Abstract
Parasites are known to be a key driving force in mate choice and are important for the expression and evolution of ornaments and behavioral traits being used. However, there is little experimental evidence on how the parasite’s burden of the choosing individual is integrated into the mate-choice process and how it affects decision-making, especially in relation to parasite infestation of potential mates. Thus, the aim of our study was to determine whether female house sparrows Passer domesticus adjust their mate preference according to their own as well as the parasite load of prospective partners. To do this, we experimentally manipulated female parasite load and determined their mate preferences prior to and after parasite treatment. We manipulated the chronic coccidian parasite burden of females either by initiating the acute infection phase via re-infecting them with coccidian or by temporally reducing the parasite load of coccidia. We then measured the effect of this manipulation on mate preference by presenting females with a choice of four stimuli: three males with similar ornaments, but unmanipulated, naturally varying chronic coccidiosis levels, and an unmanipulated control female. Additionally, we recorded some males’ behavior in relation to their infection status pointing toward an increased or reduced interest in mating. We found that females preferred highly infested males prior to manipulation, regardless of their own infestation level. However, after manipulation, infested females avoided highly infested males probably in response to the deterioration of their health condition by parasites. Our study suggests that mate-choice decisions are more complex when they are mediated by parasites. The implications of parasites for evolutionary theories of sexual signaling and mate choice are discussed.
Keywords: coccidiosis, female decision, mate choice, parasites, Passer domesticus, Isospora spp
Parasites are an important driving force for ornament evolution (Lozano 1994; Brawner et al. 2000; Fitze and Richner 2002; Hõrak et al. 2004; Mougeot et al. 2009a). Therefore, one might assume that ornaments reflect parasite infestation, although this is not necessarily the case. Whether precise information about parasites can be reflected by an ornament depends on the importance of different biotic and abiotic factors (Martin et al. 2001; Nuismer and Otto 2004; Penczykowski et al. 2016). Thus, the appearance of an ornament is more likely to be the result of a composite effect of several influential factors, so the importance of parasites may also depend on the type of ornament. For example, behavioral traits like male bird song may be affected by parasites in a different way to olfaction- or plumage-based ornaments (Garamszegi et al. 2003; Spencer et al. 2005).
Behavioral traits are probably more dynamic signals than ornaments, and the changes in signaling content, for example, infestation status, become instantaneously evident to conspecifics (Hallal-Calleros et al. 2013). In fact, changes in behavioral parameters can be detected at an earlier stage compared to clinical detection. In contrast, plumage signals reflect the individual status during the development of those feathers that constitute the ornament and last static until a new molting period. Carotenoid-based ornaments may, for example, reflect carotenoid uptake influenced by parasites over the whole molting period (Mougeot et al. 2009b; Pap et al. 2009). Nevertheless, birds can use specific plumage characteristics to gather information about factors such as parasite infestation. In this context, Griggio et al. (2010) showed that birds use plumage UV reflectance as an indicator of their current state of health. Using multiple ornaments can additionally increase the precision of the information provided when sampling different ornaments, if different ornaments convey different individual characteristics. Although there are still knowledge gaps on how different ornaments may signal host parasitization status, parasites play an important role in sexual selection, in particular, mate choice (Lozano 1994; Brawner et al. 2000; Hõrak et al. 2004; Mougeot et al. 2007, 2009a). Parasites induce the allocation of resources into immune defense, reduce growth rate, and restrain reproductive success (Sheldon and Verhulst 1996; Zuk and Stoehr 2002; Sorci and Cézilly 2008; Wisenden et al. 2009; Cantarero et al. 2013; López-Arrabé et al. 2015). Current thinking emphasizes that both sexes may be equally choosy’ however, a fact rarely considered is that parasites may influence the mating decisions of both players, namely the mate being chosen (usually the male) but also the choosing individual (usually the female), as well as the mutual interaction between the two players, depending on their individual specific parasite burdens. Relatively little work has been done on how parasites influence the mating decisions of the choosing sex, that is, how the intrinsic quality of the choosing individual is integrated into their decision-making processes regarding mate choice and preferences (Beltran-Bech and Richard 2014).
In that regard, there are at least two possible pathways. First, the cost of infections can reduce the choosiness of females resulting in random mating (Poulin 1994; Lopez 1999; Pfennig and Tinsley 2002). Alternatively, energetic stress may activate an “emergency program” and individuals may fully invest in reproduction (Fisher et al. 2013). In support of the latter, some studies have found an increase in mate sampling and a greater sexual motivation because of parasite infestation (Simmons 1994; Buchholz 2004). In line with this, Griggio and Hoi (2010) demonstrated that mate choice in female house sparrows Passer domesticus is condition dependent, with females in poor condition being more choosy than females in good condition. Therefore, poor-quality females may increase their fitness outcomes by selecting the best males.
In this study, we simultaneously evaluated the importance of parasites to both players for female mate-choice decisions. To evaluate the importance and to disentangle whether a change in mate preference is the result of behavioral plasticity of the potential mates or is caused by the parasite intensity of the choosy individual, we experimentally manipulated the parasite infestation rate of both sexes using one specific parasite. As the model system, we used house sparrows and an intestinal parasite, which can quickly affect the condition of the host (see below). Coccidia (Isospora spp.) is a common intestinal parasite of passerines (Brawner et al. 2000; Hõrak et al. 2004; Schrenzel et al. 2005; Dolnik 2006; Pap et al. 2009; Sepp et al. 2012; Surmacki and Hill 2014). The sporozoites disrupt the uptake of various food compounds via the epithelium of the small intestine (Hõrak et al. 2004), which should increase the susceptibility to other diseases and also bring about a deterioration in health. Isospora infection starts with a short acute phase that is marked by an increase in food intake and a decrease in body mass by the host (Dolnik 2002), and subsequently develops into a chronic infection. Coccidia oocysts are typically spread by fecal–oral transmission (Dolnik et al. 2009). Although direct host-to-host transmission within a flock is likely to be low, the gregarious nature of the house sparrow makes it an easy target for infection at the foraging sites of flock foragers (Dolnik et al. 2010). For infection, excreted oocysts need time for sporulation, so the exposure to live sporulated oocysts will depend on the frequency of feeding habitat use (Anna et al. 2011). As house sparrow pairs generally forage together the risk of transmission from bird to bird by ingestion of infected droppings, contaminated food or water is high. Chronic infection levels differ between individuals (Milde 1979), but under controlled experimental conditions, infection intensity remains constant so that after an acute phase caused by re-infection, infestation loads return to the original level. Dolnik and Hoi (2010) demonstrated that acute infection leads to loss of body mass and changes in the dominance hierarchy of a social group of males.
