Abstract
Purpose:
To determine risk factors and clinical course of corneal ulcers in the setting of opioid use.
Methods:
A retrospective cohort study was performed of patients presenting with bacterial or fungal keratitis at a county hospital from 2010–2021. Subjects were separated into three groups: opioid drug users (heroin, methadone, fentanyl), non-opioid drug users, and non-drug users. 24 opioid users, 77 non-opioid drug users, and 38 non-drug users were included in the study. Chi-square and t-tests were used to compare hospitalization for corneal ulcer treatment; length of hospitalization; loss to follow-up; final best corrected visual acuity (BCVA); medication noncompliance; time to ulcer resolution; and visual disability (defined either by the legal limit for driving in California or the federal limit for blindness).
Results:
Opioid users had higher rates of unemployment (p=0.002), homelessness (p=0.018), and psychiatric conditions (p=0.024) compared with non-opioid and non-drug users. They had more severe presentations, with worse initial BCVA of the affected eye (p=0.003), larger ulcer size (p=0.023), and higher rates of individuals below the legal vision thresholds for driving (p=0.009) and blindness (p=0.033) at initial presentation. Opioid use was associated with increased rate of hospitalization (p<0.001), higher fortified antibiotic use (p=0.009), worse final BCVA of the affected eye (p=0.020), and increased rates of BCVA worse than the legal vision thresholds for driving (p=0.043) and blindness (p<0.001) on final presentation.
Conclusions:
Infectious keratitis associated with opioid use is associated with more severe presentations and poorer outcomes, including higher rates of visual disability.
Keywords: opioid use disorder, infectious keratitis, county hospital, social determinants
Introduction
There is a growing epidemic of opioid use disorder (OUD) in the United States, with over 11 million active users per year.1 Analysis of eye-related emergency department visits shows that opioid users tend to have more severe ophthalmic diagnoses, such as orbital fractures, globe injury, orbital cellulitis, and endophthalmitis, as well as a higher likelihood of being admitted for ocular conditions.2
While corneal epithelial defects and infectious keratitis related to other substances of abuse, such as crack cocaine and methamphetamines, have been previously described,3–6 evidence of the impact of opioid use on the cornea is only just emerging. Endogenous opioid receptors may act as negative growth factors and inhibit re-epithelialization of abraded human corneas in vitro.7 Meanwhile, naltrexone (an opioid antagonist) has been found to facilitate corneal re-epithelialization in diabetic rat models.8,9 However, there are currently no published studies examining the association between opioid use and corneal ulcer presentations and outcomes. This study aims to determine risk factors and clinical course of corneal ulcers in the setting of opioid use at a public county hospital.
Materials and Methods
This retrospective cohort study included all patients who received a corneal culture for suspected bacterial or fungal infectious keratitis between 2010 and 2021 at the Zuckerberg San Francisco General Hospital and Trauma Center (ZSFGH), in both inpatient and outpatient settings. Exclusion criteria were patients with non-infectious or viral keratitis without suspected bacterial or fungal keratitis or absence of epithelial defect on initial presentation. The study was approved by the Institutional Review Board of the University of California, San Francisco (protocol number 19–28768) and conforms to the tenets of the Declaration of Helsinki.
The patient cohort was divided into three categories: opioid users, non-opioid drug users, and non-drug users. Opioid users included all patients with a history of opioid use, including opiates, methadone, heroin, and fentanyl. Non-opioid drug users included any patient who had a history of drug use, but no recorded history of opioid use. Other drugs that were used by patients in both opioid users and non-opioid drug users included: amphetamines/methamphetamines, tobacco, cocaine/crack cocaine, bath salts, benzodiazepines, marijuana, alcohol abuse, unspecified intravenous drug use (IVDU), and unspecified inhalants. Patients with unspecified IVDU or inhalant use were excluded from the analysis. Non-drug users were all patients who had no recorded history of drug use. Patients labeled as only former smokers were included in the non-drug users category. Drug use history was determined through chart review of physician comments within notes and/or if drug use was described in the patient’s social history within the medical chart. This information was patient report and not based on toxicology data. Due to the retrospective design of this study, the precise timing of drug use in relation to corneal ulcer development could not be ascertained.
Patient demographics, clinical characteristics, and treatment data were compared between these three groups. Demographic data included age, gender, race/ethnicity, preferred language, employment status, and housing status. Age was determined at date of initial presentation for corneal ulcer. Additional demographic data was determined using social documentation and physician notes available at the time the chart was accessed for review. Other factors included history of psychiatric conditions, contact lens use, and human immunodeficiency virus (HIV) status. Clinical characteristics included initial best-corrected visual acuity (BCVA), initial ulcer size, corneal ulcer location (central or peripheral) and laterality (unilateral or bilateral), time of symptoms prior to presentation at ZSFGH, and pathogenic organism (staphylococcal, pseudomonal, other bacterial, fungal). BCVA was measured by Snellen chart, then converted to a logMAR estimate of . Treatment data included use of fortified antibiotics (vancomycin and tobramycin), use of subconjunctival antibiotics, need for emergent penetrating keratoplasty (PKP), and need for delayed PKP (meaning PKP for optical purposes after ulcer was resolved).
The outcomes examined were: need for hospitalization for corneal ulcer treatment; length of hospitalization; loss to follow-up, defined as being out of care for at least 1 month with an active epithelial defect; final BCVA as measured on the visit that the epithelial defect was first noted to be healed, or on the final visit if the patient was lost to follow-up before the defect had healed; medication noncompliance during the timeframe an epithelial defect was present; time to ulcer resolution, defined as days between initial presentation and closure of the epithelial defect; and visual disability as defined either by the legal limit for driving in California (20/40 in one eye and at least 20/70 in the other eye)10 or the federal limit for blindness (20/200 or less in the better eye)11.
Two-tailed t-tests were used to compare means for numerical variables (initial BCVA, final BCVA, initial size, time of symptoms prior to presentation, time to ulcer resolution, and days hospitalized). Chi-square analysis was used to compare categorical variables between the three groups. A p-value of less than 0.05 was considered significant.
