Abstract
Retroviruses are capable of infectious horizontal transmission between hosts, usually between individuals within a single species, although a number of probable zoonotic infections resulting from transmission between different species of placental mammals have also been reported. Despite these data, it remains unclear how often interspecies transmission events occur or whether their frequency is influenced by the evolutionary distance between host taxa. To address this problem we used PCR to amplify and characterize endogenous retroviruses related to the murine leukemia viruses. We show that members of this retroviral genus are harbored by considerably more organisms than previously thought and that phylogenetic analysis demonstrates that viruses isolated from a particular host class generally cluster together, suggesting that infectious virus horizontal transfer between vertebrate classes occurs only rarely. However, two recent instances of transmission of zoonotic infections between distantly related host organisms were identified. One, from mammals to birds, has led to a rapid adaptive radiation into other avian hosts. The other, between placental and marsupial mammals, involves viruses clustering with recently described porcine retroviruses, adding to concerns regarding the xenotransplantation of pig organs to humans.
Retroviruses are members of a large group of transposable elements which have been isolated from bacteria, protists, insects, fungi, and plants, although retroviruses themselves have been described only within vertebrates (9). All retroviruses encode the enzyme reverse transcriptase, enabling the synthesis from their RNA genome of a DNA copy (2, 30), which can subsequently be inserted into the genome of a host organism. Germ line integration events can lead to endogenous retroviruses being passaged vertically for long periods of time (28).
Retroviruses have long been known to be capable of infecting new host species by horizontal transfer, and several examples within placental mammals have been reported (3, 13). Interest in this subject has been boosted recently because of concerns over the potential for xenotropic infection by endogenous retroviruses after xenotransplantation of animal organs into humans (1, 29). However, there is, at present, a shortage of empirical data addressing both the frequency of past retroviral interspecies transmission events and whether this frequency is influenced by the phylogenetic distance between potential hosts (11). One method by which the degree of horizontal transmission can be investigated is by constructing and comparing phylogenies derived from both retroviruses and their hosts; a high level of correlation between the viral and host phylogenies would tend to suggest that horizontal transmission has occurred infrequently in the past. We used this approach to estimate the incidence of retroviral cross-species transmission within the murine leukemia virus-related retroviruses (MLVs).
The MLVs or mammalian type C oncoviruses are known as exogenous (infectious) and endogenous agents with a widespread distribution in placental mammals, where they are associated with numerous pathogenic effects, such as malignancies, immunodeficiencies, and neurological diseases (10). Several closely related nonmammalian viruses have also been identified: these include the reticuloendotheliosis viruses (REVs) of some domestic fowl and two reptilian viruses (for which no sequence information is available), isolated from the Russell’s viper and corn snake (19, 25, 33). All these viruses have been classified into a single retroviral genus (7). The ability of certain REVs to infect some mammalian cell lines has led to the suggestion that they may originally have been of mammalian origin (15, 16).
Here, we show that endogenous MLVs are widespread within the genomes of the four classes of terrestrial vertebrates (amphibians, reptiles, birds, and mammals) and that phylogenetic distance between potential hosts may play an important role in determining the likelihood of zoonotic infection.
MATERIALS AND METHODS
Amplification and sequencing.
Genomic DNA was extracted from tissue samples of approximately 100 vertebrate taxa using a QIAamp tissue extraction kit (Qiagen). PCR amplification of retroviral fragments was performed with primers based on two highly conserved motifs within retroviral protease and reverse transcriptase proteins (32). One universal protease primer (5′ GTG/T TTI G/TTI GAC/T ACI GGI G/TC 3′, where I is inosine) was used in conjunction with three reverse transcriptase primers, i.e., two designed to amplify specifically MLV-related retroviruses (5′ AGI GTI GGI GAA/G TTC/T TTA/G AA 3′ and 5′ AGI AGG TCA/G TCI ACA/G TAG/C TG 3′) and another capable of amplifying retroviruses from each of the seven known retroviral genera (5′ ATI AGI AG/TA/G TCA/G TCI ACA/G TA 3′). At least two of the three primer pairs were used in amplification reactions with each of the taxa investigated. Sequences amplified with these primers typically range from 750 to 950 bp. Reaction conditions were as described previously (32).