In male house sparrows, a prominent plumage ornament is the melanin-based black breast patch (Figure 1), which is molted in autumn and, therefore, should not be altered by coccidia manipulation in spring. However, Dolnik and Hoi (2010) were able to demonstrate experimentally that, although the melanin-based breast patch did not change, coccidia infestation influenced male–male interactions and male dominance rank: Males with larger patches could tolerate higher infestation levels under stress conditions. In fact, female house sparrows chose mates on the basis of male badges (Møller 1988). Although badge status signals may be adaptive to reduce confrontation and concomitant costs of fights, dominant males are usually challenged in nature by subordinate or younger males. This suggests that male patch size is not a reliable signal of coccidia infestation and as such is challenged by other males. Thus, the behavior rather than the ornaments of infested males is more likely to reveal their current condition (Dolnik and Hoi 2010).
Figure 1.
Male house sparrow showing the black bib (photo credit: Rafael Palomo Santana).
To carry out the mate-choice experiments in the proposed way, we tried to control for the plumage ornament of the sex to be chosen by grouping them into three groups according to their coccidia infestation levels (low, medium, and high). In addition, we produced three female groups by manipulating their status of parasite infection by (1) maintaining their natural coccidiosis (control group), (2) re-infecting them with coccidian and thereby activating the acute infection stage, and (3) by pausing the coccidian infection with medicine. We cannot cure coccidiosis in passerines (only Eimeria in poultry) because these parasites have blood stages (Schrenzel et al. 2005). Therefore, we can only put the infection “on ice,” so that it does not develop. Thus, although oocysts stopped appearing in the feces of birds and infestation level is reduced, the birds are not cured and oocyst production resumes again within several days and the infection load increases (Brawner et al. 2000). To evaluate the effect of this conditional manipulation on female mate preference, we measured preferences before and after the manipulation. For the experiment, females could choose between three males with similar ornaments, but different levels of coccidiosis and an untreated female as control to test whether focal females were sexually motivated and did not move randomly among compartments. In this way, we aimed to clarify (1) whether females discriminate between males according to differences in parasite levels independent of ornament expression, and (2) whether female infestation level influences their decision-making processes, for example, mate choice. As a determinant of female preference, we measured the time a female spent in front of a male’s compartment. Additionally, a subsample of stimulus males was selected to test their sexual motivation based on their level of infection. We recorded some behaviors pointing toward an increased or reduced interest in mating (for details, see experimental design).
The outcome of our manipulation may depend on information females gather by visually inspecting the male, for example, the behavior of the stimulus bird. Theoretically, if the trait information suggests “no parasites,” females should always choose the male signaling “no parasites” independent of their own parasite status. However, immunity does not prevent re-infection with Isospora spp. and frequent re-infections (including self-re-infection) are possible (Dolnik 2002). Also, due to the general high infection intensity in house sparrows (e.g. 86% of the individuals in our population are infested), parasite-free males are rare, and hence, it is unlikely that a female will meet one (Milde 1979). Things become more complicated when the signal additionally reflects male quality, especially when females also rely on paternal investment. The immunocompetence handicap hypothesis (Kurtz and Sauer 1999) is based on the assumption that the immune system competes for resources with sexually selected ornaments; variation in ornaments might reflect genetic variation for immunocompetence and fight against parasites that may detract individual condition. In line with this, we predicted that (1) infested females will be more selective in favor of less parasitized or parasite-free males (prediction 1). However, this would only be true for high-pathogenic parasites. Since we work with low-pathogenic parasites and their selection pressure is constant, other predictions are also possible. We can imagine two scenarios for chronic and acute infections, for example, males who can tolerate a higher parasite burden may also be of better quality and thus this could be seen as an example of the handicap principle (Zahavi 1975). Under these circumstances, we might even predict that (2) females would prefer males with higher parasite loads (prediction 2). In contrast, an acute infection might indeed not be attractive for females, as the risk of infection and related costs may be too high.
Material and Methods
Subjects and housing
A total of 48 focal (choosing) females and 101 stimulus individuals (89 males and 12 females) were housed in 17 outdoor aviaries at the Konrad-Lorenz Institute for Ethology. All birds were older than 1 year. The house sparrow aviaries measured 3.5 m × 3.5 m × 3 m and each one housed about 15 individuals, randomly assigned to the aviaries. Each aviary was equipped in the same way with vegetation and several perches (about 7 per aviary). Commercial food for granivorous passerines (mixture of millet, canary seed, wheat and sunflower seeds, apple slices, and millet sprays) and water were provided ad libitum.
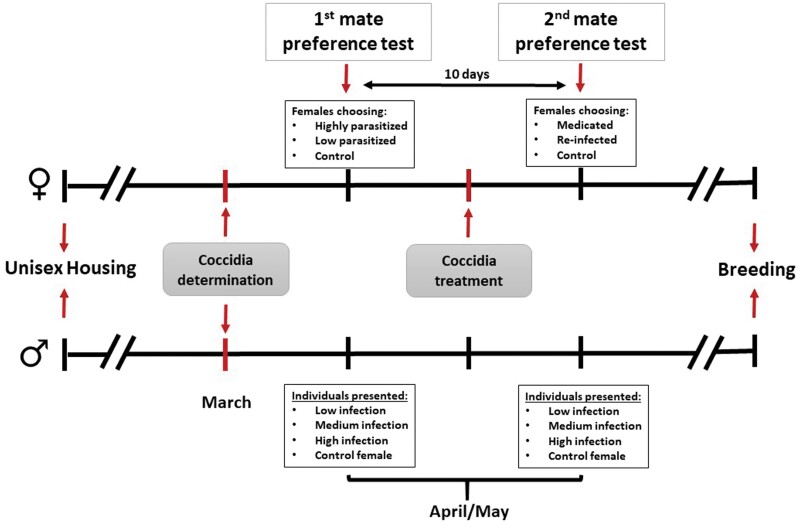
All birds were naturally infected with at least 2 Isospora spp. prior to the experiment (Milde 1979). In March 2008 (see Figure 2 for a detailed chronogram of the experimental setup), feces of all the birds were individually collected 3 times every second day and checked for coccidia oocysts (see below). Birds were placed in the dark inside a wooden box with an aluminum foil on the base and every 15 min, we checked for droppings. For chronic stable infections, this number of examinations is sufficient to describe the intensity and species composition of infection (Dolnik 2006). The birds selected for the experiment were those with a double infection (Isospora michaelbakeri [Grulet et al. 1986] were the dominant species, making up over 95% of the oocysts, plus Isospora mikei), who also presented a stable infection intensity. As an oocyst donor for artificial re-infection, we used 1 bird that was especially highly infected with I. michaelbakeri + I. mikei and was kept in a separate cage. Each bird was marked with a metal identification ring. The light/dark photoperiod was equal to the natural situation. The initiation of breeding immediately after the experimental manipulation suggests that the housing conditions and the experiment were appropriate and had no negative effect on the birds’ health or condition.
