Abstract
Introduction
Dislocation is a common complication associated with total hip replacement (THR). Dual-mobility constructs (DMC-THR) may be used in high-risk patients and have design features that may reduce the risk of dislocation. We aimed to report overall pooled estimates of all-cause construct survival for elective primary DMC-THR. Secondary outcomes included unadjusted dislocation rate, revision for instability, infection and fracture.
Methods
MEDLINE, EMBASE, Web of Science, Cochrane Library and National Joint Registry reports were systematically searched (CRD42020189664). Studies reporting revision (all-cause) survival estimates and confidence intervals by brand and construct including DMC bearings were included. A meta-analysis was performed weighting series by the standard error.
Results
Thirty-seven studies reporting 39 case series were identified; nine (10,494 DMC-THR) were included. Fourteen series (23,020 DMC-THR) from five national registries were included.
Pooled case series data for all-cause construct survival was 99.7% (95% CI 99.5–100) at 5 years, 95.7% (95% CI 94.9–96.5) at 10 years, 96.1% (95% CI 91.8–100) at 15 years and 77% (95% CI 74.4–82.0) at 20 years. Pooled joint registry data showed an all-cause construct survivorship of 97.8% (95% CI 97.3–98.4) at 5 years and 96.3% (95% CI 95.6–96.9) at 10 years.
Conclusions
Survivorship of DMC-THR in primary THR is acceptable according to the national revision benchmark published by National Institute for Clinical Excellence (NICE).
Supplementary Information
The online version contains supplementary material available at 10.1007/s00402-023-04803-3.
Keywords: Total hip replacement, Dual mobility
Introduction
Total hip replacement (THR) is common and successful [1]. Dislocation is a recognised complication, more than half occur within 3 months of primary surgery [2]. The incidence of dislocation following primary THR ranges from 0.12 to 16.13% at an average follow-up of 6 years [3]. In the National Joint Registry (NJR), dislocation or subluxation was the second most common reason for the first revision (17.4%), contributing to 361.3 revisions annually [4]. Interventions not requiring any change of implants are not captured by the NJR.
Risk factors associated with dislocation can be categorised into patient, surgical, implant and hospital related [3]. Patient-related factors include older age, high body mass index, drug use disorders, social deprivation, low income, neurological and rheumatoid disorders, increasing comorbidity indices and previous spine or hip surgery. Surgical factors include surgical approach.
To mitigate the risk of dislocation, the use of large diameter femoral heads has grown in popularity, and according to the NJR, the two most frequently used head sizes in 2020 were 32 mm and 36 mm [4]. Several studies have observed an association between larger head size and a lower dislocation rate [5, 6]. There is, however, an association between 36-mm heads and higher revision rates [4]. This observation may be explained by the proportional relationship between wear volume and sliding distance [7]. Other solutions for instability include constrained liners and may be more appropriate in the setting of complex revision [8]. An alternative is the use of a dual-mobility construct (DMC-THR). DMC-THRs were developed in the 1970s to increase the range of motion before prosthetic impingement and to increase the jump distance before dislocation [7]. DMC-THRs utilise two articulations, one between the head and polyethylene (PE) liner and another between the PE head and acetabular shell [9]. The mobile PE liner acts as a large diameter head increasing head/neck ratio, jump distance and arc of motion before prosthetic impingement. Intra-prosthetic dislocation (IAPD) is a unique complication of the DMC-THR where the head dislodges from the mobile PE component [10]. The increased sliding distance and the second bearing surface increase frictional torque which may increase wear, osteolysis and loosening [7]. This mechanism may also lead to an increased risk of periprosthetic fracture (PPF) and metallosis [11]. Despite these issues, in the USA, the use of DMC-THR has doubled in the last decade, comprising 12% of all primary THRs in 2018 [12].
A recent review article, summarising 24 case series (10,783 DMC-THRs), reported a mean survivorship of 98% (83.8–100%) at a mean follow-up of 8.5 (1.8–16.5) years [13]. Several systematic reviews have shown an association between DMC-THRs and lower dislocation rates when compared to a conventional total hip replacement (C-THR) [14–16]. Such studies are, however, susceptible to selection and publication bias and may overestimate survival [17]. These problems can be overcome by looking at national joint registries which include the entire population as its sample, making results more generalisable. In the UK, the National Institute for Clinical Excellence (NICE) and Orthopaedic Data Evaluation Panel (ODEP) recommend that THR revision rates should be 5% or lower at 10 years [18, 19].
The purpose of this review is to synthesise pooled estimates of all-cause construct survival after primary DMC-THR with the inclusion of National Joint Registry data, something that has not been done previously. In addition, we aimed to synthesise estimates of unadjusted dislocation rate (when available) and revision for instability, infection and fracture.
