Abstract
The immunological hallmarks of sepsis include the inflammation-mediated cytokine storm, apoptosis-driven lymphopenia, and prolonged immunoparalysis. While early clinical efforts were focused on increasing the survival of patients through the first phase, studies are now shifting attention to the long-term effects of sepsis on immune fitness in survivors. In particular, the most pertinent task is deciphering how the immune system becomes suppressed, leading to increased incidence of secondary infections. Here, we introduce the contribution of numerical changes and functional reprogramming within innate (NK cells, DCs) and adaptive (T cells, B cells) immune cells on the chronic immune dysregulation in the septic murine and human host. We briefly discuss how prior immunological experience in murine models impacts sepsis severity, immune dysfunction, and clinical relevance. Finally, we dive into how comorbidities, specifically autoimmunity and cancer, can influence host susceptibility to sepsis and the associated immune dysfunction.
Introduction
Sepsis is defined as an immune dysfunction and multiple organ failure stemming from dysregulated responses to an initial acute infection, which subsequently allow the instigating pathogen to go systemic (1). This disorder affects 1.7 million people on average in the U.S. annually, and leads to 300,000 deaths each year (2). Globally, ~50 million people experience a septic event each year, of which ~11 million will die (3). Additionally, sepsis continues to be the most costly inpatient hospitalization per patient, with aggregate hospital costs at $3.8B and >2000 hospital stays annually (4). Respiratory, intestinal, and genitourinary infections are responsible for the majority of sepsis cases (5). The incidence of sepsis increases in those with comorbidities, including diabetes, COPD, and cancer (6). Sepsis is diagnosed clinically when a patient reaches specific Sequential Organ Failure Assessment (SOFA) scores based on physiological responses such as blood pressure, respiratory rate, and mental awareness changes (1). However, other components of sepsis, including cellular and metabolic changes, also occur.
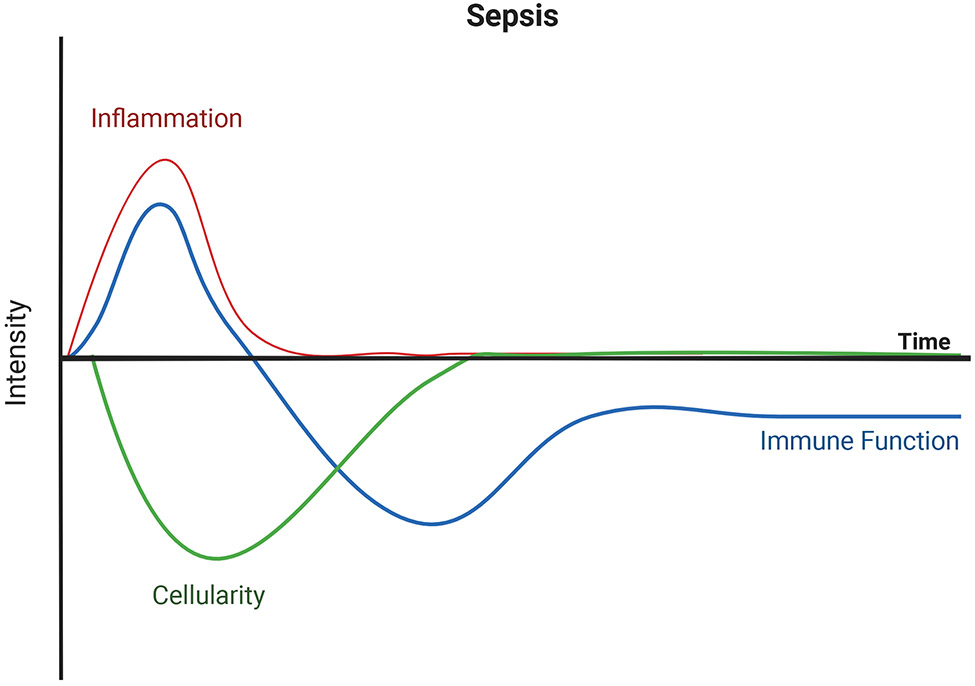
There are three canonical immunological hallmarks of sepsis (Figure 1). The first is the cytokine storm, which is a hyperinflammatory response to the disseminated infection. During this phase, both pro- and anti-inflammatory cytokines are released systemically, including TNFα, IL-6, and IL-10 (7-9). In the midst of the cytokine storm, profound lymphopenia develops – the second immunological hallmark of sepsis. Lymphocytes within the circulation and secondary lymphoid organs undergo apoptosis, resulting in septic patients experiencing a significant reduction in absolute lymphocyte count (10, 11) as well as numbers of CD4 T cells, CD8 T cells, B cells, and NK cells (12). It is believed that lymphopenia serves to help counterbalance the cytokine storm, but the extent of lymphopenia vary among hosts such that it can both predict and contribute to the poor outcomes after the septic event (13). Finally, there is a prolonged phase marked by immune suppression termed immunoparalysis which follows patients long after their time in the hospital. The immune cells that were maintained during the lymphopenia and those that recovered by post-lymphopenic homeostatic proliferation undergo a reprogramming rendering them hyporesponsive to subsequent stimulation. Clinically, this phase of sepsis is defined as persistent inflammation, immunosuppression, and catabolism syndrome (PICS). PICS is suggested to contribute to the chronic critical illness (CCI) seen in patients who recover from sepsis, wherein they exhibit increased susceptibility to secondary infections because of their immunosuppressed state. Sera from these patients with CCI indicate increased concentrations of immunosuppressive markers, such as soluble PD-L1 and IL-10, and stress metabolism markers, such as CRP and GLP-1 (8, 11). At the same time, these patients have decreased amounts of hematopoietic growth factors such as GM-CSF (8). In addition, T cells from sepsis patients are less able to produce IFNγ in response to TCR signaling (14) and IL-6, TNFα and IL-1β after LPS stimulation compared to healthy controls (15). Sepsis patients, consequently, have worse outcomes (vs. critically ill, non-septic patients) 1-year after discharge.
Figure 1.
The immunological hallmarks of the septic event. The host immune response against systemic pathogens initiates with excessive inflammation known as the cytokine storm. Concurrent declines in cellularity (lymphopenia) develop. Eventually the cytokine storm subsides, and immune cell numbers recover with subsequent poor responses from remaining cellular pools. Image created with BioRender.com.
Murine models have been developed to study the intricacies of sepsis and its impact on the immune system in ways not possible with human samples (16). One such model involves administering purified LPS to invoke systemic inflammation by stimulating Toll-like receptor (TLR) 4. This model favors innate immune system activation, but the inflammatory response to LPS is short-lived, restrictive to stimulation of a single TLR, and does not work in all murine models such as in hosts with defective TLR4 signaling (17, 18). Moreover, mice are significantly less sensitive to LPS than humans, leading some investigators to question the relevance of this model to human sepsis (19). In contrast to the sterile inflammation induced by LPS, other sepsis models inject viable microbial pathogens systemically or intraperitoneally (i.p.) into murine hosts, providing a broader repertoire of pathogen-associated molecular patterns (PAMPs) for immunological stimulation. However, the caveat to this model is that unlike human hosts, i.p. injections do not typically mirror natural primary infection sites, such as the pulmonary or urinary systems (5, 16) and human intra-abdominal sepsis is frequently polymicrobial in nature (20, 21). A third preclinical sepsis model was developed with both immunological and physiological outputs of sepsis in mind: cecal ligation and puncture (CLP) (22). The result of CLP is a polymicrobial sepsis that produces many of the same immunological (i.e., cytokine storm, lymphopenia, and immunoparalysis) and physiological (i.e., increased blood concentrations of creatine phosphokinase and blood urea nitrogen) indicators of human sepsis (23, 24). The severity of the septic event in the CLP model can be modulated depending on the length of cecum ligated, gauge of needle used to puncture the cecum, number of punctures made in the cecum, and amount of fecal material extruded and released in the peritoneum (23). CLP is currently the most used murine model to study acute sepsis and chronic immunoparalysis (22).
