Abstract
Purpose
This study aimed to investigate the ability of creatine-chemical exchange saturation transfer (Cr-CEST) technique assessed through 7-T MRI to evaluate cisplatin-induced testicular damage.
Methods
We used 8–10 weeks C57BL/6 mice (n = 10) that were divided into a control group (n = 5) and a cisplatin-treated group (n = 5). The cisplatin group received cisplatin at a dose of 15 mg/kg, via intraperitoneal injection, while the control group received saline. MR images of mouse testes were acquired under anesthesia 18 days after the injection using a horizontal 7-T scanner. The pulse sequence consisted of rapid acquisition with a relaxation enhancement (RARE) with magnetization transfer. The Z-spectra were collected using a 2000-ms saturation pulse at a B1 amplitude of 1.2 μT, with frequencies varying from −4.8 to +4.8 parts per million (ppm). Maps of magnetization transfer ratio with asymmetric analysis (MTRasym) were reconstructed at a Cr metabolite concentration of 1.8 ppm.
Results
The Cr-CEST effect was significantly reduced in the cisplatin-treated group compared to the control group (MTRasym of control mice vs. cisplatin-treated mice: 6.9 [6–7.5] vs. 5.2 [4–5.5], P = 0.008). Correlation analysis revealed a strong correlation between the Cr-CEST effect and the pathological score (ρ = 0.93, P < 0.001).
Conclusion
Cr-CEST MRI can be useful for the evaluation of cisplatin-induced testicular damage in mice.
Keywords: chemical exchange saturation transfer, cisplatin, creatine, testes
Introduction
In recent years, advances in the detection and treatment of cancer have led to an increase in the number of cancer survivors.1 However, cancer therapy is often accompanied by adverse effects, depending on the patient’s age, type of cancer, treatment method, and dosage. Specifically, testicular tissue is highly sensitive to chemotherapy, which can lead to decreased spermatogenesis and sperm quality in pre-pubescent and adult patients, thus resulting in infertility.2
Cisplatin is a platinum-derived antitumor and DNA alkylating agent used to treat various tumors, including bladder, lung, neck, cervical, ovarian, and testicular cancers.3 Although cisplatin improves the quality of life of cancer patients, its use has been limited due to its severe toxicity to healthy tissues, especially the kidneys, neurons, and male reproductive organs. Cisplatin causes severe testicular damage characterized by germ cell apoptosis, Leydig cell dysfunction, and impaired testicular steroid production. In addition, cisplatin affects spermatogenesis by inhibiting germ cell nucleic acid synthesis and testosterone production, leading to infertility.4
Although the recovery of spermatogenesis after cancer treatment has been extensively studied, no-noninvasive method for evaluating testicular function is currently available, with the exception of semen analysis. As ejaculation is difficult in pediatric cancer patients, it is important to devise a non-invasive method for evaluating testicular function.5 Previous studies have proposed various methods that employ MRI, such as MR spectroscopy (MRS).6 and apparent diffusion coefficient (ADC)-mapping.7 However, due to its technical limitations, MRS has not been widely applied in clinical practice. In fact, this technique, while effective for measuring metabolites in the brain,8 has a low spatial resolution, which limits the detailed quantification of creatine (Cr) concentration and its distribution in the testes.
Chemical exchange saturation transfer (CEST) imaging can evaluate metabolites with exchangeable protons such as amide and amine protons by using prolonged, selective saturation pulses.9–13 CEST MRI is a useful method for detecting trace molecules such as amide proton transfer in the tissues and enables the grading of brain and longitudinal tumors.14 Cr is one of the target metabolites of CEST imaging both in vivo and in vitro. Cr is an important metabolite of adenosine triphosphate (ATP) production, and its levels are known to be high in the testes.15 Moreover, Cr levels have been shown to be lowered in patients with non-obstructive azoospermia.16 Our previous study demonstrated a reduced Cr-CEST signal in both a testicular torsion model.17 and an X-ray irradiation model.18 Hence, we speculated that Cr-CEST may be useful to evaluate the testicular function of patients undergoing chemotherapy.
Therefore, we examined the potential of Cr-CEST imaging to detect testicular failure in a cisplatin-treated mouse model. Ours is the first study to evaluate the feasibility of Cr-CEST in evaluating testicular damage in mice models of chemotherapy-induced testicular damage.
