ABSTRACT
Toxoplasma gondii is an obligate intracellular parasite whose tachyzoite form causes disease via a lytic growth cycle. Its metabolic and cellular pathways are primarily designed to ensure parasite survival within a host cell. But during its lytic cycle, tachyzoites are exposed to the extracellular milieu and prolonged exposure requires activation of stress response pathways that include reprogramming the parasite proteome. Regulation of protein synthesis is therefore important for extracellular survival. We previously reported that in extracellularly stressed parasites, the elongation phase of protein synthesis is regulated by the Toxoplasma oxygen-sensing protein, PHYb. PHYb acts by promoting the activity of elongation factor eEF2, which is a GTPase that catalyzes the transfer of the peptidyl-tRNA from the A site to the P site of the ribosome. In the absence of PHYb, eEF2 is hyper-phosphorylated, which inhibits eEF2 from interacting with the ribosome. eEF2 kinases are atypical calcium-dependent kinases and BLAST analyses revealed the parasite kinase, CDPK3, as the most highly homologous to the Saccharomyces cerevisiae eEF2 kinase, RCK2. In parasites exposed to extracellular stress, loss of CDPK3 leads to decreased eEF2 phosphorylation and enhanced rates of elongation. Furthermore, co-immunoprecipitation studies revealed that CDPK3 and eEF2 interact in stressed parasites. Since CDPK3 and eEF2 normally localize to the plasma membrane and cytosol, respectively, we investigated how the two can interact. We report that under stress conditions, CDPK3 is not N-myristoylated likely leading to its cytoplasmic localization. In summary, we have identified a novel function for CDPK3 as the first protozoan extracellular stress-induced eEF2 kinase.
IMPORTANCE
Although it is an obligate intracellular parasite, Toxoplasma must be able to survive in the extracellular environment. Our previous work indicated that ensuring that elongation continues during protein synthesis is part of this stress response and that this is due to preventing phosphorylation of elongation factor 2. But the identity of the eEF2 kinase has remained unknown in Toxoplasma and other protozoan parasites. Here, we identify CDPK3 as the first protozoan eEF2 kinase and demonstrate that it is part of a stress response initiated when parasites are exposed to extracellular stress. We also demonstrate that CDPK3 engages eEF2 as a result of its relocalization from the plasma membrane to the cytosol.
KEYWORDS: Toxoplasma gondii, parasite, oxygen, protein synthesis, kinase
INTRODUCTION
Toxoplasma gondii, which is the causative agent of toxoplasmosis, is an obligate intracellular protozoan parasite that can infect almost all nucleated cells of warm-blooded mammals (1). Upon invasion, the parasite establishes and replicates within a parasitophorous vacuole until it egresses from its host cell and goes on to invade its next host cell (2). Its success as a pathogen is due to the elaborate mechanisms it uses to complete each step of its lytic cycle as well as evade host cell defenses. These include the expression of nutrient scavenging and biosynthetic pathways to satisfy its metabolic needs, the deployment of parasite-encoded host cell effectors that alter host signaling, defenses, and transcription, release of receptor complexes into host cell plasma membrane that facilitate host cell invasion, and the ability to develop into a chronic stage infection that is impervious to host immune responses and antiparasitic agents (3 - 5).
Like most other obligate intracellular pathogens, host cell egress exposes the parasite to the extracellular milieu. During extended periods of time of extracellular residence, parasite survival requires the activation of extracellular stress responses that include alterations in protein abundance (6). Thus, protein synthesis has emerged as a key extracellular stress responses pathway. Protein synthesis is composed of three stages—initiation, elongation, and termination. Initiation involves ribosome scanning identifying a start methionine AUG codon and the subsequent recruitment of a methionine-charged initiator tRNA to the A site of the ribosome. The initiation factor, eIF2α, facilitates this process by promoting initiator tRNA association with the ribosome and eIF2α phosphorylation inhibits its activity. Earlier work revealed that protein synthesis is reduced in parasites exposed to extracellular stress and that this is due, in part, to eIF2α phosphorylation and inhibition of translation initiation (6).
The Toxoplasma oxygen-sensing protein, PHYb, is important for efficient parasite lytic growth but dispensable for invasion, replication, or egress. Instead, it is required for extracellular parasite survival and does so by promoting translational elongation, which is the process of transferring the peptidyl-tRNA complex from the A site of the ribosome to the P site (7). The elongation factor, eEF2, catalyzes this process and, like eIF-2α, eEF2 phosphorylation limits its activity (8, 9). PHYb promotes elongation by limiting eEF2 phosphorylation but how PHYb regulates eEF2 phosphorylation is unknown as is the identity of an eEF2 kinase in Toxoplasma. eEF2 kinases are calcium/calmodulin-dependent kinases that have been identified in metazoans and fungi but not protozoans (10). But their identification is important due to the discovery of eEF2 as a druggable target in Plasmodium infections and likely other parasites (11 - 13).
In Toxoplasma and other apicomplexans, CDPKs are the best characterized family of calcium/calmodulin-dependent kinases (14, 15). The parasite genome predicts the presence of 15 CDPKs with most work focusing on CDPK1 and CDPK3. Both stimulate calcium-dependent exocytosis of the micronemal organelles and egress but only CDPK1 is important for invasion (16 - 21). CDPK1 and CDPK3 are both N-myristoylated but only CDPK3 is also palmitoylated and these differences in fatty acylation may be the basis for their differential localization and substrates (19, 22). But membrane association is not the only feature that defines CDPK3 substrate interactions since putative non-membrane CDPK3 substrates have also been identified (23). In addition, CDPK3 loss of function mutants inhibits death of extracellular parasites exposed to calcium-ionophore through as yet undefined mechanisms (21). In this study, we identify CDPK3 as an extracellular stress-activated eEF2 kinase that is able to interact with eEF2 in the cytoplasm due to loss of its N-myristoylation. CDPK3 phosphorylation of eEF2 leads to decreases in protein synthesis due to decreased rates of translation elongation suggesting a mechanism by which CDPK3 mediates ionophore-induced extracellular death.
RESULTS
eEF2 phosphorylation during extracellular stress is CDPK3 dependent
Using parasites engineered to conditionally express PHYb via Shield-1-mediated protein stabilization, we reported that decreased PHYb protein levels in parasites exposed to extracellular stress lead to reduced rates of elongation during protein synthesis, and this is a result of increased eEF2 phosphorylation (7). We therefore hypothesized that PHYb negatively regulates an eEF2 kinase during extracellular stress. eEF2 kinases are calcium-dependent protein kinases that have not yet been identified in protozoans. To first test whether stress-induced eEF2 phosphorylation is Ca2+-dependent, tachyzoites ±PHYb were subjected to extracellular stress for 8 h in the absence or presence of the Ca2+ chelator, BAPTA-AM for the final 2 h. Lysates were prepared and Western blotted to detect either total or phosphorylated eEF2. As previously reported, extracellular stress increased eEF2 phosphorylation in the absence of PHYb. But in the presence of BAPTA-AM, stress-induced eEF2 phosphorylation levels were significantly reduced (Fig. 1A and B).
