ABSTRACT
Extended-spectrum cephalosporin-resistant Escherichia coli (ESC-R-Ec) is an urgent public health threat with sequence type clonal complex 131 (STc131), phylogroup B2 strains being particularly concerning as the dominant cause of ESC-R-Ec infections. To address the paucity of recent ESC-R-Ec molecular epidemiology data in the United States, we used whole-genome sequencing (WGS) to fully characterize a large cohort of invasive ESC-R-Ec at a tertiary care cancer center in Houston, Texas, collected from 2016 to 2020. During the study time frame, there were 1,154 index E. coli bloodstream infections (BSIs) of which 389 (33.7%) were ESC-R-Ec. Using time series analyses, we identified a temporal dynamic of ESC-R-Ec distinct from ESC-susceptible E. coli (ESC-S-Ec), with cases peaking in the last 6 months of the calendar year. WGS of 297 ESC-R-Ec strains revealed that while STc131 strains accounted for ~45% of total BSIs, the proportion of STc131 strains remained stable across the study time frame with infection peaks driven by genetically heterogeneous ESC-R-Ec clonal complexes. blaCTX-M variants accounted for most β-lactamases conferring the ESC-R phenotype (89%; 220/248 index ESC-R-Ec), and amplification of blaCTX-M genes was widely detected in ESC-R-Ec strains, particularly in carbapenem non-susceptible, recurrent BSI strains. BlaCTX-M-55 was significantly enriched within phylogroup A strains, and we identified blaCTX-M-55 plasmid-to-chromosome transmission occurring across non-B2 strains. Our data provide important information regarding the current molecular epidemiology of invasive ESC-R-Ec infections at a large tertiary care cancer center and provide novel insights into the genetic basis of observed temporal variability for these clinically important pathogens.
IMPORTANCE
Given that E. coli is the leading cause of worldwide ESC-R Enterobacterales infections, we sought to assess the current molecular epidemiology of ESC-R-Ec using a WGS analysis of many BSIs over a 5-year period. We identified fluctuating temporal dynamics of ESC-R-Ec infections, which have also recently been identified in other geographical regions such as Israel. Our WGS data allowed us to visualize the stable nature of STc131 over the study period and demonstrate a limited but genetically diverse group of ESC-R-Ec clonal complexes are detected during infection peaks. Additionally, we provide a widespread assessment of β-lactamase gene copy number in ESC-R-Ec infections and delineate mechanisms by which such amplifications are achieved in a diverse array of ESC-R-Ec strains. These data suggest that serious ESC-R-Ec infections are driven by a diverse array of strains in our cohort and impacted by environmental factors suggesting that community-based monitoring could inform novel preventative measures.
KEYWORDS: antimicrobial resistance, extended-spectrum cephalosporin resistance, extended-spectrum beta-lactamases, molecular epidemiology, clonal complex 131, accessory genomes, copy-number variation
INTRODUCTION
Extended-spectrum β-lactamase (ESBL) producing Enterobacterales (ESBL-E) were ranked as a serious antimicrobial resistance (AMR) threat by the Centers for Disease Control and Prevention (CDC) in 2019 (1). A recent study of multi-drug resistant (MDR) infections in hospitalized patients indicated a 53.3% increased incidence rate in estimated ESBL-E cases from 2012 to 2017 in the USA (2). Other sources of US data have indicated similar trends, such as a 138% increase in resistance to third-generation cephalosporins among invasive Escherichia coli strains from 2009 to 2016 (3, 4). Furthermore, multiple population-based studies indicate that ESBL-E bloodstream infections may have a higher likelihood of adverse outcomes compared to non-ESBL-E bloodstream infections (5 - 7).
The key impact of ESBL enzymes is third-generation cephalosporin resistance (i.e., extended-spectrum cephalosporin resistance; ESC-R). Although ESBL enzymes account for the vast majority of the associated ESC-R phenotype, there are other mechanisms in Enterobacterales, such as plasmid-borne AmpC enzymes (8). Therefore, broadening our scope of potential ESC-R mechanisms provides a more holistic approach to understanding the transmission dynamics of these ESC-R genes (9). Among ESC-R Enterobacterales, some 50% or greater of infections are due to Escherichia coli (10, 11). ESC-R E. coli (ESC-R-Ec) in the 1980s and 1990s primarily harbored ESBL variants of TEM and SHV enzymes (12); however, this changed in the early 2000s with the increasing spread of CTX-M enzymes (13). The rise of CTX-M enzyme-producing E. coli coincided with a marked increase in sequence type (ST) 131 strains (14). In particular, fluoroquinolone-resistant sub-populations of ST131 (i.e., ST131-H30 subclade C) strongly associated with blaCTX-M-15 and blaCTX-M-27 carriage have been shown to be the predominant cause of ESC-R-Ec infections in the USA (15). However, whether the increasing number of ESC-R-Ec infections in the USA is driven by further expansion of ST131 strains is not well understood.
Application of whole-genome sequencing (WGS) to large cohorts of temporally diverse E. coli, including ESBL-producing isolates, from local and nationwide surveys has revealed common as well as unique molecular epidemiology features (16 - 23). Consistently, E. coli strains causing human infections are pauci-clonal with the domination of a small number of STs relative to the overall population (16 - 24 - 24). Many analyses indicate ST131 rose to become the leading E. coli ST isolated from humans by 2010 (19, 25). However, studies in England and Calgary have found that ST131 has remained at a relatively steady proportion of overall E. coli infections over the past 5–10 years (16, 19, 22), whereas investigations in France, Spain, and Norway have identified a continued increase in emergent ST131 subclades (17, 20, 21). Geographical variation in relative ST131 proportion changes may reflect differential ST131 cladal adaptation within ecological niches, with advantageous traits failing to fixate in populations due to negative frequency-dependent selection, reflected by the eventual stability of ST131 within the broader E. coli population (26).
A comprehensive US study found that ST131 accounted for 50% of all ESC-R-Ec strains in 2012 with an estimated increase of 50% from 2007 to 2011 in the percentage of ST131 E. coli (27). Given the lack of recent longitudinal data analyzing ESC-R-Ec in the USA, we sought to gain insight into the molecular epidemiology of ESC-R-Ec by analyzing bloodstream infection (BSI)–causing strains at our institution from 2016 to 2020.
RESULTS
Observed peaks in ESC-R-Ec BSIs occurred temporally within our sampling frame
We identified total Escherichia coli bloodstream infections (Ec-BSIs) at our institution that occurred from 1 March 2016 to 31 December 2020. There were 1,370 total Ec-BSIs with 84.2% (1,154/1,370) being index Ec-BSI cases and 15.8% (216/1,370) being recurrent (see Materials and Methods section for definitions). The overall ESC-R-Ec proportion was 33.7% (389/1,154) among index Ec-BSI cases. The proportion of ESC-R-Ec infections that were recurrent (121/510, 23.7%) was significantly higher compared to recurrent extended-spectrum cephalosporin-susceptible E. coli (ESC-S-Ec) cases (95/860, 11%, χ2 P-value < 0.001).
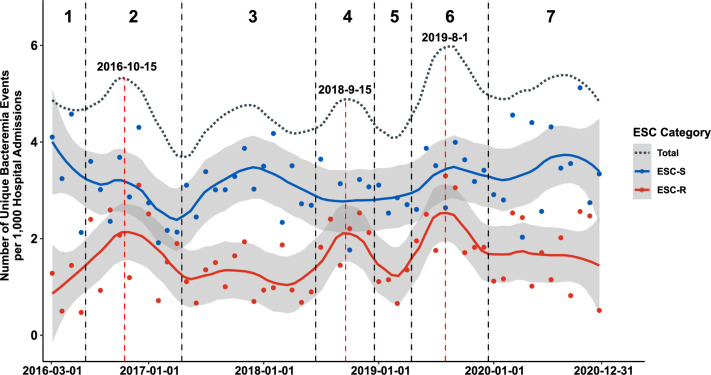
The non-normalized temporal trends of ESC-R-Ec within this 4+ year time frame are presented in Table S1. Given that changes in absolute yearly ESC-R-Ec might be reflective of variance in the total population seen at our institution, we utilized monthly admissions as a surrogate of the hospital population to calculate prevalence rates. We found no statistically significant linear increase in ESC-R-Ec rates over the course of the study (linear regression one-sample t-test P-value = 0.20). Notably, when calculating Ec-BSI prevalence rates stratified by ESC status, we identified an oscillating pattern of total Ec-BSI prevalence rates corresponding to peaks and valleys (dotted gray loess curve; Fig. 1) that primarily correlated with ESC-R-Ec prevalence rates (solid red loess curve; Fig. 1) rather than ESC-S-Ec prevalence rates (solid blue curve; Fig. 1). Stratifying the sampling frame based on these observed increased infection peaks (Fig. 1; Table 1), ESC-R-Ec prevalence rates were greatest during window 2 (1.89 BSI cases/1,000 admissions), window 4 (2.09 BSI cases/1,000 admissions), and window 6 (2.24BSI cases/1,000 admissions). The mean peak ESC-R-Ec prevalence rate (2.07 BSI cases/1,000 admissions) was 60% greater than the average of 1.29 BSI cases/1,000 admissions during non-peak periods (P < 0.001 for monthly ESC-R-Ec rates during peak versus non-peak windows by Student’s t-test).
Fig 1.
Number of bacteremia events observed per month from March 2016 to December 2020 at MD Anderson Cancer Center. Number of index E. coli bacteremia events per 1,000 hospital admissions for both ESC-R (red) and ESC-S (blue) isolates. A local regression curve for each respective ESC category is provided with 95% confidence intervals (gray area). The dotted gray line represents the prevalence trend for total E. coli bacteremia events. Windows of peak infection estimates were observed in windows 2, 4, and 6 as demarcated above by vertical dotted black lines with estimated peak date indicated within peak regions (vertical dotted red lines).
TABLE 1.
