Abstract
We determined cytokine levels in paired serum/CSF samples from first-episode schizophrenia (FES) participants (n = 20) and controls (n = 21) using a 13-plex immunoassay. Applying strictly-determined detection limits, 12 cytokines were found in serum and two in CSF. Higher serum MCP-1 levels (p = 0.007) were present in FES versus controls, which correlated with serum IgG (R = − 0.750; p = 0.013). Finally, IL-18 levels correlated with body weight in FES (R = 0.691; p = 0.041). This study demonstrates potential limitations in the sensitivity of multiplex cytokine assays for CSF studies in mental disorders and suggests that some published studies in this area should be re-evaluated.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00406-023-01569-y.
Keywords: First-episode psychosis; Schizophrenia; Serum, cerebrospinal fluid; CSF; Cytokines; Inflammation
Introduction
Increasing evidence suggests that neuroinflammation contributes to the pathogenesis of psychiatric disorders, including schizophrenia [1–6]. As many psychiatric disorders are thought of as systemic diseases, it has proved useful to integrate findings from multiple biomarker sources, such as cerebrospinal fluid (CSF) and blood [7–10], and by using multiplex immunoassays [11–13] to provide a more holistic picture of health status. However, only a few studies have applied such approaches to investigate inflammation-related biomarkers in both media in first-episode schizophrenia (FES) [6, 11].
Since multiplex immunoassays can show low sensitivity and poor correlation with corresponding singleplex methods [14, 15], it is important to characterise the performance of these platforms in clinical/laboratory-based studies. The limit of detection (LOD) defines the smallest concentration of an analyte that can be measured [16]. Although critical for discriminating between the presence or absence of low abundance analytes such as cytokines, few studies have reported that this was done correctly. This is especially true for cytokine measurements in CSF, where many of these molecules are present at < 10 pg/mL concentrations. However, most multiplex immunoassay studies have reported low concentrations of specific cytokines in CSF in mental disorders, without correctly determining the LOD [6, 17–21].
Although the companies who developed these assays may claim dynamic ranges with low LODs, none are capable of accurately measuring concentrations that give readings in the range of blank samples. Threshold levels of analytes must be present to produce signals that can be distinguished above this noise [16, 22]. We suggest adherence to the Clinical and Laboratory Standards Institute (CLSI) guideline for accurately determining the limit of the blank (LOB) and LOD to increase confidence in the results [16, 22]. Here, we carried out multiplex immunoassay analyses using the most robust assays from a 13-plex immunoassay panel to identify differences in cytokine levels in serum and CSF from FES patients and controls, following the CLSI guideline in determining the LOB and LOD for each assay.
Materials and methods
Samples
The study was performed according to German laws, the Declaration of Helsinki, and local institutional review board guidelines. Participants gave written informed consent. CSF and sera were obtained from 20 FES in-patients diagnosed according to ICD10 and AWMF-S3 guidelines [23] for whom routine differential diagnostic lumbar- and veni-puncture had been performed (Supplementary Table ST1). Samples were collected 8.0 days (median) after admission with acute psychosis. Psychopathology was assessed using the Positive and Negative Syndrome Scale (PANSS) [24]. Exclusion criteria: (a) immunological concomitant diseases, recent/current infections, trauma/systemic diseases and substance abuse, (b) treatment with cortisone or other immunosuppressive/modulating substances. Administered antipsychotics were converted to chlorpromazine (CPZ) units for statistical purposes [25].
Controls (n = 21) had headache (n = 3), pseudotumor cerebri (n = 3) or initially unclear neurological symptoms (n = 15) with no history of psychiatric disorders and underwent lumbar puncture to rule out subarachnoid hemorrhage, infectious or autoimmune central nervous system disease. They were matched for age, gender and body mass index. Routine CSF parameters were within the normal range and showed no significant group differences.
After an investigation of 36 clinical parameters (Supplementary Table ST2, column B), CSF and sera were centrifuged and the supernatants stored at − 80 °C.
