Abstract
Background:
Transfusion-associated circulatory overload (TACO) is a severe adverse reaction (AR) contributing to the leading cause of mortality associated with transfusions. As strategies to mitigate TACO have been increasingly adopted, an update of prevalence rates and risk factors associated with TACO using the growing sources of electronic health record (EHR) data can help understand transfusion safety.
Study Design and Methods:
This retrospective study aimed to provide a timely and reproducible assessment of prevalence rates and risk factors associated with TACO. Novel natural language processing (NLP) methods, now made publicly available on GitHub, were developed to extract ARs from 3,178 transfusion reaction reports. Other patient-level data were extracted computationally from UCSF EHR between 2012 and 2022. The odds ratio estimates of risk factors were calculated using a multivariate logistic regression analysis with case-to-control matched on sex and age at a ratio of 1:5.
Results:
A total of 56,208 patients received transfusions (total 573,533 units) at UCSF during the study period and 102 patients developed TACO. The prevalence of TACO was estimated to be 0.2% per patient (102/ total 56,208). Patients with a history of coagulopathy [OR, 1.36; 95% CI, 1.04–1.79] and transplant [OR, 1.99; 95% CI, 1.48–2.68] were associated with increased odds of TACO.
Discussion:
While TACO is a serious adverse reaction, events remained rare, even in populations enriched with high-risk patients. Novel computational methods can be used to find and continually surveil for transfusion ARs. Results suggest that patients with history or presence of coagulopathy and organ transplant should be carefully monitored to mitigate potential risks of TACO.
INTRODUCTION
Blood transfusions have been associated with various types of adverse reactions ranging from the most commonly reported, febrile non-hemolytic transfusion reactions (FNHTR) or allergic reactions to rare but potentially life-threatening reactions such as transfusion-related acute lung injury (TRALI) and transfusion-associated circulatory overload (TACO).1,2 TACO, described as having volume overload following transfusion, is a severe reaction that has been recognized as the leading cause of mortality associated with blood transfusions.2 While the pathophysiology of TACO has not been well-studied, it has been primarily attributed to poor adaptability to volume changes.3,4 Classic clinical presentations include hydrostatic pulmonary edema, positive fluid balance, and hypoxemia.5
Rates of TACO reported in prior published literature vary from 1 to 12% of transfused patients.5,6 Notable risk factors known to be associated with TACO include history of heart failure, renal failure, coagulopathy, and exposure to plasma or receiving multiple blood products concurrently.5,7–12 While diuretics are effective in removing fluids in patients, clinical benefits of pre-medicating patients with diuretics to prevent TACO have not been conclusive.13–15 In addition, a recent analysis published by Roubinian et al indicated the increased risks of developing TACO with the use of diuretics prior to transfusions.7 Although prior studies have shed light on rates and potential risk factors associated with TACO, many of the study populations were limited more than five to a decade ago.5,9,16–19 As awareness and strategies to mitigate TACO have been broadly adopted to improve blood transfusion safety in recent years, an update of rates and potential risk factors associated with TACO could be useful to understand blood transfusion safety in the context of current clinical practices.
This study aimed to provide a new assessment of prevalence of TACO and risks associated with development of TACO in patient populations enriched with high-risk patients, using a now nearly ubiquitous source of clinical data: electronic health records (EHR). We developed a novel natural language processing algorithm to automate the identification of TACO cases in transfusion reaction workup reports at our academic health center. Subsequently, we investigated potential risk factors with patient-level characteristics including comorbidities, medication exposures, and blood transfusion exposures using a multivariate logistic regression parsimonious model.
METHODS
Data Sources
This retrospective case-control study using clinical data collected through routine clinical care was approved by the UCSF Institutional Review Board (protocol 20–31164). All patient-level data pertinent to clinical care were extracted from the de-identified University of California San Francisco Clinical Data Warehouse (UCSF CDW) on September 6, 2022. UCSF is a tertiary academic treatment center. The CDW, which refreshes every three months, contains structured electronic health records (EHR) of approximately 5 million unique patients at UCSF.
A total of 3,178 unstructured transfusion reaction reports documenting potential reactions from 2,202 unique patients between 2012 and August 30, 2022 were retrieved by the UCSF Clinical and Translational Science Institute. These reports, known as the “Transfusion Reaction Workup”, document the reviews and assessments of any potential transfusion reactions reported at UCSF and were carried out by clinicians specialized in laboratory medicine. The assessments follow definitions described in the National Healthcare Safety Network (NHSN) Hemovigilance Module Surveillance Protocol published by the Centers for Disease Control and Prevention.20 Briefly, the definitions included case definitions that define types of transfusion reaction, severity definitions describing varying levels of reaction severity, and imputability definitions that classify the likelihood of the reaction being attributed to blood transfusion.20 The semi-structured parts of the transfusion reaction workup reports provides the diagnosis of the reaction and is amenable to regular expression-based (rule-based) extraction since it is included in all transfusion reaction workup reports.
Study Population
A total of 56,208 unique patients who received blood transfusions at least once at UCSF between January 1, 2012 and August 30, 2022, a total of 2,202 (3.9%, 2,202/56,208) unique patients developed potential reactions which subsequently lead to transfusion reaction investigations and the resulting a total of 3,178 transfusion reaction reports. The overall patient populations who received blood transfusion during the study period will be used to calculate the prevalence rate of TACO. In the case-control study investigating potential risk factors associated with TACO, we focused on patients who received inpatient transfusion as these may represent higher risk populations.