Results
Demographics and Infectious Keratitis Risk Factors Based on Opioid Use Status
A total of 174 patients with infectious keratitis were initially included in this study. Of these, 24 (13.79%) were confirmed opioid users, 74 (42.53%) non-opioid drug users, and 50 (28.74%) non-drug users (Table 1). There were also 21 (12.07%) patients with unknown drug use status and 5 (2.87%) patients with unspecified IVDU or inhalant use that were not included in this analysis. Opioid users had a mean age of 48.17 years, non-opioid drug users had a mean age of 44.77 years, and non-drug users had a mean age of 48.46 years. All groups demonstrated a male preponderance.
Table 1.
Comparison of Demographics and Risk Factors Based on Drug Use Status
| Opioid Users (n = 24) | Non-Opioid Drug Users (n = 79) | Non-Drug Users (n = 38) | |
|---|---|---|---|
|
| |||
| Age (years) | |||
| Mean ± SD | 48.2 ± 9.4 | 44.8 ± 12.4 | 48.5 ± 17.2 |
| Median (min-max) | 51.5 (32.0–63.0) | 44.5 (16.0–77.0) | 49.0 (14.0–83.0) |
| Gender | |||
| Man | 14 (58.3%) | 48 (64.9%) | 31 (62.0%) |
| Woman | 10 (41.7%) | 26 (35.1%) | 19 (38.0%) |
| Race/Ethnicity | |||
| Native American | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Asian / Pacific Islander | 0 (0.0%)* | 13 (17.5%) | 8 (16.0%) |
| Black / African American | 7 (29.2%) | 11 (14.9%) | 5 (10.0%) |
| Hispanic / Latino(a) | 1 (4.1%)* | 9 (12.2%) | 20 (40.0%)*** |
| White | 9 (37.5%) | 25 (33.8%) | 9 (18.0%) |
| Other | 7 (29.2%) | 16 (21.6%) | 8 (16.0%) |
| Housing Status | |||
| Housed | 9 (37.5%)* | 47 (63.5%) | 45 (90.0%)* |
| Homeless | 14 (58.3%)* | 23 (31.1%) | 3 (6.0%)* |
| Unknown | 1 (4.2%) | 4 (5.4%) | 2 (4.0%) |
| Employment Status | |||
| Employed / Retired | 1 (4.2%)** | 34 (46.0%) | 27 (54.0%) |
| Unemployed / Disabled | 17 (70.8%)** | 24 (32.4%) | 13 (26.0%) |
| Unknown | 6 (25.0%) | 16 (21.6%) | 10 (20.0%) |
| Non-English Preferred Language | 0 (0%) | 8 (10.8%) | 20 (40.0%)** |
| Psychiatric History | 10 (41.7%)* | 19 (25.7%) | 4 (8.0%)* |
| HIV+ Status | 2 (8.3%) | 10 (13.5%) | 4 (8.0%) |
| Contact Lens Use | |||
| Yes | 4 (16.7%) | 30 (40.6%) | 13 (26.0%) |
| No | 14 (58.3%) | 32 (43.2%) | 20 (40.0%) |
| Unknown | 6 (25.0%) | 12 (16.2%) | 17 (34.0%) |
Chi-square p-value < 0.05
Chi-square p-value < 0.01
Chi-square p-value < 0.001
Of the 24 opioid users, 17 used heroin, 11 used methadone, 4 used fentanyl, and 6 used unspecified opioids (Table 2). The forms of opioid use included: 12 intravenous, 11 oral, and 3 smoked/inhaled. Five patients had non-specified methods of use. All opioid patients reported abuse of other substances, which included various combinations of tobacco, methamphetamines/amphetamines, benzodiazepines, cocaine/crack cocaine, marijuana, and alcohol.
Table 2.
Description of the 24 opioids users included in this study
| Age (years)/ Sex | Race / Ethnicity | Housing Status | Employment Status | Type and Method of Opioid Use | Other Drug Use | Contact Lens Use | Initial Size (mm) | Initial Visual Acuity | Culture Growth | Final Visual Acuity | Fortified Antibiotics Used | Subconjunctival Antibiotics Used | Hospitalized | Lost to Follow Up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| 53/F | Latina, Native American, Pacific Islander | housed | unknown | opioids, methadone - oral | tobacco, benzodiazepines | no | 4 × 5 | OD 20/100, OS LP | Pseudomonas aeruginosa, coagulase negative Staphylococci | OD 20/400, OS CF 1’ | yes | yes | yes | no |
|
| ||||||||||||||
| 54/M | Black | housed | unemployed | heroin - IV | tobacco, cocaine | no | 3.2 × 2.2 | OD 20/20–1, OS LP | Moraxella lacunata | OD 20/20–2, OS 20/40 | yes | no | yes | no |
|
| ||||||||||||||
| 32/F | White | homeless | unknown | heroin - IV | tobacco, marijuana, methamphetamine, cocaine | unknown | 5 × 5 | OD 20/25–2, OS HM | none | OD 20/25–2, OS 20/80–1 | yes | no | yes | yes |
|
| ||||||||||||||
| 38/F | White | housed | unemployed | heroin - IV; methadone - oral | tobacco, speed, benzodiazepine, methamphetamine | no | 1.5 × 1.5 | OD 20/30–2, OS 20/200+ | Viridans group Streptococcus, coagulase negative Staphylococci | OD 20/100-, OS 20/30++ | no | no | no | yes |
|
| ||||||||||||||
| 35/M | Black | housed | disabled | heroin | tobacco, crystal meth (smokes), marijuana | no | unknown | OD 20/70, OS 20/60 | coagulase negative Staphylococci (OU), Viridans group Streptococci (OU), Gemella (OS) | OD 20/70-, OS 20/20–2 | no | no | yes | yes |
|
| ||||||||||||||
| 46/M | Latino, Black | homeless | disabled | heroin - smoke/snort; fentanyl | tobacco, marijuana, crack cocaine | no | 3.1 × 2.5 | OD CF @ 1.5 ft, OS 20/30 −1 | Staphylococcus aureus, coagulase negative Staphylococci | OD 20/150+, OS 20/20- | yes | no | unknown | no |
|
| ||||||||||||||
| 48/M | Black | homeless | unemployed | heroin - IV, fentanyl - IV, methadone - oral | tobacco, cocaine, methamphetamine (IV) | no | 1 × 1 | OD HM, OS 20/200 | coagulase negative Staphylococci, Moraxella lacunata | OD 20/60, OS 20/25–2 | yes | no | yes | yes |
