ABSTRACT
The Shigella artificial invasin complex (InvaplexAR) vaccine is a subunit approach that effectively induces robust immunogenicity directed to serotype-specific lipopolysaccharide and the broadly conserved IpaB and IpaC proteins. One advantage of the vaccine approach is the ability to adjust the constituents to address suboptimal immunogenicity and to change the Shigella serotype targeted by the vaccine. As the vaccine moves through the product development pipeline, substantial modifications have been made to address manufacturing feasibility, acceptability to regulatory authorities, and developing immunogenic and effective products for an expanded list of Shigella serotypes. Modifications of the recombinant clones used to express affinity tag-free proteins using well-established purification methods, changes to detergents utilized in the assembly process, and in vitro and in vivo evaluation of different Invaplex formulations have led to the establishment of a scalable, reproducible manufacturing process and enhanced immunogenicity of Invaplex products designed to protect against four of the most predominant Shigella serotypes responsible for global morbidity and mortality. These adjustments and improvements provide the pathway for the manufacture and clinical testing of a multivalent Invaplex vaccine.
IMPORTANCE
Shigella species are a major global health concern that cause severe diarrhea and dysentery in children and travelers to endemic areas of the world. Despite significant advancements in access to clean water, the increases in antimicrobial resistance and the risk of post-infection sequelae, including cognitive and physical stunting in children, highlight the urgent need for an efficacious vaccine. One promising vaccine approach, artificial Invaplex, delivers key antigens recognized by the immune system during infection, which results in increased resistance to re-infection. The work presented here describes novel modifications to a previously described vaccine approach resulting in improved methods for manufacturing and regulatory approvals, expansion of the breadth of coverage to all major Shigella serotypes, and an increase in the potency of artificial Invaplex.
KEYWORDS: Shigella, vaccine, efficacy, immunogenicity, Invaplex
INTRODUCTION
Diarrhea and dysentery caused by Shigella species (spp.) are major health concerns affecting children in low-middle income countries and travelers to endemic regions of the world. According to estimates from the Institute for Health Metrics and Evaluation, Shigella spp. account for 88 million infections annually and have the highest percentage of deaths (14%) among enteric bacterial pathogens (1, 2). In addition to acute diarrheal disease, Shigella infections are also associated with both physical and intellectual stunting in children and other long-term sequelae, including reactive arthritis and an increased risk of mortality due to other infectious diseases in stunted children (3 - 5). Although access to clean water and proper hygiene are effective tools to prevent infection, significant hurdles remain for these to be complete solutions. Furthermore, the increase in antimicrobial resistance in Shigella spp. has resulted in epidemiological alerts by the Pan American Health Organization and the World Health Organization (6, 7) highlighting the urgent need for an efficacious vaccine.
In non-human primate models, prior infection with Shigella provides increased resistance to re-infection with the same Shigella serotype but not un-related Shigella serotypes (8) suggesting that immune responses directed to the serotype-determinant (lipopolysaccharide, LPS) are keys to protective efficacy (PE). However, in several studies, LPS delivered alone was not capable of inducing protective immunity (9 - 11). Conjugate vaccine approaches, linking the serotype-specific O polysaccharides (O-PS) of the Shigella LPS to a protein carrier, have shown promise in adults but failed to induce protective immunity in young children (12, 13). An optimal Shigella vaccine would protect against Shigella flexneri 2a, 3a, and 6 and Shigella sonnei, which collectively account for over 80% of global infections (14 - 16).
In addition to LPS, the immune system responds to several highly-conserved protein antigens during the course of an infection. Two of the most immunogenic proteins are the invasion plasmid antigen (Ipa) proteins, IpaB and IpaC. These two proteins are essential for the virulence of shigellae playing key roles in the initial interaction and internalization of the bacterium into host cells acting as the translocon for the Type III Secretion System (T3SS) (17 - 22). As secreted effectors of the T3SS and potent immunogens in infected individuals, the highly conserved IpaB and IpaC have been proposed as key antigens and potential vaccine targets (23 - 30). Since the immune response after infection leads to protective immunity, mimicking the immune response through vaccination with the major antigens has become a viable vaccine approach.
The isolation of a complex containing IpaB, IpaC, and the LPS (termed native Invaplex or InvaplexNAT) from all Shigella species was previously described (31, 32). The InvaplexNAT product stimulated potent immune responses to LPS and water-extracted antigens, was significantly protective in small animal models (31), and was transitioned to current good manufacturing practice (cGMP) manufacture and clinical studies (33, 34). Further refinement of the product by chromatographic sizing identified a highly purified Invaplex (HP Invaplex) containing IpaB, IpaC, and LPS that was highly protective and allowed for the determination of the molar ratios of each antigen in the HP Invaplex (35). This stoichiometry was key to the further development of an Invaplex product assembled using a combination of purified recombinantly expressed IpaB and IpaC complexed with the purified LPS of the Shigella species of interest (36). Immunization of small animals with the artificially produced Invaplex (artificial Invaplex or InvaplexAR) significantly increased the immune responses to the IpaB, IpaC, and LPS antigens as compared to InvaplexNAT (36). The increase in immunogenicity translated into improved protective efficacy (PE) at a lower immunizing dose (36). However, the first generation InvaplexAR utilized a polyhistidine-tagged IpaC protein and immune responses directed to the key antigens required further optimization.
One of the significant advantages of the InvaplexAR product is the ability to alter the formulated amount of each constituent contained within the resulting Invaplex product. Using improved recombinant clones expressing both IpaB and IpaC without purification tags, efforts were taken to improve the immune response to the serotype-specific LPS antigen and the Ipa proteins by modifying the amount of LPS used in the construction of the InvaplexAR. Multiple formulations were utilized for the major Shigella serotypes of clinical interest to generate products capable of inducing robust immunogenicity and protective efficacy in small animal models. This phase of process development and preclinical evaluation is key to the successful transition of the Shigella InvaplexAR product to cGMP manufacture and clinical evaluations.
MATERIALS AND METHODS
Bacterial strains and growth
S. flexneri 2a strain 2457T, S. flexneri 3a strain J17B, S. flexneri 6 strain SSU2415, and S. sonnei strain Moseley were grown in Difco Select APS Super Broth (Becton Dickinson) and were used as the sources of LPS as previously described (36). The above-listed strains were also grown in Difco Antibiotic Medium No. 3 overnight in non-baffled shake flasks to prepare water extract for each serotype (31). Recombinant clones of Escherichia coli strain BLR(DE3) containing the pOZ-B3 or pOZ-C3 plasmids (see below) were grown in Alternative Protein Source (APS) Super Broth (Becton Dickinson) supplemented with 0.4% glucose and 50 µg/mL kanamycin at 37°C to an optical density at 600 nm (OD600) of 0.5 and induced with 1 M isopropyl β-d-1-thiogalactopyranoside (IPTG) for the time and temperatures described.
Growth and expression of the IpaB/HTIpgC and IpaC/HTIpgC clones
A description of the construction of the pOZ-B3 and p0Z-C3 plasmids for expression of, respectively, IpaB and IpaC, as well as their polyhistidine-tagged chaperone IpgC, is described in Fig. S1. An overnight culture of the pOZ-B3 recombinant clone expressing HTIpgC and IpaB was grown in APS Super Broth supplemented with 0.4% glucose (Sigma) and 50 µg/mL kanamycin (Sigma). The overnight culture was transferred to a fermenter containing fresh APS Super Broth (0.4% glucose, 50 µg/mL kanamycin) set at 37°C with a dissolved oxygen concentration set point between 20% and 40% (as controlled by an agitation cascade between 200 rpm and 500rpm) and allowed to grow to an OD600 of 3.0–5.0. The fermenter temperature was lowered to 25°C and then induced with IPTG (1 mM final concentration) and fed with additional glucose (0.2% v/v final concentration) and additional kanamycin (50 µg/mL final concentration). The fermentation/induction was allowed to continue overnight at the reduced temperature. Alternate temperatures, additives, and/or induction ODs were evaluated, but optimal expression and solubility occurred as described. The contents of the fermenter were collected by high-speed centrifugation and stored as a relatively dry paste at −30°C until further processing.
Nearly identical fermentation procedures were utilized for the pOZ-C3 clone except that optimal expression and yield of the final purified IpaC product was obtained at an induction temperature of 30°C overnight. Feeding with 0.2% v/v glucose and kanamycin at 50 µg/mL was performed, and the cell paste was harvested and stored at −30°C until further processing.
Purification of IpaB and IpaC using the chaperone IpgC
Techniques for purification of both IpaB and IpaC were nearly identical. Both proteins use the same cognate chaperone IpgC and the IpaB or IpaC/IpgC complex can be disrupted with non-ionic detergents such as n-octyl-polyoxyethylene (OPOE) (19, 36, 37). In our studies, Triton X-100 was substituted for OPOE and resulted in complete release of the Ipa proteins from IpgC.
The collected cell paste (see above) was thawed, suspended in 1× binding buffer (20 mM imidazole, 500 mM NaCl, 20 mM Tris-HCl, pH 7.9, 4 mL/g of cell paste), lysed by high pressure microfluidizer processing, and then treated with 5 µL Benzonase nuclease/50 mL lysate overnight at 4°C with gentle mixing. The lysate was centrifuged and the resulting supernatant passed through a 0.45 µm filter. This clarified supernatant was applied to a Nickel Sepharose FF column (Cytiva, formerly GE Healthcare) to capture the IpaB or IpaC/HTIpgC complex which was eluted with 100 mM imidazole in 20 mM Tris-HCl, 500 mM NaCl, pH 7.9. Fractions containing both IpaB or IpaC and HTIpgC were pooled and dialyzed against 1× binding buffer to remove imidazole, followed by addition of Triton X-100 (0.9–1% v/v final concentration; Millipore Sigma) (36) and incubated at room temperature with gentle swirling for 60 min. The Triton X-100 disrupts the IpaB or IpaC/HTIpgC complex and allows the free HTIpgC protein to bind to the metal-chelating column and the un-bound IpaB or IpaC protein to flow through the column with the void volume for collection. The Triton X-100-treated sample was then applied to a Nickel Sepharose HP column (GE Healthcare) for capture of the HTIpgC protein. Fractions were collected during the loading and initial wash buffers. Free IpaB or IpaC, not bound by the affinity column, was collected in the flow-through, and those fractions containing purified IpaB or IpaC and no residual HTIpgC [confirmed by SDS-PAGE with Coomassie staining and western blots probed with a mouse anti-HisTag mAb (Novagen)] were pooled. The final purified Ipa protein products were stored at −70°C.
LPS purification
LPS was extracted from S. flexneri 2a strain 2457T, S. flexneri 3a strain J17B, S. flexneri 6 strain SSU2415, and S. sonnei strain Moseley by the Westphal procedure as previously described (36, 38). Purified LPS was lyophilized and analyzed by SDS-PAGE (silver stained) and by enzyme-linked immunosorbent assay (ELISA) with serotype-specific monoclonal antibodies. Residual protein was assessed by the bicinchoninic acid (BCA) total protein assay (Pierce) and SDS-PAGE (Coomassie-stained). Protein levels were less than 0.15% by weight for all LPS lots.
Production of InvaplexAR using varying amounts of LPS
Using the IpaC:IpaB molar ratio previously described (36), 8 µM purified IpaC was mixed with 1 µM IpaB diluted in 20 mM Tris-HCl, 500 mM NaCl, pH 7.9, and mixed with urea buffer (20 mM Tris-HCl, 500 mM NaCl, 9 M urea, pH 7.9) to a final urea concentration of 5.1 M. The IpaB/IpaC mixture was slowly added to a vessel containing dry Shigella LPS at a ratio of 0.56 (w LPS/w protein) in the final reaction mixture (1× LPS construct), a ratio of 1.12 (2× LPS), a ratio of 2.24 (4× LPS), and a ratio of 4.48 (8× LPS). After gently swirling to solubilize the LPS, the vessel containing the reaction mixture was placed at 37°C and incubated with gentle shaking at 100–150 rpm for 2 h. The reaction mixture was diluted by addition of four volumes of 20 mM Tris-HCl and pH 7.9 at 37°C and applied to a Q Sepharose HP column (GE Healthcare). The InvaplexAR product was eluted using step gradients of the 100% Buffer B (1 M NaCl in 20 mM Tris-HCl, pH 9.0) as previously described (36). InvaplexAR elutes in the 50% Buffer B fractions. Elution fractions were analyzed for total protein by the BCA assay (Pierce). Fractions containing all three antigens contained the InvaplexAR product. The resulting InvaplexAR product was further biochemically characterized by SDS-PAGE, limulus amebocyte lysate (LAL) assay, size-exclusion chromatography (SEC)-high performance liquid chromatography (HPLC), and dynamic light scattering (DLS).