Methods
Search strategy and paper selection
The study was registered with the prospective register of systematic reviews, PROSPERO (CRD42020189664), and carried out in accordance with PRISMA guidelines [20]. Systematic searches were conducted in MEDLINE, EMBASE, Web of Science and the Cochrane Library from inception to October 2021 using OVID Silver Platter. Reference lists of included articles and bibliographies of systematic reviews were searched for additional studies. The electronic search strategy combined free and MeSH search terms related to population (e.g. “primary total hip replacement”), intervention (e.g. “dual-mobility cup”) and outcome (e.g. “dislocation”, “instability”, “revision”) (Appendix 1). The website of the International Society of Arthroplasty Registries (ISAR) was checked for a list of its members, and their most recent annual reports were scrutinised for stated outcomes to capture national joint replacement registry data.
All titles and abstracts of studies retrieved from the databases were screened. Papers were filtered by primary author (AG) using Rayyan [21]. Full texts were checked for eligibility by two independent authors (AG and HM). Papers were eligible if they were longitudinal studies, cohort, case cohort, case series or clinical trials. National Joint Registry data that reported revision as an outcome for DMC-THR were included. When specified, we treated each brand construct reported within registries as a different series. When series were not reported by brand, we included series by fixation and bearing construct. Case reports, conference abstracts, surgical technique descriptions, review articles and animal trials were excluded. Papers that included patients receiving a DMC-THR for proximal femoral fractures or revision surgery were excluded because they represent a different population with different risk and survival profile [22].
Data extraction and patients
The population included all patients undergoing elective primary THR with DMC bearing. All-cause revision of any part of the construct was the primary outcome. A revision was defined as per the NJR as “any operation performed to add, remove or modify one or more component” [4]. The secondary outcomes were unadjusted dislocation rate and revision for instability, infection and fracture.
Descriptive and quantitative information was extracted into a standardised Excel spreadsheet (version 16.45, Microsoft, USA). Data were extracted on publication date, study design, patient and implant characteristics, survival estimates at any time point, number of dislocations and number of revisions for instability, infection and fracture. When case series were published in multiple papers, the most recent report was used.
Statistical analysis
Statistical analysis was conducted using Stata IC (version 16.1, StataCorp, USA). Survival estimates were pooled with meta-analysis weighting each series on the overall pooled estimate according to its standard error (calculated from published confidence intervals). Weighted means were calculated for continuous variables. When studies did not report survival at exact time points, figures were rounded up to the nearest 5 years as a separate sensitivity analysis.
Risk-of-bias assessment
Study quality was assessed using a non-summative scoring system described by Wylde et al. [23].
Results
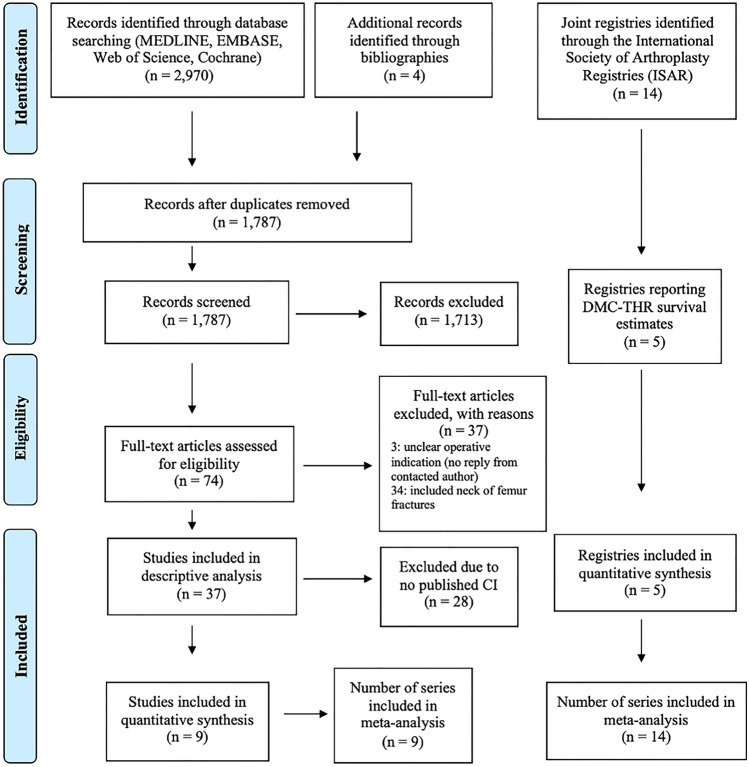
The search produced 2970 references and four additional citations through manual reference lists searches. After de-duplication, there were 1787 articles screened leaving 74 full texts for review. Thirty-seven were excluded, leaving 37 articles, reporting 39 cases series. Of these, 14 of 37 reported all-cause construct survival of which nine published confidence intervals (Fig. 1). Table 1 provides a summary of patient characteristics (individual studies: appendix 2). Of the 14 registries identified through ISAR, five published DMC-THR survival estimates and provided 14 individual brand and construct-based series.