Cytokine analysis
Concentrations of 13 cytokines [interferon (IFN)-α2, IFN-γ, interleukin (IL)-1β, IL-6, IL-8, IL-10, IL-12p70, IL-17A, IL-18, IL-23, IL-33, monocyte chemoattractant protein (MCP)-1 and tumor necrosis factor (TNF)-α] were determined in triplicate using the LEGENDplex™ Human Inflammation Panel 1 (BioVendor; Brno, Czechia) according to manufacturer instructions. This is a fluorescence–coded microsphere-based multiplex immuoassay for the detection and quantitation of analytes by flow cytometry. Sera were diluted twofold and CSF tested undiluted. Standard curves of each cytokine were analysed in duplicate on two separate occasions. The LOB and LOD were determined for each assay according to CLSI guidelines [16, 22], using the formulas below:
For statistical analysis, we used only those cytokines for which at least 2 of the 3 replicates and > 50% of samples gave readings above the LOD.
Statistical analysis
Data were analyzed using R (v4.0.5). Chi-square tests were performed to calculate group differences regarding gender and smoking. Corrected PANSS scores were derived by subtraction of minimum from raw scores [26]. Most data were not normally distributed as indicated by Shapiro–Wilk tests. Thus, group differences of continuous variables were calculated by Mann–Whitney U tests. MCP-1 serum levels (p < 0.001) were significantly higher in smokers. Therefore, diagnosis-dependent differences in MCP-1 were calculated by ART (analysis of variance using an aligned rank transformation of the data) with the covariate smoking. Due to the exploratory nature of the study, group statistics were not corrected for multiple comparisons.
We used Spearman rank tests with false discovery rate (FDR)-corrected p-values (q-values) to identify correlations of cytokines with routine blood/CSF, demographic and clinical parameters. Cliff’s delta (δ) was used to assess effect sizes (δ ≥ 0.147 = small, δ ≥ 0.330 = medium, δ ≥ 0.474 = large) [27]. All statistical tests were two-tailed with p < 0.05 considered significant.
Results and discussion
Determination of valid cytokine assays
According to our criteria, 12 out of 13 cytokine assays could be measured in serum samples with > 50% giving readings above the LOD (Table 1). The excluded cytokine was IL1β, for which only 6 out of the 21 control and 3 of the 20 FES samples had values above the LOD. In contrast to the serum results, only two, namely MCP-1 and IL-8, could be measured in CSF.
Table 1.
Valid cytokine assays
| Cytokine | Diagnosis | CSF | Serum | ||||
|---|---|---|---|---|---|---|---|
| Valid | Not valid | % Valid | Valid | Not vallid | % Valid | ||
| IFN-α2 | Cont | 0 | 21 | 0 | 20 | 1 | 95.2 |
| IFN-α2 | FES | 0 | 20 | 0 | 20 | 0 | 100 |