Windows of E. coli bloodstream infection rates per 1,000 hospital admissions from 1 March 2016 to 31 December 2020 a
| Window | 1 | 2 (ESC-R peak 1) | 3 | 4 (ESC-R peak 2) | 5 | 6 (ESC-R peak 3) | 7 |
|---|---|---|---|---|---|---|---|
| Date range (days) | 16/3/1–16/6/30 (121) |
16/7/1–17/4/30 (394) |
17/5/1–18/6/30 (425) |
18/7/1–18/12/31 (183) |
19/1/1–19/4/30 (119) |
19/5/1–19/12/31 (244) |
20/1/1–20/12/31 (365) |
| Total E. coli BSI per 1,000 admissions |
4.44 | 4.77 | 4.31 | 4.96 | 3.86 | 5.6 | 5.11 |
| ESC-R-Ec per 1,000 admissions | 0.92 | 1.89 | 1.16 | 2.09 | 1.07 | 2.24 | 1.63 |
| ESC-S-Ec per 1,000 admissions | 3.51 | 2.88 | 3.15 | 2.87 | 2.8 | 3.36 | 3.48 |
Prevalence rates calculated are based on mean monthly BSIs normalized by monthly hospital admissions occurring over the specified date ranges (see Materials and methods for details).
We performed time series analyses and found that while both ESC-R-Ec and ESC-S-Ec had non-significant monotonic trends, there was evidence of a non-monotonic trend for ESC-R-Ec that occurred up to the start of the COVID-19 pandemic in March 2020 (WAVK test-statistic = 2.7; P-value = 0.016), which was not evident in ESC-S-Ec (WAVK test-statistic = 0.2; P-value = 0.877). To determine whether ESC-R-Ec and ESC-S-Ec prevalence rates were distinct, we compared pairwise month-to-month prevalence and found no statistically significant correlation (R = 0.14, Pearson P-value = 0.31; Fig. S1A) suggesting independent temporal trends. Given the periodic trends noted in Fig. 1, we assessed whether variation in Ec-BSIs corresponded to the time of year by dividing the index infections into the first or second half of the year. We found a significantly higher average of ESC-R-Ec (7.9 ± 0.5 versus 5.5 ± 0.6 BSIs/month; P-value = 0.003) but not ESC-S-Ec (13.8 ± 0.5 versus 12.5 ± 0.6; P-value = 0.09) occurring in the last 6 months relative to the initial 6 months of each year (Fig. S1B), respectively. Taken together, these data trends suggest temporal dynamics in ESC-R-Ec independent of ESC-S-Ec strains with a potential seasonal component.
Peaks in ESC-R Ec-BSIs are composed of genetically heterogeneous strains from multiple sequence type complexes
From the 389 index and 121 recurrent ESC-R-Ec strains identified during the study period, we generated assemblies for 297 ESC-R-Ec isolates including 64% (248/389) of index and 41% (49/121) of recurrent ESC-R-Ec strains. The quality of assemblies is shown in Table S2. In total, there were seven phylogroups detected across the full sampling frame (Table 2). The majority of index ESC-R-Ec belonged to phylogroup B2 (50.8%; 126/248), followed by A (19.8%; 49/248), D (12.1%; 30/248), F (7.7%; 19/248), B1 (6.5%; 16/248), C (2.8%; 7/248), and G (0.4%; 1/248). We identified a similar distribution for recurrent ESC-R-Ec strains with B2 (38.8%; 19/49) as the most common phylogroup followed by A (34.7%; 17/49), D (12.2%; 6/49), B1 (8.2%; 4/49), C (4.1%; 2/49), and F (2.0%; 1/49). In terms of STs (Table 2), ST131 was the most common for both index (n = 102, 41%) and recurrent strains (n = 17, 35%). A further breakdown of each of the most common STs detected by phylogroup can be found in Table 2.
TABLE 2.
Overview of sequence type distribution across each phylogroup from sequenced index and recurrent ESC-R E. coli isolates
| Typing group a , b | Index (n = 248) | Recurrent (n = 49) |
|---|---|---|
| Phylogroup B2 (n = 145) | 126 (50.8%) | 19 (38.8%) |
| ST131 (n = 129) | 102 (80.9%) | 17 (89.5%) |
| ST1193 (n = 15) | 14 (11.1%) | 1 (5.3%) |
| Other ST (n = 11) | 10 (7.9%) | 1 (5.3%) |
| Phylogroup A (n = 66) | 49 (19.8%) | 17 (34.7%) |
| ST10 (n = 13) | 8 (16.3%) | 5 (29.4%) |
| ST744 (n = 11) | 9 (18.4%) | 2 (11.8%) |
| ST167 (n = 11) | 8 (16.3%) | 3 (17.6%) |
| ST44 (n = 7) | 2 (4.1%) | 5 (29.4%) |
| Other ST (n = 24) | 22 (44.9%) | 2 (11.8%) |
| Phylogroup D (n = 36) | 30 (12.1%) | 6 (12.2%) |
| ST405 (n = 19) | 16 (53.3%) | 3 (50.0%) |
| ST38 (n = 9) | 7 (23.3%) | 2 (33.3%) |
| Other ST (n = 8) | 7 (23.3%) | 1 (16.7%) |
| Phylogroup F (n = 20) | 19 (7.7%) | 1 (2.0%) |
| ST648 (n = 15) | 14 (73.7%) | 1 (100.0%) |
| Other ST (n = 5) | 5 (26.3%) | 0 (0.0%) |
| Phylogroup B1 (n = 20) | 16 (6.5%) | 4 (8.2%) |
| ST224 (n = 6) | 5 (31.3%) | 1(25.0%) |
| Other ST (n = 13) | 11 (57.9%) | 3 (75.0%) |
| Phylogroup C (n = 9) | 7 (2.8%) | 2 (4.1%) |
| ST410 (n = 7) | 5 (71.4%) | 1 (50.0%) |
| Other ST (n = 2) | 2 (28.6%) | 1 (50.0%) |
Other ST consists of sequence types detected at ≤ 5 total counts within each phylogroup.
Phylogroup G consists of one observation (ST501), thus was not included in the table.
To investigate the molecular epidemiology underlying the temporal dynamics of our ESC-R-Ec population, we analyzed isolates characterized by phylogroup across infection windows (Fig. 2). Fig. 2A and B provides an illustration of the phylogroup distribution throughout the infection windows that remained remarkably stable over time with no statistically significant trends (Fisher’s exact test simulated P-value = 0.95). There were sporadic increases in the absolute numbers of various phylogroups during the peak infection periods such as phylogroup A during windows 2 and 6, as well as phylogroup B1 and phylogroup F during window 4. We collapsed the “non-peak” (i.e., windows 1, 3, 5, and 7) and “peak” (i.e., windows 2, 4, and 6) periods together and again found no statistically significant differences in phylogroup proportions between peak and non-peak periods (Fisher’s exact test P-value = 0.42). The dominant B2 phylogroup accounted for 58% of infections during the non-peak windows and 47% during the peak windows (Fisher’s exact test P-value = 0.15).
Fig 2.
Temporal distribution of ESC-R Ec-BSI isolates. The proportional distribution and absolute frequency counts by phylogroup (A and B) and clonal complex (C and D), respectively, across each infection window. (A–C) Group totals are listed above each of the windows. (B–D) Data shown are line graphs connecting month-to-month frequency counts colored by proportions of phylogroup (B) and clonal complex (D) as indicated in the legend.
We grouped index strains by ST clonal complexes (i.e., single-locus ST variants within a 7-housekeeping gene schema) to further analyze temporal trends. The leading ST complexes (STcs) were STc131 (n = 109, 44%), STc10 (n = 35, 14%), STc405 (n = 16, 7%), STc14 (n = 14, 6%), STc648 (n = 14, 6%), and STc38 (n = 10, 4%). For testing purposes, we collapsed STcs with less than five strains into an “uncommon” category (n = 32, 13%). Similar to our phylogroup analysis, we did not identify any significant trends for a particular STc over the study period (Fisher’s exact test simulated P-value = 0.91). However, there were temporary absolute increases in specific STc infections within peak infection windows such as “uncommon” STcs within window 2, STc14 and STc648 within window 4, and STc10 within window 6 (Fig. 2C and D). Importantly, STc131 remained stable in prevalence across each of the infection windows (χ2 P-value = 0.9) with STc131 strains accounting for 50% of infections during non-peak windows compared to 40% of infections during peak infection windows (Fisher’s exact test P-value = 0.15). Temporal information regarding isolates sequenced per window including the proportion of isolates that were STc131 is provided in Table S3. There were no peak infection periods in which STc131 strains accounted for a higher percentage of infections relative to non-peak periods (Table S3). Similarly, there were no statistically significant proportion differences across windows in ST1193, the other most commonly prevalent B2 isolates (Fisher’s exact test P-value = 0.48) nor in the second most common, STc10 (Fisher’s exact test P-value = 0.74). Thus, these data indicate that peaks of ESC-R-Ec observed temporally over our study period were driven by a diverse combination of ESC-R-Ec isolates rather than the expansion of STc131 strains.
BlaCTX-M variants are dominant encoding genes conferring inferred ESC-R phenotype
We searched the WGS data for genes potentially conferring the ESC-R phenotype, which we defined as those encoding ESBLs, i.e., TEM, SHV, CTX-M-encoding genes with a 2be Jacoby-Bush classification (12), as well as CMY and carbapenemase encoding genes. Of the 248 index isolates, 236 (95%) had at least one gene expected to confer the ESC-R phenotype with CTX-M variants being present in 220 strains (89%) and CMY variants found in 21 strains (8.4%) (N.b., strains could contain multiple ESC-R encoding genes). A single ST131 strain carried an ESBL blaTEM-19, two strains carried ESBL blaSHV-12, one ST361 strain carried blaOXA-232, and three STc10 strains harbored blaNDM-5. The most common CTX-M encoding gene variants detected within index ESC-R-Ec isolates were blaCTX-M-15 (n = 134), blaCTX-M-27 (n = 29), blaCTX-M-55 (n = 26), blaCTX-M-14 (n = 19), and multiple blaCTX-M variant carriage (n = 7). A similar distribution was observed for recurrent ESC-R-Ec isolates: blaCTX-M-15 (n = 26), blaCTX-M-27 (n = 7), blaCTX-M-55 (n = 8), and blaCTX-M-14 (n = 3). Of the 12 index strains that did not carry a known ESC-R phenotype determinant, six contained blaOXA-1, five encoded non-ESBL TEM enzymes, and only one isolate lacked a known exogenous β-lactamase encoding gene. The preponderance of CTX-M with a smaller role of CMY in conferring the ESC-R phenotype in E. coli is consistent with previously published results in the USA (9, 10).
