke S, Calverley PMA, Crook AM, et al. Effect of home noninvasive ventilation with oxygen therapy vs oxygen therapy alone on hospital readmission or death after an acute COPD exacerbation: a randomized clinical trial. JAMA . 2017;317:2177–2186. doi: 10.1001/jama.2017.4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Halpin DMG, Martinez FJ. Pharmacotherapy and mortality in COPD. Am J Respir Crit Care Med . 2022 doi: 10.1164/rccm.202205-1000PP. [DOI] [PubMed] [Google Scholar]
- 34. Fuentes N, Silva Rodriguez M, Silveyra P. Role of sex hormones in lung cancer. Exp Biol Med (Maywood) . 2021;246:2098–2110. doi: 10.1177/15353702211019697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Krist AH, Davidson KW, Mangione CM, Barry MJ, Cabana M, Caughey AB, et al. US Preventive Services Task Force Screening for lung cancer: US Preventive Services Task Force recommendation statement. JAMA . 2021;325:962–970. doi: 10.1001/jama.2021.1117. [DOI] [PubMed] [Google Scholar]
- 36. Çolak Y, Nordestgaard BG, Vestbo J, Lange P, Afzal S. Prognostic significance of chronic respiratory symptoms in individuals with normal spirometry. Eur Respir J . 2019;54:1900734. doi: 10.1183/13993003.00734-2019. [DOI] [PubMed] [Google Scholar]
- 37. Balte PP, Chaves PHM, Couper DJ, Enright P, Jacobs DR, Jr, Kalhan R, et al. Association of nonobstructive chronic bronchitis with respiratory health outcomes in adults. JAMA Intern Med . 2020;180:676–686. doi: 10.1001/jamainternmed.2020.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Han MK, Muellerova H, Curran-Everett D, Dransfield MT, Washko GR, Regan EA, et al. GOLD 2011 disease severity classification in COPDGene: a prospective cohort study. Lancet Respir Med . 2013;1:43–50. doi: 10.1016/S2213-2600(12)70044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kesimer M, Ford AA, Ceppe A, Radicioni G, Cao R, Davis CW, et al. Airway mucin concentration as a marker of chronic bronchitis. N Engl J Med . 2017;377:911–922. doi: 10.1056/NEJMoa1701632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Han MK, Ye W, Wang D, White E, Arjomandi M, Barjaktarevic IZ, et al. RETHINC Study Group Bronchodilators in tobacco-exposed persons with symptoms and preserved lung function. N Engl J Med . 2022;387:1173–1184. doi: 10.1056/NEJMoa2204752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bodduluri S, Bhatt SP, Hoffman EA, Newell JD, Jr, Martinez CH, Dransfield MT, et al. COPDGene Investigators Biomechanical CT metrics are associated with patient outcomes in COPD. Thorax . 2017;72:409–414. doi: 10.1136/thoraxjnl-2016-209544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Han MK, Quibrera PM, Carretta EE, Barr RG, Bleecker ER, Bowler RP, et al. SPIROMICS investigators Frequency of exacerbations in patients with chronic obstructive pulmonary disease: an analysis of the SPIROMICS cohort. Lancet Respir Med . 2017;5:619–626. doi: 10.1016/S2213-2600(17)30207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Westreich D, Greenland S. The table 2 fallacy: presenting and interpreting confounder and modifier coefficients. Am J Epidemiol . 2013;177:292–298. doi: 10.1093/aje/kws412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ejike CO, Dransfield MT, Hansel NN, Putcha N, Raju S, Martinez CH, et al. Chronic obstructive pulmonary disease in America’s Black population. Am J Respir Crit Care Med . 2019;200:423–430. doi: 10.1164/rccm.201810-1909PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Han MK, Arteaga-Solis E, Blenis J, Bourjeily G, Clegg DJ, DeMeo D, et al. Female sex and gender in lung/sleep health and disease. Increased understanding of basic biological, pathophysiological, and behavioral mechanisms leading to better health for female patients with lung disease. Am J Respir Crit Care Med . 2018;198:850–858. doi: 10.1164/rccm.201801-0168WS. [DOI] [PMC free article] [PubMed] [Google Scholar]