This review will highlight current knowledge defining the numerical changes and functional reprogramming experienced by several immune cells during sepsis that contribute to the immunoparalysis state – with a focus on dendritic cells (DCs), T cells, B cells, and NK cells. It is important to keep in mind that there are no clinical data currently in hand that characterizes the sepsis-induced immunoparalysis state at the mechanistic level, nor is it presently possible to identify which sepsis patients will develop this condition or how long it will persist. Thus, our discussion will cover data coming from preclinical mouse and clinical studies that defines some of the immunological parameters that collectively result in the immunoparalysis state seen in sepsis survivors. We will clearly indicate the source of the data to minimize any confusion. We also realize there are some concerns when trying to equate the impact of sepsis on the murine immune system to human immune dysfunction seen during sepsis. Consequently, we have included a conversation of preclinical data describing the role of prior immune experience on the sepsis-induced acute hyperinflammation and subsequent immune dysfunction. This topic will be covered within the context of two murine models: one using hosts exposed to natural commensal and pathogenic microbes and the other using hosts sequentially infected with several well-established experimental pathogens. We will transition to the concept of comorbidities associated with sepsis, illustrating how they contribute to the severity of subsequent septic events with the examples of autoimmunity and cancer. Finally, we will return to the topic of septic immunoparalysis, flipping the context by defining its impacts on subsequent autoimmunity and cancer development. Overall, the goal of this review is to provide information and context of septic immunoparalysis coming from the evaluation of murine models and patient samples, with considerations of how the host immunological background impacts the septic event.
Cellular alterations during sepsis
Dendritic Cells
Dendritic cells (DCs) acquire, process, and present Ag to responding CD4 and CD8 T cells. Consequently, any disruption in normal DC number and function can have a significant impact on T cell priming. Pediatric septic patients display reduced frequencies of DC subsets as a result of increased apoptosis and trending declines in HLA-DR and costimulatory marker expression (24). The same is seen in adult human patients, with early numerical declines in circulating myeloid DCs and plasmacytoid DCs which are maintained in patients with subsequent ICU-acquired secondary infections (25).
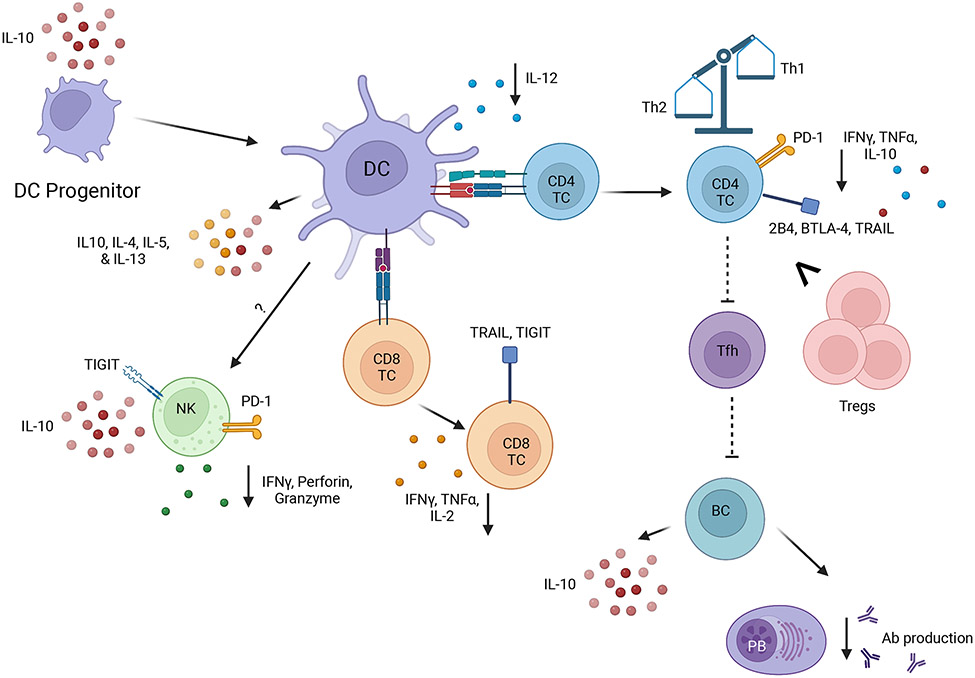
Previous work from our group and others has revealed numerical and functional deficits of multiple DC subsets in the spleen and peripheral lymph nodes of mice after CLP-mediated sepsis (26-28) (Figure 2). The remaining DC pool was altered in composition and less able to produce cytokines important for cellular immunity (e.g., IL-12) in response to secondary pathogens (26, 29). Interestingly, this inability of DC to produce IL-12 (and concomitant increased production of Th2 cytokines like IL-4, IL-5, and IL-13) result from sepsis-induced epigenetic changes in the DC mediated by the differential recruitment of histone methyltransferase complexes to the Il12 promoter regions (30). Since DCs offer early protection against excessive systemic inflammation in a murine LPS model of endotoxemia via glucocorticoid receptor-mediated inhibition of IL-12 production, this could suggest a potential mechanism for the IL-12 decline at the immunoparalysis stage (31). DC progenitors in the bone marrow of mice are also depleted during sepsis, yielding daughter cells that gain more regulatory-like functions (i.e., IL-10 production) (32). Bone marrow-derived DCs (BMDCs) contribute to the functional deficits in sepsis by not only increasing their own production of IL-10, but by decreasing total and NK-specific responses against subsequent secondary bacterial infection (32). The composition and function of murine DC progenitors is impacted by CD8 T cells in the bone marrow in a TLR2-dependent manner. These CD8 T cells influence DC capability of producing IL-12 and suppressing IL-10 and TNFα production when these DCs mature (33). These data indicate numerical and cytokine production alterations of DCs. More work is required to tease apart the contributing mechanisms to these declines in function, as well as antigen presentation, in order to inform future therapeutics.
Figure 2.
The immune dysfunctions of dendritic cells and lymphocytes during immunoparalysis. This illustrates the current understanding from combined murine and human data on the inhibitory markers, cytokines, and antibodies produced during the chronic immunoparalysis. Downward-facing arrows indicate declines, dotted lines indicate murine based mechanism. Image created with BioRender.com.
CD8 T cells
The CD8 T cell compartment experiences quantitative and qualitative changes during the lymphopenia and immunoparalysis phases of sepsis. In mice, naïve CD8 T cells observe a transient numerical reduction in the blood and secondary lymphoid organs during sepsis. When recovered, these T cells present with activated phenotypes by increased expression of CD44 and CD11a despite being naïve, or inexperienced, to their cognate antigen (34). The acquisition of the activated phenotype could be due to the generation of “virtual memory” T cells (Tvms), a population of naïve cells that express proteins typically found on Ag-experienced T cells despite the absence of cognate Ag recognition after expanding via homeostatic proliferation (35, 36). Subsequent cytokine production and Ag-specific responses from these naïve mouse CD8 T cells is low when stimulated to become effector cells (34). Additionally, while Tvms in younger mice can prove beneficial to immunity and protection (37, 38), CD8 Tvms become senescent and proliferate poorly in older hosts (39, 40), which could contribute to poor post-sepsis responses in the aged septic population. Future studies may trend in this direction by assessing not only the presence of these Tvms, but their relevance and impact especially in aged mouse and human populations. Naïve CD8 T cell expansion in the septic murine host can be further impeded by poorly responding DCs (29). Memory CD8 T cells in the blood and secondary lymphoid organs of mice also experience transient numerical reductions during sepsis. It should not be surprising to realize that the severity of sepsis dictates the magnitude of circulatory T cell lymphopenia, with higher cell loss during more severe sepsis. Interestingly, murine tissue resident memory CD8 T cell loss and endothelial permeability, in contrast, are only impacted in cases of severe sepsis (23, 41). We recently published a review detailing the myriad of acute and long-term alterations that affect mouse and human memory CD8 T cells (41), and will refer readers to that publication for more detailed information.
CD4 T Cells
A hallmark of CD4 T cells is their ability to differentiate into subsets with unique effector functions able to skew immune responses after exposure to polarizing cytokines in the context of cognate Ag presentation. Similar to CD8 T cells, mouse and human CD4 T cells experience profound lymphopenia from sepsis onset followed by qualitative changes once cell numbers have been predominantly recovered (42). Early human studies examining CD4 T cell cytokine production from septic patients suggested a subset shift from Th1 to Th2 (43-46). However, a more recent human study using freshly isolated cells from the spleen and lung found very little cytokine production (IFNγ, TNFα, or IL-10) after anti-CD3/CD28 mAb stimulation (47), suggesting post-septic CD4 T cells experience a global state of anergy (46). Additionally, post-septic CD4 T cells have enhanced expression of inhibitor receptors, including PD-1, 2B4, BTLA-4, and TRAIL (48-54), likely driven by prolonged exposure to pro- and anti-inflammatory cytokines. Another defined change in human CD4 T cell subsets following sepsis is an overrepresentation of regulatory T cells (Tregs) in the circulation (55, 56). This increase in Treg cell frequency has been shown to result from the preferential loss of other CD4 T cell subsets (e.g., Th1, Th2, Th17) (43). IL-33, a type 2 mediator released due to tissue damage, also contributes to the presence of these Tregs in mice both directly and indirectly (55). Since increased proliferation of Tregs has not been observed during sepsis, it has been suggested that human Tregs may be more resistant to sepsis-induced apoptosis than other CD4 T cell subsets (43). Post-septic murine CD4 T cells have dampened ability to provide help to other immune cells, such as in the case of T follicular helper CD4 T cell help to B cells (57), limiting the host’s capacity to effectively respond to new infections. In the immunoparalysis phase, recovered septic patients experience higher rates of CMV and HSV reaction, infections that rely on effective CD4 T cell immunity for limiting frequency and severity in humans (58). However, the mechanisms in which immunoparalysis alters latent infection control have yet to be clearly defined, thus offering further avenues of research that can help patient management after sepsis.
