Abstract
Background:
Opioid overprescribing after surgery is common. There is currently no universal predictive tool available to accurately anticipate post-discharge opioid need in a patient-specific manner. This study examined the efficacy of a patient-specific opioid prescribing framework for estimating post-discharge opioid consumption.
Methods:
A total of 149 patients were evaluated for a single-center retrospective cohort study of plastic and reconstructive surgery patients. Patients with length-of-stay of 2–8 days and quantifiable inpatient opioid consumption (n=116) were included. Each patient’s daily postoperative inpatient opioid consumption was used to generate a personalized logarithmic regression model to estimate post-discharge opioid need. The validity of the Personalized Opioid Prescription (POP) model was tested through comparison with actual post-discharge opioid consumption reported by patients 4 weeks after surgery. The accuracy of the POP model was compared with two other opioid prescribing models.
Results:
The POP model had the strongest association (R2 = 0.899, p<0.0001) between model output and post-discharge opioid consumption when compared to a Procedure-Based (R2 = 0.226, p = 0.025) or 24-Hour (R2 = 0.152, p = 0.007) models. Accuracy of the POP model was unaffected by age, gender identity, procedure type, or length-of-stay. Odds of persistent use at 4 weeks increased with post-discharge estimated opioid need at a rate of 1.16 per 37.5 OME (p=0.010, 95% CI 1.04–1.30).
Conclusions:
The POP model accurately estimates post-discharge opioid consumption and risk of developing persistent use in plastic surgery patients. Use of the POP model in clinical practice may lead to more appropriate and personalized opioid prescribing.
Introduction
Opioid dependency has been declared an epidemic in the United States,1,2 and prescriber behavior is an important consideration. Patients who are prescribed opioids have an increased risk of opioid use disorder and overdose death, with higher, longer-acting doses conferring higher risk.3–6 In addition, opioids prescribed in excess pose a risk of opioid diversion, or medication consumption by someone other than the intended recipient.1,7 In a 2019 national survey, over half of individuals who used opioids illicitly obtained the opioids from friends or family members with legitimate prescriptions.1 Among surgical patients, opioid prescribing poses a well-documented risk for new persistent use in previously opioid naïve patients.8–17 In plastic surgery, specifically, 6 to 13% of postoperative patients develop new persistent use.18–21 The risks associated with opioid prescribing, therefore, warrant more reliable and objective methods to appropriately prescribe opioids after surgery.
Current literature has focused on characterizing over-prescribing,22–29 identifying risk factors for persistent opioid use,8–11,15,16 and reducing opioid consumption after surgery.30–32 For example, Enhanced Recovery After Surgery (ERAS) protocols have been extensively investigated, are now widely implemented, and effectively reduce opioid consumption.33–38 However, few attempts have been made to synthesize these data into a clearly defined global strategy for downstream postoperative opioid prescribing at discharge. Existing prescribing models lack precision since they use aggregate inpatient opioid use patterns or patient risk factors to provide general rules for prescription ranges, rather than tools that are tailored to the individual patient.39–42
We propose a new Personalized Opioid Prescription (POP) model that incorporates patient-specific health data to estimate postoperative opioid need after discharge. The POP model employs a logarithmic regression curve that utilizes a patient’s inpatient opioid use to extrapolate an appropriate opioid prescription amount. We compared the POP model to actual prescription and post-discharge consumption to evaluate its accuracy. We then compared the POP model accuracy to other opioid prescribing models and demonstrated greater accuracy. Lastly, the POP model has the additional capability of quantifying the risk of prolonged postoperative opioid use at the time of discharge.
Methods
Population selection and study design
A retrospective review was performed for plastic surgery patients at an academic medical center between March 2018 and June 2020. Demographic information, procedure type, length-of-stay (LOS), daily inpatient opioid consumption, and post-discharge opioid prescription quantities were collected through chart review. All inpatient opioids were administered by a physician or nurse, and each dose must be recorded in the medical record along with its time of administration. Information regarding prescription opioid use, refills, and previous opioid use was gathered using patient surveys administered at 4 weeks (range 3–5 weeks) postoperatively as part of a previous quality-improvement initiative.
Patients included were required to have complete 4-week postoperative data and have LOS of 2 to 8 days. Patients with LOS beyond 8 days (>75th percentile) were excluded a priori given non-standard hospital courses. Those who did not consume any opioids while inpatient or consumed an indeterminate amount of opioids (e.g. received patient-controlled analgesia (PCA) or fentanyl-containing epidural) were also excluded since a personalized model can only be generated if patients had quantifiable opioid consumption.
Personalized Opioid Prescription (POP) model
For each patient, inpatient opioid consumption in 24-hour intervals following surgery was used as the independent variable for a logarithmic regression formula of the form Y = β*eM*X, where Y is the amount of opioid (OME) consumed on day X and β and M are patient-specific, regression-calculated constants. We prevented exponential overgrowth by limiting M to be less than zero. Since logarithmic regression cannot account for zero values (i.e. ln(0) is undefined), opioid consumption inputs of zero were converted to 0.1. We used this model to estimate daily opioid need for the 7 days post-discharge and summed the daily amounts to find the total estimated opioid need for each patient. Seven days was chosen for prescription quantity summation because Ohio law limits opioid prescribing for acute pain to 30 OME/day for 7 days in adults unless there is documented exception. To evaluate the impact of patient characteristics on model estimates, we performed covariate analyses for age, gender identity, LOS, procedure type, and patient-reported prior opioid use (opioid-naïve vs. opioid non-naïve).
Prescribing models used for comparison
We compared the utility of the POP model to two other pre-existing prescribing models: 1) Procedure-Based model and 2) 24-Hour model. This was done by first identifying the patients who, by chance, were prescribed the amount of opioids that would have been prescribed if each of these models were applied. Then, we evaluated how close to the prescribed amount patients actually reported consuming post-discharge. A range for prescribing accuracy was defined as +/− five 5mg oxycodone tablets (37.5 OME).
