Abstract
BACKGROUND:
When tested in a controlled clinic environment, individuals with neuromuscular-related symptoms may complete motor tasks within normal predicted ranges. However, measuring activity at home may better reflect typical motor performance. The accuracy of accelerometry measurements in individuals with congenital muscular dystrophy (CMD) is unknown. We aimed to compare accelerometry and manual step counts and assess free-living physical activity intensity in individuals with CMD using accelerometry.
METHODS:
Ambulatory pediatric CMD participants (n = 9) performed the 6-minute walk test in clinic while wearing ActiGraph GT3X accelerometer devices. During the test, manual step counting was conducted to assess concurrent validity of the ActiGraph step count in this population using Bland-Altman analysis. In addition, activity intensity of 6 pediatric CMD participants was monitored at home with accelerometer devices for an average of 7 days. Cut-point values previously validated for neuromuscular disorders were used for data analysis.
RESULTS:
Bland-Altman and intraclass correlation analyses showed no concurrent validity between manual and ActiGraph-recorded step counts. Fewer steps were recorded by ActiGraph step counts compared with manual step counts (411 ± 74 vs 699 ± 43, respectively; P = .004). Although improved, results were in the same direction with the application of low-frequency extension filters (587 ± 40 vs 699 ± 43, P = .03). ActiGraph step-count data did not correlate with manual step count (Spearman ρ = 0.32, P = .41; with low-frequency extension: Spearman ρ = 0.45, P = .22). Seven-day physical activity monitoring showed that participants spent more than 80% of their time in the sedentary activity level.
CONCLUSIONS:
In a controlled clinic setting, step count was significantly lower by ActiGraph GT3X than by manual step counting, possibly because of the abnormal gait in this population. Additional studies using triaxial assessment are needed to validate accelerometry measurement of activity intensity in individuals with CMD. Accelerometry outcomes may provide valuable measures and complement the 6-minute walk test in the assessment of treatment efficacy in CMD.
Keywords: accelerometry, capacity, congenital muscular dystrophy, intensity intervals, motor function, nursing, pediatric, physical performance, step counts
Congenital muscular dystrophy (CMD) encompasses a group of phenotypically and genetically heterogenous disorders characterized by hypotonia, delayed motor milestones, and progressive muscle weakness associated with a dystrophic histopathologic pattern on muscle biopsy.1 The main CMD subtypes include dystroglycanopathies, SELENON-related myopathy, and LMNA-related CMD, with LAMA2-related muscular dystrophy (LAMA2-RD) and collagen VI–related muscular dystrophy (COL6-RD) being the most common.2 Although not well documented, the point prevalence of CMD ranges from 0.5 to 2.5 per 100 000.3,4 Across all CMD subtypes, varying degrees of joint contractures and muscle weakness result in decreased mobility and impaired activities of daily living. A survey of outcome measures for individuals affected with CMD reported that achieving and retaining ambulation is of upmost priority.5 To address this concern, it is important to conduct mobility assessments of motor capacity and performance. Motor capacity refers to completion of an assigned task within a controlled environment, whereas performance measures individuals’ activity in their everyday free-living environment.6,7 The 6-minute walk test (6MWT) is an endurance test with high reliability and validity and, therefore, serves as the current standard for the assessment of motor capacity and disease progression.8,9
Assessment of performance using wearable devices has become increasingly popular, with accelerometry being the preferred method to measure free-living activity.10-13 Accelerometry assesses physical activity by measuring the person’s change in position in space. Despite the deleterious effect of reduced activity in individuals affected with CMD and the widespread research use of accelerometers, to our knowledge, no studies have investigated the accuracy of such devices at assessing step counts in individuals with CMD. Traditional accelerometry activity intervals are based on principles of cardiovascular and physical fitness measures. The traditional activity intervals are not optimal for individuals with CMD where mobility, rather than energy expenditure, is the limiting factor. However, a new set of accelerometer intervals focusing on activities recorded in the light intensity range was developed for populations with limited activity. These new intervals provide a model for objective measurement of real-life physical performance in individuals with musculoskeletal disorders. These intervals have been validated in populations with musculoskeletal disorders associated with pain and in individuals after total knee arthroplasty.14,15
ActiLife is an ActiGraph software that uses a capacitive ADXL335 sensor to record both static and dynamic movements. The ActiGraph GT3X model uses an inclinometer to monitor movement on all 3 axes and separates activity into either not wearing the monitor (0), standing (1), lying (2), or sitting (3).16 Each anatomical position has a corresponding score (0-3) recorded at a given epoch, which is the interval of time when vector magnitude counts are collected. Triaxial vector magnitudes allow for physical activity intensity measurements determined by cut-points in 3 planes. This pilot study aimed to (1) examine the validity of step-count data obtained by accelerometry and (2) explore the feasibility of using accelerometry to measure free-living physical performance in a pediatric CMD cohort.
