Abstract
Rationale
Ensifentrine is a novel, selective, dual phosphodiesterase (PDE)3 and PDE4 inhibitor with bronchodilator and antiinflammatory effects. Replicate phase III trials of nebulized ensifentrine were conducted (ENHANCE-1 and ENHANCE-2) to assess these effects in patients with chronic obstructive pulmonary disease (COPD).
Objectives
To evaluate the efficacy of ensifentrine compared with placebo for lung function, symptoms, quality of life, and exacerbations in patients with COPD.
Methods
These phase III, multicenter, randomized, double-blind, parallel-group, placebo-controlled trials were conducted between September 2020 and December 2022 at 250 research centers and pulmonology practices in 17 countries. Patients aged 40–80 years with moderate to severe symptomatic COPD were enrolled.
Measurements and Main Results
Totals of 760 (ENHANCE-1) and 789 (ENHANCE-2) patients were randomized and treated, with 69% and 55% receiving concomitant long-acting muscarinic antagonists or long-acting β2-agonists, respectively. Post-bronchodilator FEV1 percentage predicted values were 52% and 51% of predicted normal. Ensifentrine treatment significantly improved average FEV1 area under the curve at 0–12 hours versus placebo (ENHANCE-1, 87 ml [95% confidence interval, 55, 119]; ENHANCE-2, 94 ml [65, 124]; both P < 0.001). Ensifentrine treatment significantly improved symptoms (Evaluating Respiratory Symptoms) and quality of life (St. George’s Respiratory Questionnaire) versus placebo at Week 24 in ENHANCE-1 but not in ENHANCE-2. Ensifentrine treatment reduced the rate of moderate or severe exacerbations versus placebo over 24 weeks (ENHANCE-1, rate ratio, 0.64 [0.40, 1.00]; P = 0.050; ENHANCE-2, rate ratio, 0.57 [0.38, 0.87]; P = 0.009) and increased time to first exacerbation (ENHANCE-1, hazard ratio, 0.62 [0.39, 0.97]; P = 0.038; ENHANCE-2, hazard ratio, 0.58 [0.38, 0.87]; P = 0.009). Adverse event rates were similar to those for placebo.
Conclusions
Ensifentrine significantly improved lung function in both trials, with results supporting exacerbation rate and risk reduction in a broad COPD population and in addition to other classes of maintenance therapies.
Clinical trial registered with www.clinicaltrials.gov and EudraCT (ENHANCE-1, www.clinicaltrials.gov identifier NCT04535986, EudraCT identifier 2020-002086-34; ENHANCE-2, www.clinicaltrials.gov identifier NCT04542057, EudraCT identifier 2020-002069-32).
Keywords: ensifentrine, nebulized therapy, COPD, dual PDE3 and PDE4 inhibitor
At a Glance Commentary
Scientific Knowledge on the Subject
Current standard-of-care treatments for chronic obstructive pulmonary disease (COPD) have comprised the same classes of therapy for over 40 years and include inhaled short- and long-acting bronchodilators (i.e., muscarinic antagonists and β2-agonists) and inhaled corticosteroids. The ENHANCE program objectives were to evaluate the efficacy of ensifentrine, a dual inhibitor of phosphodiesterase (PDE)3 and PDE4, on lung function, symptoms, quality of life, and exacerbations in symptomatic patients with moderate to severe COPD.
What This Study Adds to the Field
The results indicate that ensifentrine, with a novel PDE3 and PDE4 inhibition mechanism, demonstrated substantial efficacy with reduction in exacerbations and improvement in lung function and had a safety profile similar to placebo. Ensifentrine would be a valuable and complementary addition to the limited available treatment mechanisms for patients with COPD.
Chronic obstructive pulmonary disease (COPD) is characterized by progressive partially reversible airflow obstruction, chronic inflammation, airway remodeling, and excessive mucus production, which lead to daily symptoms and exacerbations affecting quality of life (1, 2). Current standard-of-care treatments for COPD have comprised the same classes of therapy for over 40 years and include inhaled short- and long-acting bronchodilators (i.e., long-acting muscarinic antagonists [LAMAs] and long-acting β2-agonists [LABAs]) and inhaled corticosteroids (ICS). Despite multiple variations of approved therapies and combinations of dual and triple therapies within these classes, patients with COPD remain significantly symptomatic, with a substantial proportion of patients receiving dual or triple therapy reporting a significant burden of symptoms on everyday life and emotional well-being and persistent exacerbations (3, 4). ICS are used widely in COPD to prevent exacerbations, despite their clear association with a higher risk of pneumonia, including increased risk of hospitalized pneumonia (5, 6). Furthermore, known cardiovascular and urinary tract risks exist in a subset of patients using LABA and LAMA therapies, warranting the need for alternative personalized therapy considerations (7, 8). Novel treatments that provide additional bronchodilation, have antiinflammatory effects, reduce symptom burden, and prevent COPD exacerbations with a favorable safety profile are needed, but development of new therapies has been inadequate (9–11).
Inhibitors of phosphodiesterase (PDE)3 and PDE4 target a range of respiratory functions (12). PDE3 regulates cAMP and cGMP in airway smooth muscle, which mediates bronchial tone (13–15). PDE4 regulates cAMP and is involved in inflammatory cell activation and migration and cystic fibrosis transmembrane conductance regulator stimulation in bronchial epithelial cells (16–20). Dual inhibition of PDE3 and PDE4 has shown enhanced or synergistic effects compared with inhibition of either PDE3 or PDE4 alone on contraction of airway smooth muscle and suppression of the inflammatory response (21–23), making this dual mechanism of action a promising strategy for the treatment of obstructive and inflammatory diseases of the respiratory tract, such as COPD, cystic fibrosis, and asthma.
Ensifentrine is a novel, selective, dual inhibitor of PDE3 and PDE4 out of the 11 PDE isoforms that combines effects on airway smooth muscle, inflammation, and cystic fibrosis transmembrane conductance regulator stimulation into a single compound (24–29). Previous studies with twice-daily nebulized ensifentrine demonstrated improvements in lung function, COPD symptoms, and quality of life (30–32), together with reduction of airway inflammatory cells (33).
This report summarizes the results of two phase III clinical trials of nebulized, twice-daily ensifentrine 3 mg evaluated in the 24-week ENHANCE (Ensifentrine as a Novel Inhaled Nebulized COPD Therapy)-1 (34) (with 48-wk safety subset) and ENHANCE-2 (35) trials in patients with moderate to severe COPD. Some of the results of these trials were previously reported in the form of abstracts (36–42).
Methods
ENHANCE-1 and ENHANCE-2 were phase III, multicenter, randomized, double-blind, parallel-group, placebo-controlled trials in patients with symptomatic, moderate to severe COPD. After a 28-day run-in to ensure stable background medication use, eligible patients were randomized in a 5:3 ratio to twice-daily ensifentrine 3 mg or placebo over 24 weeks via a standard jet nebulizer (PARI). A 48-week subset in ENHANCE-1 was randomized in a 3:1 ratio. Patients were stratified by trial duration (24 or 48 wk for ENHANCE-1 only), background medication use (yes, no), and smoking status (current, former). Patients were allowed to continue participation after treatment withdrawal.
Patients
Eligible patients were aged 40–80 years, had a COPD diagnosis, had post-bronchodilator FEV1 30–70% predicted normal, had FEV1/FVC <0.7, had ⩾2 modified Medical Research Council dyspnea scale score (43), and had a smoking history ⩾10 pack-years. Patients were receiving no long-acting maintenance therapy or were receiving LABA with or without ICS or LAMA with or without ICS. Starting or stopping COPD maintenance therapy was not permitted, unless medically necessary. Patients with asthma were excluded.
