Abstract
Background and Objectives
White matter hyperintensities (WMH) are pathologic brain changes that are associated with increased age and cognitive decline. However, the association of WMH burden with amyloid positivity and conversion to dementia in people with mild cognitive impairment (MCI) is unclear. The aim of this study was to expand on this research by examining whether change in WMH burden over time differs in amyloid-negative (Aβ−) and amyloid-positive (Aβ+) people with MCI who either remain stable or convert to dementia. To examine this question, we compared regional WMH burden in 4 groups: Aβ+ progressor, Aβ− progressor, Aβ+ stable, and Aβ− stable.
Methods
Participants with MCI from the Alzheimer Disease Neuroimaging Initiative were included if they had APOE ɛ4 status and if amyloid measures were available to determine amyloid status (i.e., Aβ+, or Aβ−). Participants with a baseline diagnosis of MCI and who had APOE ɛ4 information and amyloid measures were included. An average of 5.7 follow-up time points per participant were included, with a total of 5,054 follow-up time points with a maximum follow-up duration of 13 years. Differences in total and regional WMH burden were examined using linear mixed-effects models.
Results
A total of 820 participants (55–90 years of age) were included in the study (Aβ+ progressor, n = 239; Aβ− progressor, n = 22; Aβ+ stable, n = 343; Aβ− stable, n = 216). People who were Aβ− stable exhibited reduced baseline WMH compared with Aβ+ progressors and people who were Aβ+ stable at all regions of interest (β belongs to 0.20–0.33, CI belongs to 0.03–0.49, p < 0.02), except deep WMH. When examining longitudinal results, compared with people who were Aβ− stable, all groups had steeper accumulation in WMH burden with Aβ+ progressors (β belongs to −0.03 to 0.06, CI belongs to −0.05 to 0.09, p < 0.01) having the largest increase (i.e., largest increase in WMH accumulation over time).
Discussion
These results indicate that WMH accumulation contributes to conversion to dementia in older adults with MCI who are Aβ+ and Aβ−.
Introduction
Mild cognitive impairment (MCI) is characterized by declines in cognitive functioning that are more severe than what is observed in normal aging, but do not interfere with activities of daily living.1 People with MCI experience both an increased rate of cognitive decline and convert to dementia with a higher annual conversion rate compared with healthy older adults.1,2 While not everyone with MCI converts to dementia, this stage of decline is often identified as a transitional stage between healthy aging and dementia. Much research has thus focused on studying people with MCI to identify who will convert to dementia. More specifically, researchers endeavor to find early biomarkers in people with MCI who convert to dementia (hereafter referred to as progressive MCI [pMCI]) that distinguish them from people with MCI who do not convert to dementia (hereafter referred to as stable MCI [sMCI]). Identifying who will convert from MCI to dementia has been difficult in clinical practice because of the heterogeneous nature of MCI. However, 1 approach is to examine people with MCI who have biomarkers that increase their risk of developing dementia. For example, individuals with MCI positive for β-amyloid (Aβ) or tau are at an increased risk of converting to Alzheimer disease (AD) because they may be in the initial symptomatic stages of AD (see Sperling et al.3 for a review).
Other pathologic brain changes, such as white matter hyperintensities (WMH), contribute to healthy older adults' risk of cognitive decline4 and conversion to MCI or dementia.5-7 WMH are observed as an increased signal in T2-weighted (T2w) or fluid-attenuated inversion recovery (FLAIR) MRIs. WMH are used as a proxy for cerebrovascular disease, a known contributor for cognitive decline and dementia.8-10 High WMH burden is associated with increased cognitive decline in MCI6,11-13 and increased conversion from MCI to dementia.14 Another study observed over an 18-month period that pMCI was associated with increased periventricular and deep WMH compared with sMCI.15 However, the sample size in this study was quite limited. In a much larger sample of 591 people with MCI, it was observed that those with pMCI had increases in total WMH volume compared with those with sMCI (with mean follow-up times of 2–2.5 years).14 While these studies offer insight into the association between WMH and MCI, the follow-up periods are quite short, given that the annual conversion rate from MCI to dementia in community and clinic samples is only 3% and 13%, respectively.16
Several limitations exist in the current research examining the relationship between WMH and pMCI vs sMCI. These studies used total WMH burden that captures overall lesion volume affecting the whole brain. Examining regional WMH is important when investigating conversion to dementia because different patterns of WMH accumulation are associated with different types of dementia. For example, a more widespread WMH accumulation in MCI is associated with conversion to vascular dementia,17 whereas posterior WMH are associated with AD.18,19 These findings suggest that evaluation of regional WMH differences may provide insight into the type of dementia someone with MCI will develop. Furthermore, previous studies have not examined WMH differences between pMCI and sMCI who are amyloid positive (Aβ+) and amyloid negative (Aβ−). Those with MCI who are Aβ+ are on the AD trajectory, whereas those who are Aβ− are more likely to develop other types of dementia. This difference in diagnostic outcome may influence WMH burden. Previous research examining amyloid positivity in mild amnestic dementia found that those who are Aβ+ have increased WMH compared with those who are Aβ−. Ultimately, while previous studies have observed that people with pMCI have increased WMH burden compared with those with sMCI, they have limited sample size, short follow-up times, do not examine regional WMH burden, and have not compared Aβ+ and Aβ− participants. More research is therefore needed to understand the relationship between WMH burden with amyloid positivity in those who convert from MCI to dementia.