Purification of InvaplexNAT
InvaplexNAT was purified by anion exchange chromatography of water extracts of virulent S. flexneri 2a strain 2457T, S. flexneri 3a strain J17B, S. flexneri 6 strain SSU2415, and S. sonnei strain Moseley as described earlier (31). The final InvaplexNAT product contains LPS, IpaB, IpaC, and several other proteins in low concentration (data not shown).
Electrophoresis and western blots
Polyacrylamide gel electrophoresis with Coomassie blue and silver staining and western blots were performed as previously described (36, 39). Wet SDS-PAGE gels were either photographed or scanned using a Bio-Rad GS900 calibrated flat-bed densitometer and analyzed with the Bio-Rad ImageLab software. Using the ImageLab software, IpaC:IpaB ratios were determined by dividing the “%Band” densitometric value determined for full-size IpaC (43 kDa) by the “%Band” densitometric value determined for full-size IpaB (62 kDa).
SEC-HPLC
A TSK-GEL G5000PWXL 7.8 mm × 30 cm column (TOSOH Bioscience) with a 10 µm particle size and an exclusion limit of 1 × 107 Da was connected to a Dionex UltiMate 3000 ultra-high performance liquid chromatography (UHPLC) chromatography system. The column was calibrated using thyroglobulin (669 kDa), bovine gamma-globulin (158 kDa), chicken ovalbumin (44 kDa), horse myoglobin (17 kDa), and vitamin B12 (1.35 kDa; all Bio-Rad) in 20 mM Tris-HCl, 250 mM NaCl, pH 9.0 (InvaplexAR buffer). InvaplexAR products (15 µg per injection) were applied in separate runs under the same conditions at a flow rate of 0.5 mL/min, 400 psig maximum pressure. Chromatographic traces were recorded at 215 nm using the Dionex UltiMate 3000 diode array detector and Dionex Chromeleon v7.x software.
DLS
Dynamic light scattering (DLS) experiments were performed with a Malvern Zetasizer µV system using the Zetasizer Software version 7.11 for the analysis of data (36). The final size of the product is reported as the hydrodynamic radius and is the average of five runs and represents the primary or predominate species present in the sample as determined by mass (36).
Endotoxin measurement
The amount of LPS in each InvaplexAR product was determined using samples serially 10-fold diluted in LAL Reagent Water (Charles River) and read using the kinetic colorimetric EndoSafe nexgen-PTS device and EndoSafe cartridges from 0.1 to 10 EU/mL (Charles River).
Immunogenicity and protective efficacy (PE) of InvaplexAR
Male Hartley guinea pigs (Charles River) were immunized with 25 µg total protein of each InvaplexAR product (n = 6–7 per group) or with saline (n = 10–13 per group) as a negative control. Animals were immunized intranasally three times at 2-week intervals (days 0, 14, and 28). Blood and ocular washes were collected at baseline (day 0) and 2 weeks after the third immunization (day 42) to determine vaccine induced systemic and mucosal antibody responses. Three weeks after the last immunization (day 49), guinea pigs were ocularly challenged with either S. flexneri 2a 2457T (2.7 × 108 cfu/eye), S. flexneri 3a J17B (1.2 × 108 cfu/eye), S. flexneri 6 CCHO60 (2.03 × 105 cfu/eye), or S. sonnei Moseley (1.8 × 108 cfu/eye). Each challenge strain culture, previously determined to induce a high frequency of severe disease in guinea pig eyes, was initiated from a Congo Red positive colony subcultured on tryptic soy agar (TSA) plates to prepare the challenge inoculum. Eyes of each animal were monitored daily for inflammation and keratoconjunctivitis and disease was graded as previously described (40) with scores ≥2 considered positive for disease or unprotected. For humane concerns, naïve animals are only challenged in one eye since there is no expectation of protection whereas vaccinated animals are challenged in both eyes. Each infected eye is scored separately over the course of 5 days. Results 4 days post-challenge were used to determine PE. Upon completion of all challenge studies, all animals were humanely euthanized in accordance with the American Veterinary Medicine Association Guidelines for the Euthanasia of Animals and institute approved animal use protocols.
ELISA
Serum and ocular wash samples were assayed by ELISA (31) to determine endpoint titers directed to serotype-specific LPS and InvaplexNAT for each serotype, IpaB and IpaC. Samples that were negative at the starting dilution [the assay limit of detection (LOD)] were assigned a titer corresponding to half of the starting dilution (1/2 LOD). Immune responders were defined a priori as having a ≥4-fold increase over their baseline titer.
Statistical analysis
PE was calculated as [(% disease in control animals − % disease in vaccine group)/% disease in control animals] × 100. P-values were determined by Fisher’s exact test comparing the immunized group to the saline control group.
All immune response data were assessed for normality using distribution plots. Normally distributed continuous immune response data were analyzed using appropriate parametric tests [analysis of variance (ANOVA) with Bonferroni post hoc analysis]. Non-normally distributed data were log10 transformed in order to meet the assumptions for the parametric tests. If the log transformation did not correct the distribution of the data set, then non-parametric statistical tests were used (Kruskal-Wallis test with Dunn’s post hoc correction) using GraphPad Prism 9.x software (GraphPad Software LLC, La Jolla, CA).
The relationships between the measured quantities of LPS, IpaB, IpaC to each other, various physical parameters of Invaplex, and immunological titers were evaluated by the Pearson correlation coefficient using Prism.
RESULTS
Ipa protein expression clones and purification
Previously, IpaB was co-purified with its cognate chaperone (IpgC) that was histidine-tagged (HTIpgC) followed by disassociation of the IpaB:HTIpgC complex that allowed isolation of pure IpaB. In the same study, a histidine-tagged IpaC (HTIpaC) was also used to produce the initial lots of research-grade InvaplexAR (36). With the product development goal of a safe and efficacious vaccine for human-use, transition of the recombinant clones to cGMP manufacturing and scale-up required the re-engineering of the clones to a more suitable antibiotic resistance marker, improvement of the overall yield of the recombinant products, and production of a recombinant IpaC without a histidine-tag.
IpaB and IpaC can be released from IpgC with non-ionic detergents such as the polyoxyethylene detergent OPOE (36) but OPOE is not found in any current FDA-approved vaccines. As an alternative to OPOE, the non-ionic detergent, Triton X-100 (t-octylphenoxypolyethoxyethanol; EMD Millipore) was used to completely release IpaB from the IpaB/HTIpgC complex and was comparable to the disassociation of the IpaB/HTIpgC complex achieved with OPOE. Other generally-regarded-as-safe (GRAS) listed reagents such as Tween 80 (polyoxyethylenesorbitan monooleate), arginine, CHAPS, urea, and treatment with lower pH had reduced effectiveness for release of IpaB from HTIpgC (data not shown).
Purified IpaB was soluble and stable when stored at −70°C. Using the pOZ-B3 clone, IpaB has been successfully manufactured under cGMP by the WRAIR Pilot Bioproduction Facility (PBF) at 94% purity, low contaminating endotoxin (60 EU/mL), 0.88% Triton X-100, and a yield of 0.5 mg IpaB per gram of pOZ-B3 cell paste. cGMP IpaB stored at +40°C for 72 h, 25°C for 30 days, 2–8°C for 6 months, and −70°C for more than 3 years showed no visible signs of protein aggregation or precipitation (Table S1).
The pOZ-C3 clone has also been used to successfully manufacture IpaC under cGMP by the WRAIR PBF at 95% purity, low endotoxin (0.08 EU/mL), 0.71% Triton X-100, and yield of 0.8 mg IpaC per gram of pOZ-C3 cell paste. The stability of cGMP IpaC was temperature dependent. IpaC stored at +40°C for 48 h showed significant signs of protein aggregation/precipitation; storage at 25°C led to visible aggregation or precipitation which increased over the course of 30 days; storage at 2–8°C showed no signs of visible aggregation for 3 months; and IpaC stored at −70°C for more than 3 years showed no visible signs of protein aggregation/precipitation and is thus the preferred storage temperature for cGMP purified IpaC products (Table S2).
Development of the InvaplexAR product with greater quantities of LPS
In previous clinical trials, use of the S. flexneri 2a Invaplex product purified from the wild-type organism (InvaplexNAT) stimulated limited seroconversion to the immunizing antigens (33, 34). The second-generation S. flexneri 2a InvaplexAR product (36) showed significantly improved guinea pig serum IgG responses to all antigens as compared to the InvaplexNAT product, and while the guinea pig serum IgA responses to IpaB and IpaC were higher than animals receiving S. flexneri 2a InvaplexNAT, the guinea pig anti-LPS serum IgA responses were low with little improvement over the InvaplexNAT vaccine.
Experiments that increased the amount of LPS in the InvaplexAR product were conducted to determine if the final quantity of LPS in the product effected the immune response to not only the LPS antigen but the Ipa proteins. Thus, S. flexneri 2a InvaplexAR reaction mixtures consisting of LPS:total protein weight ratios of 0.56 [defined as 1× LPS (35), 1.12 (2×), 2.24 (4×), and 4.48 (8×)] were utilized while maintaining the 8 µM IpaC:1 µM IpaB ratio in the reaction mixture. The addition of more LPS required longer anion-exchange column wash steps at 0% Buffer B prior to elution than previous chromatographic methods. Higher ratios of LPS in the reaction mixture led to increased quantities of LPS in the resulting S. flexneri 2a InvaplexAR constructs both quantitatively by LAL (Table 1) and qualitatively by SDS-PAGE with silver staining (Fig. 1). This would allow more LPS to be delivered per constant protein dose and possibly improve the immune response. Additionally, the efficiency of S. flexneri 2a product recovered from the reaction mixture was improved when a higher LPS:protein ratio was used in the reaction mixture, i.e., from 40% for 1× to 67% for 4× (Table 1).
TABLE 1.
Comparison of biochemical attributes of Shigella variable LPS InvaplexAR products
| Shigella serotype | LPS multiplier a | Endotoxin (×106 EU/mL) b |
IpaC:IpaB ratio (gel densitometry) c |
SEC-HPLC retention time (min) d | DLS (DH, nm) e | % Yield efficiency f |
|---|---|---|---|---|---|---|
| S. flexneri 2a | 1× | 15 | 3.8:1 | 16.2 | 21.4 ± 5.5 | 40.3 |
| 2× | 29 | 6.9:1 | 16.0 | 17.8 ± 4.1 | 57.1 | |
| 4× | 63 | 7.8:1 | 15.6 | 19.5 ± 7.7 | 67.2 | |
| 8× | 61 | 9.4:1 | 15.6 | 21.4 ± 26.4 | 42.5 g | |
| S. flexneri 3a | 1× | 7.3 | 2.5:1 | 16.4 | 20.7 ± 3.4 | 20.6 |
| 2× | 16 | 3.8:1 | 16.2 | 19.5 ± 4.8 | 30.8 | |
| 4× | 55 | 5.8:1 | 15.8 | 22.4 ± 3.5 | 51.8 | |
| 8× | 78 | 5.8:1 | 15.6 | 21.4 ± 2.4 | 50.3 | |
| S. flexneri 6 | 1× | 16 | 2.1:1 | Not done | 20.3 ± 1.3 | 40.0 |
| 2× | 35 | 4.1:1 | 16.3 | 21.4 ± 0.7 | 64.2 | |
| 4× | 62 | 4.0:1 | 16.2 | 25.4 ± 1.4 | 66.9 | |
| 8× | 220 | 3.9:1 | 16.0 | 28.2 ± 0.4 | 72.2 | |
| S. sonnei | 1× | 5.7 | 2.6:1 | 16.1 | 19.5 ± 4.0 | 32.4 |
| 2× | 11 | 5.6:1 | 16.0 | 19.5 ± 8.2 | 43.6 | |
| 4× | 32 | 9.4:1 | 15.8 | 19.5 ± 8.0 | 51.4 | |
| 8× | 89 | 7.2:1 | 15.7 | 22.4 ± 4.8 | 52.0 |
Lipopolysaccharide (LPS) multiplier = the fold-increase in the amount of LPS used in the initial reaction mixture of the four formulations for each serotype. The 1× amount is 0.56 mg total LPS/mg total protein.