Figure 2.
Experimental chronogram of the experiment. Coccidia infection of all birds was determined at the beginning of the experiment. Each focal female performed 2 preference trials separated 10 days; 1 before and 1 after the manipulation of its parasite infection status. Birds initiated the breeding immediately after the experiment.
Experiment scheme
We selected 3 groups of 12 males with low (0–10 oocysts), medium (20–200 oocysts), and high (350–40,000 oocysts) infestation levels. To do that, males were ranked based on their natural parasite burden, and 3 groups of equal sample size were created. The range of oocysts was established based on the incidence that each group had. Parasite burden was not manipulated in these birds or the control female offered (see below). Infestation level was expected to be stable during the course of the experiment since the infection does not change if conditions are maintained (Schröder 2019). Before the first set of mate-preference experiments (see below), females were assigned to a treatment based on their natural oocyst loads. We established 3 female groups: highly parasitized females (350–40,000 oocysts), low parasitized females (0–10 oocysts), and a group of control females with a variable range of oocysts (0–800 oocysts). After the first set of mate-preference experiments, control females (hereafter, CF; n = 15) remained sham manipulated (water, orally), highly parasitized females were medicated with Sulfadimidin (orally 0.0025 g perbird; medicated females, hereafter, MF; n = 16), and low parasitized females were artificially re-infected with I. michaelbakeri from the donor bird (orally 10,000 sporulated oocysts per bird; infected females, hereafter, IF; n = 17). By re-infecting birds, we superimposed infection of the same type that females presented naturally. Oocysts were suspended in 40 µL of water and applied with a micropipette directly into the bill (Dolnik 2002).
We collected each bird’s feces from their individual cages 3–5 h before darkness. This is the peak time of oocyst production for Isospora parasites in passerine birds (Grulet et al. 1986; Dolnik 1999a, 1999b). One fecal sample per bird was placed individually in 2.5% water solution of potassium dichromate (K2Cr2O7) and kept at room temperature to allow sporulation. Only the samples taken within the first 2 post-infection days (to check the sporulation stages of excreted oocysts) were examined immediately after sampling. All the samples were examined for Isospora spp. oocysts using flotation centrifugation in concentrated NaCl solution. The relative intensity of infection was estimated by counting the oocysts in smears (×100) and the results are shown as oocysts per defecation (opd), which was demonstrated to provide repeatable and comparable results (Dolnik 2006). The Isospora spp. were distinguished under a microscope (×1000) using immersion oil (Grulet et al. 1982).
Mate-preference experiments
In April and May 2008, we conducted a female mate-preference test using an indoor 4-choice apparatus (2 m × 2 m × 0.5 m). It consisted of 4 choice chambers, separated by opaque dividers at the 4 sides of the central choice chamber. An opaque divider was also set up in the middle of the central chamber to avoid visual interaction between the 4 stimulus individuals. The central divider also prevented the choosing females from simultaneously observing 2 or more stimuli. In 1 corner of the 4 dividers, an opening (14 cm × 14 cm) covered by a metal web allowed the female to observe the stimulus in the side chamber. During the experiment, the females could see the stimulus through these holes, but they could not physically interact. A perch was positioned in front of each of the 4 chambers. Perches had a line traced, which marked the closest distance from which a female could observe the stimulus in the nearby compartment (see Figure 3 in Griggio et al. [2011] for a schematic overview of the experimental apparatus). “Choice time” was defined as the time the focal female was present in front of the opening of each of the four perches, whereas “no-choice time” was the time a female was not present in the focus area. In accordance with the objectives of the study, females had a choice between 3 males either with low (LM), medium (MM), or high (HM) infestation levels (for group separation, see earlier). We measured the dimension of the black breast patch (“badge of dominance”) of each male with digital calipers to the nearest 0.01 mm. The badge size was estimated in mm2: badge length (mm) × badge width (mm). As a control, the fourth chamber contained a female (control group females, CGF group, n = 12). To control for potential position effects, chambers were randomly assigned to the stimulus individuals. To reduce bird stress during the trial, the position of the stimulus individuals was maintained during the test, preventing us to control for position effects within the trial. Nevertheless, the accumulated time of the females in each chamber showed that there was no side bias toward a specific compartment (ANOVA t-test for all females F3,188 = 0.149, P > 0.93). All birds were unfamiliar with each other because they originated from different visually separated housing aviaries. The stimulus individuals were presented to 3 experimental groups of females: control females (CF), medicated females (MF), and infected females (IF). Moreover, we selected a random subsample of stimulus males (12 per infection status) to test whether parasite infection impairs male motivation. Thus, we measured the number of (i) male approaches toward the focal female, (ii) coordinated movements with the female, and additionally, other behaviors pointing toward a reduced interest in mating, such as preening, drinking, or eating. The experiment consisted of 48 mate-preference trials, each with a different focal female as the subject. At the beginning of a trial, the choosing female and the stimulus individuals were placed in their experimental chambers and allowed to acclimate for at least 30 min before the trial began. After this period, the opaque separators that covered the mesh windows were removed and the position of the female was recorded every second for 1 h (all trials were video recorded and then analyzed by students, blind with respect to the infection status of the individuals). We measured the time a female spent on the perch in front of each male compartment and the female compartment. Preferences were then expressed as the total time spent in the choice area of each stimulus. All mate-preference experiments were performed from 07:30 to 11:00.