Fig 1.
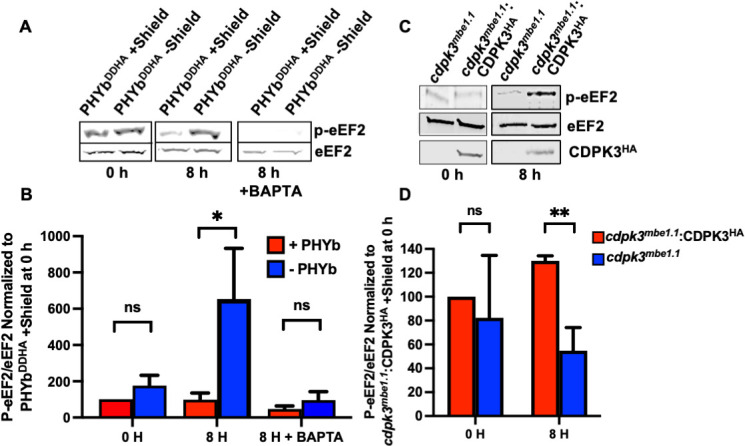
CDPK3 regulates eEF2 phosphorylation. (A and B) PHYb replete (+shield) or depleted (–shield) parasites were either unstressed (0 h) or extracellularly stressed for 8 h ±BAPTA-AM for the final 2 h. Lysates were prepared and Western blotted to detect total or phospho-eEF2. Shown are representative blots and averages ± SD of three independent experiments. (C and D) cdpk3mbe1.1 or CDPK3-overexpressing (cdpk3mbe1.1 :CDPK3HA) parasites were unstressed or stressed for 8 h. Lysates were blotted to detect total or phospho-eEF2 and HA-tagged CDPK3. Shown are representative blots and the averages±SD of three independent experiments. SD, standard deviation.
eEF2 kinases have been identified in metazoans and fungi but not protozoans. BLAST analysis of human or mouse eEF2 kinases (GenBank Accession numbers AAB58270.1 and AAH60707.1, respectively) against the Toxoplasma genome database did not identify any significant hits. The top 5 hits from a BLAST search of the Saccharomyces cerevisiae eEF2 kinase, Rck2p (24, 25), were CDPK family members with the regions of homology lying within the conserved protein kinase domain of Rck2p (Table S1 and Fig. S1). Among these, CDPK3 (TgME49_305860) was of interest because it had the highest homology (E=2e−39) and because the Plasmodium homolog of CDPK3 (PfCDPK1) was shown to regulate translation during zygote development in the mosquito midgut (26). Moreover, Toxoplasma mutants harboring loss of function mutations in CDPK3 were resistant to Ca2+ ionophore-induced death of extracellular parasites, which was hypothesized to be due to CDPK3 acting to prevent the replenishment of invasion-associated factors that were released following ionophore treatment of the extracellular parasites (20, 21).
We next compared stressed-induced eEF2 phosphorylation levels in cdpk3mbe1.1 , which is a parasite strain harboring a point mutation in CDPK3 that inhibits its kinase activity (20, 21). In these parasites, eEF2 phosphorylation was not increased after exposing the parasites to extracellular stress. In contrast, eEF2 phosphorylation was significantly increased in cdpk3mbe1.1 :CDPK3HA, a strain in which wild-type CDPK3 is overexpressed in the mutant strain (Fig. 1C and D).
CDPK3 regulates elongation during protein synthesis
Extracellular tachyzoites spontaneously secrete host cell adhesins and other factors that function in host cell invasion and other processes and these factors must be replenished for the parasites to remain invasion competent. We previously reported that PHYb-depleted parasites are susceptible to extracellular stress because increased eEF2 phosphorylation decreases rates of elongation during protein synthesis (7). Elongation rates can be determined by quantifying incorporation of puromycin into nascent polypeptides, which can be detected using puromycin antibody (27). To assess the impact CDPK3 on elongation, cdpk3mbe1.1 and cdpk3mbe1.1 :CDPK3HA parasites were incubated extracellularly for 8 h and then puromycin was added for increasing amounts of time. Lysates were prepared, separated by SDS-PAGE, and Western blotted to detect puromycylated peptides. We observed that extracellular stress increased rates of elongation only in cdpk3mbe1.1 but not cdpk3mbe1.1 :CDPK3HA parasites (Fig. 2A).
Fig 2.
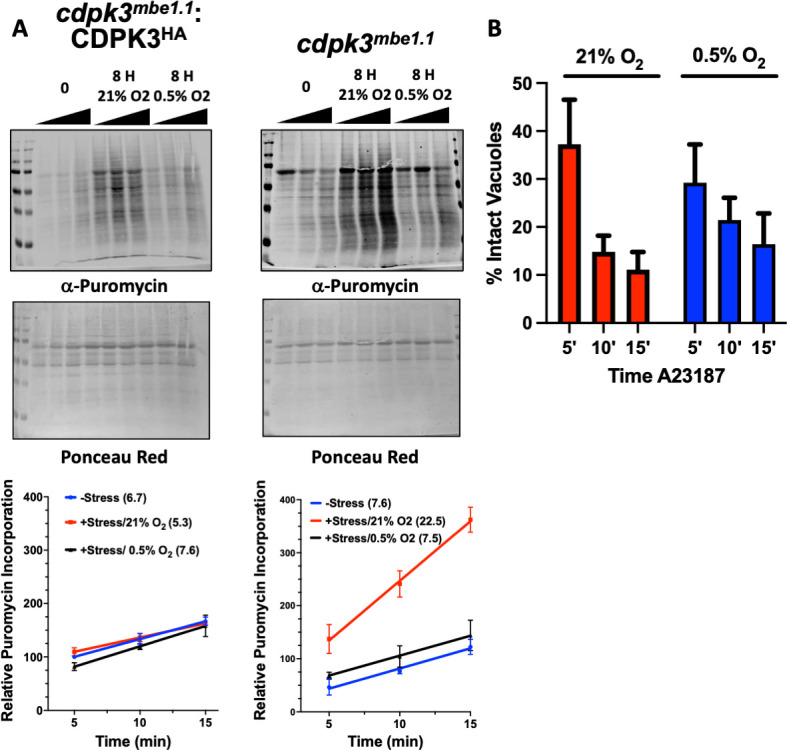
CDPK3 negatively regulates translation elongation. (A) Puromycylated peptides were detected by Western blotting lysates prepared from either freshly egressed parasites or parasites incubated extracellularly for 8 h at 21% O2 or 0.5% O2 were incubated with puromycin for the indicated times. Lysates were prepared, separated by SDS-PAGE and then either Western blotted to detect puromycylated peptides (top) or stained with Ponceau (bottom). Shown are representative blots from three independent experiments. Graphs below show averages±standard deviations of relative puromycin/ponceau labeling intensities determined for each time point. The slope of each line was calculated to determine rate of puromycin incorporation and shown in graph legend as number following each condition in parenthesis. (B) cdpk3mbe1.1 :CDPK3HA parasites were grown for on coverslips for 30 h at 21% or 0.5% O2. A23187 was then added for the indicated times and the cells were immediately fixed and stained to detect SAG1 by immunofluorescence. Shown are averages±standard deviations of two experiments performed in triplicate with at least 50 random vacuoles counted/coverslip.