B Cells
B cells are the primary immune cell type within the humoral arm of the adaptive immune system and are responsible for producing antibodies. Like T cells, B cells experience a drastic decline in numbers during the transient lymphopenia stage of sepsis. A study by Shankar et al. showed sepsis-induced lymphopenia in humans was associated with significantly lower absolute B cell counts and a selective depletion of memory B cells (59). This depletion of memory B cells contributes to the suppressed immune state experienced by septic patients (59). The B cells that are present during the immunoparalysis phase of sepsis, including both B cells that avoid acute apoptosis as well as newly replenished B cells, appear more exhausted with reduced antibody production (42, 57, 60, 61). At the same time, the functional capacity of human B cells to produce IL-10 is maintained with ex vivo production increased in septic patients compared to healthy controls (60, 62). This can alter the ability of other immune cells to mount an effective response to subsequent infection. Despite increased plasma cell numbers, the production of Ag-specific antibodies in mice is impaired following sepsis (57, 61, 63). Additionally, sepsis results in an increased representation of mature B cells and a reduced percentage of immature B cells in both mice and humans (61, 63, 64). Future lines of investigation in B cell and humoral immunity alterations during the immunoparalysis phase of sepsis may include pathogen-specific epitopes in mice and humans, and the impacts of sepsis on prior and subsequent vaccinations.
NK Cells
Natural killer cells are a TCR-independent population of lymphocytes which are understudied in the context of sepsis. NK cells provide antigen-independent help against pathogens by either secreting cytokines or lysing target cells via antibody-dependent cell-mediated cytotoxicity (ADCC). Like the cell populations discussed heretofore, NK cells are reduced in numbers and cytokine function early after a septic event. As with the previously described populations, NK cells undergo drastic numerical and functional declines in the blood of septic patients, especially in non-surviving patients where reduction of cell numbers and production of granzyme B and perforin are exacerbated (9, 42).
In mouse models, reduced DAP12 expression and poor LY49H/D signaling contributes to these altered responses by NK cells. This in turn reduces the ability of NK cells to clear viruses, such as MCMV (65). Murine NK cells become less responsive to TLR agonist stimulation after a septic event (66). One potential contributing mechanism lies in the suppressive BMDCs discussed previously. BMDCs from septic mice cultured with naïve NK cells reduced the ability of NK cells to secrete IFNγ when stimulated with Pseudomonas aeruginosa as an in vitro model of secondary infection (32). These preclinical data are corroborated by human studies where a reduced frequency of septic patient NK cells expressed CD107 and IFNγ during ex vivo culture experiments of natural and ADCC-mediate cytotoxicity (67). Murine NK cells also upregulate expression of the coinhibitory receptor TIGIT during sepsis (68). Since global TIGIT knockouts have improved sepsis outcomes, this could suggest a contribution of NK cells to the apoptotic response during sepsis. When PD-1 was analyzed in septic patients, those patients with >5% of PD-1+ NK cells in the blood were at greater risk for poor outcomes. PD-1 expression on human NK cells correlated with 28-day mortality (69). Regardless of their inhibited pro-inflammatory function at this stage, NK cells can still contribute to protective outcomes in septic hosts.
Septic patients have increased IL-10 expression compared to healthy controls 24 hours after admission (70). Depleting NK cells in mice leads to increased inflammation and mortality during CLP, owing in part to NK-specific production of anti-inflammatory IL-10 which impacts these outcomes in this model. (70). However, IL-10 can also be produced by CD169+ and M2 macrophages, and selective elimination of IL-10 production from CD169+ macrophages resulted in increased inflammation and death early after LPS injection (55, 71). Although both studies ascribed a critical role for IL-10 produced either by NK cells or tissue-resident macrophages in CLP or LPS-induced sepsis, respectively (70, 71), additional studies are needed to define the precise role of IL-10 in the development of long-lasting sepsis-induced immunoparalysis state. This also can be an example that highlight the necessity of developing (and improving) experimental models for sepsis-themed research, since it allows for well-controlled mechanistic studies that could inform and focus more challenging studies in humans. Together, these publications highlight stark alterations in multiple immune cell populations due to sepsis, providing rationale for further study of their role in sepsis, especially with such contradictory protective and exhausted contributions.
Impact of baseline immune status on sepsis immune suppression
While sepsis can dysregulate the immune system as explained above, the nearly all preclinical investigation of sepsis-induced immunoparalysis has been done using specific pathogen-free (SPF)-housed naïve mice. There is no denying the fact that the use of SPF mice in these experiments has led to a vast expansion in knowledge regarding the intricate changes that occur in the immune system following a septic event. Important differences, however, exist between the SPF mice used in most preclinical sepsis research and humans (72, 73), which has led some investigators to question the overall relevance of extensive mouse-based sepsis research. SPF housing has increased experimental reproducibility, but the immune systems of SPF mice are dominated by cells with a naïve phenotype because of the intentionally limited exposure to natural microbes (74). In contrast, humans experience daily encounters from a plethora of commensal and pathogenic microbes, which together with the various vaccinations received over time train and shape the adult human immune system to consist mostly of memory phenotype cells.
Within the last 10 years, investigators have recognized the discordance between the immune systems of laboratory mice and adult humans (75). Consequently, several mouse models have been developed that generate microbially-experienced hosts with matured immune systems to model the more prepared, Ag-experienced immune systems seen in humans. One of these “dirty” mouse models involves cohousing SPF laboratory mice with pet store mice (74). Pet store mice carry an array of microbes (many being viruses) typically excluded by SPF housing (76), and exposure of SPF mice to these microbes dramatically shifts the immune system to become skewed toward memory T cells and B cells (77). Similar to this model, some investigators have used feral mice and/or mice kept in outdoor enclosures to permit exposure to environmental agents that promote immune system maturation (78). Another microbially-experienced mouse model relies on the sequential infection of inbred murine hosts with standard mouse-adapted lab pathogens, such as influenza A and Listeria monocytogenes, which we will refer to as specific pathogen experienced (SPexp) hosts (79, 80). Though different investigators vary the pathogens used, sequence of the different infections, and timing between infections in their SPexp models, all versions generate large Ag-experiences pools of T cells and higher baseline levels of circulatory cytokines (80).
Prior pathogen exposure, either by the cohoused model (81) or sequential infection model (80), generates worse outcomes for septic mice. Baseline TLR expression is increased on myeloid cells obtained from cohoused mice compared to traditionally housed SPF mice, which helps to explain why cohoused mice are more sensitive to TLR4 stimulation leading to greater deaths using the CLP and LPS endotoxemia models of sepsis (81). Interestingly, while NK cells do provide some protection against CLP-induced sepsis mortality via IL-15 dependent IL-10 production, the protective role of NK cells seen in SPF mice is altered in SPexp mice. Depletion of NK cells improved survival of septic SPexp mice compared to control Ab-treated SPexp mice (80), suggesting an inflammatory role for NK cells in experienced hosts. Inhibitory coreceptors are also impacted in immunologically experienced murine hosts, as TIGIT actually contributes to a protective phenotype in SPexp septic hosts (82), juxtaposing its role defined in earlier studies (68). With such differences in cell populations previously investigated in sepsis, but this time in a model more immunologically alike to a multi-pathogen experienced human, more investigations of previously established immunoparalysis notions need to be re-tested with these models in mind.