The Procedure-Based model was created as part of a previous quality improvement initiative. We tracked average opioid consumption across different procedure types and recommended procedure-specific prescription quantities at discharge based on the average consumption for each procedure type.43
The 24-Hour model described by Hill et al. was based on a retrospective analysis of 234 general surgery cases at a single center. This model assays the number of pills taken in the 24-hour period prior to discharge. No pills were prescribed if no pills were taken; 15 pills were prescribed if 1–3 pills were taken, and 30 pills were prescribed if 4 or more pills were taken.41
Data analysis
Statistical regression analyses were performed using STATA16.0 (Statacorp, LLC) and GraphPad Prism version 6.00 (GraphPad Software). Linear regression analysis was performed to evaluate the following data sets: 1) comparing prescription, inpatient, and post-discharge opioid amounts, 2) comparing the POP model estimated opioid need amount and actual prescribed and post-discharge consumed amount, 3) comparing post-discharge consumption to POP estimated opioid need in patients underprescribed, appropriately prescribed, or overprescribed per model, and 4) comparing accuracy of the POP model to those of two other prescription models. Under, appropriate, and overprescribing was determined by comparing actual prescribed amount to POP estimated opioid need.
To evaluate potential confounding factors, a multiple linear regression analysis was performed using remaining OME as the dependent variable and POP model-estimated opioids remaining, gender identity, age, LOS, and previous opioid use as the independent variables. Procedure type was separately examined as a potential confounder with procedure type as a categorical variable. Models containing varying subsets of covariates were compared using likelihood ratio tests. A p-value of 0.05 was used to determine statistical significance throughout.
Finally, we evaluated the utility of the POP model for quantifying risk of persistent opioid use through logistic regression analysis with patient-reported persistent use at 4 weeks as the dependent variable and POP model estimated opioid need and previous opioid use as independent variables.
Results
Study Population
Of 642 patients with available post-discharge opioid consumption data, 149 patients had an inpatient stay between 2–8 days. From this, 19 patients who did not consume opioids while inpatient and 14 patients who consumed an indeterminate amount of opioids during their inpatient stay were excluded (Figure 1). The final cohort consisted of 116 patients with a mean age of 53 ± 12.8 (range 20–91; Table 1). Patients identifying as female represented 70.7% of the cohort (n=82; Table 1). Twenty-one patients (18.1%) reported opioid use prior to their operation (Table 1).
Figure 1.
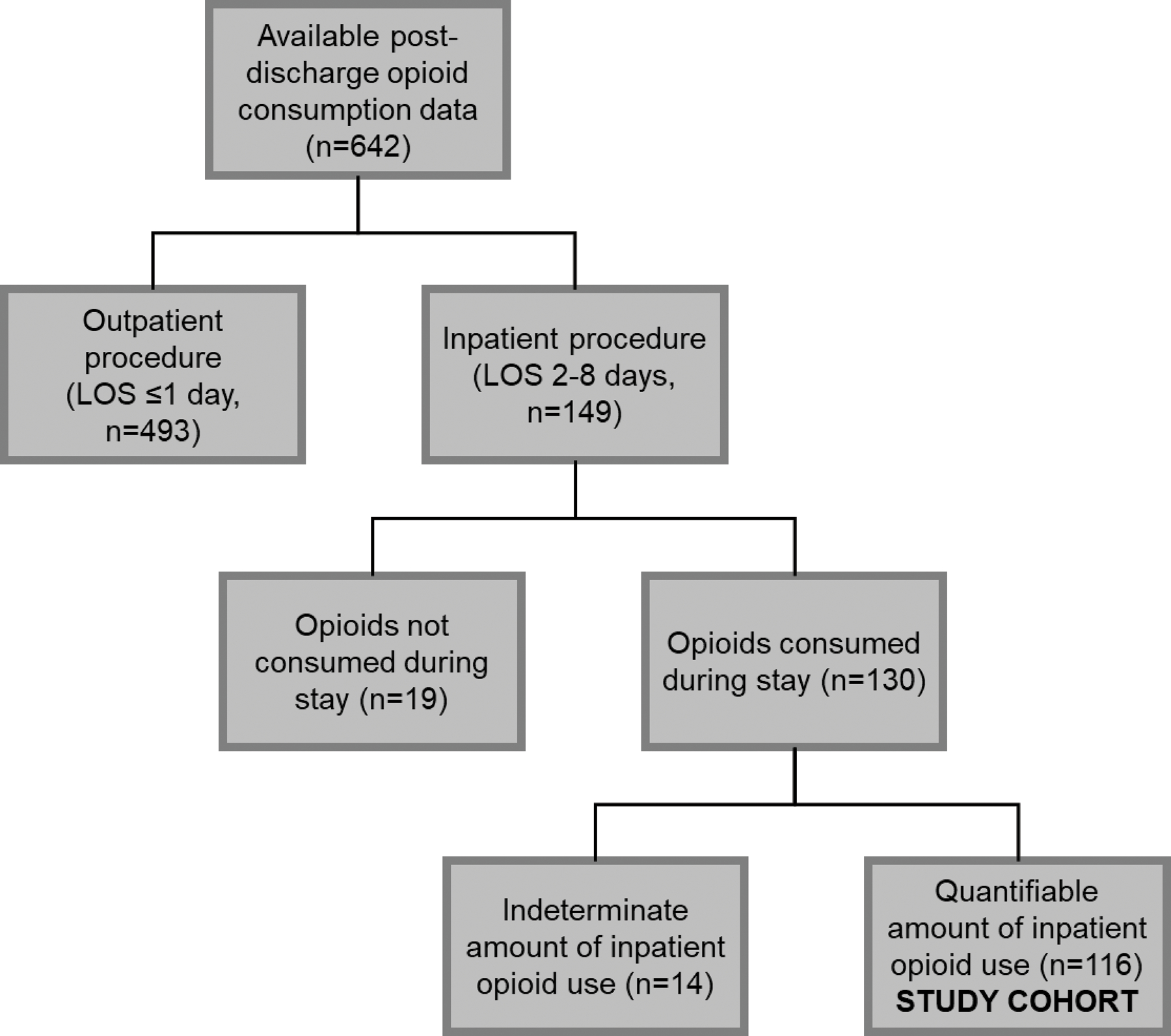
Of patients with complete 4-week post-discharge opioid consumption data (n=642), 149 patients had an inpatient stay lasting between 2–8 days (23.2%). Patients were subsequently evaluated for inpatient opioid consumption; those who did not consume opioids as an inpatient (n=19; 12.8%) or consumed an unquantifiable amount through the use of patient-controlled analgesic devices and/or epidural fentanyl (n=14; 10.8%) were excluded from this study. LOS, length of stay.