Procedures
Trial schematics are provided in Figures E1 and E2 in the online supplement. Spirometry and 12-lead electrocardiograms ( ECGs) were conducted using centralized equipment and blinded central overreads (Clario, Philadelphia, PA). Patients received an eDiary at screening for recording daily rescue medication use, Evaluating Respiratory Symptoms (E-RS) (44), and treatment adherence. The St. George’s Respiratory Questionnaire (SGRQ) and Transition Dyspnea Index (TDI) were used for assessments during clinic visits. Moderate COPD exacerbations were defined as worsening of COPD symptoms (two or more major symptoms or one major and one minor symptom) (45) for ⩾2 days requiring a minimum of 3 days of therapy with oral or systemic corticosteroids and/or antibiotics, whereas severe COPD exacerbations required worsening of symptoms and inpatient hospitalization.
Statistical Analysis
The required sample size was 500 patients in the ensifentrine group and 300 in the placebo group for 90% power to detect a treatment effect of 59 ml with a two-sided test at a 5% significance level (P < 0.05) and 250 ml estimated SD. The primary endpoint, average FEV1 area under the curve (AUC)0–12 h, was selected to assess bronchodilatory effects over 12 hours and was calculated as the AUC (predose and 30 min and 1, 2, 4, 6, 8, and 12 h postdose) using the trapezoidal method, divided by the 12 hours.
Primary and secondary endpoints were compared using an analysis of covariance model adjusting for treatment, region (North America, Europe, Asia [ENHANCE-1 only]), background medication strata, and smoking status as factors and baseline as the covariate. Data are presented as the least squares mean change from baseline. Endpoints were tested in a step-down sequential order approach to control type I error: 1) FEV1 AUC0–12 h (Week 12), 2) peak FEV1 (Week 12), 3) E-RS total score (Week 24), 4) SGRQ total score (Week 24), and 5) morning trough FEV1 (Week 12). Formal testing stopped with the first nonsignificant result. Additional secondary endpoints included Week 24 average daily rescue medication use over 7 days and TDI. Missing data were imputed using a multiple imputation approach with a missing at random assumption for continuous endpoints.
The rate for moderate or severe exacerbations was compared between treatments using a negative binomial model adjusting for treatment, region, background medication strata, and smoking status and log study time (years) as an offset. Time to first exacerbation was analyzed using the log-rank test, stratified by region, background medication strata, and smoking status. The Cox proportional hazards model was used with the same factors. Pooled analyses included a study factor. Analysis populations, modified intention to treat (mITT), and safety included all patients randomized and dosed.
Oversight
Trial conduct was approved by independent ethics committees or review boards at each institution. The list of sites and ethics committees is included in Table E1 (ENHANCE-1) and Table E2 (ENHANCE-2). The trials were performed in accordance with the Declaration of Helsinki and Good Clinical Practice (ICH/CPMP/135/95) and registered with www.clinicaltrials.gov (ENHANCE-1, NCT04535986; ENHANCE-2, NCT04542057) and EudraCT (ENHANCE-1, 2020-002086-34; ENHANCE-2, 2020-002069-32). Patients provided written informed consent before participation. Two trial sites in ENHANCE-1 and one in ENHANCE-2 were excluded from all analyses before database lock and unblinding because of significant noncompliance with Good Clinical Practice.
Results
Patients
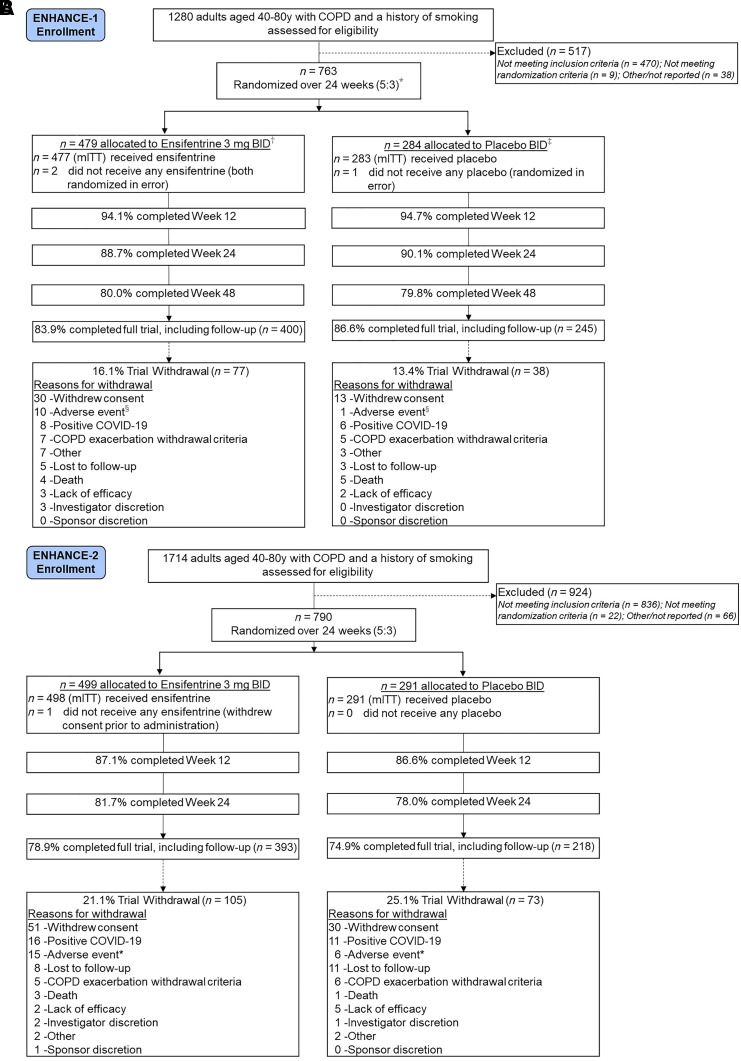
The trials were conducted between September 2020 and December 2022 in North America, Europe, Russia, and South Korea. CONSORT (Consolidated Standards of Reporting Trials) diagrams are included in Figure 1A (ENHANCE-1) and Figure 1B (ENHANCE-2).
Figure 1.
(A) Patient flow in the ENHANCE-1 trial. Proportions are based on the modified intention-to-treat population (mITT). Randomized patients excluded subjects from two trial sites because of significant noncompliance with Good Clinical Practice. *The 48-week subset in ENHANCE-1 was randomized 3:1. †n = 281 allocated to ensifentrine (n = 280 treated; mITT). ‡n = 89 allocated to placebo (n = 89 treated; mITT). §Patients were allowed to remain in the trial after discontinuing treatment. There were 18 (3.8%) ensifentrine-treated and 11 (3.9%) placebo-treated patients who discontinued treatment because of an adverse event. (B) Patient flow in the ENHANCE-2 trial. Proportions are based on the mITT. Randomized patients excluded subjects from one trial site because of significant noncompliance with Good Clinical Practice. *Patients were allowed to remain in the trial after discontinuing treatment. There were 24 (4.8%) ensifentrine-treated and 14 (4.8%) placebo-treated patients who discontinued treatment because of an adverse event. BID = twice daily; COPD = chronic obstructive pulmonary disease.
In ENHANCE-1, 763 patients were randomized, and the mITT population included 760 patients treated over 24 weeks (ensifentrine, n = 477; placebo, n = 283) with a subgroup treated for 48 weeks (ensifentrine, n = 280; placebo, n = 89). Overall, 84.5% of randomized patients completed the trial. Treatment withdrawal over 24 and 48 weeks was 20.8% for ensifentrine-treated patients and 18.0% for placebo-treated patients, including 26.3% and 24.7% in the 48-week subset, respectively.
In ENHANCE-2, 790 patients were randomized, and the mITT population included 789 patients treated over 24 weeks (ensifentrine, n = 498; placebo, n = 291). Overall, 77.3% of randomized patients completed the trial. Treatment withdrawal occurred in 24.3% of ensifentrine-treated patients and 32.6% of placebo-treated patients. A higher proportion of patients with severe COPD withdrew from treatment in the placebo group (41.9% of patients with severe COPD enrolled) versus the ensifentrine group (23.4% of severe patients with COPD enrolled). Treatment withdrawal because of COVID-19 occurred in 1.8% and 3.5% of patients in ENHANCE-1 and ENHANCE-2, respectively. Treatment adherence via returned unused vials and eDiary was high in both trials (>90%). Baseline demographic and disease characteristics of treated patients (Table 1 for ENHANCE-1 and ENHANCE-2) were well balanced between treatment groups and in each trial and were representative of patients with symptomatic, moderate, and severe COPD.