Temporal whole-genome analysis indicates a low level of clonality among ESC-R-Ec
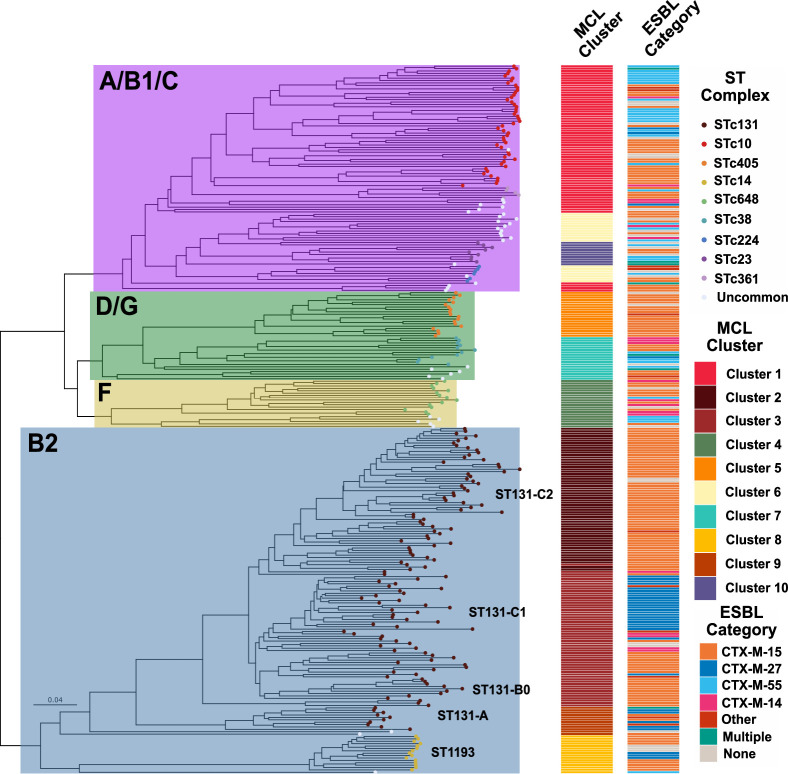
To gain further insights into the molecular epidemiology driving the temporal dynamics of ESC-R-Ec, we used WGS data to analyze the population structure for all 297 isolates. The core gene inferred maximum likelihood phylogeny based on 2,695 core genes (i.e., genes present in ≥99% of the 297 ESC-R Ec-BSI isolates) is shown in Fig. 3. Consistent with previous WGS studies of ESC-R E. coli population structure (9, 18), we observed significant variation in the degree of genetic similarity among strains of distinct STcs. For example, there was only an average of 28 core gene SNPs separating a given STc14 strain from its nearest neighbor compared to 1,518 for STc10 strains. Similarly, we identified several previously described subclades within the broader STc131 population (15, 20, 28). By overlying the infection window with our WGS phylogeny, we sought to determine whether the increased resolution would facilitate the detection of highly related strains driving infection peaks. However, in line with our phylogroup and STc-based analyses, we did not observe large numbers of phylogenetically clustered index strains occurring during infection windows (Fig. 3). Consistent with the notion of similar genetic diversity being present during peak and non-peak infection windows, there was no difference in pairwise core gene SNP distance between one isolate and its most closely related strain during the various windows (Kruskal-Wallis test P-value = 0.81; Fig. S2).
Fig 3.
Population structure of ESC-R Ec-BSI isolates. A core gene alignment inferred maximum likelihood phylogenetic tree with phylogroups highlighted within each monophyletic group. Branch tip colors indicate clonal complex numbers as shown in the figure inset with clonal complexes observed < 5 counts grouped as “Uncommon.” Rings corresponding to infection window (1), recurrence (2), and β-lactamases are labeled, respectively. Internal nodes labeled with black circles have SH-aLRT ≥ 80% and UFboot ≥ 95%.
To search for clusters of closely genetically related strains both within peak infection windows and throughout the study period, we measured the pairwise SNP distances of index isolates using recombination-free core genome alignments from the 12 most commonly observed STs in our cohort, which make up 79.8% of the total population (198/248). We identified seven groups of two or more isolates (i.e., genetic clusters) from unique patients with < 10 core genome-pairwise SNPs (Fig. S3). The maximum number of closely related isolates within a peak infection window was two strains, whereas the maximum cluster size was three strains, with the duration between isolates from the same cluster ranging from 5 days to 777 days (Fig. S3). These data indicate that even at the WGS level, peaks of ESC-R-Ec are driven by genetically heterogeneous isolates with few signals indicating clonal outbreaks across each infection window.
ESC-R encoding genes are highly enriched within Ec-BSI sub-populations
We combined the β-lactamase encoding gene data with our WGS-based phylogeny to assess the relationship between E. coli genetic content and ESC-R mechanisms (Fig. 3). There was a strong association of phylogroup B2 isolates harboring blaCTX-M-15 genes as 60.4% of (81/134) blaCTX-M-15 harboring strains were present in this phylogroup [row-wise Fisher’s exact test adjusted (adj.) P-value = 0.01]. Specifically, this association was driven by the presence of blaCTX-M-15 in 72 STc131 isolates (adj. P-value = 0.009), the vast majority of which are part of the previously identified blaCTX-M-15 carrying H30Rx lineage (15). Similarly, there was also enrichment of the second most commonly detected CTX-M encoding gene variant, blaCTX-M-27, in B2 index isolates (26/29 blaCTX-M-27 harboring strains; adj. P-value < 0.0001) again driven by STc131 isolate carriage (23/29; adj. P-value = 0.0005). Specifically, there were 19/23 blaCTX-M-27 positive ESC-R-Ec belonging to the ST131-C1-M27 clade, which has a strong association with blaCTX-M-27 carriage (28) as seen in the lower right quadrant of the core gene phylogeny (Fig. 3). The third most prevalent CTX-M encoding gene variant, blaCTX-M-55, was enriched within phylogroup A (12 phylogroup A strains out of 26 blaCTX-M-55 containing index isolates; adj. P-value = 0.004), whereas it was only present in one B2 isolate (1/26, adj. P-value < 0.0001). The final major blaCTX-M variant in our cohort, blaCTX-M-14 was carried by a diverse array of genetically heterogeneous strains (Fig. 3). In contrast to the blaCTX-M encoding genes in which there were several large clusters of genetically adjacent strains encoding the same CTX-M variant, the carriage of CMY encoding genes was sporadically observed across phylogroups. Similar to the phylogroup trend and phylogeny analyses, we did not observe any statistically significant association between ESC-R-mediating enzyme variants and infection windows (χ2 adj. P-value > 0.05).
Accessory genomes are distinct between B2 and non-B2 phylogroups
We further explored genomic differences within ESC-R-Ec by comparing accessory genome content (i.e., defined as genes carried in > 5% and < 95% of the total ESC-R-Ec population) across the full cohort. There was a total of 25,631 genes in the pangenome with 6,060 accessory genes. Through a cluster analysis using the Jaccard distance of the accessory genome between samples, we observed a strong association between phylogroup, clonal complex, and the accessory genome (Fig. 4). A neighbor joining (NJ) tree constructed from a Jaccard distance matrix shows a clear delineation of B2 and non-B2 isolates that split at the root of the tree (Fig. 4). Using a network graph analysis of the accessory genome (see Materials and Methods section) (Fig. S4), there was a separation of B2 and non-B2 isolates into 10 clusters highly correlating with STc (Fig. 4). Indeed, each of the clonal complexes with five or more isolates had at least one statistically significant association (adj. P-value < 0.05) with an accessory genome Markov Cluster group (MCL) (Fig. S5). Furthermore, the MCL clustering algorithm delineated the subclade structure of STc131 where each of the ST131-H30Rx subclades C1 (Cluster 2) and C2 (Cluster 3) were separated by the accessory genome (Fig. 4). ST131-H41-O16 subclade A (Cluster 9) was found exclusively in one MCL group (Fig. 4) demonstrating a unique accessory genome between ST131 C1/C2 and A subclades. A complementary principal component analysis demonstrated clustering of accessory content by phylogroup (Fig. S6A), STc (Fig. S6B), MCL group (Fig. S6C), and ESBL gene (Fig. S6D) further highlighting the separation of B2 and non-B2 groups by accessory genome content. The emergent ST1193 lineage, which made up all STc14 (n = 15) strains, made up a separate MCL cluster (Cluster 8) within B2 isolates; however, there were no clear associations with a particular blaCTX-M variant. These analyses indicate potential differential pathways to ESC-R development dependent on phylogroup background.
Fig 4.
Accessory genome clustering by ST131 and non-ST131 ESC-R isolates. Jaccard distance matrix inferred NJ tree of accessory genome content with metadata indicated in the legend. Phylogroups and chosen STs/clades are highlighted within tree and labeled accordingly. MCL = Markov Clustering group; STc = Sequence Type Clonal Complex.
Significant copy-number amplifications of enriched CTX-M encoding genes occur within phylogroups
Given the growing appreciation of β-lactamase gene amplification in contributing to AMR phenotypes (29 - 31), we sought to characterize gene copy-number variation of ESC-R genes to determine whether ESC-R gene amplification correlated with our previously identified gene enrichment associations. Table 3 provides copy-number estimates of blaCTX-M variants stratified by the three most common phylogroups. Table S4 and S5 provide copy numbers of other β-lactamase encoding genes and overall estimated copy numbers of AMR genes, respectively. For the 268 blaCTX-M genes, the median copy-number variant (CNV) was 1.2× [interquartile range (IQR) = 1.3] with a range of 0.4–12.9. The median CNV across CTX-M variants was similar with blaCTX-M-15 (n = 161, CNV = 1.1×; IQR = 1.2), blaCTX-M-27 (n = 37, CNV = 1.4×; IQR = 0.8), blaCTX-M-55 (n = 37, CNV = 1.5×; IQR = 1.4), and blaCTX-M-14 (n = 37, CNV = 1.4×; IQR = 2.2) having statistically significant greater than one copy (one-sample Wilcoxon signed rank adj. P-value < 0.01).