Table 1.
Demographics of Study Cohort
| Demographic and Clinical Characteristics of Study Cohort (n=116) | ||
|---|---|---|
| Characteristic | No. | % |
| Gender | ||
| Males | 34 | 29.3 |
| Females | 82 | 70.7 |
|
| ||
| Age, years (±SD) | ||
| Mean | 53.0 ± 12.8 | |
| Median | 54 | |
| Range | 20–91 | |
| Males | 54.9 ± 13.0 | |
| Females | 52.2 ± 12.7 | |
|
| ||
| Prior Opioid Use | ||
| Opioid Naive | 95 | 81.9 |
| Previous Opioid Use | 21 | 18.1 |
|
| ||
| Procedural Category | ||
| -Delayed breast reconstruction, free flap, with or without oncoplastic reduction/mastopexy | 23 | 19.8 |
| -Immediate breast reconstruction, free flap, with or without oncoplastic reduction/mastopexy | 19 | 16.4 |
| -Debridements for chronic wounds, hematomas, necrosis, seromas | 13 | 11.2 |
| -Lower or upper extremity reconstruction, local/regional flap | 12 | 10.3 |
| -Panniculectomy or abdominoplasty | 10 | 8.6 |
| -Immediate breast reconstruction, Implants/tissue expanders, with or without oncoplastic reduction/mastopexy | 5 | 4.3 |
| -Amputation with targeted muscle reinnervation | 5 | 4.3 |
| -Abdominal wall reconstruction only | 4 | 3.5 |
| -Trunk/pelvic local flaps | 4 | 3.5 |
| -Skin graft only | 3 | 2.6 |
| -Craniofacial reconstruction | 3 | 2.6 |
| -Targeted muscle reinnervation only | 3 | 2.6 |
| -Breast reconstruction revision procedures (NAR, liposuction, fat grafting, dog ear revision, scar revision, mastopexy, capsulectomy, capsulotomy, implant exchange) | 2 | 1.7 |
| -Spine closure/reconstruction | 2 | 1.7 |
| -Excision skin/soft tissue lesions/masses with reconstruction | 2 | 1.7 |
| -Delayed breast reconstruction, implants/tissue expanders, with or without oncoplastic reduction/mastopexy | 1 | 0.9 |
| -Breast reduction or mastopexy only, non-oncoplastic | 1 | 0.9 |
| -Lower or upper extremity reconstruction, Free flap | 1 | 0.9 |
| -Sternal wound reconstruction | 1 | 0.9 |
| -Vascularized lymph node transfers | 1 | 0.9 |
| -Osteocutaneous Free Fibula | 1 | 0.9 |
Associations between opioid prescribing and consumption
We first examined current opioid prescribing practices and their relationships to inpatient and post-discharge opioid consumption patterns within our patient cohort. In our current practice, post-discharge opioid prescription amount was not associated with inpatient opioid consumption (R2=0.006, p=0.394) or LOS (R2=0.002, p=0.608; Figure 2A and 2B). These findings indicate that pre-existing prescribing behaviors were not guided by these factors. However, there was a positive association (R2=0.214, β=0.411±0.074, p<0.001) between post-discharge opioid prescription and post-discharge opioid consumption, indicating the possibility that increased prescription quantities resulted in increased post-discharge consumption (Figure 2C). In addition, there was a positive association between inpatient opioid consumption and post-discharge opioid consumption (R2=0.106, β=0.374±0.102, p<0.001), indicating that inpatient opioid consumption can potentially be used to estimate post-discharge opioid need (Figure 2D).
Figure 2.

Comparison of inpatient and post-discharge opioid consumption with LOS and post-discharge opioid prescription quantity. No associations were found between A) inpatient opioid consumption and post-discharge opioid prescription or B) length of inpatient stay and post-discharge opioid prescription. However, a positive association was found between C) post-discharge opioid prescription and post-discharge opioid consumption and D) inpatient opioid consumption and post-discharge opioid consumption.
Analysis of the POP model
The POP model is a patient-specific logarithmic regression model that uses each patient’s daily inpatient opioid consumption amount to extrapolate an appropriate prescription amount for that patient (example shown in Figure 3A). After calculating an estimated opioid need with the POP model, we tested the accuracy of the estimated opioid need by comparing it to actual opioid prescribing and consumption patterns. First, we used the POP model to categorize patients into “over-prescribed per model” or “under-prescribed per model” based on their actual post-discharge opioid prescription quantity. Based on the model, most patients were substantially over-prescribed (78/116, 67.2%) or under-prescribed (23/116, 19.8%), and there was no association between the POP model estimates and the actual opioid prescription amount in current practice (R2=0.009, p=0.307; Figure 3B).
Figure 3.