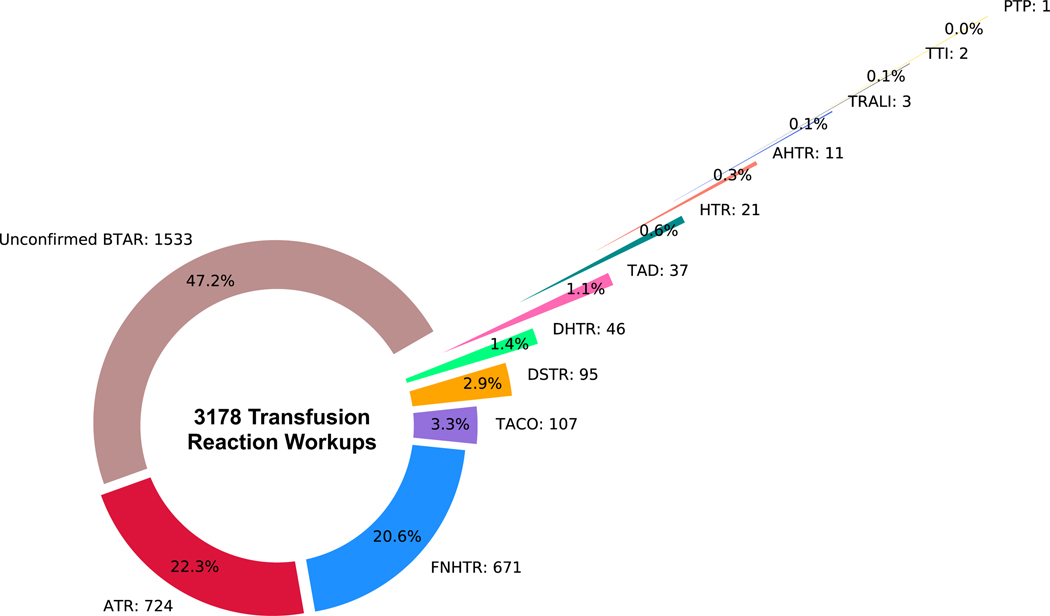
Patients with TACO were identified from the transfusion reaction report using a custom-written rule-based natural language processing (NLP) algorithm described below (hereafter termed the NLP algorithm) (Figure 1). Any transfusion reaction with imputability deemed less than “definite” or “probable” was considered not to be attributed to the blood transfusion. Hence, the true TACO cases included in this study were limited to 1) when “TACO” or “transfusion-associated circulatory overload” were diagnosed as the transfusion reaction type and 2) the imputability was deemed either definite or probable by the clinician. Transfusion reactions found in 1,533 (48.2%) of 3,178 transfusion reaction reports were deemed not attributed to blood transfusion with imputatbility less than “definite” or “probable” (Figure 2).
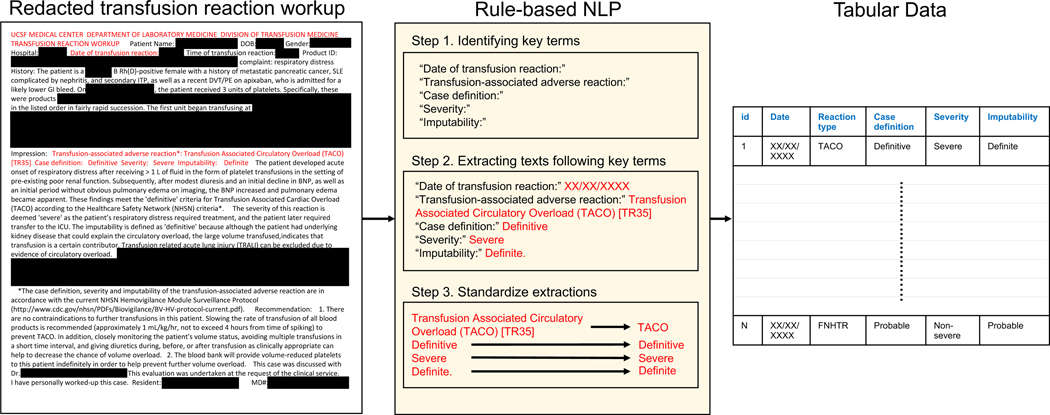
Figure 1. Blood transfusion reaction extractions using a rule-based NLP algorithm.
This illustration shows the workflow of transforming semi-structured transfusion reaction workups to tabular data using a rule-based NLP algorithm developed specifically to extract features of transfusion reactions including transfusion reaction date, type, case definition, severity, and imputability. The transfusion reaction workup report shown here has been truncated and redacted for illustration purposes. NLP: Natural language processing.
Figure 2. Adverse reactions extracted from transfusion reaction reports.
The donut chart shows the breakdown of types of adverse reactions attributed to blood transfusions found in 3178 transfusion reports from 2202 unique patients. BTAR: Blood transfusion adverse reaction, ATR: Allergic transfusion reaction, FNHTR: Febrile non-hemolytic transfusion reaction, TACO: Transfusion- associated circulatory overload, DSTR: Delayed serologic transfusion reaction, DHTR: Delayed hemolytic transfusion reaction, HTR: Hypotensive transfusion reaction, TAD: Transfusion-associated dyspnea, AHTR: Acute hemolytic transfusion reaction, TTI: Transfusion-transmitted infection, TRALI: Transfusion-related acute lung injury, PTP: Post transfusion purpura.