This study was designed to expand on the current research by examining WMH burden between Aβ+ and Aβ− pMCI and sMCI participants. The goal of this study was to examine whether WMH influence conversion to dementia in people with MCI who were either Aβ− or Aβ+. In addition, we sought to examine how much of WMH progression is associated with amyloid positivity vs vascular risk factors by including vascular risk factors into our models. This distinction between amyloid vs vascular causes of WMH is essential because many vascular risk factors associated with WMH may be treatable or preventable.20-22 Therefore, if we observe that WMH are associated with the vascular risk factors, then conversion to dementia may be mitigated or prevented by treating these underlying risk factors.
Methods
Participants
Data used in this article were obtained from the Alzheimer Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). All participants were between 55 and 90 years of age during recruitment and exhibited no evidence of depression. Participants with MCI (N = 820) with both APOE ɛ4 status and amyloid measures were included in the study. These participants had a maximum follow-up period of 13 years, with a total of 5,054 follow-up time points and an average of 5.7 follow-up time points per participant.
Participants with MCI were divided into 4 groups: (1) Aβ+ progressor, (2) Aβ− progressor, (3) Aβ+ stable, (4) Aβ− stable. Both PET and CSF values were used to determine amyloid positivity in people with MCI because some participants had only 1 measurement available. Additional participant information can be found in the eMethods (links.lww.com/WNL/C955).
WMH Measurements
In this study, WMH reflect decreased signal (i.e., hypointensities) in the white matter tissue of the brain observed on T1-weighted (T1w) images, corresponding to T2w and FLAIR hyperintensities and are used as an indicator of cerebral small vessel disease (i.e., cerebrovascular pathology). WMH volumes were computed for total brain, deep, periventricular volumes and by region for frontal, temporal, parietal, and occipital WMH volumes. Details on structural MRI acquisition and processing of WMH can be found in the eMethods (links.lww.com/WNL/C955).
Statistical Analysis
Linear regression models were used to investigate baseline group differences in total, deep, periventricular, and regional WMH loads (frontal, temporal, parietal, and occipital), including age, sex, years of education, and APOE ɛ4 status as covariates. The categorical variable of interest was group status (Aβ+ progressor, Aβ− progressor, Aβ+ stable, and Aβ− stable) to examine whether group status influenced baseline WMH (eSAP1, models 1 and 2, links.lww.com/WNL/C955).
For longitudinal assessments, WMH load at different visits was examined using linear mixed-effects models. WMH were the dependent variable of interest, and the interaction between follow-up time and group status was examined (eSAP1, model 3, links.lww.com/WNL/C955).
Additional models were added to examine the effect of continuous Aβ (extracted based on CSF Aβ) on WMH and cognition (eSAP1, models 4 and 5, links.lww.com/WNL/C955). The impact of WMH and Aβ on atrophy and conversion to dementia were also examined using linear mixed-effects models (eSAP1, models 6 and 7). Receiver operating characteristic (ROC) curve analysis was completed to examine differences between Aβ+ progressors and Aβ+ stable based on the longitudinal slope of WMH. The predictive value of total and regional WMH was assessed separately within groups with different baseline Clinical Dementia Rating–Sum of Boxes (CDR-SB) values. Detailed information is provided in eSAP for all the additional models, baseline, and longitudinal assessments.
While our T1w-based WMH have very high correlations with FLAIR-based WMH (r = 0.97, p < 0.0001), they might not capture the whole extent of WMH. To investigate whether this might affect the findings, we repeated the main analyses for whole brain WMH, separately in the ADNI1 and ADNI2/GO, which had consistently acquired T2w/PD (for ADNI1) and FLAIR (for ADNI2/GO) sequences within each study. The same WMH segmentation tool was used to extract WMH volumes based on T1w + T2w + PD sequences in the ADNI1 and T1 + FLAIR sequences in ADNI2/GO.e9 Demographic and clinical information for the ADNI1 and ADNI2/GO subsets were also compared with the full sample used to perform the primary analyses.
Results
Baseline Demographics and Cognitive Scores
eTable 1 (links.lww.com/WNL/C955) summarizes demographic information and clinical characteristics. Of the 820 individuals with MCI at baseline, 455 were diagnosed with dementia at follow-up visits. Individuals who were Aβ− stable were younger than both Aβ+ progressors (x2 = −3.48, p < 0.001) and those who were Aβ+ stable (x2 = −4.09, p < 0.001). The proportion of people with APOE ɛ4+ differed between all groups except Aβ− stable and Aβ− progressors (x2 belongs to 12–122, p < 0.001), with Aβ+ progressors having the highest proportion of people with APOE ɛ4+, followed by Aβ+ stable, Aβ− stable, and last, Aβ− progressors. Years of education and proportion of male to female individuals did not differ between any of the groups. We found significant differences in Alzheimer Disease Assessment Scale–13 (ADAS-13) scores between Aβ− stable and Aβ− progressor groups, Aβ− stable and Aβ+ stable groups, Aβ− stable and Aβ+ progressor groups, and Aβ+ stable and Aβ+ progressor groups (t belongs to 4.43–21.12, p < 0.001). For CDR-SB scores, the Aβ+ progressor group differed from Aβ+ and Aβ− stable groups (t belongs to 7.71–7.86, p < 0.001) with the Aβ+ progressor group exhibiting higher cognitive performance scores than Aβ+ and Aβ− stable groups. Body mass index (BMI) was lower in Aβ+ progressors than in the Aβ− stable group (t = −3.97, p < 0.001). As for vascular risk factors, hypertension and diabetes were not different between groups.