Methods
This pilot study was conducted at the Mark O. Hatfield Clinical Research Center at the National Institutes of Health in Bethesda, Maryland, between 2012 and 2014. Nine participants, aged 9 to 18 years, with a genetic diagnosis of COL6-RD (n = 8) or LAMA2-RD (n = 1) were recruited from a larger CMD study (NCT01568658). Ethical approval was granted by the National Institute of Neurological Disorders and Stroke Institutional Review Board (12-N-0095), assent was obtained from minors, and informed consent was obtained from legal guardians. All participants were considered ambulatory, as defined by the ability to walk 10 m without orthotics or assistive devices.
Six-Minute Walk Test and Manual Step Count
All participants completed the 6MWT for functional capacity under the supervision and guidance of a pediatric physical therapist according to the American Thoracic Society guidelines.17 Participants wore an ActiGraph device on their dominant hip during the 6MWT in the clinic. A study team member manually counted the steps during the 6MWT using a hand-counter and tally sheet. The relatively slow pace of the participants allowed the researcher to accurately record each manual step.
Observational Gait Analysis
Gait pattern videos were recorded as part of the initial study and were available for 7 of the 9 study participants. Altered gait patterns in individuals with COL6-RD and LAMA2-RD may influence the number of steps recorded by accelerometry. A physical therapist reviewed the videos for evidence of gait abnormalities that may impact accelerometer function.
ActiGraph Step Count
Of the 9 participants, 6 were equipped with ActiGraph GT3X to record their physical activities for 7 days outside the clinic setting. The accelerometer was delivered by mail, and participants received an introduction sheet, time log, and detailed instructions on how to complete a series of exercises to establish the level of maximal activity before recording free-living activities (see Supplemental Digital Content 1, available at http://links.lww.com/JNN/A247). All participants were instructed to wear the monitor around their waist for at least 10 hours a day and to record times when the monitor was put on and taken off each day.
The ActiGraph GT3X contains 3 capacitors, in which 2 capacitors move relative to a central capacitor in response to accelerations ranging between 0.5 and 2.0 g. The change in capacitance elicits specific voltage changes converted to an analog signal proportional to the detected acceleration. The signal is then filtered at a bandwidth ranging between 0.25 and 2.5 Hz, a frequency range in which most human movement occurs. If the frequency of acceleration peak occurs outside this range, the acceleration signal is diminished. A step is registered when the frequency of acceleration occurs within the bandwidth range. Acceleration data collected include vertical axis activity (axis 1), horizontal axis activity (axis 2), and perpendicular axis (axis 3). Step counts and inclinometer position (off, lying, sitting, or standing) are available for each epoch (selected time interval) of output. The size of the accelerometer is 4.6 × 3.3 × 1.5 cm, the weight is 19 g, and it is water resistant up to 1 m. Recorded data were first analyzed in ActiLife software version 6.10.2 using default settings and then repeated with a low-frequency extension (LFE) filter to enable detection of low-amplitude movements.18 Activity was captured with 60-second epochs.