Endotoxin was measured using the Charles River Laboratories EndoSafe-PTS endotoxin reader and FDA-licensed cartridges with a sensitivity of 0.001 EU/mL.
The IpaC:IpaB ratio was determined by scanning Coomassie-stained SDS-PAGE gels using a Bio-Rad GS900 calibrated flatbed scanner and the pixel densitometric ratios calculated using the Bio-Rad Image Lab software.
Size exclusion chromatography (SEC)-HPLC.
Dynamic light scattering (DLS) measurements of hydrodynamic diameter (DH) in nanometers.
Percent yield efficiency is defined as the total protein, in milligram, of the resulting InvaplexAR product divided by the total amount, in milligram, of the quantities of protein (IpaB and IpaC) used in the initial reaction mixture, multiplied by 100.
It was noted that the dialysis cassette containing the 8× product had swollen during dialysis and indeed almost twice the volume of product was recovered from the cassette as compared to the 1×, 2×, and 4× products. Total protein concentration of the 8× product was lower understandably due to this volume dilution, but the product yield efficiency was comparable to that of the 1× product. It is unclear as to the reason for this as the phenomenon was not exhibited by other 8× products of other serotypes.
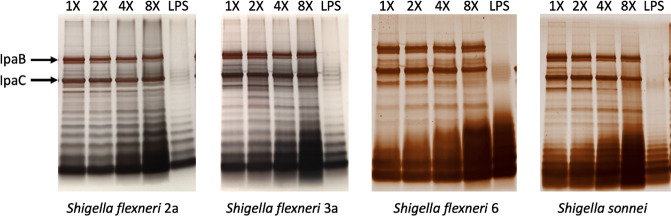
Fig 1.
Constant total protein of each Shigella InvaplexAR products for each species constructed with increasing amounts of LPS was separated and stained. Increased incorporation of LPS within the construct is seen with increased amounts of LPS used in the initial reaction mixture. Amounts of IpaB and IpaC, marked with arrows, remain relatively constant across the constructs, even with increasing LPS. LPS, lipopolysaccharide.
Silver stained SDS-PAGE gels loaded with equal amounts of total protein from each of the S. flexneri 2a products made with higher ratios of LPS were comparable for relative quantities of IpaB and IpaC (data not shown and Fig. 1). Using densitometric analysis of Coomassie-stained SDS-PAGE gels, a slight increase in the IpaC:IpaB ratios was observed in preparations made with higher quantities of LPS and more closely approximated the mass mixing ratio of 5 mg IpaC:1 mg IpaB (7.6:1 molar ratio of IpaC to IpaB; Table 1). The increase in the IpaC:IpaB ratio appeared to reflect decreased amounts of IpaB in the final products (see Table S3).
Earlier studies demonstrated that the InvaplexNAT product can be isolated from all Shigella species and enteroinvasive E. coli (31). Similarly, InvaplexAR using purified LPS from other Shigella spp. has been prepared using parameters similar to those determined for S. flexneri 2a (36, Turbyfill, unpublished data). To expand the Invaplex product family to Shigella serotypes under consideration for a multivalent vaccine, we applied the LPS ratio scheme for the construction of InvaplexAR products from other clinically relevant Shigella species, specifically S. flexneri 3a, S. flexneri 6, and S. sonnei.
InvaplexAR products formulated with increasing amounts of LPS were successfully made for S. flexneri 3a, S. flexneri 6, and S. sonnei (Fig. 1; Table 1). As noted for S. flexneri 2a, S. flexneri 3a, S. flexneri 6, and S. sonnei products demonstrated improved overall product yield efficiencies as the LPS:protein ratio increased in the reaction mixture (Fig. 1; Table 1). Higher product yield for all serotypes correlated with increasing concentrations of LPS used in the formulations (r = 0.6503, P = 0.0064). For the S. flexneri 6 products, increased silver staining of the lower molecular weight bands of S. flexneri 6 LPS that were more prominent as higher quantities of LPS were used to make the product (Fig. 1). This was consistent with a large, unexpected increase in the LAL value observed for the 8× S. flexneri 6 InvaplexAR product (Table 1) as compared to the other S. flexneri 6 products and the S. flexneri 2a, 3a, and S. sonnei products.
Overall, SEC-HPLC retention times of products from each serotype slightly decreased with increasing LPS, suggesting that the products were getting larger with increasing quantities of LPS (Table 1). The increased product size attributed to increasing LPS was more evident with the hydrodynamic diameters measured by DLS in which an increase in hydrodynamic diameter was correlated with the amount of LPS used in the formulation (r = 0.8101, P = 0.0001) (Table 1) Concurrently, as the size measured by SEC-HPLC increased (slower retention time) it was also noted that the IpaC:IpaB ratio was higher (r = −0.8077, P = 0.0003) suggesting that, for all serotypes, the increased amounts of LPS used to formulate the various products yielded products with higher quantities of LPS/mg protein and a gradually increasing IpaC:IpaB ratio as the amount of LPS increased. The net effect of these changes is the increase in the amount of LPS and IpaC and a decrease in the amounts of IpaB delivered upon immunization with each product made with more LPS (see Table S3).
Immunogenicity and efficacy of S. flexneri 2a InvaplexAR with increasing quantities of LPS
The InvaplexAR formulations produced with increasing quantities of LPS were evaluated for immunogenicity and PE in the guinea pig keratoconjunctivitis model. The 2× and 8× S. flexneri 2a constructs provided moderate levels of protection post-challenge with the 8× group (PE = 58.3%, P = 0.005; Table 2) providing slightly higher PE as compared to the 2× group (PE = 42%, P = 0.040; Table 2). The 4× construct provided the highest level of PE (75%, P = 0.0005; Table 2), while the 1× construct provided no protection (Table 2).
TABLE 2.
Protective efficacy 4 days post-challenge
| Shigella serotype | LPS multiplier | Number of eyes protected/total | Protective efficacy a (%) | P-value b |
|---|---|---|---|---|
| S. flexneri 2a | 1× | 0/12 | 0% | 1.0 |
| 2× | 5/12 | 42% | 0.040 | |
| 4× | 9/12 | 75% | 0.0005 | |
| 8× | 7/12 | 58% | 0.005 | |
| Saline | 0/10 | – | – | |
| S. flexneri 3a | 1× | 8/12 | 60% | 0.036 |
| 2× | 9/12 | 70% | 0.012 | |
| 4× | 7/12 | 50% | 0.089 | |
| 8× | 6/12 | 40% | 0.193 | |
| Saline | 2/12 | – | – | |
| S. flexneri 6 | 1× | 8/14 | 44% | 0.120 |
| 2× | 8/14 | 44% | 0.120 | |
| 4× | 7/14 | 35% | 0.237 | |
| 8× | 10/14 | 63% | 0.021 | |
| Saline | 3/13 | – | – | |
| S. sonnei | 1× | 11/12 | 92% | <0.0001 |
| 2× | 9/12 | 75% | 0.0003 | |
| 4× | 7/12 | 58% | 0.005 | |
| 8× | 9/12 | 75% | 0.0003 | |
| Saline | 0/12 | – | – |
Calculated as [(% disease in control animals − % disease in vaccine group)/% disease in control animals] × 100.
P-values determined by Fisher’s exact test of experimental group compared to saline control group.
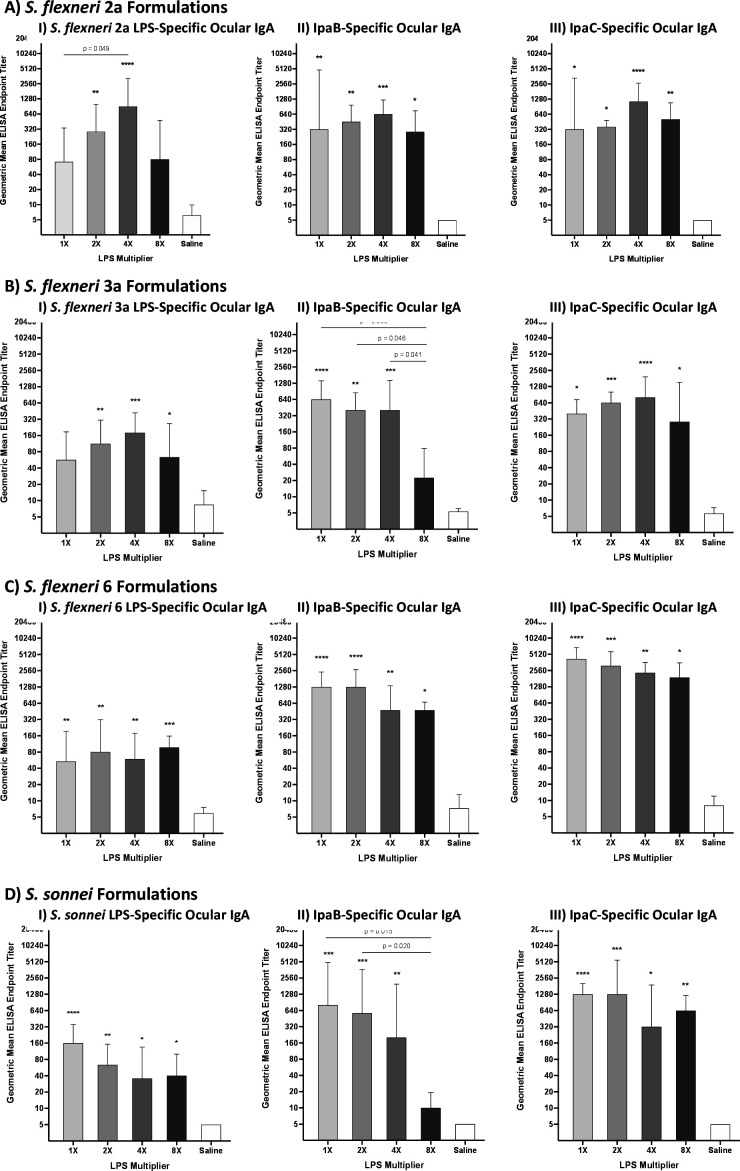
Previously, it was observed that protection in the guinea pig keratoconjunctivitis model was associated with LPS-specific ocular IgA responses (41). In the current studies, similar ocular IgA assessments on day 42 were determined to evaluate which S. flexneri 2a InvaplexAR formulation had superior immune responses. A trend similar to the previous protection results was observed with the 4× group having the highest LPS-specific ocular IgA responses (Fig. 2A). The 4× group also had significantly higher LPS-specific responses as compared to the 1× group (P = 0.049; Fig. 2A, I). When examining the IpaB and IpaC-specific ocular IgA responses, all formulation groups had significantly higher Ipa-specific ocular IgA responses as compared to saline immunized animals with the 4× group having the highest immune responses overall (Fig. 2A, II and III). There were no significant differences in the IpaB or IpaC-specific ocular IgA responses between any of the groups immunized with the different S. flexneri 2a InvaplexAR formulations (Fig. 2A, II and III).
Fig 2.
Geometric mean (with 95% confidence interval) ocular IgA responses on study day 42 of animals immunized with S. flexneri 2a (A), S. flexneri 3a (B), S. flexneri 6 (C), and S. sonnei (D) InvaplexAR formulations and the species LPS (I), IpaB (II), and IpaC (III). Stars represent significance compared to saline-treated animals (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001). P-values were determined by Kruskal-Wallis test with Dunn’s post hoc correction.
Similar trends were observed in the S. flexneri 2a LPS-specific serum antibody responses with the 4× group having the highest geometric mean titer (GMT) and percent seroconversion (PS) for IgG (GMT = 509, PS = 100%; Table 3) and IgA (GMT = 160, PS = 50%; Table 4). The S. flexneri 2a InvaplexNAT-specific serum IgG responses were relatively consistent across immunized groups; however, the Ipa-specific serum IgG responses increased with each increasing amount of LPS contained in the vaccine formulations (Table 3). Anti-IpaC serum IgG titers were significantly higher after immunization with S. flexneri 2a InvaplexAR containing 8× LPS (GMT = 29,029) as compared to IpaC-specific titers after immunization with S. flexneri 2a InvaplexAR containing 1× LPS (GMT = 8,146, P = 0.005) or 2× LPS (GMT = 10,263, P = 0.044). For IpaB, each group had significantly higher serum IgG GMT titers (as compared to saline controls) that ranged from 10,263 to 20,526 but there was no significant difference between any of the groups (Table 3). Interestingly, different trends were observed in the serum IgA responses with varied GMTs and PSs across vaccinated groups and the 2× group generally having the lowest InvaplexNAT and Ipa-specific serum IgA responses (Table 4). In consideration of the protection and ocular IgA titers, future S. flexneri 2a InvaplexAR products will be formulated using 4× purified S. flexneri 2a LPS in the reaction mixture.
