Abstract
Some viruses induce changes in membrane permeability during infection. We have shown previously that the porcine strain of rotavirus, OSU, induced an increase in the permeability to Na+, K+, and Ca2+ during replication in MA104 cells. In this work, we have characterized the divalent cation entry pathway by measuring intracellular Ca2+ in fura-2-loaded MA104 and HT29 cells in suspension. The permeability to Ca2+ and other cations was evaluated by the change of the intracellular concentration following an extracellular cation pulse. Rotavirus infection induced an increase in permeability to Ca2+, Ba2+, Sr2+, Mn2+, and Co2+. The rate of cation entry decreased over time as the intracellular concentration increased during the first 20 s. This indicates that regulatory mechanisms, including channel inactivation, are triggered. La3+ did not enter the cell and blocked the entry of the divalent cations in a dose-dependent manner. Metoxyverapamil (D600), a blocker of L-type voltage-gated channels, partially inhibited the entry of Ca2+ in virus-infected MA104 and HT29 cells. The results suggest that rotavirus infection of cultured cells activates a cation channel rather than nonspecific permeation through the plasma membrane. This activation involves the synthesis of viral proteins through mechanisms yet unknown. The increase in intracellular Ca2+ induced by the activation of this channel may be related to the increase in cytoplasmic and endoplasmic reticulum Ca2+ pools required for virus maturation and cell death.
A number of viruses induce changes in membrane permeability to ions during infection as a result of gene expression (3). Rotavirus infection of cultured cells modifies the homeostasis of both mono- and divalent cations that appears to be mediated by the synthesis of viral proteins (6, 18). Rotavirus infection provokes an increase in Ca2+ permeation, intracellular [Ca2+] ([Ca2+]i), and sequestered Ca2+ pools (17, 18). The changes in Ca2+ concentration do not seem to be the result of an effect on the Ca2+ pumps of the plasma membrane and the endoplasmic reticulum (ER) but rather seem to be the result of an increase of Ca2+ influx in excess of the regulatory capacity of the cell. Since the increase in permeability to Ca2+ appears long before a generalized modification of the permeability to other molecules, even those as small as ethidium bromide, this appears to be an early and primary effect of infection.
In a search for the viral protein related to the disturbances of Ca2+ homeostasis, Tian et al. (33, 34) studied the effects of the expression of individual recombinant proteins on the Ca2+ concentration in Spodoptera frugiperda cells (Sf9 cells). They found that only the expression of the product of gene 10, the nonstructural protein NSP4, induced a change in Ca2+ concentration in insect cells with no changes in permeability of the plasma membrane to Ca2+. Contrary to the observations of Michelangeli et al. (17, 18) they proposed that the increase in the cytosolic Ca2+ concentration brought about by rotavirus infection was not provoked by an increased Ca2+ entry. According to them, this would be due to the increase in ER membrane permeability to Ca2+ linked to the expression of NSP4 through a phospholipase C-independent pathway (33). In later work they showed that addition of exogenous NSP4 to HT29 cells induced Ca2+ release from inositol 1,4,5-triphosphate-sensitive stores and subsequent Ca2+ entry, depending on the binding of the protein (or a synthetic peptide) to a putative receptor on the plasma membrane (7, 27, 33). These effects were different from those obtained with Sf9 cells expressing NSP4.
In the present work we have characterized the permeability pathway induced by rotavirus infection after the beginning of viral protein synthesis and before the induction of cell lysis. The results indicate that infection of MA104 and HT29 cells activates a pathway of plasma membrane permeability to Ca2+. Several lines of evidence suggest that the pathway for the entry of Ca2+ has the characteristics of a Ca2+ channel rather than nonselective damage of the plasma membrane. Ca2+ entry showed an apparent saturation kinetics with respect to extracellular concentration changes. The entry of different cations followed a selectivity sequence, from Ba2+ to the impermeative La3+. The cation influx was secondarily inactivated by the intracellular cation concentration. The cation pathway was inhibited by La3+ and D600. La3+, Cr3+, and extracellular markers such as ethidium bromide as well as trypan blue did not permeate by this pathway.
MATERIALS AND METHODS
Cell cultures and virus infection.
Fetal monkey kidney cells (MA104) were used in most experiments. In some cases, the colon carcinoma HT29 cells were used. Cells were grown and maintained as previously described (18). The OSU strain of rotavirus was used in all experiments. Infection was carried out for 1 h at 37°C. The inoculum was then removed, washed with phosphate-buffered saline, and further incubated with minimal essential medium (MEM) without fetal calf serum. Infectivity titers of the preparations used were measured by titration in microplates with an anti-VP6 monoclonal antibody (4B2D2) for immunohistochemical staining after methanol fixation (4). Cell infection was performed at a multiplicity of infection of around 20. Under these conditions, all cells in the monolayer were infected during the first cycle as ascertained by immunofluorescence of VP6.
Excitation spectra of fura-2 in the presence of different cations.
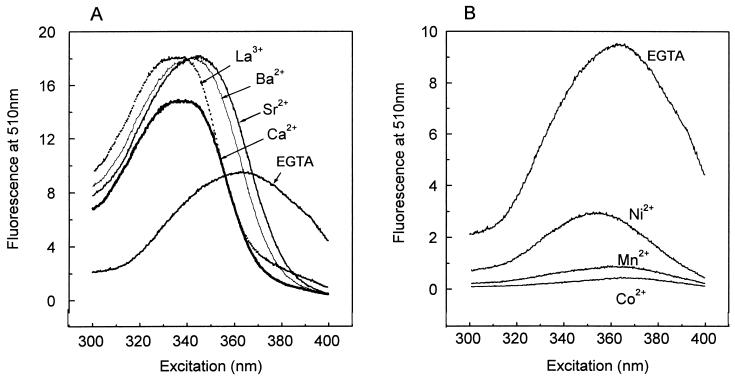
Excitation spectra of fura-2 were obtained at 37°C in a spectrofluorometer (Photon Technology International) equipped with a stirrer and temperature control. The quartz cuvette contained a solution of 10 μM fura-2 free acid, 10 mM HEPES (pH 7.2), and 100 mM KCl supplemented with a saturating concentration of the tested cation. The fluorescence spectrum of fura-2 was also determined in the absence of added cations, in the presence of 10 mM K2H2EGTA. The emission fluorescence was fixed at 510 nm. Figure 1 shows the fluorescence spectrum of fura-2 alone or complexed to the metal cations used in this study. Like binding of Ca2+, binding of Ba2+, Sr2+, and La3+ to fura-2 increases the fluorescence emission at 510 nm when excitation is at wavelengths of from 300 to 360 nm, with a maximum at around 340 nm (Fig. 1A). The isoemissive wavelengths, where fura-2 fluorescence becomes independent of cation concentration, were 356 nm for Ca2+ and La3+ and about 366 nm for Sr2+ and Ba2+ (uncorrected spectra). On the other hand, binding of Mn2+, Ni2+, and Co2+ to fura-2 quenched the fluorescence at all excitation wavelengths tested (Fig. 1B). Based on these spectra, we measured intracellular cation concentrations by using 340/380 nm ratios for Ca2+, Ba2+, Sr2+, and La3+ (see below) and measured the entry of Mn2+, Ni2+, and Co2+ as quenching of fura-2 fluorescence at 360 nm.
FIG. 1.
Fluorescence excitation spectra of fura-2 in the presence of metal cations. Fluorescence excitation spectra of fura-2 in the presence of saturating concentrations of Ca2+, Ba2+, Sr2+, and La3+ (A) or Ni2+, Co2+, and Mn2+ (B) are shown. Fluorescence emission was recorded at 510 nm. Under all conditions, the cuvette contained 1 mM cation in a solution of 10 μM fura-2 free acid, 10 mM HEPES (pH 7.2), and 100 mM KCl. The spectrum of fura-2 in the absence of any cation was obtained in the presence of 10 mM K2H2EGTA in the same medium to chelate traces of contaminant divalent cations. The y-axis scale in panel B has been increased by a factor of two.
Determination of intracellular Ca2+ and other divalent cation concentrations.