|
| ||||||||||||||
| 34/F | Asian, White | homeless | disabled | fentanyl | tobacco, methamphetamine (IV), cocaine, benzodiazepines, Gamma-hydroxybutyrate | yes | OD - 2 × 1.5; OS - 1 × 0.5 | OU HM | Staphylococcus epidermidis OU | OU HM | yes | no | no | yes |
|
| ||||||||||||||
| 53/M | Latino | homeless | unknown | heroin - IV | cocaine | unknown | 2.5 × 3.5 | OD 20/400, OS 20/25–1 | Bacillus species (not anthracis), coagulase negative Staphylococci | OD 20/60-, OS 20/25 | yes | no | yes | yes |
|
| ||||||||||||||
| 50/M | Black | incarcerated, homeless | employed | heroin - smoke/snort | tobacco, crack-cocaine | no | 6 × 4.5 | OD 20/20, OS HM | none | OD 20/20, OS HM | yes | no | no | yes |
|
| ||||||||||||||
| 56/F | Latina, White | housed | unemployed | heroin - IV, methadone - oral, prescription opioids - oral | tobacco, cocaine, benzodiazepine | no | 10 × 10 | OD LP, OS 20/20 | none | OD HM, OS 20/20 | yes | no | yes | yes |
|
| ||||||||||||||
| 37/F | White, Latina, Pacific Islander | homeless | unemployed | heroin - IV; methadone - oral; fentanyl | tobacco, crystal meth (smokes, IV) | yes | 4 × 6 | OD CF @ 1ft, OS 20/20 | Moraxella lacunata | OD 20/100, OS not measured | yes | yes | yes | yes |
|
| ||||||||||||||
| 51/M | White | homeless | unemployed | heroin - IV | none | unknown | unknown | unknown | coagulase negative Staphylococci | unknown | unknown | unknown | unknown | unknown |
|
| ||||||||||||||
| 56/M | White | homeless | unknown | methadone - oral | tobacco | unknown | 2.8 × 2.3 | OD 20/20, OS CF@3ft | Moraxella (not lacunata), Coryneform | OD 20/20, OS CF 3’ | yes | no | no | yes |
|
| ||||||||||||||
| 38/F | White | homeless | disabled | opioids, methadone - oral; heroin - IV | tobacco, methamphetamine, speed, crack cocaine | no | 1.5 × 1.3 | OD 20/25, OS 20/150–1 | Staphylococcus aureus (MRSA), coagulase negative Staphylococci | OD NLP, OS CF 6” | yes | no | yes | no |
|
| ||||||||||||||
| 56/F | Black | homeless | unemployed | opioids, methadone - oral | tobacco, methamphetamine, crack cocaine | no | 1 × 1.2 | OD 20/400+1, OS 20/20 | Corynebacteria species strain 1 and 2 | OD 20/150-, OS 20/30-- | yes | no | yes | yes |
|
| ||||||||||||||
| 57/M | White | homeless | unknown | heroin - IV | tobacco, methamphetamine, benzodiazepine | unknown | unknown | unable to assess | none | OD LP, OS LP | yes | yes | yes | yes |
|
| ||||||||||||||
| 37/M | White | unknown | unknown | heroin | methamphetamine | unknown | unknown | unknown | none | unknown | unknown | unknown | unknown | unknown |
|
| ||||||||||||||
| 63/M | Black | homeless | unemployed | heroin - IV; methadone - oral | tobacco, cocaine | no | 1 × 1 | OD 20/20-, OS 20/25- | coagulase negative Staphylococci | OD 20/20-, OS 20/25- | no | no | no | yes |
|
| ||||||||||||||
| 59/M | White | housed | disabled | opioids | tobacco, methamphetamine, cocaine, marijuana | no | 2 × 8 | OD HM, OS 20/20 −1 | coagulase negative Staphylococci | OD HM | yes | no | yes | yes |
|
| ||||||||||||||
| 52/M | White | housed | unemployed | opioids | tobacco | yes | 1 × 1 | OD 20/20–2, OS CF @ 1ft | none | OD 20/25+2, OS CF 3’ | yes | no | no | no |
|
| ||||||||||||||
| 52/M | Black, Other | homeless | disabled | methadone - oral; heroin - not IV | tobacco, cocaine | yes | 3 × 3 | unable to assess | Moraxella lacunata, Lactobacillus species | OD CF 6”, OS HM | yes | no | yes | no |
|
| ||||||||||||||
| 60/F | Black | housed | unemployed | methadone - oral | tobacco, cocaine, marijuana | no | 8 | OU NLP | none | OD NLP, OS HM | yes | no | yes | no |
|
| ||||||||||||||
| 39/F | Latino, Black, Other | homeless | unemployed | heroin - IV, inhaled | tobacco, methamphetamine | yes | 1.6 × 1.5 | OU 20/100 | coagulase negative Staphylococci, Viridans group Streptococcus | OD 20/80, OS 20/100 | yes | no | yes | yes |
On Chi-square analysis, opioid use positively correlated with homelessness (p = 0.018), unemployed/disabled status (p = 0.002), and the presence of a psychiatric history (p = 0.024); and was negatively correlated with Asian and Hispanic/Latino(a) race/ethnicity (Table 1). Non-drug users were more likely to demonstrate Hispanic/Latino(a) race/ethnicity (p < 0.001), housed status (p = 0.021), and non-English speaking preference (p = 0.006), and less likely to a psychiatric history (p = 0.017). There were no significant associations between drug use and contact lens use or HIV status.
Impact of Opioid Use on Clinical Characteristics of Infectious Keratitis
Results of Chi-square analysis for the clinical characteristics and treatment course for infectious keratitis are summarized in Table 3. There was a trend toward an association between opioid use and polymicrobial infections (p = 0.058) and bilateral ulcers (p = 0.073). There were no differences in the rates of initial ulcer location (central vs. peripheral) or positive culture growth between the three groups.