Materials and Methods
Animals
All animal procedures were approved by the Institutional Animal Care and Use Committee of Osaka University. A total of 10 healthy adult male C57BL/6 J mice aged 8–10 weeks were supplied by Japan SLC, Shizuoka, Japan. All animals were kept under standard environmental conditions (22°C, 12/12 h light/dark cycle). The mice were divided into a control (n = 5) group and a cisplatin-treated (n = 5) group. On the first day, saline solution or a single dose of cisplatin (15 mg/kg) was administered intraperitoneally to the control and cisplatin groups, respectively. On the 18th day post injection, MRI was performed under anesthesia, and the animals were subsequently sacrificed.
MRI experiments
MR images of mouse testes were acquired using a horizontal 7-T scanner (PharmaScan 70/16 US; Bruker Biospin, Ettlingen, Germany) equipped with a volume coil with a 30-mm inner diameter. To obtain MR images, the mice were positioned in a stereotaxic frame fixed at the mouth to prevent movement during acquisition. All mice were imaged in the prone position and MR images of both testes were conducted coronal slice. To prevent the testes from migrating into the abdominal cavity, imaging was performed with mild compression of the abdomen. The body temperature of the animals was maintained at 36.0°C ± 0.5°C by circulating water through heating pads, and continuously monitored using a physiological monitoring system (SA Instruments, Stony Brook, NY, USA). All MR experiments on mice were performed under general anesthesia induced and maintained with 3.0% and 2.0% isoflurane, respectively.
For evaluating changes in the CEST effect in each testis, CEST imaging was performed as described in our previous reports.17,18 The pulse sequence for CEST imaging consisted of magnetization transfer with rapid acquisition with relaxation enhancement (RARE), modified to saturate a range of frequency offsets. The sequence parameters were as follows: FOV, 32.0 × 32.0 mm2; slice thickness, 1 mm; TR, 2500 ms; effective TE, 25 ms; matrix size, 196 × 196; number of averages, 1; in-plane resolution, 163 × 163 μm2; number of slices, 1; RARE factor, 8; and K-lines order, liner order. The Z-spectra were collected using a 2000-ms saturation pulse (pulse shape = block pulse, pulse length = 100 ms, pulse band width = 12.8 Hz, number of pulses = 20, interpulse delay = 0.01 ms, and module duration = 2002 ms) at a B1 amplitude of 1.2 μT, with frequencies varying from −4.8 to +4.8 ppm (step, 0.3 ppm, 33 images) with one S0 image. S0 image is the acquired image without RF saturation as the same TR and TE of CEST images. A point-by-point B0 correction was performed from −1.0 to +1.0 ppm (step, 0.1 ppm, 21 images) with water saturation shift referencing (WASSR) method. A total of 55 images, including those for B0 mapping, were acquired in approximately 55 min.
Data processing
All image processing and data analysis were performed with in-house scripts written in MATLAB R2017b (MathWorks, Natick, MA, USA). For Z-spectrum analysis, B0 maps were used to perform pixel-by-pixel B0 correction.19 SXppm is defined as the signal intensity obtained by sequence with saturation pulse at Xppm. The CEST effect was evaluated using magnetization transfer ratio (MTR) asymmetry analysis, determined from the following equation:
The Cr-CEST effect was evaluated at 1.8 ppm based on our previous study.17,18
To quantify the signal values on the MTR map, two ROIs were drawn on the left and right whole testes.
Histological examination
The testes were collected and fixed in Bouin’s fixative. The tissues were embedded in paraffin wax and sectioned at 5 μm, stained with hematoxylin and eosin (H&E), and examined using a light microscopy. We screened up to 20 seminiferous tubules per section to calculate Johnsen’s score, a 10-point evaluation method for quantifying spermatogenesis according to the profile of the cells encountered along the seminiferous tubules. A Johnsen’s score of 10 indicates maximum spermatogenesis, while a score of 1 indicates no cells in the tubular section. The Johnsen’s score was evaluated by two urologists with more than 5 years of clinical experience.
Statistical analysis
All data are presented as mean (minimum–maximum). Data were analyzed using JMP 15 (SAS Institute, Cary, NC, USA). Mann–Whitney U test was performed to compare the two groups. Spearman’s rank correlation coefficient was used to evaluate the relationship between Cr-CEST effect and Johnsen’s score. Statistical significance was set at P < 0.05. To make a two-group comparison, we averaged two data (left and right testes) in each animal.