Table 1.
Demographic and Baseline Disease Characteristics in ENHANCE-1 and ENHANCE-2 Trials
| ENHANCE-1 |
ENHANCE-2 |
|||
|---|---|---|---|---|
| Ensifentrine 3 mg BID | Placebo BID | Ensifentrine 3 mg BID | Placebo BID | |
| Modified intention-to-treat population, n | 477 | 283 | 498 | 291 |
| Demographic characteristics | ||||
| Mean age, yr (SD) | 65.1 (7.1) | 64.9 (7.7) | 65.0 (7.4) | 65.3 (7.3) |
| Median age, yr | 65.0 | 65.0 | 65.0 | 66.0 |
| Age range, yr (min, max) | 41, 80 | 44, 79 | 42, 80 | 40, 80 |
| ⩾65 yr, n (%) | 258 (54.1) | 150 (53.0) | 274 (55.0) | 167 (57.4) |
| Sex at birth, n (%) | ||||
| Female | 203 (42.6) | 116 (41.0) | 254 (51.0) | 153 (52.6) |
| Male | 274 (57.4) | 167 (59.0) | 244 (49.0) | 138 (47.4) |
| Ethnicity, n (%) | ||||
| Hispanic or Latino | 15 (3.1) | 6 (2.1) | 26 (5.2) | 14 (4.8) |
| Not Hispanic or Latino | 462 (96.9) | 277 (97.9) | 472 (94.8) | 277 (95.2) |
| Race, n (%) | ||||
| White | 435 (91.2) | 250 (88.3) | 471 (94.6) | 276 (94.8) |
| Black or African American | 16 (3.4) | 9 (3.2) | 24 (4.8) | 11 (3.8) |
| Asian | 13 (2.7) | 11 (3.9) | 1 (0.2) | 1 (0.3) |
| American Indian or Alaska Native | 0 | 0 | 1 (0.2) | 0 |
| Other | 0 | 1 (0.4) | 1 (0.2) | 3 (1.0) |
| Not reported | 13 (2.7) | 12 (4.2) | 0 | 0 |
| North America, n (%) | 87 (18.2) | 58 (20.5) | 287 (57.6) | 180 (61.9) |
| Mean BMI, kg/m2 (SD) | 28.0 (5.3) | 27 (5.4) | 28.0 (6.0) | 29 (6.8) |
| Baseline disease characteristics | ||||
| mMRC score,* n (%) | ||||
| Grade 2 | 333 (69.8) | 197 (69.6) | 275 (55.2) | 162 (55.7) |
| Grade 3 | 137 (28.7) | 79 (27.9) | 208 (41.8) | 116 (39.9) |
| Grade 4 | 7 (1.5) | 7 (2.5) | 15 (3.0) | 13 (4.5) |
| Mean post-bronchodilator FEV1, L (SD) | 1.53 (0.46) | 1.51 (0.47) | 1.43 (0.44) | 1.42 (0.45) |
| % predicted (SD) | 52.9 (10.3) | 51.7 (10.5) | 50.8 (10.7) | 50.4 (10.7) |
| Concomitant maintenance COPD therapy use, n (%) | ||||
| Not used | 146 (30.6) | 91 (32.2) | 223 (44.8) | 131 (45.0) |
| Maintenance therapy used | 331 (69.4) | 192 (67.8) | 275 (55.2) | 160 (55.0) |
| LAMA† | 151 (31.7) | 76 (26.9) | 168 (33.7) | 90 (30.9) |
| LAMA+ICS | 4 (0.8) | 5 (1.8) | 1 (0.2) | 0 |
| LABA† | 89 (18.7) | 45 (15.9) | 34 (6.8) | 23 (7.9) |
| LABA+ICS | 87 (18.2) | 66 (23.3) | 72 (14.5) | 47 (16.2) |
| Smoking history, n (%) | ||||
| Current smokers | 268 (56.2) | 163 (57.6) | 276 (55.4) | 160 (55.0) |
| Former smokers | 209 (43.8) | 120 (42.4) | 222 (44.6) | 131 (45.0) |
| Mean pack-years (SD) | 41.1 (20.7) | 41.8 (20.6) | 42.7 (22.9) | 41.9 (20.9) |
| Mean years of smoking (SD) | 39.3 (11.3) | 39.0 (11.5) | 38.9 (10.4) | 39.9 (10.8) |
| COPD history, n (%) | ||||
| Chronic bronchitis‡ | 385 (80.7) | 215 (76.0) | 322 (64.7) | 190 (65.3) |
| Emphysema | 195 (40.9) | 146 (51.6) | 303 (60.8) | 179 (61.5) |
| COPD exacerbations, ⩽15 mo of screening | 120 (25.2) | 75 (26.5) | 102 (20.5) | 62 (21.3) |
| Severity of airflow obstruction (post-bronchodilator FEV1), n (%) | ||||
| GOLD 1 | 1 (0.2) | 0 | 1 (0.2) | 0 |
| GOLD 2 | 294 (61.6) | 164 (58.0) | 265 (53.2) | 143 (49.1) |
| GOLD 3 | 179 (37.5) | 119 (42.0) | 231 (46.4) | 148 (50.9) |
| GOLD 4 | 3 (0.6) | 0 | 1 (0.2) | 0 |
Definition of abbreviations: BID = twice daily; BMI = body mass index; COPD = chronic obstructive pulmonary disease; GOLD = Global Initiative for Chronic Obstructive Lung Disease; ICS = inhaled corticosteroids; LABA = long-acting β2-agonist; LAMA = long-acting muscarinic antagonist; mMRC = modified Medical Research Council dyspnea scale.
Proportions are based on the modified intention-to-treat population.
mMRC scored from 0 (least out of breath) to 4 (most out of breath).
The total number of patients receiving LAMAs excludes LAMA+ICS. The total number of patients receiving LABAs excludes LABA+ICS.
Chronic bronchitis was defined as regular production of sputum for ⩾3 months in two consecutive years (in the absence of other conditions that may explain it) as assessed by a medical professional.
ENHANCE-1 and ENHANCE-2 enrolled patients with mean post-bronchodilator predicted normal FEV1 of 52% and 51%, respectively. In ENHANCE-1 and ENHANCE-2, 69% and 55% of patients were receiving background LABAs or LAMAs (including 21% and 15% taking ICS), and 26% and 21% of patients reported COPD exacerbations within 15 months of screening, respectively. Because patients were not eligible if they had an exacerbation in the 3 months before screening, this was assessed 1 year plus 3 months before screening.
Primary Endpoint
Treatment with ensifentrine resulted in a significant improvement from baseline in Week 12 average FEV1 AUC0–12 h versus placebo (ENHANCE-1, 87 ml [95% confidence interval, 55, 119]; ENHANCE-2, 94 ml [65, 124]; both P < 0.001) (Table 2). The 12-hour serial FEV1 profiles (Week 12) demonstrate separation from placebo over the dosing interval (Figure E3).
Table 2.