Fig. 1.
PRISMA flow diagram of searches and included studies
Table 1.
Characteristic of contributing data sources
| Individual case series | National Joint Registry. Annual report 2021 | Australian Orthopaedic Association National Joint Replacement Registry. Annual report 2021 | Swiss National Joint Registry. Annual report 2020 | The Dutch Arthroplasty Registry. Annual report 2020 | The German Arthroplasty Registry. Annual report 2020 | |
|---|---|---|---|---|---|---|
| Study-level characteristics | ||||||
| Location | 3 countries | UK | Australia | Switzerland | The Netherlands | Germany |
| Number of series | 9 | 5 | 1 | 3 | 3 | 2 |
| Year of publication | 2011–2020 | 2021 | 2021 | 2020 | 2020 | 2021 |
| Participant-level characteristics | ||||||
| Total joint replacements (dual-mobility cups) included | 10,494 | 7569 | 10,763 | 1900 | 1355 | 1433 |
| Mean age (years) | 70.30* | > 65+ | NR | > 65+ | NR | NR |
| Number of females (%) | 6101 (58.9) | NR | NR | NR | NR | NR |
NR: not reported, +: all patients > 65
*Weighted mean for age by number in study
Case series
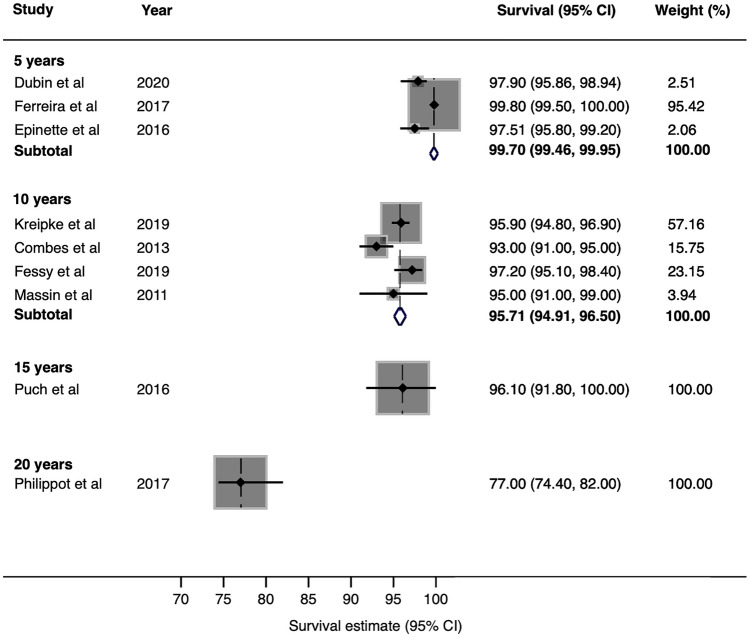
The nine case series included in the meta-analysis reported all-cause construct survival in 10,494 DMC-THR (range 119–3474) with a follow-up ranging from 4 to 20 years. Pooled analysis of data derived from case series reported at exactly 5, 10 and 20 years showed all-cause survivorship of the DMC-THR of 97.5% (95% CI 95.8–99.2) at 5 years, 95.5% (95% CI 94.3–96.7) at 10 years and 77% (95% CI 73.2–80.8) at 20 years. After rounding, pooled analysis of data extracted from case series of DMC-THR we observed a pooled all-cause construct survival of 99.7% (95% CI 99.5–100) at 5 years, 95.7% (95% CI 94.9–96.5) at 10 years, 96.1% (95% CI 91.8–100) at 15 years and 77% (95% CI 7.4–82.0) at 20 years (Fig. 2).
Fig. 2.
Estimates of survival from case series at 5 years, 10 years, 15 years and 20 years
The overall rate of dislocation, including closed reductions and revision, reported in the 39 case series (17,135 DMC-THR) was 1.1% with a mean patient age at the time of intervention to treat the dislocation of 66.5 years (weighted) at a mean follow-up of 7.3 years (2–25.3). The proportion of females was 60.8%. The overall revision estimate for DMC-THR instability, infection and fracture was 0.8%, 0.4% and 0.3%, respectively (individual studies: appendix 3).