The intracellular calcium concentration was measured by using the fluorescent indicator fura-2, which was incorporated intracellularly as its acetoxy-methyl ester (fura-2/AM). Cell monolayers were trypsinized, washed by centrifugation, and resuspended in MEM at an approximate concentration of 2 × 106 cells/ml. Aliquots of the cell suspension were incubated with 10 μM fura-2/AM with pluronic (0.02%) in a medium containing 130 mM NaCl, 5 mM KCl, 1.0 mM CaCl2, 1.0 mM MgCl2, 11 mM glucose, 10.0 mM HEPES (pH 7.4), and 0.1% (wt/vol) albumin. Cell loading of fura-2 was carried out at 37°C for 30 min to enhance dye uptake. Under these conditions it may be possible that fura-2 was taken up and sequestered into intracellular compartments, leading to an overestimation of the [Ca2+]i value. We have evaluated the contribution of this fraction by permeabilization with digitonin followed by solubilization with Triton X-100. The results show that the contribution of compartmentalized dye to the overall signal was not important under the loading conditions used for these cell types. Cells were washed twice by centrifugation to remove extracellular fluorophore and resuspended in 1.2 ml of the same medium without albumin. Fluorescence was measured at 37°C in a spectrofluorometer (Photon Technology International) equipped with a stirrer and temperature control. The excitation wavelengths were 340 and 380 nm, which were alternatively changed by computer control, allowing acquisition of one pair of data per second for experiments with fura-2. The emission wavelength was fixed at 510 nm. The ratio of the fluorescent signals measured at 340 and 380 nm was computer determined. The intracellular free Ca2+ concentration, [Ca2+]i, was evaluated by using an apparent Kd for fura-2–Ca of 224 nM (8). The maximal fluorescence ratio (Rmax) was determined by addition of digitonin (80 μg/ml) to permeabilize cells, and the minimal fluorescence ratio (Rmin) was determined by the subsequent addition of 80 mM EGTA in 0.1 M Tris (pH 7.4) buffer. The intracellular [Ca2+] was calculated according to the equation described by Grynkiewicz et al. (8). Addition of EGTA (10 mM) to the cuvette containing fura-2-loaded cells did not change the baseline fluorescence, indicating that no significant dye leaked out during the measurement period.
Intracellular Ba2+ and Sr3+ concentrations were measured by using cells loaded with fura-2. The cells were prepared as in the case of Ca2+ measurement and were incubated in a medium without Ca2+. The cation concentration was calculated with the same equation as used for Ca2+. The Kds for Ba2+ and Sr2+ used for this were 2.4 and 5.2 μM, respectively (16). The calculations have been made with Rmax obtained with digitonin. These were determined in the presence of the divalent cation and in the absence of extracellular calcium. In this way, we have corrected for differences in affinity and quantum yield, and therefore, concentrations of barium and strontium can be determined.
Assessment of membrane permeability to cations. (i) Step change of extracellular cation concentration.
The relative permeabilities of mock- and virus-infected cells to cations were evaluated by imposing a step increase in the extracellular cation concentration and measuring the rate of change in the intracellular cation concentration during the first few seconds (18). This change is the result of net cation fluxes between the cytoplasm and outside and between the cytoplasm and cation-sequestering organelles. However, the elevation of intracellular cation concentration during the first few seconds (with a linear slope) should be a measurement of cation influx and hence of permeability to the cation. Membrane permeability to Mn2+, Ni2+, and Co2+ was measured by using cells loaded with fura-2. The entry of these cations was monitored by the initial quenching of intracellular fura-2 fluorescence at excitation and emission wavelengths of 360 and 510 nm, respectively. Results are expressed as relative fluorescence quenching with respect to the maximal quenching induced by the addition of digitonin (80 μg/ml).
(ii) 45Ca2+ and 51Cr3+ uptake.
Cell monolayers were grown to confluency in 24-well Linbro plates and infected as described above. At 4, 6, or 8 postinfection, maintenance medium was removed and replaced by 200 μl of MEM containing 45Ca2+ (5 μCi/ml) or 51Cr3+ (50 μCi/ml). Uptake was stopped after 10 min by washing the cells four times by immersion in ice-cold phosphate-buffered saline. After drying, cells were dissolved with 0.25 ml of NaOH (0.1 N) and neutralized with HCl, and radioactivity was determined by liquid scintillation counting. As the number of cells per well was found to be rather constant, uptake values for single experiments are expressed as counts per minute per well.
Statistical analysis.
In most cases, when appropriate, results of representative experiments of a series are shown. In addition, mean values ± standard errors of the means (SEMs) are tabulated for calculated parameters drawn from individual curves. The level of significance of the difference for values obtained for mock- and rotavirus-infected cells was calculated by the Mann-Whitney test. Differences were considered statistically significant when P was <0.05.
RESULTS
Rotavirus infection induces an increase of plasma membrane permeability to Ca2+.
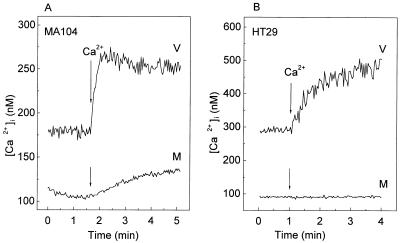
Figure 2 shows the effect of rotavirus infection on [Ca2+]i and membrane permeability to Ca2+ in MA104 and HT29 cells at 7 h postinfection. The basal [Ca2+]i in mock infected cells was around 100 nM for both cell lines. At 7 h postinfection, rotavirus infection had induced an increase in basal Ca2+ concentrations in both MA104 and HT29 cells, which attained a value two or three times higher than that in mock-infected cells.
FIG. 2.
Increase in intracellular Ca2+ concentration and plasma membrane permeability to Ca2+ in rotavirus-infected MA104 and HT29 cells. At 7 h postinfection, MA104 (A) and HT29 (B) monolayers were trypsinized, and cell suspensions were loaded with fura-2 for the measurement of [Ca2+]i (see Materials and Methods). Permeability to Ca2+ in rotavirus (V)- or mock (M)-infected cells was evaluated by the change in [Ca2+]i induced by the addition of 5 mM CaCl2 to the extracellular medium (arrow), which initially contained 1 mM Ca2+. Results of a representative experiment of a series of three for each cell type are shown.
To estimate plasma membrane permeability, a step increase of extracellular Ca2+ from 1 to 6 mM was imposed and the corresponding [Ca2+]i change was monitored. In rotavirus-infected cells, addition of 5 mM Ca2+ to the extracellular bath produced an elevation of [Ca2+]i that was higher than that in the mock-infected control for both cell lines. Elevations in [Ca2+]i and permeability to Ca2+ in rotavirus-infected cells started after 4 h postinfection and increased as infection progressed until 10 h postinfection, when plasma membrane integrity was lost and cells did not accumulate fura-2 (18). In all cases, both mock- and virus-infected MA104 cells appeared to be more permeable to Ca2+ than HT29 cells, as judged by the rate of change of concentration after the step change in the extracellular Ca2+ concentration.
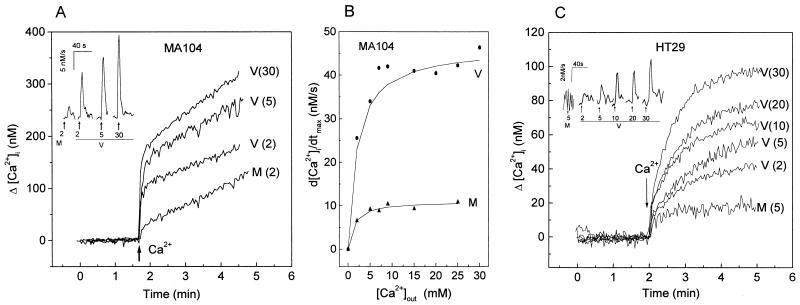
The change in [Ca2+]i as a function of the magnitude of the Ca2+ gradient was studied. Figure 3A and C show representative traces from experiments performed at different extracellular Ca2+ concentrations with virus- and mock-infected MA104 and HT29 cells. In both cell lines, the kinetics of the change in [Ca2+]i was characterized by an initial fast elevation (around 20 s) followed by a second phase with a lower rate. The concentration attained during the first 20 s was a function of the magnitude of the extracellular Ca2+ concentration applied (Table 1), suggesting that the [Ca2+]i elevation is the consequence of an influx from the extracellular space. The rapid phase should approximate the Ca2+ influx from the extracellular space to the cytoplasm, before Ca2+ extrusion mechanisms such as Ca2+ pumps and exchangers could be activated. In addition, the measured changes in concentration would be influenced by the intracellular buffer capacity, which does not permit an absolute determination of the influx rate. After the first phase, [Ca2+]i increased slowly over time and remained elevated over the basal level. At this time, the [Ca2+]i corresponded to a balance between influx and efflux of Ca2+ linked to regulatory mechanisms.