Each focal female performed a new mate-preference trial 10 days after the manipulation of its parasite infection status. The identity and the position of stimulus individuals changed between trials to avoid potential habituation effects. In any trial, we ensured that the stimulus males did not differ in body condition using size-corrected body mass and black breast patch reflecting a badge of status (ANOVA t-test: for all F2,87 < 0.54, P > 0.58). Due to the lack of appropriate stimulus males, a complete randomization of the different combinations of male traits and parasite infestation was not possible. Therefore, some males participated in several mate-preference experiments (mean 5.52 ± SD 2.63 trials), with the rule that they did not undergo more than 1 trial on the same day.
Statistical analyses
To assess the effectiveness of the experimental treatment, we compared the oocyst loads of the 3 experimental female groups before and after the manipulation. Oocyst loads did not follow a normal distribution; therefore, nonparametric tests (Wilcoxon matched-pairs test) were used.
The time the focal female spent near each stimulus male and their behavior during the presence of the focal female were both normally distributed (Shapiro–Wilk tests, P > 0.05). Therefore, ANOVA tests (SAS v9.4 software) were used to analyze differences in the time focal females invested in the choice area between stimuli and/or the experimental treatment. The alpha level for these multiple post hoc comparisons was determined using the Šidák–Bonferroni correction procedure (Abdi 2007). Differences in male behavior depending on their infection load were analyzed with generalized linear models (GLMs) with the different behaviors as response variables and infection status as a fixed factor.
Generalized mixed models (proc mixed, SAS v9.) were used to compare female preferences. The time a focal female spent near each stimulus male before or after treatment was the dependent variable and female infestation level (control, medicated, or infected), stimulus group (low-, medium-, and high-infestation level) and its interaction with female infestation level were introduced in the model as fixed factors. Trial, stimulus individual’s identity, and female identity were random factors. Degrees of freedom (df) were estimated with Satterwaite approximation, thus avoiding possible pseudoreplication. All results are presented as the mean ± SE. Random terms were always nonsignificant (P > 0.15) and their removal did not affect the results.
Results
Depending on the manipulation, the average coccidia infestation level of each female group was differently affected, while it did not change in control females (Table 1). After experimental manipulation, oocyst loads decreased in the medicated group, but increased in the infected group (Table 1).
Table 1.
Differences (means ± SD) in the number of oocysts per defecation between the 3 experimental groups of females (Wilcoxon matched-pairs test).
| Oocyst Before | Oocyst After | Z | P | |
|---|---|---|---|---|
| Control females (CF) | 150.81 ± 227.23 | 181.93 ± 258.44 | 0.783 | 0.460 |
| Medicated females (MF) | 13,806.13 ± 25,968.70 | 29.43 ± 59.07 | 3.296 | <0.001 |
| Infected females (IF) | 0.24 ± 0.56 | 3,269.35 ± 8373.63 | 3.261 | <0.001 |
P-values below 0.005 are shown in bold.
Furthermore, we found that female presence influenced male behavior toward females depending on their own infestation level. Males with high infestation levels spent more time interacting with the focal female (e.g. male approaches toward the female: F1.32 = 3.731, P = 0.035), whereas other male behaviors related to self-maintenance like preening, drinking, or eating were not significantly affected by the infestation level (for all P > 0.26).
Female investment in gathering information about mates was clearly affected by the experimental manipulation. While control or medicated females showed no difference in the total time spent in the choice area of stimulus males (CF: t1.28 = 1.178, P = 0.249; MF: t1.30 = 0.701, P = 0.489; Figure 3), infected females significantly reduced the time invested in mate-choice activities (IF: t1.32 = 2.733, P = 0.010; Figure 3).
Figure 3.
Female choice depending on her coccidian infection status in relation to total time (s) females spent in the choice area of the three stimulus males, before (black column) and after (white column) the experimental treatment. Given are averages (±SE). * indicates significant difference (P < 0.05).
In the first mate preference tests when using naturally infected females, females preferred parasitized males regardless of their own natural oocyst loads (female group: F1.183 = 0.67, P = 0.415; stimulus group: F1.183 = 3.59, P = 0.015; female group × stimulus group: F1.183 = 1.74, P = 0.161; Figure 4A). Thus, the choosing females spent less time in the choice area of the control female regardless of their natural oocyst load and showed a clear preference for highly infected males (Table S1, see ESM). This confirmed that the selected parameter adequately reflected sexual motivation (see also Table S2–S4 for results of pairwise comparisons within female groups).
Figure 4.
Time (s) focal females spent in the choice area of the 4 different choice stimuli before (A, chronic infection) and after (B, acute infection) the experimental parasite manipulation (black columns: control females; gray columns, medicated females; white columns: infected females). The 4 different choice stimuli consisted of a control female and 3 males with an either low (LM), medium (MM), or high (HM) infestation level.
After parasite manipulation, female preference was significantly affected by her own as well as the infestation level of the stimulus males (Figure 4B). There was also an interaction between female and male infestation levels (female group: F1.182 = 5.01, P = 0.026; stimulus group: F1.182 = 5.82, P < 0.001; female group × stimulus group: F1.182 = 2.81, P = 0.040). This could be mainly explained by experimentally highly infected females avoiding highly infected males (Table 2). Furthermore, female mate selection patterns revealed that the offered female (stimulus female) was less frequently visited than the highly infected male regardless of her experimental manipulation (Figure 4B; stimulus female versus pooled stimulus males, t1.190 = −3.423, P < 0.001; low + intermediate infected males versus highly infected males, t1.142 = −2.483, P = 0.014).
Table 2.
Average time (±SE) and results of paired t-tests for the time (s) females spent in the choice area of each choice stimulus, before and after the experimental treatment
| Female | Low infected male | Medium infected male | High infected male | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Control female | Before treatment | 200.9 ± 187.9 |
t
28 = 0.142 P = 0.888 |
895.7 ± 583.9 |
t
28 = 0.310 P = 0.759 |
751.5 ± 366.6 |
t
28 = 1.992 P = 0.056 |
1025.9 ± 368.6 |
t
28 = 0.002 P = 0.998 |
| After treatment | 187.9 ± 329.0 | 831.7 ± 544.8 | 461.4 ± 428.7 | 1025.5 ± 689.5 | |||||
| Medicated female | Before treatment | 478.7 ± 730.2 |
t
30 = 0.351 P = 0.728 |
472. ± 428.8 |
t
30 = -0.168 P = 0.867 |
616.6 ± 525.0 |
t
30 = 0.393 P = 0.697 |
782.0 ± 596.5 |
t
30 = 0.802 P = 0.429 |
| After treatment | 404.8 ± 418.1 | 499.2 ± 480.8 | 544.7 ± 509.2 | 617.9 ± 560.1 | |||||
| Infected female | Before treatment | 400.0 ± 433.6 |
t
32 = 0.521 P = 0.606 |
486.6 ± 552.4 |
t
32 = 0.621 P = 0.539 |
671.2 ± 660.2 |
t
32 = 1.262 P = 0.216 |
997.4 ± 582.5 |
t
32
= 2.185
P = 0.036 |
| After treatment | 334.8 ± 278.7 | 368.1 ± 560.3 | 434.2 ± 404.8 | 627.4 ± 384.9 | |||||
Note: Based on the conservative Šidák–Bonferroni correction for multiple comparisons (α = 0.041). P-values below 0.041 are shown in bold.