The quality of included case series was variable. The quality assessment showed that two (22.2%) out of nine were consecutive, seven (77.8%) were multicentre, five (55.6%) had less than 80% follow-up and two (22.2%) used multivariable analysis.
Registry series
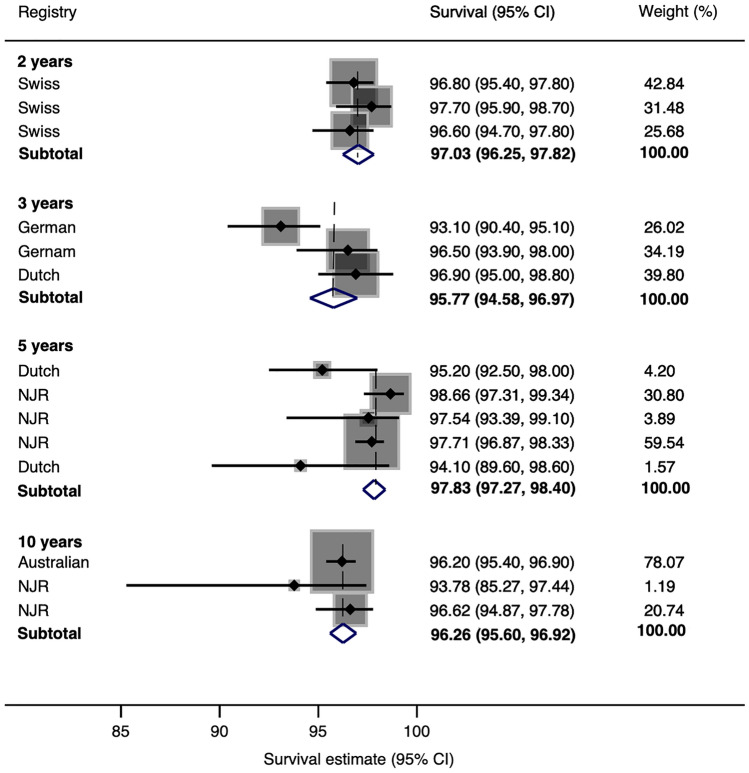
The search of joint registries revealed 14 brand and construct-based series, all of which provided confidence intervals in 23,020 (range 347–10,763) DMC-THR. Pooled analysis of data extracted from joint registries of DMC-THR showed all-cause construct survival of 97.0% (95% CI 96.3–97.8) at 2 years, 95.8 (95% CI 94.6–97.0) at 3 years, 97.8% (95% CI 97.3–98.4) at 5 years and 96.3% (95% CI 95.6–96.9) at 10 years (Fig. 3).
Fig. 3.
Estimates of survival from registries at 2 years, 3 years, 5 years and 10 years
Discussion
The pooled survival estimate for DMC-THR at 5 years was 97.8% from registry data and 99.7% from case series. The pooled survival estimate at 10 years was 96.3% from registry data and 95.7% from case series. Survival estimates at 15 and 20 years relied on case series data and were 96.1% and 77%. The unadjusted rate of DMC-THR dislocation was 1.1%. The revision estimate for DMC-THR instability, infection and fracture was 0.8%, 0.4% and 0.3%, respectively. At comparable time points, the survival estimate of DMC-THRs from case series was superior at 5 years but similar at 10 years when compared to registry series. The survival estimate of DMC-THRs at 20 years was from one case series that reported on first-generation DMC-THRs which may account for the apparent drop in survival after this time point.
The results presented are different to previously published survival estimates of primary DMC-THR. One systematic review published in 2018 reported a mean survival of DMC-THR of 98% at a mean follow-up of 8.5 years [13]. However, these figures were based on case series and survival estimates that did not include all-cause revision as an outcome. This means that the results are prone to selection and publication bias and are based on an outcome that may not be relevant to patients. These sources of bias may explain the higher survival estimates seen in case series at 5 years [17]. Jonker et al. reported all-cause revision estimates of 1.6% from case series at a minimum follow-up of 0.5–10 years and 2.7% from registry studies at a median follow-up of 2.5–3.2 years [24]. Despite the inclusion of hip fractures, this supports our theory that case series may overestimate survival and that registry data are more representative of the entire population at risk. Our study also includes much larger numbers of DMC-THR and longer follow-up times than these studies. Despite the higher revision estimates reported in this study, the estimates still fall within the acceptable thresholds set out by NICE. The observed pooled dislocation rate of our study was lower than other published estimates. Darrith et al. reported a rate of 1.5% at a mean follow-up of 8.5 years in a population that included neck of femur fractures [13]. De Martino et al. reported a dislocation rate of 0.9%, excluding IAPD, at a mean follow-up of 6.8 years which may be more representative of contemporary DMC-THR [15, 25]. A meta-analysis published in 2019 of eight comparative non-randomised studies reported that DMC-THR appears to reduce the rate of dislocation [16]. If we assume that there is selective use of DMC-THR for patients at a higher risk of dislocation, the survival estimates for dislocation and revision for dislocation observed to be the same between DMC-THR and C-THR imply that DMC-THR may be beneficial in moderating the higher risk of dislocation. However, reducing the rate of one complication may not warrant its use when there is an association with other complications such as infection and PPF [4, 26]. In addition, the cost of DMC-THR can be up to double that of a C-THR [27]. Its routine use, therefore, may not be justified if survival estimates are comparable to C-THR or potentially worse for other causes of failure.