Use of the POP regression model to measure current post-discharge over-prescribing and under-prescribing. A) An example schematic of the POP regression model. The POP regression model assumes that post-discharge opioid consumption decreases exponentially. Each patient’s daily inpatient opioid consumption is used to generate a personalized logarithmic regression formula. This formula is then used to estimate the amount of opioids this patient will need after discharge. B) There was no association between estimated post-discharge opioid need and actual prescription amount that patients historically received. C) The estimated amount that was over- or under-prescribed based on the model was compared with the actual amount of patient-reported excess opioid medication at 4 weeks postoperatively, revealing a strong positive trend (R2= 0.308, p<0.001). D) For under-prescribed per model patients, no association was found between amount prescribed and amount of opioid in excess. E) For overprescribed per model patients, the slope increased to 0.701±0.089, indicating that for every 10 OME prescribed above the estimated opioid need, 3 were consumed and 7 were in excess.
We then compared the predicted amount that was over- or under-prescribed based on the model with the actual amount of patient-reported excess opioid medication at 4 weeks postoperatively, revealing a strong positive trend (R2= 0.308, p<0.001; Figure 3C). The amount over- or under-prescribed per model was calculated by subtracting predicted opioid need from amount prescribed, while amount of excess opioids was calculated by subtracting patient-reported opioid consumption from amount prescribed. Unsurprisingly, patients who were over-prescribed by the POP model had more excess opioid tablets, and patients who were under-prescribed by the POP model had fewer excess opioid tablets.
A linear formula was also analyzed, but this version of the POP model had a weaker association (R2=0.271, p<0.001) than the logarithmic formula (Supplemental Figure 1).
Effect of opioid prescribing behavior on post-discharge opioid consumption behavior
For under-prescribed per model patients, no association was found between amount prescribed and amount of opioid in excess, as expected (R2=0.027, p=0.456; Figure 3D). For overprescribed per model patients, the slope was 0.701±0.089 (R2=0.45, p<0.001), indicating that for every 10 OME prescribed above the predicted opioid need, 3 were consumed and 7 were leftover and/or discarded (Figure 3E). Thus, opioid overprescribing by providers is associated with increased consumption by patients at a ratio of 10:3.
Accuracy of POP model estimates
In our retrospective cohort, we identified patients who, by chance, were actually prescribed within the POP model-specified range (37.5 OME or +/− five 5mg oxycodone tablets) for their estimated prescription need (Figure 4A). For these 15 patients, the POP estimated opioid need was strongly associated with actual post-discharge opioid consumption (R2 = 0.899, p<0.001; Figure 4A).
Figure 4.

Use of POP model to examine post-discharge behavior of accurately prescribed patients. A) The POP model shows a stronger association between post-discharge opioid prescription and post-discharge opioid consumption than the B) Procedure-Based or C) 24-Hour model.
We then examined the accuracy of two other prescribing models—the Procedure-Based model and the 24-Hour model—in estimating post-discharge opioid need. We identified patients in our cohort who were prescribed, by chance, within 37.5 OME of their estimated opioid need based on these two models. In total, 23 patients were accurately prescribed by the Procedure-Based model and 46 by the 24-Hour model. Unlike the POP model, the Procedure-Based model and 24-Hour model estimated opioid need was less associated with post-discharge opioid consumption (Procedure-Based: R2 = 0.226, p=0.025; 24-Hour: R2 = 0.152, p=0.007; Figure 4B and C). Although there was no association between estimated prescription size between the Procedure-Based model and the POP model (R2 = 0.0289, p=0.098; Supplemental Figure 2A), there was association between the 24-Hour model and the POP model (R2 = 0.473, p<0.001; Supplemental Figure 2B).
POP model estimates correlate with persistent use risk
We evaluated patient-reported persistent use at 4 weeks and compared it to the model estimates and patient demographics (age, gender identity, LOS, procedure type, and opioid-naïve status) using logistic regression. The only factors that correlated with persistent use at 4 weeks were non-naïve status and POP model estimated opioid need (Figure 5). Both factors were positively associated with odds of reporting persistent use at 4 weeks. Non-opioid-naïve status conferred an odds ratio of 7.70 (p<0.001, 95% CI 2.57 – 23.04; Figure 5) compared to opioid naïve patients. POP model estimated opioid need conferred an odds ratio of 1.004 per OME, or approximately 1.16 (p=0.010, 95% CI 1.04 – 1.30) per 37.5 OME. This represents a 16% increased risk of persistent opioid use at 4 weeks for every five 5mg oxycodone tablet increase in the estimated opioid need. Nonparametric receiver operating characteristic testing was found to be statistically significant, demonstrating an area under the curve of 0.662 with a standard error of 0.084, correlating with a p-value of 0.027.44
Figure 5.

Odds of reporting persistent opioid use at 4-weeks postoperatively. Opioid non-naïve patients (blue) were more likely to report persistent use than opioid naïve patients (red). Both cohorts had increased odds of reporting persistent use with higher POP model estimated prescription need. On the Y axis, patients with a value of 1.0 reported persistent opioid use at 4 weeks. Patients with a value of 0 did not report persistent opioid use at 4 weeks. POP model estimated opioid need conferred an odds ratio of 1.004 per OME, or approximately 1.16 (p=0.010, 95% CI 1.04 – 1.30) per 37.5 OME, representing a 16% increased risk of persistent opioid use at 4 weeks for every five 5mg oxycodone tablets of estimated need.