Discussion
Our results revealed that female preference is affected by infestation levels of both herself and the stimulus males. During an acute infection phase, females decrease the time they invest in mate-choice activities and avoid males with high chronic infections. Interestingly, unmanipulated females spent more time in the choice area of highly infested males, which suggests that females prefer males with a high chronic infestation rate. If males can cope with high parasite loads, that is, all males within a trial were of similar size and had similar-sized badges, one may assume that they are of better quality indicating good genes and dominance (Howard and Lively 2004). Dominant males are known to be less caring “fathers” (Forsgren 1997), and this has also been suggested for house sparrows (Václav and Hoi 2002). Depending on the female quality and environmental conditions during reproduction, paternal contribution or choice of “good genes” might be more important, and such a trade-off in relation to male quality has been found in house sparrows (Griggio and Hoi 2010). In line with this, our results show that during an acute infection, females avoided highly parasitized mates, who in our experiment were those with the highest dominance, a common trait that females use to assess the quality of their mate as a provider for offspring (Møller 1988). Furthermore, we found male behavior was influenced by coccidian infestation, which seems to have important consequences for mate choice as well, something, which is ignored in mate-choice studies.
In detail, we found support for our first prediction, namely that the level of parasite infestation of a female influences her mating behavior and her investment in mate choice, with experimentally infected females reducing the time invested in mate-choice activities (Figure 3). During their natural infection status (mate-choice trial I), there seems to be no difference in the overall time females spent in the choice area, that is, female mating behavior is not sensitive to their own coccidian infestation intensity. One reason for this could be that, to stay in the choice area per se, is probably no costlier than staying elsewhere.
In contrast, female investment in mating behavior is affected when infection in females was experimentally manipulated. In this context, a negative impact of parasites on the female condition has been specifically demonstrated for acute coccidian infections (Dolnik 2002; Knight et al. 2018). Coccidia is a common intestinal parasite, whose sporozoites impair antiparasitic defenses (Brawner et al. 2000; Hõrak et al. 2004; Dolnik 2006; Sepp et al. 2012). This can result in damage to the immune system and lead to an increase in susceptibility to infectious diseases that will be reflected phenotypically and behaviorally. The fact that only acutely infected females showed a decrease in the time invested in mate-choice activities (Figure 3) suggests that these individuals suffered a deterioration in health condition, a widely known effect of avian coccidiosis (Dolnik 2002; Hõrak et al. 2004; Knight et al. 2018). Thus, one may predict variation in mate sampling behavior because of coccidian infection. Searching for mates is costly and females seem to set a threshold regarding time and energy costs dependent on their condition. This consequently determines the number of males sampled (Burley and Foster 2006). In that regard, food deprivation has been shown to result in gonadal regression and a decrease in the excretion of sex hormones, which may in turn influence a host’s sexual motivation (Klingerman et al. 2011). Our findings are also consistent with results obtained from guppies Poecilia reticulata, where experimentally infected females were less active in mate-choice activities than healthy ones (Lopez 1999).
We found that under natural parasite burdens females preferred to mate with highly infected males (Figure 4A). According to the “parasite-mediated sexual selection” hypothesis (Hamilton and Zuk 1982), females should avoid parasite infestation by discriminating in favor of males bearing genotypes encoding resistance to parasites (Borgia and Collis 1989; Figuerola et al. 1999; Brawner et al. 2000; Worden et al. 2000; Hõrak et al. 2001; Beltran-Bech and Richard 2014). In this context, secondary sexual characteristics could act as indicators of such resistance (Potti and Merino 1996; Badás et al. 2018). In fact, it has been demonstrated that infection can have a negative impact on phenotypic traits and consequently, females should avoid less ornamented males as they may suffer a higher parasite burden (Clayton 1990; Milinski and Bakker 1990; Potti and Merino 1996; Worden et al. 2000; Aguilar et al. 2008; Beltran-Bech and Richard 2014). Although several studies have demonstrated a female preference for healthy or less-infected males (reviewed in Beltran-Bech and Richard 2014), differences in parasite pathogenicity (high or low) may be an important factor influencing mating decisions. In low-pathogenic parasites, 100% of the population can be infected, and we can speak of a chronic infection. Under such circumstances, a high infection intensity may be an indicator of high quality and a sign of strength. Thus, one explanation could be that females using ornament information are being deceived or that ornaments signal individual quality and individuals carrying high loads are able to cope with them (handicap principle; Zahavi 1975). Immunity and parasites do play a fundamental role in many biological signaling systems, but traits reflecting viability are not necessarily reflecting parasite loads (Getty 2002). For instance, from a female point of view, highly infested males might be better able to cope with infections and demonstrate improved parasite tolerance as they were able to develop similar or better secondary sexual ornaments than their competitors. In line with this, Ressel and Schall (1989) and Megía‐Palma et al. (2016) found a positive correlation between color, ornament size, and parasite load in lizard species. Producing and maintaining such ornaments while bearing parasites may be physiologically costly and only high-quality individuals will be able to afford it (Folstad and Karter 1992; McGraw and Hill 2000).