Gel-resolved amplicons were excised from 1.3% agarose gels and cleaned by using a band preparation kit (Pharmacia Biotech) before cloning and sequencing bidirectionally, using an Applied Biosystems 373 automated sequencer and a Perkin-Elmer Taq FS dye terminator kit. A minimum of five clones were sequenced for each taxon investigated. Those partial sequences with homology to MLV (38 novel viral sequences from 23 taxa [see Table 1]) were fully characterized. The origin of each viral fragment was confirmed by Southern hybridization to host genomic DNA (27). RV Koala was also hybridized to koala genomic DNA at a separate institute.
TABLE 1.
Host taxa and resultant retroviral isolates
| Host taxona | Retroviral isolate |
|---|---|
| Mammals | |
| Tachyglossus aculeatus (w) | RV Echidna |
| Phascolarctos cinereus (w) | RV Koala |
| Monodelphis ssp. (w) | RV Opossum |
| Birds | |
| Perdix perdix (w) | RV PartridgeI, II |
| Columba palumbus (w) | RV Wood pigeon |
| Sericulus bakeri (w) | RV BowerbirdII, III |
| Troglodytes troglodytes (w) | RV Wren |
| Corvus frugilegus (w) | RV Rook |
| Phasianus colchicus (w) | RV Pheasant |
| Terdus iliacus (w) | RV Redwing |
| Reptiles | |
| Tomistoma schegelii (?) | RV False gharial |
| Osteolaemus tetraspis (?) | RV African dwarf crocodile |
| Varanus komodoensis (?) | RV Komodo dragonII |
| Anolis carolinensis (c) | RV Green anole |
| Boa constrictor (w) | RV Boa constrictor |
| Bitis arietans (w) | RV Puff adder |
| Bothrops jararaca (w) | RV Pit viper |
| Thamnophilis sirtalis (w) | RV Garter snake |
| Viper berus (w) | RV European adder |
| Amphibians | |
| Rana esculenta (w) | RV Edible frogII |
| Bufo calamita (w) | RV Natterjack toad |
| Ichthyophis kohtaoensis (w) | RV Yellow striped caecilian |
| Epicrionops marmoratus (w) | RV Rhinatrematid caecilianIII, IV |
DNA was isolated from a wild-caught animal (w), a captive animal (c), or an animal of unknown status (?).
Phylogenetic analyses.
A 280-amino-acid region derived from the protease and reverse transcriptase proteins from 26 of the novel endogenous fragments was aligned with 20 previously described isolates derived from placental mammals. The data matrix consisted of 80 residues 3′ to the DTGA motif within the protease gene and 200 residues 5′ to the YVDD motif within reverse transcriptase. Sequences aligned over this region were well conserved and nearly identical in length. A DNA data set, aligned identically to the amino acid data set, was also constructed. Only one REV, the spleen necrosis virus (SNV), was included in the analysis, as little sequence information about the pol region is available for other members of this group.
Phylogenetic analyses were performed by using PAUP4 (written by D. L. Swofford) and both amino acid and DNA alignments. Most-parsimonious trees were obtained by using the PROTPARS matrix following 100 random additions with the amino acid data set. Trees based on DNA sequences were generated by using a variety of transversion/transition ratios (1:1, 5:1, and 10:1). Third codon positions were excluded from the analyses, which also included 100 random addition replicates. Single neighbor-joining trees were generated from amino acid data sets and utilized the PROTPARS matrix. Bootstrap values were generated by both the maximum-parsimony (100 replicates; simple addition by using the PROTPARS matrix) and neighbor-joining (500 replicates with the PROTPARS matrix) approaches.
Nucleotide sequence accession numbers.
The novel retrovirus (RV) sequences described here have been submitted to the EMBL/GenBank/DDLJ databases and will appear with the following accession numbers: RV Green anole, AJ236109; RV Puff adder, AJ236110; RV Boa constrictor, AJ236111; RV Pit viper, AJ236112; RV BowerbirdIII, AJ236113; RV BowerbirdII, AJ236114; RV Natterjack toad, AJ236115; RV Rhinatrematid caecilianIII, AJ236116; RV Rhinatrematid caecilianIV, AJ236117; RV Yellow-striped caecillian, AJ236118; RV Echidna, AJ236119; RV Garter snake, AJ236120; RV Komodo dragonII, AJ236121; RV Koala, AJ236122; RV Opossum, AJ236123; RV African, AJ236124; RV PartridgeI, AJ236125; RV PartridgeII, AJ236126; RV Pheasant, AJ236127; RV Edible frogII, AJ236128; RV Redwing, AJ236129; RV Rook, AJ236130; RV False gharial, AJ236131; RV European adder, AJ236132; RV Wood pigeon, AJ236133; and RV Wren, AJ236134. Previously described sequences included in the alignments can be found in reference 31 and references therein.