Data were further sorted into designated counts per minute in the following intervals: performance sedentary, 1 to 100; performance light 1, 101 to 350; performance light 2, 351 to 800; performance light 3, 801 to 2500; and performance moderate/vigorous, 2501 to 30 000.14 The LFE is suggested for individuals who take light steps or have slow movement. Because individuals with CMD present with ambulatory limitations, the triaxial cut-point with an LFE filter is crucial to capture the scope of mobility within this population. When activity exceeds 6 counts per second, the user is assumed to be standing because of the high activity values, and thus the inclination angles are ignored. Otherwise, θy<17° is considered standing, 17°<θy<65° is considered sitting, and θy > 65° is considered lying, unless θz < 22° when the unit is off (GT3X+ and wGT3X+ device manual).
Statistical analyses were performed using SPSS v.24 and Prism 8 v.8.1.2, with statistical significance set at P < .05. Because of the exploratory nature of this study, significance levels of individual tests were not adjusted with multiple testing correction. Tests were first conducted using data obtained via the accelerometer default filter and then repeated with the LFE filter applied. Strength of association between manual and ActiGraph step counts was measured using Spearman ρ nonparametric test, and Bland-Altman plot was used as the primary measure of step count agreement between the 2 recorded step counts. Paired sample t tests were used to compare mean discrepancy ± standard error of the mean (SEM) in the Bland-Altman plot between the 2 measures. Concurrent validity between manual and ActiGraph-reported step counts was also tested by intraclass correlation coefficients (ICCs).
Results
Of the 9 participants recruited for the comparison of ActiGraph and manual step counts, 3 were male, 8 were COL6-RD patients, and 1 was a LAMA2-RD patient. Performing the 6MWT while equipped with the ActiGraph GT3X in the clinic was well tolerated by the participants as they all completed the test with the device in place. The manually counted and ActiGraph-recorded steps, with and without frequency extension, are presented in Table 1.
TABLE 1.
Step Count Data
| Subject | Hand Counted |
ActiGraph Counted |
ActiGraph Counted (LFE)a |
|---|---|---|---|
| 1 | 926 | 427 | 558 |
| 2 | 685 | 601 | 681 |
| 3 | 837 | 405 | 678 |
| 4 | 699 | 597 | 692 |
| 5 | 645 | 70 | 492 |
| 6 | 615 | 403 | 597 |
| 7 | 789 | 575 | 691 |
| 8 | 524 | 24 | 330 |
| 9 | 571 | 596 | 568 |
The low-frequency extension (LFE) is a filter that enables the detection of lower amplitude motion than ActiGraph's default filter.
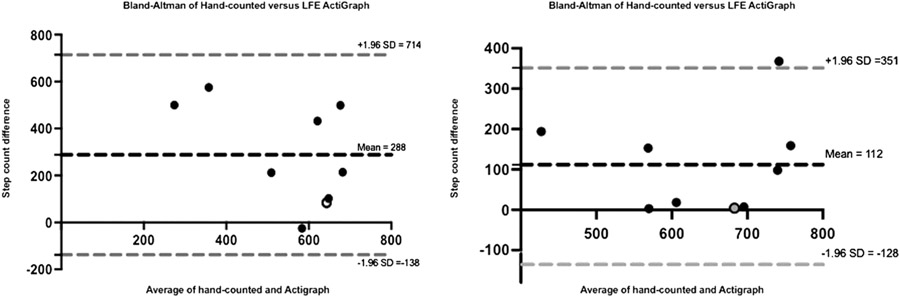
Bland-Altman plots of the mean differences between manually counted steps and both ActiGraph and LFE-ActiGraph showed consistent variability around the average discrepancy (bias, 288 steps for standard and 112 steps for LFE; Fig 1). This reflects agreement between the 2 methods, with LFE having narrower limits of agreement (−128 to 351) than the standard ActiGraph count (−138 to 714), when compared with manually counted steps. However, there was a statistically significant difference in the mean of manually counted versus ActiGraph-reported steps (699 ± 43 vs 411 ± 74 steps, respectively; P = .004). This remained the case when the LFE filter was applied, albeit to a lesser degree (699 ± 43 vs 587 ± 40 steps, respectively; P = .03). In addition, ICC analysis showed no significant concurrent validity between manual and ActiGraph-recorded step counts (ICC = 0.29, P = .21; 95% confidence interval [CI], −0.42 to 0.78) and LFE-ActiGraph (ICC = 0.52, P = .06; 95% CI, −0.16 to 0.87). There was no correlation between manual and ActiGraph (Spearman ρ = 0.32, P = .41) or LFE-ActiGraph (Spearman ρ = 0.45, P = .22) step counts.
