Abstract
Background and Objectives
Progressive focal anterior temporal lobe (ATL) neurodegeneration has been historically called semantic dementia. More recently, semantic variant primary progressive aphasia (svPPA) and semantic behavioral variant frontotemporal dementia (sbvFTD) have been linked with predominant left and right ATL neurodegeneration, respectively. Nonetheless, clinical tools for an accurate diagnosis of sbvFTD are still lacking. Expressive prosody refers to the modulation of pitch, loudness, tempo, and quality of voice used to convey emotional and linguistic information and has been linked to bilateral but right-predominant frontotemporal functioning. Changes in expressive prosody can be detected with semiautomated methods and could represent a useful diagnostic marker of socioemotional functioning in sbvFTD.
Methods
Participants underwent a comprehensive neuropsychological and language evaluation and a 3T MRI at the University of California San Francisco. Each participant provided a verbal description of the picnic scene from the Western Aphasia Battery. The fundamental frequency (f0) range, an acoustic measure of pitch variability, was extracted for each participant. We compared the f0 range between groups and investigated associations with an informant-rated measure of empathy, a facial emotion labeling task, and gray matter (GM) volumes using voxel-based morphometry.
Results
Twenty-eight patients with svPPA, 18 with sbvFTD, and 18 healthy controls (HCs) were included. f0 range was significantly different across groups: patients with sbvFTD showed reduced f0 range in comparison with both patients with svPPA (mean difference of −1.4 ± 2.4 semitones; 95% CI −2.4 to −0.4]; p < 0.005) and HCs (mean difference of −1.9 ± 3.0 semitones; 95% CI −3.0 to −0.7]; p < 0.001). A higher f0 range was correlated with a greater informant-rated empathy (r = 0.355; p ≤ 0.05), but not facial emotion labeling. Finally, the lower f0 range was correlated with lower GM volume in the right superior temporal gyrus, encompassing anterior and posterior portions (p < 0.05 FWE cluster corrected).
Discussion
Expressive prosody may be a useful clinical marker of sbvFTD. Reduced empathy is a core symptom in sbvFTD; the present results extend this to prosody, a core component of social interaction, at the intersection of speech and emotion. They also inform the long-standing debate on the lateralization of expressive prosody in the brain, highlighting the critical role of the right superior temporal lobe.
Introduction
Historically, patients with progressive focal anterior temporal lobe (ATL) neurodegeneration and semantic loss have been labeled as having semantic dementia (SD).1-4 More recently, 2 syndromes have been more extensively described to better account for the variations in clinical presentations associated with ATL degeneration.5,6 The first one, semantic variant of primary progressive aphasia (svPPA), is mainly characterized by impaired confrontation naming, loss of single-word comprehension, and bilateral but predominantly left ATL atrophy.5 The second syndrome, occurring with bilateral but predominantly right ATL atrophy, is less well characterized and has not yet been represented in consensus diagnostic criteria. Therefore, the terminology associated with this syndrome has been highly heterogeneous, with patients receiving labels of right SD, right svPPA, right behavioral variant of frontotemporal dementia (bvFTD), and right temporal frontotemporal dementia,7-12 yet a systematic investigation has shown that only a small proportion of these patients fulfill the diagnostic criteria for svPPA or bvFTD.6 Recently, the term semantic behavioral variant of frontotemporal dementia (sbvFTD) has also been coined for these patients with predominant right ATL atrophy.6 Patients with sbvFTD have been reported to have a loss of empathy for other people, which might be underpinned by a progressive loss of semantic knowledge for concepts of social-emotional relevance and difficulty identifying and naming known people, complex compulsions, or rigidity.6 In this framework, svPPA and sbvFTD would be considered the 2 clinico-anatomical extremes of the SD spectrum.6 Nonetheless, because of its more recent description, clinical tools for an accurate diagnosis of sbvFTD remain lacking with many limitations, and it is likely that a combination of many markers will be necessary. For example, empathy is most frequently assessed using informant-rated questionnaires instead of patient-facing tasks, and tests assessing recognition of known people are particularly sensitive to cultural variation.
Given the socioemotional impairments that characterize patients with sbvFTD, expressive prosody represents a good candidate for a cognitive marker in this population. Prosody is a feature of speech used to convey linguistic or affective information through the modulation of pitch, loudness, tempo, and quality of voice.13,14 In affective prosody, these acoustic modulations indicate the emotional state of the speaker invoking empathy in the listener. For example, a speaker can modulate their speech to indicate happiness or sarcasm, and in doing so, they generate a more accurate impression of their emotional state in their conversational partner.15 Those who are most able to modulate their prosody may also show strengths in other socioemotional functions, such as empathy. Thus, prosody appears at the intersection of speech and language and socioemotional cognition. While there are many aspects of expressive prosody, the modulation of pitch (as measured by the variation of the fundamental frequency of the vocal cords vibration (f0)) is often considered the most salient and most easily perceived prosody parameter for the accurate perception of emotion by communication partners, in comparison with other aspects of prosody.14,16 Expressive prosodic alterations, as measured by a reduction in f0 range, have recently been shown in patients with bvFTD and with the nonfluent/agrammatic variant of primary progressive aphasia (nfvPPA) during a picture description task.17,18 Patients with nfvPPA also showed tempo alterations as measured by pause rate. However, these changes were correlated to linguistic measures of fluency and grammaticality in all patients with PPA and therefore might not provide a pure measure of expressive prosody in these populations. In that study, while patients with svPPA showed preserved expressive prosody, patients with sbvFTD were not investigated.18
ATL degeneration has previously offered insights into the role of the temporal lobe in linguistic expression and now may offer similar insights into prosodic expression. Although damage to the ATLs may cause speech, language, and socioemotional symptoms, most of the neuroimaging literature on prosody has been conducted on stroke patients who rarely have selective ATL damage.14,19 Other studies on prosody have used fMRI in healthy individuals,20,21 which can underestimate blood-oxygen level–dependent signal in the ATL due to distortions of the magnetic field caused by the proximity of the ATL to air-filled sinuses.22 ATL degeneration may elucidate the role of the temporal lobes in prosody. The relative lateralization of svPPA and sbvFTD may further our knowledge of how prosodic expression lateralizes in the brain, which represents one of the longtime unresolved debates in the prosody literature. Historically, the right hemisphere was associated with prosody,15,23 but further studies have proposed that the left hemisphere may also play a significant role, particularly in linguistic prosody or speech changes extracted from short temporal integration windows (20–40 milliseconds); vs longer temporal integration windows (150–200 milliseconds) for the right hemisphere.24,25 Overall, lesion and neuroimaging studies of expressive prosody suggest that this function is sustained by bilateral frontotemporal regions, with a relative predominance of the right hemisphere and subcortical regions.20,26
This study investigated expressive prosody in a large sample of patients with SD, classified as svPPA or sbvFTD. The first aim was to compare expressive prosodic abilities in patients with svPPA, sbvFTD, and age-matched healthy controls (HC). We hypothesized that patients with sbvFTD would show a reduced prosodic range. The second aim was to investigate the associations between expressive prosody and empathy in SD. We hypothesized that expressive prosody would be associated with empathy. Finally, the third aim was to determine the neural correlates of expressive prosody in SD. We hypothesized that prosodic range would correlate with gray matter (GM) volume in the right ATL.