Sepsis can be exacerbated by comorbidities
Sepsis is commonly characterized in the aging population, which upon admission to the clinic or ICU frequently present with comorbidities alongside their sepsis. Examples of comorbid diseases in septic patients include hypertension, diabetes, cancer, and chronic organ diseases (83). Some comorbidities, such as obesity, yield more protective outcomes early on during sepsis though could prove detrimental long term to organ dysfunction based on both preclinical and clinical data (84-86). The impact of diabetes on septic patient outcomes is still under debate with most data pointing to no differences in survival or PD-1 expression on T cells at 28 days post enrollment (87-89). Murine models have been able to highlight some impact of diabetes on sepsis outcomes, such as altered neutrophil mobility/function, host survival, and sepsis-mediated CNS inflammation and reduced mitochondrial respiration (90, 91). However, combinations of some of these chronic diseases with an acute infection can increase the likelihood of the initial infection progressing to sepsis. A publication by Sinapidis and colleagues highlights not only the interactions of these parameters that leads to sepsis, but emphasizes that two or more comorbidities increases sepsis occurrence and 28-day mortality (92). Collectively, these data illustrate the need for further study into how these chronic diseases immunologically impact the septic host. Below we describe two such comorbidities – autoimmunity and cancer – which decrease survival in septic hosts.
Autoimmunity and sepsis: a focus on multiple sclerosis
Infections and sepsis are common diagnoses and causes for hospital mortality in autoimmune patients (93). In some instances, this leads to subsequent nosocomial infections (94). Multiple sclerosis is a debilitating neurodegenerative autoimmune disease that causes sensory, visual, and mobility issues (95). MS patients are more likely to become septic than non-MS patients and remain in the hospital longer after ICU admission (96-98). To model a similar coupled disease course, our group has utilized an experimental autoimmune encephalomyelitis (EAE) model in combination with CLP-induced sepsis. Mice experiencing EAE have increased circulatory inflammatory profiles and decreased survival rates after sepsis induction compared to non-EAE controls. This occurs regardless of when the septic event occurs after EAE induction or sepsis model used (96). Mechanistically, EAE mice with lower IL-10 in the sera prior to sepsis fared worse in terms of survival, and those that did not survive also had higher levels of IL-6 12 hours post-CLP compared to non-EAE mice and EAE survivors (96), suggesting a more severe cytokine storm. Questions remain as to which cells in EAE mice produce the inflammatory phenotypes that drive the increase in sepsis severity, as well as potential sepsis-mediated declines or alterations to EAE/MS-specific autoantibody production (99, 100) given the sepsis-mediated declines in antibodies described earlier. Importantly, applying the preclinical sepsis model to a relapsing/remitting iteration of EAE/MS would more accurately represent the MS disease course seen in human (95, 101). Another angle to consider is the link between the gut, sepsis, and autoimmunity. The microbial composition of the gut can impact outcomes in both multiple sclerosis (102, 103) and sepsis (104). Accordingly, bacterial species found detrimental to EAE (105) have been shown to be potentially helpful in CLP (106). How alterations of the gut microbiota to include more MS-protective bacterial species impacts the outcome of the septic event, or even the likelihood of an infection going systemic, remains to be defined.
Cancer and sepsis
Another comorbidity to consider with when discussing sepsis incidence and outcomes is cancer. Sepsis occurs in 4% of cancer patients, leading to a 35% chance of mortality up to 1 year after cancer diagnosis (107). Cancer patients with sepsis are more likely to undergo rehospitalization within a month after discharge and more likely to face in-hospital mortality compared to non-cancer-related sepsis (108). Murine data has shown tumor-bearing hosts fare worse in septic survival compared to tumor-free hosts despite having smaller tumor size and volume (109-112). In a model of murine pancreatic cancer, prior cancer induction resulted decreased post-sepsis survival, especially 6+ days after the septic event which presumably falls under the immunoparalysis phase. Interestingly, this combination (i.e., pancreatic cancer and sepsis) yielded increased splenic T cell counts and lower CD4 T cell apoptosis. Regardless, CD8 T cell activation was reduced and trending declines were present in splenic B cells and DCs (113). While tumor-specific CD8 T cells remain numerically unchanged in the tumor microenvironment of septic hosts compared to sham-treated hosts, a reduction in number of tumor-specific CD8 T cells was noted in the periphery of the septic mice (110, 112). Complimentary to this, there were transcriptional and protein increases of IL-10 in B16 tumors after sepsis, suggesting sepsis has the potential for expanding suppressive responses (109). The therapeutic efficacy of checkpoint inhibitors can also be altered by sepsis. PD-1 is expressed at an intermediate level in CD8 tumor-infiltrating lymphocytes in a mouse B16 melanoma/CLP model compared to high PD-1 expression in the sham counterparts. These differences between individual cancer or septic hosts compared to the comorbid model of cancer prior to sepsis suggests a need to keep in mind the differences in efficacy of therapeutics. Indeed, work from the Coopersmith and Ford groups demonstrated that although preclinical PD-1 blockade may be beneficial to sepsis alone, its impact as a therapeutic is inversed in the comorbid lung cancer cell LLC/sepsis model. Instead, 2B4 proved to be the better therapeutic target in this instance by increasing survival and reducing coinhibitory receptor expression (114). TIGIT, another coinhibitory receptor, is mildly increased in splenic Tregs and NK cells from LLC/sepsis mice compared to tumor-only mice (68). TIGIT contributes to reduced splenic T cell cytokine production, CD4 T cell expression of CTLA-4, and poorer outcomes for septic mice with preexisting malignancy (68). Interestingly, there is also preclinical data showing sepsis induction in a tumor-bearing host can promote an effective anti-tumor response, leading to reduced tumor size for those animals that survive the septic event. In this setting, the sepsis-induced anti-tumor response included increased generation of tumor-specific antigen experienced cells and IFNγ production by CD8 T cells and NK cells (110). IFNγ- and granzyme-producing NK cells also increase in frequency upon ex vivo stimulation with tumor cells with this model, suggesting a potential for increased anti-tumor immunity by NK cells after sepsis (109). TLR4 signaling during sepsis is also suggested to contribute this decrease in tumor burden, as ablation of TLR4 signaling in a mouse model of sepsis+cancer leads to trending increases in tumor volume (109).
Impact of immunoparalysis on subsequent immune disorders
When the earlier-mentioned cellular dysregulations are applied to subsequent immunological challenges, they lead to differing outcomes for those hosts that survive the septic insult. Sepsis reduces the severity of subsequent autoimmune induction in the murine EAE model of MS. Specifically, our groups noted that sepsis drove a numerical and inflammatory deficit of myelin peptide-specific CD4 T cells. This quantitative and qualitative change in myelin peptide-specific CD4 T cells reduced disease scoring in EAE mice, providing a more protective phenotype. However, this protective consequence of sepsis was temporary, as the ability of the myelin-specific T cells to proliferate was not impacted (115). Conversely, sepsis can prove detrimental in a host who develops cancer after their septic event (111, 112). B16 tumor cells from previously septic mice express lower amounts of MHC I compared to tumor cells from sham-treated mice, likely leading to the reduced CD8 T cell frequency within the tumor and IFNγ production from the CD8 T cells. This reduced cytokine function is not limited to the tumor microenvironment as there is a decrease in IFNγ and TNFα-producing tumor-specific CD8 T cells in tumor-draining lymph nodes and spleen (112). Additionally, the chronic immunoparalysis phase causes these cells to proliferate less and experience higher apoptosis rates, negatively impacting the tumor environment by reducing the efficacy of checkpoint blockade (111). The immunoparalysis phase critically subjects hosts to altered immune states, predominately in the negative sense. Further studies are required to assess the impact of immunoparalysis on other standard and opportunistic infections and immune disorders. The data discussed here suggest that while septic survivors are primed with expanded anti-tumor responses, sepsis still worsens subsequent tumors and prior malignancies worsen septic outcomes.
Conclusions
While deaths early in sepsis have declined in recent decades, those patients that survive the initial septic event are prone to succumb to secondary infections. Immunoparalysis, the long-term reduction in lymphocyte function, contributes to this susceptibility. Since the data we can derive from clinical patients can be limited, many investigators rely on murine models to further decipher the long-term impacts of sepsis on the host. DCs and lymphocytes are less able to generate protective responses against innate and antigen-specific pathogen molecules. The immunological state of the host at the time of sepsis initiation can have significant impact on the susceptibility to sepsis and the dynamics of the respective diseases/immune dysfunctions. Further preclinical efforts to study the impacts of the host immune status prior to sepsis-inducing infection, including the likelihood of an infection going septic and therapeutically modulating septic patient’s immune systems to regain functionality, can further improve patient outcomes long term.