TABLE 3.
Shigella antigen-specific serum IgG immune responses
| Shigella serotype | LPS multiplier | Antigen-specific serum IgG responses a | |||
|---|---|---|---|---|---|
| LPS b | InvaplexNAT | IpaB | IpaC | ||
| S. flexneri 2a | 1× | 227 ± 239 50% |
6,465 ± 4,336**** 100% |
10,263 ± 16,561**** 100% |
8,146 ± 3,155**** 100% |
| 2× | 454 ± 484*
c
66% |
5,132 ± 7,887**** 100% |
11,520 ± 34,448**** 100% |
10,263 ± 2,352**** 100% |
|
| 4× | 509 ± 436** 100% |
5,760 ± 3,935**** 100% |
16,292 ± 18,724**** 100% |
18,287 ± 5,949**** 100% |
|
| 8× | 454 ± 1,090* 50% |
9,143 ± 6,916**** 100% |
20,526 ± 31,426**** 100% |
29,029 ± 29,521**** 100% |
|
| Placebo | 90 ± 0 0% |
90 ± 0 0% |
90 ± 0 0% |
90 ± 0 0% |
|
| S. flexneri 3a | 1× | 202 ± 110 33% |
907 ± 478**** 100% |
5,760 ± 2,832**** 100% |
5,132 ± 3,155**** 100% |
| 2× | 286 ± 281* 50% |
1,143 ± 842**** 100% |
6,465 ± 7380**** 100% |
6,465 ± 2,352**** 100% |
|
| 4× | 509 ± 1,080** 67% |
1,018 ± 904**** 100% |
2,880 ± 8,597**** 100% |
7,257 ± 2,974**** 100% |
|
| 8× | 454 ± 461** 83% |
907 ± 478**** 100% |
808 ± 441**** 100% |
8,146 ± 3,155**** 100% |
|
| Placebo | 90 ± 0 0% |
90 ± 0 0% |
90 ± 0 0% |
90 ± 0 0% |
|
| S. flexneri 6 | 1× | 163 ± 237 29% |
720 ± 917**** 86% |
6,360 ± 7,138**** 100% |
14,043 ± 7,221**** 100% |
| 2× | 180 ± 231 29% |
1,070 ± 828**** 100% |
6,360 ± 3,266**** 100% |
15,505 ± 6,158**** 100% |
|
| 4× | 148 ± 243 29% |
720 ± 451**** 100% |
1,938 ± 1,699**** 100% |
12,719 ± 6,531**** 100% |
|
| 8× | 148 ± 231 14% |
1,181 ± 1,939**** 100% |
1,440 ± 1,894**** 100% |
14,043 ± 7,221**** 100% |
|
| Placebo | 90 ± 0 0% |
90 ± 0 0% |
90 ± 0 0% |
90 ± 0 0% |
|
| S. sonnei | 1× | 143 ± 105 17% |
1,616 ± 882**** 100% |
9,143 ± 35,273**** 100% |
4,572 ± 3,367**** 100% |
| 2× | 143 ± 105 17% |
1,814 ± 1,115**** 100% |
5,132 ± 3,155**** 100% |
8,146 ± 3,155**** 100% |
|
| 4× | 202 ± 135* 50% |
2,286 ± 1,683**** 100% |
8,146 ± 9,362**** 100% |
12,931 ± 15,456**** 100% |
|
| 8× | 101 ± 37 0% |
907 ± 478**** 100% |
404 ± 478* 83% |
8,146 ± 3,155**** 100% |
|
| Placebo | 90 ± 0 0% |
90 ± 0 0% |
90 ± 0 0% |
90 ± 0 0% |
|
Responses are shown as geometric mean endpoint titers ± standard deviation with percent responders (defined as a ≥4-fold-increase over baseline).
LPS responses are directed to the homologous serotype used for vaccination.
Significance determined by one-way ANOVA with Bonferroni post hoc test compared to placebo group *P < 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001.
TABLE 4.
Shigella antigen-specific serum IgA immune responses
| Shigella serotype | LPS multiplier | Antigen-specific serum IgA responses a | |||
|---|---|---|---|---|---|
| LPS b | InvaplexNAT | IpaB | IpaC | ||
| S. flexneri 2a | 1× | 57 ± 55 17% |
641 ± 394*** 100% |
1,018 ± 909*** 100% |
720 ± 492**** 100% |
| 2× | 90 ± 125 33% |
286 ± 519* 66% |
360 ± 487* 83% |
404 ± 483*** 100% |
|
| 4× | 160 ± 157*
c
50% |
509 ± 1,021*** 83% |
321 ± 522* 66% |
1,283 ± 1,060**** 100% |
|
| 8× | 80 ± 123 17% |
571 ± 1,008*** 100% |
360 ± 279* 83% |
1,814 ± 1,845**** 100% |
|
| Placebo | 45 ± 0 0% |
51 ± 18 0% |
51 ± 18 0% |
57 ± 55 17% |
|
| S. flexneri 3a | 1× | 71 ± 23 0% |
227 ± 231*** 83% |
641 ± 982**** 100% |
1,283 ± 789**** 100% |
| 2× | 71 ± 23 0% |
160 ± 133** 67% |
404 ± 625*** 83% |
1,143 ± 864*** 100% |
|
| 4× | 127 ± 148* 50% |
286 ± 216*** 83% |
286 ± 216** 83% |
1,440 ± 984**** 100% |
|
| 8× | 101 ± 55 33% |
202 ± 110** 83% |
90 ± 44 17% |
1,283 ± 1,060**** 100% |
|
| Placebo | 45 ± 0 0% |
45 ± 0 0% |
51 ± 18 0% |
160 ± 246 50% |
|
| S. flexneri 6 | 1× | 74 ± 64 29% |
121 ± 511 43% |
1,590 ± 1,785**** 100% |
878 ± 8,491**** 100% |
| 2× | 61 ± 24 0% |
199 ± 230* 71% |
1,304 ± 733**** 100% |
360 ± 226*** 100% |
|
| 4× | 67 ± 24 0% |
219 ± 505* 71% |
591 ± 514**** 100% |
439 ± 428*** 100% |
|
| 8× | 199 ± 102**** 86% |
163 ± 236 57% |
360 ± 1,032*** 71% |
720 ± 451**** 100% |
|
| Placebo | 45 ± 0 0% |
45 ± 0 0% |
50 ± 17 0% |
50 ± 17 0% |
|
| S. sonnei | 1× | 127 ± 109* 33% |
360 ± 478**** 100% |
321 ± 197**** 100% |
1,616 ± 4,247**** 100% |
| 2× | 143 ± 141* 50% |
509 ± 452**** 100% |
321 ± 265**** 100% |
1,440 ± 2,007**** 100% |
|
| 4× | 127 ± 49* 50% |
255 ± 226*** 83% |
101 ± 68 50% |
720 ± 957** 100% |
|
| 8× | 113 ± 120 50% |
286 ± 210*** 100% |
64 ± 54 17% |
1,018 ± 872*** 100% |
|
| Placebo | 45 ± 0 0% |
45 ± 0 0% |
45 ± 0 0% |
113 ± 46 33% |
|
Responses are shown as geometric mean endpoint titers ± standard deviation with percent responders (defined as a ≥4-fold-increase over baseline).
LPS responses are directed to the homologous serotype used for vaccination.
Significance determined by one-way ANOVA with Bonferroni post hoc test compared to placebo group *P < 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001.
Immunogenicity and protective efficacy of variable S. flexneri 3a LPS InvaplexAR formulations
S. flexneri 3a InvaplexAR constructed with increasing amounts (1×, 2×, 4×, and 8×) of purified S. flexneri 3a LPS were produced at small scale (see above) for determining the immunogenicity and PE in guinea pigs. Efficacy results using the guinea pig keratoconjunctivitis model demonstrated that the S. flexneri 3a 2× formulation provided the best, significant protection (PE = 70.0%, P = 0.012; Table 2) followed by the 1× formulation (PE = 60.0%, P = 0.036; Table 2). Modest levels of protection were observed with the 4× (PE = 50.0%, P = 0.089; Table 2) and 8× (PE = 40.0%, P = 0.193; Table 2) formulations; however, neither group reached statistical significance as compared to placebo controls.
Interestingly, the 4× group had the highest LPS-specific ocular IgA responses (P ≤ 0.001; Fig. 2B) followed by the 2× group (P ≤ 0.01; Fig. 2B, I) and the 8× group (P ≤ 0.05; Fig. 2B, I). LPS-specific ocular IgA responses in the 1× group were lower than all other groups and were not significantly higher compared to saline treated animals (Fig. 2B, I). In contrast, the IpaB-specific ocular IgA responses were highest in the 1× group (P ≤ 0.0001; Fig. 2B, II) followed by the 4× and 2× groups (Fig. 2B, II). The 8× group had minimal increases in IpaB-specific ocular IgA and all groups had significantly higher responses as compared to the 8× group (Fig. 2B, II). IpaC-specific ocular IgA responses showed yet another different pattern with all groups having significantly higher responses as compared to saline treated animals (Fig. 2B, III) with none of the immunized groups significantly different from one another.
The highest S. flexneri 3a LPS-specific serum IgG and IgA responses were observed in the 4× and 8× groups with only the 4× group having significantly higher LPS-specific serum IgA (GMT = 127, PS = 50%; Table 4) as compared to the saline treated animals. The InvaplexNAT-specific serum IgG and IgA responses were relatively consistent across immunized groups with all groups having significantly higher responses as compared to the saline group (Tables 3 and 4). The Ipa-specific serum IgG and IgA responses followed similar trends across the four S. flexneri 3a formulations as the Ipa-specific ocular IgA responses with consistent IpaC-specific responses and decreasing IpaB-specific responses (Tables 3 and 4). In consideration of the protective efficacy, future S. flexneri 3a InvaplexAR products will be formulated using 2× purified S. flexneri 3a LPS in the reaction mixture.
Immunogenicity and protective efficacy of variable S. flexneri 6 LPS InvaplexAR formulations
S. flexneri 6 InvaplexAR was produced at lab scale (see above) with increasing amounts (1×, 2×, 4×, and 8×) of purified S. flexneri 6 LPS (see Tables 1 and 2). In the guinea pig keratoconjunctivitis model, only the 8× formulation provided significant protection (PE = 62.9%, P = 0.021; Table 2) for the S. flexneri 6 products. The 1× and 2× constructs provided the same level of protection (PE = 44.3%, P = 0.120; Table 2), while the 4× construct provided the lowest level of efficacy (PE = 35.0%, P = 0.237; Table 2).
Similar levels of LPS-specific ocular IgA were induced by all four S. flexneri 6 formulations with the 8× group having the highest LPS-specific responses as compared to saline immunized animals (P ≤ 0.001; Fig. 2C, I). Robust, significant levels of Ipa-specific ocular IgA were induced across all groups with IpaB and IpaC-specific IgA levels showing a decreasing trend with increasing amounts of LPS present in the vaccine formulations (Fig. 2C, II and III).
There were no significant increases in the LPS-specific serum IgG responses across all S. flexneri 6 formulation groups; however, the 8× group induced moderate levels of serum IgA to LPS (GMT = 199, PS = 86%; Table 4) and was the only group to have significantly higher responses compared to the saline controls. The IpaC and InvaplexNAT-specific serum IgG and IgA responses were relatively consistent across all immunized groups (Tables 3 and 4), while the IpaB-specific serum IgG and IgA responses decreased with increasing amounts of LPS in the vaccine formulation. In consideration of the protection and ocular IgA titers, future S. flexneri 6 InvaplexAR products will be formulated using 8× purified S. flexneri 6 LPS in the reaction mixture.
Immunogenicity and protective efficacy of variable S. sonnei LPS InvaplexAR formulations
A series of prototype S. sonnei InvaplexAR vaccines were produced using 1×, 2×, 4×, and 8× S. sonnei LPS and 8 µM purified IpaC:1 µM purified IpaB. All four S. sonnei InvaplexAR formulations induced significant protection in the guinea pig keratoconjunctivitis model with ≥50% PE across all vaccinated groups (Table 2). The 1× formulation provided the highest level of protection (PE = 92%, P < 0.0001; Table 2) followed by the 2× and 8× groups (PE = 75%, P = 0.0003; Table 2) and finally the 4× group (PE = 58%, P = 0.005; Table 2).