TABLE 3.
Copy-number variants of β-lactamase encoding genes a
| CTX-M-15 | CTX-M-27 | CTX-M-55 | CTX-M-14 | Other CTX-M | |
|---|---|---|---|---|---|
| Total | |||||
| Count | 161 | 37 | 37 | 23 | 10 |
| Mean (SD) | 1.62 (1.29) | 1.87 (1.89) | 2.50 (2.65) | 2.61 (2.57) | 3.55 (3.35) |
| Median (IQR) | 1.11 (1.19) | 1.40 (0.80) | 1.50 (1.43) | 1.42 (2.21) | 1.31 (5.36) |
| Range | 0.37–8.72 | 0.42–9.10 | 0.62–12.9 | 0.64–10.8 | 0.60–9.45 |
| Phylogroup B2 | |||||
| Count | 92 | 33 | 1 | 6 | 3 |
| Mean (SD) | 1.41 (0.96) | 1.12 (0.36) | 3.41 | 2.53 (2.06) | 4.80 (3.66) |
| Median (IQR) | 1.03 (0.54) | 1.20 (0.354) | NA | 1.75 (1.83) | 6.54 (3.33) |
| Range | 0.44–5.84 | 0.42–9.10 | NA | 0.88–6.33 | 0.60–7.26 |
| Phylogroup A | |||||
| Count | 31 | 3 | 19 | 5 | 2 |
| Mean (SD) | 1.66 (1.72) | 1.12 (0.36) | 2.39 (2.09) | 1.65 (1.42) | 1.08 (0.13) |
| Median (IQR) | 1.07 (0.97) | 1.20 (0.35) | 1.68 (1.82) | 1.01 (0.31) | 1.08 (0.09) |
| Range | 0.50–8.72 | 0.72–1.44 | 0.62–7.20 | 0.78–4.16 | 0.98–1.17 |
| Phylogroup D | |||||
| Count | 21 | 1 | 5 | 4 | 3 |
| Mean (SD) | 2.02 (1.48) | 1.8 | 1.25 (0.49) | 1.80 (0.98) | 3.90 (4.81) |
| Median (IQR) | 2.00 (1.62) | NA | 1.30 (0.53) | 1.43 (0.54) | 1.31 (4.25) |
| Range | 0.37–5.14 | NA | 1.00–1.96 | 1.10–3.26 | 0.94–9.45 |
SD, standard deviation; IQR, interquartile range; NA, not applicable. Copy-number variants are estimated based on CONVICT results.
We and others have previously identified that copy number increases in narrow-spectrum and ESBL encoding genes contribute to the development of carbapenem resistance (32, 33). Therefore, we stratified our ESC-R-Ec population by carbapenem susceptibility (i.e., carbapenem non-susceptible versus susceptible) to determine if there were statistically significant amplifications in carbapenem-susceptible ESC-R-Ec isolates. The carbapenem non-susceptible ESC-R-Ec had greater estimated copy numbers of blaCTX-M (median CNV = 2.7×; IQR = 4.2) compared to carbapenem-susceptible ESC-R-Ec (median CNV = 1.1 ×; IQR = 1.0; two-sample Wilcoxon rank sum test P-value = 3e-7). Thus, we further characterized blaCTX-M gene amplification across phylogroups solely within ESC-R-Ec that are carbapenem susceptible (Fig. 5A). We found statistically significant amplifications for blaCTX-M-15 and blaCTX-M-27 in phylogroup B2 isolates and blaCTX-M-55 in phylogroup A isolates, which aligned with the enrichment analysis. These statistically significant trends persisted specifically within STc131 for blaCTX-M-15/blaCTX-M-27 and STc10 for blaCTX-M-55 (Fig. S7). To assess whether blaCTX-M amplifications occurred as an adaptive response, we sought to identify isolates from the same patient that were the same clonal complex and had the same blaCTX-M variants, i.e., were likely clonal, recurrent infections (Fig. 5B). We identified 16 pairs from 14 patients in which 56% (9/16) second ESC-R Ec-BSI isolates were carbapenem non-susceptible following their antecedent carbapenem-susceptible isolate. We found that there was a statistically significant increase (Wilcoxon signed rank test P-value = 0.03) in blaCTX-M copy numbers from the first isolate (median CNV = 1.02; IQR = 0.86) to the second isolate (median CNV = 1.80; IQR = 3.32). Notably, these blaCTX-M amplifications occurring within sequential isolates were observed for blaCTX-M-15/blaCTX-M-27 and blaCTX-M-55 in STc131 and STc10, respectively (Fig. 5B). To gain insights into how β-lactamase gene amplification affected AMR phenotypes, we characterized the MIC distribution for recurrent versus non-recurrent (i.e., first isolate) populations for piperacillin-tazobactam (TZP), ceftazidime (CAZ), cefepime (FEP), ertapenem (ETP), and meropenem (MEM) antimicrobial susceptibility testing (Fig. S8A through D). For all tested β-lactams, the MIC90 was higher for the recurrent relative to the non-recurrent isolates (Fig. S8). Thus, there was a general shift in MIC distributions, which correlated with recurrent isolates that had increased blaCTX-M gene copy numbers.
Fig 5.
BlaCTX-M amplification occurring within ESC-R-Ec isolates. (A) Copy-number estimates of blaCTX-M variants stratified by phylogroup indicated in facetted plots that are ESC-R and carbapenem-susceptible. One-sample Wilcoxon rank sum test for amplification (i.e., copy number > 1) performed across each blaCTX-M variant with * indicating P-value < 0.05. (B) Estimated copy number of blaCTX-M variants of paired ESC-R serial isolates (selection criteria as outlined in the text). Shapes and colors indicate clonal complex and CTX-M variant as noted in the legend. Dotted lines connect paired isolates. Paired Wilcoxon signed rank test was statistically significant with * indicating P-value < 0.05.
F-type plasmids are primary vectors of blaCTX-M-55 in non-STc131 strains
Previous studies have focused largely on ST131 subclade associations with blaCTX-M-15 and blaCTX-M-27 (15, 28) including our characterization of blaCTX-M-15 and blaOXA-1 co-carriage on similar, multireplicon F-type plasmids contributing to carbapenem resistance (32, 33). Given the rarity of blaCTX-M-55 molecular epidemiology data from North America (34), we focused on blaCTX-M-55 inasmuch as it was highly prevalent throughout our sampling frame. We used a long-read approach to complete assemblies of nine blaCTX-M-55 positive strains and found that most (n = 7) carried blaCTX-M-55 in a highly conserved ISEcp1 context on ~100 kb IncFIB/IncFIC plasmids (Fig. 6A). Whereas the cargo gene region was relatively variable, the F-type plasmid regions responsible for transfer, replication, and stability were highly conserved (blastn ID/coverage (COV) ≥ 99%). For the full cohort of blaCTX-M-55 isolates (n = 38), 31 isolates had IncFIB/IncFIC amplicon hits from PlasmidFinder (> 80% ID; > 80% COV). Short-read pile-up analysis to pMB8960_1 (i.e., the most conserved IncFIB/IncFIC blaCTX-M-55 positive isolate) showed that 30/31 isolates had > 80% breadth of coverage and > 100 × coverage depth for pMB8960_1.
Fig 6.
Characterization of mobile genetic elements associated with transmission and amplification of blaCTX-M-55 within non-B2 isolates. (A) BRIGs output of similar IncFIB/IncFIC plasmids with conserved carriage of ISEcp1-blaCTX-M-55 carriage (underlined) in four different non-B2 phylogroups. Inner ring indicates GC content/skew with coding DNA sequences in reverse/forward orientation. The next seven rings indicate blastn identities to pMB6206_1 as indicated in the legend. Outer ring includes plasmid, mobile genetic element, and antimicrobial resistance gene content as identified by the Comprehensive Antimicrobial Resistance Database (CARD). (B) Blast nucleotide and gene amplification comparisons of differential blaCTX-M-55 vectors responsible for plasmid transmission as well as potential vertical transmission via chromosomal insertion. Normalized coverage depth of conserved ~10-kb region that harbors ISEcp1-blaCTX-M-55 (~10 kb) as demarcated by dotted black lines in (A). Inferred plasmid copy number versus plasmid-to-chromosome events as indicated by tracking target site duplications of ISEcp1-blaCTX-M-55 on complete assemblies as well as mean coverage depths of full-length plasmid. Coding sequences within blastn comparisons are identified as followed: ISEcp1 (purple), blaCTX-M-55 (green), AMR genes (red), IS26 (white), IS26-v1 (beige), other MGE (black), and other CDS (gray). Black horizontal line demarcates IncFIB/IncFIC-positive isolates from high copy number IncX1 plasmids harboring blaCTX-M-55 from ST167 isolates. Parentheticals following the plasmid label indicate plasmid length and copy number. CDS with striped, purple label indicates ISEcp1 truncated by IS26-v1 upstream of blaCTX-M-55.
Our long-read analyses allowed for the identification of blaCTX-M-55 amplification arising from plasmid copy-number variation (MB8960 and MB9029) as well as plasmid-to-chromosome transfer (i.e., segmental duplication) from two isolates (MB6206 and MB7355) as evidenced by tracking target site duplications (TSDs) and plasmid content from ISEcp1-blaCTX-M-55 (Fig. 6B). This included an isolate in which ISEcp1-blaCTX-M-55 disrupts the envZ gene, which is part of a two-component regulatory system (i.e., EnvZ-OmpR) that regulates the expression of outer membrane porin genes (35). A carbapenem-susceptible ESC-R-Ec isolate (MB7355) was identified with two independent insertions within the chromosome likely originating from the IncFIB/IncFIC plasmid as evidenced through TSD tracking (Fig. 6B; one insertion into fhuA gene demonstrated). Another vector of blaCTX-M-55 was identified in two ST167 isolates in a substantially differing context with blaCTX-M-55 upstream of IS26-v1 on smaller IncX1 plasmids (Fig. 6B). The IS26-v1 upstream of blaCTX-M-55 had truncated ISEcp1 (Fig. 6B; purple-striped CDS) and were within unique antimicrobial resistance gene transposons. These analyses together show that blaCTX-M-55 is a major contributor to the ESC-R-Ec phenotype in our cohort with predominantly IncFIB/IncFIC carriage.