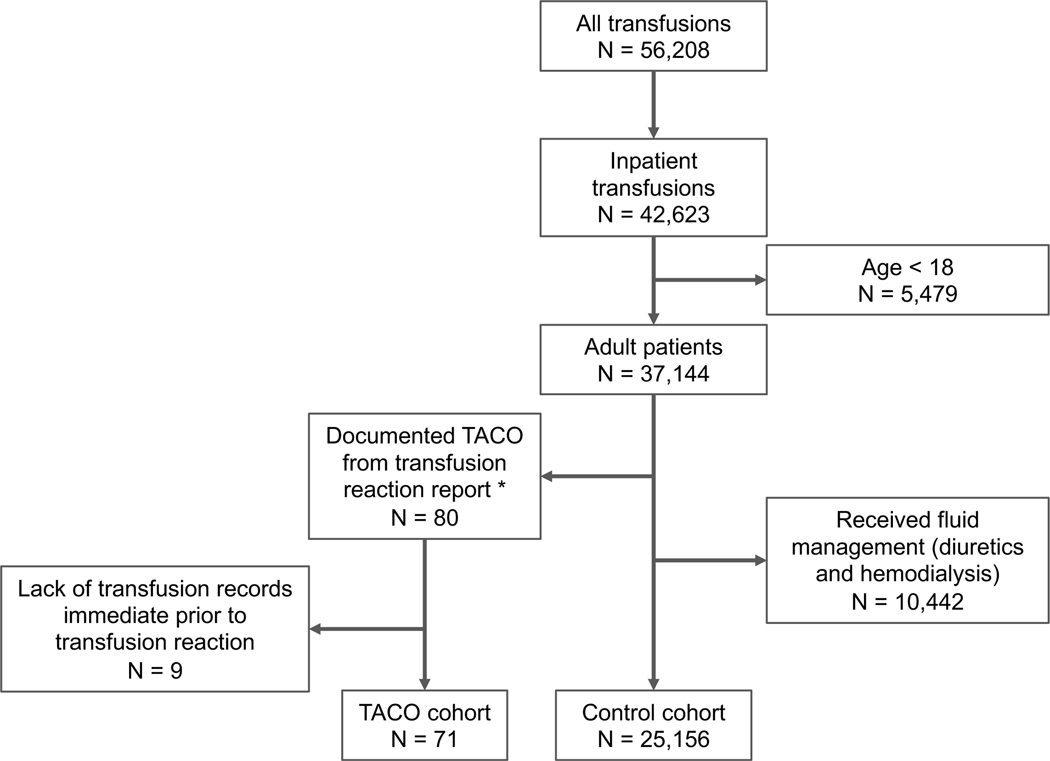
In the retrospective case-control study, the TACO case and control cohorts were constructed from the overall patient population receiving transfusion at UCSF (Figure 3). Specifically, the inclusion criteria for the TACO case cohort were adult patients who received inpatient transfusion at UCSF during the study period (between 2012 and August 2022), with at least one documented confirmed TACO in a transfusion reaction report, and have transfusion records available immediately (within 3 days) prior to the onset of TACO. Of the 102 unique patients with TACO identified from the transfusion reaction workup reports, 91 patients were retrievable in the de-identified UCSF CDW. 71 of the 91 patients met the inclusion criteria and were included in the TACO study cohort. The inclusion criteria for the overall control cohort (N = 25,156) were any adult patients who received inpatient transfusion during the study period, did not receive fluid management (e.g. diuretics treatments) immediately (within 3 days) after transfusion, and did not develop any other transfusion reaction documented in transfusion reaction reports. Patients who received fluid managements after blood transfusion may be highly indicative of developing volume overload symptoms which is a key representing symptom of TACO. Due to the potential of under-reporting and under-diagnosing of TACO, these patients were not included in the control cohort for this analysis. To construct the final control cohort for analysis, patients in the overall control cohort were randomly selected and matched to each TACO case with age and sex at a ratio of 5 to 1.
Figure 3. Study cohort selection.
The overall control cohort included all adult patients receiving transfusions inpatient between 2012 and July 2022. Patients who have had prior transfusion reactions or receive fluid management after transfusions were excluded. TACO: Transfusionassociated circulatory overload.
* 91 of 102 patients identified from transfusion reaction reports were retrievable from the UCSF CDW. 80 of 91 patients were adults and received inpatient transfusion at UCSF during the study period.
Variables and End Points
The end points included in this study were the rates of TACO in our patient population and the odds ratio (OR) estimates of covariates associated with TACO. To identify the confirmed TACO cases, we extracted variables included reaction date, reaction type, case definition, severity, and imputability of the corresponding transfusion reactions from each transfusion reaction reports. To understand the clinical presentation and management of TACO, we extracted additional variables from the UCSF CDW including the variables related to the transfusion itself such as blood product type and volume transfused. The total units transfused for each patient was calculated by dividing the sum of the total volume transfused for each blood type by the average volume per unit for each blood product type (300 ml/unit for red blood cell, 250 ml/unit for plasma, 250 ml/unit for platelet, and 100 ml/unit for cryoprecipitate). Other variables such as maximum of laboratory and flowsheet values for blood pressure, body temperature, heart rate, respiratory rate, radiology scores from chest X-ray, and the B-type natriuretic peptide (BNP) (if measured) within one day following the onset of TACO were extracted to characterize the clinical presentation of TACO. For clinical management, we focused on the use of diuretics, hemodialysis, oxygen device support, and the fraction of inspired oxygen.
Furthermore, additional covariates including comorbidities, medications exposures, and blood transfusions were extracted from the UCSF CDW and included in the case-control analysis. Specifically, the history or presence of comorbidities were identified based on the International Classification of Diseases (ICD)10 codes and the Elixhauser Comorbidity Index was adopted for the disease classifications.21 For covariates related to medication exposures, we focused on the identifying the inpatient administration of diuretics within three days prior to blood transfusions. When the ICD10 diagnosis codes for comorbidities or medication administration records for diuretics were not found, we assumed patient did not have such comorbidities or treatment. For blood transfusion related covariates, we focused on the blood product type and if patients received multiple blood product types at once. Missing values were found in laboratory values including BNP and x-ray scores (Table 1). Missing value imputation was not needed as these laboratory values were not included in the multivariate logistic regression analysis.
Table 1.