Figure 1.
Bland-Altman Plots Comparing Manual and ActiGraph Step Counts. Note. Bland-Altman (B-A) analyses of hand-counted and ActiGraph-counted steps. Individual B-A analyses of each participant's results were performed to determine the level of agreement between the hand-counted and ActiGraph-counted (with/without low-frequency extension [LFE]) methods. The mean value from both methods for each participant (x-axis) is plotted against the difference between the same results (y-axis). The limit of agreement shows consistent variability around the average discrepancy (bias, 288 steps for standard ActiGraph and 112 steps for LFE-ActiGraph) and, therefore, agreement between the 2 methods. Gray-colored dot indicates participant with LAMA2-related disorder
Accelerometry and Physical Performance
The number of epochs during a 7-day period outside the clinic setting was collected and converted to a daily average for each subject and intensity interval (see Supplemental Digital Content 2, available at http://links.lww.com/JNN/A248). For days with a valid wear time of 10 or more hours, more than 80% of the free-living motion intensity of all 6 pediatric CMD participants was in the sedentary physical performance interval (performance sedentary) (mean [SEM], 86.88% [2.24]), with less than 4% motion intensity in the moderate to vigorous (performance moderate/vigorous) interval (mean [SEM], 1.92 [0.51]) (see Supplemental Digital Content 3, available at http://links.lww.com/JNN/A249). While wearing the ActiGraph device, participants recorded the highest counts for axis 2 (horizontal axis) during the scored time (mean [SEM], 2175204.5 counts [497041.3]) (see Supplemental Digital Content 4, available at http://links.lww.com/JNN/A250).
Feasibility
Participants or their parents/caregivers kept a daily log to document periods (start and end times) during which the ActiGraph device was not worn (eg, during water-related activities, bedtime, or out-of-the-ordinary activities). In addition, a mail-in exit survey was given to participants and their parents to assess the feasibility of wearing and using the device from the participant and caregiver perspectives (see Supplemental Digital Content 5, available at http://links.lww.com/JNN/A251). Overall, it was easy to persuade the child to wear the device. The ActiGraph device was easy to wear without discomfort for 10 hours a day for 7 consecutive days outside the clinic setting, and participants did not feel embarrassed wearing the device around others. Parents and caregivers did not report any inconveniences while the child was wearing the device.
Discussion
Free-living physical performance is an important health outcome that is yet to be characterized in the CMD population. Performance can present a greater challenge to individuals with neuromuscular disorders such as CMD than motor capacity. Physical activity assessments in daily life should be performed over periods long enough to be representative of the habitual activity level and with minimal discomfort to the subject.19 Waist-worn accelerometer protocols have been used to determine levels of physical performance and sedentary behavior in various populations, including children.20-22
In this pilot study, we assessed capacity and free-living physical performance in a group of participants with CMD using waist-worn accelerometry. Concurrent validity between manually counted and ActiGraph-counted steps was not demonstrated in this study. Although there was agreement in step count measurement between the 2 methods, the limits of agreement from the Bland-Altman plots were relatively wide (standard ActiGraph, −138 to 714; LFE-ActiGraph, −128 to 351), which introduced ambiguity into the observed variability around the mean difference. Therefore, on the basis of our findings, the 2 methods (manual count vs ActiGraph or LFE-ActiGraph) should not be considered equivalent. There were statistically significant difference and no correlation between manually counted and ActiGraph-recorded steps in this cohort. In addition, we did not obtain a statistically significant ICC to establish the concurrent validity of ActiGraph (ICC = 0.29, P = .21; 95% CI, −0.42 to 0.78) and LFE-ActiGraph (ICC = 0.52, P = .06; 95% CI, −0.16 to 0.87) step counts with manual step count. The difference in step counts between manual and ActiGraph is likely due to a combination of Trendelenburg gait and slow walking speed in CMD-affected individuals, relative to the general population,23,24 which introduces a systematic bias to the ActiGraph count, especially when the LFE option is not activated. All participants presented with a bilateral compensatory Trendelenburg gait (lateral trunk lean), with varying degrees of hip flexion. As such, a measure that uses uniaxial data may not accurately assess the movement of an ambulatory individual with CMD. The difference between manual and ActiGraph counts was smaller when using the LFE option on the ActiGraph device. The LFE option increases sensitivity to very low–amplitude activities, allowing for the capture of slow or very light steps.