| IFN-γ | Cont | 0 | 21 | 0 | 13 | 8 | 61.9 |
| IFN-γ | FES | 0 | 20 | 0 | 14 | 6 | 70 |
| IL-10 | Cont | 0 | 21 | 0 | 20 | 1 | 95.2 |
| IL-10 | FES | 2 | 18 | 10 | 20 | 0 | 100 |
| IL-12p70 | Cont | 0 | 21 | 0 | 17 | 4 | 81 |
| IL-12p70 | FES | 0 | 20 | 0 | 17 | 3 | 85 |
| IL-17A | Cont | 0 | 21 | 0 | 19 | 2 | 90 |
| IL-17A | FES | 1 | 19 | 5 | 19 | 1 | 95 |
| IL-18 | Cont | 2 | 19 | 10 | 21 | 0 | 100 |
| IL-18 | FES | 3 | 17 | 15 | 20 | 0 | 100 |
| IL-1β | Cont | 0 | 21 | 0 | 6 | 15 | 29 |
| IL-1β | FES | 0 | 20 | 0 | 2 | 18 | 10 |
| IL-23 | Cont | 0 | 21 | 0 | 18 | 3 | 86 |
| IL-23 | FES | 0 | 20 | 0 | 17 | 3 | 85 |
| IL-33 | Cont | 0 | 21 | 0 | 19 | 2 | 90.5 |
| IL-33 | FES | 0 | 20 | 0 | 20 | 0 | 100 |
| IL-6 | Cont | 7 | 14 | 33.3 | 20 | 1 | 95.2 |
| IL-6 | FES | 12 | 8 | 60 | 17 | 3 | 85 |
| IL-8 | Cont | 20 | 1 | 95.2 | 19 | 2 | 90.48 |
| IL-8 | FES | 20 | 0 | 100 | 20 | 0 | 100 |
| MCP-1 | Cont | 21 | 0 | 100 | 20 | 1 | 95 |
| MCP-1 | FES | 20 | 0 | 100 | 20 | 0 | 100 |
| TNF-α | Cont | 0 | 21 | 0 | 16 | 5 | 76.2 |
| TNF-α | FES | 0 | 20 | 0 | 15 | 5 | 75 |
Cytokine assays for which at least 2 out of the 3 replicates and > 50% of the samples gave readings above the LOD are indicated for CSF and serum control (CONT) and FES samples
The assays which passed these criteria are indicated in bold font
Diagnosis-specific differences in cytokine levels
Neither of the two measurable CSF cytokines showed significant differences in concentrations between the groups (Table 2). The use of the current stringent approach might explain why we did not find some of the cytokine increases reported in a previous meta-analysis [6]. For the serum analysis, the levels of MCP-1 were significantly higher with a large effect size in FES (n = 20) [327.2 (225; 463.8) pg/mL] compared to controls (n = 19) [220.0 (108.5; 265.9) pg/mL, p = 0.007, δ = 0.495]. This was confirmed by ART with the covariate smoking [p = 0.024, δ = 0.479].
Table 2.
Cytokine levels in serum and CSF from FES patients and controls
| Cytokine | Cont [mean (Q1;Q2;n)] | FES [ean (Q1;Q2;n)] | U test | Cliff’s delta |
|---|---|---|---|---|
| Serum | ||||
| IFN-α2 | 9.213 (5.928;23.863;20) | 11.46 (5.52;29.21;20) | 0.698 | − 0.075 |
| IFN-γ | 9.981 (7.183;30.639;13) | 15.37 (5.56;40.08;14) | 0.616 | − 0.121 |
| IL-10 | 13.36 (7.19;33.38;20) | 21.32 (13.02;39.67;20) | 0.218 | − 0.230 |
| IL-12p70 | 13.02 (9.95;39.49;17) | 14.36 (8.32;23.80;17) | 0.730 | 0.073 |
| IL-17A | 4.854 (2.895;13.419;19) | 3.731 (2.997;6.110;19) | 0.201 | 0.247 |
| IL-18 | 345.3 (242.1;417.3;21) | 358.6 (240.0;565.3;20) | 0.561 | − 0.110 |
| IL-23 | 47.84 (25.85;100.07;18 | 49.18 (35.71;79.19;17) | 0.883 | − 0.033 |
| IL-33 | 369.7 (115.0;555.0;19) | 306.9 (135.8;409.6;20) | 0.380 | 0.168 |