Methods
Participants
Patients were recruited through the University of California San Francisco (UCSF) Memory and Aging Center (MAC) between 2003 and 2020. Patients with svPPA fulfilled the current diagnostic criteria for imaging-supported svPPA,5 while patients with sbvFTD fulfilled the proposed criteria for probable sbvFTD suggested by Younes et al.6 The diagnosis was made after a comprehensive evaluation (neurologic history and examination, standardized neuropsychological and language evaluations) and a review of this evaluation at a consensus diagnostic meeting at the UCSF MAC. The predominance of atrophy (left, right) was confirmed using an atrophy lateralization index measured from structural brain MRI, as described in Section Atrophy Predominance Characterization. HCs who were neurologically normal as attested by a neurologic examination, neuropsychological evaluation, and MRI were also included in the sample as a comparison group.
General inclusion criteria for this study were as follows: (1) being a native English speaker and (2) availability of a recorded picture description from the Western Aphasia Battery27 of sufficient quality (i.e., no prominent background noise, few examiner interventions) and length (>50 words) for acoustic analysis. Patients also needed an MRI scan available within 6 months of completing the picture description task. In total, 42 HCs and 70 patients fulfilled these criteria. Six HCs and 11 patients were excluded after the blind quality check of the pitch tracking (described in Section Automatic Extraction of Prosody). From that sample, we constructed 3 demographically (age, sex, and education) and clinically (disease duration, Mini-Mental State Examination [MMSE], Clinical Dementia Rating [CDR] scale total, and CDR box score for patients with svPPA and sbvFTD only) matched groups. Our final sample comprised 18 HCs, 28 patients with svPPA, and 18 with sbvFTD.
Standard Protocol Approvals, Registrations, and Patient Consents
Written informed consent was obtained from all participants (or legally authorized representatives of participants) in the study, and the institutional review board approved the study.
Procedure
General Neuropsychological and Language Assessment
All participants underwent cognitive and language evaluation, as previously described.28,29
Prosody Assessment
Picture Description Task
A picture description task of the picnic scene from the Western Aphasia Battery27 was administered to each participant. Participants were instructed to describe the picture in as much detail as possible with the following instructions: “I'm going to show you a picture. Tell me what you see. Talk in sentences.” Brief and noninformative prompting was used when participants paused for more than a few seconds.
Automatic Extraction of Prosody
Recordings were performed in a clinical research setting on a digital video camcorder. The audio files were then reviewed and manually edited to exclude interviewer speech, background noises that could confound pitch tracking, and periods during which the patient was not describing the picture (e.g., asking questions about the task, off-topic comments). Audacity (version 2.3.3) was used to reduce background noise on the obtained files through its “Noise Reduction” function. Pitch tracking (fundamental frequency (f0) extraction in time) was performed with Praat (version 6.1.09). To remove extreme values, pitch tracking boundaries were adjusted for each participant according to their own pitch distribution using a previously defined formula.30 The octave jump cost was also adjusted (value = 0.9) to correct for abrupt artifactual pitch variations.
Because pitch tracking in Praat may sometimes lead to artifacts such as doubling of the f0 line,31 all pitch curves were blindly and independently inspected by 2 of the authors (M.M. and A.G.) and rated as “normal” or “with doubling artefact.” When the 2 raters disagreed, they reviewed the curves together to reach a consensus. Six (14.3%) HCs and 11 (15.7%) patients were excluded because of this artefact.
We measured f0 range with a method previously used to investigate prosody in patients with bvFTD and PPA.17,18 An open script32 modified by A.G. was used to extract f0 estimates at each 10 milliseconds of each participant's speech sample. Percentile values were estimated for each f0 estimate, and an optimized f0 range was then calculated using the following formula: f0 range = 12 × log2(“f0 at 90th percentile”/“f0 at 10th percentile”). First, this formula transforms f0 from Hz to semitones (ST), a unit commonly used in music and speech analysis and closer to human perception of pitch. Second, it centers each participant's f0 range to its individual 10th f0 percentile, thus controlling for individual pitch differences. Therefore, the 90th f0 percentile represents the f0 range in ST.
Empathy Assessment
Empathy was measured using the Revised Self-Monitoring Scale (RSMS),33 a 13-item informant-rated questionnaire that uses a 6-point Likert scale ranging from “totally disagree” to “totally agree.”34 It includes items on the individual's sensitivity to expressive behavior (sample item: “The subject is often able to correctly read people's true emotions through their eyes”) and ability to modify their self-presentation based on their observations of others' social feedback (sample item: “The subject has the ability to control the way he/she comes across to people, depending on the impression he/she wants to give them”). Although no patient-facing empathy tasks were available, some items of the RSMS might be sensitive to reduced prosody expression, such as “The subject can adjust his behavior to meet the requirements of any situation he finds himself in”; however, this scale also covers a broad range of empathy-related behaviors. Responses were summed to provide a total score between 13 and 78.