RESULTS AND DISCUSSION
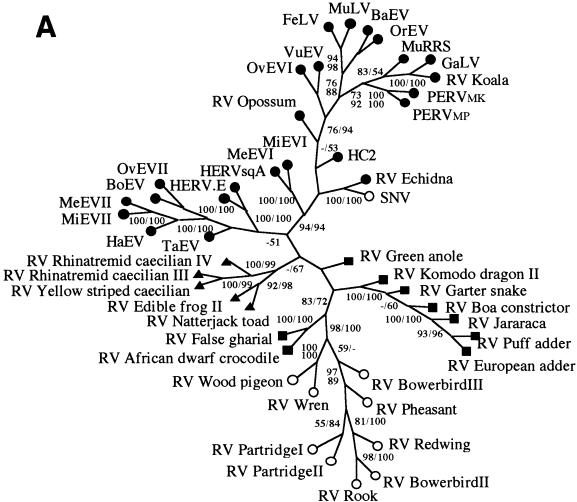
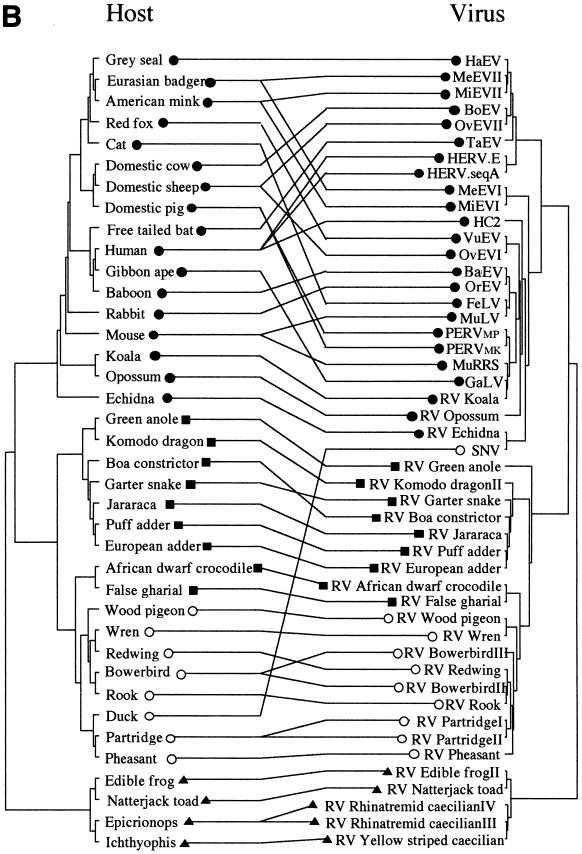
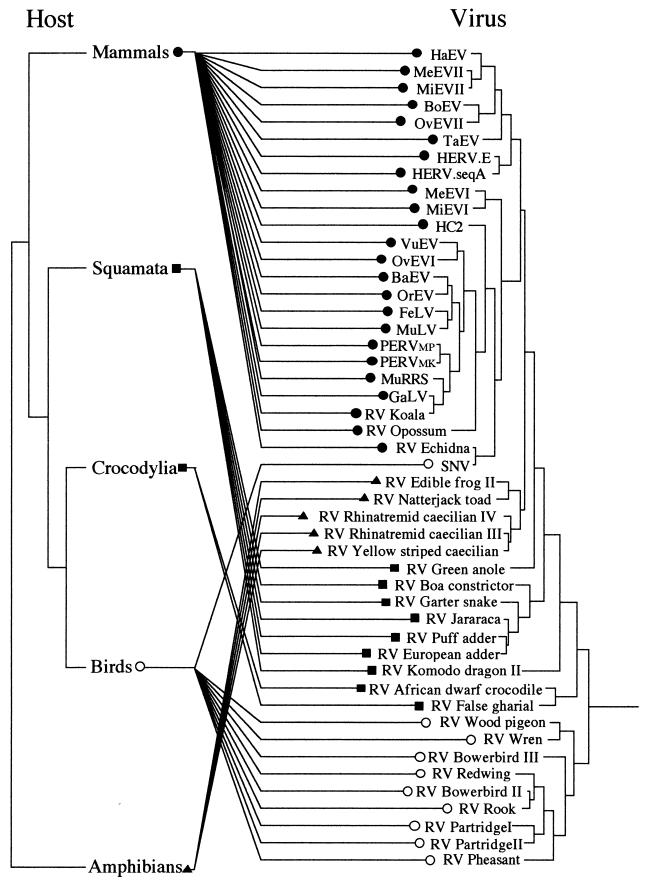
PCR amplification, cloning, and subsequent sequencing of endogenous MLV-related viruses from vertebrate genomes showed them to be widespread within the four classes of terrestrial vertebrates (amphibians, reptiles, birds, and mammals); we were unable to identify examples within fish or other basal chordates, despite screening some 30 species (Table 1). A total of 38 novel endogenous fragments were isolated from 23 taxa. In most cases where more than one virus was isolated from a taxon (nine host species), nucleotide sequences were identical or at least 90% similar. In three cases in which one host harbored viruses showing less than 70% homology, multiple isolates were fully characterized. An amino acid alignment was constructed (see Fig. 1 for a representative sample of the sequences) and was used as the basis for phylogenetic analyses with both the maximum-parsimony and distance-based approaches (26). Various sequence addition and character-weighting options were also investigated. Figure 2A shows an unrooted maximum-parsimony tree of the MLVs described in this report, together with a number of previously described isolates. In general, viruses isolated from a given vertebrate class clustered together into discrete monophyletic groups, as would be expected if zoonotic infections between distantly related hosts occurred only rarely. Furthermore, the monophyletic amphibian virus clade was subdivided into two well-supported groups derived from the orders Anura (frogs and toads) and Gymnophiona (caecilians), and the viral sequences from snakes were monophyletic with respect to those obtained from lizards. Unrooted trees also placed the two crocodilian MLVs as sister taxa to a number of endogenous viruses derived from birds. All these associations are congruent with known