Previously, we reported that translation elongation was inhibited in parasites exposed to extracellular stress at 21% O2 but not at 0.5% O2 and that this phenotype was mediated by PHYb (7). Thus, we used puromycin incorporation to assess translation elongation at 0.5% O2. At low O2, rates of translation elongation were not changed in either CDPK3-deficient or overexpressing parasites (Fig. 2A). The inability for CDPK3 to regulate translation at low O2 was not due to a defect in its inherent kinase activity since Ca2+ ionophore induced egress, which requires CDPK3, was functional at low O2 (Fig. 2B). Taken together, these data indicate that CDPK3 regulates translation elongation as part of an extracellular stress response that functions upstream of O2/PHYb signaling.
Extracellular stress promotes CDPK3-eEF2 interactions
Having established that extracellular stress increased CDPK3-dependent eEF2 phosphorylation, we next sought to determine whether the two proteins interact. We prepared extracts from either freshly egressed or extracellularly stressed cdpk3mbe1.1 and cdpk3mbe1.1 :CDPK3HA parasites. Lysates were then incubated with anti-HA antibodies, immune complexes separated by SDS-PAGE, and CDPK3HA and eEF2 detected by Western blotting. It was observed that eEF2 only interacted with CDPK3 in stressed parasites (Fig. 3A). Our attempts at reciprocal IPs were unsuccessful due to our inability to efficiently IP eEF2 (not shown).
Fig 3.

Extracellular stress promotes CDPK3/eEF2 interactions. (A) CDPK3Δ or CDPK3Δ:CDPK3HA parasites were incubated extracellularly for 0 or 8 h. Whole cell lysates were prepared and either Western blotted to detect CDPK3 and eEF2 or incubated with anti-HA/Protein A beads and the immune complexes blotted to detect CDPK3HA and eEF2. Shown are representative blots from three independent experiments. (B–D) CDPK3Δ:CDPK3HA parasites were incubated extracellularly for 0 or 8 h and then stained to detect CDPK3HA, eEF2, IMC3, or GAP45. Plots on right represent staining intensities of each protein as shown by the white line in the overlay images. Shown are representative images. Scale bar=1 μm.
In neurons and other cell types, eEF2 is a cytoplasmically enriched, soluble nucleocytoplasmic protein (28 - 30) and HYPERLOPIT analyses predicted Toxoplasma eEF2 to also be a highly enriched in the cytoplasm (31). On the other hand, CDPK3 is embedded in the inner leaflet of the parasite’s plasma membrane (19, 21). Because the inner membrane complex (IMC) is directly opposed to the plasma membrane, cytoplasmic proteins do not have direct access to the plasma membrane. Thus, for CDPK3 and eEF2 to interact, one of the proteins would likely have to alter its localization. We therefore used immunofluorescence assays to examine localization of each protein in freshly egressed and stressed parasites. eEF2 was observed to maintain its cytoplasmic localization regardless of stress (Fig. 3B). On the other hand, extracellular stress induced a significant fraction of CDPK3 to relocalize from the plasma membrane to the cytoplasm. The altered localization was not due to a breakdown of the parasite plasma membrane or underlying IMC as localization of either GAP45 (plasma membrane associated) or IMC3 (a resident IMC protein) was unaltered by stress (Fig. 3C and D).
Extracellular stress alters CDPK3 N-myristoylation
CDPK3 is embedded in the plasma membrane by virtue of its myristoylation and palmitoylation at positions 2Gly and 3Cys, respectively (18, 19, 21, 32). We therefore hypothesized that stress-induced CDPK3 cytoplasmic localization was due to alterations in its fatty acylation. To test this hypothesis, stressed and unstressed cdpk3mbe1.1 and cdpk3mbe1.1 :CDPK3HA parasites were incubated in the presence of azido-myristic acid or azido-palmitic acid for 2 h. Lysates were then prepared and incubated with alkyne-conjugated biotin, separated by SDS-PAGE, and Western blotted to detect total protein fatty acylation. We found that extracellular stress had no significant impact on either global myristoylation or palmitoylation (Fig. 4A and B). Next, CDPK3HA was immunoprecipitated from the azido-labeled cdpk3mbe1.1 :CDPK3HA lysates and the immune complexes were Western blotted using streptavidin to specifically assess CDPK3 myristoylation or palmitoylation. We found that while extracellular stress had no apparent impact on its palmitoylation, CDPK3 myristoylation was significantly reduced (Fig. 4C and D).
Fig 4.

Extracellular stress alters CDPK3 myristoylation. Freshly egressed (−stress) or extracellularly stressed (+stress) CDPK3Δ:CDPK3HA parasites were incubated with azido-myristate (A, C) or azido-palmitate (B, D) for 4 h. Whole-cell lysates (1/10 or total) were incubated with alkyne-biotin, separated by SDS-PAGE, and Western blotted with streptavidin to detect total myristoylated (A) or palmitoylated (B) proteins or total CDPK3 (⍺-HA WCL (whole-cell lysate) in (C and D). The remainder of lysates were incubated with anti-HA beads to capture CDPK3HA and immune complexes incubated with alkyne-biotin to biotinylate the myristate (C) or palmitate (D) analogs. The reactions were separated by SDS-PAGE and Western blotted to detect total CDPK3 (αHA) or and acylated CDPK3 (streptavidin). Shown are representative blots from three independent experiments and graphs represent average±SD of the ratios of fatty acylated CDPK3HA:total CDPK3HA.
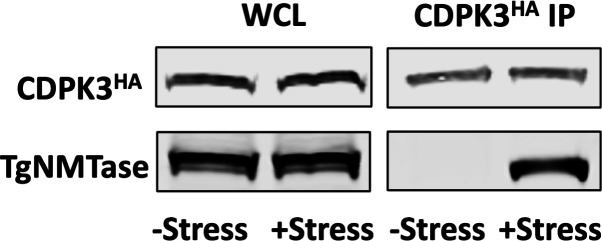
N-myristoylation is catalyzed by protein N-myristoyltransferase (NMTase) that in Toxoplasma is encoded by a single essential gene (TGME49_209160). TgNMTase is predicted to be important for tachyzoite fitness (33) and migrates as a ~57 kDa protein in SDS-PAGE (34). Western blot analysis indicated that decreased CDPK3 myristoylation was not due to a stress-induced decrease in TgNMTase expression, which is consistent with the observation that extracellular stress did not have an apparent global impact on protein myristoylation (Fig. 5A). The NMTase reaction is initiated by the enzyme first complexing with myristoyl-CoA followed by binding of the protein substrate whose 2Gly has been exposed by release of the N-terminal methionine by a methionine aminopeptidase. A series of conformational changes brings the reactants near enough to allow conjugation of the myristic acid to 2Gly after which the myristoylated protein is released. We therefore assessed whether extracellular stress affected CDPK3/TgNMTase interactions by immunoprecipitating CDPK3HA from unstressed and extracellularly stressed parasites and then Western blotted to detect CDPK3HA or TgNMTase. In unstressed parasites, the enzyme:substrate complex was undetectable indicating that the N-myristoylation reaction was efficient (Fig. 5). In contrast, TgNMTase could be detected in the CDPK3HA immunoprecipitates from the stressed parasites. These data together suggest that defects in CDPK3 myristoylation were due to TgNMTase being unable to properly interact with CDPK3.