Covariate analyses
Procedure type, patient age, LOS, gender identity, and previous opioid use were evaluated as potential variables that could influence model estimates (Supplemental Figure 3). The effects of procedure type (multiple linear regression with categorical variable p=0.210–1.000 depending on procedure type), patient age (p=0.266, LR test p=0.252), and LOS (p=0.164, LR test p=0.154) were determined to be insignificant. Gender identity was found to have a significant effect on the amount of opioids remaining (p=0.027, LR test p=0.024). Compared to women, men received larger opioid prescriptions on average (p=0.026) but consumed a similar amount of opioids (p=0.944), and this discrepancy resulted in a difference in the amount of pills remaining. However, gender identity did not affect POP model predicted need or how patients’ predicted needs compared to actual use (interaction term p value = 0.136; Supplemental Figure 4A). Non-naïve patients were found to consume more opioids on average (p<0.001) while receiving similar prescription sizes as naïve patients (p=0.066); this also did not affect POP model predictive ability of patient use (interaction term p value=0.091; Supplemental Figure 4B).
Discussion
Physician prescribing practices after surgery are an important consideration in the midst of a national opioid epidemic1,2. Despite robust strategies to reduce reliance on opioid-predominant pain management regimens, there has been little progress in translating these successes into a universal strategy for more appropriate and patient-specific opioid prescribing after discharge. The downstream effect of this is substantial overprescribing and highly variable prescribing patterns that are largely based on provider behavior. At our own institution, we have previously demonstrated that 52% of opioid tablets prescribed at discharge go unused for plastic and reconstructive surgery patients, culminating in over 30,000 excess pills per year from a single surgical service.43 In the present study, we demonstrate that historically prescribed opioid quantities were disconnected from potentially relevant clinical characteristics, such as inpatient opioid consumption and LOS. Simultaneously, we found that inpatient opioid consumption was positively associated with post-discharge opioid consumption and could be objectively measured to better estimate prescription quantities. To this end, we hypothesized that a personalized opioid prescribing model that utilizes daily inpatient opioid consumption can accurately estimate post-discharge opioid requirements. The use of this model in clinical practice may reduce opioid overprescribing by tailoring prescription amounts to anticipated need, with the added benefit of identifying patients at risk of new persistent use.
Previously proposed opioid prescribing protocols have relied on aggregate patient data or arbitrary cut-offs to categorize patient opioid requirements. We demonstrate that these models have significantly lower accuracy, posing a greater likelihood of under- or over-prescribing for the individual patient who may have unique and perhaps unmeasurable characteristics or circumstances that influence opioid need. We propose that the best way to anticipate what a patient will need at discharge is to examine the trend of data they have already provided. We determined that the accuracy of the POP model was not influenced by age, gender identity, procedure type, LOS, or preoperative opioid use, supporting the potential for generalizing this model to many surgical patient types. Because the accuracy of the POP model was not impacted by these additional variables, the model stands as a single variable input, significantly improving ease-of-use for the prescriber.
In subsequent analyses, we identified two additional important findings. First, we found that patient opioid consumption behavior was directly related to provider overprescribing behavior. In our study, patients consumed 3 out of 10 OME that was overprescribed above their estimated need. This finding supports other studies that have also found post-discharge opioid consumption to be a function of prescription amount.17,18 In particular, Howard et al. reported a similar relationship of 5 pills consumed for every 10 additionally prescribed for laparoscopic cholecystectomy patients.17 These findings demonstrate the importance of evidence-based opioid stewardship.
Second, we uncovered the utility of the POP model for evaluating risk of persistent opioid use 4 weeks after surgery. Despite the clear link that has been described across numerous surgical specialties between acute postoperative opioid prescriptions and new persistent opioid use in previously opioid naïve patients,8–17 there remains no effective method to preemptively identify patients at risk beyond recognizing a subset of limited predisposing characteristics.12,19,20 Using the POP model, we determined that the odds of persistent use increased by 0.16 for every 37.5 OME of predicted post-discharge need. Therefore, a potential strength of the POP model is its ability to calculate this risk before the patient leaves the hospital. Future prospective work will be conducted to evaluate its predictive value and determine thresholds for identifying high risk patients for early intervention.
Our goal is to integrate the POP model into the electronic health record and this work is presently underway at our institution. This automated, provider-facing tool will generate inpatient opioid consumption trends for clinical interpretation, predicted post-discharge opioid need, and risk scores for new persistent use.
We recognize limitations within this study. In order to create a personalized logarithmic regression formula for a patient, we needed the patient to have at least 2 data points. This means that we were unable to generate a formula for patients who did not consume opioids as an inpatient, who had outpatient procedures, or hospital stays less than 2 days. Additionally, patients with shorter length of stay, and therefore, fewer data points, may have less accurate formulas. Although we did not see a difference in logarithmic regression accuracy for patients with a two-day inpatient stay versus a longer stay, the limited number of patients available for this analysis limits the power of that investigation. This exclusion criterion also means that a large portion of plastic surgery patients were excluded in our study; however, other surgical specialties with fewer outpatient or observational procedures may not be as significantly affected. Work is underway to investigate the utility of an adapted model with single data point input or shorter-interval opioid consumption reporting (it is presently modeled in 24-hour intervals). Additionally, the sample size of 116 limits the conclusions that can be drawn from the data; a number of potential confounders could not be assessed, such as the impact of adjunct analgesics. Lastly, we utilized data that had been previously collected and retrofit into the model to determine its efficacy. Future work will include prospective validation of the model across a broader range of disciplines.
Conclusion
Opioid dependency and opioid diversion are serious public health concerns and surgeons have an opportunity for improved opioid stewardship. Presently, there is no universal strategy for accurately prescribing postoperative opioids for surgical patients at discharge. This study presents a solution to this problem through development of the POP model, which utilizes patient-specific health data to estimate opioid prescription quantities more accurately. The potential benefits of this solution include decreased overprescribing, opioid waste and risk of opioid diversion, decreased risk of under-prescribing and poorly controlled patient pain, and an improved ability to detect patients at risk of new persistent use at an early time point. Further prospective analysis of these benefits can be performed with clinical integration of the POP model using electronic health record technology, which may ultimately improve patient outcomes and save lines through more precise prescribing recommendations that are personalized to each patient’s needs.
