Abstract
Tat expression is required for efficient human immunodeficiency virus type 1 (HIV-1) reverse transcription. In the present study, we generated a series of 293 cell lines that contained a provirus with a tat gene deletion (Δtat). Cell lines that contained Δtat and stably transfected vectors containing either wild-type tat or a number of tat mutants were obtained so that the abilities of these tat genes to stimulate HIV-1 gene expression and reverse transcription could be compared. tat genes with mutations in the amino terminus did not stimulate either viral gene expression or HIV-1 reverse transcription. In contrast, tat mutants in the activation, core, and basic domains of Tat did not stimulate HIV-1 gene expression but markedly stimulated HIV-1 reverse transcription. No differences in the levels of virion genomic RNA or tRNA3Lys were seen in the HIV-1 Δtat viruses complemented with either mutant or wild-type tat. Finally, overexpression of the Tat-associated kinases CDK7 and CDK9, which are involved in Tat activation of HIV-1 transcription, was not able to complement the reverse transcription defects associated with the lack of a functional tat gene. These results indicate that the mechanism by which tat modulates HIV-1 reverse transcription is distinct from its ability to activate HIV-1 gene expression.
Reverse transcription is the process by which retroviruses synthesize a double-stranded DNA provirus from their positive-strand RNA genomes (4, 71). Studies involving the analysis of human immunodeficiency virus type 1 (HIV-1) reverse transcription have demonstrated that this process is subject to complex regulation by both viral and cellular factors. For example, the virally encoded heterodimeric reverse transcriptase (RT) p51/p66 (6) and nucleocapsid protein (NCp7) (51) interact with a cellular tRNA3Lys, which is preferentially imported into virion particles (32), by complementary base pairing with a region of HIV-1 genomic RNA known as the primer-binding site (64). Interactions between the cellular tRNA3Lys, RT (5, 63), and NCp7 (52, 54, 57) may help influence the specific reverse transcription initiation complex. Other RNA structures, including TAR RNA (31, 35) and an A-rich loop outside the primer-binding site within U5 (40, 42, 44), have also been reported to be necessary and may promote a structurally favored initiation complex required for efficient reverse transcription.
In addition to those in the genes for RT and NCp7, mutations in other HIV-1 structural, regulatory, and accessory genes have resulted in viruses that are defective for reverse transcription. These include the nef (1, 67) and vif (75) genes, which have been shown to affect HIV-1 reverse transcription by influencing virus particle formation. HIV-1 matrix protein (11, 74) and Vpr (37) may influence reverse transcription efficiency by directing nuclear import of the reverse transcription complex, in addition to having effects on early steps in the life cycle prior to reverse transcription (47). HIV-1 integrase (56) and the viral transactivator Tat (34) are also required for efficient HIV-1 reverse transcription. Cellular proteins, including cyclophilin A (23, 72), DNA topoisomerase I (66), and ERK2 (45), which are specifically incorporated into HIV-1 virions, have also been suggested to play either a direct or indirect role in the process of reverse transcription.
The HIV-1 transactivator Tat is required for efficient viral replication by stimulating HIV-1 transcriptional activation. Tat activation requires a double-stranded RNA structure known as TAR, extending from the transcription initiation site to position +57. Tat directly interacts with at least two cellular kinases, CDK7 (14, 28) and CDK9 (55, 76), to stimulate hyperphosphorylation of the C-terminal domain of RNA polymerase II and increase the processivity of the elongating transcription complexes. Both the activation and basic domains of Tat are required for this function. Specifically, Tat binds through an interaction between its basic domain and the bulge region of its effector molecule TAR RNA (18, 21). The activation domain interacts with the cellular kinases and may direct them into the transcription complexes that are assembling on the HIV-1 promoter and therefore bypass the normal recruitment mechanisms (76).