There is a paucity of randomised comparative trials in this area and designing such a study to provide evidence of causation is difficult due to cost, the large sample sizes required and the challenges of long-term follow-up of joint replacement [28]. One meta-analysis of five comparative studies reported no difference in the all-cause risk of revision between DMC-THR and C-THR [14]. However, only one study was prospective and none were randomised. A proposed nested registry trial may go some way in providing higher-quality evidence [29]. However, concerns have been raised about the trial’s generalizability because several patient groups that are generally regarded as high risk for dislocation are to be excluded. The study may also be underpowered as the power calculation was based on studies that included such patients [30].
The results of this study must be interpreted in the context of its limitations. A total of 6,315 DMC-THRs had to be excluded from the quantitative analysis because the authors did not provide all-cause DMC-THR survival estimates or confidence intervals [31–35]. Some authors chose to publish survival estimates for specific end points: aseptic cup loosening only [36–41], all-cause cup failure only [42] or cup failure after removing patients who were revised for sepsis [43]. Reporting survival of part of the DMC-THR does not match the lived patient experience or patient preference for defining revision outcomes of THR and may bias any conclusion made, falsely suggesting a positive association between DMC-THR and superior survival estimates. All-cause construct survival is closer to what is an acceptable outcome for patients and is recommended by ISAR as the principal outcome measure for both early and late benchmarking [44]. Reporting survival of an implant only is, in itself, also a limitation [45]. Qualitative studies have shown that function and being able to engage in valued everyday activities matters most to patients [46]. Most studies included in this review were retrospective case series and are exposed to confounding and bias. For example, a proportion of patients such as those who are older with multiple comorbidities may be less likely to be offered revision surgery. This also highlights a limitation of NJR data in that it does not report on closed reductions, PFF treated with interventions that do not involve revision and infections that are not revised. These factors may bias the outcome away from the null hypothesis. Only two studies included in the final analysis adjusted their results for any potential confounders which may lead to a conclusion of a false association between DMC-THR and lower dislocations rates [37, 47]. A strength of our study is the inclusion of 23,020 DMC-THRs from national registries, which reduces one source of bias and may better reflect survival in the general population. In addition, it is also the largest study of survival estimates of primary DMC-THRs.
The results in our study suggest that selective use of DMC-THR in primary THR may be justified to reduce the risk of dislocation. However, increased costs and other causes of failure must be taken into consideration with its use. In-depth scrutiny of generalizable early warnings will be paramount to mitigate against potentially higher rates of early revision surgery.
In conclusion, pooled survival estimates of the DMC-THR in primary THR at 5 and 10 years reported in this study are acceptable according to the revision threshold set out by NICE but its use should be carefully considered in light of its cost and outcomes.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
AG: contributed to conceptualization, data curation, formal analysis, and writing—original draft. HM: contributed to conceptualization and data curation. JTE: contributed to conceptualization, methodology, supervision, and writing—review and editing. AS: contributed to supervision and writing—review and editing. MRW: contributed to conceptualization, supervision, and writing—review and editing.
Funding
None.
Data availability
The authors declare that the data supporting the findings of this study are available within the article (and its supplementary information files).
Declarations
Conflict of interest
The institution of one or more of the authors (MW) has received funding from Heraeus. MW has or may receive payments or benefits from Taylor & Francis (for editing of orthopaedic textbooks) related to this work. MW is the associate editor of Hip International; Editorial board of Bone and Joint Journal and Bone and Joint 360. JTE is the associate editor of BJ360. AS is the Chairman of the ISAR benchmarking working group.
Ethical approval
None required.
Informed consent
None required.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Andrew Gardner, Email: a.gardner5@nhs.net.
Hamish Macdonald, Email: hamish.macdonald@nhs.net.