Table 3.
Clinical Course of Infectious Keratitis Stratified by Drug Use Status
| Opioid Users (n = 24) | Non-Opioid Drug Users (n = 74) | Non-Drug Users (n = 50) | |
|---|---|---|---|
|
| |||
| Initial Ulcer Location | |||
| Central | 9 (37.5%) | 28 (37.8%) | 16 (32.0%) |
| Peripheral | 7 (29.2%) | 22 (29.7%) | 14 (28.0%) |
| Unknown | 8 (33.3%) | 24 (32.4%) | 20 (40.0%) |
| Hospitalized | |||
| Yes | 15 (62.5%)*** | 24 (32.4%) | 6 (12.0%)** |
| No | 7 (29.2%)*** | 40 (54.1%) | 39 (78.0%)** |
| Unknown | 2 (8.3%) | 10 (13.5%) | 5 (10.0%) |
| Lost to Follow-Up | |||
| Yes | 15 (62.5%) | 37 (50.0%) | 12 (24.0%)* |
| No | 7 (29.2%) | 28 (37.8%) | 30 (60.0%)* |
| Unknown | 2 (8.3%) | 9 (12.2%) | 8 (16.0%) |
| Compliant with Medications | |||
| Yes | 9 (37.5%) | 36 (48.6%) | 24 (48.0%) |
| No | 9 (37.5%) | 18 (24.3%) | 15 (30.0%) |
| Unknown | 6 (25.0%) | 20 (27.0%) | 11 (22.0%) |
| Bilateral Ulcers | 3 (12.5%) | 3 (4.1%) | 1 (2.0%) |
| Positive Culture Growth | 17 (70.8%) | 51 (68.9%) | 26 (52.0%) |
| Polymicrobial Infection | 11 (45.8%) | 22 (29.7%) | 14 (28.0%) |
| Subconjunctival Antibiotics Used | 3 (12.5%) | 10 (13.5%) | 6 (12.0%) |
| Fortified Topical Antibiotics Used | 19 (79.2%)** | 44 (59.5%) | 19 (8.0%)* |
| Emergent Penetrating Keratoplasty or Glue | 2 (8.3%) | 6 (8.1%) | 2 (4.0%) |
Chi-square p-value < 0.05
Chi-square p-value < 0.01
Chi-square p-value < 0.001
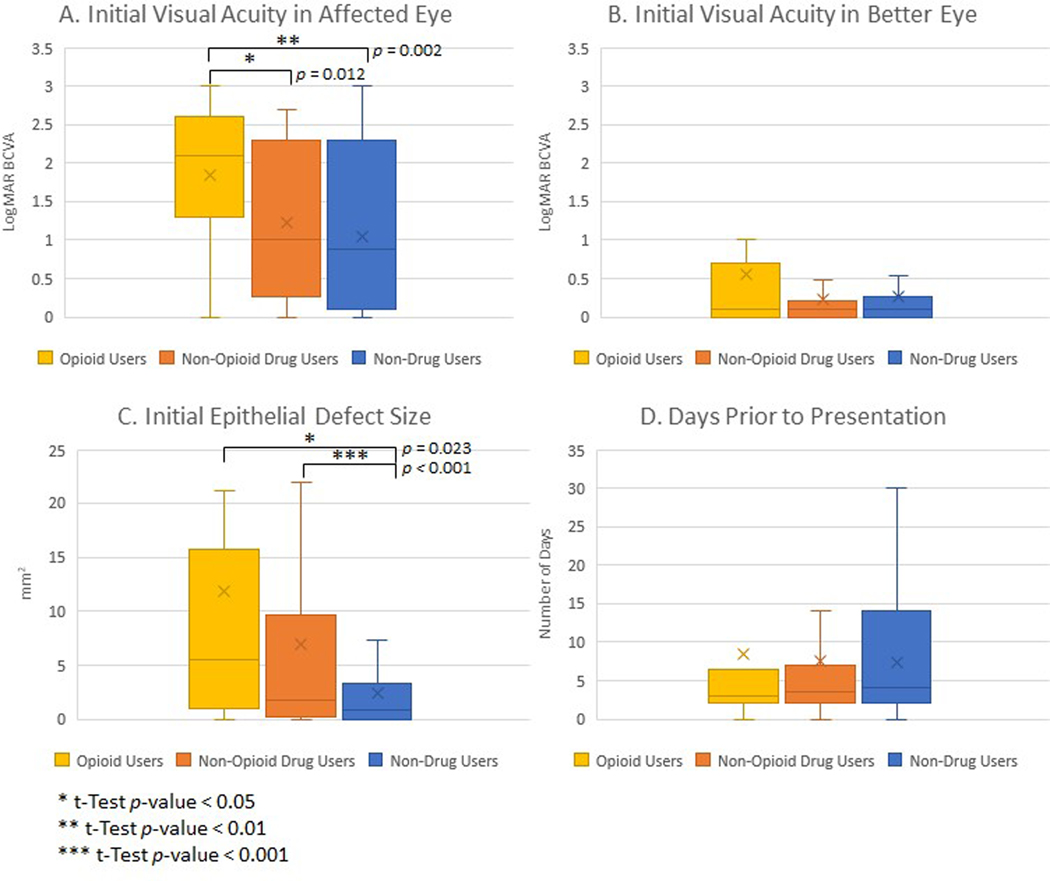
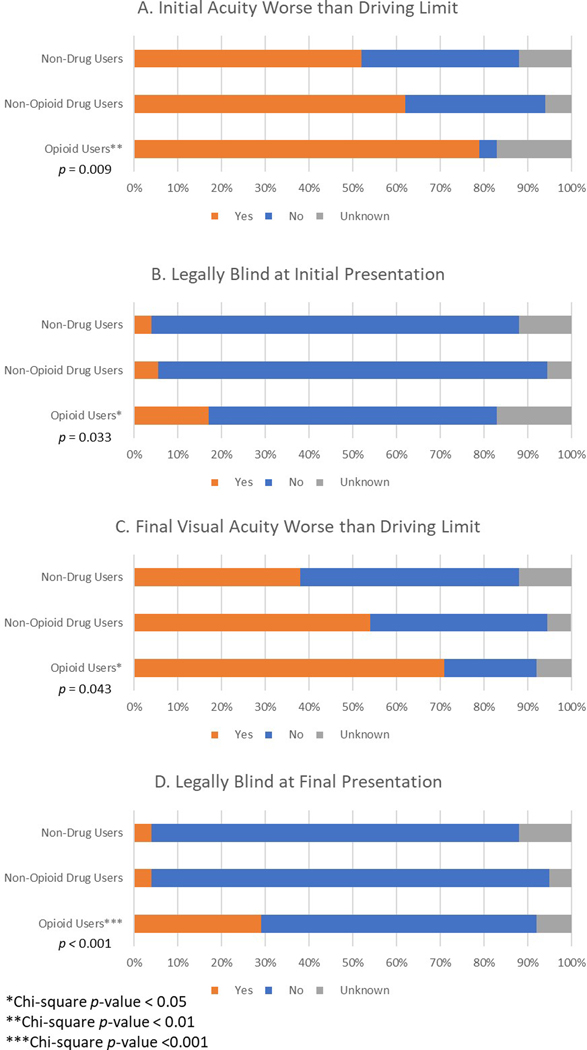
Opioid users presented with significantly worse visual acuity in the affected eye, with an average logMAR initial BCVA of 1.84 compared to 1.22 for non-opioid drug users (p = 0.013) and 1.04 for non-drug users (p = 0.003) (Figure 1). Additionally, opioid users trended toward worse initial visual acuity in the better eye (meaning the eye with better visual acuity, regardless of infection status), with an average logMAR BCVA of 0.55 compared to 0.23 in non-opioid drug users (p = 0.139). There was no significant difference between non-opioid drug users and non-drug users in initial visual acuity of the affected eye or better eye. Opioid users had higher proportions of visual disability at initial presentation, with 79.17% below the California visual limit for driving (p = 0.009) and 16.67% below the federal limit for legal blindness (p = 0.033) (Figure 2). In comparison, 62.16% of non-opioid drug users (p = 0.694) and 52.00% of non-drug users (p = 0.210) had initial visual acuities worse than the driving limit, and 5.41% of non-opioid drug users (p = 0.578) and 4.00% of non-drug users (p = 0.462) were legally blind on initial presentation.