Primary, Key Secondary, and Additional Endpoint Results in ENHANCE-1 and ENHANCE-2 Trials (mITT Population)
| Treatment Group | ENHANCE-1 |
ENHANCE-2 |
||
|---|---|---|---|---|
| Ensifentrine 3 mg BID (n = 477) | Placebo BID (n = 283) | Ensifentrine 3 mg BID (n = 498) | Placebo BID (n = 291) | |
| Primary endpoint | ||||
| Mean baseline FEV1, ml (SD) | 1,420 (487) | 1,403 (468) | 1,285 (451) | 1,279 (473) |
| Week 12 average FEV1 AUC0–12 h | ||||
| LS mean change from baseline, ml (95% CI) | 61 (25, 97) | −26 (−64, 13) | 48 (30, 66) | −46 (−70, −22) |
| Ensifentrine vs. placebo, ml (95% CI) | 87 (55, 119) | — | 94 (65, 124) | — |
| P value | <0.001 | — | <0.001 | — |
| Key secondary endpoints | ||||
| Week 12 peak FEV1 | ||||
| LS mean change from baseline, ml (95% CI) | 204 (165, 244) | 57 (15, 100) | 195 (175, 214) | 48 (22, 75) |
| Ensifentrine vs. placebo, ml (95% CI) | 147 (111, 183) | — | 146 (113, 179) | — |
| P value | <0.001 | — | <0.001 | — |
| Week 12 morning trough FEV1 | ||||
| LS mean change from baseline, ml (95% CI) | 8 (−30, 45) | −27 (−67, 13) | 6 (−13, 24) | −44 (−68, −19) |
| Ensifentrine vs. placebo, ml (95% CI) | 35 (1, 68) | — | 49 (19, 80) | — |
| P value | 0.041 | — | 0.002 | — |
| Week 24 E-RS total score | ||||
| Mean baseline (SD) | 14.1 (6.8) | 13.3 (6.1) | 13.3 (6.7) | 13.3 (6.2) |
| LS mean change from baseline (95% CI) | −2.2 (−3.1, −1.4) | −1.3 (−2.2, −0.4) | −2.1 (−2.6, −1.6) | −1.5 (−2.2, −0.9) |
| Ensifentrine vs. placebo (95% CI) | −1.0 (−1.7, −0.2) | — | −0.6 (−1.4, 0.2) | — |
| P value | 0.011 | — | 0.134 | — |
| Week 24 SGRQ total score | ||||
| Mean baseline (SD) | 48.1 (18.3) | 46.9 (17.1) | 50.6 (17.4) | 51.2 (16.4) |
| LS mean change from baseline (95% CI) | −6.2 (−8.4, −3.9) | −3.9 (−6.3, −1.5) | −4.5 (−5.9, −3.2) | −4.1 (−5.8, −2.3) |
| Ensifentrine vs. placebo (95% CI) | −2.3 (−4.3, −0.3) | — | −0.5 (−2.7, 1.7) | — |
| P value | 0.025 | — | 0.669 | — |
| Additional endpoints | ||||
| Week 24 average daily rescue medication use over 7 d | ||||
| Mean baseline (SD) | 1.54 (2.40) | 1.52 (2.23) | 1.86 (2.35) | 1.93 (2.43) |
| LS mean change from baseline (95% CI) | −0.51 (−0.79, −0.22) | −0.05 (−0.36, 0.25) | −0.49 (−0.66, −0.31) | −0.35 (−0.57, −0.12) |
| Ensifentrine vs. placebo (95% CI) | −0.45 (−0.70, −0.20) | — | −0.14 (−0.41, 0.14) | — |
| P value | <0.001 | — | 0.320 | — |
| Week 24 TDI | ||||
| Mean baseline (SD) | 5.9 (1.1) | 5.9 (1.1) | 5.9 (1.3) | 5.9 (1.2) |
| LS mean change from baseline (95% CI) | 1.9 (1.4, 2.3) | 0.8 (0.3, 1.4) | 2.2 (1.9, 2.5) | 1.3 (0.9, 1.7) |
| Ensifentrine vs. placebo (95% CI) | 1.0 (0.6, 1.5) | — | 0.9 (0.4, 1.4) | — |
| P value | <0.001 | — | <0.001 | — |
Definition of abbreviations: AUC = area under the curve; BID = twice daily; CI = confidence interval; E-RS = Evaluating-Respiratory Symptoms; LS = least squares; mITT = modified intention-to-treat; SGRQ = St. George’s Respiratory Questionnaire; SD = standard deviation; TDI = Transition Dyspnea Index.
Average FEV1 AUC0–12 h is defined as the AUC over 12 hours of the FEV1, divided by 12 hours. Peak FEV1 is defined as the maximum value in the 4 hours after dosing. Morning trough FEV1 is defined as the last value collected before the morning dose. Primary and key secondary endpoints were compared using an analysis of covariance model adjusting for treatment, region (North America, Europe, Asia [ENHANCE-1 only]), background medication strata (yes, no), and smoking status (current, former) as factors and baseline as the covariate.
Secondary Endpoints
Ensifentrine treatment demonstrated a significant improvement in Week 12 peak FEV1 from baseline versus placebo (ENHANCE-1, 147 ml [111, 183]; ENHANCE-2, 146 ml [113, 179]; both P < 0.001) (Table 2). The increase in peak FEV1 was of a consistent magnitude and statistically significant on Day 1 and at Weeks 6, 12, and 24, demonstrating durability of effect (Figure E4).
In ENHANCE-1, mean E-RS and SGRQ total scores were significantly improved versus placebo (E-RS, −1.0 [−1.7, −0.2], P = 0.011; SGRQ, −2.3 [−4.3, −0.3]; P = 0.025) (Table 2). The mean scores in the ensifentrine treatment group exceeded the minimal clinically important differences (MCIDs) of −2 (46) and −4 (47), respectively. In ENHANCE-2, numerical improvements were observed that were not significant at Week 24, which concluded the formal testing hierarchy for this trial. Significant or nominally significant reduction in symptoms was observed at Weeks 6 and 12 in both trials (Figure E5 [E-RS], Figure E6 [SGRQ]). In ENHANCE-2, the mean scores in the ensifentrine treatment group also exceeded the MCIDs for both the E-RS and SGRQ, respectively, and the mean score in the placebo group also exceeded the MCID for the SGRQ at Week 24. Treatment with ensifentrine demonstrated improvement from baseline in Week 12 morning trough FEV1 versus placebo (ENHANCE-1, 35 ml [1, 68]; P = 0.041; ENHANCE-2, 49 ml [19, 80]; P = 0.002) (Table 2).
At Week 24, mean daily rescue medication use was reduced versus placebo in ENHANCE-1 (−0.45 puffs/d [−0.70, −0.20]; P < 0.001) with a numerical decrease versus placebo in ENHANCE-2 (−0.14 [−0.41, 0.14]; P = 0.320) (Table 2). Less rescue medication use was observed in the ensifentrine group at Weeks 6, 12, and 24 in both trials (Figure E7). Dyspnea measured by TDI (MCID, 1) (48) was also improved versus placebo with ensifentrine in both trials at Week 24 (ENHANCE-1, 1.0 [0.6, 1.5]; ENHANCE-2, 0.9 [0.4, 1.4]; both P < 0.001) (Table 2), which was also observed at Weeks 6 and 12 in both trials (Figure E8).
COPD Exacerbations
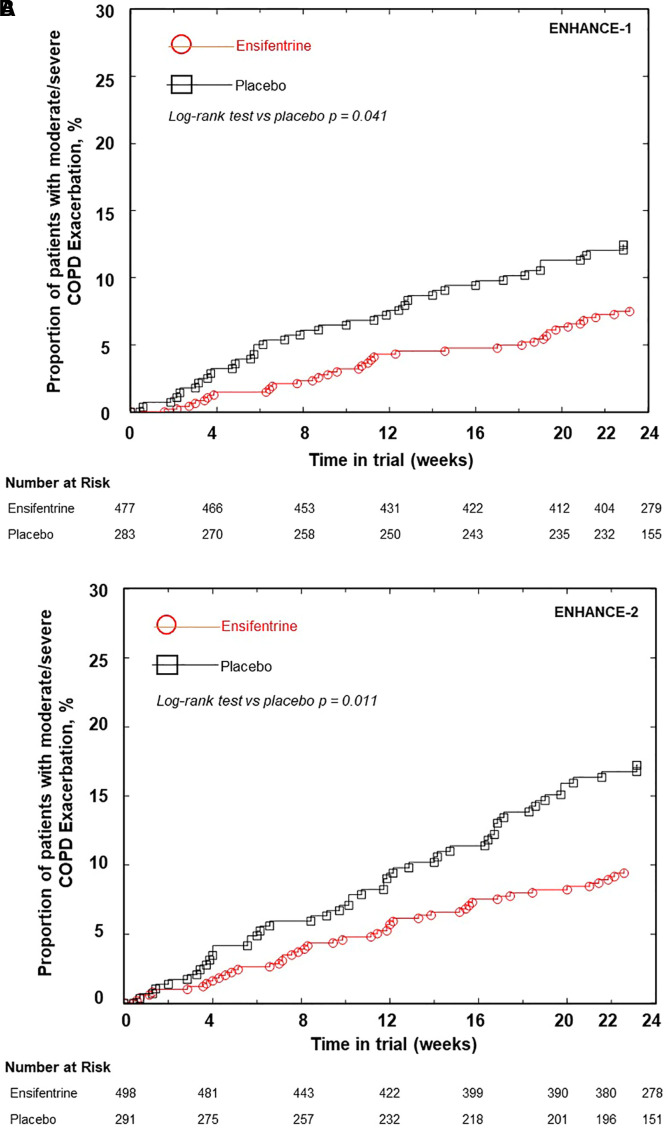
A reduction in the moderate or severe exacerbation rate and a delay in the time to first moderate or severe exacerbation over 24 and 48 weeks were observed with a similar magnitude in both trials (Table 3). In ENHANCE-1, ensifentrine reduced the annualized moderate or severe exacerbation rate over 24 weeks by 36% (24-wk rate ratio [RR], 0.64 [0.40, 1.00]; P = 0.050) and over 48 weeks (48-wk subset) by 44% (48-wk RR, 0.56 [0.32, 1.00], P = 0.052). A longer time to first event was also demonstrated over 24 and 48 weeks of treatment with ensifentrine versus placebo (24-wk hazard ratio [HR], 0.62 [0.39, 0.97]; P = 0.038; Figure 2A; 48-wk HR, 0.48 [0.28, 0.82]; P = 0.007).