Facial emotion labeling was assessed using the Comprehensive Affect Testing System (CATS)—Emotion labeling subtask.35 In this task, participants must match 16 serially presented facial expressions of emotion with the correct written label (7 choices: neutral, happiness, sadness, anger, fear, surprise, and disgust). Responses were summed to provide a total score between 0 and 16.
Neuroimaging
MRI Acquisition
MRI data were collected within 6 months of the picture description task. Eight HCs had no MRI scan available and were therefore excluded from imaging analyses. T1 images were acquired with either a 1.5T, 3T, or 4T scanner, as previously described.36.
MRI Processing
Image preprocessing for voxel-based morphometry (VBM) was performed using CAT12, which is an SPM12 (Statistical Parametric Mapping) toolbox within the MATLAB environment (Mathworks, Natick, MA). All T1-weighted images were corrected for bias (i.e., field inhomogeneities and noise). They were then segmented into GM, white matter, and CSF, spatially normalized to the standard template provided in SPM12, and modulated by the Jacobian determinant to preserve the relative GM volume. After preprocessing, all scans passed a visual check for artifacts and the automated CAT12 quality check protocol. The modulated and normalized GM images were then smoothed with a Gaussian kernel of 8 mm FWHM.
Atrophy Predominance Characterization
We measured the predominance of left or right ATL atrophy using a lateralization index based on the GM images calculated as described earlier. Estimations of volumes of left and right ATLs were performed using the modulated GM images in SPM12 as the sum of voxel within a mask specific to this region.36 Z-scores of these volumes were calculated using HC volumes as reference. The lateralization indexes were then extracted subtracting the z-score corresponding to the left and right ATL (left ATL z-score − right ATL z-score). Positive values indicated a right predominant atrophy, while negative indicated a left predominant atrophy. These values were used to provide imaging-supported diagnoses of svPPA and sbvFTD.
Statistical Analyses
To compare f0 ranges between the 3 groups, an ANCOVA (controlling for age and sex) followed by a Tukey post hoc test was conducted. To examine the clinical utility of expressive prosodic range at the individual level, we calculated the percentage of patients whose score fell in a quantitatively clinically abnormal range, an approach commonly used by neuropsychologists and other clinicians in clinical practice to interpret scores at the individual level. Each patient's prosodic range was standardized to a z-score using the mean and standard deviation of the HC group. We used a cutoff of −1.6 (>95th percentile) to determine whether a patient's prosodic range was abnormally low and of clinical significance.
To determine the association between empathy and the f0 range, we ran Pearson correlations with CATS affect matching and RSMS33 total scores. To determine the GM correlates of expressive prosody, we used a VBM approach to examine the correlation between each voxel of the whole brain and f0 range. We entered f0 range in a regression model as a variable of interest, with the normalized, modulated, smoothed GM images as inputs and including age, sex, disease severity (CDR box score), intracranial volume, and MRI magnet strength as covariates of no interest. Contrasts were set to examine the hypothesis that a reduced f0 range would be associated with decreased GM volume. These association analyses were conducted on both patients with svPPA and sbvFTD combined. This is consistent with the recent framework highlighting these groups as 2 clinico-anatomical extremes of the SD spectrum. Therefore, we wanted to take advantage of the variance offered by the SD spectrum as a whole for behavioral measures (patients showing varying degrees and predominance of language and socioemotional behavior impairments) and imaging measures (patients showing varying degrees and predominance of left and right ATL atrophies). It is also consistent with the aim of the analysis, which is to inform the long-standing debate on the lateralization (right vs left hemisphere) of expressive prosody in the brain, more specifically in the temporal lobes.
Data Availability
While we can share anonymized data, public archiving is not yet permitted under the study's IRB approval due to the sensitive nature of patient data. Specific requests can be submitted through the UCSF-MAC Resource (Request form: memory.ucsf.edu/resources/data). Following a UCSF-regulated procedure, access will be granted to designated individuals in line with ethical guidelines on the reuse of sensitive data. This would require submission of a Material Transfer Agreement, available at: icd.ucsf.edu/material-transfer-and-data-agreements. Commercial use will not be approved.
Results
Characterization of HCs, Patients With svPPA, and Patients With sbvFTD
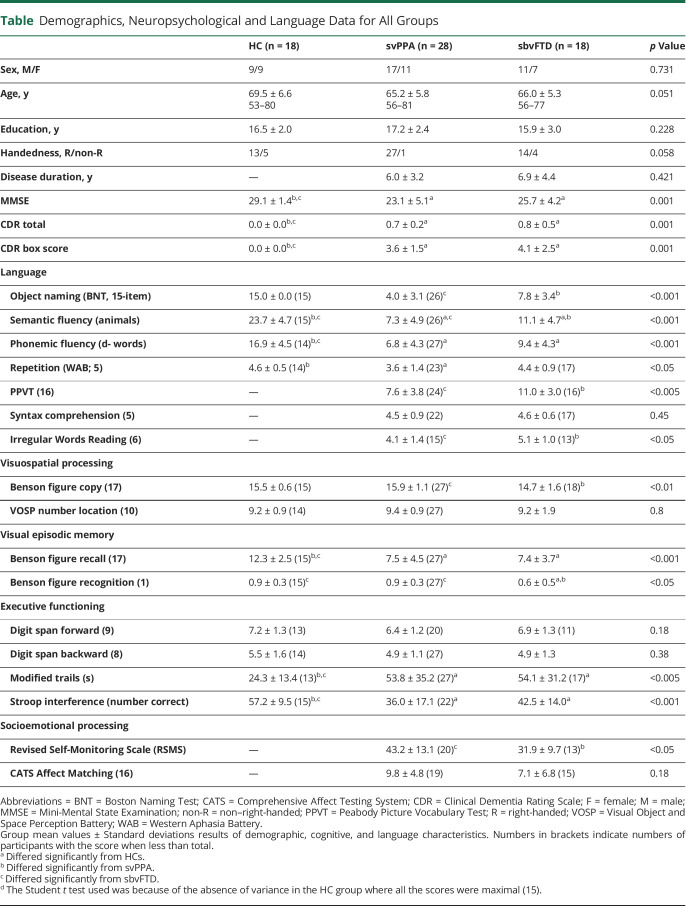
The sample comprises 3 demographically (age, sex, and education) and clinically (disease duration, MMSE, CDR total, and CDR box score for patients with svPPA and sbvFTD only) matched groups of 18 HCs, 28 patients with svPPA, and 18 patients with sbvFTD. Summary statistics for demographic characteristics, cognitive, and language testing for all 3 groups are summarized in the Table. Whole-brain GM comparisons were performed between the 3 groups (Figure 1).