Supplementary Material
Supplemental Figure 1: Linear Opioid Prediction Model A) Visual description of linear regression model, using patient inpatient opioid use (blue) to create a patient-specific trendline (dashed line) and estimate outpatient opioid need (red). B) Comparison of linear POP model-estimated overprescription vs actual unused opioids remaining for dataset of analyzed patients. Total model R2 value of 0.271 was less than that for the logarithmic model R2 of 0.308.
Supplemental Figure 2: Comparison of Prescribing Models A) POP Model and Procedure-Based Model estimated prescription sizes for the same patients showed no association (R2=0.029). B) POP Model and Final 24-Hour Model estimated prescription sizes for the same patients showed a positive association (R2=0.473), however there was much more variation in the POP model-estimated prescription sizes.
Supplemental Figure 3: Effects of Incorporating Multivariate Predictors into the POP Model A) Regression of remaining OME versus POP model-estimated overprescription and procedure type, documented as a categorical variable. Likelihood-ratio test of the model with and without the inclusion of procedure type found that procedure type was not a significant variable in the model (p = 0.061). B) Regressions of remaining OME versus POP model-estimated overprescription with sequential removal of confounding factors: age, length of stay, gender identify, and previous use, with likelihood ratio tests of the model with and without the factor inclusion are shown on right. Patient-identified gender (p=0.024) and previous opioid use (p=0.005) were found to have significant effects on total remaining opioids, explored further in Supplemental Figure 4.
Supplemental Figure 4: Variations in Prescribing and Consumption Patterns A) Males were prescribed more opioids than females, with similar amounts of opioids consumed after discharge. However, gender did not affect the accuracy of POP model estimates. B) Patients with previous opioid use were found to be prescribed similar amounts of opioids and consumed larger amounts of opioids on average. Previous opioid use did not affect the accuracy of POP model estimates. *p<0.05, ***p<0.001.
Acknowledgments
KMB was supported by the Tau Beta Pi 35th Centennial Fellowship. JCB was supported by NIH F32HL144120, NIH T32AI106704 and the Ohio State University President’s Postdoctoral Scholar Program.
Footnotes
Financial Disclosure Statement: Dr. Janis receives royalties from Thieme and Springer Publishing. None of the other authors have a financial interest in any of the products, devices, or drugs mentioned in this manuscript.
References
- 1.SAMHSA. Key Substance Use and Mental Health Indicators in the United States: Results from the 2019 National Survey on Drug Use and Health 2020. https://www.samhsa.gov/data/report/2019-nsduh-annual-national-report
- 2.Hedegaard H, Minino AM, Warner M. Drug overdose deaths in the United States, 1999–2018. Vol. 356. 2020. NCHS Data Brief January 2020. https://www.cdc.gov/nchs/data/databriefs/db356-h.pdf [PubMed] [Google Scholar]
- 3.Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA Apr 6 2011;305(13):1315–21. doi: 10.1001/jama.2011.370 [DOI] [PubMed] [Google Scholar]
- 4.Edlund MJ, Martin BC, Russo JE, DeVries A, Braden JB, Sullivan MD. The role of opioid prescription in incident opioid abuse and dependence among individuals with chronic noncancer pain: the role of opioid prescription. Clin J Pain Jul 2014;30(7):557–64. doi: 10.1097/AJP.0000000000000021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chua KP, Brummett CM, Conti RM, Bohnert A. Association of Opioid Prescribing Patterns With Prescription Opioid Overdose in Adolescents and Young Adults. JAMA Pediatr Feb 1 2020;174(2):141–148. doi: 10.1001/jamapediatrics.2019.4878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller M, Barber CW, Leatherman S, et al. Prescription opioid duration of action and the risk of unintentional overdose among patients receiving opioid therapy. JAMA Intern Med Apr 2015;175(4):608–15. doi: 10.1001/jamainternmed.2014.8071 [DOI] [PubMed] [Google Scholar]
- 7.Khan NF, Bateman BT, Landon JE, Gagne JJ. Association of Opioid Overdose With Opioid Prescriptions to Family Members. JAMA Intern Med Jun 24 2019;doi: 10.1001/jamainternmed.2019.1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sekhri S, Arora NS, Cottrell H, et al. Probability of Opioid Prescription Refilling After Surgery: Does Initial Prescription Dose Matter? Ann Surg Aug 2018;268(2):271–276. doi: 10.1097/SLA.0000000000002308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown CR, Chen Z, Khurshan F, Groeneveld PW, Desai ND. Development of Persistent Opioid Use After Cardiac Surgery. JAMA Cardiol Jun 17 2020;doi: 10.1001/jamacardio.2020.1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clarke H, Soneji N, Ko DT, Yun L, Wijeysundera DN. Rates and risk factors for prolonged opioid use after major surgery: population based cohort study. BMJ Feb 11 2014;348:g1251. doi: 10.1136/bmj.g1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Page MG, Kudrina I, Zomahoun HTV, et al. A Systematic Review of the Relative Frequency and Risk Factors for Prolonged Opioid Prescription Following Surgery and Trauma Among Adults. Ann Surg May 2020;271(5):845–854. doi: 10.1097/SLA.0000000000003403 [DOI] [PubMed] [Google Scholar]