Another possible explanation could be that female house sparrows are manipulated by the behavior of highly infected males. The fact that infected males spent more time interacting with focal females suggests that these males increased their mating investment toward the choosing female, which may in turn influence the female’s choice. Thus, when ornaments do not reflect infections, male behavior such as the number of approaches to the female could be an important factor in mate-choice decisions of female house sparrows, and this could imply that they are selecting highly infected males. In fact, it has been suggested that parasitized males may increase their behavioral display intensity to compensate for a potential loss in attractiveness (Reynolds 1993) or to secure the current reproduction (Polak and Starmer 1998; Candolin 2000). Females may get an advantage to pair with those males if they are resistant or tolerant to infection (Zahavi 1975). Namely, males with high parasite loads are of higher quality and are dominant but are not as good at feeding nestlings (Howard and Lively 2004). Accordingly, females with acute infection may need more energy and resources for themselves and also more assistance with offspring care. Thus, females should prefer subdominant individuals with medium-chronic-level infestation and better paternal skills (Forsgren 1997).
The experimental results also support our second prediction; medicated females did not avoid parasitized mates, but infected females did (Figure 4B). This means that female house sparrows seem to be able to discriminate between males according to their infection status based on their behavior and independent of their ornament expression. Based on this, females can adopt preference strategies specific to their own parasite status. Their ability to recognize male parasite status is likely given that males seem to be able to recognize the parasite status of conspecifics (see Dolnik and Hoi 2010). Males with high parasite loads are of higher quality and are dominant but are not as good at feeding nestlings (Howard and Lively 2004). Accordingly, females with an acute infection may need more energy and resources for themselves and also more assistance with offspring care. Thus, females should prefer subdominant individuals with a medium-chronic-level infestation and better paternal skills (Forsgren 1997). Since ornament size is controlled for in this study, it is very likely that behavioral signals play a role in determining parasite infestation and influencing female mate choice. We were also able to show that males adjust their behavior in response to their own chronic coccidia infection levels. Thus, in both studies, evidence for parasite discrimination has been found based on experimental manipulation of infection (either cured or additionally infected). However, the rather extreme experimental change in parasite loads may mean that we cannot draw conclusions about behavior under natural circumstances. It remains to be investigated how important male behavior is to gather relevant information about parasite intensity under chronic infection conditions.
Highly infected females may not benefit from mating with a highly infested male, because mating with such a male may increase the risk of infection with additional coccidia species. Alternatively, as we have already mentioned, experimentally infected females, in particular, may suffer from elevated coccidian loads resulting in a reduced health condition and motivation to invest in mate choice. Early exposure to coccidian species might be beneficial for the developing offspring. The chances of surviving an infection might be higher when offspring are still being fed by the parents (nestling period) compared to the first time of independence (early fledgling period), when their own feeding skills are still poor. Furthermore, one may predict that the developing immune system may better cope and adapt to challenges when resources are not constrained (food provided by the parents). These early immune system adaptations may further increase the likelihood of an appropriate immune response when challenged later on. Therefore, females should not necessarily try to protect their offspring from infections; rather they should try to facilitate early infections with diverse coccidian species.
Further studies are necessary to address the importance of behavior in signaling parasite infection and how accurately parasite infestation might be reflected. Furthermore, it is important to understand in more detail, how infestation rate and parasite species diversity may influence the risk of novel infections or re-infection.
Supplementary Material
Acknowledgments
We thank Christa Grabmayer and Wolfgang Pegler who coordinated the care of the house sparrows. We thank the anonymous reviewers for their careful reading of our manuscript and their many insightful comments and suggestions.
This paper is dedicated to the memory of Professor Matteo Griggio who passed suddenly away on May 15, 2020.
Contributor Information
Alejandro Cantarero, Department of Physiology, Veterinary School, Complutense University of Madrid, Avenida Puerta de Hierro s/n, 28040 Madrid, Spain.
Olga V Dolnik, Department of Integrative Biology and Evolution, Konrad Lorenz Institute of Ethology (KLIVV), University of Veterinary Medicine Vienna, Savoyenstraße 1a, A-1160 Vienna, Austria.
Matteo Griggio, Dipartimento di Biologia, Università di Padova, Via U. Bassi 58/B, I-35131 Padova, Italy.
Herbert Hoi, Department of Integrative Biology and Evolution, Konrad Lorenz Institute of Ethology (KLIVV), University of Veterinary Medicine Vienna, Savoyenstraße 1a, A-1160 Vienna, Austria.
Conflict of Interest statement
The authors declare no conflicts of interest.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were strictly followed. All animal sample collection protocols complied with the current laws of Austria.
Author Contributions
HH and MG conceived the study. MG and OD performed the experiment and conducted lab analyses. ACC and HH analyzed the data. ACC wrote the manuscript with input from all coauthors. All authors approve the publication and are accountable for this work.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- Abdi H, 2007. The Bonferroni and Sidák corrections for multiple comparisons. In: Salkind N editor. Encyclopedia of Measurement and Statistics. Thousand Oaks (CA): Sage, 1–9. [Google Scholar]
- Aguilar TM, Maia R, Santos ESA, Macedo RH, 2008. Parasite levels in blue-black grassquits correlate with male displays but not female mate preference. Behav Ecol 19:292–301. [Google Scholar]