FIG. 3.
Changes of intracellular Ca2+ concentration as a function of the magnitude of the extracellular Ca2+ increase. Intracellular Ca2+ concentration and permeability in rotavirus- and mock-infected MA104 (A) and HT29 (C) cells were determined at 7 h postinfection as detailed in the legend to Fig. 2. Membrane permeability to Ca2+ was evaluated by the change of [Ca2+]i induced by the addition of different Ca2+ concentrations (shown in parentheses, in millimolar) to the extracellular medium, which initially did not contain Ca2+, in rotavirus (V)- and mock (M)-infected cells. The insets in panels A and C show the first derivatives (d[Ca2+]i/dt) of these traces right after the Ca2+ pulse application (arrows). The computer traces for the derivatives have been offset to be able to observe the different curves. The peak value corresponds to the maximal rate of [Ca2+]i increase after Ca2+ addition (d[Ca2+]i/dtmax), which is plotted in panel B as a function of the magnitude of the extracellular step change for a range of Ca2+ concentrations from 2 to 30 mM in virus- and mock-infected MA104 cells. The data points from one experimental series were fitted to a hyperbolic curve by using Microcal Origin 4.00 software. See Table 1 for statistics.
TABLE 1.
Changes of intracellular Ca2+ concentration following an extracellular Ca2+ increase in mock- and rotavirus-infected MA104 cellsa
| Infection | [Ca2+]out addition (mM) | [Ca2+]i change
|
Time to d[Ca2+]i/ dtmax (s) | n | |
|---|---|---|---|---|---|
| Δ amplitude at 20 s (nM) | d[Ca2+]i/dtmax (nM/s) | ||||
| Mock | 2 | 35 ± 2 | 7.2 ± 1.0 | 8.6 ± 0.4 | 3 |
| 5 | 48 ± 4 | 8.2 ± 0.5 | 9.7 ± 0.4 | 8 | |
| 30 | 63 ± 6 | 10.5 ± 1.1 | 9.8 ± 0.6 | 5 | |
| Virus | 2 | 98 ± 5* | 23.6 ± 1.4* | 7.5 ± 0.4† | 3 |
| 5 | 188 ± 21* | 39.7 ± 4.2* | 9.8 ± 0.9† | 8 | |
| 30 | 214 ± 47* | 50.2 ± 10.8* | 9.2 ± 0.4† | 5 | |
Intracellular Ca2+ concentration and permeability in mock- and rotavirus-infected MA104 cells were determined at 7 h postinfection as detailed in the legend to Fig. 3, with extracellular Ca2+ concentration changes of 2, 5, and 30 mM. The change in [Ca2+]i was determined in each individual experiment at 20 s after the change in extracellular Ca2+ concentration (Δ amplitude). The peak value of the first derivative of the original traces corresponds to the maximal rate of [Ca2+]i increase after Ca2+ addition (d[Ca2+]i/dtmax). The time from Ca2+ addition to attain this last value was computer determined in each experiment from derivative curves like the ones shown in Fig. 3. Values are means ± SEMs from n experiments in each case. *, significantly different from the corresponding value for mock-infected cells by the Mann-Whitney test (P < 0.05); †, not significantly different from the corresponding value for mock-infected cells.
In order to estimate the initial rates of Ca2+ entry, we have calculated the first derivatives of the [Ca2+]i signals as a function of time, up to 30 s after the change in extracellular Ca2+ concentration. This is shown in the insets of Fig. 3A and C, and the average values for the experimental series are given in Table 1. The maximum rate of change (d[Ca2+]i/dt) was attained in the first 8 to 10 s in both virus- and mock-infected cells for all Ca2+out step changes in concentration tested. In Fig. 3B and Table 1, maximal values of the slope of the increase in [Ca2+]i for MA104 cells are given as a function of the magnitude of the extracellular step change for a range of Ca2+ concentrations from 2 to 30 mM. Both mock- and virus-infected cells show apparent saturation kinetics. For virus-infected cells, an apparent maximal velocity of change of 47 nM · s−1 and an apparent affinity of 0.72 mM were estimated from double-reciprocal plots (not shown). The values for mock-infected cells were 11 nM · s−1 and 0.61 mM, respectively. The differences between mock- and virus-infected cells in [Ca2+]i attained and maximal velocity of [Ca2+]i change after the extracellular concentration increase were statistically significant (Table 1). Therefore, virus infection seems to induce a change in maximal velocity without a modification of the apparent affinity.
La3+ and D600 block Ca2+ entry stimulated by infection.
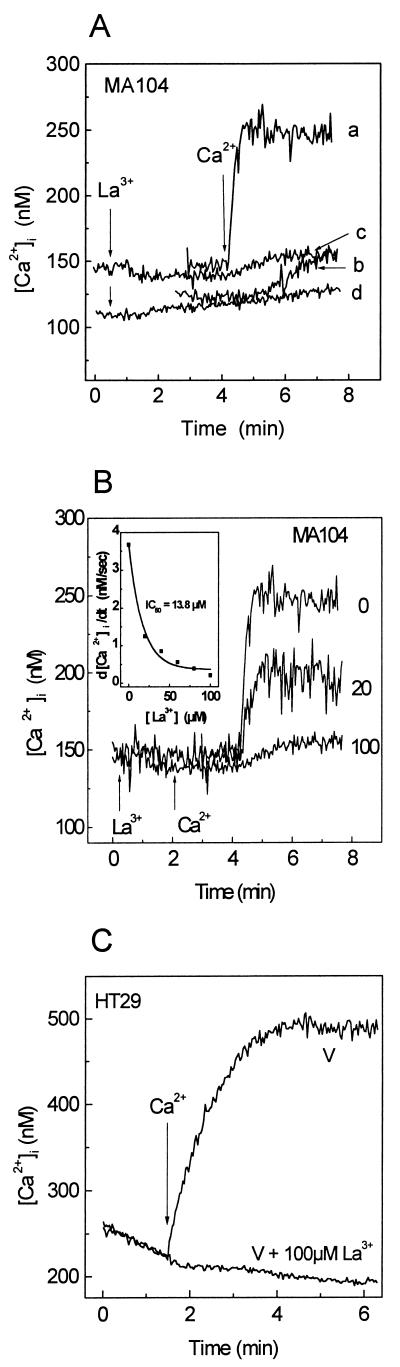
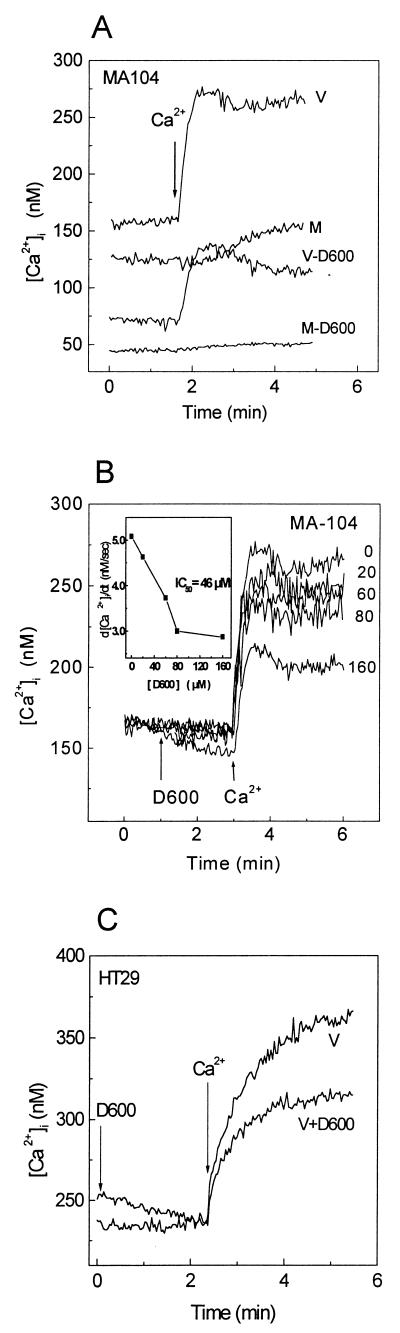
As lanthanum is a well-known inorganic calcium channel blocker, we have tested its effects on Ca2+ entry. As shown in Fig. 4A, addition of 100 μM LaCl3 to mock- or virus-infected MA104 cells did not alter fura-2 fluorescence, indicating that this cation did not enter these cells. Addition of 5 mM Ca2+ to virus- and mock-infected cells in the presence of La3+ did not induce the intracellular Ca2+ rise observed in controls. These results indicate that extracellular La3+ blocked calcium entry in both mock- and virus-infected cells. Similar results were obtained with HT29 cells (Fig. 4C). Removal of extracellular La3+ resulted in the complete reversal of the inhibitory effect (not shown). The blockade of Ca2+ influx by La3+ was concentration dependent, affecting the initial rate of Ca2+ entry (d[Ca2+]i/dt) and the maximal concentration attained after the change (Fig. 4B), with a calculated 50% inhibitory concentration of 13.8 μM (Fig. 4B, inset).