- 12.Gil JA, Gunaseelan V, DeFroda SF, Brummett CM, Bedi A, Waljee JF. Risk of Prolonged Opioid Use Among Opioid-Naive Patients After Common Shoulder Arthroscopy Procedures. Am J Sports Med Apr 2019;47(5):1043–1050. doi: 10.1177/0363546518819780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JS, Hu HM, Edelman AL, et al. New Persistent Opioid Use Among Patients With Cancer After Curative-Intent Surgery. J Clin Oncol Dec 20 2017;35(36):4042–4049. doi: 10.1200/JCO.2017.74.1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harbaugh CM, Lee JS, Hu HM, et al. Persistent Opioid Use Among Pediatric Patients After Surgery. Pediatrics Jan 2018;141(1)doi: 10.1542/peds.2017-2439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lawal OD, Gold J, Murthy A, et al. Rate and Risk Factors Associated With Prolonged Opioid Use After Surgery: A Systematic Review and Meta-analysis. JAMA Netw Open Jun 1 2020;3(6):e207367. doi: 10.1001/jamanetworkopen.2020.7367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peahl AF, Dalton VK, Montgomery JR, Lai YL, Hu HM, Waljee JF. Rates of New Persistent Opioid Use After Vaginal or Cesarean Birth Among US Women. JAMA Netw Open Jul 3 2019;2(7):e197863. doi: 10.1001/jamanetworkopen.2019.7863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brummett CM, Waljee JF, Goesling J, et al. New Persistent Opioid Use After Minor and Major Surgical Procedures in US Adults. JAMA Surg Jun 21 2017;152(6):e170504. doi: 10.1001/jamasurg.2017.0504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olds C, Spataro E, Li K, Kandathil C, Most SP. Assessment of Persistent and Prolonged Postoperative Opioid Use Among Patients Undergoing Plastic and Reconstructive Surgery. JAMA Facial Plast Surg Jul 1 2019;21(4):286–291. doi: 10.1001/jamafacial.2018.2035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson SP, Chung KC, Zhong L, et al. Risk of Prolonged Opioid Use Among Opioid-Naive Patients Following Common Hand Surgery Procedures. J Hand Surg Am Oct 2016;41(10):947–957 e3. doi: 10.1016/j.jhsa.2016.07.113 [DOI] [PubMed] [Google Scholar]
- 20.Marcusa DP, Mann RA, Cron DC, et al. Prescription Opioid Use among Opioid-Naive Women Undergoing Immediate Breast Reconstruction. Plast Reconstr Surg Dec 2017;140(6):1081–1090. doi: 10.1097/PRS.0000000000003832 [DOI] [PubMed] [Google Scholar]
- 21.Bennett KG, Kelley BP, Vick AD, et al. Persistent Opioid Use and High-Risk Prescribing in Body Contouring Patients. Plast Reconstr Surg Jan 2019;143(1):87–96. doi: 10.1097/PRS.0000000000005084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bicket MC, Long JJ, Pronovost PJ, Alexander GC, Wu CL. Prescription Opioid Analgesics Commonly Unused After Surgery: A Systematic Review. JAMA Surg Nov 1 2017;152(11):1066–1071. doi: 10.1001/jamasurg.2017.0831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bicket MC, White E, Pronovost PJ, Wu CL, Yaster M, Alexander GC. Opioid Oversupply After Joint and Spine Surgery: A Prospective Cohort Study. Anesth Analg Feb 2019;128(2):358–364. doi: 10.1213/ANE.0000000000003364 [DOI] [PubMed] [Google Scholar]
- 24.Rodgers J, Cunningham K, Fitzgerald K, Finnerty E. Opioid consumption following outpatient upper extremity surgery. J Hand Surg Am Apr 2012;37(4):645–50. doi: 10.1016/j.jhsa.2012.01.035 [DOI] [PubMed] [Google Scholar]
- 25.Rose KR, Christie BM, Block LM, Rao VK, Michelotti BF. Opioid Prescribing and Consumption Patterns following Outpatient Plastic Surgery Procedures. Plast Reconstr Surg Mar 2019;143(3):929–938. doi: 10.1097/PRS.0000000000005351 [DOI] [PubMed] [Google Scholar]
- 26.Hanson KT, Thiels CA, Polites SF, et al. The opioid epidemic in acute care surgery-Characteristics of overprescribing following laparoscopic cholecystectomy. J Trauma Acute Care Surg Jul 2018;85(1):62–70. doi: 10.1097/TA.0000000000001834 [DOI] [PubMed] [Google Scholar]
- 27.Fujii MH, Hodges AC, Russell RL, et al. Post-Discharge Opioid Prescribing and Use after Common Surgical Procedure. J Am Coll Surg Jun 2018;226(6):1004–1012. doi: 10.1016/j.jamcollsurg.2018.01.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pruitt LCC, Swords DS, Russell KW, Rollins MD, Skarda DE. Prescription vs. consumption: Opioid overprescription to children after common surgical procedures. J Pediatr Surg Nov 2019;54(11):2195–2199. doi: 10.1016/j.jpedsurg.2019.04.013 [DOI] [PubMed] [Google Scholar]
- 29.Thiels CA, Anderson SS, Ubl DS, et al. Wide Variation and Overprescription of Opioids After Elective Surgery. Ann Surg Oct 2017;266(4):564–573. doi: 10.1097/SLA.0000000000002365 [DOI] [PubMed] [Google Scholar]
- 30.Shah A, Rowlands M, Krishnan N, Patel A, Ott-Young A. Thoracic Intercostal Nerve Blocks Reduce Opioid Consumption and Length of Stay in Patients Undergoing Implant-Based Breast Reconstruction. Plast Reconstr Surg Nov 2015;136(5):584e–591e. doi: 10.1097/PRS.0000000000001717 [DOI] [PubMed] [Google Scholar]
- 31.Barker JC, DiBartola K, Wee C, et al. Preoperative Multimodal Analgesia Decreases Postanesthesia Care Unit Narcotic Use and Pain Scores in Outpatient Breast Surgery. Plast Reconstr Surg Oct 2018;142(4):443e–450e. doi: 10.1097/PRS.0000000000004804 [DOI] [PubMed] [Google Scholar]