Patient baseline characteristics and clinical presentations following development of TACO.
| Patient Characteristics | Overall (n = 71) |
|---|---|
| Sex – n (%) | |
| Female – n (%) | 40 (56.3) |
| Male – n (%) | 31 (43.7) |
| Race – n (%) | |
| American Indian or Alaska Native | 1 (1.4) |
| Asian | 19 (26.8) |
| Black or African American | 3 (4.2) |
| White or Caucasian | 33 (46.5) |
| Other or Unknown | 15 (21.1) |
| Age – median (IQR) | 62.0 (51.0, 69.8) |
| Age ≧ 65 years – n (%) | 28 (39.4) |
| Any High Risk Comorbidities – n (%) | 54 (76.1) |
| Congestive Heart Failure | 16 (22.5) |
| Coagulopathy | 47 (66.2) |
| Renal Failure | 18 (25.4) |
| Organ Transplant | 24 (33.8) |
| Blood Transfusion Product Type and Volume | |
| Multiple Blood Product Types – n (%) | 49 (69.0) |
| RBC + Platelets | 31 (43.7) |
| RBC + Platelets + Plasma | 14 (19.7) |
| Platelets + Plasma | 2 (2.8) |
| RBC + Plasma | 1 (1.4) |
| RBC + Platelets + Plasma + Cryoprecipitate | 1 (1.4) |
| Single Blood Product Type – n (%) | 22 (31.0) |
| RBC | 14 (19.7) |
| Platelets | 6 (8.5) |
| Plasma | 2 (2.8) |
| Transfusion Volume (mL) – median (IQR) | 1262.5 (558.3, 2540.8) |
| Transfusion Unit – median (IQR) | 4.6 (2.0, 9.5) |
| TACO Management | |
| Any Fluid management - n (%) | 63 (88.7) |
| Bumetanide | 4 (5.6) |
| Furosemide | 47 (66.2) |
| Combination of pharmacological therapies | 7 (9.9) |
| Hemodialysis | 7 (9.9) |
| Any Oxygen Device – n (%) | 57 (80.3) |
| Oxygen Device FIO2 – median (IQR) | 100 (100, 100) |
| Ventilator – n (%) | 8 (11.3) |
| Hospital Service | |
| General Surgery | 3 (4.2) |
| Hospital Medicine | 13 (18.3) |
| Liver Transplant | 7 (9.9) |
| Malignant Hematology | 34 (47.9) |
| Neurosurgery | 5 (7.0) |
| Other | 9 (12.7) |
| Laboratory Measures – median (IQR) | |
| B-type natriuretic peptide (pg/mL) (n = 38) | 396.0 (177, 1270.0) |
| Systolic Blood Pressure (mmHg) | 159.5 (141.8, 182.8) |
| Diastolic Blood Pressure (mmHg) | 81.0 (70.5, 94.8) |
| Body Temperature (°F) | 100.9 (99.3, 102.6) |
| Body Temperature ≧ 100.4 °F – n (%) | 41 (57.7) |
| Chest X-Ray Radiology Score (n= 20) | 3.0 (3.0, 3.0) |
| Weight Changes (L) | +1.35 (+0.2, +4.0) |
Regular Expression-Based NLP
We developed a rule-based NLP method to extract key features of transfusion reactions including reaction dates, types, case definitions, severities, and imputabilities from the semi-structured portion of the transfusion reaction reports (Figure 1). NLP algorithms are sets of algorithms that can process language texts to perform tasks including information extraction, relation extraction, name entity identification, and language understanding. Our rule-based NLP algorithm specializes in information extraction, specifically the key transfusion reaction features from the reaction reports. The algorithm first identifies the key mentions in the text, including phrases such as “transfusion reaction dates”, “transfusion reaction impression”, “case definitions”, “severity”, and “imputability”. These key mentions were identified in an automated manner by pattern matching. For instance, the specific text “Imputability:” was considered a key mention, over any other mention of “imputability” in the document.
The algorithm then subsequently extracts and standardizes text following the key mentions as the outputs. For instance, “severe” following the key mention of “Severity:” was extracted and standardized to “Severe” as the final output. Any dates extracted following the “transfusion reaction date:” were standardized into “mm/dd/yyyy” format. All standardized extractions (dates, reaction types, case definitions, severity, and imputabilities) were collected into a tabular data format. All corresponding features for each transfusion reaction were extracted if more than one reaction was identified in the transfusion reaction report. The performance of the NLP algorithm was validated manually by reviewing the transfusion reaction reports. Specifically, we assessed the algorithm’s specificity and sensitivity at identifying TACO cases by reviewing a total of randomly sampled 50 transfusion reports, of which 25 were TACO-positive and 25 were TACO-negative reaction reports for a confidence interval of 85% and a margin of error of 10%. The performances of the algorithm for reaction dates and imputabilities corresponding to each reaction achieved 100% accuracies (eFigure 1). For reaction types, the algorithm achieved 100% accuracy in identifying TACO cases and ranged between 95 and 100% for other reaction types. This rule-based NLP algorithm has been made openly available on GitHub (https://github.com/m10wang/btar_rulebasedNLP). 22
Statistical Analysis
Median, range, and percentage were provided to describe the clinical representations and management of patients following the onset of TACO (Table 1). The prevalence rates of TACO per patient was calculated based on the total number of distinct patients who received blood transfusions as the denominator.
In the retrospective case-control study, patients in the control cohort were matched to each TACO case with age and sex at a ratio of 5 to 1 using the exact-matching method. The odds ratios and 95% confidence intervals (CI) of covariates were estimated using the multivariate logistic regression model. The multivariate logistic regression model was fitted in 355 control patients versus 71 patients who developed TACO (5 control to 1 case). The multivariate model was a parsimonious model and the covariates in the model were selected based on domain knowledge and prior literature of potential biological relevance to TACO (eFigure 2).4,9,17,24–26 The covariates included patient demographics (age and gender), comorbidities (congestive heart failure, renal failure, liver disease, organ transplant, cancer, hypertension, coagulopathy, pulmonary disease, and anemia), and medication exposures (diuretics), and blood product exposures. Specific blood product exposure covariates such as receiving multiple blood product types, red blood cells as single blood product type, platelets as single blood product type, and transfusion volume were included in the multivariate analysis to account for the exposure differences (eTable 1). Transfusion unit was not included as a covariate as it is correlated with transfusion volume. Potential collinearity of the covariates was measured with variance inflation factors (eFigure 3). We have also conducted sensitivity analysis to explore the variability of OR estimates in case to control matched at ratios of 1:7 and 1:10 (eFigure 4).