Figure 1:
Initial clinical presentation of infectious keratitis stratified by drug use status. Box and whisker plots of initial best corrected visual acuity (BCVA) in affected eye (A) and better eye (B), initial epithelial defect size (C), and days prior to presentation (D), showing median value line and upper and lower quartiles, with errors bars as minimum and maximum values and x as mean marker. BCVA was measured in logMAR estimate of , with higher logMAR indicating worse BCVA. *t-Test p-value < 0.05. **t-Test p-value < 0.01.
Figure 2.
Incidence of visual disability stratified by drug use status. California driving limit (A,C) was defined as 20/40 in one eye and at least 20/70 in the other eye. Federal legal blindness (B,D) was defined as 20/200 or less in the better eye. *Chi-square p-value < 0.05, **Chi-square p-value < 0.01, ***Chi-square p-value < 0.001.
Non-drug users had smaller initial epithelial defects, averaging 2.39 mm2, compared to 11.23 mm2 for opioid users (p = 0.023) and 6.92 mm2 for non-opioid drug users (p < 0.001), with no significant difference between opioid users and non-opioid drug users (p = 0.235). Symptom days prior to presentation did not show significant variation between opioid users and non-opioid drug users (p = 0.828) or non-drug users (p = 0.764), with an average of 8.36 days for opioid users, 7.60 days for non-opioid drug users, and 7.39 days for non-drug users.
Impact of Opioid Use Status on Treatment and Outcomes for Infectious Keratitis
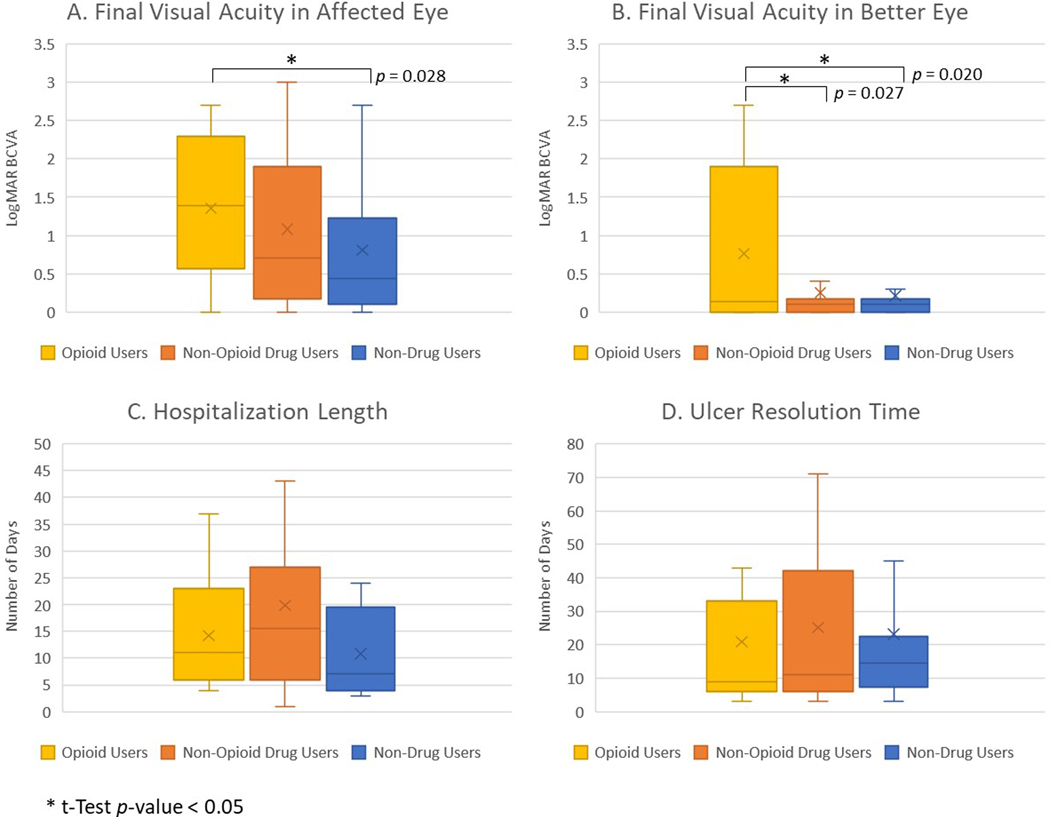
Opioid users had a higher rate of hospitalization (62.50%) for infectious keratitis (p < 0.001), while non-drug users had a lower rate of hospitalization (12.00%) (p = 0.003). Of the patients that were hospitalized, there were no significant differences between opioid users, average 14.20 days, compared to non-opioid drug users, average 19.86 days (p = 0.245), and non-drug users, average 10.8 days (p = 0.495) (Figure 3).