Jonathan T. Evans, Email: j.t.evans@bristol.ac.uk
Adrian Sayers, Email: adrian.Sayers@bristol.ac.uk.
Michael R. Whitehouse, Email: michael.whitehouse@bristol.ac.uk
References
- 1.Learmonth ID, Young C, Rorabeck C. The operation of the century. Lancet. 2007;370(9597):1508–1519. doi: 10.1016/S0140-6736(07)60457-7. [DOI] [PubMed] [Google Scholar]
- 2.Fessy MH, Putman S, Viste A, Isida R, Ramdane N, Ferreira A, et al. What are the risk factors for dislocation in primary total hip arthroplasty? Orthop Traumatol Surg Res. 2017;103(5):663–668. doi: 10.1016/j.otsr.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 3.Kunutsor SK, Barrett MC, Beswick AD, Judge A, Blom AW, Wylde V, et al. Risk factors for dislocation after primary total hip replacement. Lancet Rheumatol. 2019;1(2):e111–e121. doi: 10.1016/S2665-9913(19)30045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Joint Registry for England, Wales, Northern Ireland and the Isle of Man. 18th Annual Report. 2021. https://reports.njrcentre.org.uk/Portals/0/PDFdownloads/NJR%2018th%20Annual%20Report%202021.pdf. Accessed 24 Jan 2022
- 5.Kostensalo I, Junnila M, Virolainen P, Remes V, Matilainen M, Vahlberg T, et al. Effect of femoral head size on risk of revision for dislocation after total hip arthroplasty A population-based analysis of 42,379 primary procedures from the Finnish Arthroplasty Register. Acta Orthop. 2013;84(4):342–347. doi: 10.3109/17453674.2013.810518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zijlstra WP, De Hartog B, Van Steenbergen LN, Scheurs BW, Nelissen R. Effect of femoral head size and surgical approach on risk of revision for dislocation after total hip arthroplasty. Acta Orthop. 2017;88(4):395–401. doi: 10.1080/17453674.2017.1317515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laura AD, Hothi H, Battisti C, Cerquiglini A, Henckel J, Skinner J, et al. Wear of dual-mobility cups. Int Orthop. 2017;41(3):625–633. doi: 10.1007/s00264-016-3326-9. [DOI] [PubMed] [Google Scholar]
- 8.Unter Ecker N, Piakong P, Delgado G, Gehrke T, Citak M, Ohlmeier M. What is the failure rate of constrained liners in complex revision total hip arthroplasty? Arch Orthop Trauma Surg. 2022 doi: 10.1007/s00402-022-04419-z. [DOI] [PubMed] [Google Scholar]
- 9.Blakeney WG, Epinette JA, Vendittoli PA. Dual mobility total hip arthroplasty: should everyone get one? EFORT Open Rev. 2019;4(9):541–547. doi: 10.1302/2058-5241.4.180045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Philippot R, Boyer B, Farizon F. Intraprosthetic dislocation: a specific complication of the dual-mobility system. Clin Orthop Relat Res. 2013;471(3):965–970. doi: 10.1007/s11999-012-2639-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sappey-Marinier E, Viste A, Blangero Y, Desmarchelier R, Fessy MH. A comparative study about the incidence of dislocation and peri-prosthetic fracture between dual mobility versus standard cups after primary total hip arthroplasty. Int Orthop. 2019;43(12):2691–2695. doi: 10.1007/s00264-018-4279-y. [DOI] [PubMed] [Google Scholar]
- 12.Heckmann N, Weitzman DS, Jaffri H, Berry DJ, Springer BD, Lieberman JR. Trends in the use of dual mobility bearings in hip arthroplasty. Bone Joint J. 2020;102(7):27–32. doi: 10.1302/0301-620X.102B7.BJJ-2019-1669.R1. [DOI] [PubMed] [Google Scholar]
- 13.Darrith B, Courtney PM, Della Valle CJ. Outcomes of dual mobility components in total hip arthroplasty. Bone Joint J. 2018;100B(1):11–19. doi: 10.1302/0301-620X.100B1.BJJ-2017-0462.R1. [DOI] [PubMed] [Google Scholar]
- 14.Reina N, Pareek A, Krych AJ, Pagnano MW, Berry DJ, Abdel MP. Dual-mobility constructs in primary and revision total hip arthroplasty. J Arthroplasty. 2019;34(3):594–603. doi: 10.1016/j.arth.2018.11.020. [DOI] [PubMed] [Google Scholar]
- 15.De Martino I, D'Apolito R, Soranoglou VG, Poultsides LA, Sculco PK, Sculco TP. Dislocation following total hip arthroplasty using dual mobility acetabular components. Bone Joint J. 2017;99-B(5):703. doi: 10.1302/0301-620X.99B1.BJJ-2016-0398.R1. [DOI] [PubMed] [Google Scholar]