Results
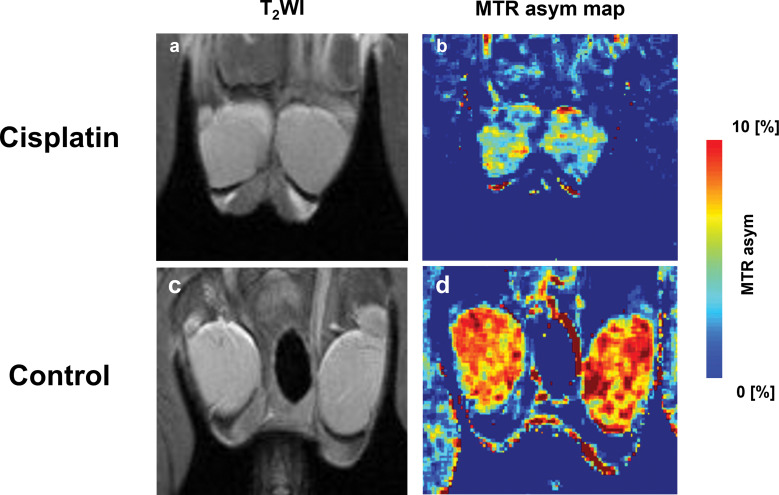
Representative MR images are shown in Fig. 1a–1d. The T2-weighted images of 18-day cisplatin-treated and control mice are shown in Fig. 1a and 1c. There was no difference on T2-weighted images. For testicular Cr-CEST imaging, an MTR asymmetry map at 1.8 ppm was generated (Fig. 1b and 1d). The MTR asymmetry at 1.8 ppm in the testes of cisplatin-treated mice was much lower than that in the control mice.
Fig. 1.
In vivo Cr-CEST imaging of mice testes. (a and c) Anatomical imaging and (b and d) MTR asym map at 1.8 ppm. MTR asym map showed lower Cr-CEST effect in a cisplatin-treated mouse than a control mouse. asym, asymmetry; Cr-CEST, creatine-chemical exchange saturation transfer; MTR, magnetization transfer ratio; T2WI, T2-weighted imaging.
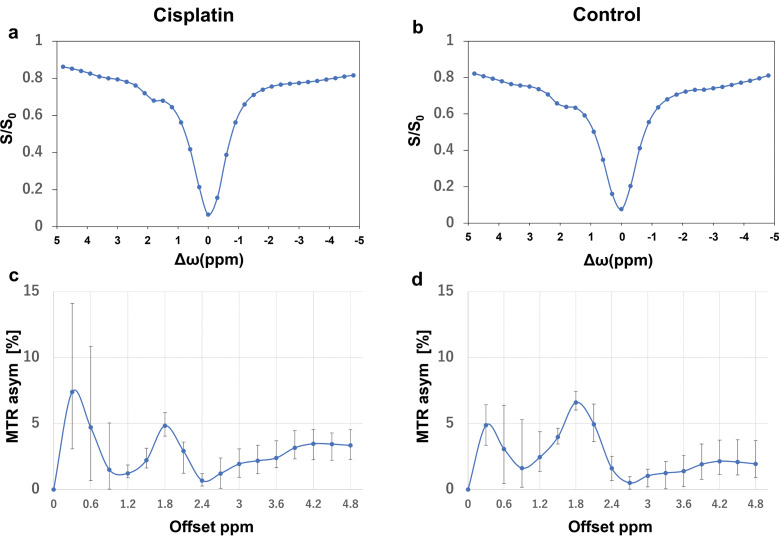
Figure 2 shows the in vivo Z-spectrum analysis and MTR asymmetry plot of the testes of cisplatin-treated and control mice. The Z spectrum was asymmetric at 1.8 ppm on CEST MRI, with 1.8 ppm being the likely CEST effect of creatine (Fig. 2a and 2b). The MTR asymmetry at 1.8 ppm was significantly decreased in cisplatin-treated testes compared to the control testes (P = 0.008) (Fig. 2c and 2d).
Fig. 2.
(a and b) In vivo Z-spectrum analysis and (c and d) magnetization transfer ratio asymmetry plot of the testes of cisplatin-treated and control mice (5 animals in each group). The variation bars in the figures are maximum and minimum. asym, asymmetry; MTR, magnetization transfer ratio.