LPS-specific ocular IgA responses were consistent with protection data in that the 1× S. sonnei group had the highest LPS-specific ocular IgA responses as compared to the saline group (P ≤ 0.001; Fig. 2D, I) followed by the 2× group (P ≤ 0.01; Fig. 2D, I) and then the 4× and 8× groups having similar levels of LPS-specific ocular IgA (P ≤ 0.05; Fig. 2D, I). The Ipa-specific ocular IgA responses were also highest in the 1× group (Fig. 2D, II and III). While all groups had significantly higher IpaC-specific ocular IgA responses as compared to saline treated animals (Fig. 2D, III), the 8× group had minimal increases in IpaB-specific ocular IgA with both the 1× group (P = 0.015; Fig. 2D, II) and the 2× group (P = 0.02; Fig. 2D, II) having significantly higher responses as compared to the 8× group.
Interestingly, the highest serum IgG responses were observed in the 4× group with this being the only group to have significantly higher LPS-specific serum IgG as compared to saline immunized animals (GMT = 202, PS = 50%; Table 3). Similar to the ocular IgA responses, the 8× group had lower IpaB-specific serum IgG responses as compared to other vaccinated groups; however, all groups had significantly higher responses as compared to the saline group (Table 3). Antigen-specific serum IgA responses varied across groups and the 1× and 2× groups had the highest serum IgA responses for all antigens as compared to all other groups (Table 4). In consideration of the protection and ocular IgA titers, future S. sonnei InvaplexAR products will be formulated using 1× purified S. sonnei LPS in the reaction mixture.
DISCUSSION
Despite efforts toward clean water and proper hygiene, infectious diarrhea caused by Shigella spp. remains a significant cause of morbidity and mortality in children of low and middle income countries as well as in travelers (1) to those regions of the world. Furthermore, the increasing prevalence of antimicrobial resistance often exacerbates treatment and management efforts. The continued global burden of shigellosis has been recognized by the World Health Organization (7) which has recommended an urgent need for additional control measures such as vaccines.
Shigella vaccine approaches that are in clinical development include live attenuated vaccines (NCT04242264; 42) and subunit vaccine efforts (43, 44). For the most part, Shigella vaccine approaches focus on the delivery of the serotype-defining Shigella LPS or O-SP, which has been suggested as a protective antigen (45, 46). Building on earlier clinical studies by Robbins and co-workers using O-SP conjugate vaccines, are recent approaches delivering the O-specific polysaccharide with either bioconjugates (47) or synthetic conjugates (48) which have advanced to clinical studies in target populations (NCT02646371 and NCT04602975).
The immune response post-infection with Shigella spp. not only recognizes the LPS component of the bacteria, but also the highly conserved T3SS effectors IpaB, IpaC, and IpaD proteins (8, 49). In controlled human infections models, immune responses directed to the Ipa proteins and LPS more closely correlated with resistance to infection as compared to LPS alone (49), suggesting that Ipa-specific immune responses play a critical role in protection. The only vaccine that effectively delivers measured amounts of IpaB and IpaC together with LPS were previous iterations of the Invaplex vaccine, which can induce potent immune responses to the major antigens involved in protective efficacy (36).
The original Invaplex vaccine product isolated from all species of virulent shigellae (InvaplexNAT), followed by the isolation of the well-defined, functional HP Invaplex vaccine product (35). This laid the groundwork for the product to be assembled from the individual purified constituents and was termed InvaplexAR (36). The historical development of the Shigella Invaplex product has been recently reviewed (32).
Preclinical studies on InvaplexAR clearly showed improved definition of product composition, immunogenicity, and efficacy as compared to InvaplexNAT, but the transition to cGMP and human studies required several modifications to improve yields and satisfy regulatory guidelines. To address some of the anticipated regulatory hurdles such as biosimilarity, use of acceptable antibiotics and GRAS-listed reagents during manufacture, new Ipa-expression recombinants were developed that led to greatly improved yields of non-tagged IpaB and IpaC products co-expressed with his-tagged-IpgC under the control of a common promoter, and the use of a kanamycin resistance marker, which is more favorable for products intended for human use.
The inherent hydrophobicity of IpaB and IpaC results in poor solubility in aqueous buffers (50, 51), but the use of Triton X-100 and other detergents improves solubility and stability of the purified recombinant proteins (28, 29, 37, 52 - 56). The use of Triton X-100 for complete Ipa protein release from the histidine-tagged IpgC chaperone during purification resulted in soluble and stable solutions of the Ipa proteins that were useful for InvaplexAR production and various antigen-specific immunoassays such as ELISAs (49, 57). Both purified IpaB and IpaC, as prepared for our studies, maintain all known epitopes in that they react with previously defined mAbs (58, 59) (Turbyfill, unpublished data). The stability of IpaC in the presence of ≥0.8% Triton X-100 is comparable to that exhibited by histidine-tagged IpaC in the presence of lauryldimethylamine-N-oxide (LDAO) (29) and the stability of IpaB in the presence of Triton X-100 appears to be even more thermally stable. Furthermore, once the assembly process for making InvaplexAR is complete, solubility and freeze-thawing sensitivity are no longer a problem. InvaplexAR is stable when stored frozen at −70°C at least 60 months (Duplessis, manuscript in preparation). These significant improvements in the stability of non-tagged IpaB or IpaC are important considerations for future vaccine storage and distribution. Finally, the use of Triton X-100, which is listed on the Institute for Vaccine Safety vaccine excipients list (January 2021; https://www.hopkinsvaccine.org/components-Excipients.htm) and is a component of US human influenza vaccines (Fluraix, Flublok, and Fluzone) (FDA Appendix B Vaccine Excipient Table, November 2021), substitutes as an established reagent for human vaccines for earlier reagents that had not been previously used to manufacture human vaccines.
The LPS component of InvaplexAR presents serotype-specific epitopes to immune cells that are associated with protective immunity (60 - 62). By utilizing overnight (stationary phase) cultures for the production of the LPS component of InvaplexAR products, maintenance of multiple lengths of the O-polysaccharide chain was achieved, which is described as being crucial to O-antigen-focused Shigella vaccine products (43, 48, 63 - 68) and presentation of the T3SS and invasiveness of the wild-type organism (69). The polydispersity of the chain lengths and other factors such as O-acylation are often serotype specific and may impact the frequency of immunogenic and protective epitopes (70). Using LPS vaccine components that contain a broad population of O-PS side chains to prepare the InvaplexAR products likely preserves the O-antigen as it would be natively presented by the wild-type organism.
The more potent InvaplexAR product provides comparable disease protection and immune stimulation at lower intranasal doses as compared to the InvaplexNAT product (36). The assembly process used to produce InvaplexAR allowed us to determine the effect that increased LPS component quantities would have on the vaccine’s overall composition and more importantly, its immunogenicity and potency.
Varying LPS amounts were evaluated in the assembly of InvaplexAR products for the clinically relevant S. flexneri 2a, 3a, 6, and S. sonnei. A trend evident for all four serotypes was that increased amounts of LPS in the initial reaction mixture resulted in increased product yield (r = 0.6503; P = 0.0064) and all yields were greater than the previously reported high of 22% (36). The InvaplexAR products for all four Shigella species had comparable sizes as assessed by SEC-HPLC and DLS (Table 1). Throughout these reported studies on the effect of varying amounts of LPS on the assembly process, the quantities and lots of IpaB and IpaC were held constant during the assembly step. Even so, it appeared that as the quantity of LPS increased, the IpaC:IpaB ratio, as measured by gel densitometry (Table 1), almost doubled for each serotype over the four LPS amounts. The higher IpaC:IpaB ratio resulted in an increase in the amount of IpaC and a decrease in the quantity of IpaB delivered with higher LPS formulations (r = −0.9403; P = <0.0001) for each serotype. The retention times of the InvaplexAR products (measured by SEC) were inversely correlated with the ratio of IpaC:IpaB (r = −0.8077, P = 0.0003) although the size relationship with the IpaC:IpaB ratio was not evident by DLS measurements. Compared to the previously reported S. flexneri 2a InvaplexAR product made with histidine-tagged IpaC, which had a hydrodynamic diameter of 44.9 nm (36), the new generation of products is approximately twofold smaller in hydrodynamic diameter (Table 1). It is unclear what has caused the size difference and whether the histidine tagged IpaC is a factor. By comparison, a 1 mg/mL solution of S. flexneri 2a LPS in water for injection (WFI) examined by DLS under these same conditions has a major complex with a hydrodynamic diameter of 132 nm in size, roughly six times larger than the S. flexneri 2a InvaplexAR complex (data not shown) suggesting that either the addition of the proteins, the overall manufacturing process, or both, may result in the smaller hydrodynamic diameters of the resulting InvaplexAR complexes. The DLS also shows that the products are polydisperse in size (data not shown). This polydispersity of the sizes of individual InvaplexAR complexes made with different serotypes or amounts of LPS suggests that each InvaplexAR has a distribution of individual complex sizes that are the roughly same.
The InvaplexAR products are more stable than the individual component proteins. The lipid A portion of the LPS most likely helps stabilize the hydrophobic IpaB and IpaC in the context of the InvaplexAR product. IpaC, in particular, is the less stable of the two proteins and is prone to self-aggregation (29, 36, 37, 50, 71). cGMP manufactured InvaplexAR products, which have approximately 100-fold lower quantities of residual detergent (Triton X-100), often used to stabilize IpaB and IpaC, show no signs of protein aggregation once complexed with LPS (manuscript in preparation). Monitoring the size of the InvaplexAR by DLS while heating to 95°C shows aggregation of the complex which is reversible upon cooling to 4°C (data not shown) which is supportive of a stable complex.
The approach used to expand the InvaplexAR technology to new serotypes suggested that optimizing the new products’ assembly process, with respect to the quantities of LPS, was necessary to produce new InvaplexAR products for different serotypes that were comparable both biochemically and immunologically. It was not known if LPS prepared from different serotypes interact with IpaB and IpaC in a similar, predictable manner. However, the physical parameters tested (see above) suggest there are consistent trends that lead to reproducible and stable products. Even so, testing each preparation for immunogenicity and efficacy provided key information that aided in the selection of an assembly process for each of the four serotypes.
The immunogenicity and efficacy of InvaplexAR formulated using increasing amounts of LPS were evaluated in the guinea pig keratoconjunctivitis model, which has been used successfully to support potency assessments (immunogenicity and efficacy measures) as part of Investigational New Drug applications. Overall, the potency of InvaplexAR in the model confirmed that LPSs isolated from different Shigella serotypes in the context of the InvaplexAR product have different inherent immunogenicities, with S. sonnei being the most potent and S. flexneri 6 being the least potent as evidenced with the level of protective efficacy. The anti-IpaC ocular IgA responses were comparable for each serotype across the variable amount of LPS used to produce InvaplexAR but interestingly, with S. sonnei and S. flexneri 3a, the IpaB-specific ocular responses were lower at higher amounts of LPS used for manufacture, which translated into lower levels of efficacy at the 4× and 8× LPS products for these two serotypes. This result suggests that the structure of the LPS may have more to do with the immunogenicity than the relative amount present (72).
The lower amounts of IpaB delivered in products with more LPS and higher IpaC:IpaB ratios are likely the source of the lower immune responses to IpaB, although other structural differences in the Invaplex complex as compared to S. flexneri 2a and 6 could play a role. While the InvaplexAR product provided a slight increase in the amount of O-antigen as compared to InvaplexNAT, serum IgG and IgA responses were not significantly different (36). When examining the potency of the new InvaplexAR products, increasing the yield efficiencies, increasing the IpaC:IpaB ratio, or the LPS content did not necessarily result in a concomitant increase in protective efficacy. Each serotype of Shigella InvaplexAR had varying protective efficacies (Table 2). Thus, the variability in protective efficacy may not be solely dependent on the quantity of any single antigen in the vaccine product but may also involve the presentation of each antigen as part of the complex to the host’s immune system as well as the potential adjuvant properties of each individual LPS antigen (73). Also, while increasing amounts of LPS resulted in increases in the total protein yield of InvaplexAR product, the LPS increase may be at the cost of a decrease in the exposure of the Ipa proteins to the host, potentially blocking important epitopes crucial to the immune response or functional domains of the Ipa proteins involved in host cell interactions.