Fig 5.
CDPK3/TgNMTase interactions are enhanced in parasites exposed of extracellular stress. Whole-cell lysates (WCL) from freshly egressed (−stress) or extracellularly stressed (+stress) CDPK3Δ:CDPK3HA parasites were either Western blotted to detect total CDPK3HA and TgNMTase or incubated with anti-HA/Protein A beads and the immune complexes blotted to detect CDPK3HA and TgNMTase. Shown are representative blots from three independent experiments.
DISCUSSION
Metabolic and other essential processes of obligate intracellular pathogens are designed for their intracellular lifestyle. But these pathogens are exposed to the extracellular milieu as they move between host cells and how they survive under these conditions is largely unknown. In a previous study, we identified elongation as a process that is modulated as part of this stress response (7). We further demonstrated that eEF2 phosphorylation is negatively regulated by PHYb and that this negatively regulatory loop is required to allow parasites to persist under prolonged exposure to extracellular stress. In this study, we sought to identify the stress-activated eEF2 kinase and based upon results from co-immunoprecipitation, eEF2 phosphorylation status, and localization studies; we conclude that CDPK3 functions as a eEF2 kinase and that PHYb is required to modulate this activity to ensure protein synthesis can continue (Fig. 6).
Fig 6.

Model for PHYb/CDPK3 promoting extracellular survival via eEF2 phosphorylation.
CDPK3 was first identified in a genetic screen designed to identify parasite mutants that were resistant to parasites egressing from host cells upon exposure to the Ca2+ ionophore A23187 (20, 21). In addition, this mutant (named MBE1.1) were also resistant parasite death induced when extracellular parasites were exposed for prolonged times to the Ca2+ ionophore. CDPK3 substrates have been identified including a myosin isoform whose phosphorylation likely underlies the egress phenotype (23, 35). But neither microneme secretion nor motility was affected in extracellular CDPK3 mutants leading to the suggestion that the CDPK3 mutants are unable to replenish those invasion-associated factors secreted upon Ca2+ ionophore exposure (20, 21). Our findings here support such a model in which CDPK3 negatively regulates protein synthesis in extracellular parasites and that loss of CDPK3 removes this repression. We do note, however, previous work did not identify eEF2 as a CDPK3 substrate (23). This, however, was expected since that study did not utilize stressed parasites and we found that CDPK3 had no apparent effect on eEF2 phosphorylation in unstressed parasites.
Stress responses modulate protein synthesis to allow a cell to reprogram its proteome. In most organisms including Toxoplasma and other apicomplexans, translation initiation is the best characterized mechanism for stress responses to regulate protein synthesis (36) with eIF2α phosphorylation as a key target. In Toxoplasma, distinct kinases phosphorylate eIF2α in response to oxidative, endoplasmic reticulum, nutritional, and extracellular stresses (37 - 40). Elongation, on the other hand, is a less wellunderstood though recognized stress prokaryotic and eukaryotic response target (41 - 43). Unlike eIF2α as the primary target for regulating translation initiation, numerous elongation factors can be modulated by stress and these include eEF2 (7, 43 - 47). Our identification of CDPK3 as an eEF2 kinase represents, to our knowledge, the first protozoan eEF2 kinase. Our data showing that CDPK3 only impacts eEF2 phosphorylation in extracellularly stressed parasites suggests the presence of additional eEF2 kinases. Whether other CDPK family members serve this function remains to be determined but given that eEF2 kinases are not essential in Saccharomyces, Caenorhabditis elegans, or mice (48, 49), we predict that like CDPK3 that non-essential Toxoplasma CDPKs would most likely act as eEF2 kinases. Identification of these kinases is important given the increasing focus on eEF2 as a drug target in related parasites such as Plasmodium and Eimeria (12, 13, 49).
The IMC in Toxoplasma and other apicomplexans is a specialized double-membrane organelle that lies directly underneath the plasma membrane and functions in parasite motility, secretion, and replication (2, 50 - 52). Its close apposition to the plasma membrane limits contact between plasma membrane and cytosolic factors. Thus, we predicted that marked relocalization of either eEF2 or CDPK3 would be required for the two proteins to interact. Our finding CDPK3 that relocalizes under extracellular stress was reminiscent of glycolytic enzyme relocalization in extracellular parasites (53), although there are significant differences exist between the two. First, glycolytic enzymes relocalize from the cytosol to the periphery. Second, glycolytic enzymes associate with membranes via electrostatic interactions rather than embedment via fatty acylation. Finally, glycolytic enzymes associate with the cytoplasmic face of the IMC, a finding further supporting our observations that the IMC remains intact upon exposure to extracellular stress.
Mechanistically, CDPK3 relocalization is likely the result of newly synthesized CDPK3 from becoming N-myristoylated since N-myristoylation is considered an irreversible posttranslational modification (54). On the other hand, we found that CDPK3 was palmitoylated, which was initially surprising given that S-palmityl acyltransferase (PAT) is membrane-associated and 2Gly myristoylation is thought to mediate PAT access to N-terminal cysteines in substrates, like CDPK3 that is palmitoylated at 3Cys (19, 21). One explanation for this finding is that 3Cys-palmitoylation is independent of 2Gly myristoylation. Alternatively, CDPK3 was reported to also be Ser-palmitoylated (32) and our assays cannot discriminate between single or multiple palmitoylation events nor can it discriminate between the modified amino acids. Regardless, these data suggest that CDPK3 utilizes multiple membrane targeting mechanisms that is likely a binding protein since the protein lacks apparent regions of positively charged amino acids.
The ability for CDPK3 and NMTase to interact in response to extracellular stress suggests that CDPK3 becomes “trapped” with NMTase in an intermediate complex, which represents to our knowledge the first example of such an interaction Based on structural studies of the NMTase reaction (55), we hypothesize that CDPK3 associates with NMTase-bound myristyl-CoA in a manner that 2Gly cannot interact with the carbonyl group of myristyl-CoA. It is also unclear what triggers this block in CDPK3 N-myristoylation. Our future studies will address these questions.