Although mutations in the tat gene reduce viral replication several thousandfold (16, 22), heterologous viral transactivators, which restore HIV-1 gene expression, only partially offset the severe defects in viral replication and cytopathicity (39). This result suggested that Tat might function in steps of the viral life cycle other than increasing transcription. Previously, we have demonstrated that HIV-1 virions with tat gene deletion (Δtat) produce levels of negative-strand strong-stop DNA at least 10-fold lower than those wild-type HIV-1 (34). Also, HIV-1 proviruses that lack tat can be complemented by the expression of a functional tat gene in the cell lines producing the mutant HIV-1. This defect in reverse transcription was also seen in endogenous reverse transcription assays. Thus, HIV-1 Tat is required at early stages of reverse transcription, although its exact role in this process has not been determined.
Tat may be involved in the initiation of reverse transcription prior to the subsequent switch to elongation (42, 43). The kinetics of this process have been studied (41, 65) and found to be close to the overall rate of DNA synthesis for other polymerases, with initiation being the rate-limiting step (50). It should be mentioned that reverse transcription can occur in the absence of a functional tat gene but that the accumulation of proviral DNA intermediates is greatly reduced (34). These results suggest that in the absence of Tat, an optimal reverse transcription complex is not formed. It is possible that Tat may be directly involved in these early steps and/or that Tat may interact with a cellular factor(s) during initiation to enhance the processivity of HIV-1 RT. Finally, Tat could also function during viral assembly by either recruiting a cellular factor or modifying an existing viral protein.
To better define the role of Tat in reverse transcription, we studied a panel of tat mutants to define domains that are required to support efficient HIV-1 reverse transcription. In addition, we wished to identify tat mutants that could stimulate reverse transcription but not viral gene expression. We performed both single-cycle infection and natural endogenous reverse transcription (NERT) assays with viruses produced from 293 cells expressing HIV-1 with a tat gene deletion and expressed a panel of tat mutants both stably and transiently. Our results suggest that the mechanism by which Tat stimulates HIV-1 reverse transcription can be separated from its role in activating HIV-1 gene expression.
MATERIALS AND METHODS
Plasmids and constructs.
The amino acids in Tat at positions 3, 5, and 9 were mutated to glycine, and lysine 41 was mutated to alanine, by site-directed mutagenesis by the QuikChange method (Stratagene, Inc.). The wild-type and mutated tat genes have been previously described (27, 73). The tat genes were ligated into pBK-RSV (Stratagene, Inc.) or pDex (27) and verified by sequencing. Plasmids expressing either CDK7, CDK9, or Cdc5 were the generous gift of León F. Garcia-Martínez. Plasmid pCH110, which expressed the β-galactosidase (β-Gal) gene, was obtained from Amersham Pharmacia Biotech. The positive control tRNA3Lys plasmid was the generous gift of J. Pata, Yale University.
Transfections and CAT assays.
HeLa cells were transfected with an HIV-1 long terminal repeat (LTR)-chloramphenicol acetyltransferase (CAT) reporter plasmid, a eucaryotic expression plasmid driven by Rous sarcoma virus (RSV) promoter and containing either the wild-type or mutant tat gene, a simian virus 40 β-Gal control plasmid. For each transfection, HeLa cells were grown to 30 to 50% confluence and transfected by using the Lipofectamine transfection protocol (Life Technologies) with 2 μg of each of the eucaryotic expression plasmids containing the tat genes, 3 μg of HIV-1 LTR-CAT reporter plasmid and 2 μg of pCH110. At 48 h posttransfection, the cells were washed with phosphate-buffered saline (PBS), resuspended in 500 μl of 0.25 M Tris-HCl (pH 7.8), and lysed by repeated freezing and thawing. The protein content of cell lysates was measured by using the Bio-Rad protein assay. β-Gal activity was determined by using a chlorophenol red galactopyranoside assay with standardized β-Gal concentrations. CAT protein levels were determined with extracts standardized for transfection efficiency according to β-Gal activity by using the Roche Diagnostics CAT enzyme-linked immunosorbent assay (ELISA) kit.