Table 3.
Moderate or Severe Exacerbation Rate and Time to First Event Results in ENHANCE-1 and ENHANCE-2 Trials (mITT Population)
| Treatment Group | ENHANCE-1 |
ENHANCE-2 |
||
|---|---|---|---|---|
| Ensifentrine 3 mg BID (n = 477) | Placebo BID (n = 283) | Ensifentrine 3 mg BID (n = 498) | Placebo BID (n = 291) | |
| Moderate or severe COPD exacerbations over 24 wk | ||||
| Annualized exacerbation event rate, LS mean (95% CI) | 0.26 (0.17, 0.40) | 0.41 (0.27, 0.63) | 0.24 (0.18, 0.32) | 0.42 (0.30, 0.57) |
| Rate ratio (95% CI) | 0.64 (0.40, 1.00) | — | 0.57 (0.38, 0.87) | — |
| P value | 0.050 | — | 0.009 | — |
| Time to first event | ||||
| Log-rank test vs. placebo | P = 0.041 | — | P = 0.011 | — |
| Hazard ratio (95% CI) | 0.62 (0.39, 0.97) | — | 0.58 (0.38, 0.87) | — |
| P value | 0.038 | — | 0.009 | — |
| Moderate or severe COPD exacerbations over 48 wk | Ensifentrine (n = 280) | Placebo (n = 89) | — | — |
| Annualized exacerbation event rate, LS mean (95% CI) | 0.25 (0.13, 0.48) | 0.44 (0.22, 0.87) | — | — |
| Rate ratio (95% CI) | 0.56 (0.32, 1.00) | — | — | — |
| P value | 0.052 | — | — | — |
| Time to first event | ||||
| Log-rank test vs. placebo | P = 0.014 | — | — | — |
| Hazard ratio (95% CI) | 0.48 (0.28, 0.82) | — | — | — |
| P value | 0.007 | — | — | — |
Definition of abbreviations: BID = twice daily; CI = confidence interval; COPD = chronic obstructive pulmonary disease; LS = least squares; mITT = modified intention-to-treat.
Figure 2.
(A) Kaplan-Meier plot of time to first moderate or severe chronic obstructive pulmonary disease (COPD) exacerbation over 24 weeks in the ENHANCE-1 trial (modified intention-to-treat population [mITT]). Hazard ratio versus placebo (95% confidence interval [CI]), 0.62 (0.39, 0.97); P = 0.038. (B) Kaplan-Meier plot of time to first moderate or severe COPD exacerbation over 24 weeks in the ENHANCE-2 trial (mITT). Hazard ratio versus placebo (95% CI), 0.58 (0.38, 0.87); P = 0.009.
In ENHANCE-2, the annualized event rate of moderate or severe COPD exacerbations was also reduced with ensifentrine versus placebo, by 43% (RR, 0.57 [0.38, 0.87]; P = 0.009), together with delayed time to first moderate or severe exacerbation versus placebo (HR, 0.58 [0.38, 0.87]; P = 0.009) (Figure 2B). Nonproportional hazards were assessed in both trials, and interaction tests were not significant.
Results in patients receiving concomitant LABA with or without ICS and LAMA with or without ICS for each trial were generally consistent with the mITT analyses for lung function improvement, symptoms, quality of life, and exacerbation reduction (Tables E3 and E4). A similar effect on exacerbation endpoints was observed in patients with and without chronic bronchitis and with and without ICS use (Table E5).
Safety
Ensifentrine was well tolerated in both trials, with a similar proportion of patients reporting adverse events (AEs) in both treatment groups (Table 4). In ENHANCE-1, 38.4% of ensifentrine patients and 36.4% of placebo patients reported treatment-emergent adverse events (TEAEs). TEAEs leading to treatment withdrawal occurred in 6.1% and 6.4% of ensifentrine-treated and placebo-treated patients, respectively. Non–COVID-19 TEAEs leading to trial withdrawal occurred in 2.1% of patients. Serious TEAEs were reported in 6.7% of both groups. Over 24–48 weeks, the AE profile was similar to that observed in the first 24 weeks.
Table 4.
Adverse Event Summary in ENHANCE-1 and ENHANCE-2 Trials (Safety Population)
| ENHANCE-1 |
ENHANCE-2 |
|||
|---|---|---|---|---|
| Ensifentrine 3 mg BID | Placebo BID | Ensifentrine 3 mg BID | Placebo BID | |
| TEAEs over 24 wk (including follow-up period), no. of patients reported (%) | n = 477 | n = 283 | n = 498 | n = 291 |
| Any TEAE | 183 (38.4) | 103 (36.4) | 176 (35.3) | 103 (35.4) |
| Any TEAE (by preferred term) that occurred >1% in ensifentrine group and greater than placebo | ||||
| Nasopharyngitis | 13 (2.7) | 16 (5.7) | 9 (1.8) | 3 (1.0) |
| Hypertension | 12 (2.5) | 4 (1.4) | 5 (1.0) | 1 (0.3) |
| Back pain | 10 (2.1) | 1 (0.4) | 8 (1.6) | 5 (1.7) |
| COPD | 7 (1.5) | 6 (2.1) | 11 (2.2) | 5 (1.7) |
| Toothache | 6 (1.3) | 2 (0.7) | 0 | 1 (0.3) |
| Pneumonia* | 6 (1.3) | 2 (0.7) | 4 (0.8) | 5 (1.7) |
| Urinary tract infection | 5 (1.0) | 1 (0.4) | 8 (1.6) | 5 (1.7) |
| Diarrhea | 2 (0.4) | 2 (0.7) | 8 (1.6) | 2 (0.7) |
| Sinusitis | 1 (0.2) | 1 (0.4) | 6 (1.2) | 0 |
| Any TEAE causally related to treatment | 24 (5.0) | 11 (3.9) | 20 (4.0) | 12 (4.1) |
| Any serious TEAE | 32 (6.7) | 19 (6.7) | 28 (5.6) | 17 (5.8) |
| Any TEAE with an outcome of death | 2 (0.4) | 4 (1.4) | 4 (0.8) | 1 (0.3) |
| Any TEAE leading to discontinuation of treatment | 29 (6.1) | 18 (6.4) | 45 (9.0) | 29 (10.0) |
| Any TEAE leading to withdrawal from trial | 19 (4.0) | 10 (3.5) | 35 (7.0) | 20 (6.9) |
| No. with a diagnosis of COVID-19 | 8 (1.7) | 5 (1.8) | 16 (3.2) | 10 (3.4) |
| No. with no diagnosis of COVID-19 | 11 (2.3) | 5 (1.8) | 19 (3.8) | 10 (3.4) |
| Any severe TEAE | 27 (5.7) | 15 (5.3) | 22 (4.4) | 12 (4.1) |
| TEAEs from Week 24 to Week 48 (including follow-up),† no. of patients reported (%) | n = 228 | n = 70 | — | — |
| Any TEAE | 58 (25.4) | 19 (27.1) | — | — |
| Any TEAE (by preferred term) that occurred >1% in ensifentrine group and greater than placebo | ||||
| Nasopharyngitis | 6 (2.6) | 0 | — | — |
| Upper respiratory tract infection | 4 (1.8) | 0 | — | — |
| Any TEAE causally related to treatment | 2 (0.9) | 0 | — | — |
| Any serious TEAE | 11 (4.8) | 5 (7.1) | — | — |
| Any TEAE with an outcome of death | 2 (0.9) | 1 (1.4) | — | — |
| Any TEAE leading to discontinuation of treatment | 5 (2.2) | 2 (2.9) | — | — |
| Any TEAE leading to withdrawal from trial | 4 (1.8) | 1 (1.4) | — | — |
| No. with a diagnosis of COVID-19 | 2 (0.9) | 0 | — | — |
| No. with no diagnosis of COVID-19 | 2 (0.9) | 1 (1.4) | — | — |
| Any severe TEAE | 5 (2.2) | 3 (4.3) | — | — |
Definition of abbreviations: BID = twice daily; COPD = chronic obstructive pulmonary disease; TEAE = treatment-emergent adverse event.