To examine potential selection biases in our sample, participants who were excluded because of missing measurements (i.e., amyloid, APOE ɛ4 status, or MRI scans) were compared with our full sample. Demographic and clinical characteristics are summarized in eTable 2 (links.lww.com/WNL/C955). The sample that was excluded was similar in terms of age, sex, and education levels, but had higher CDR-SB (1.6 vs 1.4, t = 2.85, p = 0.004) and ADAS-13 scores (17.1 vs 15.9, t = 2.98, p = 0.002) compared with the participants included in our sample.
Finally, we compared the demographic and clinical information from the full sample (i.e., those with T1w images) with the ADNI1 (i.e., those with T1w + T2w + PD) and ADNI2/GO (i.e., those with T1w + FLAIR). Demographic and clinical characteristics were not different across the 3 groups (eTables 3 and 4, links.lww.com/WNL/C955).
Baseline Assessments
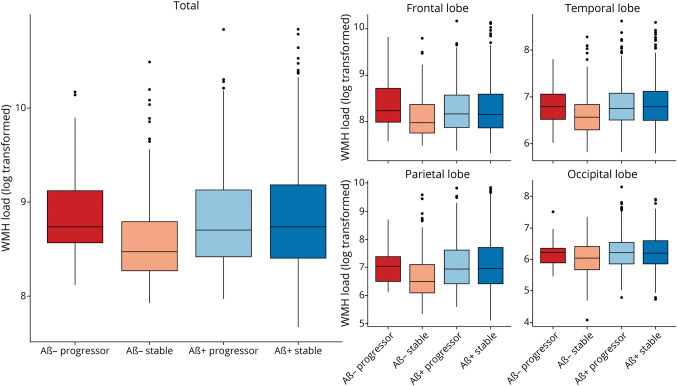
Figure 1 shows boxplots of baseline total WMH load and separately for each lobe for all groups. eTables 5 and 6 (links.lww.com/WNL/C955) summarize the linear regression model results for baseline WMH for all groups across all regions. Both Aβ+ groups exhibited greater WMH burden than the Aβ− stable group (Aβ+ progressor: t belongs to 2.65–3.73, p < 0.001, and Aβ+ stable: t belongs to 2.35–3.94, p < 0.01) at all regions of interest, except for deep WMH.
Figure 1. Boxplots Showing Baseline WMH Distributions (Log Transformed) Across Diagnostic Groups for Each Lobe.
Baseline WMH distributions (log transformed) across diagnostic groups for each lobe. The first and second rows show the log-transformed WMH loads for each group by lobe. Aβ+ progressor, Aβ− progressor, Aβ+ stable, and Aβ− stable. Aβ− = amyloid negative; Aβ+ = amyloid positive; WMH = white matter hyperintensity.
Longitudinal Assessments
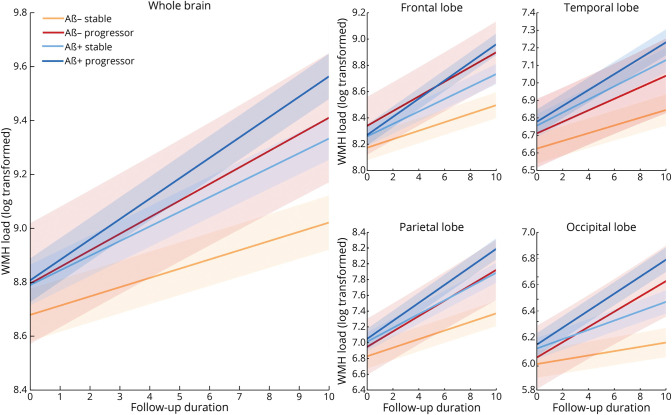
Figure 2 shows the longitudinal WMH distributions separately for each lobe for all groups. eTables 7 and 8 (links.lww.com/WNL/C955) summarize the results of the longitudinal linear mixed-effects models for all groups contrasted against the Aβ− stable group across all WMH regions. Follow-up time was associated with increase in WMH load in the Aβ− stable group (t belongs to 7.15–21.33, p < 0.001). Aβ− progressors (t belongs to 4.45–5.22, p < 0.001) had increased WMH load change over time compared with the Aβ− stable group at all regions except the temporal region. Individuals who were Aβ+ stable (t belongs to 6.12–9.30, p < 0.001) and Aβ+ progressors (t belongs to 10.77–19.17, p < 0.001) had increased WMH load change over time in all regions compared with the Aβ− stable group. Aβ+ progressors (t belongs to 3.87–11.01, p < 0.001) had increased WMH load change over time compared with the Aβ+ stable group. Aβ− progressors (t = 2.89, p = 0.004) had increased WMH load change over time compared with the Aβ+ stable group at only the occipital region. Aβ+ progressors (t belongs to 5.07–6.45, p < 0.02) had increased WMH load change over time in all regions compared with the Aβ− progressor group at total, frontal, and parietal regions only. That is, compared with the Aβ− stable group, increasingly steeper change over time was observed in Aβ− progressors, followed by the Aβ+ stable group and Aβ+ progressors. To ensure that the minimum duration of follow-up time did not affect these results, the models were repeated including only participants with a minimum of 1, 2, and 3 years of follow-up, yielding similar results in terms of effect size and significance (eTables 9 and 10).