| IL-6 | 23.27 (14.27;70.30;20) | 25.32 (14.13;63.47;17) | 0.964 | 0.012 |
| IL-8 | 130.5 (62.3;221.1;19) | 76.46 (60.63;150.68;20) | 0.396 | 0.163 |
| MCP-1 | 220.0 (108.5;265.9;19) | 327.2 (225.7;463.8;20) | 0.007# | − 0.495 |
| TNF-α | 32.01 (15.29;228.23;16) | 27.16 (18.34;62.01;15) | 0.495 | 0.150 |
| CSF | ||||
| IL-8 | 118.0 (65.0;144.6;20) | 93.77 (67.78;107.23;20) | 0.429 | 0.150 |
| MCP-1 | 457.5 (401.8;595.7;21) | 452.1 (393.8;568.9;20) | 0.847 | 0.038 |
| CSF/serum | ||||
| IL-8 | 1.031 (0.485;1.567;18) | 1.117 (0.494;1.810;20) | 0.393 | 0.167 |
| MCP-1 | 1.934 (1.401;4.065;20) | 1.285 (0.931;2.393;20) | 0.030+ | 0.400 |
Mean (Q1;Q3) values are in pg/mL
Significant differences between FES and controls (Cont) are indicated in bold
#ART with covariate smoking: p = 0.024
+ART with covariate smoking: p = 0.114
Correlation of cytokines with other parameters
Serum MCP-1
Of the 36 parameters determined (Supplementary Table ST2), there were no correlations with serum-MCP-1 apart from a significant negative correlation with serum IgG in FES (R = − 750; p < 0.001; q = 0.013) compared to controls (R = − 0.004; p = 0.987; q = 0.987) (Fig. 1A, Supplementary Table ST2). This supports previous findings of immunodeficiency-like and inflammatory phenotypes in schizophrenia [28–32]. Also, the finding of increased levels of MCP-1 in FES is consistent with previous studies which showed that schizophrenia patients have elevated serum MCP-1 levels in association with metabolic syndrome [33]. Although these physiological changes can be a side effect of antipsychotics, there have also been reports of such metabolic dysfunctions in first-onset patients prior to receiving medication [34]. Of note, serum MCP-1 levels did not correlate with PANSS scores (Supplementary Table ST3), CPZ units (R = − 0.154, p = 0.741, q = 0.919), albumin quotient (R = 0.579, p = 0.012; q = 0.169) or IgG index (R = 0.155, p = 0.538; q = 0.848).
Fig. 1.
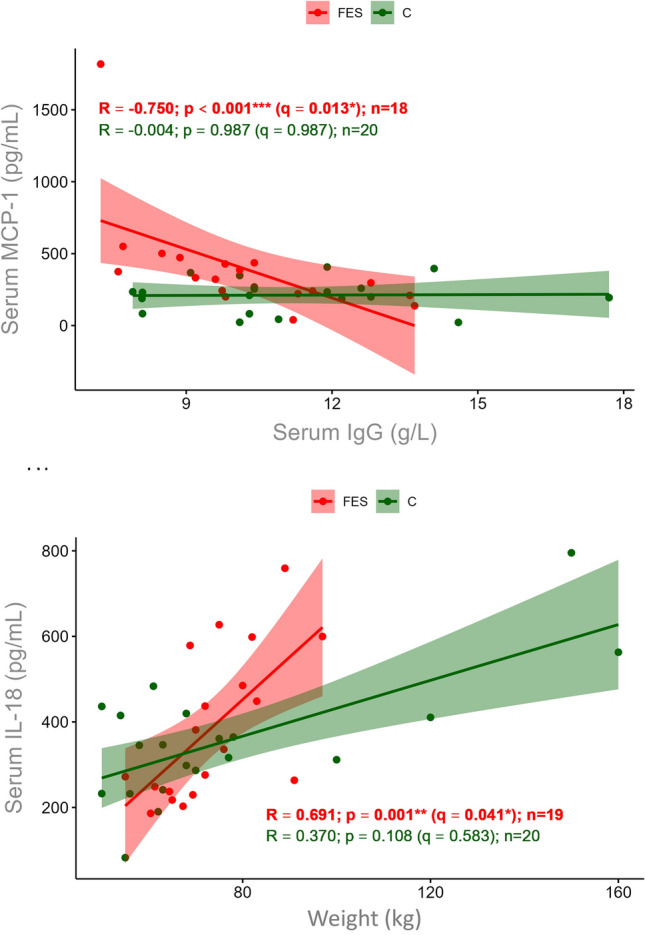
A Scatter plot showing Spearman rank correlation analysis of serum MCP-1 and IgG levels in FES (n = 18; red) and controls (C, n = 20; green). B Scatter plot showing Spearman rank correlation analysis of serum IL-18 levels with body weight (FES: n = 19, red; C: n = 20, green)
Serum IL-18
Although serum IL-18 levels showed no significant differences between groups (p = 0.561; U test), IL-18 was significantly correlated with body weight (R = 0.691; p < 0.001; q = 0.041) in FES but not controls (R = 0.370; p = 0.108; q = 0.583) (Fig. 1B, Supplementary Table ST1). This is consistent with previous studies showing that, like MCP-1, IL-18 is linked to metabolic disorders including diabetes and insulin resistance [35–40] (Supplementary Table ST2).