- 16.Romagnoli M, Grassi A, Costa GG, Lazaro LE, Lo Presti M, Zaffagnini S. The efficacy of dual-mobility cup in preventing dislocation after total hip arthroplasty. Int Orthop. 2019;43(5):1071–1082. doi: 10.1007/s00264-018-4062-0. [DOI] [PubMed] [Google Scholar]
- 17.Evans JT, Evans JP, Walker RW, Blom AW, Whitehouse MR, Sayers A. How long does a hip replacement last? Lancet. 2019;393(10172):647–654. doi: 10.1016/S0140-6736(18)31665-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.No authors listed. Total hip replacement and resurfacing arthroplasty for end-stage arthritis of the hip (TA304). National Institute for Clinical Excellent. 2014. https://www.nice.org.uk/guidance/ta304/resources/total-hip-replacement-and-resurfacing-arthroplasty-for-endstage-arthritis-of-the-hip-pdf-82602365977285. Accessed 24 Jan 2022
- 19.No authors listed. ODEP Benchmarks Developments 2017. Orthopaedic Data Evaluation Panel. 2017. https://www.odep.org.uk/Portals/0/ODEPStatements/ODEP%20Benchmarks%202017_rev.6.pdf. Accessed 24 Jan 2022
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Sys Rev. 2016;5(1):210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le Manach Y, Collins G, Bhandari M, Bessissow A, Boddaert J, Khiami F, et al. Outcomes after hip fracture surgery compared with elective total hip replacement. JAMA. 2015;314(11):1159–1166. doi: 10.1001/jama.2015.10842. [DOI] [PubMed] [Google Scholar]
- 23.Wylde V, Beswick AD, Dennis J, Gooberman-Hill R. Post-operative patient-related risk factors for chronic pain after total knee replacement. BMJ Open. 2017;7(11):e018105. doi: 10.1136/bmjopen-2017-018105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jonker RC, Van Beers L, Van der Wal BCH, Vogely HC, Parratte S, Castelein RM, et al. Can mobility cups prevent dislocation without increasing revision rates in primary total hip arthroplasty? Orthop Traumatol Surg Res. 2020;106(3):509–517. doi: 10.1016/j.otsr.2019.12.019. [DOI] [PubMed] [Google Scholar]
- 25.Pai F-Y, Ma H-H, Chou T-FA, Huang T-W, Huang K-C, Tsai S-W, et al. Risk factors and modes of failure in the modern dual mobility implant. BMC Musculoskelet Disord. 2021;22(1):541. doi: 10.1186/s12891-021-04404-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee G-C, Kamath A, Courtney PM. Clinical concerns with dual mobility—should I avoid it when possible? J Arthroplasty. 2021;36(7):S88–S91. doi: 10.1016/j.arth.2021.01.006. [DOI] [PubMed] [Google Scholar]
- 27.Khoshbin A, Haddad FS, Ward S, Oh S, Wu J, Nherera L, et al. A cost-effectiveness assessment of dual-mobility bearings in revision hip arthroplasty. Bone Joint J. 2020;102-b(9):1128–1135. doi: 10.1302/0301-620X.102B9.BJJ-2019-1742.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verhaar J. Hope for better days. Acta Orthop. 2020;91(5):501–502. doi: 10.1080/17453674.2020.1817663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Beers L, Van Der Wal BCH, Van Loon TG, Moojen DJF, Van Wier MF, Klaassen AD, et al. Study protocol: effectiveness of dual-mobility cups compared with uni-polar cups for preventing dislocation after primary total hip arthroplasty in elderly patients. Acta Orthop. 2020;91(5):514–519. doi: 10.1080/17453674.2020.1798658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Evans JT, Whitehouse MR. A review of registry research from 2020. Bone Joint 360. 2020;9(6):47–49. doi: 10.1302/2048-0105.96.360818. [DOI] [Google Scholar]
- 31.Assi C, Kheir N, Samaha C, Kouyoumdjian P, Yammine K. Early results with total hip arthroplasty using dual-mobility cup in patients with osteonecrosis of the femoral head. SICOT J. 2018;4:4. doi: 10.1051/sicotj/2018001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laurendon L, Philippot R, Neri T, Boyer B, Farizon F. Ten-year clinical and radiological outcomes of 100 total hip arthroplasty cases with a modern cementless dual mobility cup. Surg Technol Int. 2018;32:331–336. [PubMed] [Google Scholar]