- Anna MP, Rafal K, Aleksander WD, 2011. The annual cycle of shedding Eimeria oocysts by European bison Bison bonasus in the Bialowieza Primeval Forest, Poland. J Parasitol 97:737–739. [DOI] [PubMed] [Google Scholar]
- Badás EP, Martínez J, Rivero-de Aguilar J, Ponce C, Stevens Met al. , 2018. Colour change in a structural ornament is related to individual quality, parasites and mating patterns in the blue tit. Sci Nat 105:17. [DOI] [PubMed] [Google Scholar]
- Beltran-Bech S, Richard F-J, 2014. Impact of infection on mate choice. Anim Behav 90:159–170. [Google Scholar]
- Borgia G, Collis K, 1989. Female choice for parasite-free male satin bowerbirds and the evolution of bright male plumage. Behav Ecol Sociobiol 25:445–453. [Google Scholar]
- Brawner WR, Hill GE, Sundermann CA, 2000. Effects of coccidial and mycoplasmal infections on carotenoid-based plumage pigmentation in male house finches. Auk 117:952–963. [Google Scholar]
- Buchholz R, 2004. Effects of parasitic infection on mate sampling by female wild turkeys Meleagris gallopavo: Should infected females be more or less choosy? Behav Ecol 15:687–694. [Google Scholar]
- Burley NT, Foster VS, 2006. Variation in female choice of mates: Condition influences selectivity. Anim Behav 72:713–719. [Google Scholar]
- Candolin U, 2000. Increased signalling effort when survival prospects decrease: Male-male competition ensures honesty. Anim Behav 60:417–422. [DOI] [PubMed] [Google Scholar]
- Cantarero A, López-Arrabé J, Redondo AJ, Moreno J, 2013. Behavioural responses to ectoparasites in Pied Flycatchers Ficedula hypoleuca: An experimental study. J Avian Biol 44:591–599. [Google Scholar]
- Clayton DH, 1990. Mate choice in experimentally parasitized rock doves: Lousy males lose. Integr Comp Biol 30:251–262. [Google Scholar]
- Dolnik OV, 1999a. Diurnal oocyst periodicity in Isospora dilatata (Sporozoa, Eimeriidae) from the common starling Sturnus vulgaris in nature. Parasitologiya 33:74–80. [Google Scholar]
- Dolnik OV, 1999b. Diurnal periodicity in appearance of Isospora (Protozoa: Coccidea) oocysts from some passerine birds. Proc Zool Inst RAS 281:113–118. [Google Scholar]
- Dolnik OV, 2002. Some Aspects of the Biology and Host-Parasite Interactions of Isospora Spp. (Protozoa: Coccidiida) of Passerine Birds. Dissertation For Doctoral Degree, Oldenburg University. [Google Scholar]
- Dolnik OV, 2006. The relative stability of chronic Isospora sylvianthina (Protozoa: Apicomplexa) infection in blackcaps Sylvia atricapilla: Evaluation of a simplified method of estimating isosporan infection intensity in passerine birds. Parasitol Res 100:155–160. [DOI] [PubMed] [Google Scholar]
- Dolnik OV, Dolnik VR, Bairlein F, 2010. The effect of host foraging ecology on the prevalence and intensity of coccidian infection in wild passerine birds. Ardea 98:97–103, 107. [Google Scholar]
- Dolnik OV, Hoi H, 2010. Honest signalling, dominance hierarchies and body condition in house sparrows Passer domesticus (Aves: Passeriformes) during acute coccidiosis. Biol J Linn Soc 99:718–726. [Google Scholar]
- Dolnik OV, Palinauskas V, Bensch S, 2009. Individual oocysts of Isospora (Apicomplexa: Coccidia) parasites from avian feces: From photo to sequence. J Parasitol 95:169–174. [DOI] [PubMed] [Google Scholar]
- Figuerola J, Muñoz E, Gutiérrez R, Ferrer D, 1999. Blood parasites, leucocytes and plumage brightness in the cirl bunting Emberiza cirlus. Funct Ecol 13:594–601. [Google Scholar]
- Fisher DO, Dickman CR, Jones ME, Blomberg SP, 2013. Sperm competition drives the evolution of suicidal reproduction in mammals. Proc Natl Acad Sci USA 110:17910–17914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitze PS, Richner H, 2002. Differential effects of a parasite on ornamental structures based on melanins and carotenoids. Behav Ecol 13:401–407. [Google Scholar]
- Folstad I, Karter AJ, 1992. Parasites, bright males, and the immunocompetence handicap. Am Nat 139:603–622. [Google Scholar]
- Forsgren E, 1997. Female sand gobies prefer good fathers over dominant males. Proc R Soc Lond B 264:1283–1286. [Google Scholar]
- Garamszegi LZ, Møller AP, Erritzoe J, 2003. The evolution of immune defense and song complexity in birds. Evolution 57:905–912. [DOI] [PubMed] [Google Scholar]
- Getty T, 2002. Signaling health versus parasites. Am Nat 159:363–371. [DOI] [PubMed] [Google Scholar]
- Griggio M, Biard C, Penn DJ, Hoi H, 2011. Female house sparrows “count on” male genes: Experimental evidence for MHC-dependent mate preference in birds. BMC Evol Biol 11:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griggio M, Hoi H, 2010. Only females in poor condition display a clear preference and prefer males with an average badge. BMC Evol Biol 10:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griggio M, Hoi H, Pilastro A, 2010. Plumage maintenance affects ultraviolet colour and female preference in the budgerigar. Behav Processes 84:739–744. [DOI] [PubMed] [Google Scholar]
- Grulet O, Landau I, Baccam D, 1982. Isospora from the domestic sparrow: Multiplicity of species. Ann Parasitol Hum Comp 57:209–235. [PubMed] [Google Scholar]
- Grulet O, Landau I, Millet P, Baccam D, 1986. Les Isospora du Moineau. Ann Parasitol Hum Comp 61:161–192. [PubMed] [Google Scholar]
- Hallal-Calleros C, Morales-Montor J, Vázquez-Montiel JA, Hoffman KL, Nieto-Rodríguez Aet al. , 2013. Hormonal and behavioral changes induced by acute and chronic experimental infestation with Psoroptes cuniculi in the domestic rabbit Oryctolagus cuniculus. Parasit Vectors 6:361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton WD, Zuk M, 1982. Heritable true fitness and bright birds: A role for parasites? Science 218:384–387. [DOI] [PubMed] [Google Scholar]
- Hõrak P, Ots I, Vellau H, Spottiswoode C, Møller AP, 2001. Carotenoid-based plumage coloration reflects hemoparasite infection and local survival in breeding great tits. Oecologia 126:166–173. [DOI] [PubMed] [Google Scholar]
- Hõrak P, Saks L, Karu U, Ots I, Surai PFet al. , 2004. How coccidian parasites affect health and appearance of greenfinches. J Anim Ecol 73:935–947. [Google Scholar]
- Howard RS, Lively CM, 2004. Good vs complementary genes for parasite resistance and the evolution of mate choice. BMC Biol 4:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingerman CM, Patel A, Hedges VL, Meisel RL, Schneider JE, 2011. Food restriction dissociates sexual motivation, sexual performance, and the rewarding consequences of copulation in female Syrian hamsters. Behav Brain Res 223:356–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight A, Ewen JG, Brekke P, Santure AW.. 2018. The evolutionary biology, ecology and epidemiology of coccidia of passerine birds. In: Rollinson D, Stothard JR editors. Advances in Parasitology. London: Academic Press, 35–60. [DOI] [PubMed] [Google Scholar]