Figure 2. Longitudinal Change in Total and Regional WMH Volume by Group.
Longitudinal WMH distributions (log transformed) across diagnostic groups for each lobe. The first and second rows show the log-transformed WMH loads for each group by lobe. Aβ+ progressor, Aβ− progressor, Aβ+ stable, and Aβ− stable. Aβ− = amyloid negative; Aβ+ = amyloid positive; WMH = white matter hyperintensity.
To investigate whether using T1w-based WMH biased our results, we repeated the longitudinal WMH analyses for whole brain WMH with T2w/PD-based (for ADNI1) and FLAIR-based (for ADNI2/GO) WMH. Our analyses showed similar results to the T1w-based analyses (eTables 11 and 12, links.lww.com/WNL/C955).
Continuous Amyloid Measure
eTable 13 (links.lww.com/WNL/C955) summarizes the linear mixed-model results for WMH burden and Aβ across all regions. There was a significant association between Aβ and WMH (t belongs to −4.43 to −2.11, p < 0.05) for all regions of interest.
There was a significant interaction between regional WMH and Aβ on cognition. Higher WMH and lower CSF Aβ (i.e., increased levels of Aβ in the brain) was associated with higher CDR-SB score (i.e., worse cognitive performance) at total, frontal, and parietal regions (t belongs to −3.26 to −2.52, p < 0.01). These results are summarized in eTable 14 (links.lww.com/WNL/C955).
WMH, Amyloid, and Brain Atrophy
We observed a significant impact of WMH (t = −2.03, p = 0.04) and a significant interaction between Aβ positivity and WMH burden (t = −4.97, p < 0.001) on hippocampal atrophy. For the entorhinal cortex, we observed independent impacts of WMH (t = −2.32, p = 0.02) and Aβ positivity (t = −3.18, p = 0.001), but not an interaction. For the medial temporal lobe, we observed both significant impacts of WMH (t = −2.14, p = 0.03) and Aβ positivity (t = −3.03, p = 0.002) and a significant interaction (t = −6.58, p < 0.001). Results are summarized in eTable 15 (links.lww.com/WNL/C955).
Conversion
The generalized mixed-effects models showed independent impacts of WMH (t = 3.68, p < 0.001), and Aβ positivity (t = 5.32, p < 0.001), but not their interaction (t = −1.60, p = 0.11) on conversion to dementia.
Vascular Risk Factors
At baseline assessment, BMI was not significantly associated with WMH at any region. Diabetes was observed to be associated with WMH only in the occipital region (t = −2.12, p = 0.03) showing no relationship in the other regions. Hypertension was found to be associated with WMH in total volume (t = 3.38, p < 0.001) at all regions (t belongs to 2.14–3.70, p < 0.035) except the occipital region.
At longitudinal assessment, BMI and diabetes were not associated with WMH at any region (p > 0.05). Hypertension was found to be associated with total WMH volume (t = 4.00, p < 0.001) and at all regions (t belongs to 2.50–4.33, p < 0.015) except the occipital region. Although not significant, the strongest association between WMH and diabetes was observed in the occipital region (t = −1.90, p = 0.056).
The Aβ+ progressors had a longer maximum follow-up year compared with Aβ+ stable and Aβ− stable groups. To ensure group differences were not driven by the variation in maximum follow-up year, all analyses were rerun matching a subsample of the Aβ+ progressors to the other groups in terms of maximum follow-up year. All results remained the same in terms of effect size and significance. Additional secondary analyses were completed on the subset of participants (N = 767) with baseline triglyceride, smoking, serum glucose, and cholesterol levels and indication of cardiovascular disease (identified as 1 = yes, 0 = no). This information was downloaded from the ADNI. We observed that only serum glucose levels were associated with baseline WMH burden at all measures and regions except occipital and periventricular regions (t belongs to 2.28–2.89, p < 0.05). The main effects of these factors on WMH burden by region are summarized in eTables 16 and 17 (links.lww.com/WNL/C955). Of importance, including these extravascular risk factors did not change the effect size or significance of the main effects of interest (group status).