Table.
Demographics, Neuropsychological and Language Data for All Groups
Figure 1. Atrophy Profiles Comparison Between the 3 Participant Groups (p < 0.05 FWE Corrected).
Light green represents areas where patients with sbvFTD have more atrophy than HCs and svPPA and dark green those where patients with svPPA have more atrophy than HCs and patients with sbvFTD.
Patients With sbvFTD Present a Decreased Expressive Prosodic Range
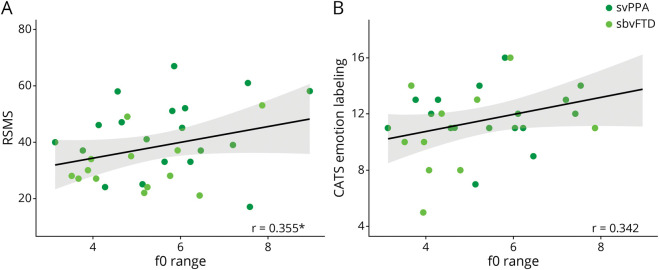
The analysis of covariance controlling for sex and age showed differences in f0 range across the 3 groups (Figure 2) (p < 0.005; ω2 = 0.19, indicating a large effect size). Specifically, Tukey post hoc tests indicated that patients with sbvFTD had reduced f0 range in comparison with both HCs (p < 0.001) and patients with svPPA (p < 0.005) (mean ± standard deviation f0 range in HC: 6.6 ± 1.1; in svPPA: 6.2 ± 2.0; in sbvFTD: 4.8 ± 1.2). Patients with svPPA and HCs did not differ significantly. Examining individual patients' expressive prosodic ranges indicated that 9 of 28 patients with svPPA (32.1%) fell in the abnormally clinically low range, while 11 of 18 patients with sbvFTD (61.1%) did.
Figure 2. Fundamental Frequency (f0) Range in Each Group of Participants.
f0 = fundamental frequency; * indicating p < 0.005; ** indicating p < 0.001.
Expressive Prosody Correlated With Informant-Rated Empathy in Patients
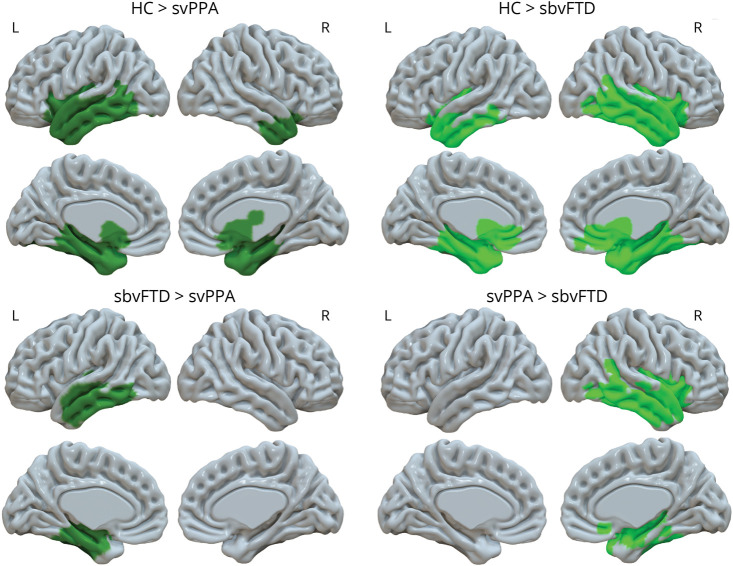
f0 range correlated with informant-rated empathy, as measured with the RSMS, in patients with svPPA and sbvFTD combined (Figure 3A; r = 0.355; p ≤ 0.05). Although a positive trend was observed, emotion recognition (as measured with the CATS emotion labeling subtask) did not significantly correlate with f0 range (Figure 3B; r = 0.342; p = 0.075).
Figure 3. Correlations Between Socioemotional Measures and f0 Range in Patients With svPPA and sbvFTD.
(A) Correlation between f0 range and informant-rated empathy assessed by RSMS; (B) Correlation between f0 range and facial emotion labelling measured by the CATS. CATS = Comprehensive Affect Testing System35; f0 = fundamental frequency, RSMS = Revised Self-Monitoring Scale33; * indicating p < 0.05.