As mentioned above, the different serotypes of LPS may each have an inherently different immunogenicity as compared to each other. This difference may be due to the sugar composition, non-stoichiometric modifications that may be strain specific (74 - 77), and/or due to the different multimeric packing of LPS around/with the protein antigens yielding different antigen presentation. Additional studies to evaluate the immunogenicity of a multivalent InvaplexAR vaccine may contribute to a better understanding of the interactions between the immune system and LPS from various Shigella serotypes being presented in the context of the Ipa proteins.
The successful construction of effective InvaplexAR products has been achieved for S. flexneri 2a, 3a, 6, and S. sonnei, all leading candidate serotypes for a multivalent Shigella vaccine. These studies indicate that the immunogenicity and efficacy of various Shigella serotype vaccines may not be entirely predictable and will require evaluation of each vaccine in available models before full development in clinical trials. Each of the InvaplexAR serotype formulations evaluated resulted in a strong response to IpaB and IpaC but varying response to LPS, with at least one formulation for each serotype that was protective in the guinea pig model. Of note, S. flexneri 6, a serotype not evaluated very often with respect to immunogenicity or protective antigens (77 - 79), may require unique strategies to optimize the response to the LPS component. The InvaplexAR products developed not only provide a vaccine approach to deliver both serotype specific and Shigella genus conserved antigens but have overcome some of the manufacturing hurdles facing vaccine developers. To date, a successful cGMP S. flexneri 2a InvaplexAR product has been manufactured using the formulation outlined herein.
Although clinical grade InvaplexAR products have been made, and one has been successfully transitioned to cGMP manufacture and phase 1 trials, it is clear that additional improvements in the vaccine formulations and delivery could provide increased beneficial immune responses in human patients. For example, delivery by a non-mucosal route, the addition of a safe adjuvant, and possibly altering the amounts of IpaB and IpaC in the final formulation could all positively impact a more protective immune response. As preliminary studies using parenteral immunizations with an InvaplexAR formulated with an under-acylated S. flexneri 2a LPS (InvaplexAR-Detox) have increased the immune responses to all three antigens (32, 80, Guiterrez, manuscript in preparation), this work can also serve as a promising foundation for the development of better, clinically-ready, multivalent, Shigella Invaplex vaccines in the future.
ACKNOWLEDGMENTS
The authors wish to thank Bill Picking for the original Ipa expression clones. We thank the excellent biochemical technical assistance of Dianaly Au, Michael Pratt, Jacob Reinhart, and Michael Millett and the excellent immunology technical assistance of Hailey Weerts, Brielle Barnard, Rachel Kasmerski, Michael Marll, and Nyssa Bryant. The authors would also like to thank R. J. Cybulski for his review of this manuscript.
K. R. Turbyfill and D. V. Zurawski are employees of the U.S. Government. Material has been reviewed by the Walter Reed Army Institute of Research. There is no objection to its presentation and/or publication. The opinions or assertions contained herein are the private views of the author and are not to be construed as official or as reflecting true views of the Department of the Army, the Department of Defense, or the U.S. Government.
None of the authors have a conflict of interest for any of the materials presented in the manuscript.
Contributor Information
K. Ross Turbyfill, Email: kevin.r.turbyfill.civ@health.mil.
Alfredo G. Torres, The University of Texas Medical Branch, Galveston, Texas, USA
ETHICS APPROVAL
The research was conducted under an IACUC-approved animal use protocol in an AAALAC International-accredited facility with a Public Health Services Animal Welfare Assurance and in compliance with the Animal Welfare Act and other federal statutes and regulations relating to laboratory animals.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/msphere.00073-23.
Plasmid maps for described recombinant clones and protein stability results.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Troeger C, Blacker BF, Khalil IA, Rao PC, Cao S, Zimsen SR, Albertson SB, Stanaway JD, Deshpande A, Abebe Z, Alvis-Guzman N, Amare AT, Asgedom SW, Anteneh ZA, Antonio CAT, Aremu O, Asfaw ET, Atey TM, Atique S, Avokpaho E, Awasthi A, Ayele HT, Barac A, Barreto ML, Bassat Q, Belay SA, Bensenor IM, Bhutta ZA, Bijani A, Bizuneh H, Castañeda-Orjuela CA, Dadi AF, Dandona L, Dandona R, Do HP, Dubey M, Dubljanin E, Edessa D, Endries AY, Eshrati B, Farag T, Feyissa GT, Foreman KJ, Forouzanfar MH, Fullman N, Gething PW, Gishu MD, Godwin WW, Gugnani HC, Gupta R, Hailu GB, Hassen HY, Hibstu DT, Ilesanmi OS, Jonas JB, Kahsay A, Kang G, Kasaeian A, Khader YS, Khalil IA, Khan EA, Khan MA, Khang Y-H, Kissoon N, Kochhar S, Kotloff KL, Koyanagi A, Kumar GA, Magdy Abd El Razek H, Malekzadeh R, Malta DC, Mehata S, Mendoza W, Mengistu DT, Menota BG, Mezgebe HB, Mlashu FW, Murthy S, Naik GA, Nguyen CT, Nguyen TH, Ningrum DNA, Ogbo FA, Olagunju AT, Paudel D, Platts-Mills JA, Qorbani M, Rafay A, Rai RK, Rana SM, Ranabhat CL, Rasella D, Ray SE, Reis C, Renzaho AM, Rezai MS, Ruhago GM, Safiri S, Salomon JA, Sanabria JR, Sartorius B, Sawhney M, Sepanlou SG, Shigematsu M, Sisay M, Somayaji R, Sreeramareddy CT, Sykes BL, Taffere GR, Topor-Madry R, Tran BX, Tuem KB, Ukwaja KN, Vollset SE, Walson JL, Weaver MR, Weldegwergs KG, Werdecker A, Workicho A, Yenesew M, Yirsaw BD, Yonemoto N, El Sayed Zaki M, Vos T, Lim SS, Naghavi M, Murray CJ, Mokdad AH, Hay SI, Reiner RC. 2018. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the global burden of disease study 2016. Lancet Infect Dis 18:1211–1228. doi: 10.1016/S1473-3099(18)30362-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Khalil IA, Troeger C, Blacker BF, Rao PC, Brown A, Atherly DE, Brewer TG, Engmann CM, Houpt ER, Kang G, Kotloff KL, Levine MM, Luby SP, MacLennan CA, Pan WK, Pavlinac PB, Platts-Mills JA, Qadri F, Riddle MS, Ryan ET, Shoultz DA, Steele AD, Walson JL, Sanders JW, Mokdad AH, Murray CJL, Hay SIReiner Jr RC. 2018. Morbidity and mortality due to shigella and enterotoxigenic Escherichia coli diarrhoea: the global burden of disease study 1990-2016. Lancet Infect Dis 18:1229–1240. doi: 10.1016/S1473-3099(18)30475-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nataro JP, Guerrant RL. 2017. Chronic consequences on human health induced by microbial pathogens: growth faltering among children in developing countries. Vaccine 35:6807–6812. doi: 10.1016/j.vaccine.2017.05.035 [DOI] [PubMed] [Google Scholar]
- 4. Rogawski ET, Guerrant RL. 2017. The burden of Enteropathy and "Subclinical" infections. Pediatr Clin North Am 64:815–836. doi: 10.1016/j.pcl.2017.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Anderson JD, Bagamian KH, Muhib F, Amaya MP, Laytner LA, Wierzba T, Rheingans R. 2019. Burden of enterotoxigenic Escherichia coli and shigella non-fatal diarrhoeal infections in 79 low-income and lower middle-income countries: a modelling analysis. Lancet Glob Health 7:e321–e330. doi: 10.1016/S2214-109X(18)30483-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Organization PAHOWH . 2022. Epidemiological alert emergence and spread of Shigella Sonnei with extreme resistance to antibiotics. In Potential risk for Latin America and the Caribbean. Pan American Health Organization. [Google Scholar]
- 7. Organization WH. 2022. Bacterial vaccines in clinical and preclinical development: an overview and analysis, Geneva: [Google Scholar]
- 8. Formal SB, Oaks EV, Olsen RE, Wingfield-Eggleston M, Snoy PJ, Cogan JP. 1991. Effect of prior infection with virulent Shigella flexneri 2a on the resistance of monkeys to subsequent infection with Shigella sonnei. J Infect Dis 164:533–537. doi: 10.1093/infdis/164.3.533 [DOI] [PubMed] [Google Scholar]
- 9. Fries LF, Montemarano AD, Mallett CP, Taylor DN, Hale TL, Lowell GH. 2001. Safety and immunogenicity of a proteosome-Shigella flexneri 2a lipopolysaccharide vaccine administered Intranasally to healthy adults. Infect Immun 69:4545–4553. doi: 10.1128/IAI.69.7.4545-4553.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mallett CP, Hale TL, Kaminski RW, Larsen T, Orr N, Cohen D, Lowell GH. 1995. Intransal or intragastric immunization with proteosome-Shigella lipopolysaccharide vaccines protects against lethal pneumonia in a murine model of Shigella infection. Infect Immun 63:2382–2386. doi: 10.1128/iai.63.6.2382-2386.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Orr N, Arnon R, Rubin G, Cohen D, Bercovier H, Lowell GH. 1994. Enhancement of anti-Shigella lipopolysaccharide (LPS) response by addition of the cholera toxin B subunit to oral and intranasal proteosome-Shigella flexneri 2a LPS vaccines. Infect Immun 62:5198–5200. doi: 10.1128/iai.62.11.5198-5200.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cohen D, Ashkenazi S, Green MS, Gdalevich M, Robin G, Slepon R, Yavzori M, Orr N, Block C, Ashkenazi I, Shemer J, Taylor DN, Hale TL, Sadoff JC, Pavliakova D, Schneerson R, Robbins JB. 1997. Double-blind vaccine-controlled randomised efficacy trial of an investigational Shigella sonnei conjugate vaccine in young adults. Lancet 349:155–159. doi: 10.1016/S0140-6736(96)06255-1 [DOI] [PubMed] [Google Scholar]
- 13. Passwell JH, Ashkenazi S, Banet-Levi Y, Ramon-Saraf R, Farzam N, Lerner-Geva L, Even-Nir H, Yerushalmi B, Chu C, Shiloach J, Robbins JB, Schneerson R, Israeli Shigella Study Group . 2010. Age-related efficacy of Shigella O-specific polysaccharide conjugates in 1-4-year-old Israeli children. Vaccine 28:2231–2235. doi: 10.1016/j.vaccine.2009.12.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque AS, Zaidi AK, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acácio S, Biswas K, O’Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins-Browne RM, Levine MM. 2013. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the global enteric multicenter study, GEMS): a prospective, case-control study. Lancet 382:209–222. doi: 10.1016/S0140-6736(13)60844-2 [DOI] [PubMed] [Google Scholar]