Figure 3:
Treatment outcomes for infectious keratitis stratified by drug use status. Box and whisker plots of final best corrected visual acuity (BCVA) in affected eye (A) and better eye (B), hospitalization length (C), and ulcer resolution time (D), showing median value line and upper and lower quartiles, with errors bars as minimum and maximum values and x as mean marker. BCVA was measured in logMAR estimate of , with higher logMAR indicating worse BCVA. *t-Test p-value < 0.05.
Opioid users had increased rates of fortified antibiotic use (p = 0.009), while non-drug users had decreased rates (p = 0.015). There were similar rates of subconjunctival antibiotic use, emergent PKP, and gluing among the three groups. Non-drug users were less likely to be lost to follow-up (p = 0.011) and there was a trend toward an association between opioid users and loss to follow-up (p = 0.082). There were no significant differences in medication compliance rates between the three groups. There were no significant differences in the average ulcer resolution time between opioid users and non-opioid drug users (p = 0.635) or non-drug users (p = 0.805), with an average of 20.91 days for opioid users, 25.18 days for non-opioid drug users, and 23.09 days for non-drug users.
Opioid users had a worse final visual acuity of the affected eye compared to non-drug users (p = 0.028), and no significant difference compared to non-opioid drug users (p = 0.230), with an average final logMAR BCVA of 1.35 in opioids users, 1.08 in non-opioid drug users, and 0.81 in non-drug users. Similarly, when comparing the cohorts excluding all lost to follow-up patients, both opioid users and non-opioid drug users demonstrated worse final BCVA in the affected eye compared to non-drug users (p = 0.012 and p = 0.046, respectively.) Opioid users also had higher rates of visual disability at final presentation; 70.83% of patients had worse acuity than the legal limit for driving in California (p = 0.043) and 29.17% of patients met criteria for legal blindness (p < 0.001) (Figure 2). In comparison, 54.05% of non-opioid drug users (p = 0.832) and 38.00% of non-drug users (p = 0.090) had final visual acuities worse than the driving limit, and only 4.05% of non-opioid drug users (p = 0.181) and 4.00% of non-drug users (p = 0.317) were legally blind on final presentation.
Discussion
In this retrospective cohort study of patients presenting with infectious keratitis to a public county hospital, opioid use was found to correlate with a unique pattern of demographic risk factors, clinical characteristics, and treatment profiles. Significant associations were seen between opioid use and unemployment, homelessness, and psychiatric history, Several of these patterns have been observed in other studies. Unemployment is known to increase the risk for OUDs,12 and cross-sectional analysis of state-level data on OUD prevalence found that unemployment was significantly associated with increased opioid dependence.13 Patients who are homeless are more likely to experience substance use disorders, including opioid and heroin use.14 OUD has been associated with a variety of psychiatric conditions, including posttraumatic stress disorder, depressive disorders, bipolar disorder, and personality disorders;12,15 this spectrum of conditions is reflected in this study’s patient cohort.
The initial presentations for infectious keratitis in opioid users were more severe than in non-opioid drug users and non-drug users. Opioid users were found to have a higher number of bilateral ulcers, worse initial visual acuity in the affected eye than all non-opioid users, and larger initial epithelial defect sizes than non-drug users. Opioid users also showed worse visual disability at initial presentation, with a higher proportion of patients below the legal driving and legal blindness visual acuity thresholds. This parallels analyses of case reports of infectious keratitis associated with other drugs. In one systematic review of case reports for “crack eye,” a syndrome of corneal disease associated with crack cocaine smoking that includes microbial keratitis and corneal epithelial defects, a majority of cases had bilateral involvement.17 The cases of “crack eye” all showed markedly diminished visual acuity in the affected eye(s) at initial presentation.17 There are also multiple case reports of methamphetamine-induced corneal ulcers that similarly show reduced initial visual acuity at presentation, with one case of bilateral involvement.4,6
Opioid users also required increased rates of fortified antibiotic use and hospitalization. Hospitalizations are more likely in patients who present with larger ulcers and worse initial visual acuity,18 which may explain the higher hospitalization rates seen with opioid users in this analysis. Additionally, Usmani et al. found that patients with an OUD had a higher hospitalization rate when presenting with a primary ophthalmic diagnosis to emergency departments compared to those without opioid abuse,2 suggesting that opioid use in itself is an independent risk factor for admission. Of note, the opioid user group had higher rates of homelessness, and being unhoused has been found to be a significant risk factor of hospitalization due to infectious keratitis in the county hospital population (unpublished data). Previous studies have shown that fortified antibiotics are generally indicated in more severe presentations of infectious keratitis19,20 and that fortified antibiotic use increases with inpatient treatment.21 Our findings concur with those of previous students that opioid use is associated with increased severity of infectious keratitis, hospital admission, and fortified antibiotic use.