FIG. 4.
La3+ blocks Ca2+ entry induced by rotavirus infection. MA104 and HT29 cell monolayers were trypsinized at 7 h postinfection and loaded with fura-2. The cells were resuspended in 1 ml of medium containing 1 mM Ca2+. (A) Effect of La3+ on Ca2+ entry in rotavirus (a and c)- and mock (b and d)-infected cells. Addition of 100 μM LaCl3 (c and d) and 5 mM Ca2+ (a, b, c, and d) to cell suspensions is indicated by arrows. (B) Dose-response curve for inhibition of Ca2+ entry by La3+ in rotavirus-infected MA104 cells. La3+ concentrations (in micromolar) are indicated at the right of each curve. The inset corresponds to the peak rate of change in [Ca2+]i during the first 15 s following addition of extracellular Ca2+ (5 mM) in the presence of different La3+ concentrations (d[Ca2+]i/dtmax), calculated as described for Fig. 3. (C) Inhibition of Ca2+ entry by La3+ in rotavirus-infected HT29 cells. La3+ (100 μM) was added at time zero, and 5 mM Ca2+ was added to the medium for both untreated and La3+-treated infected cells. Results of a representative experiment of a series of three are shown in each case. IC50, 50% inhibitory concentration.
Metoxyverapamil (D600) blocks L-type voltage-dependent Ca2+ channels (10). This compound (80 μM) added for 30 min to MA104 cells infected for 7 h, induced a decrease in basal [Ca2+]i (Fig. 5A). Furthermore, D600 inhibited permeability to Ca2+ as evidenced by the addition of an extracellular Ca2+ pulse (5 mM) in both mock- and virus-infected cells, reducing the initial rate of Ca2+ entry (d[Ca2+]i/dt) and the plateau level. These effects were dose dependent, with a 50% inhibitory concentration of 46 μM (Fig. 5B). The decrease in basal [Ca2+]i suggests that after the reduction of membrane permeability by D600, cytoplasmic Ca2+ was brought to a new steady state by regulatory mechanisms still operative at these times postinfection. Partial inhibition of Ca2+ entry by D600 (80 μM) was also observed in rotavirus-infected HT29 cells (Fig. 5C).
FIG. 5.
Metoxyverapamil (D600) inhibits Ca2+ entry induced by rotavirus infection. MA104 and HT29 cell monolayers were trypsinized at 7 h postinfection and loaded with fura-2. (A) Cells were preincubated for 30 min with 160 μM D600 (during fura-2 loading), and then the cells were washed and resuspended in 1 ml of medium containing 1 mM Ca2+ and 160 μM D600 and the free Ca2+ concentration was measured. Addition of 5 mM Ca2+ to evaluate membrane permeability is indicated by an arrow. (B) Effect of different concentrations of D600 (added at the time indicated by the arrow) on the change in [Ca2+]i induced by the addition of 5 mM Ca2+. The inset corresponds to peak values of the first derivatives (d[Ca2+]i/dtmax), calculated as described for Fig. 3, plotted as a function of D600 concentration. The experimental points are joined by straight lines. (C) Inhibition of Ca2+ entry by D600 in rotavirus-infected HT29 cells. Addition of 80 μM D600 and 5 mM Ca2+ is indicated by arrows. Results of a single experiment representative of two are shown in each case. V, virus-infected cells; M, mock-infected cells; IC50, 50% inhibitory concentration.
Permeability of the plasma membrane to metal cations in virus-infected MA104 cells.
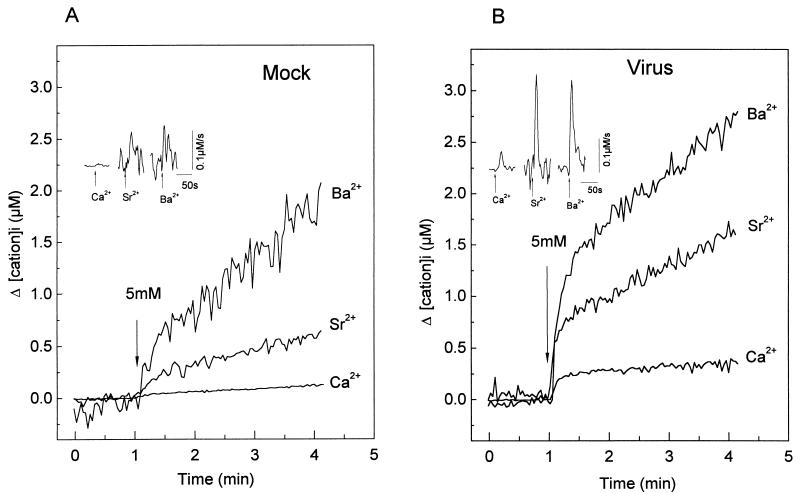
Having demonstrated a Ca2+ permeability pathway induced by infection, we studied its selectivity for other divalent cations. For this purpose, we took advantage of the changes in fluorescence of fura-2 upon binding to various metal cations. Figure 6 shows the comparison of the changes in intracellular Ca2+, Ba2+, and Sr2+ concentrations induced by an extracellular cation pulse (5 mM) in suspensions of mock- and virus-infected MA104 cells preincubated in the absence of nominal free Ca2+. The intracellular concentrations presented in the curves correspond to the change induced by the pulse and were calculated by using the respective Kds for the cations. The basal level before addition of the pulse, corresponding to the intracellular free Ca2+ concentration, was subtracted from the curve.
FIG. 6.
Comparison of the permeabilities of the plasma membrane to Ca2+, Ba2+, and Sr2+ in mock- and rotavirus-infected MA104 cells. At 7 h postinfection, the intracellular cation concentration in mock (A)- or rotavirus (B)-infected MA104 cells loaded with fura-2 was measured. The cells were suspended in a medium (1 ml) without added Ca2+ prior to the experiment. Cations were added to the cuvette at a final concentration of 5 mM at the time indicated by the arrows. The intracellular cation concentration was calculated by using fura-2 fluorescence ratios monitored at 340- and 380-nm excitation wavelengths and by using 224 nM, 2.4 μM, and 5.2 μM as Kds for Ca2+, Ba2+, and Sr2+, respectively, and the Rmax was determined by addition of digitonin in the presence of the cation. Variations in cation concentration are shown and were calculated by subtracting the initial basal value, just before the addition of the cation. The insets show the first derivatives of these traces (d[Ca2+]i/dtmax) right after cation addition (arrows). The computer traces for the derivatives have been offset to be able to observe the different curves. The peak value corresponds to the maximal rate of intracellular concentration change after cation addition. The experiments shown were performed with the same batch of cells for all ions and both mock- and virus-infected cells. See Table 2 for statistics.