Cell lines, viruses, and infections.
The isolation and characterization of the 293 cell lines (30) producing HIV-1 Δtat and wild-type HIV-1, designated Δtat and wild type, respectively, have been previously described (33, 34). The Δtat cell line was grown in Iscove’s modified Dulbecco’s medium (IMDM) supplemented with 5% newborn calf serum, 2% fetal calf serum, 1% penicillin-streptomycin, 1% GlutaMax (Life Technologies), and 0.25 μg of puromycin (Sigma) per ml. HIV-1 Δtat cells were transfected by using Lipofectamine (Life Technologies) with either the parental vector pBK-RSV or the same plasmid containing a wild-type or mutated tat gene. Cells were serially diluted at 48 h posttransfection and cultured in complete IMDM with the addition of 1 mg of G418 per ml. Next, 36 to 48 individual foci were randomly selected and clones were expanded in 24-well plates. Cell lines were assessed for growth characteristics, cell morphology, and HIV-1 production. Tat expression was determined by RT-PCR as described below. Three individual cell lines were chosen from each stably transfected 293 Δtat cell line.
Peripheral blood mononuclear cells (PBMCs) were obtained from HIV-1-seronegative donors and isolated on a Ficoll-Plaque (Amersham Pharmacia Biotech) gradient as previously described (33). PBMCs were activated in RPMI 1640 medium supplemented with 20% fetal bovine serum, 1% GlutaMax, 1% penicillin-streptomycin, and 1% KaryoMAX phytohemagglutinin (M form) (Life Technologies) for 72 h. The PBMCs were maintained in complete RPMI 1640 medium containing 10 U of interleukin-2 (Roche Diagnostics) per ml and lacking phytohemagglutinin.
Virus stocks were produced and assayed as previously described (34). Briefly, each 293 Δtat cell line containing either a wild-type or mutant tat gene was grown in 100-mm-diameter tissue culture dishes in complete IMDM supplemented with 1 mg of G418 per ml and 0.25 μg of puromycin per ml. The supernatant was removed when the cells were 50% confluent, replaced with complete IMDM lacking both puromycin and G418, and cultured for 18 h at 37°C with 5% CO2. The medium was removed, filtered through a 0.45-μm-pore-size PES membrane, and stored in 10-ml aliquots at −80°C. Each virus stock was assayed for HIV-1 p24 antigen (Ag) by ELISA (NEN Life Science Products) and for RT activity by the RT Detect Assay (Roche Diagnostics).
Cell-free supernatant containing 90 mU of RT activity was adjusted to 45 ml with cell-conditioned culture medium and supplemented with 10 mM MgCl2 and 300 U of DNase I (Worthington Biochemical). The viral supernatants were incubated at 37°C for 30 min, after which a 15-ml aliquot of each was heat-inactivated at 60°C for 20 min. Each DNase I-treated viral supernatant was then incubated with 2 × 107 activated PBMCs for 2 h. The infected PBMCs were washed three times with culture medium to remove residual virus, and low-molecular-weight nucleic acids were isolated from half of the cells by the Hirt lysis method (38). The remaining infected cells, as well as cells infected with heat-inactivated virus, were harvested after an additional 22 h in culture.
To measure virus replication kinetics, 107 PBMCs were infected with 30 ml of HIV-1 supernatant containing 60 mU of total RT activity. The residual virus was removed by washing the cells with complete RPMI 1640 medium, and the infected cells were cultured in 10 ml of complete RPMI 1640 medium supplemented with 10 U of interleukin-2 per ml (infection day 0). The infected cells were passaged every 3 to 4 days for a total of 21 days and supplemented once weekly with newly activated PBMCs at a 1:1 ratio. Cells were removed by centrifugation, and the culture supernatant was assayed for p24 Ag by ELISA.
NERT assay.