DISCUSSION
The past 20 years in the USA have seen a consistent rise in ESC-R-Ec causing infection, primarily due to ESBL enzymes (2 - 4). While the high prevalence of ST131 observed in the USA is concerning, it is not clear if these pathogens are responsible for the increased frequency of ESC-R-Ec (2). We sought to leverage our longitudinal collection of bloodstream isolates to address the recent temporal dynamics of E. coli at our tertiary care cancer center. Although we observed that STc131 strains accounted for nearly 50% of infecting isolates, the STc131 prevalence rates remained fairly stable over time with multiple MDR clones accounting for peaks in infection rates observed during our study period.
We chose to focus on BSIs because of the clear distinction between colonization and infection relative to other potential sample sites. Our large number of fully sequenced isolates and extended study time frame provide critical information regarding contemporary molecular epidemiology of invasive ESC-R-Ec infections in a major US city. Although our finding of ~35% Ec-BSIs being ESC-R is high relative to other studies from the USA (4), it is consistent with the ~40% proportion of ESC-R in Ec-BSI occurring in solid-organ transplant recipients (36). Whereas recent longitudinal ESC-R-Ec epidemiological studies from the USA are lacking, we can compare our findings to those recently published from Europe (16 - 21). A similar theme has emerged from these investigations where for several years following introduction into the population, STc131 strains dramatically increase before becoming a stable proportion of overall E. coli infections (16 - 21). Given that STc131 had been identified as the dominant antimicrobial-resistant E. coli STc in the USA for at least 10 years prior to the start of our sampling (27, 37), our finding of a relatively stable contribution of STc131 is consistent with other studies (16 - 21) and suggest that, at least for our population, STc131 may have reached a stable level in the broader ESC-R-Ec population.
A key strength of our study was our ability to investigate the molecular epidemiology underlying the temporal variability of ESC-R-Ec prevalence rates. We found that ESC-R-Ec rates generally peaked within the last 6 months of the calendar year and were independent of ESC-S-Ec rates. Seasonality of both ESC-R-Ec infection and colonization has been previously reported with generally higher rates identified during warmer months (38 - 40). For example, a recent pooled observational cohort analysis of ESBL-positive E. coli and Klebsiella pneumoniae (ESBL-E/K) carriage in the Netherlands found that the highest prevalence occurred in August [odds ratio (OR) 1.88, 95% confidence interval (CI) 1.02–3.49] and September (OR 2.25, 95% CI 1.30–3.89) compared to January (39). In Houston, the highest temperatures of the year are generally observed between June and September (https://www.weather.gov/hgx/climate_iah_normals_summary), which might facilitate the proliferation of E. coli strains leading to colonization and subsequent infection at a delayed interval. Consistent with environmental factors being a major driver of the temporal variability, we observed low genetic relatedness among E. coli strains during the peak windows of infection rather than closely related clones. On a few occasions, we identified strains from distinct patients who were closely related (i.e., < 10 SNPs) consistent with hospital acquisition, but most strains were genetically diverse suggesting a community source. Given the recent success of wastewater surveillance in identifying severe acute respiratory syndrome coronavirus 2 and influenza transmission dynamics (41, 42), we envision that a similar approach could be used to improve understanding of how community prevalence of ESC-R pathogens impacts subsequent infections.
In previous works, we have identified that non-carbapenemase-producing, carbapenem-resistant Enterobacterales (non-CP-CRE) have increased copy numbers of genes encoding ESC-R-mediating enzymes such as blaCTX-M variants (32, 33). This study expands on our previous copy-number assessment of β-lactamase encoding genes mediating the ESC-R phenotype in carbapenem-susceptible E. coli strains. This allowed us to find that carbapenem-susceptible ESC-R-Ec strains have lower blaCTX-M copy numbers relative to carbapenem-resistant ESC-R-Ec strains consistent with an important role of blaCTX-M amplification in the transition from ESC-R to the non-CP-CRE phenotype. Moreover, via an analysis of paired isolates from the same patients, we found significant blaCTX-M amplifications suggesting that such amplifications are likely an adaptive mechanism to antimicrobial exposure in the host as has been observed by our group and others in the laboratory environment (32, 33, 43). Together with recently published data from ESBL-positive E. coli strains isolated in St. Louis (34), our long-read analyses demonstrate the diverse mechanisms by which E. coli can increase blaCTX-M encoding gene copy numbers including augmented plasmid copy numbers, multiple chromosomal copies in distinct locations, and in situ amplifications mediated by insertion sequences such as ISEcp1 and IS26. Although gene amplifications have long been thought to confer a major fitness cost and thus be transient in response to antimicrobials, incorporation of blaCTX-M encoding genes into particular chromosomal locations has been found to have a low fitness cost, allowing for stable amplifications across many generations in the absence of antimicrobial pressure (44, 45). We hypothesize that the high rates of recurrence observed among ESC-R-Ec relative to ESC-S-Ec strains might, in part, be due to blaCTX-M amplifications occurring among colonizing strains in the gastrointestinal tract, which would allow for their persistence during carbapenem treatment and subsequent re-infection. Such an amplification of blaCTX-M-14 was recently described in an E. coli strain recurrently isolated from a patient’s urine over many months (34).
Furthermore, we were able to identify IncFIB/IncFIC plasmids as the primary vector of ISEcp1-blaCTX-M-55 carriage in our cohort where amplification was occurring through both increase in plasmid copy numbers as well as plasmid-to-chromosome horizontal gene transfer. Although we did not identify a “clonal expansion” of chromosomal ISEcp1-blaCTX-M-55, the independent transfer of these vectors into two different phylogroup chromosomal backgrounds suggests the potential for stable chromosomal propagation. A recent study in China found a temporal trend with increasing chromosomal blaCTX-M-55 albeit without clarity as to whether this was occurring within clonal populations (46). While little is known regarding blaCTX-M-55 prevalence in North America (34), the enrichment of this blaCTX-M variant in the second most commonly detected phylogroup/STc in our cohort suggests these IncFIB/IncFIC::ISEcp1-blaCTX-M-55 plasmids are a significant contributor to ESC-R-Ec in our region.
Although our study has many strengths, there are important limitations. First, this was a single-center investigation at a hospital serving an immunocompromised population and thus external generalizability is unclear. This weakness is somewhat mitigated by our center serving a geographically dispersed group of patients and because the impact of ESC-R-Ec is particularly severe among immunocompromised persons. Moreover, our finding of temporal variability among E. coli infections has been observed among broader populations such as a recent nationwide report from Israel (47). Second, the diverse nature of observed clonal complexes and subclones meant that there were often small numbers of non-STc131 clonal complexes during specific infection windows. Larger sampling from a more geographically dispersed area is needed to address these issues. A final weakness is that the beginning of the COVID-19 epidemic likely led to a major disruption in infection epidemiology at our institution given the nearly 50% decrease in admissions observed in the spring and summer of 2020. Whereas both the absolute numbers and proportion of ESC-R-Ec BSIs had been steadily increasing from 2017 through 2019, both significantly decreased during 2020. The reasons for these findings are not clear, but they stand in contrast to preliminary data released by the CDC indicating an increase in ESBL Enterobacterales in 2020 nationwide (48).
In summary, we present a comprehensive whole genome-based analyses of ESC-R-Ec from the USA analyzing recently collected strains. These data show that STc131 strains have reached a stable proportion of our ESC-R-Ec population. Additionally, we provide molecular insights into the temporal variability of ESC-R-Ec prevalence which is being increasingly appreciated but is not well understood mechanistically (16 - 21). The genetically diverse nature of ESC-R-Ec strains identified in our study indicates that efforts to mitigate serious ESC-R-Ec infections likely will need to address broad environmental reservoirs such as through vaccination and highlight the need to better understand ESC-R-Ec transmission and temporal dynamics outside of the hospital setting.
MATERIALS AND METHODS
Study design and sample collection
Escherichia coli bloodstream infections (Ec-BSIs) occurred from 1 March 2016 to 31 December 2020 at MD Anderson Cancer Center (MDACC) in Houston, TX, with 1 March 2016, chosen given this was the date of implementation of the Epic Electronic Health Record system. We defined an index Ec-BSI isolate as the first occurrence of a positive blood culture and a recurrent Ec-BSI isolate as a positive blood culture that occurred at least 14 days from a previous Ec-BSI. Antibiotic susceptibility testing of all Ec-BSI isolates was performed by the MDACC microbiology laboratory using the Accelerate PhenoTest BC (Accelerate Diagnostics), ETEST (bioMérieux), and VITEK 2 (bioMérieux). Ec-BSI isolates were considered extended-spectrum cephalosporin-resistant (ESC-R) if they had a ceftriaxone (CRO) MIC ≥ 4 µg/mL and/or a predicted ESBL phenotype.
Genomic sequencing
ESC-R-Ec isolates were sequenced by Illumina NovaSeq 6000 or Illumina MiSeq as previously described (33). There were 297 ESC-R isolates that had paired-end 150-base pair (bp) reads that passed quality control assessed using fastqc-v0.11.9. We performed long-read sequencing on nine isolates using the Oxford Nanopore Technologies GridION platform as described previously (33).
Genome analysis and molecular typing
Quality assessed, paired-end 150-bp reads were assembled via SPAdes-v3.15.2 (49) with default parameters and the inclusion of the isolate option. Quality assessment of output SPAdes assembly contigs was performed via QUAST-v5.2.0 (50) to determine N50 and contiguity. Assemblies with (i) genomes < 4 Mb or > 6 Mb or (ii) contigs > 500 were excluded from the analysis. The EzClermont in silico phylotyping tool was utilized with the 2013 Clermont PCR typing method (Waters, N EzClermont Github: https://github.com/nickp60/EzClermont). E. coli multi-locus sequence typing using the Achtman definition was performed with the mlst-v2.19.0 in silico contig scanning tool (Seemann T mlst GitHub: https://github.com/tseemann/mlst), which features the PUBMLST database (51). The COpy Number Variant quantifICation Tool (convict-v1.0) was used to identify AMR genes and estimate gene copy numbers as described previously (Shropshire, W convict GitHub: https://github.com/wshropshire/convict) (33). Briefly, short-read data are used as input to KmerResistance-v2.2.0 (52, 53) to identify potential AMR genes from the resfinder database (accessed 1 October 2022). Through a coverage depth ratio of AMR gene to housekeeping gene that accounts for variance in coverage depths, convict estimates gene copy numbers. The high-performance computing cluster, Seadragon, which is hosted through MDACC was used to perform genomic analyses.