As shown for Ca2+, addition of a 5 mM Ba2+ or Sr2+ pulse induced a fast increase in intracellular concentration in virus-infected cells, whereas this change was notably smaller in mock-infected cells. The kinetics of [Ba2+]i and [Sr2+]i elevation in virus-infected cells were biphasic and similar to that observed for Ca2+. The first derivatives of the curves show that the maximum rate of increase was attained 8 to 12 s following the pulse for all cations in both mock- and virus-infected cells (Fig. 6, insets; Table 2). The maximal slope (dCation/dtmax) attained was higher in virus-infected than in mock-infected cells. Furthermore, the magnitudes of the intracellular concentration changes during the first phase differed among the divalent cations tested. The [Sr2+]i and [Ba2+]i reached at 20 s after the pulse were approximately 10 times higher than that observed with Ca2+. Also, the slope of the change was significantly higher for Sr2+ and Ba2+ than for Ca2+ in both mock- and virus-infected cells (Table 2). This could be explained by differences in permeability to cations of the virus-activated entry pathway. As in the case of Ca2+ influx, a second, slow phase of increase of intracellular cation concentration was observed. The change in slope should be due to the activation of cation removal mechanisms and/or reduction of cation entry. For each of the three ions, the contributions of these mechanisms of regulation might be different.
TABLE 2.
Comparison of the permeabilities of the plasma membrane to Ca2+, Ba2+, and Sr2+ in mock-and rotavirus-infected MA104 cellsa
| Infection | Cation | [Cation2+]i change
|
Time to d [Cation2+]i/ dtmax (s) | n | |
|---|---|---|---|---|---|
| Δ amplitude at 20 s (nM) | d[Cation2+]i/ dtmax (nM/s) | ||||
| Mock | Ca2+ | 44 ± 3 | 7.7 ± 0.5 | 9.8 ± 0.8 | 4 |
| Ba2+ | 431 ± 63 | 77.8 ± 15.3 | 9.0 ± 0.1 | 3 | |
| Sr2+ | 365 ± 92 | 113.0 ± 18.6 | 9.0 ± 0.7 | 3 | |
| Virus | Ca2+ | 228 ± 23* | 48.0 ± 4.7* | 11.3 ± 1.9† | 4 |
| Ba2+ | 1,226 ± 144* | 298.8 ± 92.2* | 10.7 ± 1.7† | 3 | |
| Sr2+ | 894 ± 55* | 204.7 ± 34.9* | 11.7 ± 2.2† | 3 | |
At 7 h postinfection, changes in intracellular cation concentration in mock- and rotavirus-infected MA104 cells were measured as detailed in the legend to Fig. 6. The change in intracellular cation concentration was determined in each individual experiment at 20 s after a 5 mM change in extracellular cation concentration (Δ amplitude). The peak value of the first derivative of the original traces corresponds to the maximal rate of [Cation2+]i increase after cation addition (d[Cation2+]i/dtmax). The time from the cation addition to attain this last value was computer determined in each experiment from derivative curves like the ones shown in Fig. 6. Values are means ± SEMs from n experiments in each case. *, significantly different from the corresponding value for mock-infected cells by the Mann-Whitney test (P < 0.05); †, not significantly different from the corresponding value for mock-infected cells.
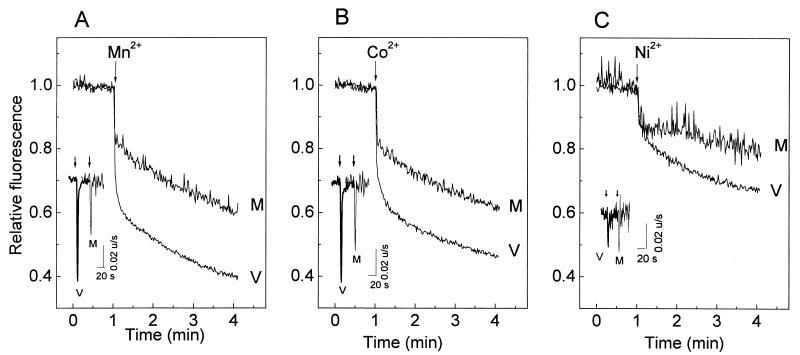
Intracellular fura-2 fluorescence quenching due to the entry of Mn2+, Co2+, and Ni2+ into the cytoplasm of MA104 cells is presented as relative changes, with maximal quenching estimated with permeabilization by digitonin (Fig. 7). This permits correction for differences in fura-2 loading and quantum efficiency of the fluorophore in the presence of different cations. Mn2+ and Co2+ induced a biphasic quenching of fura-2 fluorescence. The kinetics of this response was characterized by an initial fast fluorescence quench which was larger in virus-infected than in mock-infected cells. The second phase had a lower rate and was similar in both cases. The first derivatives of the fluorescence signals indicate that maximum rates of fluorescence quenching were attained in the first 8 to 12 s following the cation pulse (Fig. 7, insets). The similarity of the changes and kinetics described for the cations tested (Mn2+, Co2+, Ba2+, Sr2+, and Ca2+) suggests that all of these divalent cations enter the cytoplasm of virus-infected cells by the same pathway. On the other hand, no significant differences between virus- and mock-infected cells were observed in fluorescence quenching with Ni2+, suggesting that the pathway induced by the infection is less permeable to this cation. As in the case of Ca2+, La3+ blocked Ba2+ and Mn2+ entry in both mock- and virus-infected cells (Fig. 8).
FIG. 7.
Comparison of the permeabilities of the plasma membrane to Mn2+, Ni2+, and Co2+ in rotavirus- and mock-infected MA104 cells. At 7 h postinfection, MA104 cells were trypsinized, loaded with fura-2, washed, and resuspended in medium (1 ml) without added Ca2+. Cations were added to the cuvette at a final concentration of 5 mM (5 μl of a 1 M solution) at the times indicated by the arrows. Mn2+, Ni2+, and Co2+ entry was measured by the quenching of fluorescence with an excitation wavelength of 356 nm, the isoemissive wavelength for Ca2+, and an emission wavelength of 510 nm. The curves represent fluorescence relative to total quenching attained by the addition of digitonin to saturate fura-2 with the indicated quenching cation. The insets show the first derivatives of these traces right after the cation pulse application (arrows). The computer traces for the derivatives have been offset to be able to observe the different curves. The peak value corresponds to the maximal rate of intracellular fluorescence change after cation addition. The experiments shown were performed with the same batch of cells for all ions and for both mock (M)- and virus (V)-infected cells. Results of a representative experiment of a series of four are shown.
FIG. 8.
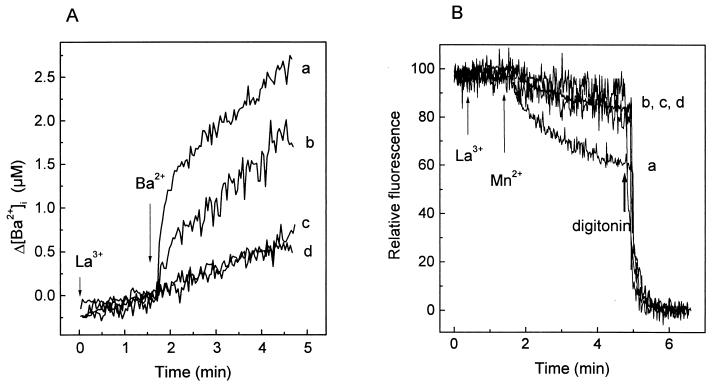
Effect of La3+ on Ba2+ and Mn2+ entry in mock- and virus-infected MA104 cells. MA104 cell monolayers were trypsinized at 7 h postinfection, loaded with fura-2, washed, and resuspended in 1 ml of medium without added Ca2+ (nominally Ca2+ free). (A) Inhibition of Ba2+ entry by La3+ in mock- and virus-infected cells. Addition of 100 μM LaCl3 (c and d) and 5 mM Ba2+ (a, b, c, and d) to rotavirus (a and c)- and mock (b and d)-infected MA104 cells is indicated by the arrows. The variation in cation concentration is shown and was calculated by subtracting the initial basal value, just before the addition of the cation. (B) Inhibition of Mn2+ entry by La3+ in virus- and mock-infected cells. Experiments were performed as described for panel A. The addition of 100 μM LaCl3 (c and d) and 5 mM Mn2+ (a, b, c, and d) is indicated by the arrows. Maximal quenching was attained by addition of 10 μM digitonin in all cases.