Supplementary Material
Acknowledgments
Supported by NIH Grants T32AI007511 (E.E.S), GM134880 (V.P.B), AI114543 (V.P.B.), R35GM140881 (T.S.G.) and a Veterans Administration Merit Review Award I01BX001324 (T.S.G.). V.P.B. is a University of Iowa Distinguished Scholar. T.S.G. is the recipient of a Research Career Scientist award (IK6BX006192) from the Department of Veterans Affairs.
References:
- 1.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche J-D, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent J-L, and Angus DC. 2016. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315: 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prevention, C. f. D. C. a. 2021. U.S. Department of Health and Human Services. Sepsis: clinical information; 2020 Dec 7. Atlanta (GA). [Google Scholar]
- 3.Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, Colombara DV, Ikuta KS, Kissoon N, Finfer S, Fleischmann-Struzek C, Machado FR, Reinhart KK, Rowan K, Seymour CW, Watson RS, West TE, Marinho F, Hay SI, Lozano R, Lopez AD, Angus DC, Murray CJL, and Naghavi M. 2020. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. The Lancet 395: 200–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liang L 2020. National Inpatient Hospital Costs: The Most Expensive Conditions by Payer, 2017. Agency for Healthcare Research and Quality (US); 2006 Statistical Brief #261. [PubMed] [Google Scholar]
- 5.Vincent J-L, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, Moreno R, Carlet J, Le Gall J-R, Payen D, and o. b. o. t. S. O. i. A. I. P. Investigators. 2006. Sepsis in European intensive care units: Results of the SOAP study*. Critical Care Medicine 34: 344–353. [DOI] [PubMed] [Google Scholar]
- 6.Esper AM, Moss M, Lewis CA, Nisbet R, Mannino DM, and Martin GS. 2006. The role of infection and comorbidity: Factors that influence disparities in sepsis. Critical Care Medicine 34: 2576–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gogos CA, Drosou E, Bassaris HP, and Skoutelis A. 2000. Pro- versus Anti-inflammatory Cytokine Profile in Patients with Severe Sepsis: A Marker for Prognosis and Future Therapeutic Options. The Journal of Infectious Diseases 181: 176–180. [DOI] [PubMed] [Google Scholar]
- 8.Darden DB, Brakenridge SC, Efron PA, Ghita GL, Fenner BP, Kelly LS, Mohr AM, Moldawer LL, and Moore FA. 2021. Biomarker Evidence of the Persistent Inflammation, Immunosuppression and Catabolism Syndrome (PICS) in Chronic Critical Illness (CCI) After Surgical Sepsis. Annals of Surgery 274: 664–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen R, Qin S, Zhu H, Chang G, Li M, Lu H, Shen M, Gao Q, and Lin X. 2022. Dynamic monitoring of circulating CD8+ T and NK cell function in patients with septic shock. Immunology Letters 243: 61–68. [DOI] [PubMed] [Google Scholar]
- 10.Hotchkiss RS, Monneret G, and Payen D. 2013. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. The Lancet Infectious Diseases 13: 260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stortz JA, Mira JC, Raymond SL, Loftus TJ, Ozrazgat-Baslanti T, Wang Z, Ghita GL, Leeuwenburgh C, Segal MS, Bihorac A, Brumback BA, Mohr AM, Efron PA, Moldawer LL, Moore FA, and Brakenridge SC. 2018. Benchmarking clinical outcomes and the immunocatabolic phenotype of chronic critical illness after sepsis in surgical intensive care unit patients. Journal of Trauma and Acute Care Surgery 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hotchkiss RS, Tinsley KW, Swanson PE, Schmieg RE, Hui JJ, Chang KC, Osborne DF, Freeman BD, Cobb JP, Buchman TG, and Karl IE. 2001. Sepsis-Induced Apoptosis Causes Progressive Profound Depletion of B and CD4+ T Lymphocytes in Humans. The Journal of Immunology 166: 6952–6963. [DOI] [PubMed] [Google Scholar]
- 13.Drewry AM, Samra N, Skrupky LP, Fuller BM, Compton SM, and Hotchkiss RS. 2014. Persistent Lymphopenia After Diagnosis of Sepsis Predicts Mortality. Shock 42: 383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mazer MB, Caldwell CC, Hanson J, Mannion D, Turnbull IR, Drewry A, Osborne D, Walton A, Blood T, Moldawer LL, Brakenridge S, Remy KE, and Hotchkiss RS. 2021. A Whole Blood Enzyme-Linked Immunospot Assay for Functional Immune Endotyping of Septic Patients. The Journal of Immunology 206: 23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ertel W, Kremer J-P, Kenney J, Steckholzer U, Jarrar D, Trentz O, and Schildberg FW. 1995. Downregulation of Proinflammatory Cytokine Release in Whole Blood From Septic Patients. Blood 85: 1341–1347. [PubMed] [Google Scholar]
- 16.Lewis AJ, Seymour CW, and Rosengart MR. 2016. Current Murine Models of Sepsis. Surgical Infections 17: 385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong PMC, Kang A, Chen H, Yuan Q, Fan P, Sultzer BM, Kan YW, and Chung S-W. 1999. Lpsd/Ran of endotoxin-resistant C3H/HeJ mice is defective in mediating lipopolysaccharide endotoxin responses. Proceedings of the National Academy of Sciences 96: 11543–11548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poltorak A, He X, Smirnova I, Liu M-Y, Huffel CV, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, and Beutler B. 1998. Defective LPS Signaling in C3H/HeJ and C57BL/10ScCr Mice: Mutations in Tlr4 Gene. Science 282: 2085–2088. [DOI] [PubMed] [Google Scholar]
- 19.Fink MP 2014. Animal models of sepsis. Virulence 5: 143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fukushima S, Hagiya H, Fujita K, Kamiyama S, Yamada H, Kishida M, and Otsuka F. 2022. Clinical and microbiological characteristics of polymicrobial bacteremia: a retrospective, multicenter study. Infection 50: 1233–1242. [DOI] [PubMed] [Google Scholar]
- 21.Nichols RL 1977. Intraabdominal Sepsis: Characterization and Treatment. The Journal of Infectious Diseases 135: S54–S57. [DOI] [PubMed] [Google Scholar]
- 22.Sjaastad FV, Jensen IJ, Berton RR, Badovinac VP, and Griffith TS. 2020. Inducing Experimental Polymicrobial Sepsis by Cecal Ligation and Puncture. Current Protocols in Immunology 131: e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moioffer SJ, Danahy DB, van de Wall S, Jensen IJ, Sjaastad FV, Anthony SM, Harty JT, Griffith TS, and Badovinac VP. 2021. Severity of Sepsis Determines the Degree of Impairment Observed in Circulatory and Tissue-Resident Memory CD8 T Cell Populations. The Journal of Immunology 207: 1871–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu J, Sun K, Yang H, Fan D, Huang H, Hong Y, Wu S, Zhou H, Fang F, Li Y, Meng L, Huang J, and Bai Z. 2021. Sepsis Inflammation Impairs the Generation of Functional Dendritic Cells by Targeting Their Progenitors. Frontiers in Immunology 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grimaldi D, Louis S, Pène F, Sirgo G, Rousseau C, Claessens YE, Vimeux L, Cariou A, Mira JP, Hosmalin A, and Chiche JD. 2011. Profound and persistent decrease of circulating dendritic cells is associated with ICU-acquired infection in patients with septic shock. Intensive Care Medicine 37: 1438–1446. [DOI] [PubMed] [Google Scholar]
- 26.Flohé SB, Agrawal H, Schmitz D, Gertz M, Flohé S, and Schade FU. 2005. Dendritic cells during polymicrobial sepsis rapidly mature but fail to initiate a protective Th1-type immune response. Journal of Leukocyte Biology 79: 473–481. [DOI] [PubMed] [Google Scholar]