Expressive Prosody Correlated With GM Volume in the Right Superior Temporal Lobe in Patients
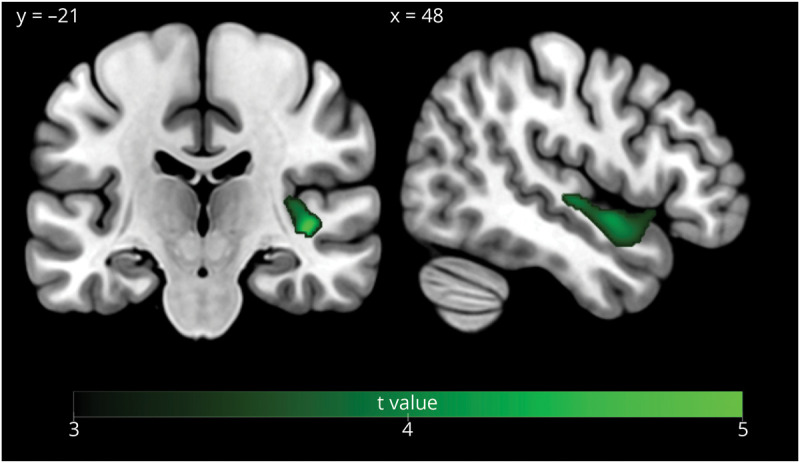
The regression voxel-based analysis (controlling for age, sex, disease severity, total intracranial volume, and MRI magnet strength) showed a positive association between f0 range and GM volume in patients with svPPA and sbvFTD combined in a cluster centered in the right superior temporal gyrus (p < 0.05 FWE cluster corrected; cluster size = 1,173 voxels; x = 42, y = −21, z = −2) and extending into the right anterior superior temporal gyrus (x = 48, y = 0, z = −9) (Figure 4).
Figure 4. Gray Matter Correlates of f0 Range in Patients With svPPA and sbvFTD (p < 0.05 FWE Corrected).
Discussion
In this study, we found that patients with sbvFTD presented with a restricted prosodic range in comparison with patients with svPPA or HCs. Expressive prosody was associated with informant-rated empathy and with GM volume in the right superior temporal gyrus in patients with SD. These findings suggest that expressive prosody could be a useful clinical marker used to support the diagnosis of sbvFTD and for the differential diagnosis with svPPA. Furthermore, changes in expressive prosody could be associated with the wide array of socioemotional behavior deficits observed in sbvFTD and coincides with reduction in empathy, as observed by caregiver informants. Finally, this study also informs the neural bases of prosody production, highlighting the critical role of the right superior temporal lobe.
Expressive prosody is restricted in patients with sbvFTD in comparison with HCs and those with svPPA. It may therefore serve as a useful clinical marker for the diagnosis of sbvFTD and better characterization of these patients. This result aligns with the clinical description of this syndrome, in which a combination of socioemotional and linguistic deficits is reported.7-12 More specifically, loss of empathy, reflected, for example, in increased interpersonal coldness, is most often the earliest, and overall, most frequent clinical feature in patients with sbvFTD.6 In our sample, patients with sbvFTD presented with lower empathy, as measured by informant report on the RSMS, in comparison with patients with svPPA. Patients with a reduced prosodic range were also described as being less empathetic by their relatives. These results are in line with previous studies establishing clear relationships between expressive prosody and empathy.21,37 One of the empathy facets captured by the RSMS is the ability of patients to appropriately modify their self-presentation according to the social context. While there are many channels through which one can modify behavior to convey socioemotional meaning (e.g., facial expressions, body movement and postures, verbal content), our results could suggest that the inability to modulate expressive prosody (due to a focal emotional or speech deficit) might contribute to the lower empathy ratings made by observers describing patients with sbvFTD. On the contrary a lack of empathy might lead patients with sbvFTD to not care about communicating clearly, and these patients might simply not use prosody management as much in their own speech to convey emotion. Future studies should clarify this relationship between expressive prosody and empathy.
Impaired prosodic production may also be a useful clinical marker for the differential diagnosis between sbvFTD and svPPA syndromes because our results show that it distinguishes these 2 forms of anterior temporal degeneration. Previously, Pressman et al. compared prosody production in patients with right or left anterior temporal degeneration and did not find any difference between these 2 groups. These conflicting results might be due to the use of a different task (conversational speech) and measure (coefficient of variation of f0) of prosody and a smaller sample size of patients with sbvFTD than in this study.38 Clinically differentiating patients with sbvFTD and svPPA can remain challenging,39 especially at the individual level, and only a few clinical markers have been previously reported as discriminant in group-level analyses, specifically naming, repetition, and word comprehension (worse in svPPA) and empathy, emotion reading, difficulty identifying known people, and visuospatial abilities (worse in sbvFTD).40 Expressive prosody may therefore represent an additional tool in clinical settings to better differentiate these 2 syndromes: a combination of several clinical markers might be optimal in differentiating patients with sbvFTD at the individual level in clinical practice, not only with patients with svPPA but also with patients with bvFTD. Regarding intervention, our results also draw attention to potential social communication intervention approaches to be used in patients with expressive prosodic impairments. These approaches could be added to a portfolio of interventions used by speech and language pathologists, such as lexical retrieval treatment for patients with verbal semantic impairments, the efficacy of which has been demonstrated in clinical trials.41 Furthermore, brain localization of symptoms remains a key goal of neuropsychology and behavioral neurology, and using patients with ATL degeneration as a novel framework improves the localization of expressive prosodic impairments.