Other cytokines
No other cytokines were correlated with the other measured parameters, disease duration or CPZ after FDR correction (q-values, Supplementary Tables ST2, ST4).
Limitations
This study was limited as we only detected 2 out of 13 cytokines in CSF samples, obviating comparisons with cytokine readings in serum samples. Also, the small sample size of this exploratory investigation necessitates validation in larger cohorts. Regarding the biological findings, we did not assess the potential links of MCP-1 and IL-18 with metabolism-related disturbances as no measures of insulin resistance or visceral fat accumulation were available. Finally, although we controlled for the potential effects of antipsychotic medication, disease duration and smoking, we could not account for other influences like nutrition and sleep, and the use of headache patients as controls might bias the results as inflammatory causes may play a role in different forms of headache.
Conclusions
To our knowledge, this is the first multiplex cytokine immunoassay study of paired serum/CSF samples from FES patients and controls using criteria based on strictly determined LODs. The main advantage of using the CLSI guideline is a reduction of false positives as this approach takes into account the variance of both the blank and lowest concentration samples1. The potential disadvantages include the possibility of not detecting low-concentration cytokines close to these variable regions. Using this stringent approach, we detected 12 cytokines in serum and only two in CSF using a 13-plex panel. The undetectable cytokine assays all gave signals that were indistinguishable from the blank readings. This revealed potential limitations in the sensitivity of multiplex cytokine assays of CSF. This calls attention to the need for more sensitive assays that can be used to obtain reliable readings in CSF, such as gold nanoparticle immuno-PCR [41, 42] and single molecule arrays [43, 44]. Both methods can provide sensitivities approximately 100- to 1000-fold greater than conventional multiplexed immunoassay systems.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This study was funded in part by a grant of the European Research Area Network/ERA-NET-Neuron grant of the European Commission and German Federal Ministry of Education and Research (BMBF) to JS (project NicAb, funding code 01EW2012).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Other methods like bead count/median fluorescence intensity approaches do not account for variance.
The authors Deepti Singh and Paul C. Guest contributed equally to this work.
References
- 1.Reale M, Patruno A, De Lutiis MA, Pesce M, Felaco M, Di Giannantonio M, Di Nicola M, Grilli A. Dysregulation of chemo-cytokine production in schizophrenic patients versus healthy controls. BMC Neurosci. 2011;12:13. doi: 10.1186/1471-2202-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ezeoke A, Mellor A, Buckley P, Miller BJ. A systematic quantitative review of blood autoantibody elevations in schizophrenia. Schizophr Res. 2013;150:245–251. doi: 10.1016/j.schres.2013.07.029. [DOI] [PubMed] [Google Scholar]
- 3.Gibney SM, Drexhage HA. Evidence for a dysregulated immune system in the etiology of psychiatric disorders. J Neuroimmune Pharmacol. 2013;8:900–920. doi: 10.1007/s11481-013-9462-8. [DOI] [PubMed] [Google Scholar]