Translation elongation is a recognized target for hypoxic stress responses (56, 57) and in Toxoplasma our collective data points to a model in which PHYb negatively regulates CDPK3 phosphorylation of eEF2. This is most likely achieved by CDPK3 and/or eEF2 serving as substrates for PHYb’s prolyl hydroxylase activity. In murine cells, eEF2 kinase is a prolyl hydroxylase substrate and hydroxylation of 98Pro blocks eEF2 kinase activity (58). This would contrast with Toxoplasma PHYb that is active at high oxygen and thus PHYb-mediated prolyl hydroxylation of CDPK3 would be expected to promote CDPK3 activity. In addition, 98Pro from metazoan eEF2 kinase is not conserved in CDPK3. It is also possible that PHYb acts by preventing CDPK3 from binding its substrates or by promoting the activity of an eEF2 phosphatase.
In summary, we identified CDPK3 as a kinase that regulates the phosphorylation state and activity of eEF2 in response to Toxoplasma exposure to extracellular stress. We further determined that interactions between CDPK3 and eEF2 are mediated by reduced N-myristoylation and that is due an apparent defect in the NMTase reaction. Given the conservation of CDPK3 and eEF2 among other apicomplexan parasites, we expect that this signaling axis will be conserved in these parasites as well.
MATERIALS AND METHODS
Cells and parasites
The type I ckpk3mbe1.1 and ckpk3mbe1.1:CDPK3HA Toxoplasma strains (21) were obtained from Dr. Gustavo Arrizabalaga (Indiana University School of Medicine) and maintained by passage in human foreskin fibroblasts (HFFs) in complete medium (Dulbecco’s modified Eagle’s medium supplemented with 10% heat-inactivated fetal calf serum, L-glutamine, and penicillin/streptomycin) as previously described (59). Parasites and host cells were regularly tested for Mycoplasma contamination using the Mycoalert detection kit (Lonza, Basel, Switzerland). For all assays, parasites harvested from unlysed HFF monolayers were released by syringe lysis by passage through a 27-gauge needle followed by washing in serum-free media. Extracellular stress was applied by incubating the parasites in complete medium at 37°C at either 21% O2 or 0.5% O2 (Invivo2 Baker, Sanford, ME). Calcium-ionophore induced egress assays were performed essentially as described (20). Briefly, parasites were grown in HFFs on coverslips for 30 h at 21% or 0.5% O2. The medium was then replaced with prewarmed Hanks buffered saline solution containing 8 µM A23187 for the indicated times and then ice-cold methanol was added to fix the cells. Lysed and intact vacuoles were identified by immunofluorescence staining of the parasite surface protein SAG1 (Novus, Centennial, CO).
Western blotting
Parasites were pelleted by centrifugation 2000×g for 8 min at 4°C and lysed in boiling SDS-PAGE sample buffer containing 2-β mercaptoethanol. Equivalent cell volume of lysates was separated by SDS-PAGE gels, transferred to nitrocellulose membranes, and blocked with Odyssey Blocking Buffer (LI-COR Biosciences, Lincoln, NE), blotted using antibodies to detect total eEF2 (Abcam #33523, Cambridge, UK) phospho-eEF2 (Cell Signaling Technology #2331S, Danvers, MA), anti-HA (Sigma-Aldrich #12CA5, St. Louis, MO), and TgNMTase (34). Blots were imaged using an LI-COR Odyssey scanner and analyzed using Image Studio software (LI-COR, Omaha, NE).
Immunoprecipitation
Freshly egressed or extracellularly stressed parasites were lysed in 50 mM Tris-HCl pH 7.4 with 1% Triton X-100, 150 mM NaCl, 1 mM NaF, 1 mM CaCl2, 0.2 mM Na3VO4, 1× protease inhibitor cocktail (Thermo Fisher Scientific, Waltham, MA), incubated on ice for 30 min and then subjected to sonication. Lysates were clarified by centrifugation at 16,000×g, incubated with mouse α-anti-HA clone 12CA5 conjugated with agarose beads (Abcam #214758) for 16 h at 4˚C. Immune complexes were separated by SDS-PAGE, transferred to nitrocellulose, and processed for Western blotting.
Immunofluorescence microscopy
Freshly egressed or extracellularly stressed parasites (107) were fixed with 4% w/v paraformaldehyde in phosphate-buffered saline (PBS) for 20 min at room temperature, permeabilized with 0.1% Triton X-100 in PBS for 10 min, and blocked 5% w/v bovine serum albumin (BSA) in PBS for 20 min. Parasites were then incubated with a combination of antibodies to detect HA, and either eEF2, IMC3 (from Dr. Marc-Jan Gubbels, Boston College, MA) or GAP45 (gift from Dr. Gary Ward, University of Vermont, VT) for 16 h at 4°C and followed by secondary Alexa-Fluor 488- or Alexa Fluor-594-conjugated antibodies for another 60 min (Thermo Fisher Scientific).
For image acquisition, a 0.2-µm z-increment serial image stacks were collected with capture times from 100 to 500 ms (100× Plan Apo oil immersion lens, 1.46 numerical aperture) on a motorized Zeiss Axioimager M2 stand equipped with a rear-mounted excitation filter wheel, a triple pass [DAPI/fluorescein isothiocyanate (FITC)/Texas Red] emission cube, differential interference contrast (DIC) optics, and an Orca ER charge-coupled-device (CCD) camera (Hamamatsu, Bridgewater, NJ). Images were collected with Volocity, version 6.1, Acquisition Module (Improvision Inc., Lexington, MA). Individual channel stacks were deconvolved by a constrained iterative algorithm, pseudo-colored, and merged using the Volocity, version 6.1, Restoration Module. All images presented here are summed stack projections of merged channels.
Translation elongation assay
Puromycin incorporation was performed essentially as described (7). Briefly, freshly harvested parasites (3 × 106) were resuspended in 1 mL of complete DMEM and incubated at 21% or 0.5% O2 for 0 or 8 h at 37°C. Puromycin (10 µg/mL) (Sigma-Aldrich) was added for the indicated times and the parasites were pelleted, washed in ice-cold PBS, and analyzed by Western blotting using an antipuromycin antibody. Each lane was scanned and quantified densitometrically. Puromycin incorporation was normalized to the Ponceau S stain intensity of the membranes, and translation rates were calculated from slopes of the lines generated using linear regression analysis with Prism Software (GraphPad, La Jolla, CA).