ROC for Classification: Aβ+ Converters vs Nonconverters
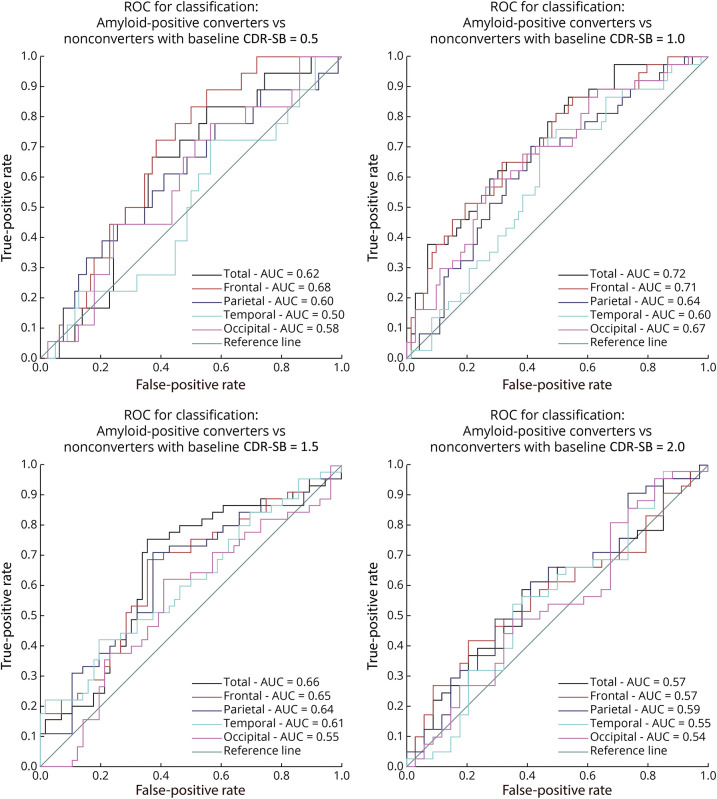
To distinguish Aβ+ progressors from those who were Aβ+ stable based on the longitudinal WMH slope, we compared the predictive value of total and regional WMH for each baseline CDR-SB value (0.5, 1, 1.5, and 2). As expected, conversion ratios were very different across groups with different baseline CDR-SB scores. For baseline CDR-SB value of 0.5 (N = 111), conversion ratio was observed at 0.1875. For CDR-SB of 1 (N = 118), 1.5 (N = 109), and 2 (N = 85), conversion ratios were observed at 0.3364, 0.4455, and 0.5467, respectively. ROC curve analyses showed that WMH slopes were able to differentiate between stable and progressor individuals. Classification performance was highest for the group with baseline CDR-SB value of 1, with best differentiation seen for the frontal region (area under the ROC curve [AUC] = 0.71, Figure 3) and total WMH (AUC = 0.72, Figure 3). Overall, total and frontal WMH slopes were very good differentiators of conversion, suggesting that WMH accumulation may contribute to AD conversion, particularly in individuals in the initial stages of cognitive impairment (i.e., CDR-SB < 2).
Figure 3. ROC Analysis to Compare the AUC of Amyloid Positive Converters With Nonconverters.
ROC analysis to compare the AUC of amyloid-positive converters with nonconverters at 0.5, 1.0, 1.5, and 2.0 CDR-SB scores for regional and total WMH. AUC = area under the curve; CDR-SB = Clinical Dementia Rating–Sum of Boxes; ROC = receiver operating characteristic; WMH = white matter hyperintensity.
Discussion
Previous research has identified an association between MCI and WMH, with pMCI showing increased WMH loads compared with those with sMCI.14,15 This association, however, has not been examined in studies with long follow-up periods to determine whether rate of WMH load change over time differs between the groups. Findings have also yet to explore whether amyloid positivity or negativity influences rate of change in WMH burden in pMCI and sMCI. This study investigated regional baseline and longitudinal WMH burden in 4 subtypes of MCI: Aβ+ progressors, Aβ+ stable, Aβ− progressors, and Aβ− stable. We have 4 main findings: (1) WMH burden was related to conversion to both AD and dementia (i.e., associated with an increased rate of change in both Aβ+ progressors and Aβ− progressors), (2) Aβ+ individuals have increased WMH progression compared with Aβ− individuals, (3) hypertension was significantly associated with baseline and longitudinal progression of WMH burden in total volume and all regions except occipital, and (4) slope of change in frontal and total WMH can differentiate between Aβ+ stable and progressor individuals with similar baseline cognitive status (CDR-SB), particularly in earlier stages of cognitive decline.
We observed lower baseline WMH in the Aβ− stable group compared with that in Aβ+ progressors and the Aβ+ stable group across all regions. In line with previous reports, which had a shorter follow-up period, we found a disproportionately greater prevalence of WMH in Aβ+ progressors with an increased rate of conversion from MCI to dementia.14,15 When examining the longitudinal data, increased follow-up time was associated with increased WMH in all regions. Furthermore, in the longitudinal data, compared with the Aβ− stable group, all groups had significantly steeper change in WMH progression. The largest change (in slope) was observed for Aβ+ progressors compared with the Aβ− stable group. Compared with Aβ+ stable, Aβ− progressors only had steeper slopes (i.e., increased change in WMH over time) in the occipital region. Posterior WMH accumulation has previously been associated with AD-associated degenerative mechanisms.18,19,23 Thus, although these Aβ− individuals are converting to non-AD dementia, they are exhibiting some WMH burden that resembles the pattern seen in individuals with AD.
In such cases, accumulation of WMH in the occipital region is an indicator of dementia conversion for Aβ− individuals. The Aβ+ progressors exhibited a steeper slope over time than Aβ− progressors (in total volume, frontal, and temporal regions). This difference between Aβ− and Aβ+ progressors suggests that WMH rate of change in these areas in people who develop dementia may be partly accounted for by amyloid positivity. The Aβ+ progressors exhibited an increased rate of WMH change compared with Aβ+ stables in the same regions as Aβ− progressors (total, frontal, and temporal) and in the parietal and occipital regions, indicating that the parietal and occipital changes might be associated with conversion to dementia, whereas the total, frontal, and temporal WMH may be associated with other factors (e.g., amyloid or vascular risk factors). This interpretation coincides with previous research suggesting that frontal white matter lesions are partly associated with small vessel disease.23 These findings suggest that an increase in widespread WMH pathology occurs in MCI who convert to dementia.