- 4.Sommer IE, van Westrhenen R, Begemann MJ, de Witte LD, Leucht S, Kahn RS. Efficacy of anti-inflammatory agents to improve symptoms in patients with schizophrenia: an update. Schizophr Bull. 2014;40:181–191. doi: 10.1093/schbul/sbt139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Girgis RR, Kumar SS, Brown AS. The cytokine model of schizophrenia: emerging therapeutic strategies. Biol Psychiatry. 2014;75(4):292–299. doi: 10.1016/j.biopsych.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang AK, Miller BJ. Meta-analysis of cerebrospinal fluid cytokine and tryptophan catabolite alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder, and depression. Schizophr Bull. 2018;44(1):75–83. doi: 10.1093/schbul/sbx035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harris LW, Pietsch S, Cheng TM, Schwarz E, Guest PC, Bahn S. Comparison of peripheral and central schizophrenia biomarker profiles. PLoS ONE. 2012;7(10):e46368. doi: 10.1371/journal.pone.0046368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guest PC, Guest FL, Martins-de Souza D. Making sense of blood-based proteomics and metabolomics in psychiatric research. Int J Neuropsychopharmacol. 2016;19(6):pyv138. doi: 10.1093/ijnp/pyv138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Misra BB, Langefeld CD, Olivier M, Cox LA. Integrated omics: tools, advances, and future approaches. J Mol Endocrinol. 2018 doi: 10.1530/JME-18-0055. [DOI] [PubMed] [Google Scholar]
- 10.Subramanian I, Verma S, Kumar S, Jere A, Anamika K. Multi-omics data integration, interpretation, and its application. Bioinform Biol Insights. 2020;14:1177932219899051. doi: 10.1177/1177932219899051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gallego JA, Blanco EA, Husain-Krautter S, Madeline Fagen E, Moreno-Merino P, Del Ojo-Jiménez JA, Ahmed A, Rothstein TL, Lencz T, Malhotra AK. Cytokines in cerebrospinal fluid of patients with schizophrenia spectrum disorders: new data and an updated meta-analysis. Schizophr Res. 2018;202:64–71. doi: 10.1016/j.schres.2018.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu Q, Zhu J, Van Eyk JE. Comparison of multiplex immunoassay platforms. Clin Chem. 2010;56(2):314–318. doi: 10.1373/clinchem.2009.135087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stephen L, Schwarz E, Guest PC. Multiplex immunoassay profiling of serum in psychiatric disorders. Adv Exp Med Biol. 2017;974:149–156. doi: 10.1007/978-3-319-52479-5_10. [DOI] [PubMed] [Google Scholar]
- 14.Liu MY, Xydakis AM, Hoogeveen RC, Jones PH, Smith EO, Nelson KW, Ballantyne CM. Multiplexed analysis of biomarkers related to obesity and the metabolic syndrome in human plasma, using the Luminex-100 system. Clin Chem. 2005;51(7):1102–1109. doi: 10.1373/clinchem.2004.047084. [DOI] [PubMed] [Google Scholar]
- 15.Codorean E, Nichita C, Albulescu L, Răducan E, Popescu ID, Lonită AC, Albulescu R. Correlation of XMAP and ELISA cytokine profiles; development and validation for immunotoxicological studies in vitro. Roum Arch Microbiol Immunol. 2010;69(1):13–19. [PubMed] [Google Scholar]
- 16.Clinical and Laboratory Standards Institute. Protocols for Determination of Limits of Detection and Limits of Quantitation, Approved Guideline. CLSI document EP17. Wayne, PA USA: CLSI; 2004. https://webstore.ansi.org/preview-pages/CLSI/preview_EP17-A.pdf. Accessed 7 Sept 2022.