Opioid use strongly correlated with worse outcomes in infectious keratitis. Though there was no significant difference in the final visual acuity of affected eyes, opioid users suffered higher visual disability and worse vision in the better eye compared to non-opioid users. This increased visual disability among opioid users parallels results from Han et al. that demonstrated a higher prevalence of substance use disorders, including opioid misuse, among adults with visual impairment compared to non-visually impaired individuals in the United States.22 Though not analyzed in this study, the high propensity for visual disability in opioid users likely translates to significant socioeconomic costs. Rein et al. estimated the annual economic burden of vision loss (blindness or difficulty seeing even with glasses) in the United States as $134.2 billion, with $98.7 billion in direct costs (medical, nursing home, and supportive services) and $35.5 billion in indirect costs (absenteeism, lost household production, reduced labor force participation, and informal care).23
Given the worse outcomes seen in this study of opioid users with infectious keratitis, there are possible mechanisms that could explain the negative effects of opioid exposure on corneal wound healing. The opioid growth factor receptor (OGFr) is a non-classical opioid receptor found on the corneal epithelium and corneal terminal nerves that plays a role in corneal homeostasis.24 Multiple studies have examined the effects of this receptor and opioid exposure on corneal wound healing. Zagon et al. (2006) used plasmid amplification to overexpress OGFr in rat eyes, causing larger corneal defects than control eyes; conversely, DNA synthesis was increased in eyes that had decreased expression of OGFr.25 Another study found that transgenic mice with overexpression of OGFr had decreased DNA synthesis in the corneal epithelium, an effect that was amplified with addition of exogenous and reversed by naloxone. These mice also had a 75% slower healing rate time of full thickness corneal wounds compared to wild-type mice.26 Wenk et al. (2003) used a rat cornea model of acute chemical injury to examine the effects of morphine sulfate eye drops, showing that there were significant decrease and delay in immune cell infiltration into the corneal stroma after chemical injury in eyes exposed to exogeneous morphine sulfate.27
In addition to delays in corneal wound healing, decreased tear production related to opioid exposure may also be a factor. Topical naltrexone application has been shown to restore corneal sensation and improve tear function in rats with induced diabetes mellitus.28 Additionally, topical opioids have been shown to decrease tear production in rats.29 However, in rabbits and dogs, topical morphine was not shown to delay corneal epithelial wound healing.30,31 Areas for further study include the effect of opioids on corneal sensation, tear production, and wound healing in humans.
One important limitation to this study is the unreliability of patient reporting regarding their true drug use status, especially since data collection relied on patient report data and not on toxicology reports. It is possible patients with opioid abuse did not report this information in the medical record and were incorrectly placed in the non-opioid drug use or non-drug use category. The retrospective nature of this study limited further collection of information regarding drug use status. Additionally, we were unable to clarify temporal associations between the timing of opioid use and the development of infectious keratitis.
In summary, opioid use is associated with social risk factors, increased clinical severity, and worse visual outcomes, including visual disability. Opioid use as a causative factor for worse presentation and outcomes cannot be inferred from this study. However, at the very least, opioid use indicates a suboptimal social and physiologic environment for recovery from infectious keratitis. Physicians should assess patients for opioid abuse during the evaluation of infectious keratitis, especially in severe, bilateral cases. There should be a low threshold for diagnosis and treatment of infectious keratitis in opioid users, and a multi-disciplinary approach, including concurrent substance abuse management, should be considered for these patients. Opioid-associated infectious keratitis is an emerging clinical entity that is gaining importance in the context of the rising opioid epidemic, and further study is required to elucidate the optimal treatment approach for this especially vulnerable patient population.
Acknowledgements
Funding:
This study was supported by funds from the National Institutes of Health (R01 R01EY032161 to MFC and NIH-NEI P30 EY002162 - Core Grant for Vision Research), Research to Prevent Blindness (an unrestricted grant to the UCSF Department of Ophthalmology) and All May See Foundation. The sponsor or funding organization had no role in the design or conduct of this research.
Footnotes
Conflicts of Interest:
M.F.C. has consulted for Surrozen, Inc. and XCaliber Biotechnology, Inc. and is currently receiving a grant (R01EY032161) from the NIH/NEI. M.Y. has consulted for Iota Biosciences, Inc. G.D.S. has consulted for Dompé. For the remaining authors none were declared.
References
- 1.Lyden J, Binswanger IA. The United States opioid epidemic. Seminars in Perinatology. 2019;43(3):123–131. doi: 10.1053/j.semperi.2019.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Usmani B, Latif A, Amarasekera S, et al. Eye-Related Emergency Department Visits and The Opioid Epidemic: a 10-Year Analysis. Ophthalmic Epidemiol. 2020;27(4):300–309. doi: 10.1080/09286586.2020.1744165 [DOI] [PubMed] [Google Scholar]
- 3.Colatrella N, Daniel TE. Crack eye syndrome. J Am Optom Assoc. 1999;70(3):193–197. [PubMed] [Google Scholar]
- 4.Franco J, Bennett A, Patel P, Waldrop W, McCulley J. Methamphetamine-Induced Keratitis Case Series. Cornea. 2022;41(3):367–369. doi: 10.1097/ICO.0000000000002764 [DOI] [PubMed] [Google Scholar]
- 5.Ghosheh FR, Ehlers JP, Ayres BD, Hammersmith KM, Rapuano CJ, Cohen EJ. Corneal ulcers associated with aerosolized crack cocaine use. Cornea. 2007;26(8):966–969. doi: 10.1097/ICO.0b013e3180cfe716 [DOI] [PubMed] [Google Scholar]