Pangenome analysis
Draft and complete genomes were annotated using the Prokka-v1.14.6 (54) using default parameters. Annotated gff files from prokka were used as input for pangenome analysis using Roary-v3.13.0 (55) using a protein family ID threshold of 95%. A core gene alignment was completed using Mafft-v7.508 with the Roary pipeline using a core gene threshold of 99%. This core gene alignment file was used as input to IQTree2-v2.2.0-beta to create a core gene inferred maximum likelihood phylogeny. ModelFinder was utilized to determine the most appropriate DNA model with the best-fit model: UNREST + FO + I + G4 chosen according to BIC. A final bootstrap consensus tree was created using 1,000 SH-like aLRT replicates in addition to 1,000 ultrafast bootstrap (UFboot) support values. A core genome alignment was performed on the top 12 STs observed in our cohort using Snippy-v4.6.0 using default variant calling parameters. The following were the chosen references for alignment: (ST131: CP049085.2; ST405: CP103633.1; ST1193: MB11100; ST648: CP103657.1; ST744: MB7355; ST10: CP049081.1; ST167: CP103540.1; ST38: CP103559.1; ST617: CP103645.1; ST410: CP103533.1; ST224: CP103609.1; ST361: CP103704.1). A core genome alignment was used as input for Gubbins (56) to create a recombination-free, maximum likelihood tree using IQTree20v.2.2.0 using the aforementioned parameters. The recombination-free fasta output was used to extract pairwise SNP sites (snp-sites-v2.5.1) (57) and SNP distances (snp-dists-v0.8.2), respectively. A pairwise SNP distance cutoff of 10 for window-independent analyses of transmission was based on the recombination-free, core-genome SNP differences of eight pairwise comparisons of ST131 recurrent isolates, where the median pairwise SNP difference was 5.5 SNPs and the maximum observed was 9 SNPs.
The accessory genome was assessed using the gene_presence_absence.csv file output from Roary. A Jaccard distance matrix was created using this binary accessory genome file after removing genes present in < 5% and > 95% of the total population. This distance matrix was used as input to create a NJ tree using the BIONJ algorithm (58). We performed a principal component analysis of this distance matrix using base R functions. The graph network analysis was accomplished using the same gene_presence_absence.csv input using Graphia (59, 60). Edge transformations using a k-nearest neighbors algorithm (n = 8) were employed to reduce the number of edges from 44k to 1,724. Clustering was performed using the Markov Cluster algorithm (MCL) with an inflation value = 1.5 (61). Visualization of data were accomplished using ggplot2-v3.3.5 (62) and ggtree-v.3.3.1 (63).
Long-read analysis
A short- and long-read assembly pipeline (Shropshire, W flye_hybrid_assembly_pipeline GitHub: https://github.com/wshropshire/flye_hybrid_assembly_pipeline) was used to close complete genomes of ONT-sequenced data as described previously (33). Incomplete assemblies were re-assembled using Unicycler-v0.5.0 and manually curated for errors using short- and long-read pile-ups and visualizing with the integrated genome browser (IGV-v2.14.1). Comparison of plasmid vectors with BLAST ring image generator (BLAST) was performed using the Proksee server while querying AMR genes with the Comprehensive Antimicrobial Resistance Database (CARD) (64).
Statistical analysis
Prevalence rates were plotted across time using ggplot2 with a smoothing curve (i.e., a loess function) to visualize prevalence trends. Time series data were assessed for monotonic trends using the Mann-Kendall trend test and non-monotonic using the WAVK statistic. Correlations between ESC-R-Ec and ESC-S-Ec were measured with a Pearson’s product-moment correlation test. We used χ2 test (Fisher’s exact when observations < 5) to determine statistical differences in proportions across phylogroup/clonal complexes. A Kruskal-Wallis global test to determine differences in gene copy numbers across groups. Pairwise and one-way Wilcoxon rank sum tests were to determine group-level and single-mean differences, respectively, with a Bonferroni correction for multiple comparisons. All statistics were computed on R (4.0.4), and α = 0.05 parameter was used across all tests.
ACKNOWLEDGMENTS
We would like to thank the MDACC clinical microbiology lab for all their hard work in identifying, handling, and transferring these pathogenic strains of interest to us for our research projects. The authors acknowledge the support of the High-Performance Computing for research facility at the University of Texas MDACC for providing computational resources that have contributed to the research results reported in this paper.
Core grant CA016672(ATGC) and National Institutes of Health 1S10OD024977-01 grant provide funding for the Advanced Technology Genomics Core (ATGC) sequencing facility at MDACC. W.C.S. is supported through the National Institute of Allergy and Infectious Diseases (NIAID) T32 AI141349 Training Program in Antimicrobial Resistance. Support for this study was also provided by the NIAID R21AI151536 and P01AI152999 for S.A.S. The research in the A.K. laboratory is supported by NIGMS 1R01GM133904-01 and the Welch Foundation Research Grant AU-1998-20190330.
Contributor Information
Samuel A. Shelburne, Email: sshelburne@mdanderson.org.
Ana Cristina Gales, Escola Paulista de Medicina/Universidade Federal de São Paulo, São Paulo, Brazil .
DATA AVAILABILITY
WGS data sequenced during this study period was submitted to National Center for Biotechnology Information and can be accessed within BioProject PRJNA924946. WGS data from previous studies can be accessed from BioProject no. PRJNA603908 and PRJNA836696 (33, 65). All R Scripts can be made available through the request to the corresponding author.
ETHICS APPROVAL
The MDACC IRB approved the collection of clinical isolates with de-identified data on 7/5/2022 (IRB No. 2021-0487_MODCR001).
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/msphere.00183-23.
Fig. S1 to S8.
Tables S1 to S5.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. CDC . 2019. Antibiotic resistance threats in the united states,2019. U.S. Department of Health and Human Services, CDC, Atlanta, GA. [Google Scholar]
- 2. Jernigan JA, Hatfield KM, Wolford H, Nelson RE, Olubajo B, Reddy SC, McCarthy N, Paul P, McDonald LC, Kallen A, Fiore A, Craig M, Baggs J. 2020. Multidrug-resistant bacterial infections in U.S. hospitalized patients, 2012. N Engl J Med 382:1309–1319. doi: 10.1056/NEJMoa1914433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kaye KS, Gupta V, Mulgirigama A, Joshi AV, Scangarella-Oman NE, Yu K, Ye G, Mitrani-Gold FS. 2021. Antimicrobial resistance trends in urine Escherichia coli isolates from adult and adolescent females in the United States from 2011 to 2019: rising ESBL strains and impact on patient management. Clin Infect Dis 73:1992–1999. doi: 10.1093/cid/ciab560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Begier E, Rosenthal NA, Gurtman A, Kartashov A, Donald RGK, Lockhart SP. 2021. Epidemiology of invasive Escherichia coli infection and antibiotic resistance status among patients treated in US hospitals: 2009-2016. Clin Infect Dis 73:565–574. doi: 10.1093/cid/ciab005 [DOI] [PubMed] [Google Scholar]
- 5. MacKinnon MC, McEwen SA, Pearl DL, Lyytikäinen O, Jacobsson G, Collignon P, Gregson DB, Valiquette L, Laupland KB. 2021. Mortality in Escherichia coli bloodstream infections: a multinational population-based cohort study. BMC Infect Dis 21:606. doi: 10.1186/s12879-021-06326-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. MacKinnon MC, McEwen SA, Pearl DL, Parfitt EC, Pasquill K, Steele L, Laupland KB. 2021. Escherichia coli bloodstream infections in the western interior of British Columbia, Canada: a population-based cohort study. Epidemiol Infect 149:e195. doi: 10.1017/S0950268821001874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Trecarichi EM, Giuliano G, Cattaneo C, Ballanti S, Criscuolo M, Candoni A, Marchesi F, Laurino M, Dargenio M, Fanci R, Cefalo M, Delia M, Spolzino A, Maracci L, Nadali G, Busca A, Del Principe MI, Daffini R, Simonetti E, Dragonetti G, Zannier ME, Pagano L, Tumbarello M, Haematologic Malignancies Associated Bloodstream Infections Surveillance (HEMABIS) registry–Sorveglianza Epidemiologica Infezioni Fungine in Emopatie Maligne (SEIFEM) group, Italy . 2019. Bloodstream infections caused by Escherichia coli in onco-haematological patients: risk factors and mortality in an Italian prospective survey. PLoS ONE 14:e0224465. doi: 10.1371/journal.pone.0224465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Philippon A, Arlet G, Jacoby GA. 2002. Plasmid-determined AmpC-type β-lactamases. Antimicrob Agents Chemother 46:1–11. doi: 10.1128/AAC.46.1.1-11.