vertebrate relationships. Indeed, if the network shown in Fig. 2A was rooted at the point where the novel amphibian viruses branch from the other MLVs, it would be almost entirely congruent with the vertebrate tree of life, as shown in Fig. 2B. If this were the case, most of the transmission events that occurred in the evolutionary history of this retroviral genus would have been vertical (germ line), and zoonotic infections would have been largely limited to hosts within the same vertebrate class. Comparison of host and virus phylogenies by using the computer program Treemap (22) confirmed that the level of congruence between the two trees was higher than that obtained from each of 1,000 randomly generated viral phylogenies (P < 0.001). This congruence is consistent with either an evolutionary history dominated by cospeciation of the viruses with their hosts or one in which horizontal transmission was strongly bounded by the level of genetic distance between potential hosts, resulting in apparent cocladogenesis at higher taxonomic levels. At a lower taxonomic level, within host classes, the level of congruence between mammalian viruses and their hosts appears lower than that observed with isolates from other classes of vertebrates. This either reflects a higher level of horizontal transmission between mammalian host taxa or results from viral lineage duplication at an early stage of mammalian evolution.
FIG. 1.
Multiple alignment of a representative sample of the MLV-related viruses used in subsequent phylogenetic analyses. The values in brackets are the numbers of amino acid residues omitted from the alignment. Asterisks represent stop codons, whereas question marks indicate missing data (due either to postinsertion deletion events or, in the cases of RV Rh. caecilian, RV Natterjack toad, and RV Pit viper, to the use of alternative oligonucleotide primers in the amplification reactions). Rh., rhinatrematid.
FIG. 2.