The rule-based NLP algorithm and logistic regression were performed in Python 3.7.3. Python modules, statsmodels version 0.11.1 and re version 2.2.1, were used.
RESULTS
TACO Patient Characteristics
The clinical characteristics and treatments for a total of 71 patients with TACO were summarized in Table 1. The median age was 62 with 28 (39.4%) patients older than 65 years old. 54 (76.1%) of these patients were found with a history or presence of comorbidities previously described as associated with TACO such as congestive heart failure (22.5%), renal failure (25.4%), coagulopathy (66.2%), and organ transplant (33.8%). A total of 49 (69.0%) patients received more than one type of blood product on the day of TACO onset. The median total cumulative transfusion volume three days prior to TACO onset was 1.15 L with high variability amongst patients. Nearly half (37, 47.9%) of TACO patients were admitted by malignant hematology hospital services, followed by hospital medicine (13, 18.3%) and liver transplant services (7, 9.9%).
Following the onset of TACO, the median BNP value was found elevated at 300 pg/mL and ranged between 40 and 3270 pg/mL. The median of radiology scores for chest X-rays was 3, systolic and diastolic blood pressures were 159 and 79 mm Hg, respectively. In addition, the median peak body temperature was 100.6 °F and a total of 41 (57.7%) patients developed fever. We observed a positive median 1.8 L fluid changes during the hospital stay starting three days prior to until the date of TACO. A total of 63 (88.7%) of patients received diuretics and/or hemodialysis to remove fluid following the onset of TACO. Of the 63 patients, the majority of patients (47, 74.6%) received furosemide as a monotherapy. A total of 57 (80.3%) patients required oxygen support to alleviate hypoxemia, and 8 (11.3%) patients received oxygen through ventilators.
Rates of TACO
Across all the transfusion reaction reports, only a total of 107 cases (102 unique patient) of TACO were identified. As a result, the prevalence rate of TACO overall was estimated to be 0.18% (102/ total 56,208 patients) per patient.
Risk Factor Analysis
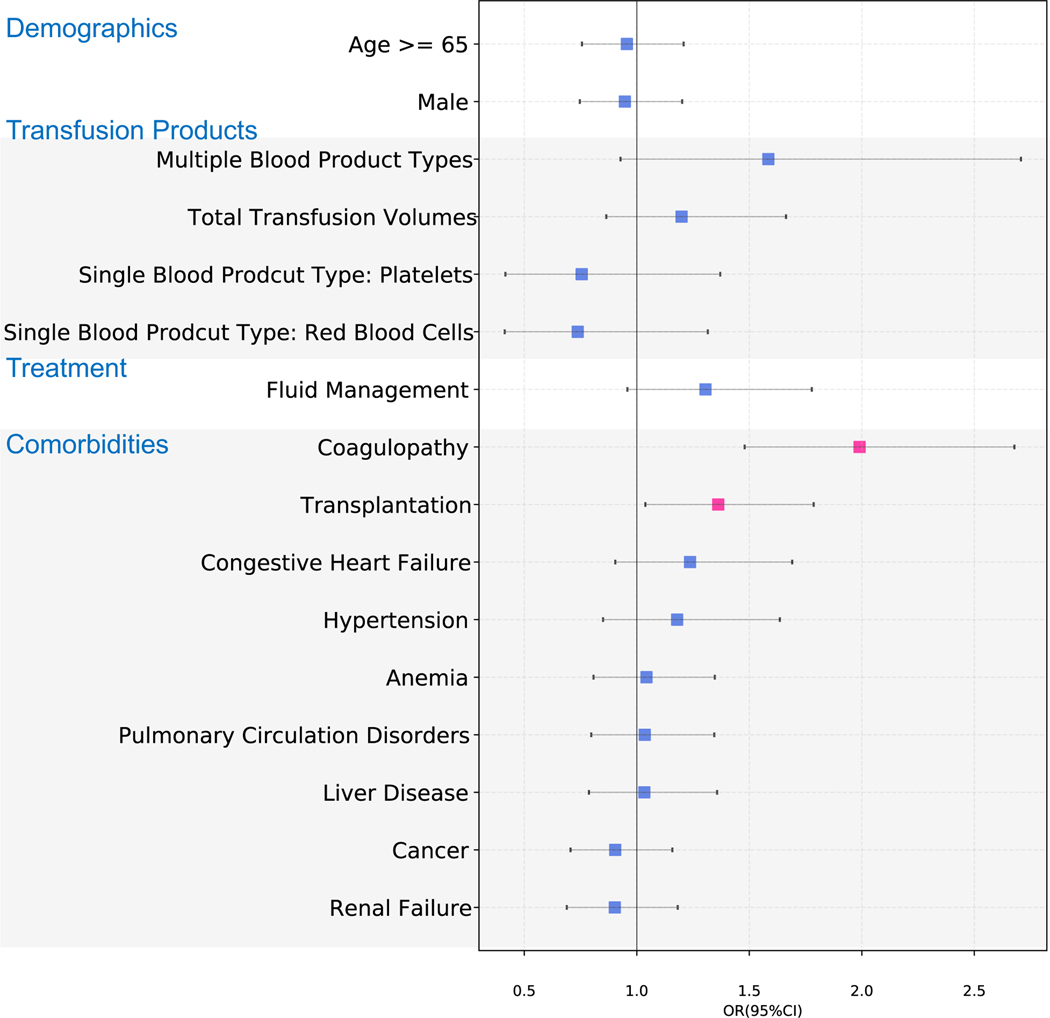
The multivariate logistic regression found increased odds of TACO associated with having history or presence of comorbidities of coagulopathy [OR, 1.36; 95% CI, 1.04–1.79], transplant [OR, 1.99; 95% CI, 1.48–2.68] (Figure 4). Patients who required diuretic therapies [OR, 1.31; 95% CI, 0.96–1.78] prior to transfusion, receiving multiple types of blood transfusion products [OR, 1.58; 95% CI, 0.93–2.71], higher transfusion volumes [OR, 1.20; 95% CI, 0.86–1.66], and having comorbidities of congestive heart failure [OR, 1.24; 95% CI, 0.90 to 1.69] or hypertension [OR, 1.18 ; 95% CI, 0.85 to 1.64] were associated with increased odds of TACO but did not reach statistical significance.