- 15. Liu J, Platts-Mills JA, Juma J, Kabir F, Nkeze J, Okoi C, Operario DJ, Uddin J, Ahmed S, Alonso PL, Antonio M, Becker SM, Blackwelder WC, Breiman RF, Faruque ASG, Fields B, Gratz J, Haque R, Hossain A, Hossain MJ, Jarju S, Qamar F, Iqbal NT, Kwambana B, Mandomando I, McMurry TL, Ochieng C, Ochieng JB, Ochieng M, Onyango C, Panchalingam S, Kalam A, Aziz F, Qureshi S, Ramamurthy T, Roberts JH, Saha D, Sow SO, Stroup SE, Sur D, Tamboura B, Taniuchi M, Tennant SM, Toema D, Wu Y, Zaidi A, Nataro JP, Kotloff KL, Levine MM, Houpt ER. 2016. Use of quantitative molecular diagnostic methods to identify causes of diarrhoea in children: a reanalysis of the GEMS case-control study. Lancet 388:1291–1301. doi: 10.1016/S0140-6736(16)31529-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Platts-Mills JA, Liu J, Rogawski ET, Kabir F, Lertsethtakarn P, Siguas M, Khan SS, Praharaj I, Murei A, Nshama R, Mujaga B, Havt A, Maciel IA, McMurry TL, Operario DJ, Taniuchi M, Gratz J, Stroup SE, Roberts JH, Kalam A, Aziz F, Qureshi S, Islam MO, Sakpaisal P, Silapong S, Yori PP, Rajendiran R, Benny B, McGrath M, McCormick BJJ, Seidman JC, Lang D, Gottlieb M, Guerrant RL, Lima AAM, Leite JP, Samie A, Bessong PO, Page N, Bodhidatta L, Mason C, Shrestha S, Kiwelu I, Mduma ER, Iqbal NT, Bhutta ZA, Ahmed T, Haque R, Kang G, Kosek MN, Houpt ER, Acosta AM, Rios de Burga R, Chavez CB, Flores JT, Olotegui MP, Pinedo SR, Trigoso DR, Vasquez AO, Ahmed I, Alam D, Ali A, Rasheed M, Soofi S, Turab A, Yousafzai A, Zaidi AK, Shrestha B, Rayamajhi BB, Strand T, Ammu G, Babji S, Bose A, George AT, Hariraju D, Jennifer MS, John S, Kaki S, Karunakaran P, Koshy B, Lazarus RP, Muliyil J, Ragasudha P, Raghava MV, Raju S, Ramachandran A, Ramadas R, Ramanujam K, Rose A, Roshan R, Sharma SL, Sundaram S, Thomas RJ, Pan WK, Ambikapathi R, Carreon JD, Doan V, Hoest C, Knobler S, Miller MA, Psaki S, Rasmussen Z, Richard SA, Tountas KH, Svensen E, Amour C, Bayyo E, Mvungi R, Pascal J, Yarrot L, Barrett L, Dillingham R, Petri WA, Scharf R, Ahmed AS, Alam MA, Haque U, Hossain MI, Islam M, Mahfuz M, Mondal D, Nahar B, Tofail F, Chandyo RK, Shrestha PS, Shrestha R, Ulak M, Bauck A, Black R, Caulfield L, Checkley W, Lee G, Schulze K, Scott S, Murray-Kolb LE, Ross AC, Schaefer B, Simons S, Pendergast L, Abreu CB, Costa H, Di Moura A, Filho JQ, ÁM L, Lima NL, Lima IF, Maciel BL, Medeiros PH, Moraes M, Mota FS, Oriá RB, Quetz J, Soares AM, Mota RM, Patil CL, Mahopo C, Maphula A, Nyathi E. 2018. Use of quantitative molecular diagnostic methods to assess the Aetiology, burden, and clinical characteristics of diarrhoea in children in low-resource settings: a reanalysis of the MAL-ED cohort study. Lancet Glob Health 6:e1309–e1318. doi: 10.1016/S2214-109X(18)30349-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Picking WL, Coye L, Osiecki JC, Serfis AB, Schaper E, Picking WD. 2001. Identification of functional regions within invasion plasmid antigen C (Ipac) of Shigella Flexneri. Mol Microbiol 39:100–111. doi: 10.1046/j.1365-2958.2001.02210.x [DOI] [PubMed] [Google Scholar]
- 18. Veenendaal AKJ, Hodgkinson JL, Schwarzer L, Stabat D, Zenk SF, Blocker AJ. 2007. The type III secretion system needle tip complex mediates host cell sensing and translocon insertion. Mol Microbiol 63:1719–1730. doi: 10.1111/j.1365-2958.2007.05620.x [DOI] [PubMed] [Google Scholar]
- 19. Page AL, Ohayon H, Sansonetti PJ, Parsot C. 1999. The secreted IpaB and IpaC Invasins and their cytoplasmic chaperone IpgC are required for intercellular dissemination of Shigella flexneri. Cell Microbiol 1:183–193. doi: 10.1046/j.1462-5822.1999.00019.x [DOI] [PubMed] [Google Scholar]
- 20. Roehrich AD, Martinez-Argudo I, Johnson S, Blocker AJ, Veenendaal AKJ. 2010. The extreme C terminus of Shigella flexneri IpaB is required for regulation of type III secretion, needle tip composition, and binding. Infect Immun 78:1682–1691. doi: 10.1128/IAI.00645-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Muthuramalingam M, Whittier SK, Picking WL, Picking WD. 2021. The Shigella type III secretion system: an overview from top to bottom. Microorganisms 9:451. doi: 10.3390/microorganisms9020451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Russo BC, Duncan JK, Wiscovitch AL, Hachey AC, Goldberg MB. 2019. Activation of Shigella Flexneri type 3 secretion requires a host-induced conformational change to the translocon pore. PLoS Pathog 15:e1007928. doi: 10.1371/journal.ppat.1007928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shimanovich AA, Buskirk AD, Heine SJ, Blackwelder WC, Wahid R, Kotloff KL, Pasetti MF. 2017. Functional and antigen-specific serum antibody levels as correlates of protection against Shigellosis in a controlled human challenge study. Clin Vaccine Immunol 24:e00412-16. doi: 10.1128/CVI.00412-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ndungo E, Randall A, Hazen TH, Kania DA, Trappl-Kimmons K, Liang X, Barry EM, Kotloff KL, Chakraborty S, Mani S, Rasko DA, Pasetti MF. 2018. A novel Shigella proteome microarray discriminates targets of human antibody reactivity following oral vaccination and experimental challenge. mSphere 3:e00260-18. doi: 10.1128/mSphere.00260-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Heine SJ, Franco-Mahecha OL, Chen X, Choudhari S, Blackwelder WC, van Roosmalen ML, Leenhouts K, Picking WL, Pasetti MF. 2015. Shigella IpaB and IpaD displayed on L. lactis bacterium-like particles induce protective immunity in adult and infant mice. Immunol Cell Biol 93:641–652. doi: 10.1038/icb.2015.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Martinez-Becerra FJ, Kissmann JM, Diaz-McNair J, Choudhari SP, Quick AM, Mellado-Sanchez G, Clements JD, Pasetti MF, Picking WL. 2012. Broadly protective Shigella vaccine based on type III secretion apparatus proteins. Infect Immun 80:1222–1231. doi: 10.1128/IAI.06174-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Malaei F, Hesaraki M, Saadati M, Ahdi AM, Sadraeian M, Honari H, Nazarian S. 2013. Immunogenicity of a new recombinant IpaC from Shigella dysenteriae type I in guinea pig as a vaccine candidate. Iran J Immunol 10:110–117. [PubMed] [Google Scholar]
- 28. MacRae AF, Preiszner J, Ng S, Bolla RI. 2004. Expression of his-tagged Shigella IpaC in arabidopsis. J Biotechnol 112:247–253. doi: 10.1016/j.jbiotec.2004.04.012 [DOI] [PubMed] [Google Scholar]
- 29. Baruah N, Ahamad N, Maiti S, Howlader DR, Bhaumik U, Patil VV, Chakrabarti MK, Koley H, Katti DS. 2021. Development of a self-adjuvanting, cross-protective, stable intranasal recombinant vaccine for Shigellosis. ACS Infect Dis 7:3182–3196. doi: 10.1021/acsinfecdis.1c00345 [DOI] [PubMed] [Google Scholar]
- 30. Bârzu S, Arondel J, Guillot S, Sansonetti PJ, Phalipon A. 1998. Immunogenicity of IpaC-hybrid proteins expressed in the Shigella flexneri 2a vaccine candidate SC602. Infect Immun 66:77–82. doi: 10.1128/IAI.66.1.77-82.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Turbyfill KR, Hartman AB, Oaks EV. 2000. Isolation and characterization of a Shigella Flexneri Invasin complex subunit vaccine. Infect Immun 68:6624–6632. doi: 10.1128/IAI.68.12.6624-6632.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Turbyfill KR, Clarkson KA, Oaks EV, Kaminski RW. 2022. From concept to clinical product: a brief history of the novel Shigella invaplex vaccine’s refinement and evolution. Vaccines (Basel) 10:548. doi: 10.3390/vaccines10040548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tribble D, Kaminski R, Cantrell J, Nelson M, Porter C, Baqar S, Williams C, Arora R, Saunders J, Ananthakrishnan M, Sanders J, Zaucha G, Turbyfill R, Oaks E. 2010. Safety and immunogenicity of a Shigella flexneri 2a Invaplex 50 intranasal vaccine in adult volunteers. Vaccine 28:6076–6085. doi: 10.1016/j.vaccine.2010.06.086 [DOI] [PubMed] [Google Scholar]
- 34. Riddle MS, Kaminski RW, Williams C, Porter C, Baqar S, Kordis A, Gilliland T, Lapa J, Coughlin M, Soltis C, Jones E, Saunders J, Keiser PB, Ranallo RT, Gormley R, Nelson M, Turbyfill KR, Tribble D, Oaks EV. 2011. Safety and immunogenicity of an intranasal Shigella flexneri 2a Invaplex 50 vaccine. Vaccine 29:7009–7019. doi: 10.1016/j.vaccine.2011.07.033 [DOI] [PubMed] [Google Scholar]
- 35. Turbyfill KR, Kaminski RW, Oaks EV. 2008. Immunogenicity and efficacy of highly purified Invasin complex vaccine from Shigella flexneri 2a. Vaccine 26:1353–1364. doi: 10.1016/j.vaccine.2007.12.040 [DOI] [PubMed] [Google Scholar]
- 36. Turbyfill KR, Clarkson KA, Vortherms AR, Oaks EV, Kaminski RW. 2018. Assembly, biochemical characterization, immunogenicity, adjuvanticity, and efficacy of Shigella artificial invaplex. mSphere 3:e00583-17. doi: 10.1128/mSphere.00583-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Birket SE, Harrington AT, Espina M, Smith ND, Terry CM, Darboe N, Markham AP, Middaugh CR, Picking WL, Picking WD. 2007. Preparation and characterization of translocator/chaperone complexes and their component proteins from Shigella flexneri. Biochemistry 46:8128–8137. doi: 10.1021/bi700099c [DOI] [PubMed] [Google Scholar]
- 38. Westphal O, Jann K. 1965. Bacterial lipopolysaccarides. Extraction with phenolwater and further applications of the procedure. Methods in Carbohydrate Chemistry. [Google Scholar]
- 39. Oaks EV, Hale TL, Formal SB. 1986. Serum immune response to Shigella protein antigens in rhesus monkeys and humans infected with Shigella spp. Infect Immun 53:57–63. doi: 10.1128/iai.53.1.57-63.1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hartman AB, Powell CJ, Schultz CL, Oaks EV, Eckels KH. 1991. Small-animal model to measure efficacy and immunogenicity of Shigella vaccine strains. Infect Immun 59:4075–4083. doi: 10.1128/iai.59.11.4075-4083.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kaminski RW, Wu M, Turbyfill KR, Clarkson K, Tai B, Bourgeois AL, Van De Verg LL, Walker RI, Oaks EV. 2014. Development and preclinical evaluation of a trivalent, formalin-inactivated Shigella whole-cell vaccine. Clin Vaccine Immunol 21:366–382. doi: 10.1128/CVI.00683-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Harutyunyan S, Neuhauser I, Mayer A, Aichinger M, Szijártó V, Nagy G, Nagy E, Girardi P, Malinoski FJ, Henics T. 2020. Characterization of ShigETEC, a novel live attenuated combined vaccine against Shigellae and ETEC. Vaccines (Basel) 8:689. doi: 10.3390/vaccines8040689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Barel LA, Mulard LA. 2019. Classical and novel strategies to develop a Shigella glycoconjugate vaccine: from concept to efficacy in human. Hum Vaccin Immunother 15:1338–1356. doi: 10.1080/21645515.2019.1606972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Martin P, Alaimo C. 2022. The ongoing journey of a Shigella bioconjugate vaccine. Vaccines 10:212. doi: 10.3390/vaccines10020212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Robbins JB, Chu C, Schneerson R. 1992. Hypothesis for vaccine development: protective immunity to enteric diseases caused by nontyphoidal Salmonellae and Shigellae may be conferred by serum IgG antibodies to the O-specific polysaccharide of their lipopolysaccharides. Clin Infect Dis 15:346–361. doi: 10.1093/clinids/15.2.346 [DOI] [PubMed] [Google Scholar]