- 27.Benjamim CF, Lundy SK, Lukacs NW, Hogaboam CM, and Kunkel SL. 2005. Reversal of long-term sepsis-induced immunosuppression by dendritic cells. Blood 105: 3588–3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wen H, Hogaboam CM, Gauldie J, and Kunkel SL. 2006. Severe Sepsis Exacerbates Cell-Mediated Immunity in the Lung Due to an Altered Dendritic Cell Cytokine Profile. The American Journal of Pathology 168: 1940–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strother RK, Danahy DB, Kotov DI, Kucaba TA, Zacharias ZR, Griffith TS, Legge KL, and Badovinac VP. 2016. Polymicrobial Sepsis Diminishes Dendritic Cell Numbers and Function Directly Contributing to Impaired Primary CD8 T Cell Responses In Vivo. The Journal of Immunology 197: 4301–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wen H, Dou Y, Hogaboam CM, and Kunkel SL. 2008. Epigenetic regulation of dendritic cell–derived interleukin-12 facilitates immunosuppression after a severe innate immune response. Blood 111: 1797–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li CC, Munitic I, Mittelstadt PR, Castro E, and Ashwell JD. 2015. Suppression of Dendritic Cell-Derived IL-12 by Endogenous Glucocorticoids Is Protective in LPS-Induced Sepsis. PLOS Biology 13: e1002269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pastille E, Didovic S, Brauckmann D, Rani M, Agrawal H, Schade FU, Zhang Y, and Flohé SB. 2011. Modulation of Dendritic Cell Differentiation in the Bone Marrow Mediates Sustained Immunosuppression after Polymicrobial Sepsis. The Journal of Immunology 186: 977–986. [DOI] [PubMed] [Google Scholar]
- 33.Antoni A-C, Pylaeva E, Budeus B, Jablonska J, Klein-Hitpaß L, Dudda M, and Flohé SB. 2022. TLR2-induced CD8+ T-cell deactivation shapes dendritic cell differentiation in the bone marrow during sepsis. Frontiers in Immunology 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Condotta SA, Rai D, James BR, Griffith TS, and Badovinac VP. 2013. Sustained and Incomplete Recovery of Naive CD8+ T Cell Precursors after Sepsis Contributes to Impaired CD8+ T Cell Responses to Infection. The Journal of Immunology 190: 1991–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haluszczak C, Akue AD, Hamilton SE, Johnson LDS, Pujanauski L, Teodorovic L, Jameson SC, and Kedl RM. 2009. The antigen-specific CD8+ T cell repertoire in unimmunized mice includes memory phenotype cells bearing markers of homeostatic expansion. Journal of Experimental Medicine 206: 435–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thiele D, La Gruta NL, Nguyen A, and Hussain T. 2020. Hiding in Plain Sight: Virtually Unrecognizable Memory Phenotype CD8+ T cells. International Journal of Molecular Sciences 21: 8626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee J-Y, Hamilton SE, Akue AD, Hogquist KA, and Jameson SC. 2013. Virtual memory CD8 T cells display unique functional properties. Proceedings of the National Academy of Sciences 110: 13498–13503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hou S, Shao T, Mao T, Shi J, Sun J, Mei M, Tan X, and Qi H. 2021. Virtual memory T cells orchestrate extralymphoid responses conducive to resident memory. Science Immunology 6: eabg9433. [DOI] [PubMed] [Google Scholar]
- 39.Renkema KR, Li G, Wu A, Smithey MJ, and Nikolich-Žugich J. 2014. Two Separate Defects Affecting True Naive or Virtual Memory T Cell Precursors Combine To Reduce Naive T Cell Responses with Aging. The Journal of Immunology 192: 151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quinn KM, Fox A, Harland KL, Russ BE, Li J, Nguyen THO, Loh L, Olshanksy M, Naeem H, Tsyganov K, Wiede F, Webster R, Blyth C, Sng XYX, Tiganis T, Powell D, Doherty PC, Turner SJ, Kedzierska K, and La Gruta NL. 2018. Age-Related Decline in Primary CD8+ T Cell Responses Is Associated with the Development of Senescence in Virtual Memory CD8+ T Cells. Cell Reports 23: 3512–3524. [DOI] [PubMed] [Google Scholar]
- 41.Heidarian M, Griffith TS, and Badovinac VP. 2023. Sepsis-induced changes in differentiation, maintenance, and function of memory CD8 T cell subsets. Frontiers in Immunology 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang H, Qin S, Li Z, Gao W, Tang M, and Dong X. 2023. Early immune system alterations in patients with septic shock. Frontiers in Immunology 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Venet F, Pachot A, Debard A-L, Bohé J, Bienvenu J, Lepape A, and Monneret G. 2004. Increased percentage of CD4+CD25+ regulatory T cells during septic shock is due to the decrease of CD4+CD25− lymphocytes. Critical Care Medicine 32: 2329–2331. [DOI] [PubMed] [Google Scholar]
- 44.Roth G, Moser B, Krenn C, Brunner M, Haisjackl M, Almer G, Gerlitz S, Wolner E, Boltz-Nitulescu G, and Ankersmit HJ. 2003. Susceptibility to programmed cell death in T-lymphocytes from septic patients: a mechanism for lymphopenia and Th2 predominance. Biochemical and Biophysical Research Communications 308: 840–846. [DOI] [PubMed] [Google Scholar]
- 45.Heidecke C-D, Hensler T, Weighardt H, Zantl N, Wagner H, Siewert J-R, and Holzmann B. 1999. Selective defects of T lymphocyte function in patients with lethal intraabdominal infection. The American Journal of Surgery 178: 288–292. [DOI] [PubMed] [Google Scholar]
- 46.De AK, Kodys KM, Pellegrini J, Yeh B, Furse RK, Bankey P, and Miller-Graziano CL. 2000. Induction of Global Anergy Rather Than Inhibitory Th2 Lymphokines Mediates Posttrauma T Cell Immunodepression. Clinical Immunology 96: 52–66. [DOI] [PubMed] [Google Scholar]
- 47.Boomer JS, To K, Chang KC, Takasu O, Osborne DF, Walton AH, Bricker TL, Jarman SD, Kreisel D, Krupnick AS, Srivastava A, Swanson PE, Green JM, and Hotchkiss RS. 2011. Immunosuppression in Patients Who Die of Sepsis and Multiple Organ Failure. JAMA 306: 2594–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Unsinger J, Kazama H, McDonough JS, Griffith TS, Hotchkiss RS, and Ferguson TA. 2010. Sepsis-Induced Apoptosis Leads to Active Suppression of Delayed-Type Hypersensitivity by CD8+ Regulatory T Cells through a TRAIL-Dependent Mechanism. The Journal of Immunology 184: 6766–6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shubin NJ, Monaghan SF, Heffernan DS, Chung C-S, and Ayala A. 2013. B and T lymphocyte attenuator expression on CD4+ T-cells associates with sepsis and subsequent infections in ICU patients. Critical Care 17: R276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen C. w., Mittal R, Klingensmith NJ, Burd EM, Terhorst C, Martin GS, Coopersmith CM, and Ford ML. 2017. Cutting Edge: 2B4-Mediated Coinhibition of CD4+ T Cells Underlies Mortality in Experimental Sepsis. The Journal of Immunology 199: 1961–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Y, Li J, Lou J, Zhou Y, Bo L, Zhu J, Zhu K, Wan X, Cai Z, and Deng X. 2011. Upregulation of programmed death-1 on T cells and programmed death ligand-1 on monocytes in septic shock patients. Critical Care 15: R70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guignant C, Lepape A, Huang X, Kherouf H, Denis L, Poitevin F, Malcus C, Chéron A, Allaouchiche B, Gueyffier F, Ayala A, Monneret G, and Venet F. 2011. Programmed death-1 levels correlate with increased mortality, nosocomial infection and immune dysfunctions in septic shock patients. Critical Care 15: R99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang X, Venet F, Wang YL, Lepape A, Yuan Z, Chen Y, Swan R, Kherouf H, Monneret G, Chung C-S, and Ayala A. 2009. PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proceedings of the National Academy of Sciences 106: 6303–6308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shubin NJ, Chung CS, Heffernan DS, Irwin LR, Monaghan SF, and Ayala A. 2012. BTLA expression contributes to septic morbidity and mortality by inducing innate inflammatory cell dysfunction. Journal of Leukocyte Biology 92: 593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nascimento DC, Melo PH, Piñeros AR, Ferreira RG, Colón DF, Donate PB, Castanheira FV, Gozzi A, Czaikoski PG, Niedbala W, Borges MC, Zamboni DS, Liew FY, Cunha FQ, and Alves-Filho JC. 2017. IL-33 contributes to sepsis-induced long-term immunosuppression by expanding the regulatory T cell population. Nature Communications 8: 14919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Monneret G, Debard A-L, Venet F, Bohe J, Hequet O, Bienvenu J, and Lepape A. 2003. Marked elevation of human circulating CD4+CD25+ regulatory T cells in sepsis-induced immunoparalysis. Critical Care Medicine 31. [DOI] [PubMed] [Google Scholar]