Figure 4. Risk factors associated with TACO.
The potential risk factors with mean odds ratio and confidence intervals estimated using the multivariable logistic regression model are shown. Significant risk factors are highlighted in pink markers. The markers are grouped into separate categories for drug exposures, comorbidities, transfusion products, and demographics. TACO: Transfusion-associated circulatory overload.
DISCUSSION
We found the prevalence rate of 0.2% per patient of TACO as much as five-fold lower than previously reported rates in existing literature, despite that more than half of the patient population at our tertiary-care academic medical center have a history or presence of comorbidities associated with higher odds of developing TACO (eFigure 5). The rates we observed are likely stemming from the different approaches we took to identify “true” TACO cases compared to other existing literature. At UCSF, any suspected transfusion reaction reported to blood bank prompts the investigation and documentation of the potential transfusion reaction. In each transfusion reaction workup report, the transfusion reaction date and diagnosis of the type of transfusion reaction will be documented along with assessment of if the reaction was caused by blood transfusion (imputability). We excluded any TACO cases where the imputability was deemed “possible”, “ruled out”, or “not determined”. Our methods, while are reproducible and can ensure that the TACO cases identified were true TACO cases, could lead to under-estimation of rates of TACO compared to rates reported in existing literature. Existing literature primarily identified TACO cases through individual chart reviews, which may possibly contribute to over-estimation as criteria used to identify TACO could be different and the imputability aspect was often not considered. In addition, in our logistic regression analysis, we further excluding patients, in the control cohort, who received fluid managements immediately after receiving transfusions. These patients could potentially have had TACO but were not reported to blood bank.
These adult patients with TACO were characterized based on demographics, comorbidities, transfusion exposures, hospital services, laboratory measurements, vital signs, fluid balance, and medical interventions following the onset of TACO. It is worth noting that a high percentage (76.1%) of TACO patients were found to have comorbidities of congestive heart failure, renal failure, coagulopathy, and organ transplant. The higher number of patients receiving combination of blood products, hospital services in malignant hematology, and organ transplant reflect that many of these patients may be patients with cancer or undergoing transplantation who may often require larger transfusion volumes and multiple types of blood products. The laboratory measurements, clinical management, and vital signs summarized in Table 1 shows the common clinical presentations of TACO which include elevated body temperature, blood pressure, positive fluid balance, pulmonary edema, and hypoxemia. In addition, although the median BNP value was elevated, high variabilities observed indicating the need for better characterization of BNP changes in patients with TACO which was highlighted by a recent study validating definitions of TACO by Wiersum-Osselton et al.27 Interestingly, the median peak of body temperature was 100.6 °F and more than half (57.7%) of patients developed fever following the onset of TACO. While fever was previously reported in roughly one third of TACO patients28, it was found with higher prevalence in our patient cohort which further coincided the possible role of inflammation in the pathophysiology of TACO, or may reflect the underlying high rate of fever in malignant hematology and post-transplant inpatients due to their underlying diseases. The primary clinical management strategies for symptomatic alleviations were diuretics or hemodialysis for fluid removal and oxygen support for hypoxemia.
Patients with history or presence of comorbidities such as coagulopathy and organ transplant were found associated with increased odds of TACO highlighted the need of careful monitoring for these patients. Interestingly, though did not reach statistical significance, receiving diuretic therapies prior to transfusions was also found associated with increased odds of TACO. Although pre-medicating patients with diuretics to prevent TACO has not yet been widely recommended due to unclear clinical benefit,15 it is not impossible to have scenarios where patients were already presenting characteristics such as shortness of breath and positive fluid balance, which could prompt the decision to preemptively initiate fluid managements strategies (diuretics) prior to transfusions. Consequently, the clinical implication of such observed association of the use of diuretics and the increased odds of TACO could potentially imply that the use of diuretics in removing excessive fluids prior to transfusions may not suffice or not aggressive enough to mitigate TACO effectively in the small percentage of patients that experienced TACO. However, if we believe that diuresis is an effective method of preventing TACO in at risk patients and that provider education has led to improved management of these patients and lower rates of TACO, we would expect the minority of patients who still experience TACO to be those for whom diuresis was ineffective or insufficient. Additional studies are warranted to further validate our hypothesis and investigate potential subgroup of patients who may need more aggressive preventative strategies to effectively mitigate TACO. Other comorbidity factors such as congestive heart failure and hypertension did not significantly increased odds of TACO in our study. We hypothesize that this observation could be due to the awareness of TACO risks in high-risk patients especially with congestive heart failure and hypertension leading to providers to pay extra attention to mitigate risk of TACO when administering transfusions. However, this hypothesis requires additional studies with larger sample sizes to confirm. Preferably, cross-institution studies can help support investigating impact of different clinical management strategies to risk of TACO in sub-populations prior to transfusions.
LIMITATION
Our study represents an updated assessment of TACO prevalence rate and risk factor analysis using an efficient and reproducible approach to extract the outcomes from blood transfusion reaction reports using an NLP algorithm. This retrospective study is subject to selection bias as all study cohort and covariates were retrospectively identified using the EHR data at UCSF. As previously mentioned, we focused on the “true” TACO cases by extracting clinician’s assessments from transfusion reaction reports. Although true cases of TACO can be extracted accurately and reliably, this approach may introduce selection bias as well as recording bias by limiting to reactions reported by clinicians. A computational algorithm that can accurately identify and distinguish the “true” TACO cases using structured EHR data may reduce such selection bias and could further enable better generalizability for future active surveillance system as electronic health record systems have been widely adopted across the United States.