- 46. Cohen D, Meron-Sudai S, Bialik A, Asato V, Ashkenazi S. 2022. Detoxified O-specific polysaccharide (O-SP)–protein conjugates: emerging approach in the Shigella vaccine development scene. Vaccines 10:675. doi: 10.3390/vaccines10050675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ravenscroft N, Braun M, Schneider J, Dreyer AM, Wetter M, Haeuptle MA, Kemmler S, Steffen M, Sirena D, Herwig S, Carranza P, Jones C, Pollard AJ, Wacker M, Kowarik M. 2019. Characterization and immunogenicity of a Shigella flexneri 2a O-antigen bioconjugate vaccine candidate. Glycobiology 29:669–680. doi: 10.1093/glycob/cwz044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Phalipon A, Mulard LA. 2022. Toward a multivalent synthetic oligosaccharide-based conjugate vaccine against Shigella: State-of-the-art for a monovalent prototype and challenges. Vaccines (Basel) 10:403. doi: 10.3390/vaccines10030403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Clarkson KA, Frenck RW, Dickey M, Suvarnapunya AE, Chandrasekaran L, Weerts HP, Heaney CD, McNeal M, Detizio K, Parker S, Hoeper A, Bourgeois AL, Porter CK, Venkatesan MM, Kaminski RW, Pascual DW. 2020. Immune response characterization after controlled infection with lyophilized Shigella Sonnei 53G. mSphere 5. doi: 10.1128/mSphere.00988-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Picking WL, Mertz JA, Marquart ME, Picking WD. 1996. Cloning, expression, and affinity purification of recombinant Shigella flexneri invasion plasmid antigens IpaB and IpaC. Protein Express Purif 8:401–408. doi: 10.1006/prep.1996.0117 [DOI] [PubMed] [Google Scholar]
- 51. Ptak-Kaczor M, Banach M, Stapor K, Fabian P, Konieczny L, Roterman I. 2021. Solubility and aggregation of selected proteins interpreted on the basis of hydrophobicity distribution. Int J Mol Sci 22:5002. doi: 10.3390/ijms22095002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bernard AR, Duarte SM, Kumar P, Dickenson NE. 2016. Detergent isolation stabilizes and activates the Shigella type III secretion system translocator protein IpaC. J Pharm Sci 105:2240–2248. doi: 10.1016/j.xphs.2016.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chen X, Choudhari SP, Martinez-Becerra FJ, Kim JH, Dickenson NE, Toth RT, Joshi SB, Greenwood JC, Clements JD, Picking WD, Middaugh CR, Picking WL. 2015. Impact of detergent on biophysical properties and immune response of the IpaDB fusion protein, a candidate subunit vaccine against Shigella species. Infect Immun 83:292–299. doi: 10.1128/IAI.02457-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Barta ML, Adam PR, Dickenson NE. 2017. Recombinant expression and purification of the Shigella translocator IpaB. Methods Mol Biol 1531:173–181. doi: 10.1007/978-1-4939-6649-3_15 [DOI] [PubMed] [Google Scholar]
- 55. Kapoor N, Ndungo E, Pill L, Desalegn G, Berges A, Oaks EV, Fairman J, Pasetti MF. 2022. Efficient production of immunologically active Shigella invasion plasmid antigens IpaB and IpaH using a cell-free expression system. Appl Microbiol Biotechnol 106:401–414. doi: 10.1007/s00253-021-11701-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mobilon C, de Toledo MAS, Paganelli FL, dos Santos CA, de Pace F, de Paiva JB, Stehling EG, Nakazato G, Balieiro AG, Airoldi F da S, Reis F de A, da Silveira WD. 2013. Cloning and purification of Ipac antigen from Shigella Flexneri: proposal of a new methodology. Protein Pept Lett 20:133–139. doi: 10.2174/092986613804725316 [DOI] [PubMed] [Google Scholar]
- 57. Clarkson KA, Talaat KR, Alaimo C, Martin P, Bourgeois AL, Dreyer A, Porter CK, Chakraborty S, Brubaker J, Elwood D, Frölich R, DeNearing B, Weerts HP, Feijoo B, Halpern J, Sack D, Riddle MS, Fonck VG, Kaminski RW. 2021. Immune response characterization in a human challenge study with a Shigella Flexneri 2a bioconjugate vaccine. EBioMedicine 66:103308. doi: 10.1016/j.ebiom.2021.103308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Oaks EV, Turbyfill KR. 1992. Myosin-cross-reactive EPITOPE of Shigella flexneri invasion plasmid antigen B. Infect Immun 60:557–564. doi: 10.1128/iai.60.2.557-564.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Turbyfill KR, Joseph SW, Oaks EV. 1995. Recognition of three epitopic regions on invasion plasmid antigen C by immune sera of rhesus monkeys infected with Shigella flexneri 2a. Infect Immun 63:3927–3935. doi: 10.1128/iai.63.10.3927-3935.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rasolofo-Razanamparany V, Cassel-Beraud AM, Roux J, Sansonetti PJ, Phalipon A. 2001. Predominance of serotype-specific mucosal antibody response in Shigella flexneri-infected humans living in an area of endemicity. Infect Immun 69:5230–5234. doi: 10.1128/IAI.69.9.5230-5234.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hu Z, Benkoulouche M, Barel L-A, Le Heiget G, Ben Imeddourene A, Le Guen Y, Monties N, Guerreiro C, Remaud-Siméon M, Moulis C, André I, Mulard LA. 2021. A convergent chemoenzymatic strategy to deliver a diversity of Shigella flexneri serotype-specific O-antigen segments from a unique lightly protected tetrasaccharide core. J Org Chem 86:2058–2075. doi: 10.1021/acs.joc.0c00777 [DOI] [PubMed] [Google Scholar]
- 62. Cohen D, Meron-Sudai S, Bialik A, Asato V, Goren S, Ariel-Cohen O, Reizis A, Hochberg A, Ashkenazi S. 2019. Serum IgG antibodies to Shigella lipopolysaccharide antigens - a correlate of protection against shigellosis. Hum Vaccin Immunother 15:1401–1408. doi: 10.1080/21645515.2019.1606971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Orr N, Robin G, Cohen D, Arnon R, Lowell GH. 1993. Immunogenicity and efficacy of oral or intranasal Shigella flexneri 2a and Shigella sonnei proteosome-lipopolysaccharide vaccines in animal models. Infect Immun 61:2390–2395. doi: 10.1128/iai.61.6.2390-2395.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Simon JK, Wahid R, Maciel M, Picking WL, Kotloff KL, Levine MM, Sztein MB. 2009. Antigen-specific B memory cell responses to lipopolysaccharide (LPS) and invasion plasmid antigen (Ipa) B elicited in volunteers vaccinated with live-attenuated Shigella flexneri 2a vaccine candidates. Vaccine 27:565–572. doi: 10.1016/j.vaccine.2008.10.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hoare A, Bravo D, Martinić M, Valvano MA, Contreras I, Álvarez SA. 2012. The normal chain length distribution of the O antigen is required for the interaction of Shigella flexneri 2a with polarized Caco-2 cells. Biol Res 45:21–26. doi: 10.4067/S0716-97602012000100003 [DOI] [PubMed] [Google Scholar]
- 66. Barman S, Koley H, Ramamurthy T, Chakrabarti MK, Shinoda S, Nair GB, Takeda Y. 2013. Protective immunity by oral immunization with heat-killed Shigella strains in a guinea pig colitis model. Microbiol Immunol 57:762–771. doi: 10.1111/1348-0421.12095 [DOI] [PubMed] [Google Scholar]
- 67. Gasperini G, Raso MM, Arato V, Aruta MG, Cescutti P, Necchi F, Micoli F. 2021. Effect of O-antigen chain length regulation on the immunogenicity of Shigella and Salmonella generalized modules for membrane antigens (GMMA). Int J Mol Sci 22:1309. doi: 10.3390/ijms22031309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. van der Put RMF, Smitsman C, de Haan A, Hamzink M, Timmermans H, Uittenbogaard J, Westdijk J, Stork M, Ophorst O, Thouron F, Guerreiro C, Sansonetti PJ, Phalipon A, Mulard LA. 2022. The first-in-human synthetic glycan-based conjugate vaccine candidate against Shigella. ACS Cent Sci 8:449–460. doi: 10.1021/acscentsci.1c01479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. West NP, Sansonetti P, Mounier J, Exley RM, Parsot C, Guadagnini S, Prévost M-C, Prochnicka-Chalufour A, Delepierre M, Tanguy M, Tang CM. 2005. Optimization of virulence functions through glucosylation of Shigella LPS. Science 307:1313–1317. doi: 10.1126/science.1108472 [DOI] [PubMed] [Google Scholar]
- 70. Kubler-Kielb J, Vinogradov E, Chu C, Schneerson R. 2007. O-acetylation in the O-specific polysaccharide isolated from Shigella flexneri serotype 2a. Carbohydr Res 342:643–647. doi: 10.1016/j.carres.2006.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Davis R, Marquart ME, Lucius D, Picking WD. 1998. Protein-protein interactions in the assembly of Shigella flexneri invasion plasmid antigens Ipab and Ipac into protein complexes. Biochim Biophys Acta 1429:45–56. doi: 10.1016/s0167-4838(98)00213-1 [DOI] [PubMed] [Google Scholar]
- 72. Kubler-Kielb J, Vinogradov E, Mocca C, Pozsgay V, Coxon B, Robbins JB, Schneerson R. 2010. Immunochemical studies of Shigella flexneri 2a and 6, and Shigella dysenteriae type 1 O-specific polysaccharide-core fragments and their protein conjugates as vaccine candidates. Carbohydr Res 345:1600–1608. doi: 10.1016/j.carres.2010.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zariri A, van der Ley P. 2015. Biosynthetically engineered lipopolysaccharide as vaccine adjuvant. Expert Rev Vaccines 14:861–876. doi: 10.1586/14760584.2015.1026808 [DOI] [PubMed] [Google Scholar]
- 74. Clément MJ, Imberty A, Phalipon A, Pérez S, Simenel C, Mulard LA, Delepierre M. 2003. Conformational studies of the O-specific polysaccharide of Shigella flexneri 5a and of four related synthetic pentasaccharide fragments using NMR and molecular modeling. J Biol Chem 278:47928–47936. doi: 10.1074/jbc.M308259200 [DOI] [PubMed] [Google Scholar]
- 75. Perepelov AV, Shekht ME, Liu B, Shevelev SD, Ledov VA, Senchenkova SN, L’vov VL, Shashkov AS, Feng L, Aparin PG, Wang L, Knirel YA. 2012. Shigella flexneri O-antigens revisited: final elucidation of the O-acetylation profiles and a survey of the O-antigen structure diversity. FEMS Immunol Med Microbiol 66:201–210. doi: 10.1111/j.1574-695X.2012.01000.x [DOI] [PubMed] [Google Scholar]
- 76. Boutet J, Blasco P, Guerreiro C, Thouron F, Dartevelle S, Nato F, Cañada FJ, Ardá A, Phalipon A, Jiménez-Barbero J, Mulard LA. 2016. Detailed investigation of the immunodominant role of O-antigen stoichiometric O-acetylation as revealed by chemical synthesis, immunochemistry, solution conformation and STD-NMR spectroscopy for Shigella flexneri 3a. Chemistry 22:10892–10911. doi: 10.1002/chem.201600567 [DOI] [PubMed] [Google Scholar]
- 77. Richardson NI, Ravenscroft N, Arato V, Oldrini D, Micoli F, Kuttel MM. 2021. Conformational and immunogenicity studies of the Shigella flexneri serogroup 6 O-antigen: the effect of O-acetylation. Vaccines (Basel) 9:432. doi: 10.3390/vaccines9050432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Noriega FR, Liao FM, Maneval DR, Ren S, Formal SB, Levine MM. 1999. Strategy for cross-protection among Shigella flexneri serotypes. Infect Immun 67:782–788. doi: 10.1128/IAI.67.2.782-788.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Farzam N, Ramon-Saraf R, Banet-Levi Y, Lerner-Geva L, Ashkenazi S, Kubler-Kielb J, Vinogradov E, Robbins JB, Schneerson R. 2017. Vaccination with Shigella flexneri 2a conjugate induces type 2a and cross-reactive type 6 antibodies in humans but not in mice. Vaccine 35:4990–4996. doi: 10.1016/j.vaccine.2017.07.070 [DOI] [PubMed] [Google Scholar]
- 80. Clarkson K, Gutierrez R, Turbyfill KR, Detizio K, Vortherms AR, Lynen A, Barnard B, Weerts H, Porter CK, Maier N, Erdem A, Bourgeois AL, Kaminski RW. Clinical evaluation of Shigella flexneri 2a detoxified artificial invaplex, abstr vaccines against Shigella and ETEC (VASE) conference, p 28–30, Washington, DC, USA: [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Plasmid maps for described recombinant clones and protein stability results.