(A) Unrooted maximum-parsimony tree based on a 280-amino-acid region of retroviral protease and reverse transcriptase proteins. Symbols on each terminal branch represent the host class from which the virus was isolated, as follows: ●, mammals; ○, birds; ■, reptiles; ▴, amphibians. The values on each branch represent percentage bootstrap support determined by using the maximum-parsimony (to the left or top) and neighbor-joining (to the right or bottom) approaches. (B) Virus versus host species phylogeny. The viral phylogeny is the same as that shown in panel A; the host phylogeny was derived from the literature. OvEV, ovine endogenous retrovirus; BoEV, bovine endogenous retrovirus; MiEV, mink endogenous retrovirus; MeEV, Meles endogenous retrovirus; VuEV, Vulpes endogenous retrovirus; FeLV, feline leukemia retrovirus; HaEV, Halichoerus endogenous retrovirus; TaEV, Tadarida endogenous retrovirus; BaEV, baboon endogenous retrovirus; OrEV, Oryctolagus endogenous retrovirus; and HC2, HERV HC2.
There were some differences in the viral topology when a number of other retroviral sequences, such as the human endogenous retrovirus (HERV.I)-related viruses and ERV-9 were included for rooting purposes (20), as shown in Fig. 3, and we also observed slight differences when using tree building with the neighbor-joining approach or when using maximum parsimony based on DNA data. For example, in some trees the amphibian clade appeared as a sister group to a clade containing the HERV.E subgroup of mammalian MLVs, and the location of RV Komodo was also inconsistent. These alternative topologies can be explained by a few ancient interclass horizontal transmission events, followed by the evolution of particular retroviral groups largely within their respective host classes. Nevertheless, all our analyses indicate that viral interclass transmission is infrequent and suggest that the MLV genus is likely to be of nonmammalian origin.
FIG. 3.
Rooted virus versus host class phylogeny. A viral tree like that shown in Fig. 2 but with the addition of a number of other retroviral sequences for rooting purposes was generated. Symbols represent host classes as described in the legend for Fig. 2. Viral branch lengths are proportional to the degree of divergence between the sequences. Abbreviations are as defined in the legend for Fig. 2.
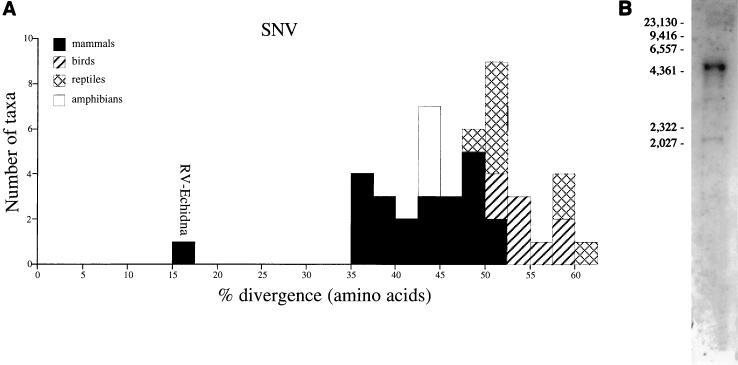
Although our data indicate that most transmission is constrained within rather than between host classes, we have found strong evidence for two relatively recent instances of transmission of zoonotic infections between distantly related host taxa. It seems highly probable that an event of horizontal transmission from mammals to birds explains the position of the SNV in our phylogenies. SNV is the only virus to cluster robustly with viral sequences derived from a different host class. It is clearly far more similar to mammalian MLVs than to other bird viruses and is most closely related to an endogenous virus we identified in a monotreme, the short-beaked echidna (Fig. 4). SNV is a member of the REV group, which also comprises the duck infectious anemia virus, the chicken syncytial virus (CSV), REV itself from turkeys, and several other isolates from geese and pheasants (6, 25). Of these, sequence information from the polymerase region is available only for SNV, but fluorescent antibody tests, antigenic subtyping, and other sequence data all suggest that they form a monophyletic group (6, 25). An alignment of a 350-bp region of the long terminal repeat regions from several of these viruses, including SNV, CSV, and REV-A, also demonstrated their close relationships; all had at least 90% nucleotide identity with other members of the REV group (unpublished data). Therefore, it appears that a single event of horizontal transfer from mammals has been followed by a retroviral adaptive radiation within gallinaceous and anseriform birds. Consistent with this, no naturally occurring endogenous REVs have been identified (24), suggesting that their transfer from mammals was recent enough that they have not yet integrated into germ lines. The identification of REVs inserted into the genomes of fowlpox and herpesviruses suggests one possible mechanism by which these viruses have infected several new species within a short period (12, 14). In our analyses, SNV showed only 17% amino acid divergence across regions of the protease and reverse transcriptase proteins from the echidna MLV. However, it is unlikely that the echidna virus is the direct source of the REV group; the number of MLV-related viruses described to date comprises only a small fraction of the total diversity present in mammals, and thus it is probable that the actual source remains to be identified.