- Kurtz J, Sauer KP, 1999. The immunocompetence handicap hypothesis: Testing the genetic predictions. Proc Biol Sci 266:2515–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Arrabé J, Cantarero A, Pérez-Rodríguez L, Palma A, Alonso-Alvarez Cet al. , 2015. Nest-dwelling ectoparasites reduce antioxidant defences in females and nestlings of a passerine: A field experiment. Oecologia 179:29–41. [DOI] [PubMed] [Google Scholar]
- Lopez S, 1999. Parasitized female guppies do not prefer showy males. Anim Behav 57:1129–1134. [DOI] [PubMed] [Google Scholar]
- Lozano GA, 1994. Carotenoids, parasites, and sexual selection. Oikos 70:309–311. [Google Scholar]
- Martin TE, Møller AP, Merino S, Clobert J, 2001. Does clutch size evolve in response to parasites and immunocompetence? Proc Natl Acad Sci USA 98:2071–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw KJ, Hill GE, 2000. Differential effects of endoparasitism on the expression of carotenoid- and melanin-based ornamental coloration. Proc R Soc Lond B 267:1525–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megía-Palma R, Martínez J, Merino S, 2016. A structural colour ornament correlates positively with parasite load and body condition in an insular lizard species. Sci Nat 103:52. [DOI] [PubMed] [Google Scholar]
- Milde K, 1979. Light and electron microscopic studies on isosporan parasites (Sporozoa) in sparrows (Passer domesticus L.). Protistologica 15:607–627. [Google Scholar]
- Milinski M, Bakker TCM, 1990. Female sticklebacks use male coloration in mate choice and hence avoid parasitized males. Nature 344:330–333. [Google Scholar]
- Møller AP, 1988. Badge size in the house sparrow Passer domesticus. Behav Ecol Sociobiol 22:373–378. [Google Scholar]
- Mougeot F, Martínez-Padilla J, Webster LMI, Blount JD, Pérez-Rodríguez Let al. , 2009a. , Honest sexual signalling mediated by parasite and testosterone effects on oxidative balance. Proc R Soc B 276:1093–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mougeot F, Pérez-Rodríguez L, Martínez-Padilla J, Leckie F, Redpath SM, 2007. Parasites, testosterone and honest carotenoid-based signalling of health. Funct Ecol 21:886–898. [Google Scholar]
- Mougeot F, Pérez-Rodríguez L, Sumozas N, Terraube J, 2009b. Parasites, condition, immune responsiveness and carotenoid-based ornamentation in male red-legged partridge Alectoris rufa. J Avian Biol 40:67–74. [Google Scholar]
- Nuismer SL, Otto SP, 2004. Host-parasite interactions and the evolution of ploidy. Proc Natl Acad Sci USA 101:11036–11039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pap PL, Csongor István V, Gábor Árpád C, Adriana T, Adela Pet al. , 2009. Carotenoids modulate the effect of coccidian infection on the condition and immune response in moulting house sparrows. J Exp Biol 212:3228–3235. [DOI] [PubMed] [Google Scholar]
- Penczykowski RM, Laine A-L, Koskella B, 2016. Understanding the ecology and evolution of host-parasite interactions across scales. Evol Appl 9:37–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfennig KS, Tinsley RC, 2002. Different mate preferences by parasitized and unparasitized females potentially reduces sexual selection. J Evol Biol 15:399–406. [Google Scholar]
- Polak M, Starmer WT, 1998. Parasite–induced risk of mortality elevates reproductive effort in male Drosophila. Proc R Soc Lond B 265:2197–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potti J, Merino S, 1996. Decreased levels of blood trypanosome infection correlate with female expression of a male secondary sexual trait: Implications for sexual selection. Proc R Soc Lond B 263:1199–1204. [Google Scholar]
- Poulin R, 1994. Mate choice decisions by parasitized female upland bullies Gobiomorphus breviceps. Proc R Soc Lond B 256:183–187. [Google Scholar]
- Ressel S, Schall JJ, 1989. Parasites and showy males: Malarial infection and color variation in fence lizards. Oecologia 78:158–164. [DOI] [PubMed] [Google Scholar]
- Reynolds JD, 1993. Should attractive individuals court more? Theory and a test. Am Naturalist 141:914–927. [DOI] [PubMed] [Google Scholar]
- Schrenzel MD, Maalouf GA, Gaffney PM, Tokarz D, Keener LLet al. , 2005. Molecular characterization of isosporoid Coccidia (Isospora and Atoxoplasma spp.) in passerine birds. J Parasitol 91:635–647. [DOI] [PubMed] [Google Scholar]
- Schröder I, 2019. Biological safety: Principles and practices, 5th edition. Emerg Infect Dis 2019 25:195. [Google Scholar]
- Sepp T, Karu U, Blount JD, Sild E, Männiste Met al. , 2012. Coccidian infection causes oxidative damage in greenfinches. PLoS ONE 7:e36495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon BC, Verhulst S, 1996. Ecological immunology: Costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol Evol 11:317–321. [DOI] [PubMed] [Google Scholar]
- Simmons LW, 1994. Courtship role reversal in bush crickets: Another role for parasites? Behav Ecol 5:259–266. [Google Scholar]
- Sorci G, Cézilly F, 2008. Interspecific parasitism and mutualism. In: Danchin É, Giraldeau LA, Cézilly F editors. Behavioural Ecology: An Evolutionary Perspective on Behaviour. New York: Oxford Univeristy Press, 5–17. [Google Scholar]
- Spencer KA, Buchanan KL, Leitner S, Goldsmith AR, Catchpole CK, 2005. Parasites affect song complexity and neural development in a songbird. Proc Biol Sci 272:2037–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmacki A, Hill GE, 2014. Coccidial infection does not influence preening behavior in American goldfinches. Acta Ethol 17:107–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Václav R, Hoi H, 2002. Different reproductive tactics in house sparrows signalled by badge size: Is there a benefit to being average? J Ethol 108:569–582. [Google Scholar]
- Wisenden BD, Goater CP, James CT, 2009. Behavioral defenses against parasites and pathogens. In: Zaccone G, Perrière C, Mathis A, Kapoor BG editors. Fish Defenses. Enfield: Science Publishers, 151–168. [Google Scholar]
- Worden BD, Parker PG, Pappas PW, 2000. Parasites reduce attractiveness and reproductive success in male grain beetles. Anim Behav 59:543–550. [DOI] [PubMed] [Google Scholar]
- Zahavi A, 1975. Mate selection: A selection for a handicap. J Theor Biol 53:205–214. [DOI] [PubMed] [Google Scholar]
- Zuk M, Stoehr AM, 2002. Immune defense and host life history. Am Nat 160:S9–S22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.