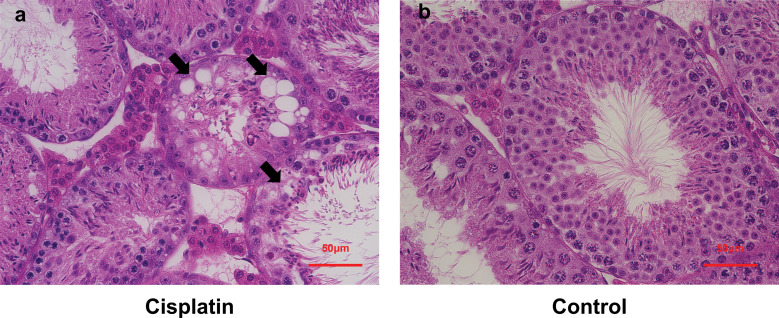
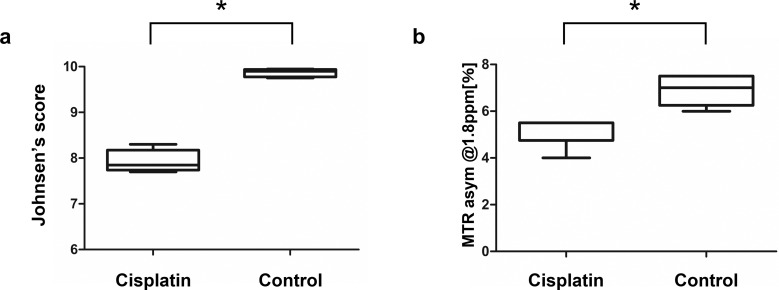
Histopathological analyses of the seminiferous epithelium in the cisplatin-treated and control mice are shown in Fig. 3a and 3b. The cisplatin-treated mice showed disorganization of the cytoarchitecture of the seminiferous epithelium (Fig. 3a) and a significant decrease in Johnsen’s score compared to the control group (control mice vs. cisplatin-treated mice: 9.87 (9.75–9.95) vs. 7.94 (7.7–8.3), P = 0.008, Fig. 4a). The cisplatin-treated group also showed a 20% decrease in the MTR asymmetry signal compared to the control group (control mice vs. cisplatin-treated mice: 6.9 (6.0–7.5) vs. 5.2 (4–5.5), P = 0.008, Fig. 4b).
Fig. 3.
Histopathological analysis of the seminiferous epithelium in (a) cisplatin-treated mice and (b) controls. Cisplatin-treated mice (a) show disorganization of the cytoarchitecture of the seminiferous epithelium, vacuolization (arrows).
Fig. 4.
Box plots showing the differences in Johnsen’s score and MTR asymmetry in control and cisplatin-treated mice (5 animals in each group). (a) A significantly lower Johnsen’s score is seen in cisplatin-treated mice. (b) A significantly lower Cr-CEST effect is seen in cisplatin-treated testes than that in controls. *P = 0.008. Statistical analysis was performed using Mann–Whitney U test. asym, asymmetry; Cr-CEST, creatine-chemical exchange saturation transfer; MTR, magnetization transfer ratio.
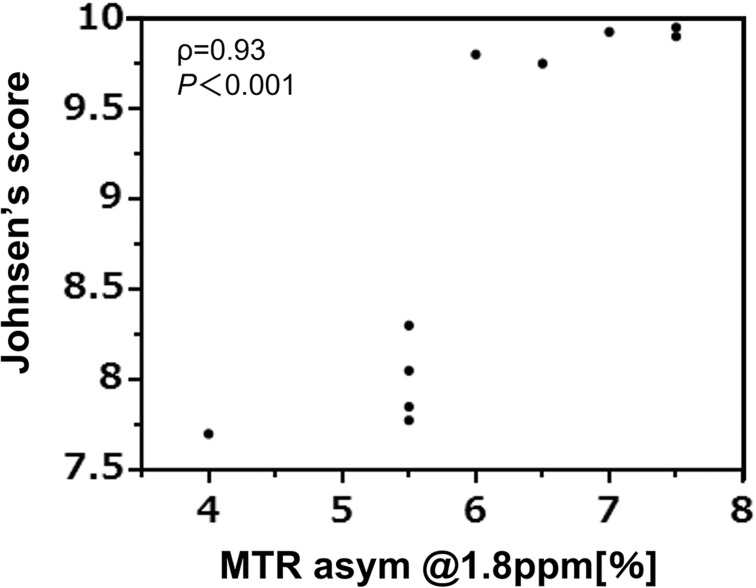
Figure 5 shows the correlation analysis between the MTR asymmetry at 1.8 ppm from the Cr-CEST effect and the Johnsen’s score: A positive correlation was detected between the cisplatin-induced pathological score and the MTR signal (ρ = 0.93, P < 0.001).
Fig. 5.