FIG. 4.
(A) Bar chart showing the percentage of amino acid divergence between SNV and other retroviral sequences, calculated from the same regions as were used for phylogenetic reconstruction. (B) Southern hybridization of EcoRI-digested genomic DNA of short-beaked echidna performed by using RV Echidna as the probe. The filter was hybridized overnight at 65°C and washed with 0.5% sodium dodecyl sulfate–0.5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) at 63°C. Marker sizes are expressed in kilobase pairs.
A second horizontal transfer event illustrates a zoonotic infection transmitted between marsupial and placental mammals. We have identified an endogenous virus in the koala which is remarkably similar (93% amino acid similarity and 85% nucleic acid similarity across the amplified region) to a highly oncogenic exogenous virus (GaLV) that causes leukemia in gibbons (Fig. 5A and B). The transfer probably occurred recently, because the level of sequence divergence between GaLV and the koala virus is comparable to that observed between different strains of GaLV. For example, two strains (GaLVSEATO and GaLVSF) have previously been shown to have 87% nucleotide identity for a region of their pol genes (8). Furthermore, the simian sarcoma virus (SSV) of woolly monkeys, which probably represents a third strain of GaLV (a gibbon ape is likely to have infected the woolly monkey in captivity) has 91% and 94% nucleotide similarities to the SEATO strain for the regions across gag and pol, respectively (8, 21). RV Koala was isolated from DNA obtained from a wild-caught koala, and an uncharacterized type C oncovirus has been implicated in the development of spontaneous lymphoid neoplasia within noncaptive members of this species (4, 5). Since gibbons (found in southeast Asia) and koalas (found in Australia) are not common to the same continent, this proposed mode of zoonosis almost certainly involves an intermediate vector. Several southeast Asian mice harbor viruses which cross-hybridize to GaLV at high stringency (18), and rodents may therefore be potential candidates for such vectors.
FIG. 5.
(A) RV Koala compared with other MLV-related viruses, highlighting the position of the pig endogenous isolates PERVMP and PERVMK. Percentage identity was calculated as described for Fig. 4A. (B) Southern hybridization of BglII-digested koala genomic DNA performed by using RV Koala as the probe. The filter was hybridized by using Church and Gilbert buffer at 55°C and washed down to 1× SSC–0.1% sodium dodecyl sulfate, also at 55°C. Marker sizes are expressed in kilobase pairs. Negative results were obtained after hybridization to the following eight additional marsupial and monotreme species: stripe faced dunnart (Sminthopsis macroura), tammar (Macropus eugenii), Godman’s Rock wallaby (Petrogale godmani), common brushtail possum (Trichosaurus vulpecula), brindled bandicoot (Isoodon macrourus), opossum (Monodelphis sp.), short-beaked echidna (Tachyglossus aculeatus), and platypus (Ornithorhynchus anatinus) (unpublished data).
The evolutionary tree shown in Fig. 2A demonstrates that both GaLV and RV Koala are relatively closely related to the recently described endogenous porcine retroviruses PERVMP and PERVPK. These pig viruses have previously been shown to be capable of infecting human cells in vitro, raising concerns about reactivation of endogenous viruses after grafting of porcine organs into human patients (17, 23). The existence of a viral subgroup comprising these isolates and murine retrovirus-related sequence (MuRRS) (a defective endogenous virus within mice) was well supported by bootstrap analysis (SSV could not be included in these analyses since it is defective in the region of the pol gene used). Calculation of the percentages of divergence between the koala isolate and other MLVs (Fig. 5A) also demonstrates the similarities among these viruses. It is therefore clear that viruses present in the genomes of animals proposed as organ donors for humans are clustering with others for which there is compelling evidence of recent infectious spread between disparate host species. This may add further weight to recently expressed reservations regarding xenotransplantation of pig organs into humans (29).