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zamudio R, Boerlin P, Beyrouthy R, Madec J-Y, Schwarz S, Mulvey MR, Zhanel GG, Cormier A, Chalmers G, Bonnet R, Haenni M, Eichhorn I, Kaspar H, Garcia-Fierro R, Wood JLN, Mather AE. 2022. Dynamics of extended-spectrum cephalosporin resistance genes in Escherichia coli from Europe and North America. Nat Commun 13:7490. doi: 10.1038/s41467-022-34970-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tamma PD, Sharara SL, Pana ZD, Amoah J, Fisher SL, Tekle T, Doi Y, Simner PJ. 2019. Molecular epidemiology of ceftriaxone-nonsusceptible enterobacterales isolates in an academic medical center in the United States. Open Forum Infect Dis 6:ofz353. doi: 10.1093/ofid/ofz353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Castanheira M, Deshpande LM, Mendes RE, Canton R, Sader HS, Jones RN. 2019. Variations in the occurrence of resistance phenotypes and carbapenemase genes among Enterobacteriaceae isolates in 20 years of the SENTRY antimicrobial surveillance program. Open Forum Infect Dis 6:S23–S33. doi: 10.1093/ofid/ofy347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bush K, Jacoby GA. 2010. Updated functional classification of β-lactamases. Antimicrob Agents Chemother 54:969–976. doi: 10.1128/AAC.01009-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Perez F, Endimiani A, Hujer KM, Bonomo RA. 2007. The continuing challenge of ESBLs. Curr Opin Pharmacol 7:459–469. doi: 10.1016/j.coph.2007.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stoesser N, Sheppard AE, Pankhurst L, De Maio N, Moore CE, Sebra R, Turner P, Anson LW, Kasarskis A, Batty EM, Kos V, Wilson DJ, Phetsouvanh R, Wyllie D, Sokurenko E, Manges AR, Johnson TJ, Price LB, Peto TEA, Johnson JR, Didelot X, Walker AS, Crook DW, Rasko DA, Keim PS, Modernizing Medical Microbiology Informatics Group (MMMIG) . 2016. Evolutionary history of the global emergence of the Escherichia coli epidemic clone ST131. mBio 7:e02162. doi: 10.1128/mBio.02162-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Price LB, Johnson JR, Aziz M, Clabots C, Johnston B, Tchesnokova V, Nordstrom L, Billig M, Chattopadhyay S, Stegger M, Andersen PS, Pearson T, Riddell K, Rogers P, Scholes D, Kahl B, Keim P, Sokurenko EV. 2013. The epidemic of extended-spectrum-β-Lactamase-producing Escherichia Coli St131 is driven by a single highly pathogenic Subclone. mBio 4:e00377-13. doi: 10.1128/mBio.00377-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lipworth S, Vihta K-D, Chau K, Barker L, George S, Kavanagh J, Davies T, Vaughan A, Andersson M, Jeffery K, Oakley S, Morgan M, Hopkins S, Peto TEA, Crook DW, Walker AS, Stoesser N. 2021. Ten-year longitudinal molecular epidemiology study of Escherichia coli and Klebsiella species bloodstream infections in Oxfordshire, UK. Genome Med 13:144. doi: 10.1186/s13073-021-00947-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Royer G, Darty MM, Clermont O, Condamine B, Laouenan C, Decousser J-W, Vallenet D, Lefort A, de Lastours V, Denamur E, COLIBAFI and SEPTICOLI groups . 2021. Phylogroup stability contrasts with high within sequence type complex dynamics of Escherichia coli bloodstream infection isolates over a 12-year period. Genome Med 13:77. doi: 10.1186/s13073-021-00892-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Verschuuren TD, van Hout D, Arredondo-Alonso S, Fluit AC, Reuland EA, Top J, Schürch AC, Bosch T, Bonten MJM, Kluytmans JAJW, Willems RJL. 2021. Comparative genomics of ESBL-producing Escherichia coli (ESBL-Ec) reveals a similar distribution of the 10 most prevalent ESBL-EC clones and ESBL genes among human community faecal and extra-intestinal infection isolates in the Netherlands (2014-17). J Antimicrob Chemother 76:901–908. doi: 10.1093/jac/dkaa534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kallonen T, Brodrick HJ, Harris SR, Corander J, Brown NM, Martin V, Peacock SJ, Parkhill J. 2017. Systematic longitudinal survey of invasive Escherichia Coli in England demonstrates a stable population structure only transiently disturbed by the emergence of ST131. Genome Res 27:1437–1449. doi: 10.1101/gr.216606.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gladstone RA, McNally A, Pöntinen AK, Tonkin-Hill G, Lees JA, Skytén K, Cléon F, Christensen MOK, Haldorsen BC, Bye KK, Gammelsrud KW, Hjetland R, Kümmel A, Larsen HE, Lindemann PC, Löhr IH, Å M, Nilsen E, Noer MT, Simonsen GS, Steinbakk M, Tofteland S, Vattøy M, Bentley SD, Croucher NJ, Parkhill J, Johnsen PJ, Samuelsen Ø, Corander J. 2021. Emergence and dissemination of antimicrobial resistance in Escherichia Coli causing bloodstream infections in Norway in 2002–17: a nationwide, longitudinal, microbial population genomic study. Lancet Microbe 2:e331–e341. doi: 10.1016/S2666-5247(21)00031-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rodríguez I, Figueiredo AS, Sousa M, Aracil-Gisbert S, Fernández-de-Bobadilla MD, Lanza VF, Rodríguez C, Zamora J, Loza E, Mingo P, Brooks CJ, Cantón R, Baquero F, Coque TM. 2021. A 21-year survey of Escherichia coli from bloodstream infections (BSI) in a tertiary hospital reveals how community-hospital dynamics of B2 phylogroup clones influence local BSI rates. mSphere 6:e0086821. doi: 10.1128/msphere.00868-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Peirano G, Lynch T, Matsumara Y, Nobrega D, Finn TJ, Devinney R, Pitout JDD. 2020. Trends in population dynamics of Escherichia coli sequence type 131. Emerging Infect Dis 26:2907-2915. doi: 10.3201/eid2612.201221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mills EG, Martin MJ, Luo TL, Ong AC, Maybank R, Corey BW, Harless C, Preston LN, Rosado-Mendez JA, Preston SB, Kwak YI, Backlund MG, Bennett JW, Mc Gann PT, Lebreton F. 2022. A one-year genomic investigation of Escherichia coli epidemiology and nosocomial spread at a large US healthcare network. Genome Med 14:147. doi: 10.1186/s13073-022-01150-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Manges AR, Geum HM, Guo A, Edens TJ, Fibke CD, Pitout JDD. 2019. Global extraintestinal pathogenic Escherichia coli (ExPEC) lineages. Clin Microbiol Rev 32:e00135-18. doi: 10.1128/CMR.00135-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Banerjee R, Johnson JR. 2014. A new clone sweeps clean: the enigmatic emergence of Escherichia coli sequence type 131. Antimicrob Agents Chemother 58:4997–5004. doi: 10.1128/AAC.02824-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McNally A, Kallonen T, Connor C, Abudahab K, Aanensen DM, Horner C, Peacock SJ, Parkhill J, Croucher NJ, Corander J. 2019. Diversification of colonization factors in a multidrug-resistant Escherichia coli lineage evolving under negative frequency-dependent selection. mBio 10:e00644-19. doi: 10.1128/mBio.00644-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Johnson JR, Porter S, Thuras P, Castanheira M. 2017. The pandemic H30 subclone of sequence type 131 (ST131) as the leading cause of multidrug-resistant Escherichia coli infections in the United States. Open Forum Infect Dis 4:ofx089. doi: 10.1093/ofid/ofx089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Matsumura Y, Pitout JDD, Gomi R, Matsuda T, Noguchi T, Yamamoto M, Peirano G, DeVinney R, Bradford PA, Motyl MR, Tanaka M, Nagao M, Takakura S, Ichiyama S. 2016. Global Escherichia coli sequence type 131 clade with blaCTX-M-27 gene. Emerg Infect Dis 22:1900–1907. doi: 10.3201/eid2211.160519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hansen KH, Andreasen MR, Pedersen MS, Westh H, Jelsbak L, Schønning K. 2019. Resistance to piperacillin/tazobactam in Escherichia coli resulting from extensive IS26-associated gene amplification of blaTEM-1. J Antimicrob Chemother 74:3179–3183. doi: 10.1093/jac/dkz349 [DOI] [PubMed] [Google Scholar]
- 30. Rodríguez-Villodres Á, Gil-Marqués ML, Álvarez-Marín R, Bonnin RA, Pachón-Ibáñez ME, Aguilar-Guisado M, Naas T, Aznar J, Pachón J, Lepe JA, Smani Y. 2019. Extended-spectrum resistance to beta-lactams/ beta-lactamase inhibitors (ESRI) evolved from low-level resistant Escherichia coli. J Antimicrob Chemother 75:77–85. doi: 10.1093/jac/dkz393 [DOI] [PubMed] [Google Scholar]
- 31. Nicoloff H, Hjort K, Levin BR, Andersson DI. 2019. The high prevalence of antibiotic heteroresistance in pathogenic bacteria is mainly caused by gene amplification. Nat Microbiol 4:504–514. doi: 10.1038/s41564-018-0342-0 [DOI] [PubMed] [Google Scholar]
- 32. Shropshire WC, Aitken SL, Pifer R, Kim J, Bhatti MM, Li X, Kalia A, Galloway-Peña J, Sahasrabhojane P, Arias CA, Greenberg DE, Hanson BM, Shelburne SA. 2021. IS26-mediated amplification of blaOXA-1 and blaCTX-M-15 with concurrent outer membrane porin disruption associated with de novo carbapenem resistance in a recurrent bacteraemia cohort. J Antimicrob Chemother 76:385–395. doi: 10.1093/jac/dkaa447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shropshire WC, Konovalova A, McDaneld P, Gohel M, Strope B, Sahasrabhojane P, Tran CN, Greenberg D, Kim J, Zhan X, Aitken S, Bhatti M, Savidge TC, Treangen TJ, Hanson BM, Arias CA, Shelburne SA. 2022. Systematic analysis of mobile genetic elements mediating beta-lactamase gene amplification in noncarbapenemase-producing carbapenem-resistant enterobacterales bloodstream infections. mSystems 7:e0047622. doi: 10.1128/msystems.00476-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mahmud B, Wallace MA, Reske KA, Alvarado K, Muenks CE, Rasmussen DA, Burnham C-A, Lanzas C, Dubberke ER, Dantas G. 2022. Epidemiology of plasmid lineages mediating the spread of extended-spectrum beta-lactamases among clinical Escherichia coli. mSystems 7:e0051922. doi: 10.1128/msystems.00519-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cai SJ, Inouye M. 2002. EnvZ-OmpR interaction and osmoregulation in Escherichia coli. J Biol Chem 277:24155–24161. doi: 10.1074/jbc.M110715200 [DOI] [PubMed] [Google Scholar]