It is important to validate objective means of measuring free-living physical activity for CMD patients, as effective therapeutics transition to clinical trials.25 In this study, we demonstrated the feasibility of using an accelerometry device to capture free-living physical activity in a pediatric cohort of participants with CMD. Participants spent more than 80% of their ActiGraph-recorded performance in the sedentary intensity interval. The cut-points used for this study were able to capture intensities in the light range intervals (performance light 1 to performance light 3), which have been associated with reduction in mortality risk26 but were commonly difficult to measure in patients with mobility limitations and neuromuscular disorders.14 The focus on light-intensity activity and sedentary behavior, in contrast to moderate to vigorous physical activity, provides greater discrimination and better characterizes activity levels in mobility-limited populations such as CMD patients. The physical performance intervals used for this study have been validated in adults with musculoskeletal pain and mobility limitations, but not in a pediatric population.
An acknowledged limitation of the ActiGraph protocol in this study is the requirement that participants take off the device before bedtime to improve wear time compliance, which led to missing bedtime and wake-time information. Accelerometers are typically worn at the wrist or ankle to assess and accurately record bedtimes and wake times in younger children.27 Wrist-worn devices capture data from sedentary behavior, physical activity of all intensities, and sleep-period time, while increasing compliance with the wearing regimen and ensuring minimum daily wear-time duration necessary to measure habitual behavior.28,29
Given the small number of patients, we suggest repeating and reproducing the findings of this study with a larger sample size and age-matched controls, with considerations for seasonal confounders such as school breaks and weather conditions. A future study should explore and validate ActiGraph as a measure of activity intensity in CMD, in addition to assessing energy expenditure in the CMD pediatric population using indirect calorimetry.30 However, activity measurement, rather than energy expenditure, is of greater focus in musculoskeletal disorders.14
Conclusion
This pilot study shows that waist-worn accelerometers can be used to capture free-living physical activity intensity in children affected with CMD. The ActiGraph device was well tolerated and may provide an added practical approach to measuring and quantifying therapeutic effects in this group of neuromuscular disorders. The use of accelerometry intervals previously optimized for musculoskeletal disorders provides an opportunity to quantify free-living physical performance in individuals with CMD. Participants in this study were nonactive during a large part of their wear time, and step count was not captured adequately, likely because of Trendelenburg gait. This improved with the use of the LFE but remained suboptimal. Accelerometers are able to accurately estimate the duration, frequency, and intensity of physical activity in nondisabled populations by measuring activity across several planes. A known disadvantage is their inability to accurately capture events where an individual is not moving the body part being monitored by the accelerometer.31 Further research is needed to validate these findings. With disease- and age-appropriate cut-points, ActiGraph has the potential to serve individuals with CMD and their clinicians by providing a feasible and affordable noninvasive tool for use in clinical practice along with the 6MWT, including the potential for monitoring physical performance patterns associated with treatment response.
Supplementary Material
Footnotes
The authors declare no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.jnnonline.com).
Contributor Information
Tokunbor A. Lawal, National Institute of Nursing Research, NIH, Bethesda, MD..
Joshua J. Todd, National Institute of Nursing Research, NIH, Bethesda, MD..
Jeffrey S. Elliott, National Institute of Nursing Research, NIH, Bethesda, MD..
Melody M. Linton, National Institute of Nursing Research, NIH, Bethesda, MD..
Megan Andres, National Institute of Nursing Research, NIH, Bethesda, MD..
Jessica W. Witherspoon, National Institute of Nursing Research, NIH, Bethesda, MD..