Although the neural correlates of prosodic production are still debated, it is believed to be sustained by bilateral frontotemporal regions, with a relative predominance of the right hemisphere and subcortical regions.20,26 This study emphasizes the specific role of the right superior temporal gyrus in expressive prosody, providing clarification in both the interhemispheric and intrahemispheric localization of this function. The right interhemispheric lateralization of prosody production is consistent with most previously published studies19,23,25,42 and has been previously related to the role of the right hemisphere in prosody as a whole,15,23 in affective prosody,42 or in the processing of specific prosody cues with longtime changes.24,25 Of interest, the pattern observed in our study mirrors the pattern observed in stroke patients. In this population, these impairments are mostly reported in right hemisphere strokes,23,43 and some studies have even shown that impaired emotional prosody is more common than objectively measured impairments in emotional empathy.44 The predominant role of the right ATL in expressive prosody was also supported in regression-based neuroimaging analyses when acknowledging the overlap between patients with svPPA and patients with sbvFTD and therefore considering them as being part of a continuous SD spectrum. Furthermore, at the intrahemispheric level, the involvement of the superior temporal gyrus in prosody is consistent with functional MRI investigations in HCs and studies of patients with vascular lesions.20,21,45,46 This region includes both the superior part of the right ATL and extends posteriorly to the superior posterior temporal lobe. Three hypotheses may be considered for the involvement of right superior temporal regions in expressive prosody. First, the right ATL is involved in socioemotional semantic processing,47 and prosody models suggest that it could underlie access to semantic representations of emotions themselves.26 Therefore, the severe ATL atrophy and progressive loss of semantic knowledge for concepts of social-emotional relevance experienced by patients with sbvFTD might affect the expression of emotion through prosody. Second, the superior temporal region could be the host of a lexicon of abstract representations of acoustic characteristics that convey emotions linking semantic representations of emotions (e.g., sadness) to the motor programming needed for expression in the frontal lobes (in the case of sadness, articulatory muscles modulating pitch, intensity, pauses producing low pitch, slow speech rate, and low volume).26,46,48 Due to dysfunction in this region, patients with sbvFTD might have difficulty accessing these abstract representations, which could limit their prosodic range. Third, the right superior posterior temporal region could support sensorimotor loops allowing for online monitoring of prosody production between the right superior temporal gyrus and the frontal regions.20 Indeed, middle and posterior temporal regions have been previously described as auditory regions involved in the perception and integration of prosodic acoustic cue combinations even when attention is not directed to the stimulus.45 Thus, patients with sbvFTD might have an altered perception of their own voice during online monitoring of prosodic production. These hypotheses are not mutually exclusive. Of interest, in patients with bvFTD, limited prosodic range is associated with GM volume in bilateral inferior and dorsomedial frontal regions,17 suggesting that the prosodic impairments in these 2 populations may be due to distinct underlying mechanisms. Further studies using more specific experimental tasks will be needed to discriminate between these possible mechanisms in both patients with sbvFTD and patients with bvFTD.
This study has a few limitations. Although the picnic picture description task is ecological, it may not induce as much emotion as a more emotionally evocative picture. While in the expected direction, the association between expressive prosody and a facial emotion labeling task did not surpass the threshold for statistical significance. Real-world measures of behavior may be more sensitive to empathy deficits in patients with sbvFTD than experimental tasks. Moreover, because the RSMS is an informant-rated questionnaire, no patient-facing empathy tasks were used. Furthermore, our study does not allow conclusions to be drawn about how early in the disease course this clinical marker appears in sbvFTD. In the future, longitudinal studies with larger sample sizes that conduct head-to-head comparisons between multiple clinical markers will be essential to determine which markers are most useful for differential diagnosis and at which stage of the disease, while also considering patients with bvFTD, in which differential diagnosis can also be very challenging, as shown in previous studies.11 Finally, while SD represents a powerful and novel model to assess the role of temporal regions in expressive prosody, we may have overlooked the role of other brain regions, which show less GM volume loss in patients with svPPA and sbvFTD. For example, bilateral frontal regions and subcortical regions have been described as implicated in prosody production.14,17,20,49 The brain region found in our study is probably a core node of a larger, bilateral network involved in prosody, and connectivity-based imaging approaches (such as resting-state fMRI or structural covariance analyses) could be used in future studies involving multiple neurodegenerative conditions, especially patients with bvFTD, because they may be clinically difficult to distinguish from sbvFTD because of their similar impairments in social cognition.
In conclusion, this study identified expressive prosodic impairments in patients with sbvFTD and their association with empathy deficits and GM atrophy in the right superior temporal lobe. Our results highlight the importance of investigating the interaction between language and socioemotional symptoms in neurodegenerative diseases because they can greatly affect communication between patients and their loved ones. Finally, the study supports the use of neurodegenerative diseases as a framework to investigate prosody, in complement to the more extensive literature on stroke patients and functional neuroimaging of neurotypical individuals.
Glossary
- ATL
anterior temporal lobe
- CATS
Comprehensive Affect Testing System
- CDR
Clinical Dementia Rating Scale
- GM
gray matter
- HCs
healthy controls
- MAC
Memory and Aging Center
- MMSE
Mini-Mental State Examination
- nfvPPA
nonfluent/agrammatic variant of primary progressive aphasia
- RSMS
Revised Self-Monitoring Scale
- sbvFTD
semantic behavioral variant frontotemporal dementia
- SD
semantic dementia
- svPPA
semantic variant primary progressive aphasia
- UCSF
University of California San Francisco
- VBM
voxel-based morphometry
Appendix. Authors
Study Funding
This work was funded by the NIH (NINDS R01NS050915, NIDCD K24DC015544, NIA P50AG023501, NIA P01AG019724, NIA R01AG038791, NIH/NIDCD R01DC016291, NINDS U54NS092089, NINDS R01AG029577, NIA K08AG052648, NIA R01AG029577, NIA P50AG023501), Alzheimer Disease Research Center of California (03–75271 DHS/ADP/ARCC); Larry L. Hillblom Foundation; John Douglas French Alzheimer Foundation; Koret Family Foundation; Consortium for Frontotemporal Dementia Research; and McBean Family Foundation.
Disclosure
A. Geraudie received an internship grant from the French Society of Neurology. M. Montembeault is supported by postdoctoral funding from Canadian Institutes of Health Research (CIHR) and Fonds Québécois de Recherche en Santé (FRQS). Go to Neurology.org/N for full disclosures.