Fatty acylation assays
CDPK3 myristoylation and palmitoylation were assessed using the CLICK-iT kit following the manufacturer’s protocol (Thermo Fisher Scientific). Briefly, intracellular (−stress) or freshly egressed (+stress) parasites were incubated with Click-iT myristic acid azide (25 µM) or palmitic acid azide (50 µM) for 4 h at 37˚C. Parasites were collected and lysed by sonication in RIPA buffer (150 mM NaCl, 50 mM Tris-HCl pH 7.4, 10 mM MgCl, 0.5% sodium deoxycholate, 0.1% SDS, and 1% Triton with protease inhibitor cocktail [Roche]). To assess global fatty acylation, lysates were incubated with biotin alkyne (20 µM) using the Click-iT Protein Reaction Buffer protocol and the proteins were precipitated and then resuspended and boiled for 10 min in 2× Laemmli buffer. To assess CDPK3 fatty acylation, lysates were incubated with rabbit α-HA antibody and then captured using Protein G Agarose (Pierce, Rockford, IL). Immune complexes were then incubated with biotin alkyne (20 µM) in the Click-iT Protein Reaction Buffer Kit. Lysates and immune complexes were separated by SDS-PAGE and blotted to detect either biotinylated proteins (IRDye 680LT streptavidin (Li-Cor)) or CDPK3HA (rat-anti-HA)/anti-rat DyLight 800 (Invitrogen).
Statistics
Unless otherwise noted, all experiments were repeated a minimum of three times. Data were expressed as mean±SD, plotted and tested for significance using two-tailed Student’s t test with Prism Software (GraphPad, La Jolla, CA).
ACKNOWLEDGMENTS
We thank Drs. Gustavo Arrizabalaga, Gary Ward, and Marc Jan Gubbels for supplying critical reagents and members of our lab for helpful discussions.
Contributor Information
Ira J. Blader, Email: iblader@buffalo.edu.
William J. Sullivan, Indiana University School of Medicine, Indianapolis, Indiana, USA
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/msphere.00156-23.
Blast results.
Blader results.
Legends for Fig. S1 and Table S1.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Kim K, Weiss LM. 2004. Toxoplasma ondii: the model apicomplexan. Int J Parasitol 34:423–432. doi: 10.1016/j.ijpara.2003.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blader IJ, Coleman BI, Chen C-T, Gubbels M-J. 2015. Lytic cycle of Toxoplasma gondii: 15 years later. Annu Rev Microbiol 69:463–485. doi: 10.1146/annurev-micro-091014-104100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blader IJ, Saeij JP. 2009. Communication between Toxoplasma gondii and its host: impact on parasite growth, development, immune evasion, and virulence. APMIS 117:458–476. doi: 10.1111/j.1600-0463.2009.02453.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Augusto L, Wek RC, Sullivan WJ. 2021. Host sensing and signal transduction during Toxoplasma stage conversion. Mol Microbiol 115:839–848. doi: 10.1111/mmi.14634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang Y, Sangaré LO, Paredes-Santos TC, Saeij JPJ. 2020. Toxoplasma mechanisms for delivery of proteins and uptake of nutrients across the host-pathogen interface. Annu Rev Microbiol 74:567–586. doi: 10.1146/annurev-micro-011720-122318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Joyce BR, Queener SF, Wek RC, Sullivan WJ. 2010. Phosphorylation of eukaryotic initiation Factor-2{Alpha} promotes the extracellular survival of obligate intracellular parasite Toxoplasma gondii. Proc Natl Acad Sci U S A 107:17200–17205. doi: 10.1073/pnas.1007610107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Florimond C, Cordonnier C, Taujale R, van der Wel H, Kannan N, West CM, Blader IJ. 2019. A Toxoplasma prolyl hydroxylase mediates oxygen stress responses by regulating translation elongation. mBio 10: e00234-19. doi: 10.1128/mBio.00234-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ryazanov AG, Davydova EK. 1989. Mechanism of elongation factor 2 (EF-2) inactivation upon phosphorylation. Phosphorylated EF-2 is unable to catalyze translocation. FEBS lett 251:187–190. doi: 10.1016/0014-5793(89)81452-8 [DOI] [PubMed] [Google Scholar]
- 9. Ryazanov AG, Shestakova EA, Natapov PG. 1988. Phosphorylation of elongation factor 2 by EF-2 kinase affects rate of translation. Nature 334:170–173. doi: 10.1038/334170a0 [DOI] [PubMed] [Google Scholar]
- 10. Proud CG. 2015. Regulation and roles of elongation factor 2 kinase. Biochem Soc Trans 43:328–332. doi: 10.1042/BST20140323 [DOI] [PubMed] [Google Scholar]
- 11. Baragaña B, Hallyburton I, Lee MCS, Norcross NR, Grimaldi R, Otto TD, Proto WR, Blagborough AM, Meister S, Wirjanata G, Ruecker A, Upton LM, Abraham TS, Almeida MJ, Pradhan A, Porzelle A, Luksch T, Martínez MS, Luksch T, Bolscher JM, Woodland A, Norval S, Zuccotto F, Thomas J, Simeons F, Stojanovski L, Osuna-Cabello M, Brock PM, Churcher TS, Sala KA, Zakutansky SE, Jiménez-Díaz MB, Sanz LM, Riley J, Basak R, Campbell M, Avery VM, Sauerwein RW, Dechering KJ, Noviyanti R, Campo B, Frearson JA, Angulo-Barturen I, Ferrer-Bazaga S, Gamo FJ, Wyatt PG, Leroy D, Siegl P, Delves MJ, Kyle DE, Wittlin S, Marfurt J, Price RN, Sinden RE, Winzeler EA, Charman SA, Bebrevska L, Gray DW, Campbell S, Fairlamb AH, Willis PA, Rayner JC, Fidock DA, Read KD, Gilbert IH. 2015. A novel multiple-stage antimalarial agent that inhibits protein synthesis. Nature 522:315–320. doi: 10.1038/nature14451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tamaki F, Fisher F, Milne R, Terán F-R, Wiedemar N, Wrobel K, Edwards D, Baumann H, Gilbert IH, Baragana B, Baum J, Wyllie S. 2022. High-throughput screening platform to identify inhibitors of protein synthesis with potential for the treatment of malaria. Antimicrob Agents Chemother 66: e0023722. doi: 10.1128/aac.00237-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhou B-H, Jia L-S, Guo H-W, Ding H-Y, Yang J-Y, Wang H-W. 2019. Eukaryotic elongation factor 2 is involved in the anticoccidial action of diclazuril in the second-generation merozoites of Eimeria tenella. Vet Parasitol 276:108991. doi: 10.1016/j.vetpar.2019.108991 [DOI] [PubMed] [Google Scholar]