- 36. Anesi JA, Lautenbach E, Tamma PD, Thom KA, Blumberg EA, Alby K, Bilker WB, Werzen A, Tolomeo P, Omorogbe J, Pineles L, Han JH. 2021. Risk factors for extended-spectrum beta-Lactamase-producing enterobacterales bloodstream infection among solid-organ transplant recipients. Clin Infect Dis 72:953–960. doi: 10.1093/cid/ciaa190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Johnson JR, Urban C, Weissman SJ, Jorgensen JH, Lewis JS, Hansen G, Edelstein PH, Robicsek A, Cleary T, Adachi J, Paterson D, Quinn J, Hanson ND, Johnston BD, Clabots C, Kuskowski MA, AMERECUS Investigators . 2012. Molecular Epidemiological analysis of Escherichia coli sequence type St131 (O25:H4) and blaCTX-M-15 among extended-spectrum-Β-Lactamase-producing E. coli from the United States, 2000 to 2009. Antimicrob Agents Chemother 56:2364–2370. doi: 10.1128/AAC.05824-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gradel KO, Nielsen SL, Pedersen C, Knudsen JD, Østergaard C, Arpi M, Jensen TG, Kolmos HJ, Søgaard M, Lassen AT, Schønheyder HC, Danish Collaborative Bacteraemia Network (DACOBAN) and the Danish Observational Registry of Infectious Syndromes (DORIS) . 2016. Seasonal variation of Escherichia coli, Staphylococcus aureus, and Streptococcus pneumoniae bacteremia according to acquisition and patient characteristics: a population-based study. Infect Control Hosp Epidemiol 37:946–953. doi: 10.1017/ice.2016.89 [DOI] [PubMed] [Google Scholar]
- 39. Wielders CCH, Van Duijkeren E, Van Den Bunt G, Meijs AP, Dierikx CM, Bonten MJM, Van Pelt W, Franz E, De Greeff SC. 2020. Seasonality in carriage of extended-spectrum β-lactamase-producing Escherichia Coli and Klebsiella pneumoniae in the general population: a pooled analysis of nationwide cross-sectional studies. Epidemiol Infect 148:e68. doi: 10.1017/S0950268820000539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Deeny SR, van Kleef E, Bou-Antoun S, Hope RJ, Robotham JV. 2015. Seasonal changes in the incidence of Escherichia coli bloodstream infection: variation with region and place of onset. Clin Microbiol Infect 21:924–929. doi: 10.1016/j.cmi.2015.06.023 [DOI] [PubMed] [Google Scholar]
- 41. Maryam S, Ul Haq I, Yahya G, Ul Haq M, Algammal AM, Saber S, Cavalu S. 2022. COVID-19 surveillance in wastewater: an epidemiological tool for the monitoring of SARS-CoV-2. Front Cell Infect Microbiol 12:978643. doi: 10.3389/fcimb.2022.978643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wolken M, Sun T, McCall C, Schneider R, Caton K, Hundley C, Hopkins L, Ensor K, Domakonda K, Kalvapalle P, Persse D, Williams S, Stadler LB. 2023. Wastewater surveillance of SARS-CoV-2 and influenza in preK-12 schools shows school, community, and citywide infections. Water Res 231:119648. doi: 10.1016/j.watres.2023.119648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Johnson TJ, Danzeisen JL, Youmans B, Case K, Llop K, Munoz-Aguayo J, Flores-Figueroa C, Aziz M, Stoesser N, Sokurenko E, Price LB, Johnson JR, Castanheira M. 2016. Separate F-type plasmids have shaped the evolution of the H 30 subclone of Escherichia coli sequence type 131. mSphere 1:e00121-16. doi: 10.1128/mSphere.00121-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yoon E-J, Gwon B, Liu C, Kim D, Won D, Park SG, Choi JR, Jeong SH, Bulman Z. 2020. Beneficial chromosomal integration of the genes for CTX-M extended-spectrum β-lactamase in Klebsiella pneumoniae for stable propagation. mSystems 5:e00459-20. doi: 10.1128/mSystems.00459-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yoon E-J, Choi YJ, Kim D, Won D, Choi JR, Jeong SH, Garcia-Solache MA. 2022. Amplification of the chromosomal bla CTX-M-14 gene in Escherichia coli expanding the spectrum of resistance under antimicrobial pressure. Microbiol Spectr 10:e0031922. doi: 10.1128/spectrum.00319-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zeng S, Luo J, Li X, Zhuo C, Wu A, Chen X, Huang L. 2021. Molecular epidemiology and characteristics of CTX-M-55 extended-spectrum beta-Lactamase-producing Escherichia coli from Guangzhou, China. Front Microbiol 12:730012. doi: 10.3389/fmicb.2021.730012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Feldman SF, Temkin E, Wulffhart L, Nutman A, Schechner V, Shitrit P, Shvartz R, Schwaber MJ, Carmeli Y. 2022. Effect of temperature on Escherichia coli bloodstream infection in a nationwide population-based study of incidence and resistance. Antimicrob Resist Infect Control 11:1–9. doi: 10.1186/s13756-022-01184-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. CDC . 2022. COVID-19: US impact on antimicrobial resistance, special report 2022. U.S. Department of Health and Human Services, CDC, Atlanta, GA. [Google Scholar]
- 49. Prjibelski A, Antipov D, Meleshko D, Lapidus A, Korobeynikov A. 2020. Using SPAdes de novo assembler. Curr Protoc Bioinformatics 70:e102. doi: 10.1002/cpbi.102 [DOI] [PubMed] [Google Scholar]
- 50. Mikheenko A, Prjibelski A, Saveliev V, Antipov D, Gurevich A. 2018. Versatile genome assembly evaluation with QUAST-LG. Bioinformatics 34:i142–i150. doi: 10.1093/bioinformatics/bty266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jolley KA, Maiden MCJ. 2010. BIGSdb: scalable analysis of bacterial genome variation at the population level. BMC Bioinformatics 11:595. doi: 10.1186/1471-2105-11-595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Clausen PTLC, Zankari E, Aarestrup FM, Lund O. 2016. Benchmarking of methods for identification of antimicrobial resistance genes in bacterial whole genome data. J Antimicrob Chemother 71:2484–2488. doi: 10.1093/jac/dkw184 [DOI] [PubMed] [Google Scholar]
- 53. Clausen PTLC, Aarestrup FM, Lund O. 2018. Rapid and precise alignment of raw reads against redundant databases with KMA. BMC Bioinformatics 19:307. doi: 10.1186/s12859-018-2336-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Seemann T. 2014. Prokka: rapid prokaryotic genome annotation. Bioinformatics 30:2068–2069. doi: 10.1093/bioinformatics/btu153 [DOI] [PubMed] [Google Scholar]
- 55. Page AJ, Cummins CA, Hunt M, Wong VK, Reuter S, Holden MTG, Fookes M, Falush D, Keane JA, Parkhill J. 2015. Roary: rapid large-scale prokaryote pan genome analysis. Bioinformatics 31:3691–3693. doi: 10.1093/bioinformatics/btv421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Croucher NJ, Page AJ, Connor TR, Delaney AJ, Keane JA, Bentley SD, Parkhill J, Harris SR. 2015. Rapid phylogenetic analysis of large samples of recombinant bacterial whole genome sequences using gubbins. Nucleic Acids Res 43:e15. doi: 10.1093/nar/gku1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Page AJ, Taylor B, Delaney AJ, Soares J, Seemann T, Keane JA, Harris SR. 2016. SNP-sites: rapid efficient extraction of SNPs from multi-FASTA alignments. Microb Genom 2:e000056. doi: 10.1099/mgen.0.000056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gascuel O. 1997. BIONJ: an improved version of the NJ algorithm based on a simple model of sequence data. Mol Biol Evol 14:685–695. doi: 10.1093/oxfordjournals.molbev.a025808 [DOI] [PubMed] [Google Scholar]
- 59. Freeman TC, Horsewell S, Patir A, Harling-Lee J, Regan T, Shih BB, Prendergast J, Hume DA, Angus T. 2022. Graphia: a platform for the graph-based visualisation and analysis of high dimensional data. PLOS Comput Biol 18:e1010310. doi: 10.1371/journal.pcbi.1010310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Harling-Lee JD, Gorzynski J, Yebra G, Angus T, Fitzgerald JR, Freeman TC. 2022. A graph-based approach for the visualisation and analysis of bacterial pangenomes. BMC Bioinformatics 23:1–15. doi: 10.1186/s12859-022-04898-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Van Dongen S. 2008. Graph clustering via a discrete uncoupling process. SIAM J Matrix Anal & Appl 30:121–141. doi: 10.1137/040608635 [DOI] [Google Scholar]
- 62. Wickham H. 2016. ggplot2: elegant graphics for data analysis. Springer-Verlag, New York. [Google Scholar]
- 63. Yu G, Smith DK, Zhu H, Guan Y, TTY L. 2017. ggtree: package for visualization and annotation of phylogenetic trees with their covariates and other associated data. Methods Ecol Evol 8:28–36. doi: 10.1111/2041-210X.12628 [DOI] [Google Scholar]
- 64. Jia B, Raphenya AR, Alcock B, Waglechner N, Guo P, Tsang KK, Lago BA, Dave BM, Pereira S, Sharma AN, Doshi S, Courtot M, Lo R, Williams LE, Frye JG, Elsayegh T, Sardar D, Westman EL, Pawlowski AC, Johnson TA, Brinkman FSL, Wright GD, McArthur AG. 2017. CARD 2017: expansion and model-centric curation of the comprehensive antibiotic resistance database. Nucleic Acids Res 45:D566–D573. doi: 10.1093/nar/gkw1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Shelburne SA, Kim J, Munita JM, Sahasrabhojane P, Shields RK, Press EG, Li X, Arias CA, Cantarel B, Jiang Y, Kim MS, Aitken SL, Greenberg DE. 2017. Whole-genome sequencing accurately identifies resistance to extended-spectrum beta-lactams for major gram-negative bacterial pathogens. Clin Infect Dis 65:738–745. doi: 10.1093/cid/cix417 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 to S8.
Tables S1 to S5.
Data Availability Statement
WGS data sequenced during this study period was submitted to National Center for Biotechnology Information and can be accessed within BioProject PRJNA924946. WGS data from previous studies can be accessed from BioProject no. PRJNA603908 and PRJNA836696 (33, 65). All R Scripts can be made available through the request to the corresponding author.