COPD includes exacerbation or worsening of COPD reported as an adverse event. The safety population was randomized patients who received at least one dose (or partial dose) of treatment. Patients are classified according to actual treatment group.
Includes all reports of non–COVID-19 pneumonia (i.e., bacterial, chlamydial, Proteus).
Patients in the 48-week subset were randomized 3:1 to ensifentrine:placebo; thus, patient-years in the trial were higher in the ensifentrine group than in the placebo group. The number of patients in the analysis set includes patients who had their last treatment dose date after Week 24.
In ENHANCE-2, the proportion of patients with TEAEs was similar in the ensifentrine (35.3%) and placebo (35.4%) groups. TEAEs leading to treatment withdrawal occurred in 9.0% and 10.0% of ensifentrine-treated and placebo-treated patients, respectively. Non–COVID-19 TEAEs leading to trial withdrawal occurred in 3.7% of patients. Serious TEAEs were reported in 5.6% of ensifentrine-treated patients versus 5.8% of placebo-treated patients.
Overall gastrointestinal events were similar in the ensifentrine and placebo groups. Diarrhea events in the ensifentrine groups were not temporally associated with dosing, with most occurring more than 85 days after the start of treatment. The overall incidence of pneumonia events was low and similar in the ensifentrine and placebo treatment groups. In both trials, there were no relevant treatment group differences in safety laboratory tests, ECG parameters, or vital signs.
Discussion
Ensifentrine, a novel, selective, dual inhibitor of PDE3 and PDE4, has demonstrated consistent improvements in lung function (30, 32) as well as broad antiinflammatory effects in clinical trials (33). In these phase III trials of ensifentrine 3 mg twice daily in symptomatic, predominantly Global Initiative for Chronic Obstructive Lung Disease (GOLD) group B patients with moderate to severe COPD, the primary endpoint was met, demonstrating significant improvement in average FEV1 AUC0–12 h compared with placebo at Week 12.
A large magnitude of effect on exacerbations was demonstrated with ensifentrine compared with placebo in both trials over 24 weeks and in the subset of ENHANCE-1 patients treated over 48 weeks, which is notable, given that 55–69% of patients were receiving background medication (LAMA or LABA with or without ICS). Effects were consistent in patients with and without chronic bronchitis, in contrast to oral PDE4 inhibitors. Although not part of the formal testing hierarchy, these data were consistent across rate and time to first event endpoints across both trials over 24 and 48 weeks and across important subgroups, supporting effects of ensifentrine treatment complementary to existing classes of bronchodilatory and antiinflammatory therapy.
Over 24 weeks in ENHANCE-1, ensifentrine-treated patients reported significant improvements from baseline in symptoms that were consistent across E-RS, TDI, and rescue medication use measures, as well as quality of life (SGRQ). In ENHANCE-2, these endpoints were all numerically larger than placebo at all weeks, but they were not significant at Week 24. In ENHANCE-2, progressive improvement from baseline in the placebo group in all patient-reported endpoints was evident and in contrast with ENHANCE-1. The observation of improved symptoms and quality of life from baseline in the placebo group may be a result of the higher proportion of patients with severe COPD receiving placebo who withdrew from treatment and the trial than the proportion of ensifentrine-treated patients before Week 24, which was not observed in ENHANCE-1. In ENHANCE-2, this may have resulted in a less severe placebo group at Week 24 than at baseline. The totality of data supports a directional effect of ensifentrine versus placebo that was consistent across all symptom and quality-of-life endpoints at all time points assessed.
Rates of AEs were similar in the ensifentrine and placebo groups. There were no trends or differences in the incidence or type of AEs comparing patients treated with ensifentrine or placebo, including pneumonia. Gastrointestinal disorders, frequently seen with oral PDE4 inhibitors within 4 weeks of starting treatment (18, 49), were low and similar in both treatment groups and were not temporally associated with ensifentrine treatment.
Patients with COPD are still symptomatic and experience significant impairment in their activities of daily living despite the use of currently available medications. It is important to note that ensifentrine was not studied in all possible combinations of medications that could be used to currently treat COPD and excluded patients receiving concurrent dual LAMA/LABA or triple therapy. Despite recent GOLD and American Thoracic Society (50) clinical practice guideline recommendations for the use of dual bronchodilator therapy, most patients in the United States are still receiving single bronchodilator therapies for COPD maintenance (51, 52). GOLD also recognizes significant benefit/risk concerns regarding the use of ICS-containing therapies, including triple therapy, in patients with eosinophils <300 cells/μl. Thus, the population enrolled in the ENHANCE program was intended to reflect a broad moderate to severe COPD population receiving maintenance therapy still commonly used and to inform on the efficacy and safety of ensifentrine with a novel mechanism as a monotherapy and when added to these existing classes. Additional data with ensifentrine added to dual bronchodilators will further inform treatment decision making. Finally, the trials were performed within the entirety of the global COVID-19 pandemic, and the effect of quarantines or other pandemic-related practices in high-risk patients on patient-reported outcomes and safety results cannot be predicted.
Reductions in exacerbation rate and increased time to first exacerbation with ensifentrine were observed, even though the population was not enhanced for patients with a recent history of an exacerbation (53, 54). Exacerbation rates observed during the trials are consistent with population analyses describing exacerbation rates in a broad population of patients with moderate to severe COPD before the pandemic, with exacerbations occurring in 29–47% of patients per year (55, 56). Given this, the overall impact on the healthcare system may in fact be substantial, given the consistent effect identified in a broad COPD population in replicate trials.
The ENHANCE program provides evidence supporting the efficacy and safety of ensifentrine in symptomatic patients with moderate to severe COPD consistent with GOLD group B, including when added to current standard-of-care therapy: LAMA, LABA, and LABA/ICS. A significant improvement in bronchodilation; evidence of improvement in symptoms and quality of life; and, notably, a clinically important reduction in moderate or severe COPD exacerbations were demonstrated. Ensifentrine was well tolerated and not associated with gastrointestinal tolerability issues related to oral PDE4 therapy or to an increase in pneumonia associated with ICS-containing therapies. These results indicate that ensifentrine, with a novel PDE3 and PDE4 inhibition mechanism, would be a valuable and complementary addition to the limited available treatment mechanisms for patients with COPD.
Footnotes
Supported by Verona Pharma plc, London, United Kingdom.
Author Contributions: Substantial contributions to the conception or design of the work or the acquisition, analysis, or interpretation of data for the work: all authors. Drafting the work or revising it critically for important intellectual content: all authors. Final approval of the version to be published: all authors. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: all authors.