For the multivariate logistic regression analysis, we constructed a directed acyclic diagram (DAG) to identify key covariates to adjust in order to minimize potential biases (eFigure2). The DAG was constructed based on domain knowledge and prior literature to depict the potential correlations between key covariates affecting the exposure (transfusion) and the outcome (TACO). However, it is possible that the proposed DAG could be incomplete resulting in missing potential (unknown) confounding covariates. Hence, our findings cannot support causal relations between risk factors and TACO.
Lastly, this retrospective study represents experiences from a single tertiary treatment center with a relatively small sample size for number of TACO cases. As a result, the generalizability of our findings may be limited. However, the study method, specifically, the NLP algorithms that can reliably extract transfusion reactions from the transfusion reaction reports, are made available on GitHub for other researchers to adopt and study the outcome and prevention of TACO. However, the trends and associations identified may be worth exploring in future multi-institutional studies with larger cohort size from a more diverse patient population.
CONCLUSION
In summary, we developed novel computational methods to better identify rare adverse reactions associated with blood transfusions and the findings could provide an updated view of prevalence of TACO in high-risk patient populations. We found much lower prevalence rate of TACO in UCSF transfusion populations highlighting that while TACO is the most common severe blood transfusion reaction, it remained rare even in high-risk patient populations. This research could provide an updated view of the prevalence of TACO in high-risk patient populations receiving blood transfusions. The risk factors we identified associated with TACO were largely aligned with previous studies. Patients with history or presence of coagulopathy and organ transplant should be carefully monitored to mitigate potential risks of TACO. Additionally, the increased odds of TACO found in patients requiring diuretics prior to blood transfusions could suggest that more aggressive fluid management strategy may be one of several strategies warranted in a small minority of patients to prevent risks of developing TACO. Our analysis provides important insights into identifying patients who may be at increased odds of developing TACO as well as improving clinical management through its mitigation. Finally, our work shows the utility of EHR data to find and monitor for rare blood-product based adverse events.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank the staff of the Bakar Computational Health Sciences Institute, UCSF Information Commons, and the Center for Data-Driven Insights and Innovation within University of California Health. We thank UCSF Clinical and Translational Science Institute (UL1TR001872) for their help on retrieving UCSF clinical notes for chart review and validation. GMG was supported by the US National Institutes of Health NHLBI grants R38HL143581 and NHLBI 1K38HL159128-01. MW was supported by the US National Institutes of Health T32 training grants (5T32GM007175-43). Partial grant support was provided through the FDA U01FD005978 to the UCSF-Stanford Center of Excellence in Regulatory Sciences and Innovation (CERSI). The content reflects the views of the authors and should not be construed to represent NIH’s or FDA’s views or policies.
Funding support:
Partial grant support was provided through the FDA U01FD005978 to the UCSF-Stanford Center of Excellence in Regulatory Sciences and Innovation (CERSI).
Footnotes
Disclaimer: This article reflects the views of the authors and should not be construed to represent FDA’s views or policies.
A.J.B. is a co-founder and consultant to Personalis and NuMedii; consultant to Samsung, Mango Tree Corporation, and in the recent past, 10x Genomics, Helix, Pathway Genomics, and Verinata (Illumina); has served on paid advisory panels or boards for Geisinger Health, Regenstrief Institute, Gerson Lehman Group, AlphaSights, Covance, Novartis, Genentech, and Merck, and Roche; is a shareholder in Personalis and NuMedii; is a minor shareholder in Apple, Facebook, Alphabet (Google), Microsoft, Amazon, Snap, 10x Genomics, Illumina, CVS, Nuna Health, Assay Depot, Vet24seven, Regeneron, Sanofi, Royalty Pharma, AstraZeneca, Moderna, Biogen, Paraxel, and Sutro, and several other non-health related companies and mutual funds; and has received honoraria and travel reimbursement for invited talks from Johnson and Johnson, Roche, Genentech, Pfizer, Merck, Lilly, Takeda, Varian, Mars, Siemens, Optum, Abbott, Celgene, AstraZeneca, AbbVie, Westat, and many academic institutions, medical or disease specific foundations and associations, and health systems. Atul Butte receives royalty payments through Stanford University, for several patents and other disclosures licensed to NuMedii and Personalis. Atul Butte’s research has been funded by NIH, Peraton (as the prime on an NIH contract), Genentech, Johnson and Johnson, FDA, Robert Wood Johnson Foundation, Leon Lowenstein Foundation, Intervalien Foundation, Priscilla Chan and Mark Zuckerberg, the Barbara and Gerson Bakar Foundation, and in the recent past, the March of Dimes, Juvenile Diabetes Research Foundation, California Governor’s Office of Planning and Research, California Institute for Regenerative Medicine, L’Oreal, and Progenity.
DECLARATION OF INTERESTS
M.W., G.M.G, A.P., B.W., A.B., B.C., E.P., B.R., S.A. do not have any conflict of interest to disclose.