- 32.Wick EC, Grant MC, Wu CL. Postoperative Multimodal Analgesia Pain Management With Nonopioid Analgesics and Techniques: A Review. JAMA Surg Jul 1 2017;152(7):691–697. doi: 10.1001/jamasurg.2017.0898 [DOI] [PubMed] [Google Scholar]
- 33.Ljungqvist O, Scott M, Fearon KC. Enhanced Recovery After Surgery: A Review. JAMA Surg Mar 1 2017;152(3):292–298. doi: 10.1001/jamasurg.2016.4952 [DOI] [PubMed] [Google Scholar]
- 34.Greco M, Capretti G, Beretta L, Gemma M, Pecorelli N, Braga M. Enhanced recovery program in colorectal surgery: a meta-analysis of randomized controlled trials. World J Surg Jun 2014;38(6):1531–41. doi: 10.1007/s00268-013-2416-8 [DOI] [PubMed] [Google Scholar]
- 35.Batdorf NJ, Lemaine V, Lovely JK, et al. Enhanced recovery after surgery in microvascular breast reconstruction. J Plast Reconstr Aesthet Surg Mar 2015;68(3):395–402. doi: 10.1016/j.bjps.2014.11.014 [DOI] [PubMed] [Google Scholar]
- 36.Engelman RM, Rousou JA, Flack JE 3rd, et al. Fast-track recovery of the coronary bypass patient. Ann Thorac Surg Dec 1994;58(6):1742–6. doi: 10.1016/0003-4975(94)91674-8 [DOI] [PubMed] [Google Scholar]
- 37.Soffin EM, YaDeau JT. Enhanced recovery after surgery for primary hip and knee arthroplasty: a review of the evidence. Br J Anaesth Dec 2016;117(suppl 3):iii62–iii72. doi: 10.1093/bja/aew362 [DOI] [PubMed] [Google Scholar]
- 38.Daneshmand S, Ahmadi H, Schuckman AK, et al. Enhanced recovery protocol after radical cystectomy for bladder cancer. J Urol Jul 2014;192(1):50–5. doi: 10.1016/j.juro.2014.01.097 [DOI] [PubMed] [Google Scholar]
- 39.Thiels CA, Ubl DS, Yost KJ, et al. Results of a Prospective, Multicenter Initiative Aimed at Developing Opioid-prescribing Guidelines After Surgery. Ann Surg Sep 2018;268(3):457–468. doi: 10.1097/SLA.0000000000002919 [DOI] [PubMed] [Google Scholar]
- 40.Carrico JA, Mahoney K, Raymond KM, et al. Predicting Opioid Use Following Discharge After Cesarean Delivery. Ann Fam Med Mar 2020;18(2):118–126. doi: 10.1370/afm.2493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hill MV, Stucke RS, Billmeier SE, Kelly JL, Barth RJ Jr. Guideline for Discharge Opioid Prescriptions after Inpatient General Surgical Procedures. J Am Coll Surg Jun 2018;226(6):996–1003. doi: 10.1016/j.jamcollsurg.2017.10.012 [DOI] [PubMed] [Google Scholar]
- 42.Wyles CC, Hevesi M, Ubl DS, et al. Implementation of Procedure-Specific Opioid Guidelines: A Readily Employable Strategy to Improve Consistency and Decrease Excessive Prescribing Following Orthopaedic Surgery. JB JS Open Access Jan-Mar 2020;5(1):e0050. doi: 10.2106/JBJS.OA.19.00050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chu JJ, Janis JE, Skoracki R, Barker JC. Opioid Overprescribing and Procedure-Specific Opioid Consumption Patterns for Plastic and Reconstructive Surgery Patients. Plast Reconstr Surg Apr 1 2021;147(4):669e–679e. doi: 10.1097/PRS.0000000000007782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mandrekar JN. Receiver operating characteristic curve in diagnostic test assessment. J Thorac Oncol Sep 2010;5(9):1315–6. doi: 10.1097/JTO.0b013e3181ec173d [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Linear Opioid Prediction Model A) Visual description of linear regression model, using patient inpatient opioid use (blue) to create a patient-specific trendline (dashed line) and estimate outpatient opioid need (red). B) Comparison of linear POP model-estimated overprescription vs actual unused opioids remaining for dataset of analyzed patients. Total model R2 value of 0.271 was less than that for the logarithmic model R2 of 0.308.
Supplemental Figure 2: Comparison of Prescribing Models A) POP Model and Procedure-Based Model estimated prescription sizes for the same patients showed no association (R2=0.029). B) POP Model and Final 24-Hour Model estimated prescription sizes for the same patients showed a positive association (R2=0.473), however there was much more variation in the POP model-estimated prescription sizes.
Supplemental Figure 3: Effects of Incorporating Multivariate Predictors into the POP Model A) Regression of remaining OME versus POP model-estimated overprescription and procedure type, documented as a categorical variable. Likelihood-ratio test of the model with and without the inclusion of procedure type found that procedure type was not a significant variable in the model (p = 0.061). B) Regressions of remaining OME versus POP model-estimated overprescription with sequential removal of confounding factors: age, length of stay, gender identify, and previous use, with likelihood ratio tests of the model with and without the factor inclusion are shown on right. Patient-identified gender (p=0.024) and previous opioid use (p=0.005) were found to have significant effects on total remaining opioids, explored further in Supplemental Figure 4.
Supplemental Figure 4: Variations in Prescribing and Consumption Patterns A) Males were prescribed more opioids than females, with similar amounts of opioids consumed after discharge. However, gender did not affect the accuracy of POP model estimates. B) Patients with previous opioid use were found to be prescribed similar amounts of opioids and consumed larger amounts of opioids on average. Previous opioid use did not affect the accuracy of POP model estimates. *p<0.05, ***p<0.001.