John P. Collins, Mark O. Hatfield Clinical Research Center, NIH, Bethesda, MD; Department of Rehabilitation Science, George Mason University, Fairfax, VA..
Irene C. Chrismer, National Institute of Nursing Research, NIH, Bethesda, MD..
Fatoumata Tounkara, National Institute of Nursing Research, NIH, Bethesda, MD..
Melissa R. Waite, Mark O. Hatfield Clinical Research Center, NIH, Bethesda, MD..
Carmel Nichols, Mark O. Hatfield Clinical Research Center, NIH, Bethesda, MD..
Carsten G. Bönnemann, National Institute of Neurological Disorders and Stroke, NIH, Bethesda, MD..
Carole Vuillerot, Service de Médecine Physique et de Réadaptation Pédiatrique, Hôpital Femme-Mère-Enfant, Hospices Civils de Lyon, Bron, France..
Roxanna Bendixen, Department of Occupational Therapy, University of Pittsburgh, Pittsburgh, PA..
Minal S. Jain, Mark O. Hatfield Clinical Research Center, NIH, Bethesda, MD..
Katherine G. Meilleur, National Institute of Nursing Research, NIH, Bethesda, MD..
References
- 1.Reed UC. Congenital muscular dystrophy. Part I: a review of phenotypical and diagnostic aspects. Arq Neuropsiquiatr. 2009;67(1):144–168. [DOI] [PubMed] [Google Scholar]
- 2.Muntoni F, Voit T. The congenital muscular dystrophies in 2004: a century of exciting progress. Neuromuscul Disord. 2004;14(10):635–649. [DOI] [PubMed] [Google Scholar]
- 3.Graziano A, Bianco F, D’Amico A, et al. Prevalence of congenital muscular dystrophy in Italy: a population study. Neurology. 2015;84(9):904–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Darin N, Tulinius M. Neuromuscular disorders in childhood: a descriptive epidemiological study from western Sweden. Neuromuscul Disord. 2000;10(1):1–9. [DOI] [PubMed] [Google Scholar]
- 5.Smith-Hoban D, Sklaroff-Van Hook S, Rutkowski A. A congenital muscular dystrophy quality of life and caregiver assessment survey. Neuromuscul Disord. 2010;20(8):564–565. [DOI] [PubMed] [Google Scholar]
- 6.Agarwal V, Smuck M, Tomkins-Lane C, Shah NH. Inferring physical function from wearable activity monitors: analysis of free-living activity data from patients with knee osteoarthritis. JMIR Mhealth Uhealth. 2018;6(12):e11315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holsbeeke L, Ketelaar M, Schoemaker MM, Gorter JW. Capacity, capability, and performance: different constructs or three of a kind? Arch Phys Med Rehabil. 2009;90(5):849–855. [DOI] [PubMed] [Google Scholar]
- 8.Andersen LK, Knak KL, Witting N, Vissing J. Two- and 6-minute walk tests assess walking capability equally in neuromuscular diseases. Neurology. 2016;86(5):442–445. [DOI] [PubMed] [Google Scholar]
- 9.Witherspoon JW, Vasavada RP, Waite MR, et al. 6-minute walk test as a measure of disease progression and fatigability in a cohort of individuals with RYR1-related myopathies. Orphanet J Rare Dis. 2018;13(1):105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang CC, Hsu YL. A review of accelerometry-based wearable motion detectors for physical activity monitoring. Sensors (Basel). 2010;10(8):7772–7788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrari GL, Araujo TL, Oliveira L, et al. Association between television viewing and physical activity in 10-year-old Brazilian children. J Phys Act Health. 2015;12(10):1401–1408. [DOI] [PubMed] [Google Scholar]