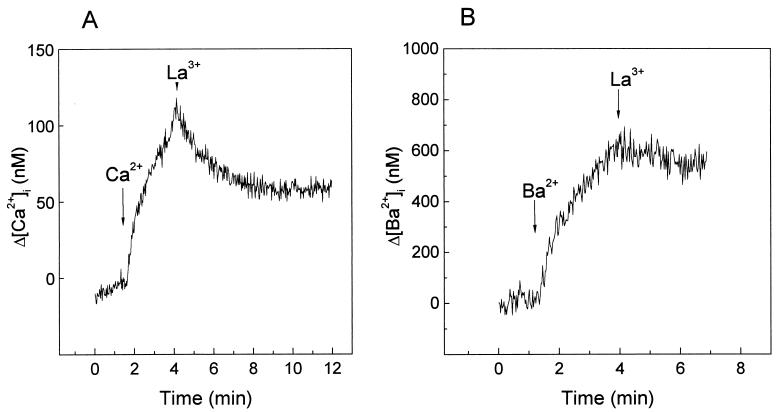
As La3+ blocked divalent cation entry in infected MA104 and HT29 cells (Fig. 4 and 8), we have used this property to further study Ca2+ and Ba2+ regulation. Previous addition of La3+ to the incubation medium also blocked Ba2+ entry in both mock- and virus-infected HT29 cells (not shown). La3+ added to HT29 cells after the fast increase in Ca2+ concentration induced by the elevation of extracellular Ca2+ stopped influx and induced a decrease in [Ca2+]i. This decay should correspond to the removal of excess cytoplasmic Ca2+ by the activation of regulatory mechanisms (Fig. 9A). In the case of Ba2+, La3+ also stopped the increase by blocking entry, but its concentration stayed high and constant (Fig. 9B). This indicates that, in contrast to Ca2+, Ba2+ was not removed by extrusion mechanisms. Thus, the change in the slope of Ba2+ entry after about 10 s (Fig. 6) should not be due to the regulation of concentration by pumps and exchangers. Rather, this change of slope may correspond to a closure of the cation entry pathway.
FIG. 9.
Effect of La3+ on the regulation of Ca2+ and Ba2+ intracellular concentrations in rotavirus-infected HT29 cells. HT29 cell monolayers were trypsinized at 7 h postinfection and loaded with fura-2. The cells were washed and resuspended in 1 ml of medium containing 1 mM Ca2+. Arrows indicate the time of addition of 5 mM CaCl2 (A) or BaCl2 (B) and of 100 μM LaCl3 (A and B). Variations in cation concentrations are shown and were calculated by subtracting the initial basal value, just before the addition of the cation. Results of a representative experiment in a series of two are shown.
To further study the specificity of the divalent cation pathway we measured 51Cr3+ uptake, which cannot be detected by fura-2 fluorescence. Long incubation times were needed to detect 51Cr3+ uptake in both mock- and virus-infected cells. Accumulation of 51Cr3+ during a period of 10 min was not different in both mock- and virus-infected cells at 4, 6, and 8 h postinfection. By contrast, 45Ca2+ uptake (measured by the same technique) increased in virus-infected cells as infection progressed (Table 3). The fact that the cell membrane is impermeable to chromium and other molecules, such as ethidium bromide, at these times postinfection (18) indicates that Ca2+ entry activated by rotavirus infection is not due to damage of the cell membrane.
TABLE 3.
Uptake of 45Ca2+ and 51Cr3+ in mock- and rotavirus-infected MA104 cellsa
| Infection | Cation | cpm at:
|
||
|---|---|---|---|---|
| 4 hpi | 6 hpi | 8 hpi | ||
| Mock | 45Ca2+ | 223 ± 9.7 | 174 ± 13.9 | 234 ± 21.0 |
| 51Cr3+ | 118 ± 4.3 | 89.3 ± 4.6 | 102 ± 4.6 | |
| Virus | 45Ca2+ | 225 ± 15.4† | 256 ± 11.5* | 532 ± 21.0* |
| 51Cr3+ | 104 ± 1.9† | 105 ± 4.8† | 117 ± 8.3† | |
| Ratio (virus/mock) | 45Ca2+ | 1.0 | 1.5 | 2.3 |
| 51Cr3+ | 0.9 | 1.2 | 1.1 | |
MA104 cell monolayers were grown to confluency in 24-well Linbro plates and infected. At 4, 6, or 8 h postinfection (hpi), 45Ca2+ or 51Cr3+ uptake was determined (see Materials and Methods for details). The time of uptake was 10 min in each case. Values correspond to the means ± SEMs from four independent measurements in each case. The virus/mock ratio was calculated from the mean values for each ion at the different times postinfection. *, significantly different from the corresponding value for mock-infected cells by the Mann-Whitney test (P < 0.05); †, not significantly different from the corresponding value for mock-infected cells.
DISCUSSION
We have previously reported that during the course of rotavirus infection of MA104 cells, there was a progressive increase in membrane permeability to molecules of increasing size. At early times after initiation of viral protein synthesis (4 to 6 h postinfection), permeability to monovalent cations such as Na+ and K+, as well as Ca2+, increased. Later, after 8 h postinfection, molecules which are normally impermeative in an uninfected cell, such as ethidium bromide and trypan blue, entered (17, 18).
The permeability pathway for Ca2+ in infected cells was studied by using step changes of extracellular Ca2+ concentration. Studies using fluorescent indicators have shown that the initial transient increase in [Ca2+]i following the extracellular Ca2+ change reflects the influx of Ca2+ from the external compartment (31). The fast increase in [Ca2+]i in response to Ca2+ addition appeared to be directly related to the influx pathway, since it varied with the driving force for Ca2+ entry. The elevation of the apparent Vmax for Ca2+ entry in the virus-infected cell without a change in the apparent affinity constant for Ca2+ may suggest that infection induced the activation of a cellular Ca2+ channel.
The selectivity of the Ca2+ entry pathway induced by rotavirus infection was characterized by using a set of cations that are known to permeate different Ca2+ channels in other systems (12, 23, 31). We took advantage of the ability of fura-2 to complex to metal cations other than Ca2+ and the possibility of calculating actual intracellular concentrations with the known Kds (16). The Ca2+ pathway in both mock- and rotavirus-infected cells was permeable to other divalent cations, such as Ba2+, Sr2+, Mn2+, and Co2+. However, it was poorly permeable to Ni2+ and impermeable to the trivalent cations La3+ and Cr3+. These characteristics are common to numerous divalent cation channels in both excitable and nonexcitable cells (10, 14, 19, 24, 31). The influx pathway exhibited an apparent sequence of Sr2+ ≈ Ba2+ > Ca2+, which may reflect the actual permeability sequence. No quantitative comparison was possible with the quenching cations Mn2+ and Co2+, since determination of concentrations was not feasible. In all cases rotavirus-infected cells showed a much higher permeability than mock-infected ones.
The intracellular concentrations of all permeative cations increased upon addition of an extracellular pulse according to a similar temporal pattern. After an initially fast (1 to 2 min) elevation, the increase of intracellular concentration attained a new rate characterized by a smaller slope, in both mock- and virus-infected cells. The second phase should be the result of a balance between cation influx and efflux, both of which are subject to regulation. The rate of influx can be modulated by a change in the intracellular cation concentration which may lead to a closure of the pathway (10, 24). At the same time, regulatory mechanisms governing the efflux would be activated. When we compared the slopes of the second phase, that for Ba2+ was much higher than that for Ca2+. It has been shown that Ba2+ does not replace Ca2+ as a substrate for the Ca2+-ATPases of the ER or the plasma membrane or for the Na+/Ca2+ exchanger (35). The results obtained with the addition of La3+ after the pulse of Ba2+ and Ca2+ further support this interpretation. La3+ added after the peak of the response to an extracellular Ca2+ pulse induced a decrease in fluorescence. Since La3+ blocks cation influx, this decrease should reveal the removal of excess Ca2+ from the cytosol by regulatory mechanisms. In the case of Ba2+, La3+ blocked the entry and the concentration remained constant. Excess Ba2+ that entered before the addition of La3+ was not removed from the cytoplasm by the pumps. Therefore, the change in the kinetics of [Ba2+]i should reflect a time-dependent inhibition of the influx pathway, as has been described for other systems (31). In the case of Ca2+, reduction in the influx pathway as well as activation of the efflux component results in regulation of [Ca2+]i to a new steady state.