ACKNOWLEDGMENTS
We are grateful to D. L. Swofford for permission to publish results from PAUP4. We thank D. Quicke, A. Purvis, R. Belshaw, and R. Page for discussion and comments on the manuscript. We also thank M. Hayler, A. Taylor, R. Kusmierski, J. Gatesy, A. Flavell, M. Paine, H. Tegelström, B. Cohen, and J. Sheps for providing the DNA samples used in this study.
This work was supported by the NERC Initiative in Taxonomy, a NERC studentship (J.M.), and the Royal Society.
REFERENCES
- 1.Bach F H, Fishman J A, Daniels N, Proimos J, Anderson B, Carpenter C B, Forrow L, Robson S C, Fineberg H V. Uncertainty in xenotransplantation: individual benefit versus collective risk. Nat Med. 1998;4:141–142. doi: 10.1038/nm0298-141. [DOI] [PubMed] [Google Scholar]
- 2.Baltimore D. RNA-dependent DNA polymerase in virions of RNA tumor viruses. Nature. 1970;226:1209–1211. doi: 10.1038/2261209a0. [DOI] [PubMed] [Google Scholar]
- 3.Benveniste R E, Todaro G J. Evolution of C-type viral genes: inheritance of exogenously acquired viral genes. Nature. 1974;252:456–459. doi: 10.1038/252456a0. [DOI] [PubMed] [Google Scholar]
- 4.Canfield P J, Brown A S, Kelly W R, Sutton R H. Spontaneous lymphoid neoplasia in the koala (Phascolarctos cinereus) J Comp Pathol. 1987;97:171–178. doi: 10.1016/0021-9975(87)90037-5. [DOI] [PubMed] [Google Scholar]
- 5.Canfield P J, Sabine J M, Love D N. Virus particles associated with leukaemia in a koala. Aust Vet J. 1988;65:327–328. doi: 10.1111/j.1751-0813.1988.tb14518.x. [DOI] [PubMed] [Google Scholar]
- 6.Chen P-Y, Cui Z, Lee L-F, Witter R L. Serologic differences among nondefective reticuloendotheliosis viruses. Arch Virol. 1987;93:233–245. doi: 10.1007/BF01310977. [DOI] [PubMed] [Google Scholar]
- 7.Coffin J M. Structure and classification of retroviruses. In: Levy J A, editor. The Retroviridae. Vol. 1. New York, N.Y: Plenum Press; 1992. pp. 313–362. [Google Scholar]
- 8.Delassus S, Sonigo P, Wain-Hobson S. Genetic organization of gibbon ape leukemia virus. Virology. 1989;173:205–213. doi: 10.1016/0042-6822(89)90236-5. [DOI] [PubMed] [Google Scholar]
- 9.Eickbush T H. Origin and evolutionary relationships of retroelements. In: Morse S S, editor. The evolutionary biology of viruses. New York, N.Y: Raven Press; 1994. pp. 121–157. [Google Scholar]
- 10.Fan H. Retroviruses and their role in cancer. In: Levy J A, editor. The Retroviridae. Vol. 3. New York, N.Y: Plenum Press; 1992. pp. 313–362. [Google Scholar]
- 11.Herniou E, Martin J, Miller K, Cook J, Wilkinson M, Tristem M. Retroviral diversity and distribution in vertebrates. J Virol. 1998;72:5955–5966. doi: 10.1128/jvi.72.7.5955-5966.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hertig C H, Coupar B E H, Gould A R, Boyle D B. Field and vaccine strains of fowlpox virus carry integrated sequences from the avian retrovirus, reticuloendotheliosis virus. Virology. 1997;235:367–376. doi: 10.1006/viro.1997.8691. [DOI] [PubMed] [Google Scholar]
- 13.Hirsch V M, Dapolito G, Goeken R, Campbell B J. Phylogeny and natural history of the primate lentiviruses, SIV and HIV. Curr Opin Genet Dev. 1995;5:798–806. doi: 10.1016/0959-437x(95)80014-v. [DOI] [PubMed] [Google Scholar]