Virus stocks were prepared from 293 cells expressing wild-type HIV-1, HIV-1 Δtat, or HIV-1 Δtat complemented with either wild-type or mutant tat genes. These stocks were assayed for total RT activity on a synthetic template according to the directions of the manufacturer (Roche Diagnostics, Inc.). For each NERT assay, virus stock containing 0.75 mU of RT activity was supplemented with 10 mM MgCl2 and incubated for 30 min at 37°C with 100 U of DNase I in a final volume of 200 μl of IMDM. Enzymatic activity was terminated in half of the DNase I-treated virus stock by the addition of 150 μl of stop solution (10 mM Tris-HCl [pH 7.4], 10 mM EDTA, 20 μg of sheared salmon sperm DNA per ml, and 50 μg of proteinase K per ml) followed by incubation at 37°C for 10 min and then boiling for 10 min. The remaining 100 μl was supplemented with 50 μM deoxynucleoside triphosphates (dNTPs) and incubated at 37°C for 90 min before the activity was stopped as described above. The stopped reaction mixtures were centrifuged briefly in a microcentrifuge at 14,000 × g, and 10 μl of each was assayed for negative-strand strong-stop DNA by 34 cycles of PCR as described except for the addition of 3.5 mM MgCl2 to compensate for EDTA present in the stop mix.
PCR and RT-PCR analysis.
Analysis of low-molecular weight nucleic acids by PCR was as previously described (36, 38, 78). All HIV-1-specific oligonucleotides are denoted numerically by using the HIV-1 transcription start site as +1 (genomic RNA). Briefly, an oligonucleotide (5′-ATGCAGCGCAAGTAGGT) complementary to the sense strand of the mitochondrial cytochrome c-oxidase II (Cyt-OxyII) gene was end labeled to a specific activity of greater than 108 cpm/μg by using T4 polynucleotide kinase (New England BioLabs) and [γ-32P]ATP (>7,000 Ci/mmol) (ICN). Hirt lysates were serially diluted in fivefold increments and assayed for Cyt-OxyII levels by 20 cycles of PCR (65°C for 2 min and 93°C for 1 min) with 25 ng of the 32P-labeled oligonucleotide, 50 ng of an unlabeled oligonucleotide (5′-GGAAAATGATTATGAGGGCGTG) complementary to the antisense strand, 1.5 mM MgCl2, 1× reaction buffer as supplied, and 0.25 U of Platinum Taq DNA polymerase (Life Technologies). The PCR products were resolved on 9% polyacrylamide gels, and the dried gels were visualized and analyzed with a Molecular Dynamics PhosphorImager. All samples were assayed within the linear range of the PCR. The Hirt lysates, which were normalized for equivalent Cyt-OxyII levels, were assayed by PCR for HIV-1 reverse transcription products corresponding to negative-strand strong-stop DNA by using 32P-labeled oligonucleotides complementary to sequences between +96 and +118 (5′-CAAGTAGTGTGTGCCCGTCTGTT, sense) and +182 and +158 (5′-CTGCTAGAGATTTTTCCACACTGAC, antisense). Full-length HIV-1 DNA was detected by using 25 ng of 32P-labeled +96/+118 HIV-1 oligonucleotide and 50 ng of an oligonucleotide complementary to HIV-1 sequences located downstream from the primer-binding site between +242 and +219 (5′-CCTGCGTCGAGAGAGCTCCTCTGG, antisense). PCR products were resolved by 9% polyacrylamide gel electrophoresis. The gels were dried and analyzed on a Molecular Dynamics PhosphorImager.