Calcium channel blockers are commonly used to characterize the nature of Ca2+ pathways. The majority of normal epithelial cells do not contain voltage-gated calcium channels. However, L-type Ca2+ channels have been detected in renal cells of the proximal and distal tubules and Henle’s loop (25, 26, 28, 37), and malignant transformation seems to induce the expression of this type of channel in the pancreatic tumor cell line AR4-2J (2, 5). Also, Ca2+ influx induced by carbachol is inhibited by verapamil in the intestinal carcinoma HT29 cell line (21). We do not know if mock-infected cells contain a channel sensitive to D600. The Ca2+ influx pathway in rotavirus-infected cells was partially inhibited by D600. We cannot claim at this point that an L-type Ca2+ channel is involved in the Ca2+ influx pathway induced by rotavirus, because the concentrations of D600 required to cause inhibition are higher than those required for the inhibition of L-type channels. However, infection may have induced the expression or activation of such a channel. Preliminary results obtained by using the patch clamp technique with MA104 cells suggest that infection activates a voltage-dependent Ca2+ channel (24a). If this is confirmed, infection may have induced depolarization by increasing the permeability of the cell membrane to Na+ and K+ and thereby increasing a subsequent Ca2+ influx (6).
It has been proposed that the increased Ca2+ uptake in virus-infected cells could be secondary to depletion of ER stores through the so-called capacitative pathway induced by the expression of NSP4 (7, 27, 33). However, evidence argues against this possibility: (i) the emptying of Ca2+ from the ER would not be compatible with rotavirus maturation and stability (17, 29); (ii) there is an increase in radioactive Ca2+ pools, sensitive to thapsigargin, in rotavirus-infected cells (17, 18); (iii) depletion of the stores by thapsigargin in mock-infected cells provoked a capacitative entry of Ca2+ much smaller than that induced by rotavirus infection (17); and (iv) the cation selectivity profile of the capacitative channel, Ca2+ > Ba2+ = Sr2+, is not exhibited by the rotavirus-activated pathway (24). Therefore, the Ca2+ pathway activated during rotavirus infection appears to be different from the capacitative one. A possible explanation for the discrepancy is that NSP4 expression in Sf9 cells may have effects on ER calcium different from those induced by the expression of the entire genome in infected mammalian cell lines. However, at later times postinfection, when cytoplasmic Ca2+ has already increased and pool depletion may have occurred, both mechanisms may be operative.
Several lines of evidence suggest that the pathway for the entry of Ca2+ has the characteristics of a channel rather than of unspecific damage: (i) apparent saturation kinetics with respect to extracellular concentration; (ii) entry of cations following an apparent selectivity sequence, from Ba2+ to the impermeative La3+; (iii) inactivation of the influx induced by the cation; (iv) inhibition of Ca2+ influx by La3+ and D600; and (v) lack of permeability of the pathway to La3+, Cr3+, and extracellular markers such as ethidium bromide as well as trypan blue. Although a cellular Ca2+ channel preexisting in the cell might be activated by rotavirus infection, it is also possible that a viral protein synthesized during infection and inserted in the membrane acts as a Ca2+ channel. Viral proteins in other systems, such as the M2 protein of influenza A virus, function as ion channels (15).
Changes in the permeability of the plasma membrane to Ca2+ have been shown to exist in other viral infections and may be a general mechanism for the induction of cytotoxicity. An increase in calcium permeation and/or cytosolic concentration has been found to occur as a result of infection by cytomegalovirus (22), measles and vaccinia viruses (30, 32), coxsackievirus (36), and poliovirus (11). In some cases this could be linked to viral gene expression (22, 32). The expression of recombinant viral proteins such as the A38L protein of vaccinia virus (30), the 2B protein of cosxackievirus (36), or the 2BC protein of poliovirus (1) induced alterations in intracellular Ca2+ homeostasis. In the case of coxsackievirus, it appears that Ca2+ entry is linked to depletion of ER stores (36). Also, poliovirus infection activates phospholipase C, inositol 1,4,5-triphosphate synthesis, and probably the capacitative entry of Ca2+ (1, 9). The external addition of individual viral proteins like gp120 and gp160 of human immunodeficiency virus also potentiates agonist-induced Ca2+ entry (13). The sensitivity of this permeability to verapamil and inhibitors of the dihydropyridine family may suggest the participation of voltage-gated channels in some systems (11, 13, 32).
Regardless of the molecular mechanism of Ca2+ entry, alterations of membrane permeability to Ca2+ of the rotavirus-infected cell could have important physiological consequences. Increases in [Ca2+]i during rotavirus infection have been associated with cytotoxicity in MA104 cells (17, 18, 24a). Also, the expression of NSP4 in mammalian cells, using a vaccinia virus vector, induced cytotoxicity (20). This effect may have resulted from an increase in [Ca2+]i. Whether the cytotoxic effects of NSP4 expression and those provoked by increases in [Ca2+]i are related is not yet known. The viral protein involved in the chain of events leading to the activation of permeability to Ca2+ during infection of cultured cells remains to be defined.
ACKNOWLEDGMENTS
This work was supported in part by grants from CONICIT (S1-95000520) Venezuela and the INCO program of the European Community (ERB3514PL950019).
We thank Ferdinando Liprandi for constructive criticisms and Aleida Sanchez for technical assistance.