- 57.Sjaastad FV, Condotta SA, Kotov JA, Pape KA, Dail C, Danahy DB, Kucaba TA, Tygrett LT, Murphy KA, Cabrera-Perez J, Waldschmidt TJ, Badovinac VP, and Griffith TS. 2018. Polymicrobial Sepsis Chronic Immunoparalysis Is Defined by Diminished Ag-Specific T Cell-Dependent B Cell Responses. Frontiers in Immunology 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martin MD, Badovinac VP, and Griffith TS. 2020. CD4 T Cell Responses and the Sepsis-Induced Immunoparalysis State. Frontiers in Immunology 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shankar-Hari M, Fear D, Lavender P, Mare T, Beale R, Swanson C, Singer M, and Spencer J. 2017. Activation-Associated Accelerated Apoptosis of Memory B Cells in Critically Ill Patients With Sepsis. Critical Care Medicine 45. [DOI] [PubMed] [Google Scholar]
- 60.Gustave C-A, Gossez M, Demaret J, Rimmelé T, Lepape A, Malcus C, Poitevin-Later F, Jallades L, Textoris J, Monneret G, and Venet F. 2018. Septic Shock Shapes B Cell Response toward an Exhausted-like/Immunoregulatory Profile in Patients. The Journal of Immunology 200: 2418–2425. [DOI] [PubMed] [Google Scholar]
- 61.Mohr A, Polz J, Martin EM, Grießl S, Kammler A, Pötschke C, Lechner A, Bröker BM, Mostböck S, and Männel DN. 2012. Sepsis leads to a reduced antigen-specific primary antibody response. European Journal of Immunology 42: 341–352. [DOI] [PubMed] [Google Scholar]
- 62.Brinkhoff A, Zeng Y, Sieberichs A, Dolff S, Shilei X, Sun M, Engler H, Benson S, Korth J, Schedlowski M, Kribben A, Witzke O, and Wilde B. 2019. B-cell dynamics during experimental endotoxemia in humans. Bioscience Reports 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pötschke C, Kessler W, Maier S, Heidecke C-D, and Bröker BM. 2013. Experimental Sepsis Impairs Humoral Memory in Mice. PLOS ONE 8: e81752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Monserrat J, de Pablo R, Diaz-Martín D, Rodríguez-Zapata M, de la Hera A, Prieto A, and Alvarez-Mon M. 2013. Early alterations of B cells in patients with septic shock. Critical Care 17: R105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jensen IJ, Winborn CS, Fosdick MG, Shao P, Tremblay MM, Shan Q, Tripathy SK, Snyder CM, Xue H-H, Griffith TS, Houtman JC, and Badovinac VP. 2018. Polymicrobial sepsis influences NK-cell-mediated immunity by diminishing NK-cell-intrinsic receptor-mediated effector responses to viral ligands or infections. PLOS Pathogens 14: e1007405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Souza-Fonseca-Guimaraes F, Parlato M, Fitting C, Cavaillon J-M, and Adib-Conquy M. 2012. NK Cell Tolerance to TLR Agonists Mediated by Regulatory T Cells after Polymicrobial Sepsis. The Journal of Immunology 188: 5850–5858. [DOI] [PubMed] [Google Scholar]
- 67.Forel J-M, Chiche L, Thomas G, Mancini J, Farnarier C, Cognet C, Guervilly C, Daumas A, Vély F, Xéridat F, Vivier E, and Papazian L. 2012. Phenotype and Functions of Natural Killer Cells in Critically-Ill Septic Patients. PLOS ONE 7: e50446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang W, Anyalebechi JC, Ramonell KM, Chen C.-w., Xie J, Liang Z, Chihade DB, Otani S, Coopersmith CM, and Ford ML. 2021. TIGIT modulates sepsis-induced immune dysregulation in mice with preexisting malignancy. JCI Insight 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jiang W, Li X, Wen M, Liu X, Wang K, Wang Q, Li Y, Zhou M, Liu M, Hu B, and Zeng H. 2020. Increased percentage of PD-L1+ natural killer cells predicts poor prognosis in sepsis patients: a prospective observational cohort study. Critical Care 24: 617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jensen IJ, McGonagill PW, Butler NS, Harty JT, Griffith TS, and Badovinac VP. 2021. NK Cell–Derived IL-10 Supports Host Survival during Sepsis. The Journal of Immunology 206: 1171–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yeung ST, Ovando LJ, Russo AJ, Rathinam VA, and Khanna KM. 2023. CD169+ macrophage intrinsic IL-10 production regulates immune homeostasis during sepsis. Cell Reports 42: 112171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Masopust D, Sivula CP, and Jameson SC. 2017. Of Mice, Dirty Mice, and Men: Using Mice To Understand Human Immunology. The Journal of Immunology 199: 383–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mestas J, and Hughes CCW. 2004. Of Mice and Not Men: Differences between Mouse and Human Immunology. The Journal of Immunology 172: 2731–2738. [DOI] [PubMed] [Google Scholar]
- 74.Beura LK, Hamilton SE, Bi K, Schenkel JM, Odumade OA, Casey KA, Thompson EA, Fraser KA, Rosato PC, Filali-Mouhim A, Sekaly RP, Jenkins MK, Vezys V, Haining WN, Jameson SC, and Masopust D. 2016. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature 532: 512–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huggins MA, Jameson SC, and Hamilton SE. 2018. Embracing microbial exposure in mouse research. Journal of Leukocyte Biology 105: 73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fay EJ, Balla KM, Roach SN, Shepherd FK, Putri DS, Wiggen TD, Goldstein SA, Pierson MJ, Ferris MT, Thefaine CE, Tucker A, Salnikov M, Cortez V, Compton SR, Kotenko SV, Hunter RC, Masopust D, Elde NC, and Langlois RA. 2021. Natural rodent model of viral transmission reveals biological features of virus population dynamics. Journal of Experimental Medicine 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Labuda JC, Fong KD, and McSorley SJ. 2022. Cohousing with Dirty Mice Increases the Frequency of Memory T Cells and Has Variable Effects on Intracellular Bacterial Infection. ImmunoHorizons 6: 184–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Arnesen H, Knutsen LE, Hognestad BW, Johansen GM, Bemark M, Pabst O, Storset AK, and Boysen P. 2021. A Model System for Feralizing Laboratory Mice in Large Farmyard-Like Pens. Frontiers in Microbiology 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reese Tiffany A., Bi K, Kambal A, Filali-Mouhim A, Beura Lalit K., Bürger Matheus C., Pulendran B, Sekaly R-P, Jameson Stephen C., Masopust D, Haining WN, and Virgin Herbert W.. 2016. Sequential Infection with Common Pathogens Promotes Human-like Immune Gene Expression and Altered Vaccine Response. Cell Host & Microbe 19: 713–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Berton RR, Jensen IJ, Harty JT, Griffith TS, and Badovinac VP. 2022. Inflammation Controls Susceptibility of Immune-Experienced Mice to Sepsis. ImmunoHorizons 6: 528–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huggins MA, Sjaastad FV, Pierson M, Kucaba TA, Swanson W, Staley C, Weingarden AR, Jensen IJ, Danahy DB, Badovinac VP, Jameson SC, Vezys V, Masopust D, Khoruts A, Griffith TS, and Hamilton SE. 2019. Microbial Exposure Enhances Immunity to Pathogens Recognized by TLR2 but Increases Susceptibility to Cytokine Storm through TLR4 Sensitization. Cell Reports 28: 1729–1743.e1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sun Y, Anyalebechi JC, Sun H, Yumoto T, Xue M, Liu D, Liang Z, Coopersmith CM, and Ford ML. 2021. Anti-TIGIT differentially affects sepsis survival in immunologically experienced versus previously naive hosts. JCI Insight 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rhee C, Jones TM, Hamad Y, Pande A, Varon J, O’Brien C, Anderson DJ, Warren DK, Dantes RB, Epstein L, Klompas M, f. t. C. f. D. Control, and P. P. E. Program. 2019. Prevalence, Underlying Causes, and Preventability of Sepsis-Associated Mortality in US Acute Care Hospitals. JAMA Network Open 2: e187571–e187571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lewis ED, Williams HC, Bruno MEC, Stromberg AJ, Saito H, Johnson LA, and Starr ME. 2022. Exploring the Obesity Paradox in A Murine Model of Sepsis: Improved Survival Despite Increased Organ Injury in Obese Mice. Shock 57: 151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yeo HJ, Kim TH, Jang JH, Jeon K, Oh DK, Park MH, Lim C-M, Kim K, Cho WH, and o. b. o. t. K. S. A. investigators. 2023. Obesity Paradox and Functional Outcomes in Sepsis: A Multicenter Prospective Study. Critical Care Medicine: 10.1097/CCM.0000000000005801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Robinson J, Swift-Scanlan T, and Salyer J. 2020. Obesity and 1-Year Mortality in Adults After Sepsis: A Systematic Review. Biological Research For Nursing 22: 103–113. [DOI] [PubMed] [Google Scholar]