Overall, our results highlight that regardless of amyloid status, older adults who convert to dementia have more WMH progression than those who remain stable. Thus, WMH progression contributes to dementia conversion in people with MCI who are both Aβ− and Aβ+. These findings indicate that WMH are important for progression to dementia/AD. In addition, people with MCI who are Aβ+ exhibited more WMH progression than people with MCI who are Aβ−. This finding suggests that amyloid positivity is related to the rate of progression of WMH across brain regions and that at least a portion of WMH progression can be explained by amyloid positivity. When examining the interaction between WMH burden and continuous amyloid levels, we observed significant associations between WMH loads and continuous CSF Aβ values for total and all regional WMH indicating an association between WMH and CSF Aβ. This result suggests that higher WMH burden in participants with MCI (both progressor and stable) is linked to the accumulation of Aβ in the brain (corresponding to lower CSF Aβ values). We further found a significant association with WMH and Aβ, affecting cognitive performance, suggesting a combined effect of higher WMH burden and lower CSF Aβ (i.e., higher Aβ levels in the brain) on cognitive decline in participants with MCI. These results show that there is an interaction between WMH and Aβ positivity in defining the future risk of dementia.
We also examined the relationship between WMH burden and vascular risk factors as the direct underlying mechanism involved in WMH accumulation. The importance of examining these factors is that vascular risk factors are potentially preventable/treatable, and with appropriate intervention, WMH progression might be controlled, which in turn could slow down the conversion to dementia. To examine the association with vascular factors, diabetes, BMI, and hypertension were added to the baseline and longitudinal models. Our findings suggest that hypertension is strongly associated with both baseline and rate of accumulation of total and frontal WMH. In this sample, approximately 48% of participants had hypertension, which allows for stronger conclusions regarding the influence of hypertension on WMH burden. This finding follows past research indicating the association between small vessel disease and anterior WMH burden.23 Given that hypertension is a modifiable condition, it may be possible to prevent some of the WMH accumulation through blood pressure management. Prevention of WMH burden may in turn mitigate or slow conversion to dementia.24,25 Diabetes showed to be marginally associated with baseline occipital WMH burden, which was no longer significant in the longitudinal findings when including other risk factors as covariates in the models. These limited findings are likely because few participants identified as having diabetes (only 69 of the entire 820 participants included). Therefore, these results need to be replicated in a larger sample of diabetic patients to make generalizable conclusions on the influence of diabetes on WMH burden in people with MCI. BMI was not significantly associated with baseline or longitudinal WMH accumulation. Other factors such as waist-to-hip ratio, which has previously shown to be associated with WMH burden,26 may be a more sensitive risk factor that should be examined in future MCI studies.
The ROC analysis (Figure 3) displays that total and frontal longitudinal WMH slopes of participants with baseline CDR-SB score of 1 surpassed all other regions and baseline CDR-SB scores at successfully classifying Aβ+ pMCI from Aβ+ sMCI (total AUC = 0.72; frontal AUC = 0.71). Although most individuals with Aβ positivity will convert to AD eventually,3 screening using Aβ status tends to result in the inclusion of a considerable percentage of people who remain stable.27 Our results indicate that WMH burden can increase classification of Aβ+ people who either progress to dementia or remain stable with MCI with moderately high accuracy (AUC = 0.72, Figure 3). These findings suggest that total and regional WMH can aid in future research and clinical settings to assess trajectories of patients with MCI who are Aβ+ on the AD continuum. The AUC is commonly used as a measure of discriminative performance. However, while our ROC results are promising, they lack external validation. Future studies investigating the discriminative performance of WMH measurements in other independent cohorts are therefore warranted to confirm the generalizability of these findings.
The first limitation of this study is that participants in the ADNI data set have relatively high education levels; future research should aim to examine the influence of education levels on WMH in more representative populations to determine whether high education levels are protective against WMH progression and the related conversion to dementia. Furthermore, the Aβ− progressor group had a smaller sample size compared with the other groups, limiting the statistical power and generalizability of its results. Although linear mixed models are robust regarding unbalanced data and optimally use all information together without reducing power, a larger sample is warranted and would make the results comparing the baseline and longitudinal data involving Aβ− progressor individuals more conclusive.
Another limitation is that other variables might influence WMH burden and conversion from MCI to dementia that were not examined in this study. For example, social status, atrial fibrillation, other MRI markers of small vessel disease (e.g., lacunes, microbleeds), and medications for risk factors and comorbidities such as antihypertensives, blood glucose–lowering treatments, statins, or antiplatelets could influence outcomes. Future research should examine whether these variables influence WMH progression and/or conversion from MCI to dementia. In addition, many health conditions (e.g., stroke) had limited occurrences in the follow-ups and other measures (e.g., triglyceride levels) that may influence WMH progression were measured at baseline and not during follow-up assessments. Previous research has shown that WMH severity is associated with increased blood-brain barrier permeability and abnormal white matter microstructural integrity28; therefore, future research should also examine the influence of change in health status (e.g., development of strokes) across follow-up visits on WMH burden. This study was unable to examine the proportion of people who progressed to AD vs other types of dementia because the ADNI only provides a diagnosis of “dementia” for those who convert from MCI to dementia during the study. Associations between baseline WMH and change in WMH burden over time may differ between the types of dementia. Not all participants with MCI had APOE ɛ4 status, MRI scans, and amyloid measurements available from the ADNI. Given that the cognitive status of the subset of participants that had missing measurements was significantly worse than those that had these measurements available (eTable 2, links.lww.com/WNL/C955), it is possible that our findings might underestimate the levels of pathology in those participants. CSF-based amyloid measures were used in the continuous models because they were consistently available for a larger subset of the participants. While the CSF amyloid measures had high correlations with both Pittsburgh compound B (PiB) and florbetapir (AV45) PET amyloid measures (PiB: r = −0.61, p < 0.001; AV45: r = −0.58, p < 0.001) in the subsets of participants that had both CSF and these PET measures available, lack of consistently acquired amyloid PET measures for the entire data set remains a limitation of this study. Last, we did not have consistently acquired FLAIR images for all participants included in this study. Therefore, we used T1w images to segment WMH and compared our findings with T2w/PD (for ADNI1) and FLAIR (for ADNI2/GO). Our main analysis did, however, show similar results to the T2w/PD and FLAIR sub-analyses.