Data sharing statement: No individual deidentified participant data (including data dictionaries) will be shared. Results will be posted on public registries as required by law.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202306-0944OC on June 26, 2023
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2023. https://goldcopd.org/2023-gold-report-2/
- 2. Quaderi SA, Hurst JR. The unmet global burden of COPD. Glob Health Epidemiol Genom . 2018;3:e4. doi: 10.1017/gheg.2018.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen S, Small M, Lindner L, Xu X. Symptomatic burden of COPD for patients receiving dual or triple therapy. Int J Chron Obstruct Pulmon Dis . 2018;13:1365–1376. doi: 10.2147/COPD.S163717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phreesia Life Sciences. Patients in focus: COPD treatment and perceptions. Wilmington, DE: Phreesia Life Sciences; 2023. [Google Scholar]
- 5. Kew KM, Seniukovich A. Inhaled steroids and risk of pneumonia for chronic obstructive pulmonary disease. Cochrane Database Syst Rev . 2014;2014:CD010115. doi: 10.1002/14651858.CD010115.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tashkin DP, Miravitlles M, Celli BR, Metzdorf N, Mueller A, Halpin DMG, et al. Concomitant inhaled corticosteroid use and the risk of pneumonia in COPD: a matched-subgroup post hoc analysis of the UPLIFT trial. Respir Res . 2018;19:196–207. doi: 10.1186/s12931-018-0874-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stephenson A, Seitz D, Bell CM, Gruneir A, Gershon AS, Austin PC, et al. Inhaled anticholinergic drug therapy and the risk of acute urinary retention in chronic obstructive pulmonary disease: a population-based study. Arch Intern Med . 2011;171:914–920. doi: 10.1001/archinternmed.2011.170. [DOI] [PubMed] [Google Scholar]
- 8. Gershon A, Croxford R, Calzavara A, To T, Stanbrook MB, Upshur R, et al. Cardiovascular safety of inhaled long-acting bronchodilators in individuals with chronic obstructive pulmonary disease. JAMA Intern Med . 2013;173:1175–1185. doi: 10.1001/jamainternmed.2013.1016. [DOI] [PubMed] [Google Scholar]
- 9. Cazzola M, Rogliani P, Matera MG. The future of bronchodilation: looking for new classes of bronchodilators. Eur Respir Rev . 2019;28:190095. doi: 10.1183/16000617.0095-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Matera MG, Cazzola M, Page C. Prospects for COPD treatment. Curr Opin Pharmacol . 2021;56:74–84. doi: 10.1016/j.coph.2020.11.003. [DOI] [PubMed] [Google Scholar]
- 11. Stolz D, Mkorombindo T, Schumann DM, Agusti A, Ash SY, Bafadhel M, et al. Towards the elimination of chronic obstructive pulmonary disease: a Lancet Commission. Lancet . 2022;400:921–972. doi: 10.1016/S0140-6736(22)01273-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zuo H, Cattani-Cavalieri I, Musheshe N, Nikolaev VO, Schmidt M. Phosphodiesterases as therapeutic targets for respiratory diseases. Pharmacol Ther . 2019;197:225–242. doi: 10.1016/j.pharmthera.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 13. Banner KH, Press NJ. Dual PDE3/4 inhibitors as therapeutic agents for chronic obstructive pulmonary disease. Br J Pharmacol . 2009;157:892–906. doi: 10.1111/j.1476-5381.2009.00170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Boer J, Philpott AJ, van Amsterdam RG, Shahid M, Zaagsma J, Nicholson CD. Human bronchial cyclic nucleotide phosphodiesterase isoenzymes: biochemical and pharmacological analysis using selective inhibitors. Br J Pharmacol . 1992;106:1028–1034. doi: 10.1111/j.1476-5381.1992.tb14451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Page CP, Spina D. Phosphodiesterase inhibitors in the treatment of inflammatory diseases. Handb Exp Pharmacol . 2011;204:391–414. doi: 10.1007/978-3-642-17969-3_17. [DOI] [PubMed] [Google Scholar]
- 16. Calverley PM, Rabe KF, Goehring UM, Kristiansen S, Fabbri LM, Martinez FJ, M2-124 and M2-125 study groups Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet . 2009;374:685–694. doi: 10.1016/S0140-6736(09)61255-1. [DOI] [PubMed] [Google Scholar]
- 17. Singh D, Emirova A, Francisco C, Santoro D, Govoni M, Nandeuil MA. Efficacy and safety of CHF6001, a novel inhaled PDE4 inhibitor in COPD: the PIONEER study. Respir Res . 2020;21:246. doi: 10.1186/s12931-020-01512-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.2018.
- 19. Thomas B, Koh MS, O’Callaghan C, Allen JC, Jr, Rutman A, Hirst RA, et al. Dysfunctional bronchial cilia are a feature of chronic obstructive pulmonary disease (COPD) COPD . 2021;18:657–663. doi: 10.1080/15412555.2021.1963695. [DOI] [PubMed] [Google Scholar]
- 20. Raju SV, Rasmussen L, Sloane PA, Tang LP, Libby EF, Rowe SM. Roflumilast reverses CFTR-mediated ion transport dysfunction in cigarette smoke-exposed mice. Respir Res . 2017;18:173. doi: 10.1186/s12931-017-0656-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schmidt DT, Watson N, Dent G, Rühlmann E, Branscheid D, Magnussen H, et al. The effect of selective and non-selective phosphodiesterase inhibitors on allergen- and leukotriene C4-induced contractions in passively sensitized human airways. Br J Pharmacol . 2000;131:1607–1618. doi: 10.1038/sj.bjp.0703725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Challiss RA, Adams D, Mistry R, Nicholson CD. Modulation of spasmogen-stimulated Ins(1,4,5)P3 generation and functional responses by selective inhibitors of types 3 and 4 phosphodiesterase in airways smooth muscle. Br J Pharmacol . 1998;124:47–54. doi: 10.1038/sj.bjp.0701792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Milara J, Navarro A, Almudéver P, Lluch J, Morcillo EJ, Cortijo J. Oxidative stress-induced glucocorticoid resistance is prevented by dual PDE3/PDE4 inhibition in human alveolar macrophages. Clin Exp Allergy . 2011;41:535–546. doi: 10.1111/j.1365-2222.2011.03715.x. [DOI] [PubMed] [Google Scholar]
- 24. Boswell-Smith V, Spina D, Oxford AW, Comer MB, Seeds EA, Page CP. The pharmacology of two novel long-acting phosphodiesterase 3/4 inhibitors, RPL554 [9,10-dimethoxy-2(2,4,6-trimethylphenylimino)-3-(N-carbamoyl-2-aminoethyl)-3,4,6,7-tetrahydro-2H-pyrimido[6,1-a]isoquinolin-4-one] and RPL565 [6,7-dihydro-2-(2,6-diisopropylphenoxy)-9,10-dimethoxy-4H-pyrimido[6,1-a]isoquinolin-4-one] J Pharmacol Exp Ther . 2006;318:840–848. doi: 10.1124/jpet.105.099192. [DOI] [PubMed] [Google Scholar]
- 25. Calzetta L, Page CP, Spina D, Cazzola M, Rogliani P, Facciolo F, et al. Effect of the mixed phosphodiesterase 3/4 inhibitor RPL554 on human isolated bronchial smooth muscle tone. J Pharmacol Exp Ther . 2013;346:414–423. doi: 10.1124/jpet.113.204644. [DOI] [PubMed] [Google Scholar]
- 26. Calzetta L, Cazzola M, Page CP, Rogliani P, Facciolo F, Matera MG. Pharmacological characterization of the interaction between the dual phosphodiesterase (PDE) 3/4 inhibitor RPL554 and glycopyrronium on human isolated bronchi and small airways. Pulm Pharmacol Ther . 2015;32:15–23. doi: 10.1016/j.pupt.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 27. Venkatasamy R, Spina D. Novel relaxant effects of RPL554 on guinea pig tracheal smooth muscle contractility. Br J Pharmacol . 2016;173:2335–2351. doi: 10.1111/bph.13512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Turner MJ, Matthes E, Billet A, Ferguson AJ, Thomas DY, Randell SH, et al. The dual phosphodiesterase 3 and 4 inhibitor RPL554 stimulates CFTR and ciliary beating in primary cultures of bronchial epithelia. Am J Physiol Lung Cell Mol Physiol . 2016;310:L59–L70. doi: 10.1152/ajplung.00324.2015. [DOI] [PubMed] [Google Scholar]