- 17.Maxeiner HG, Marion Schneider E, Kurfiss ST, Brettschneider J, Tumani H, Bechter K. Cerebrospinal fluid and serum cytokine profiling to detect immune control of infectious and inflammatory neurological and psychiatric diseases. Cytokine. 2014;69(1):62–67. doi: 10.1016/j.cyto.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 18.Hestad KA, Engedal K, Whist JE, Aukrust P, Farup PG, Mollnes TE, Ueland T. Patients with depression display cytokine levels in serum and cerebrospinal fluid similar to patients with diffuse neurological symptoms without a defined diagnosis. Neuropsychiatr Dis Treat. 2016;12:817–822. doi: 10.2147/NDT.S101925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuzior H, Fiebich BL, Yousif NM, Saliba SW, Ziegler C, Nickel K, Maier SJ, Süß P, Runge K, Matysik M, Dersch R, Berger B, Robinson T, Venhoff N, Kessler F, Blank T, Domschke K, Tebartz van Elst L, Endres D. Increased IL-8 concentrations in the cerebrospinal fluid of patients with unipolar depression. Compr Psychiatry. 2020;102:152196. doi: 10.1016/j.comppsych.2020.152196. [DOI] [PubMed] [Google Scholar]
- 20.Hidese S, Hattori K, Sasayama D, Tsumagari T, Miyakawa T, Matsumura R, Yokota Y, Ishida I, Matsuo J, Yoshida S, Ota M, Kunugi H. Cerebrospinal fluid inflammatory cytokine levels in patients with major psychiatric disorders: a multiplex immunoassay study. Front Pharmacol. 2021;11:594394. doi: 10.3389/fphar.2020.594394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Runge K, Fiebich BL, Kuzior H, Saliba SW, Yousif NM, Meixensberger S, Nickel K, Denzel D, Schiele MA, Maier SJ, Berger B, Dersch R, Domschke K, Tebartz van Elst L, Endres D. An observational study investigating cytokine levels in the cerebrospinal fluid of patients with schizophrenia spectrum disorders. Schizophr Res. 2021;231:205–213. doi: 10.1016/j.schres.2021.03.022. [DOI] [PubMed] [Google Scholar]
- 22.Armbruster DA, Pry T. Limit of blank, limit of detection and limit of quantitation. Clin Biochem Rev. 2008;29(Suppl 1):S49–52. [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization (2015) The international statistical classification of diseases and related health problems, ICD-10: 10th Revision (ICD-10). World Health Organization; Geneva, Switzerland. ISBN-13: 978–9241549165.
- 24.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 25.Atkins M, Burgess A, Bottomley C, Riccio M. Chlorpromazine equivalents: a consensus of opinion for both clinical and research applications. Psychiatr Bull. 1997;21(4):224–226. doi: 10.1192/pb.21.4.224. [DOI] [Google Scholar]
- 26.Obermeier M, Schennach-Wolff R, Meyer S, Möller HJ, Riedel M, Krause D, Seemüller F. Is the PANSS used correctly? A systematic review. BMC Psychiatry. 2011;11:113. doi: 10.1186/1471-244X-11-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen J (1988) Statistical power analysis for the behavioral sciences. Routledge; 2nd edition; Abingdon-on-Thames, Oxfordshire, England, UK. ISBN-13: 978-0805802832
- 28.Schwarz E, van Beveren NJ, Ramsey J, Leweke FM, Rothermundt M, Bogerts B, Steiner J, Guest PC, Bahn S. Identification of subgroups of schizophrenia patients with changes in either immune or growth factor and hormonal pathways. Schizophr Bull. 2014;40(4):787–795. doi: 10.1093/schbul/sbt105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steiner J, Bernstein HG, Schiltz K, Müller UJ, Westphal S, Drexhage HA, Bogerts B. Immune system and glucose metabolism interaction in schizophrenia: a chicken-egg dilemma. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:287–294. doi: 10.1016/j.pnpbp.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 30.Klaus F, Mitchell K, Liou SC, Eyler LT, Nguyen TT. Chemokine MCP1 is associated with cognitive flexibility in schizophrenia: a preliminary analysis. J Psychiatr Res. 2021;138:139–145. doi: 10.1016/j.jpsychires.