RT-PCR to determine the amount of tat RNA produced by each of the 293 cell lines was performed on total RNA isolated from 293 stable cell lines by using TriPure reagent (Roche Diagnostics). For each reaction, 10 μg of total RNA, 0.5 μg of an antisense oligonucleotide complementary to β-actin mRNA (BA3, 5′-GGCGTACAGGGACAGCACA), and 0.5 μg of an antisense oligonucleotide complementary to pBK-RSV-directed tat mRNA (M13 forward, 5′-GTTTTCCCAGTCACGAC) were heated at 75°C for 15 min and placed on ice. cDNA synthesis reaction mixtures containing the reaction buffer provided, 10 mM dithiothreitol, 2 mM dNTPs, 20 U of RnaseOut (Life Technologies), and 200 U of Moloney murine leukemia virus (M-MLV) RT (Life Technologies) were incubated at 37°C for 1 h. Each reaction mixture was serially diluted in fivefold increments and assayed by PCR with 100 ng of BA3 and 100 ng of a β-actin sense primer, BA4 (5′-GGCGTACAGGGACAGCACA). PCR was performed for 25 cycles at 53, 72, and 94°C for 1 min at each temperature, and the DNA products were resolved on a 1.5% agarose gel. The cDNA reaction mixtures were normalized to β-actin mRNA levels and then assayed for pBK-RSV tat cDNA by PCR with a nested tat primer, TA3 (5′-AGATCTATACACTCGCACGCC, antisense), and a primer complementary to vector sequences (5′-AGCGGATAACAATTTCACACAGGA, sense) for 35 cycles at 50, 72, and 94°C for 1 min at each temperature. The products were separated on a 1.5% agarose gel, stained with ethidium bromide, and visualized on a UV transilluminator.
The positive control tRNA3Lys plasmid was linearized with the restriction enzyme NsiI. In vitro-synthesized RNA was obtained by using T7 RNA polymerase, treated with RQ-DNase I, and gel purified. Also, an HIV-1 DNA fragment that contained sequences from −22 to +517, and a deletion of sequences from +80 to +151, was ligated into pGem4z (Promega). This was linearized with EcoRI, and in vitro-transcribed RNA was made by using T7 AmpliScribe reagents (Epicentre Technologies), treated with RQ-DNase I, and gel purified.
To detect tRNA3Lys incorporation into virions, DNase I-treated virus stocks of either the wild-type, Δtat, or Δtat complemented viruses were subjected to centrifugation at 22,000 × g for 90 min and resuspended in 1× PBS–1% bovine serum albumin (BSA) (PBS-BSA). The viral suspensions were assayed for p24 Ag and RT activity. Exactly 100 ng of p24 Ag of each virus was extracted by using TRIzol reagent (Life Technologies) according to the manufacturer’s recommendations. Nucleic acids were precipitated overnight (−20°C) and recovered by centrifugation at 15,000 × g at 4°C for 60 min. A visible pellet was washed with 70% ethanol and centrifuged as before, and the pellet was resuspended in 30 μl of TE (10 mM Tris-HCl [pH 7.8], 0.1 mM EDTA). Total HeLa cell RNA (7.5 μg) and an in vitro-transcribed tRNA3Lys molecule (0.5 μg) were used as positive controls. Duplicate reactions with 5 μl of each viral RNA and the positive control were set up and included 20 U of RNasin (Promega), 0.5 μl of dimethyl sulfoxide, 50 ng of either a tRNA3Lys-specific antisense oligonucleotide (5′-TGGCGCCCGAACAGGGACTTGA) or an HIV-1-specific antisense oligonucleotide (5′-CCTGCGTCGAGAGAGCTCCTCTGG), and 3 μl of H2O. These reaction mixtures were heated to 75°C for 10 min and placed on ice. In vitro reverse transcription reactions were performed in the presence and absence of avian myeloblastosis (AMV) RT (Promega) with buffers provided by the manufacturer plus 0.2 mM dNTPs at 42°C for 1 h followed by 72°C for 5 min. The reverse transcription reactions were amplified by PCR with primer pairs specific for tRNA3Lys (5′-ATAGCTCAGTCGGTAGAGCAT [sense] and 5′-GCCGAACAGGGACTTGAT [antisense]) and HIV-1 genomic RNA (5′-CAAGTAGTGTGTGCCCGTCTGTT [sense] and 5′-CGAGAGAGCTCCTCTGGTTCTAC [antisense]). The PCR products were separated on a 9% polyacrylamide gel matrix in 1× Tris-borate-EDTA, dried, and quantitated on a Molecular Dynamics PhosphorImager.