- 33.Martz P, Maczynski A, Elsair S, Labattut L, Viard B, Baulot E. Total hip arthroplasty with dual mobility cup in osteonecrosis of the femoral head in young patients. Int Orthop. 2017;41(3):605–610. doi: 10.1007/s00264-016-3344-7. [DOI] [PubMed] [Google Scholar]
- 34.Neri T, Philippot R, Farizon F, Boyer B. Results of primary total hip replacement with first generation Bousquet dual mobility socket with more than twenty five years follow up. Int Orthop. 2017;41(3):557–561. doi: 10.1007/s00264-016-3373-2. [DOI] [PubMed] [Google Scholar]
- 35.Vermersch T, Viste A, Desmarchelier R, Fessy MH. Prospective longitudinal study of one hundred patients with total hip arthroplasty using a second-generation cementless dual-mobility cup. Int Orthop. 2015;39(11):2097–2101. doi: 10.1007/s00264-015-2985-2. [DOI] [PubMed] [Google Scholar]
- 36.Bauchu P, Bonnard O, Cypres A, Fiquet A, Girardin P, Noyer D. The dual-mobility POLARCUP: first results from a multicenter study. Orthopedics. 2008;31(12 Suppl 2):1663–1672. [PubMed] [Google Scholar]
- 37.Fessy MH, Jacquot L, Rollier JC, Chouteau J, Ait-Si-Selmi T, Bothorel H, et al. Midterm clinical and radiographic outcomes of a contemporary monoblock dual-mobility cup in uncemented total hip arthroplasty. J Arthroplasty. 2019;34(12):2983–2991. doi: 10.1016/j.arth.2019.07.026. [DOI] [PubMed] [Google Scholar]
- 38.Fresard PL, Alvherne C, Cartier JL, Cuinet P, Lantuejoul JP. Seven-year results of a press-fit hydroxyapatite-coated double mobility acetabular component in patients aged 65 years or older. Eur J Orthop Surg Traumatol. 2012;23(4):425–429. doi: 10.1007/s00590-012-0991-2. [DOI] [PubMed] [Google Scholar]
- 39.Epinette JA, Beracassat R, Tracol P, Pagazani G, Vandenbussche E. Are modern dual mobility cups a valuable option in reducing instability after primary hip arthroplasty, even in younger patients? J Arthroplasty. 2014;29(6):1323–1328. doi: 10.1016/j.arth.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 40.Leclercq S, Lavigne M, Girard J, Chiron P, Vendittoli PA. Durom hip resurfacing system. Orthop Traumatol Surg Res. 2013;99(3):273–279. doi: 10.1016/j.otsr.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 41.Philippot R, Adam P, Reckhaus M, Delangle F, Verdot FX, Curvale G, et al. Prevention of dislocation in total hip revision surgery using a dual mobility design. Orthop Traumatol Surg Res. 2009;95(6):407–413. doi: 10.1016/j.otsr.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 42.Harwin SF, Mistry JB, Chughtai M, Khlopas A, Gwam C, Newman JM, et al. Dual mobility acetabular cups in primary total hip arthroplasty in patients at high risk for dislocation. Surg Technol Int. 2017;30:251–258. [PubMed] [Google Scholar]
- 43.Farizon F, De Lavison R, Azoulai JJ, Bousquet G. Results with a cementless alumina-coated cup with dual mobility. Int Orthop. 1998;22(4):219–224. doi: 10.1007/s002640050246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.No Authors listed. International Prosthesis Benchmarking Working Group Guidance Document. Internation Society of Arthroplasty Registries. 2018. https://drive.google.com/file/d/0BwKvdROo5Eg-MjZYc2VHQUZGYzNJMlRaenZEVUN3cTdMYlBj/view?resourcekey=0-OtTMX1RmF7E-HAZgxXiNZg. Accessed 24 Jan 2022
- 45.Wylde V, Blom AW. The failure of survivorship. J Bone Joint Surg Br. 2011;93(5):569–570. doi: 10.1302/0301-620X.93B5.26687. [DOI] [PubMed] [Google Scholar]
- 46.Carroll FE, Gooberman-Hill R, Strange S, Blom AW, Moore AJ. What are patients' preferences for revision surgery after periprosthetic joint infection? A discrete choice experiment. BMJ Open. 2020;10(1):e031645. doi: 10.1136/bmjopen-2019-031645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kreipke R, Rogmark C, Pedersen AB, Karrholm J, Hallan G, Havelin LI, et al. Dual mobility cups: Effect on risk of revision of primary total hip arthroplasty due to osteoarthritis. J Bone Joint Surg Am. 2019;101(2):169–176. doi: 10.2106/JBJS.17.00841. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the article (and its supplementary information files).