- 14. Gaji RY, Sharp AK, Brown AM. 2021. Protein kinases in Toxoplasma gondii. Int J Parasitol 51:415–429. doi: 10.1016/j.ijpara.2020.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Peixoto L, Chen F, Harb OS, Davis PH, Beiting DP, Brownback CS, Ouloguem D, Roos DS. 2010. Integrative genomic approaches highlight a family of parasite-specific kinases that regulate host responses. Cell Host Microbe 8:208–218. doi: 10.1016/j.chom.2010.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sugi T, Kato K, Kobayashi K, Watanabe S, Kurokawa H, Gong H, Pandey K, Takemae H, Akashi H. 2010. Use of the kinase inhibitor analog 1Nm-PP1 reveals a role for Toxoplasma gondii CDPK1 in the invasion step. Eukaryot Cell 9:667–670. doi: 10.1128/EC.00351-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lourido S, Shuman J, Zhang C, Shokat KM, Hui R, Sibley LD. 2010. Calcium-dependent protein kinase 1 is an essential regulator of exocytosis in Toxoplasma. Nature 465:359–362. doi: 10.1038/nature09022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lourido S, Tang K, Sibley LD. 2012. Distinct signalling pathways control Toxoplasma Egress and host-cell invasion. Embo J 31:4524–4534. doi: 10.1038/emboj.2012.299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McCoy JM, Whitehead L, van Dooren GG, Tonkin CJ. 2012. Tgcdpk3 regulates calcium-dependent Egress of Toxoplasma gondii from host cells. PLoS Pathog 8: e1003066. doi: 10.1371/journal.ppat.1003066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Black MW, Arrizabalaga G, Boothroyd JC. 2000. Ionophore-resistant mutants of Toxoplasma gondii reveal host cell Permeabilization as an early event in egress. Mol Cell Biol 20:9399–9408. doi: 10.1128/MCB.20.24.9399-9408.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Garrison E, Treeck M, Ehret E, Butz H, Garbuz T, Oswald BP, Settles M, Boothroyd J, Arrizabalaga G. 2012. A forward genetic screen reveals that calcium-dependent protein kinase 3 regulates egress in Toxoplasma. PLoS Pathog 8: e1003049. doi: 10.1371/journal.ppat.1003049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Broncel M, Dominicus C, Vigetti L, Nofal SD, Bartlett EJ, Touquet B, Hunt A, Wallbank BA, Federico S, Matthews S, Young JC, Tate EW, Tardieux I, Treeck M. 2020. Profiling of myristoylation in Toxoplasma gondii reveals an N-myristoylated protein important for host cell penetration. Elife 9: e57861. doi: 10.7554/eLife.57861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Treeck M, Sanders JL, Gaji RY, LaFavers KA, Child MA, Arrizabalaga G, Elias JE, Boothroyd JC. 2014. The calcium-dependent protein kinase 3 of Toxoplasma influences basal calcium levels and functions beyond egress as revealed by quantitative Phosphoproteome analysis. PLoS Pathog 10: e1004197. doi: 10.1371/journal.ppat.1004197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Teige M, Scheikl E, Reiser V, Ruis H, Ammerer G. 2001. Rck2, a member of the calmodulin-protein kinase family, links protein synthesis to high osmolarity MAP kinase signaling in budding yeast. Proc Natl Acad Sci U S A 98:5625–5630. doi: 10.1073/pnas.091610798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Swaminathan S, Masek T, Molin C, Pospisek M, Sunnerhagen P. 2006. Rck2 is required for reprogramming of ribosomes during oxidative stress. Mol Biol Cell 17:1472–1482. doi: 10.1091/mbc.e05-07-0632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sebastian S, Brochet M, Collins MO, Schwach F, Jones ML, Goulding D, Rayner JC, Choudhary JS, Billker O. 2012. A Plasmodium calcium-dependent protein kinase controls zygote development and transmission by translationally activating repressed mRNAs. Cell Host Microbe 12:9–19. doi: 10.1016/j.chom.2012.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schmidt EK, Clavarino G, Ceppi M, Pierre P. 2009. SUnSET, a Nonradioactive method to monitor protein synthesis. Nat Methods 6:275–277. doi: 10.1038/nmeth.1314 [DOI] [PubMed] [Google Scholar]
- 28. Argüelles S, Camandola S, Hutchison ER, Cutler RG, Ayala A, Mattson MP. 2013. Molecular control of the amount, subcellular location, and activity state of translation elongation factor 2 in neurons experiencing stress. Free Radic Biol Med 61:61–71. doi: 10.1016/j.freeradbiomed.2013.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Argüelles S, Camandola S, Cutler RG, Ayala A, Mattson MP. 2014. Elongation factor 2 diphthamide is critical for translation of two IRES-dependent protein targets, XIAP and FGF2, under oxidative stress conditions. Free Radic Biol Med 67:131–138. doi: 10.1016/j.freeradbiomed.2013.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hekman KE, Yu G-Y, Brown CD, Zhu H, Du X, Gervin K, Undlien DE, Peterson A, Stevanin G, Clark HB, Pulst SM, Bird TD, White KP, Gomez CM. 2012. A conserved eEF2 coding variant in SCA26 leads to loss of translational fidelity and increased susceptibility to proteostatic insult. Hum Mol Genet 21:5472–5483. doi: 10.1093/hmg/dds392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Barylyuk K, Koreny L, Ke H, Butterworth S, Crook OM, Lassadi I, Gupta V, Tromer E, Mourier T, Stevens TJ, Breckels LM, Pain A, Lilley KS, Waller RF. 2020. A comprehensive subcellular atlas of the Toxoplasma proteome via hyperlopit provides spatial context for protein functions. Cell Host Microbe 28:752–766. doi: 10.1016/j.chom.2020.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Foe IT, Child MA, Majmudar JD, Krishnamurthy S, van der Linden WA, Ward GE, Martin BR, Bogyo M. 2015. Global analysis of palmitoylated proteins in Toxoplasma gondii. Cell Host Microbe 18:501–511. doi: 10.1016/j.chom.2015.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sidik SM, Hackett CG, Tran F, Westwood NJ, Lourido S. 2014. Efficient genome engineering of Toxoplasma gondii using CRISPR/Cas9. PLoS One 9: e100450. doi: 10.1371/journal.pone.0100450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Alonso AM, Turowski VR, Ruiz DM, Orelo BD, Moresco JJ, Yates JR, Corvi MM. 2019. Exploring protein myristoylation in Toxoplasma gondii. Exp Parasitol 203:8–18. doi: 10.1016/j.exppara.2019.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gaji RY, Johnson DE, Treeck M, Wang M, Hudmon A, Arrizabalaga G. 2015. Phosphorylation of a myosin motor by TgCDPK3 facilitates rapid initiation of motility during Toxoplasma gondii egress. PLoS Pathog 11: e1005268. doi: 10.1371/journal.ppat.1005268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang M, Joyce BR, Sullivan WJ, Nussenzweig V. 2013. Translational control in Plasmodium and Toxoplasma parasites. Eukaryot Cell 12:161–167. doi: 10.1128/EC.00296-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Konrad C, Wek RC, Sullivan WJ. 2011. A Gcn2-like Eukaryotic initiation factor 2 kinase increases the viability of extracellular Toxoplasma gondii parasites. Eukaryot Cell 10:1403–1412. doi: 10.1128/EC.05117-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Konrad C, Wek RC, Sullivan WJ. 2014. Gcn2-like Eif2Α kinase manages the amino acid starvation response in Toxoplasma gondii. Int J Parasitol 44:139–146. doi: 10.1016/j.ijpara.2013.