This study observed that WMH accumulation is an important factor in MCI conversion to dementia for both Aβ+ and Aβ− individuals. This association was strongest in those who were Aβ+, indicating that WMH accumulation is partly related to amyloid deposition. Furthermore, the use of WMH progression to track conversion to dementia proved to be successful with relatively high accuracies obtained in the ROC analysis. This finding suggests that WMH have the potential to improve clinical predictions of trajectories of patients with MCI to AD and dementia. Hypertension was also found to be associated with increased WMH burden and rate of change in total volume and all regions except the occipital region, suggesting that conversion from MCI to dementia may be mitigated through hypertension prevention and treatment.
Glossary
- Aβ
β-amyloid
- Aβ−
amyloid negative
- Aβ+
amyloid positive
- AD
Alzheimer disease
- ADAS-13
Alzheimer Disease Assessment Scale–13
- ADNI
Alzheimer Disease Neuroimaging Initiative
- AUC
area under the ROC curve
- AV45
florbetapir
- BMI
body mass index
- CDR-SB
Clinical Dementia Rating–Sum of Boxes
- FLAIR
fluid-attenuated inversion recovery
- MCI
mild cognitive impairment
- PiB
Pittsburgh compound B
- pMCI
progressive MCI
- ROC
receiver operating characteristic
- sMCI
stable MCI
- T1w
T1 weighted
- T2w
T2 weighted
- WMH
white matter hyperintensity
Appendix. Authors
Study Funding
Data collection and sharing for this project was funded by the Alzheimer Disease Neuroimaging Initiative (ADNI) (NIH grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). The ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer Association; Alzheimer Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd. and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC; Johnson & Johnson Pharmaceutical Research & Development LLC; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Disclosure
F. Kamal is supported by the Quebec Bio-Imaging Network (QBIN) Recruitment Scholarship for Postdoctoral fellowship. C. Morrison is supported by a postdoctoral fellowship from Canadian Institutes of Health Research, Funding Reference Number: MFE-176608. M. Dadar reports receiving research funding from the Healthy Brains for Healthy Lives (HBHL), Alzheimer Society Research Program (ASRP), and Douglas Research Center (DRC). Go to Neurology.org/N for full disclosures.
References
- 1.Petersen RC, Caracciolo B, Brayne C, Gauthier S, Jelic V, Fratiglioni L. Mild cognitive impairment: a concept in evolution. J Intern Med. 2014;275(3):214-228. doi: 10.1111/joim.12190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petersen RC. Mild cognitive impairment. Contin Lifelong Learn Neurol. 2016;22(2):404. doi: 10.1002/9781118656082.ch6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sperling R, Mormino E, Johnson K. The evolution of preclinical Alzheimer's disease: implications for prevention trials. Neuron. 2014;84(3):608-622. doi: 10.1016/j.neuron.2014.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morrison C, Dadar M, Villeneuve S, Louis Collins D. White matter hyperintensities may be an early marker for age-related cognitive decline. Neuroimage Clin. 2022;35:103096. doi: 10.1016/j.nicl.2022.103096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bangen KJ, Preis SR, Delano-Wood L, et al. Baseline white matter hyperintensities and hippocampal volume are associated with conversion from normal cognition to mild cognitive impairment in the Framingham Offspring Study. Alzheimer Dis Assoc Disord. 2018;32(1):50-56. doi: 10.1097/WAD.0000000000000215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim S, Choi SH, Lee YM, et al. Periventricular white matter hyperintensities and the risk of dementia: a CREDOS study. Int Psychogeriatrics. 2015;27(12):2069-2077. doi: 10.1017/S1041610215001076 [DOI] [PubMed] [Google Scholar]
- 7.Yoshita M, Fletcher E, Harvey D, et al. Extent and distribution of white matter hyperintensities in normal aging, MCI, and AD. Neurology. 2006;67(12):2192-2198. doi: 10.1212/01.wnl.0000249119.95747.1f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Der Flier WM, Skoog I, Schneider JA, et al. Vascular cognitive impairment. Nat Rev Dis Prim. 2018;4:18003. doi: 10.1038/nrdp.2018.3 [DOI] [PubMed] [Google Scholar]
- 9.Abraham HMA, Wolfson L, Moscufo N, Guttmann CRG, Kaplan RF, White WB. Cardiovascular risk factors and small vessel disease of the brain: blood pressure, white matter lesions, and functional decline in older persons. J Cereb Blood Flow Metab. 2016;36(1):132-142. doi: 10.1038/jcbfm.2015.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tamura Y, Araki A. Diabetes mellitus and white matter hyperintensity. Geriatr Gerontol Int. 2015;15:34-42. doi: 10.1111/ggi.12666 [DOI] [PubMed] [Google Scholar]