- 14.Isfort R, Jones D, Kost R, Witter R, Kung H-J. Retrovirus insertion into herpesvirus in vitro and in vivo. Proc Natl Acad Sci USA. 1992;89:991–995. doi: 10.1073/pnas.89.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kewalramani V N, Panganiban A T, Emerman M. Spleen necrosis virus, an avian immunosuppressive retrovirus, shares a receptor with the type D simian retroviruses. J Virol. 1992;66:3026–3031. doi: 10.1128/jvi.66.5.3026-3031.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koo H-M, Brown A M C, Ron Y, Dougherty J P. Spleen necrosis virus, an avian retrovirus, can infect primate cells. J Virol. 1991;65:4769–4776. doi: 10.1128/jvi.65.9.4769-4776.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le Tissier P, Stoye J P, Takeuchi Y, Patience C, Weiss R A. Two sets of human-tropic pig retrovirus. Nature. 1997;389:681–682. doi: 10.1038/39489. [DOI] [PubMed] [Google Scholar]
- 18.Lieber M M, Sherr C J, Todaro G J, Benveniste R E, Callahan R, Coon H G. Isolation from the Asian mouse Mus caroli of an endogenous type C virus related to infectious primate type C viruses. Proc Natl Acad Sci USA. 1975;72:2315–2319. doi: 10.1073/pnas.72.6.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lunger P D, Hardy W D, Clark H F. C type virus particles in a reptilian tumor. J Natl Cancer Inst. 1974;52:1231–1235. doi: 10.1093/jnci/52.4.1231. [DOI] [PubMed] [Google Scholar]
- 20.Martin J, Herniou E, Cook J, Waugh O’Neill R, Tristem M. Human endogenous retrovirus type I-related viruses have an apparently widespread distribution within vertebrates. J Virol. 1997;71:437–443. doi: 10.1128/jvi.71.1.437-443.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oroszlan S, Copeland T, Smythers G, Summers M R, Gilden R V. Comparative primary structure analysis of the p30 protein of the woolly monkey and gibbon C type viruses. Virology. 1977;77:413–417. doi: 10.1016/0042-6822(77)90438-x. [DOI] [PubMed] [Google Scholar]
- 22.Page R D M. Parallel phylogenies: reconstructing the history of host-parasite assemblages. Cladistics. 1994;10:155–173. [Google Scholar]
- 23.Patience C, Takeuchi Y, Weiss R A. Infection of human cells by an endogenous retrovirus of pigs. Nat Med. 1997;3:282–286. doi: 10.1038/nm0397-282. [DOI] [PubMed] [Google Scholar]
- 24.Payne L N. Biology of avian retroviruses. In: Levy J A, editor. The Retroviridae. Vol. 2. New York, N.Y: Plenum Press; 1992. pp. 299–404. [Google Scholar]
- 25.Purchase H G, Ludford C, Nazerian K, Cox H W. A new group of oncogenic viruses: reticuloendotheliosis viruses, chick syncytial, duck infectious anemia, and spleen necrosis viruses. J Natl Cancer Inst. 1973;51:489–499. [PubMed] [Google Scholar]
- 26.Saito N, Nei M. The neighbour joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 27.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 28.Shih A, Misra R, Rush M G. Detection of multiple, novel reverse transcriptase coding sequences in human nucleic acids: relation to primate retroviruses. J Virol. 1989;63:64–75. doi: 10.1128/jvi.63.1.64-75.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stoye J P, Coffin J M. The dangers of xenotransplantation. Nat Med. 1995;1:1100. doi: 10.1038/nm1195-1100a. [DOI] [PubMed] [Google Scholar]
- 30.Temin H M, Mizutani S. RNA-dependent DNA polymerase in virions of Rous sarcoma virus. Nature. 1970;226:1211–1213. doi: 10.1038/2261211a0. [DOI] [PubMed] [Google Scholar]
- 31.Tristem M, Kabat P, Lieberman L, Linde S, Karpas A, Hill F. Characterization of a novel murine leukemia virus-related subgroup within mammals. J Virol. 1996;70:8241–8246. doi: 10.1128/jvi.70.11.8241-8246.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tristem M. Amplification of divergent retroelements by PCR. BioTechniques. 1996;20:608–612. doi: 10.2144/19962004608. [DOI] [PubMed] [Google Scholar]
- 33.Zeigel R F, Clark H F. Histologic and electron microscopic observations on a tumor-bearing viper: establishment of a C type virus-producing cell line. J Natl Cancer Inst. 1971;46:309. [PubMed] [Google Scholar]