References
- 1.Narayan DS, Bellamy PM, Spinks C, et al. ANNUAL SHOT REPORT 2018. Published online 2018:210. [Google Scholar]
- 2.Fatalities Reported to FDA Following Blood Collection and Transfusion: Annual Summary for FY2018. :17. [Google Scholar]
- 3.Transfusion-associated circulatory overload and transfusion-related acute lung injury | Blood | American Society of Hematology. Accessed March 16, 2021. https://ashpublications.org/blood/article/133/17/1840/275907/Transfusion-associated-circulatory-overload-and
- 4.Roubinian N TACO and TRALI: biology, risk factors, and prevention strategies. Hematology. 2018;2018(1):585–594. doi: 10.1182/asheducation-2018.1.585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bosboom JJ, Klanderman RB, Migdady Y, et al. Transfusion-Associated Circulatory Overload: A Clinical Perspective. Transfusion Medicine Reviews. 2019;33(2):69–77. doi: 10.1016/j.tmrv.2019.01.003 [DOI] [PubMed] [Google Scholar]
- 6.Goel R, Tobian AAR, Shaz BH. Noninfectious transfusion-associated adverse events and their mitigation strategies. Blood. 2019;133(17):1831–1839. doi: 10.1182/blood-2018-10-833988 [DOI] [PubMed] [Google Scholar]
- 7.Roubinian NH, Hendrickson JE, Triulzi DJ, et al. Contemporary risk factors and outcomes of transfusion-associated circulatory overload. Crit Care Med. 2018;46(4):577–585. doi: 10.1097/CCM.0000000000002948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clifford L, Jia Q, Yadav H, et al. Characterizing the Epidemiology of Perioperative Transfusion-associated Circulatory Overload. Anesthesiology. 2015;122(1):21–28. doi: 10.1097/ALN.0000000000000513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy EL, Kwaan N, Looney MR, et al. Risk Factors and Outcomes in Transfusion-associated Circulatory Overload. Am J Med. 2013;126(4):357.e29–357.e38. doi: 10.1016/j.amjmed.2012.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lieberman L, Maskens C, Cserti-Gazdewich C, et al. A retrospective review of patient factors, transfusion practices, and outcomes in patients with transfusion-associated circulatory overload. Transfus Med Rev. 2013;27(4):206–212. doi: 10.1016/j.tmrv.2013.07.002 [DOI] [PubMed] [Google Scholar]
- 11.Risk Factors and Clinical Outcomes Associated with Perioperative Transfusion-associated Circulatory Overload | Anesthesiology | American Society of Anesthesiologists. Accessed March 16, 2021. https://pubs.asahq.org/anesthesiology/article/126/3/409/19745/Risk-Factors-and-Clinical-Outcomes-Associated-with [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daurat A, Grenie J, Roger C, et al. Outcomes and risk factors of transfusion-associated circulatory overload: a case control study. Transfusion. 2019;59(1):191–195. doi: 10.1111/trf.15040 [DOI] [PubMed] [Google Scholar]
- 13.Andrzejewski C, Casey MA, Popovsky MA. How we view and approach transfusion-associated circulatory overload: pathogenesis, diagnosis, management, mitigation, and prevention. Transfusion. 2013;53(12):3037–3047. doi: 10.1111/trf.12454 [DOI] [PubMed] [Google Scholar]
- 14.Grey S, Bolton‐Maggs P. Pulmonary complications of transfusion: Changes, challenges, and future directions. Transfusion Medicine. 2020;30(6):442–449. doi: 10.1111/tme.12709 [DOI] [PubMed] [Google Scholar]
- 15.Sarai M, Tejani AM. Loop diuretics for patients receiving blood transfusions. Cochrane Database of Systematic Reviews. 2015;(2). doi: 10.1002/14651858.CD010138.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li G, Rachmale S, Kojicic M, et al. Incidence and transfusion risk factors for transfusion-associated circulatory overload among medical intensive care unit patients. Transfusion. 2011;51(2):338–343. doi: 10.1111/j.1537-2995.2010.02816.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bosboom JJ, Klanderman RB, Zijp M, et al. Incidence, risk factors, and outcome of transfusion-associated circulatory overload in a mixed intensive care unit population: a nested case-control study. Transfusion. 2018;58(2):498–506. doi: 10.1111/trf.14432 [DOI] [PubMed] [Google Scholar]
- 18.Hendrickson JE, Roubinian NH, Chowdhury D, et al. Incidence of transfusion reactions: a multicenter study utilizing systematic active surveillance and expert adjudication. Transfusion. 2016;56(10):2587–2596. doi: 10.1111/trf.13730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bojanić I, Lukić M, Plenković F, Raos M, Medenjak M, Ćepulić BG. Changes in the incidence of transfusion reactions in hematological patients over the past 30 years. Transfusion. 2022;62(3):600–611. doi: 10.1111/trf.16816 [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. National Healthcare Safety Network Biovigilance Component Hemovigilance Module Surveillance Protocol. Accessed March 16, 2021. https://www.cdc.gov/nhsn/PDFs/Biovigilance/BV-HV-protocol-current.pdf
- 21.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83 [DOI] [PubMed] [Google Scholar]
- 22.GitHub. Blood Transfusion Reaction Rule-Based NLP. https://github.com/m10wang/btar_rulebasedNLP.
- 23.Wasserman L The Bootstrap. In: Wasserman L, ed. All of Statistics: A Concise Course in Statistical Inference. Springer Texts in Statistics. Springer; 2004:107–118. doi: 10.1007/978-0-387-21736-9_8 [DOI] [Google Scholar]
- 24.Menis M, Anderson SA, Forshee RA, et al. Transfusion-associated circulatory overload (TACO) and potential risk factors among the inpatient US elderly as recorded in Medicare administrative databases during 2011. Vox Sang. 2014;106(2):144–152. doi: 10.1111/vox.12070 [DOI] [PubMed] [Google Scholar]
- 25.Piccin A, Spizzo G, Popovski MA, et al. Transfusion-associated circulatory overload in gastroenterology. Blood Transfus. 2021;19(3):197–204. doi: 10.2450/2020.0025-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Transfusion-associated circulatory overload (TACO) Definition - 2018. :5. [Google Scholar]
- 27.Wiersum-Osselton JC, Whitaker B, Grey S, et al. Revised international surveillance case definition of transfusion-associated circulatory overload: a classification agreement validation study. Lancet Haematol. 2019;6(7):e350–e358. doi: 10.1016/S2352-3026(19)30080-8 [DOI] [PubMed] [Google Scholar]
- 28.Parmar N, Pendergrast J, Lieberman L, Lin Y, Callum J, Cserti‐Gazdewich C. The association of fever with transfusion-associated circulatory overload. Vox Sanguinis. 2017;112(1):70–78. doi: 10.1111/vox.12473 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.