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dunleavy C, Elsworthy RJ, Upthegrove R, Wood SJ, Aldred S. Inflammation in first-episode psychosis: the contribution of inflammatory biomarkers to the emergence of negative symptoms, a systematic review and meta-analysis. Acta Psychiatr Scand. 2022;146(1):6–20. doi: 10.1111/acps.13416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ermakov EA, Melamud MM, Buneva VN, Ivanova SA. Immune system abnormalities in schizophrenia: an integrative view and translational perspectives. Front Psychiatry. 2022;13:880568. doi: 10.3389/fpsyt.2022.880568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drexhage RC, Padmos RC, de Wit H, Versnel MA, Hooijkaas H, van der Lely AJ, van Beveren N, deRijk RH, Cohen D. Patients with schizophrenia show raised serum levels of the pro-inflammatory chemokine CCL2: association with the metabolic syndrome in patients? Schizophr Res. 2008;102(1–3):352–355. doi: 10.1016/j.schres.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 34.Harris LW, Guest PC, Wayland MT, Umrania Y, Krishnamurthy D, Rahmoune H, Bahn S. Schizophrenia: metabolic aspects of aetiology, diagnosis and future treatment strategies. Psychoneuroendocrinology. 2013;38(6):752–766. doi: 10.1016/j.psyneuen.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 35.Leick L, Lindegaard B, Stensvold D, Plomgaard P, Saltin B, Pilegaard H. Adipose tissue interleukin-18 mRNA and plasma interleukin-18: effect of obesity and exercise. Obesity (Silver Spring) 2007;15(2):356–363. doi: 10.1038/oby.2007.528. [DOI] [PubMed] [Google Scholar]
- 36.Bruun JM, Stallknecht B, Helge JW, Richelsen B. Interleukin-18 in plasma and adipose tissue: effects of obesity, insulin resistance, and weight loss. Eur J Endocrinol. 2007;157(4):465–471. doi: 10.1530/EJE-07-0206. [DOI] [PubMed] [Google Scholar]
- 37.Jung C, Gerdes N, Fritzenwanger M, Figulla HR. Circulating levels of interleukin-1 family cytokines in overweight adolescents. Mediat Inflamm. 2010;2010:958403. doi: 10.1155/2010/958403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahmad R, Thomas R, Kochumon S, Sindhu S. Increased adipose tissue expression of IL-18R and its ligand IL-18 associates with inflammation and insulin resistance in obesity. Immun Inflamm Dis. 2017;5(3):318–335. doi: 10.1002/iid3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bankul A, Mitra P, Suri S, Saxena I, Shukla R, Shukla K, Sharma P. Increased serum IL-18 levels and IL-18R expression in newly diagnosed type 2 diabetes mellitus. Minerva Endocrinol. 2020 doi: 10.23736/S0391-1977.20.03271-X. [DOI] [PubMed] [Google Scholar]
- 40.Nedeva I, Gateva A, Assyov Y, Karamfilova V, Hristova J, Yamanishi K, Kamenov Z, Okamura H. IL-18 serum levels in patients with obesity, prediabetes and newly diagnosed type 2 diabetes. Iran J Immunol. 2022;19(2):199–206. doi: 10.22034/iji.2022.90095.1987. [DOI] [PubMed] [Google Scholar]
- 41.Potůčková L, Franko F, Bambousková M, Dráber P. Rapid and sensitive detection of cytokines using functionalized gold nanoparticle-based immuno-PCR, comparison with immuno-PCR and ELISA. J Immunol Methods. 2011;371(1–2):38–47. doi: 10.1016/j.jim.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 42.Dahiya B, Mehta PK. Detection of potential biomarkers associated with outrageous diseases and environmental pollutants by nanoparticle-based immuno-PCR assays. Anal Biochem. 2019;587:113444. doi: 10.1016/j.ab.2019.113444. [DOI] [PubMed] [Google Scholar]
- 43.Lee S, Cho NP, Kim JD, Jung H, Kang SH. An ultra-sensitive nanoarray chip based on single-molecule sandwich immunoassay and TIRFM for protein detection in biologic fluids. Analyst. 2009;134(5):933–938. doi: 10.1039/b822094h. [DOI] [PubMed] [Google Scholar]
- 44.Rissin DM, Kan CW, Song L, Rivnak AJ, Fishburn MW, Shao Q, Piech T, Ferrell EP, Meyer RE, Campbell TG, Fournier DR, Duffy DC. Multiplexed single molecule immunoassays. Lab Chip. 2013;13(15):2902–2911. doi: 10.1039/c3lc50416f. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