References
- 1.Warrington EK. The selective impairment of semantic memory. Q J Exp Psychol. 1975;27(4):635-657. doi: 10.1080/14640747508400525 [DOI] [PubMed] [Google Scholar]
- 2.Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51(6):1546-1554. doi: 10.1212/wnl.51.6.1546 [DOI] [PubMed] [Google Scholar]
- 3.Hodges JR, Patterson K, Oxbury S, Funnell E. Semantic dementia. Progressive fluent aphasia with temporal lobe atrophy. Brain J Neurol. 1992;115 (Pt 6):1783-1806. doi: 10.1093/brain/115.6.1783 [DOI] [PubMed] [Google Scholar]
- 4.Snowden JS, Goulding PJ, Neary D. Semantic dementia: a form of circumscribed cerebral atrophy. Behav Neurol. 1989;2(3):167-182. [Google Scholar]
- 5.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006-1014. doi: 10.1212/WNL.0b013e31821103e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Younes K, Borghesani V, Montembeault M, et al. Right temporal degeneration and socioemotional semantics: semantic behavioural variant frontotemporal dementia. Brain. 2022;145(11):4080-4096. doi: 10.1093/brain/awac217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ulugut Erkoyun H, Groot C, Heilbron R, et al. A clinical-radiological framework of the right temporal variant of frontotemporal dementia. Brain J Neurol. 2020;143(9):2831-2843. doi: 10.1093/brain/awaa225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards-Lee T, Miller BL, Benson DF, et al. The temporal variant of frontotemporal dementia. Brain J Neurol. 1997;120 (Pt 6):1027-1040. doi: 10.1093/brain/120.6.1027 [DOI] [PubMed] [Google Scholar]
- 9.Gorno-Tempini ML, Rankin KP, Woolley JD, Rosen HJ, Phengrasamy L, Miller BL. Cognitive and behavioral profile in a case of right anterior temporal lobe neurodegeneration. Cortex J Devoted Study Nerv Syst Behav. 2004;40(4-5):631-644. doi: 10.1016/s0010-9452(08)70159-x [DOI] [PubMed] [Google Scholar]
- 10.Chan D, Anderson V, Pijnenburg Y, et al. The clinical profile of right temporal lobe atrophy. Brain J Neurol. 2009;132(Pt 5):1287-1298. doi: 10.1093/brain/awp037 [DOI] [PubMed] [Google Scholar]
- 11.Kamminga J, Kumfor F, Burrell JR, Piguet O, Hodges JR, Irish M. Differentiating between right-lateralised semantic dementia and behavioural-variant frontotemporal dementia: an examination of clinical characteristics and emotion processing. J Neurol Neurosurg Psychiatry. 2015;86(10):1082-1088. doi: 10.1136/jnnp-2014-309120 [DOI] [PubMed] [Google Scholar]
- 12.Thompson SA, Patterson K, Hodges JR. Left/right asymmetry of atrophy in semantic dementia: behavioral-cognitive implications. Neurology. 2003;61(9):1196-1203. doi: 10.1212/01.wnl.0000091868.28557.b8 [DOI] [PubMed] [Google Scholar]
- 13.Monrad-Krohn GH. Dysprosody or altered melody of language. Brain J Neurol. 1947;70(Pt 4):405-415. doi: 10.1093/brain/70.4.405 [DOI] [PubMed] [Google Scholar]
- 14.Ross, Shayya L, Rousseau JF. Prosodic stress: acoustic, aphasic, aprosodic and neuroanatomic interactions. J Neurolinguist. 2013;26(5):526-551. doi: 10.1016/j.jneuroling.2013.02.003 [DOI] [Google Scholar]
- 15.Ross ED, Monnot M. Neurology of affective prosody and its functional-anatomic organization in right hemisphere. Brain Lang. 2008;104(1):51-74. doi: 10.1016/j.bandl.2007.04.007 [DOI] [PubMed] [Google Scholar]
- 16.Monnot M, Orbelo D, Riccardo L, Sikka S, Rossa E. Acoustic analyses support subjective judgments of vocal emotion. Ann N Y Acad Sci. 2003;1000:288-292. doi: 10.1196/annals.1280.027 [DOI] [PubMed] [Google Scholar]
- 17.Nevler N, Ash S, Jester C, Irwin DJ, Liberman M, Grossman M. Automatic measurement of prosody in behavioral variant FTD. Neurology. 2017;89(7):650-656. doi: 10.1212/WNL.0000000000004236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nevler N, Ash S, Irwin DJ, Liberman M, Grossman M. Validated automatic speech biomarkers in primary progressive aphasia. Ann Clin Transl Neurol. 2019;6(1):4-14. doi: 10.1002/acn3.653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wright AE, Davis C, Gomez Y, et al. Acute ischemic lesions associated with impairments in expression and recognition of affective prosody. Perspect ASHA Spec Interest Groups. 2016;1(2):82-95. doi: 10.1044/persp1.SIG2.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pichon S, Kell CA. Affective and sensorimotor components of emotional prosody generation. J Neurosci. 2013;33(4):1640-1650. doi: 10.1523/JNEUROSCI.3530-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aziz-Zadeh L, Sheng T, Gheytanchi A. Common premotor regions for the perception and production of prosody and correlations with empathy and prosodic ability. PLoS One. 2010;5(1):e8759. doi: 10.1371/journal.pone.0008759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Devlin JT, Russell RP, Davis MH, et al. Susceptibility-induced loss of signal: comparing PET and fMRI on a semantic task. Neuroimage. 2000;11(6):589-600. doi: 10.1006/nimg.2000.0595 [DOI] [PubMed] [Google Scholar]
- 23.Ross ED, Mesulam MM. Dominant language functions of the right hemisphere? Prosody and emotional gesturing. Arch Neurol. 1979;36(3):144-148. doi: 10.1001/archneur.1979.00500390062006 [DOI] [PubMed] [Google Scholar]
- 24.Poeppel D. The analysis of speech in different temporal integration windows: cerebral lateralization as “asymmetric sampling in time”. Speech Commun. 2003;41(1):245-255. doi: 10.1016/S0167-6393(02)00107-3 [DOI] [Google Scholar]
- 25.Van Lancker D, Sidtis JJ. The identification of affective-prosodic stimuli by left- and right-hemisphere-damaged subjects: all errors are not created equal. J Speech Hear Res. 1992;35(5):963-970. doi: 10.1044/jshr.3505.963 [DOI] [PubMed] [Google Scholar]