- 29. Turner MJ, Dauletbaev N, Lands LC, Hanrahan JW. The phosphodiesterase inhibitor ensifentrine reduces production of proinflammatory mediators in well differentiated bronchial epithelial cells by inhibiting PDE4. J Pharmacol Exp Ther . 2020;375:414–429. doi: 10.1124/jpet.120.000080. [DOI] [PubMed] [Google Scholar]
- 30. Singh D, Martinez FJ, Watz H, Bengtsson T, Maurer BT. A dose-ranging study of the inhaled dual phosphodiesterase 3 and 4 inhibitor ensifentrine in COPD. Respir Res . 2020;21:47. doi: 10.1186/s12931-020-1307-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Watz H, Rickard K, Rheault T, Bengtsson T, Singh D. symptom improvement following treatment with the inhaled dual phosphodiesterase 3 and 4 inhibitor ensifentrine in patients with moderate to severe COPD—a detailed analysis. Int J Chron Obstruct Pulmon Dis . 2020;15:2199–2206. doi: 10.2147/COPD.S263025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ferguson GT, Kerwin EM, Rheault T, Bengtsson T, Rickard K. A dose-ranging study of the novel inhaled dual PDE 3 and 4 inhibitor ensifentrine in patients with COPD receiving maintenance tiotropium therapy. Int J Chron Obstruct Pulmon Dis . 2021;16:1137–1148. doi: 10.2147/COPD.S307160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Franciosi LG, Diamant Z, Banner KH, Zuiker R, Morelli N, Kamerling IM, et al. Efficacy and safety of RPL554, a dual PDE3 and PDE4 inhibitor, in healthy volunteers and in patients with asthma or chronic obstructive pulmonary disease: findings from four clinical trials. Lancet Respir Med . 2013;1:714–727. doi: 10.1016/S2213-2600(13)70187-5. [DOI] [PubMed] [Google Scholar]
- 34.Verona Pharma plc. 2023. https://clinicaltrials.gov/ct2/show/NCT04535986
- 35.Verona Pharma plc. 2022. https://clinicaltrials.gov/ct2/show/NCT04542057
- 36.Anzueto A, Barjaktarevic IZ, Siler TM, Rheault T, Bengtsson T, Rickard K, et al.
- 37. Anzueto A, Barjaktarevic IZ, Rheault T, Bengtsson T, Rickard K. Ensifentrine, a novel dual phosphodiesterase (PDE) 3 and 4 inhibitor, improves lung function and reduces exacerbation rate and risk in phase 3 Enhance-2 trial [abstract] Am J Respir Crit Care Med . 2023;207:A4494. doi: 10.1164/rccm.202306-0944OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rheault T, Bengtsson T, Rickard K. Ensifentrine, a novel dual phosphodiesterase (PDE) 3 and 4 inhibitor, in moderate and severe COPD: symptoms, quality of life and health status from the phase 3 trial Enhance-2 [abstract] Am J Respir Crit Care Med . 2023;207:A5000. [Google Scholar]
- 39. Siler TM, Rickard K, Bengtsson T, Rheault T. Safety results from dual PDE3/4 inhibitor ensifentrine: gastrointestinal and cardiovascular safety from a 24-week phase 3 trial, Enhance-2 [abstract] Am J Respir Crit Care Med . 2023;207:A5003. [Google Scholar]
- 40. Sciurba FC, Anzueto A, Rheault T, Bengtsson T, Rickard K. Ensifentrine, a novel dual phosphodiesterase (PDE) 3 and 4 inhibitor, improves lung function, symptoms, quality of life and reduces exacerbation rate and risk in patients with COPD: results from replicate phase 3 trials [abstract] Am J Respir Crit Care Med . 2023;207:A5005. [Google Scholar]
- 41. Sciurba FC, Wise R, Rheault T, Bengtsson T, Rickard K. Ensifentrine, a novel dual phosphodiesterase (PDE) 3 and 4 inhibitor, improves lung function, symptoms, quality of life and reduces exacerbation rate and risk in the Enhance-1 phase 3 trial of ensifentrine in COPD [abstract] Am J Respir Crit Care Med . 2023;207:A5006. [Google Scholar]
- 42. Sciurba FC, Rheault T, Bengtsson T, Rickard K. Ensifentrine, a novel dual phosphodiesterase (PDE) 3 and 4 inhibitor, significantly improves COPD symptoms and quality of life in the phase 3 Enhance-1 trial [abstract] Am J Respir Crit Care Med . 2023;207:A5007. doi: 10.1164/rccm.202306-0944OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest . 1988;93:580–586. doi: 10.1378/chest.93.3.580. [DOI] [PubMed] [Google Scholar]
- 44.Evidera, Inc. EXACT-Respiratory Symptoms user manual. Bethesda, MD: Evidera, Inc; 2014. [Google Scholar]
- 45. Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GK, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med . 1987;106:196–204. doi: 10.7326/0003-4819-106-2-196. [DOI] [PubMed] [Google Scholar]
- 46. Leidy NK, Murray LT, Monz BU, Nelsen L, Goldman M, Jones PW, et al. Measuring respiratory symptoms of COPD: performance of the EXACT-Respiratory Symptoms Tool (E-RS) in three clinical trials. Respir Res . 2014;15:124. doi: 10.1186/s12931-014-0124-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jones PW, Quirk FH, Baveystock CM. The St George’s Respiratory Questionnaire. Respir Med . 1991;85:25–31. doi: 10.1016/s0954-6111(06)80166-6. [DOI] [PubMed] [Google Scholar]
- 48. Mahler DA, Witek TJ., Jr The MCID of the transition dyspnea index is a total score of one unit. COPD . 2005;2:99–103. doi: 10.1081/copd-200050666. [DOI] [PubMed] [Google Scholar]
- 49. Fabbri LM, Calverley PM, Izquierdo-Alonso JL, Bundschuh DS, Brose M, Martinez FJ, et al. M2-127 and M2-128 study groups Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with long acting bronchodilators: two randomised clinical trials. Lancet . 2009;374:695–703. doi: 10.1016/S0140-6736(09)61252-6. [DOI] [PubMed] [Google Scholar]
- 50. Nici L, Mammen MJ, Charbek E, Alexander PE, Au DH, Boyd CM, et al. Pharmacologic management of chronic obstructive pulmonary disease. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med . 2020;201:e56–e69. doi: 10.1164/rccm.202003-0625ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.2023.
- 52.Mannino D, Siddall J, Small M, Haq A, Stiegler M, Bogart M. Treatment patterns for chronic obstructive pulmonary disease (COPD) in the United States: results from an observational cross-sectional physician and patient survey. Int J Chron Obstruct Pulmon Dis. 2022;17:749–761. doi: 10.2147/COPD.S340794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lipson DA, Barnhart F, Brealey N, Brooks J, Criner GJ, Day NC, et al. IMPACT Investigators Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med . 2018;378:1671–1680. doi: 10.1056/NEJMoa1713901. [DOI] [PubMed] [Google Scholar]
- 54. Rabe KF, Martinez FJ, Ferguson GT, Wang C, Singh D, Wedzicha JA, et al. ETHOS Investigators Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N Engl J Med . 2020;383:35–48. doi: 10.1056/NEJMoa1916046. [DOI] [PubMed] [Google Scholar]
- 55. Sethi S, Make BJ, Robinson SB, Kumar S, Pollack M, Moretz C, et al. Relationship of COPD exacerbation severity and frequency on risks for future events and economic burden in the Medicare fee-for-service population. Int J Chron Obstruct Pulmon Dis . 2022;17:593–608. doi: 10.2147/COPD.S350248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hurst JR, Vestbo J, Anzueto A, Locantore N, Müllerova H, Tal-Singer R, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med . 2010;363:1128–1138. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]