Correlation analysis of the Cr-CEST effect and the Johnsen’s score. A strong correlation was observed between the Cr-CEST effect and Johnsen’s score (ρ = 0.93, P < 0.001, 5 animals in each group). Cr-CEST, creatine-chemical exchange saturation transfer.
Discussion
In this study, we revealed that cisplatin-induced testicular damage correlates with a decrease in MTR asymmetry at 1.8 ppm from Cr in the testes. We have already shown in a previous study that it is possible to assess Cr in the testes using CEST-MRI, and that duration of testicular ischemia correlates with a decrease in MTR.17 Our results suggest that Cr-CEST can be used to evaluate alterations occurring due to testicular damage. Therefore, this method may be used for the detection of testicular failure due to chemotherapy, such as that occurring with cisplatin.
Many childhood cancer survivors experience both testicular and sexual dysfunctions, such as erectile dysfunction and ejaculation disorder.20 Currently, there are no reports of testicular function evaluation in cancer survivors using methods other than semen analysis. However, since children are unable to ejaculate, evaluation of testicular function cannot be performed through this method. Therefore, this study suggests that CEST-MRI can be successfully used to assess testicular function in cancer survivors, which may lead to the diagnosis and treatment of testicular dysfunction.
In addition, this study showed that cisplatin treatment caused a 20% decrease in the Cr-CEST effect after 18 days of cisplatin treatment, a result that correlated with pathological findings. The effects of a single dose of cisplatin have been shown to improve over time,21 and a previous report showed that testicular findings improved after 60 days of cisplatin administration in a model of testicular damage.22 In the preliminary study, we found that the Cr-CEST effect improved with the improvement of pathological findings after 98 days of cisplatin treatment (data not shown). Therefore, although further investigation is necessary due to the small sample size, it is possible that creatine concentration in the testes may be an indicator of spermatogenesis.
In the testes, unlike in skeletal muscle, conversion enzymes for Cr production are present in the Sertoli cells.23 Here, contrary to what is observed in skeletal muscle, Cr is mostly present as Cr rather than Cr phosphate. In addition, the testes not only have the second-highest amount of Cr and Cr phosphate combined after skeletal muscle but also have the highest amount of free Cr.23 However, the role of Cr within the testes, apart from its function as ATP storage, is not clear, but Cr levels are reduced in patients with nonobstructive azoospermia.16 We have already shown that Cr levels are decreased in models of testicular ischemia and irradiation, and this study suggests that Cr levels are also decreased in the cisplatin-treated model. On the other hand, we have not been able to determine why creatine levels in the testes decrease, so further studies investigating the association between creatine levels and testicular function are necessary.
The present study has several limitations. First, the spermatogenesis of mice lasts 35 days, but imaging was performed on day 18 of cisplatin administration. We may have needed to consider the timing of imaging. Nonetheless, because all cisplatin-treated mice showed pathological findings in the testes, we believe that the timing was appropriate. Second, we did not examine other methods of assessing testicular function, such as MRS, diffusion MRS, and ADC mapping. In the future, it will be necessary to understand the difference between these methods and Cr-CEST.
Conclusion
We demonstrated that cisplatin-induced testicular damage caused a decrease in the Cr-CEST effect. This may be useful for the diagnosis of chemotherapy-induced testicular damage.
Funding Statement
This research was supported by the Japan Agency for Medical Research and Development (AMED) (grant number: J210705035). This work was supported by “MRI platform” as a program of the Project for Promoting public Utilization of Advanced Research Infrastructure of the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan.