- 12.Jefferis BJ, Merom D, Sartini C, et al. Physical activity and falls in older men: the critical role of mobility limitations. Med Sci Sports Exerc. 2015;47(10):2119–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joseph RP, Keller C, Adams MA, Ainsworth BE. Print versus a culturally-relevant Facebook and text message delivered intervention to promote physical activity in African American women: a randomized pilot trial. BMC Womens Health. 2015;15:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smuck M, Tomkins-Lane C, Ith MA, Jarosz R, Kao MJ. Physical performance analysis: a new approach to assessing free-living physical activity in musculoskeletal pain and mobility-limited populations. PloS One. 2017;12(2):e0172804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agarwal V, Smuck M, Shah NH. Quantifying the relative change in physical activity after total knee arthroplasty using accelerometer based measurements. AMIA Jt Summits Transl Sci Proc. 2017;2017:463–472. [PMC free article] [PubMed] [Google Scholar]
- 16.John D, Freedson P. ActiGraph and Actical physical activity monitors: a peek under the hood. Med Sci Sports Exerc. 2012;44(1, suppl 1):S86–S89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. [DOI] [PubMed] [Google Scholar]
- 18.Hjorth MF, Chaput JP, Damsgaard CT, et al. Measure of sleep and physical activity by a single accelerometer: can a waist-worn Actigraph adequately measure sleep in children? Sleep Biol Rhythms. 2012;10(4):328–335. [Google Scholar]
- 19.Plasqui G, Westerterp KR. Physical activity assessment with accelerometers: an evaluation against doubly labeled water. Obesity. 2007;15(10):2371–2379. [DOI] [PubMed] [Google Scholar]
- 20.Freedson P, Pober D, Janz KF. Calibration of accelerometer output for children. Med Sci Sports Exerc. 2005;37(11 suppl):S523–S530. [DOI] [PubMed] [Google Scholar]
- 21.Troiano RP, Berrigan D. Physical activity in the United States measured by accelerometer: comment-response. Med Sci Sports Exerc. 2008;40(6):1189. [DOI] [PubMed] [Google Scholar]
- 22.McClain JJ, Tudor-Locke C. Objective monitoring of physical activity in children: considerations for instrument selection. J Sci Med Sport. 2009;12(5):526–533. [DOI] [PubMed] [Google Scholar]
- 23.Oftedal S, Bell KL, Davies PS, Ware RS, Boyd RN. Validation of accelerometer cut points in toddlers with and without cerebral palsy. Med Sci Sports Exerc. 2014;46(9):1808–1815. [DOI] [PubMed] [Google Scholar]
- 24.Feng J, Wick J, Bompiani E, Aiona M. Applications of gait analysis in pediatric orthopaedics. Current Orthopaedic Practice. 2016;27(4):455–464. [Google Scholar]
- 25.Leach M, Foley A, Averion G, et al. Congenital muscular dystrophy ascending multiple dose cohort study analyzing pharmacokinetics at three dose levels in children and adolescents with assessment of safety and tolerability of omigapil (CALLISTO) trial update. Neuromuscul Disord. 2017;27:S107. [Google Scholar]
- 26.Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health—updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116(9):1081–1093. [DOI] [PubMed] [Google Scholar]
- 27.Kinder JR, Lee KA, Thompson H, Hicks K, Topp K, Madsen KA. Validation of a hip-worn accelerometer in measuring sleep time in children. J Pediatr Nurs. 2012;27(2):127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tudor-Locke C, Barreira TV, Schuna JM Jr., Mire EF, Katzmarzyk PT. Fully automated waist-worn accelerometer algorithm for detecting children’s sleep-period time separate from 24-h physical activity or sedentary behaviors. Appl Physiol Nutr Metab. 2014;39(1):53–57. [DOI] [PubMed] [Google Scholar]
- 29.Cain KL, Sallis JF, Conway TL, Van Dyck D, Calhoon L. Using accelerometers in youth physical activity studies: a review of methods. J Phys Act Health. 2013;10(3):437–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santos-Lozano A, Santin-Medeiros F, Cardon G, et al. Actigraph GT3X: validation and determination of physical activity intensity cut points. Int J Sports Med. 2013;34(11):975–982. [DOI] [PubMed] [Google Scholar]
- 31.Crouter SE, Churilla JR, Bassett DR Jr. Accuracy of the Actiheart for the assessment of energy expenditure in adults. Eur J Clin Nutr. 2008;62(6):704–711. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.