- 11.Hirao K, Yamashita F, Tsugawa A, et al. Association of white matter hyperintensity progression with cognitive decline in patients with amnestic mild cognitive impairment. J Alzheimers Dis. 2021;80(2):877-883. doi: 10.3233/JAD-201451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li JQ, Tan L, Wang HF, et al. Risk factors for predicting progression from mild cognitive impairment to Alzheimer's disease: a systematic review and meta-analysis of cohort studies. J Neurol Neurosurg Psychiatry. 2016;87(5):476-484. doi: 10.1136/jnnp-2014-310095 [DOI] [PubMed] [Google Scholar]
- 13.Kamal F, Morrison C, Maranzano J, Zeighami Y, Dadar M. Topographical differences in white matter hyperintensity burden and cognition in aging, MCI, and AD. Geroscience. 2023;45(1):1-16. doi: 10.1007/s11357-022-00665-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dadar M, Maranzano J, Ducharme S, Collins DL. White matter in different regions evolves differently during progression to dementia. Neurobiol Aging. 2019;76:71-79. doi: 10.1016/j.neurobiolaging.2018.12.004 [DOI] [PubMed] [Google Scholar]
- 15.Prasad K, Wiryasaputra L, Ng A, Kandiah N. White matter disease independently predicts progression from mild cognitive impairment to Alzheimer's disease in a clinic cohort. Dement Geriatr Cogn Disord. 2011;31(6):431-434. doi: 10.1159/000330019 [DOI] [PubMed] [Google Scholar]
- 16.Farias ST, Mungas D, Reed BR, Harvey D, DeCarli C. Progression of mild cognitive impairment to dementia in clinic- vs community-based cohorts. Arch Neurol. 2009;66(9):1151-1157. doi: 10.1001/archneurol.2009.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bombois S, Debette S, Bruandet A, et al. Vascular subcortical hyperintensities predict conversion to vascular and mixed dementia in MCI patients. Stroke. 2008;39(7):2046-2051. doi: 10.1161/STROKEAHA.107.505206 [DOI] [PubMed] [Google Scholar]
- 18.Brickman AM. Contemplating Alzheimer's disease and the contribution of white matter hyperintensities. Curr Neurol Neurosci Rep. 2013;13(12):415. doi: 10.1007/s11910-013-0415-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Habes M, Erus G, Toledo JB, et al. Regional tract-specific white matter hyperintensities are associated with patterns to aging-related brain atrophy via vascular risk factors, but also independently. Alzheimers Dement (Amst). 2018;10:278-284. doi: 10.1016/j.dadm.2018.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scott JA, Braskie MN, Tosun D, et al. Cerebral amyloid and hypertension are independently associated with white matter lesions in elderly. Front Aging Neurosci. 2015;7:221. doi: 10.3389/fnagi.2015.00221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Snyder HM, Corriveau RA, Craft S, et al. Vascular contributions to cognitive impairment and dementia including Alzheimer's disease. Alzheimers Dement. 2015;11(6):710-717. doi: 10.1016/j.jalz.2014.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamada M, Naiki H. Cerebral Amyloid Angiopathy. Vol. 107. 1st ed. Elsevier Inc.; 2012. doi: 10.1016/B978-0-12-385883-2.00006-0 [DOI] [PubMed] [Google Scholar]
- 23.McAleese KE, Miah M, Graham S, et al. Frontal white matter lesions in Alzheimer's disease are associated with both small vessel disease and AD-associated cortical pathology. Acta Neuropathol. 2021;142(6):937-950. doi: 10.1007/s00401-021-02376-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kjeldsen SE, Narkiewicz K, Burnier M, Oparil S. Intensive blood pressure lowering prevents mild cognitive impairment and possible dementia and slows development of white matter lesions in brain: the SPRINT Memory and Cognition IN Decreased Hypertension (SPRINT MIND) study. Blood Press. 2018;27(5):247-248. doi: 10.1080/08037051.2018.1507621 [DOI] [PubMed] [Google Scholar]
- 25.Williamson JD, Pajewski NM, Auchus AP, et al. Effect of intensive vs standard blood pressure control on probable dementia: a randomized clinical trial. JAMA. 2019;321(6):553-561. doi: 10.1001/jama.2018.21442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Veldsman M, Kindalova P, Husain M, Kosmidis I, Nichols TE. Spatial distribution and cognitive impact of cerebrovascular risk-related white matter hyperintensities. Neuroimage Clin. 2020;28:102405. doi: 10.1016/j.nicl.2020.102405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ossenkoppele R, Jansen WJ, Rabinovici GD, et al. Prevalence of amyloid PET positivity in dementia syndromes: a meta-analysis. JAMA. 2015;313(19):1939-1950. doi: 10.1001/jama.2015.4669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rost NS, Cougo P, Lorenzano S, et al. Diffuse microvascular dysfunction and loss of white matter integrity predict poor outcomes in patients with acute ischemic stroke. J Cereb Blood Flow Metab. 2018;38(1):75-86. doi: 10.1177/0271678X17706449 [DOI] [PMC free article] [PubMed] [Google Scholar]