Footnotes
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin 2014; 64:252–271. [DOI] [PubMed] [Google Scholar]
- 2.Wasilewski-Masker K, Seidel KD, Leisenring W, et al. Male infertility in long-term survivors of pediatric cancer: a report from the childhood cancer survivor study. J Cancer Surviv 2014; 8:437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tian En L, Brougham MFH, Wallace WHB, Mitchell RT. Impacts of platinum-based chemotherapy on subsequent testicular function and fertility in boys with cancer. Hum Reprod Update 2020; 26:874–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howell SJ, Shalet SM. Spermatogenesis after cancer treatment: damage and recovery. J Natl Cancer Inst Monogr 2005; 2005:12–17. [DOI] [PubMed] [Google Scholar]
- 5.Schlegel PN, Sigman M, Collura B, et al. Diagnosis and treatment of infertility in men: AUA/ASRM guideline part I. Fertil Steril 2021; 115:54–61. [DOI] [PubMed] [Google Scholar]
- 6.Ntorkou A, Tsili AC, Astrakas L, et al. In vivo biochemical investigation of spermatogenic status: 1H-MR spectroscopy of testes with nonobstructive azoospermia. Eur Radiol 2020; 30:4284–4294. [DOI] [PubMed] [Google Scholar]
- 7.Tsili AC, Ntorkou A, Goussia A, et al. Diffusion tensor imaging parameters in testes with nonobstructive azoospermia. J Magn Reson Imaging 2018; 48:1318–1325. [DOI] [PubMed] [Google Scholar]
- 8.Takanashi J. Neurochemistry of hypomyelination investigated with MR spectroscopy. Magn Reson Med Sci 2015; 14:85–91. [DOI] [PubMed] [Google Scholar]
- 9.Saito S, Mori Y, Tanki N, Yoshioka Y, Murase K. Factors affecting the chemical exchange saturation transfer of Creatine as assessed by 11.7 T MRI. Radiol Phys Technol 2015; 8:146–152. [DOI] [PubMed] [Google Scholar]
- 10.Saito S, Takahashi Y, Ohki A, Shintani Y, Higuchi T. Early detection of elevated lactate levels in a mitochondrial disease model using chemical exchange saturation transfer (CEST) and magnetic resonance spectroscopy (MRS) at 7T-MRI. Radiol Phys Technol 2019; 12:46–54. [DOI] [PubMed] [Google Scholar]
- 11.Saito S, Takahashi Y, Ohki A, Shintani Y, Higuchi T. Early detection of elevated lactate levels in a mitochondrial disease model using chemical exchange saturation transfer (CEST) and magnetic resonance spectroscopy (MRS) with 7T MR imaging. Radiol Phys Technol 2019; 12:232–233. [DOI] [PubMed] [Google Scholar]
- 12.Tanoue M, Saito S, Takahashi Y, et al. Amide proton transfer imaging of glioblastoma, neuroblastoma, and breast cancer cells on a 11.7T magnetic resonance imaging system. Magn Reson Imaging 2019; 62:181–190. [DOI] [PubMed] [Google Scholar]
- 13.Ohki A, Saito S, Hirayama E, et al. Comparison of chemical exchange saturation transfer imaging with diffusion-weighted imaging and magnetic resonance spectroscopy in a rat model of hypoxic-ischemic encephalopathy. Magn Reson Med Sci 2020; 19:359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dou W, Lin CE, Ding H, et al. Chemical exchange saturation transfer magnetic resonance imaging and its main and potential applications in pre-clinical and clinical studies. Quant Imaging Med Surg 2019; 9:1747–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev 2000; 80:1107–1213. [DOI] [PubMed] [Google Scholar]
- 16.Storey P, Gonen O, Rosenkrantz AB, et al. Quantitative proton spectroscopy of the testes at 3 T: Toward a noninvasive biomarker of spermatogenesis. Invest Radiol 2018; 53:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takahashi Y, Kioka H, Saito S, et al. Accurate estimation of the duration of testicular ischemia using creatine chemical exchange saturation transfer (CrCEST) imaging. J Magn Reson Imaging 2021; 53:1559–1567. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi Y, Kioka H, Fukuhara S, et al. Visualization of spatial distribution of spermatogenesis in mouse testes using creatine chemical exchange saturation transfer imaging. J Magn Reson Imaging 2021; 54:1457–1465. [DOI] [PubMed] [Google Scholar]
- 19.Kim M, Gillen J, Landman BA, Zhou J, van Zijl PC. Water saturation shift referencing (WASSR) for chemical exchange saturation transfer (CEST) experiments. Magn Reson Med 2009; 61:1441–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hovén E, Fagerkvist K, Jahnukainen K, et al. Sexual dysfunction in young adult survivors of childhood cancer - A population-based study. Eur J Cancer 2021; 154:147–156. [DOI] [PubMed] [Google Scholar]
- 21.Perše M, Večerić-Haler Z. Cisplatin-induced rodent model of kidney injury: Characteristics and challenges. BioMed Res Int 2018; 2018:1462802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adler ID, el Tarras A. Clastogenic effects of cis-diamminedichloroplatinum. II. Induction of chromosomal aberrations in primary spermatocytes and spermatogonial stem cells of mice. Mutat Res 1990; 243:173–178. [DOI] [PubMed] [Google Scholar]
- 23.Moore NP. The distribution, metabolism and function of creatine in the male mammalian reproductive tract: a review. Int J Androl 2000; 23:4–12. [DOI] [PubMed] [Google Scholar]