- 87.Sathananthan M, Sathananthan A, and Jeganathan N. 2020. Characteristics and Outcomes of Patients With and Without Type 2 Diabetes Mellitus and Pulmonary Sepsis. Journal of Intensive Care Medicine 35: 836–843. [DOI] [PubMed] [Google Scholar]
- 88.Jia Y, Zhao Y, Li C, and Shao R. 2016. The Expression of Programmed Death-1 on CD4+ and CD8+ T Lymphocytes in Patients with Type 2 Diabetes and Severe Sepsis. PLOS ONE 11: e0159383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Esper AM, Moss M, and Martin GS. 2009. The effect of diabetes mellitus on organ dysfunction with sepsis: an epidemiological study. Critical Care 13: R18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Insuela DBR, Ferrero MR, Gonçalves-de-Albuquerque CF, Chaves A. d. S., da Silva AYO, Castro-Faria-Neto HC, Simões RL, Barja-Fidalgo TC, Silva P. M. R. e., Martins MA, Silva AR, and Carvalho VF. 2021. Glucagon Reduces Neutrophil Migration and Increases Susceptibility to Sepsis in Diabetic Mice. Frontiers in Immunology 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.de Souza Stork S, Hübner M, Biehl E, Danielski LG, Bonfante S, Joaquim L, Denicol T, Cidreira T, Pacheco A, Bagio E, Lanzzarin E, Bernades G, de Oliveira MP, da Silva LE, Mack JM, Bobinski F, Rezin GT, Barichello T, Streck EL, and Petronilho F. 2022. Diabetes Exacerbates Sepsis-Induced Neuroinflammation and Brain Mitochondrial Dysfunction. Inflammation 45: 2352–2367. [DOI] [PubMed] [Google Scholar]
- 92.Sinapidis D, Kosmas V, Vittoros V, Koutelidakis IM, Pantazi A, Stefos A, Katsaros KE, Akinosoglou K, Bristianou M, Toutouzas K, Chrisofos M, and Giamarellos-Bourboulis EJ. 2018. Progression into sepsis: an individualized process varying by the interaction of comorbidities with the underlying infection. BMC Infectious Diseases 18: 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Manadan AM, Kambhatla S, Gauto-Mariotti E, Okoli C, and Block JA. 2020. Reasons for Hospitalization and In-Hospital Mortality in Adult Systemic Lupus Erythematosus. ACR Open Rheumatology 2: 683–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Maksimowicz-McKinnon K, Zhou J, Hudy J, Hegab S, and McKinnon JE. 2021. Subclinical CMV viremia is associated with increased nosocomial infections and prolonged hospitalization in patients with systemic autoimmune diseases. Journal of Clinical Virology 140: 104849. [DOI] [PubMed] [Google Scholar]
- 95.Pöllinger B, Krishnamoorthy G, Berer K, Lassmann H, Bösl MR, Dunn R, Domingues HS, Holz A, Kurschus FC, and Wekerle H. 2009. Spontaneous relapsing-remitting EAE in the SJL/J mouse: MOG-reactive transgenic T cells recruit endogenous MOG-specific B cells. Journal of Experimental Medicine 206: 1303–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jensen IJ, Jensen SN, McGonagill PW, Griffith TS, Mangalam AK, and Badovinac VP. 2021. Autoimmunity Increases Susceptibility to and Mortality from Sepsis. ImmunoHorizons 5: 844–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ernst FR, Pocoski J, Cutter G, Kaufman DW, and Pleimes D. 2016. Analysis of Diagnoses Associated with Multiple Sclerosis–Related In-Hospital Mortality Using the Premier Hospital Database. International Journal of MS Care 18: 154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Oud L, and Garza J. 2022. The burden of sepsis in critically ill patients with multiple sclerosis: A population-based cohort study. Journal of Critical Care 69: 153985. [DOI] [PubMed] [Google Scholar]
- 99.Ohtani S, Kohyama K, and Matsumoto Y. 2011. Autoantibodies recognizing native MOG are closely associated with active demyelination but not with neuroinflammation in chronic EAE. Neuropathology 31: 101–111. [DOI] [PubMed] [Google Scholar]
- 100.Höftberger R, Lassmann H, Berger T, and Reindl M. 2022. Pathogenic autoantibodies in multiple sclerosis — from a simple idea to a complex concept. Nature Reviews Neurology 18: 681–688. [DOI] [PubMed] [Google Scholar]
- 101.Klineova S, and Lublin FD. 2018. Clinical Course of Multiple Sclerosis. Cold Spring Harbor Perspectives in Medicine 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Freedman SN, Shahi SK, and Mangalam AK. 2018. The “Gut Feeling”: Breaking Down the Role of Gut Microbiome in Multiple Sclerosis. Neurotherapeutics 15: 109–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mangalam A, Shahi SK, Luckey D, Karau M, Marietta E, Luo N, Choung RS, Ju J, Sompallae R, Gibson-Corley K, Patel R, Rodriguez M, David C, Taneja V, and Murray J. 2017. Human Gut-Derived Commensal Bacteria Suppress CNS Inflammatory and Demyelinating Disease. Cell Reports 20: 1269–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fay KT, Klingensmith NJ, Chen C-W, Zhang W, Sun Y, Morrow KN, Liang Z, Burd EM, Ford ML, and Coopersmith CM. 2019. The gut microbiome alters immunophenotype and survival from sepsis. The FASEB Journal 33: 11258–11269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shahi SK, Ghimire S, Lehman P, and Mangalam AK. 2022. Obesity induced gut dysbiosis contributes to disease severity in an animal model of multiple sclerosis. Frontiers in Immunology 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wilmore JR, Gaudette BT, Gomez Atria D, Hashemi T, Jones DD, Gardner CA, Cole SD, Misic AM, Beiting DP, and Allman D. 2018. Commensal Microbes Induce Serum IgA Responses that Protect against Polymicrobial Sepsis. Cell Host & Microbe 23: 302–311.e303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Van de Louw A, Cohrs A, and Leslie D. 2021. Incidence of sepsis and associated mortality within the first year after cancer diagnosis in middle aged adults: A US population based study. PLOS ONE 15: e0243449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hensley MK, Donnelly JP, Carlton EF, and Prescott HC. 2019. Epidemiology and Outcomes of Cancer-Related Versus Non–Cancer-Related Sepsis Hospitalizations*. Critical Care Medicine 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vigneron C, Mirouse A, Merdji H, Rousseau C, Cousin C, Alby-Laurent F, Mira J-P, Chiche J-D, Llitjos J-F, and Pène F. 2019. Sepsis inhibits tumor growth in mice with cancer through Toll-like receptor 4-associated enhanced Natural Killer cell activity. OncoImmunology 8: e1641391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Danahy DB, Jensen IJ, Griffith TS, and Badovinac VP. 2019. Cutting Edge: Polymicrobial Sepsis Has the Capacity to Reinvigorate Tumor-Infiltrating CD8 T Cells and Prolong Host Survival. The Journal of Immunology 202: 2843–2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Danahy DB, Kurup SP, Winborn CS, Jensen IJ, Harty JT, Griffith TS, and Badovinac VP. 2019. Sepsis-Induced State of Immunoparalysis Is Defined by Diminished CD8 T Cell–Mediated Antitumor Immunity. The Journal of Immunology 203: 725–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen C. w., Bennion KB, Swift DA, Morrow KN, Zhang W, Oami T, Coopersmith CM, and Ford ML. 2021. Tumor-Specific T Cells Exacerbate Mortality and Immune Dysregulation during Sepsis. The Journal of Immunology 206: 2412–2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lyons JD, Chen C-W, Liang Z, Zhang W, Chihade DB, Burd EM, Farris AB, Ford ML, and Coopersmith CM. 2019. Murine Pancreatic Cancer Alters T Cell Activation and Apoptosis and Worsens Survival After Cecal Ligation and Puncture. Shock 51: 731–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen C. w., Xue M, Zhang W, Xie J, Coopersmith CM, and Ford ML. 2019. 2B4 but not PD-1 blockade improves mortality in septic animals with preexisting malignancy. JCI Insight 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jensen IJ, Jensen SN, Sjaastad FV, Gibson-Corley KN, Dileepan T, Griffith TS, Mangalam AK, and Badovinac VP. 2020. Sepsis impedes EAE disease development and diminishes autoantigen-specific naive CD4 T cells. eLife 9: e55800. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.