- 26.Wright A, Saxena S. Sheppard SM, Hillis AE. Selective impairments in components of affective prosody in neurologically impaired individuals. Brain Cogn. 2018;124:29-36. doi: 10.1016/j.bandc.2018.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kertesz A. Western Aphasia Battery Test Manual. Grune&Stratton; 1982. [Google Scholar]
- 28.Gorno-Tempini ML, Dronkers NF, Rankin KP, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol. 2004;55(3):335-346. doi: 10.1002/ana.10825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kramer JH, Jurik J, Sha SJ, et al. Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cogn Behav Neurol. 2003;16(4):211-218. [DOI] [PubMed] [Google Scholar]
- 30.De Looze C Analyse et Interprétation de l'Empan Temporel Des Variations Prosodiques En Français et En Anglais. Sciences de l'Homme et Société. Université de Provence - Aix-Marseille I; 2010. [Google Scholar]
- 31.Styler W Using Praat for Linguistic Research. 2017. Accessed January 2022 savethevowels.org/praat [Google Scholar]
- 32.Lennes M. Collect_Pitch_Data_From_Files. 2003. Accessed January 2022 github.com/FieldDB/Praat%02Scripts/blob/master/collect_pitch_data_from_files.praat [Google Scholar]
- 33.Lennox RD, Wolfe RN. Revision of the self-monitoring scale. J Pers Soc Psychol. 1984;46(6):1349-1364. doi: 10.1037/0022-3514.46.6.1349 [DOI] [PubMed] [Google Scholar]
- 34.Toller G, Brown J, Sollberger M, et al. Individual differences in socioemotional sensitivity are an index of salience network function. Cortex J Devoted Study Nerv Syst Behav. 2018;103:211-223. doi: 10.1016/j.cortex.2018.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Froming KB, Weiner SG, Gregory AL, Levy CM, Ekman P. The Comprehensive Affect Testing System: An Emotion Processing System Clinical Version. Psychology Software, Inc. 2006. [Google Scholar]
- 36.Borghesani V, Battistella G, Mandelli ML, et al. Regional and hemispheric susceptibility of the temporal lobe to FTLD-TDP type C pathology. Neuroimage Clin. 2020;28:102369. doi: 10.1016/j.nicl.2020.102369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Regenbogen C, Schneider DA, Finkelmeyer A, et al. The differential contribution of facial expressions, prosody, and speech content to empathy. Cogn Emot. 2012;26(6):995-1014. doi: 10.1080/02699931.2011.631296 [DOI] [PubMed] [Google Scholar]
- 38.Pressman PS, Ross ED, Cohen KB, et al. Interpersonal prosodic correlation in frontotemporal dementia. Ann Clin Transl Neurol. 2019;6(7):1352-1357. doi: 10.1002/acn3.50816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumfor F, Landin-Romero R, Devenney E, et al. On the right side? A longitudinal study of left- versus right-lateralized semantic dementia. Brain J Neurol. 2016;139(Pt 3):986-998. doi: 10.1093/brain/awv387 [DOI] [PubMed] [Google Scholar]
- 40.Binney RJ, Henry ML, Babiak M, et al. Reading words and other people: a comparison of exception word, familiar face and affect processing in the left and right temporal variants of primary progressive aphasia. Cortex J Devoted Study Nerv Syst Behav. 2016;82:147-163. doi: 10.1016/j.cortex.2016.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Henry ML, Hubbard M, Grasso SM, et al. Treatment for word retrieval in semantic and logopenic variants of primary progressive aphasia: immediate and long-term outcomes. J Speech Lang Hear Res. 2019;62(8). doi: 10.1044/2018_JSLHR-L-18-0144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Lancker. Cerebral lateralization of pitch cues in the linguistic signal. Pap Linguist. 1980;13(2):201-277. doi: 10.1080/08351818009370498 [DOI] [Google Scholar]
- 43.Dara C, Kirsch-Darrow L, Ochfeld E, et al. Impaired emotion processing from vocal and facial cues in frontotemporal dementia compared to right hemisphere stroke. Neurocase. 2013;19(6):521-529. doi: 10.1080/13554794.2012.701641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leigh R, Oishi K, Hsu J, et al. Acute lesions that impair affective empathy. Brain. 2013;136(8):2539-2549. doi: 10.1093/brain/awt177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ethofer T, Anders S, Erb M, et al. Cerebral pathways in processing of affective prosody: a dynamic causal modeling study. Neuroimage. 2006;30(2):580-587. doi: 10.1016/j.neuroimage.2005.09.059 [DOI] [PubMed] [Google Scholar]
- 46.Wildgruber D, Ackermann H, Kreifelts B, Ethofer T. Cerebral processing of linguistic and emotional prosody: fMRI studies. Prog Brain Res. 2006;156:249-268. doi: 10.1016/S0079-6123(06)56013-3 [DOI] [PubMed] [Google Scholar]
- 47.Lambon Ralph MA, Jefferies E, Patterson K, Rogers TT. The neural and computational bases of semantic cognition. Nat Rev Neurosci. 2017;18(1):42-55. doi: 10.1038/nrn.2016.150 [DOI] [PubMed] [Google Scholar]
- 48.Schirmer A, Kotz SA. Beyond the right hemisphere: brain mechanisms mediating vocal emotional processing. Trends Cogn Sci. 2006;10(1):24-30. doi: 10.1016/j.tics.2005.11.009 [DOI] [PubMed] [Google Scholar]
- 49.Cancelliere AE, Kertesz A. Lesion localization in acquired deficits of emotional expression and comprehension. Brain Cogn. 1990;13(2):133-147. doi: 10.1016/0278-2626(90)90046-q [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
While we can share anonymized data, public archiving is not yet permitted under the study's IRB approval due to the sensitive nature of patient data. Specific requests can be submitted through the UCSF-MAC Resource (Request form: memory.ucsf.edu/resources/data). Following a UCSF-regulated procedure, access will be granted to designated individuals in line with ethical guidelines on the reuse of sensitive data. This would require submission of a Material Transfer Agreement, available at: icd.ucsf.edu/material-transfer-and-data-agreements. Commercial use will not be approved.