REFERENCES
- 1.Aldabe R, Irurzun A, Carrasco L. Poliovirus protein 2BC increases cytosolic free calcium concentrations. J Virol. 1997;71:6214–6217. doi: 10.1128/jvi.71.8.6214-6217.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertrand V, Bastie M J, Bouisson M, Vaysse N, Pradayrol L. AR4-2J cell line coexpresses dihydropyridine and omega-conotoxin sensitive Ca2+ channels. Cell Calcium. 1996;19:495–500. doi: 10.1016/s0143-4160(96)90058-5. [DOI] [PubMed] [Google Scholar]
- 3.Carrasco L. Modification of membrane permeability by animal viruses. Adv Virus Res. 1995;45:61–112. doi: 10.1016/S0065-3527(08)60058-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ciarlet M, Ludert J E, Liprandi F. Comparative amino acid sequence analysis of the major outer capsid protein (VP7) of porcine rotaviruses with G3 and G5 serotype specificities isolated in Venezuela and Argentina. Arch Virol. 1995;140:437–451. doi: 10.1007/BF01718422. [DOI] [PubMed] [Google Scholar]
- 5.Cui Z J. Types of voltage-dependent calcium channels involved in high potassium depolarization-induced amylase secretion in the exocrine pancreatic tumour cell line AR4-2J. Cell Res. 1998;8:23–31. doi: 10.1038/cr.1998.3. [DOI] [PubMed] [Google Scholar]
- 6.del Castillo J R, Ludert J E, Sanchez A, Ruiz M C, Michelangeli F, Liprandi F. Rotavirus infection alters Na+ and K+ homeostasis in MA-104 cells. J Gen Virol. 1991;72:541–547. doi: 10.1099/0022-1317-72-3-541. [DOI] [PubMed] [Google Scholar]
- 7.Dong Y, Zeng C Q, Ball J M, Estes M K, Morris A P. The rotavirus enterotoxin NSP4 mobilizes intracellular calcium in human intestinal cells by stimulating phospholipase C-mediated inositol 1,4,5-triphosphate production. Proc Natl Acad Sci USA. 1997;94:3960–3965. doi: 10.1073/pnas.94.8.3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grynkiewicz G, Poenie M, Tsien R Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 9.Guinea R, Carrasco L. Phospholipid biosynthesis and poliovirus genome replication, two coupled phenomena. EMBO J. 1990;9:2011–2016. doi: 10.1002/j.1460-2075.1990.tb08329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hille B. Ionic channels of excitable membranes. 2nd ed. Sunderland, Mass: Sinauer Associates Inc.; 1992. [Google Scholar]
- 11.Irurzun A, Arroyo J, Alvarez A, Carrasco L. Enhanced intracellular calcium concentration during poliovirus infection. J Virol. 1995;69:5142–5146. doi: 10.1128/jvi.69.8.5142-5146.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones K T, Sharpe G R. Ni2+ blocks the Ca2+ influx in human keratinocytes following a rise in extracellular Ca2+ Exp Cell Res. 1994;212:409–413. doi: 10.1006/excr.1994.1161. [DOI] [PubMed] [Google Scholar]
- 13.Lannuzel A, Lledo P M, Lamghitnia H O, Vincent J D, Tardieu M. HIV-1 envelope proteins gp120 and gp160 potentiate NMDA-induced [Ca2+]i increase, alter [Ca2+]i homeostasis and induce neurotoxicity in human embryonic neurons. Eur J Neurosci. 1995;7:2285–2293. doi: 10.1111/j.1460-9568.1995.tb00649.x. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y J, Gylfe E. Store-operated Ca2+ entry in insulin-releasing pancreatic beta-cells. Cell Calcium. 1997;22:277–286. doi: 10.1016/s0143-4160(97)90066-x. [DOI] [PubMed] [Google Scholar]
- 15.Marsh M. Keeping the viral coat on. Curr Biol. 1992;2:379–381. doi: 10.1016/0960-9822(92)90080-t. [DOI] [PubMed] [Google Scholar]
- 16.McCormack J G, Osbaldeston N J. The use of the Ca2(+)-sensitive intramitochondrial dehydrogenases and entrapped fura-2 to study Sr2+ and Ba2+ transport across the inner membrane of mammalian mitochondria. Eur J Biochem. 1990;192:239–244. doi: 10.1111/j.1432-1033.1990.tb19221.x. [DOI] [PubMed] [Google Scholar]
- 17.Michelangeli F, Liprandi F, Chemello M E, Ciarlet M, Ruiz M C. Selective depletion of stored calcium by thapsigargin blocks rotavirus maturation but not the cytopathic effect. J Virol. 1995;69:3838–3847. doi: 10.1128/jvi.69.6.3838-3847.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michelangeli F, Ruiz M C, del Castillo J R, Ludert J E, Liprandi F. Effect of rotavirus infection on intracellular calcium homeostasis in cultured cells. Virology. 1991;181:520–527. doi: 10.1016/0042-6822(91)90884-e. [DOI] [PubMed] [Google Scholar]
- 19.Negulescu P A, Machen T E. La3+ and pH sensitivity of Ca2+ entry and intracellular store filling in gastric parietal cells. Am J Physiol. 1995;269:G770–G778. doi: 10.1152/ajpgi.1995.269.5.G770. [DOI] [PubMed] [Google Scholar]
- 20.Newton K, Meyer J C, Bellamy A R, Taylor J A. Rotavirus nonstructural glycoprotein NSP4 alters plasma membrane permeability in mammalian cells. J Virol. 1997;71:9458–9465. doi: 10.1128/jvi.71.12.9458-9465.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nitschke R, Leipziger J, Greger R. Agonist-induced intracellular Ca2+ transients in HT29 cells. Pflugers Arch. 1993;423:519–526. doi: 10.1007/BF00374950. [DOI] [PubMed] [Google Scholar]
- 22.Nokta M A, Eaton D, Steinsland O S, Albrecht T. Ca2+ responses in cytomegalovirus-infected fibroblasts of human origin. Virology. 1987;157:259–267. doi: 10.1016/0042-6822(87)90268-6. [DOI] [PubMed] [Google Scholar]
- 23.Ozaki Y, Yatomi Y, Kume S. Evaluation of platelet calcium ion mobilization by the use of various divalent ions. Cell Calcium. 1992;13:19–27. doi: 10.1016/0143-4160(92)90026-o. [DOI] [PubMed] [Google Scholar]
- 24.Parekh A B, Penner R. Store depletion and calcium influx. Physiol Rev. 1997;77:901–930. doi: 10.1152/physrev.1997.77.4.901. [DOI] [PubMed] [Google Scholar]
- 24a.Pérez J F, Chemello M E, Liprandi F, Ruiz M C, Michelangeli F. Oncosis in MA-104 cells is induced by rotavirus infection through an increase in intracellular Ca2+ concentration. Virology. 1998;252:17–27. doi: 10.1006/viro.1998.9433. [DOI] [PubMed] [Google Scholar]
- 25.Peters S M, Tijsen M J, van Os C H, Wetzels J F, Bindels R J. Hypoxia decreases calcium influx into rat proximal tubules. Kidney Int. 1998;53:703–708. doi: 10.1046/j.1523-1755.1998.00816.x. [DOI] [PubMed] [Google Scholar]
- 26.Poncet V, Merot J, Poujeol P. A calcium-permeable channel in the apical membrane of primary cultures of the rabbit distal bright convoluted tubule. Pflugers Arch. 1992;422:112–119. doi: 10.1007/BF00370410. [DOI] [PubMed] [Google Scholar]
- 27.Putney J W. Capacitative calcium entry revisited. Cell Calcium. 1990;11:611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- 28.Rose U M, Hartog A, Jansen J W, Van Os C H, Bindels R J. Anoxia-induced increases in intracellular calcium concentration in primary cultures of rabbit thick ascending limb of Henle’s loop. Biochim Biophys Acta. 1994;1226:291–299. doi: 10.1016/0925-4439(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 29.Ruiz M C, Charpilienne A, Liprandi F, Gajardo R, Michelangeli F, Cohen J. Concentration of Ca2+ that solubilizes outer capsid proteins from rotavirus particles is dependent on the strain. J Virol. 1996;70:4877–4883. doi: 10.1128/jvi.70.8.4877-4883.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanderson C M, Parkinson J E, Hollinshead M, Smith G L. Overexpression of the vaccinia virus A38L integral membrane protein promotes Ca2+ influx into infected cells. J Virol. 1996;70:905–914. doi: 10.1128/jvi.70.2.905-914.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schilling W P, Rajan L, Strobl-Jager E. Characterization of the bradykinin-stimulated calcium influx pathway of cultured vascular endothelial cells. Saturability, selectivity, and kinetics. J Biol Chem. 1989;264:12838–12848. [PubMed] [Google Scholar]
- 32.Shainkin-Kestenbaum R, Winikoff Y, Chaimovitz C, Zimlichman S, Sarov I. Inhibitory effect of the calcium antagonist, verapamil, on measles and vaccinia replication in cell culture. Isr J Med Sci. 1993;29:2–6. [PubMed] [Google Scholar]
- 33.Tian P, Estes M K, Hu Y, Ball J M, Zeng C Q, Schilling W P. The rotavirus nonstructural glycoprotein NSP4 mobilizes Ca2+ from the endoplasmic reticulum. J Virol. 1995;69:5763–5772. doi: 10.1128/jvi.69.9.5763-5772.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tian P, Hu Y, Schilling W P, Lindsay D A, Eiden J, Estes M K. The nonstructural glycoprotein of rotavirus affects intracellular calcium levels. J Virol. 1994;68:251–257. doi: 10.1128/jvi.68.1.251-257.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vanderkooi J M, Martonosi A. Sarcoplasmic reticulum. XVI. The permeability of phosphatidyl choline vesicles for calcium. Arch Biochem Biophys. 1971;147:632–646. doi: 10.1016/0003-9861(71)90422-x. [DOI] [PubMed] [Google Scholar]
- 36.van Kuppeveld F J, Hoenderop J G, Smeets R L, Willems P H, Dijkman H B, Galama J M, Melchers W J. Coxsackievirus protein 2B modifies endoplasmic reticulum membrane and plasma membrane permeability and facilitates virus release. EMBO J. 1997;16:3519–3532. doi: 10.1093/emboj/16.12.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang M I N, O’Neil R G. An L-type calcium channel in renal epithelial cells. J Membr Biol. 1996;154:259–266. doi: 10.1007/s002329900150. [DOI] [PubMed] [Google Scholar]