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Augusto L, Martynowicz J, Amin PH, Carlson KR, Wek RC, Sullivan WJ. 2021. TgIF2K-B is an eIF2alpha kinase in Toxoplasma gondii that responds to oxidative stress and optimizes pathogenicity. mBio 12: e03160-20. doi: 10.1128/mBio.03160-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Joyce BR, Tampaki Z, Kim K, Wek RC, Sullivan WJ. 2013. The unfolded protein response in the protozoan parasite Toxoplasma Gondii features Translational and transcriptional control. Eukaryot cell 12:979–989. doi: 10.1128/EC.00021-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ude S, Lassak J, Starosta AL, Kraxenberger T, Wilson DN, Jung K. 2013. Translation elongation factor EF-P alleviates ribosome stalling at polyproline stretches. Science 339:82–85. doi: 10.1126/science.1228985 [DOI] [PubMed] [Google Scholar]
- 42. Olarewaju O, Ortiz PA, Chowdhury WQ, Chatterjee I, Kinzy TG. 2004. The translation elongation factor eEF1B plays a role in the oxidative stress response pathway. RNA Biol 1:89–94. doi: 10.4161/rna.1.2.1033 [DOI] [PubMed] [Google Scholar]
- 43. Liu L, Wise DR, Diehl JA, Simon MC. 2008. Hypoxic reactive oxygen species regulate the integrated stress response and cell survival. J Biol Chem 283:31153–31162. doi: 10.1074/jbc.M805056200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Knebel A, Haydon CE, Morrice N, Cohen P. 2002. Stress-Induced regulation of eukaryotic elongation factor 2 kinase by SB 203580-sensitive and -insensitive pathways. Biochem J 367:525–532. doi: 10.1042/BJ20020916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kruiswijk F, Yuniati L, Magliozzi R, Low TY, Lim R, Bolder R, Mohammed S, Proud CG, Heck AJR, Pagano M, Guardavaccaro D. 2012. Coupled activation and degradation of eEF2K regulates protein synthesis in response to genotoxic stress. Sci Signal 5: ra40. doi: 10.1126/scisignal.2002718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Leprivier G, Remke M, Rotblat B, Dubuc A, Mateo A-R, Kool M, Agnihotri S, El-Naggar A, Yu B, Somasekharan SP, Faubert B, Bridon G, Tognon CE, Mathers J, Thomas R, Li A, Barokas A, Kwok B, Bowden M, Smith S, Wu X, Korshunov A, Hielscher T, Northcott PA, Galpin JD, Ahern CA, Wang Y, McCabe MG, Collins VP, Jones RG, Pollak M, Delattre O, Gleave ME, Jan E, Pfister SM, Proud CG, Derry WB, Taylor MD, Sorensen PH. 2013. The eEF2 kinase confers resistance to nutrient deprivation by blocking translation elongation. Cell 153:1064–1079. doi: 10.1016/j.cell.2013.04.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wu C-C, Zinshteyn B, Wehner KA, Green R. 2019. High-resolution ribosome profiling defines discrete ribosome elongation states and translational regulation during cellular stress. Mol Cell 73:959–970. doi: 10.1016/j.molcel.2018.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dahlkvist A, Sunnerhagen P. 1994. Two novel deduced serine/threonine protein kinases from Saccharomyces cerevisiae. Gene 139:27–33. doi: 10.1016/0378-1119(94)90519-3 [DOI] [PubMed] [Google Scholar]
- 49. Ryazanov AG. 2002. Elongation factor-2 kinase and its newly discovered relatives. FEBS Lett 514:26–29. doi: 10.1016/s0014-5793(02)02299-8 [DOI] [PubMed] [Google Scholar]
- 50. Harding CR, Meissner M. 2014. The inner membrane complex through development of Toxoplasma gondii and Plasmodium. Cell Microbiol 16:632–641. doi: 10.1111/cmi.12285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Back PS, O’Shaughnessy WJ, Moon AS, Dewangan PS, Hu X, Sha J, Wohlschlegel JA, Bradley PJ, Reese ML. 2020. Ancient MAPK ERK7 is regulated by an unusual inhibitory scaffold required for Toxoplasma apical complex biogenesis. Proc Natl Acad Sci U S A 117:12164–12173. doi: 10.1073/pnas.1921245117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tosetti N, Dos Santos Pacheco N, Bertiaux E, Maco B, Bournonville L, Hamel V, Guichard P, Soldati-Favre D. 2020. Essential function of the alveolin network in the subpellicular microtubules and conoid assembly in Toxoplasma gondii. Elife 9: e56635. doi: 10.7554/eLife.56635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pomel S, Luk FCY, Beckers CJM. 2008. Host cell egress and invasion induce marked relocations of glycolytic enzymes in Toxoplasma gondii tachyzoites. PLoS Pathog 4: e1000188. doi: 10.1371/journal.ppat.1000188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Farazi TA, Waksman G, Gordon JI. 2001. The biology and enzymology of protein N-myristoylation. J Biol Chem 276:39501–39504. doi: 10.1074/jbc.R100042200 [DOI] [PubMed] [Google Scholar]
- 55. Dian C, Pérez-Dorado I, Rivière F, Asensio T, Legrand P, Ritzefeld M, Shen M, Cota E, Meinnel T, Tate EW, Giglione C. 2020. High-resolution snapshots of human N-myristoyltransferase in action illuminate a mechanism promoting N-terminal Lys and Gly myristoylation. Nat Commun 11:1132. doi: 10.1038/s41467-020-14847-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Liu L, Cash TP, Jones RG, Keith B, Thompson CB, Simon MC. 2006. Hypoxia-Induced energy stress regulates mRNA translation and cell growth. Mol Cell 21:521–531. doi: 10.1016/j.molcel.2006.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Connolly E, Braunstein S, Formenti S, Schneider RJ. 2006. Hypoxia inhibits protein synthesis through a 4E-BP1 and elongation factor 2 kinase pathway controlled by mTOR and uncoupled in breast cancer cells. Mol Cell Biol 26:3955–3965. doi: 10.1128/MCB.26.10.3955-3965.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Moore CEJ, Mikolajek H, Regufe da Mota S, Wang X, Kenney JW, Werner JM, Proud CG. 2015. Elongation factor 2 kinase is regulated by proline hydroxylation and protects cells during hypoxia. Mol Cell Biol 35:1788–1804. doi: 10.1128/MCB.01457-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wiley M, Sweeney KR, Chan DA, Brown KM, McMurtrey C, Howard EW, Giaccia AJ, Blader IJ. 2010. Toxoplasma gondii activates hypoxia-inducible factor (HIF) by stabilizing the HIF-1alpha subunit via type I activin-like receptor kinase receptor signaling. J Biol Chem 285:26852–26860. doi: 10.1074/jbc.M110.147041 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Blast results.
Blader results.
Legends for Fig. S1 and Table S1.