- 6.Poulsen EJ, Mannis MJ, Chang SD. Keratitis in methamphetamine abusers. Cornea. 1996;15(5):477–482. [PubMed] [Google Scholar]
- 7.Zagon IS, Sassani JW, McLaughlin PJ. Reepithelialization of the Human Cornea Is Regulated by Endogenous Opioids. Investigative Ophthalmology & Visual Science. 2000;41(1):73–81. [PubMed] [Google Scholar]
- 8.Zagon IS, Jenkins JB, Sassani JW, et al. Naltrexone, an opioid antagonist, facilitates reepithelialization of the cornea in diabetic rat. Diabetes. 2002;51(10):3055–3062. doi: 10.2337/diabetes.51.10.3055 [DOI] [PubMed] [Google Scholar]
- 9.Klocek MS, Sassani JW, McLaughlin PJ, Zagon IS. Topically applied naltrexone restores corneal reepithelialization in diabetic rats. J Ocul Pharmacol Ther. 2007;23(2):89–102. doi: 10.1089/jop.2006.0111 [DOI] [PubMed] [Google Scholar]
- 10.Standards Vision. California DMV. Accessed May 11, 2022. https://www.dmv.ca.gov/portal/driver-education-and-safety/educational-materials/fast-facts/vision-standards-ffdl-14/ [Google Scholar]
- 11.Low Vision Information - Clearinghouse for Specialized Media & Technology (CA Dept of Education). Accessed May 11, 2022. https://www.cde.ca.gov/re/pn/sm/lowvision.asp [Google Scholar]
- 12.Blanco C, Volkow ND. Management of opioid use disorder in the USA: present status and future directions. The Lancet. 2019;393(10182):1760–1772. doi: 10.1016/S0140-6736(18)33078-2 [DOI] [PubMed] [Google Scholar]
- 13.Leung J, Chan GCK, Tan SX, McClure-Thomas C, Degenhardt L, Hall W. State-Level Prevalence and Associates of Opioid Dependence in the USA. Int J Environ Res Public Health. 2022;19(7):3825. doi: 10.3390/ijerph19073825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doran KM, Rahai N, McCormack RP, et al. Substance use and homelessness among emergency department patients. Drug and Alcohol Dependence. 2018;188:328–333. doi: 10.1016/j.drugalcdep.2018.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saha TD, Kerridge BT, Goldstein RB, et al. Nonmedical Prescription Opioid Use and DSM-5 Nonmedical Prescription Opioid Use Disorder in the United States. J Clin Psychiatry. 2016;77(6):772–780. doi: 10.4088/JCP.15m10386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siddiqui N, Urman RD. Opioid Use Disorder and Racial/Ethnic Health Disparities: Prevention and Management. Curr Pain Headache Rep. 2022;26(2):129–137. doi: 10.1007/s11916-022-01010-4 [DOI] [PubMed] [Google Scholar]
- 17.Gohil H, Miskovic M, Buxton JA, Holland SP, Strike C. Smoke Gets in the Eye: A systematic review of case reports of ocular complications of crack cocaine use. Drug and Alcohol Review. 2022;41(2):347–355. doi: 10.1111/dar.13366 [DOI] [PubMed] [Google Scholar]
- 18.Wong T, Ormonde S, Gamble G, McGhee CNJ. Severe infective keratitis leading to hospital admission in New Zealand. Br J Ophthalmol. 2003;87(9):1103–1108. doi: 10.1136/bjo.87.9.1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kenia VP, Kenia RV, Pirdankar OH . Diagnosis and Management Protocol of Acute Corneal Ulcer. International Journal of Health Sciences and Research. 2020;10(3):69–78. [Google Scholar]
- 20.Austin A, Lietman T, Rose-Nussbaumer J. Update on the Management of Infectious Keratitis. Ophthalmology. 2017;124(11):1678–1689. doi: 10.1016/j.ophtha.2017.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Truong DT, Bui MT, Memon P, Cavanagh HD. Microbial Keratitis at an Urban Public Hospital: A 10-Year Update. J Clin Exp Ophthalmol. 2015;6(6):498. doi: 10.4172/2155-9570.1000498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han BH, Leddy JF, Lopez FA, Palamar JJ. Prevalence of Psychoactive Substance Use Among Middle-aged and Older Adults With Visual Impairment in the US. JAMA Ophthalmology. 2022;140(1):94–95. doi: 10.1001/jamaophthalmol.2021.4667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rein DB, Wittenborn JS, Zhang P, et al. The Economic Burden of Vision Loss and Blindness in the United States. Ophthalmology. 2022;129(4):369–378. doi: 10.1016/j.ophtha.2021.09.010 [DOI] [PubMed] [Google Scholar]
- 24.García-López C, Gómez-Huertas C, Sánchez-González JM, et al. Opioids and Ocular Surface Pathology: A Literature Review of New Treatments Horizons. Journal of Clinical Medicine. 2022;11(5):1424. doi: 10.3390/jcm11051424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zagon IS, Sassani JW, Malefyt KJ, McLaughlin PJ. Regulation of corneal repair by particle-mediated gene transfer of opioid growth factor receptor complementary DNA. Arch Ophthalmol. 2006;124(11):1620–1624. doi: 10.1001/archopht.124.11.1620 [DOI] [PubMed] [Google Scholar]
- 26.McLaughlin PJ, Keiper CL, Verderame MF, Zagon IS. Targeted overexpression of OGFr in epithelium of transgenic mice suppresses cell proliferation and impairs full-thickness wound closure. Am J Physiol Regul Integr Comp Physiol. 2012;302(9):R1084–1090. doi: 10.1152/ajpregu.00670.2011 [DOI] [PubMed] [Google Scholar]
- 27.Wenk NH, Nannenga NM, Honda NC. Effect of morphine sulphate eye drops on hyperalgesia in the rat cornea. Pain. 2003;105(3):455–465. doi: 10.1016/S0304-3959(03)00260-4 [DOI] [PubMed] [Google Scholar]
- 28.Zagon IS, Klocek MS, Sassani JW, McLaughlin PJ. Topical Naltrexone Reverses Dry Eye and Restores Corneal Sensation in Diabetes Mellitus. Arch Ophthalmol. 2009;127(11):1468–1473. doi: 10.1001/archophthalmol.2009.270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zagon IS, Campbell AM, Sassani JW, McLaughlin PJ. Spontaneous Episodic Decreased Tear Secretion in Rats Is Related to Opioidergic Signaling Pathways. Invest Ophthalmol Vis Sci. 2012;53(6):3234–3240. doi: 10.1167/iovs.11-9051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peyman GA, Rahimy MH, Fernandes ML. Effects of morphine on corneal sensitivity and epithelial wound healing: implications for topical ophthalmic analgesia. Br J Ophthalmol. 1994;78(2):138–141. doi: 10.1136/bjo.78.2.138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stiles J, Honda CN, Krohne SG, Kazacos EA. Effect of topical administration of 1% morphine sulfate solution on signs of pain and corneal wound healing in dogs. Am J Vet Res. 2003;64(7):813–818. doi: 10.2460/ajvr.2003.64.813 [DOI] [PubMed] [Google Scholar]