Similarly, filtered viral supernatants containing wild-type, Δtat, or complemented Δtat viruses were treated with 300 U of DNase I, and the virus particles were pelleted through 20% sucrose at 75,000 × g for 2 h. The pellets were suspended in 0.5 ml of PBS-BSA. The viral suspensions were assayed for p24 Ag. Supernatant containing 60 ng of p24 Ag was treated with TriPure reagent (Roche Diagnostics), 0.5 pg of in vitro-synthesized HIV-1 RNA was added, and total virion RNA was isolated according to the manufacturer’s recommendations. Total viral RNA was annealed to an oligonucleotide (5′-GACTGCGAATCGTTCTAG-3′, antisense) complementary to sequences in the gag open reading frame at 75°C for 10 min and placed on ice, and cDNA was made by using the supplied buffers, 0.2 mM dNTPs, and M-MLV RT (Life Technologies) at 37°C for 60 min. Each cDNA reaction was assayed by PCR for the internal control (IC) cDNA (reverse transcribed from IC RNA) by using a 32P-labeled oligonucleotide specific for pGem4z sequences (5′-GGGAGACAAGCTTGCATGCCTG, sense) and an unlabeled HIV-1-specific oligonucleotide (5′-GCAGTGGGTTCCCTAGTTAGC, antisense) for 25 cycles at 93°C for 1 min and 65°C for 2 min. The normalized cDNA reaction mixtures were serially diluted and assayed for HIV-1 DNA by using HIV-1-specific primers (32P-labeled +96/+118 and unlabeled +182/+158) for 30 cycles with the same cycling parameters. The PCR DNA products were separated on a 9% polyacrylamide gel, dried, and visualized and quantitated on a PhosphorImager (Molecular Dynamics).
RESULTS
Isolation of clonal 293 cell lines containing tat deletion HIV-1 and stably transfected wild-type or mutant tat genes.
We previously demonstrated that tat was required for efficient HIV-1 reverse transcription (34), in addition to its well-characterized role in activating HIV-1 gene expression (reviewed in references 29 and 46). Transient transfection of a wild-type tat gene into cells containing an integrated HIV-1 provirus with a tat gene deletion produced virus that was fully competent for reverse transcription upon infection of PBMCs. Several tat mutants which were defective in activating HIV-1 gene expression were also unable to complement HIV-1 reverse transcription, while tat genes that stimulated high levels of HIV-1 gene expression correlated with efficient reverse transcription. Thus, it was important to address whether we could identify tat mutants that were defective in transactivation yet were able to stimulate HIV-1 reverse transcription.
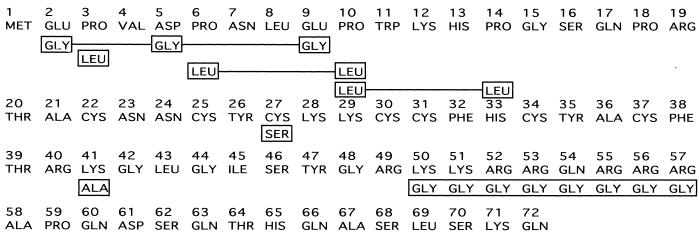
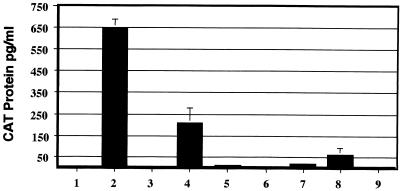
To determine whether we could separate these functions of tat, we used a panel of mutated tat genes that were defective for the activation of viral gene expression (Fig. 1). These included mutants coding for substitutions of three acidic residues in the amino terminus ([E2G, D5G, E9G]), a mutation of proline residue 3 to leucine (P3L), a mutation of proline residues 6 and 10 or 10 and 14 to leucine residues (P[6, 10]L or P[10, 14]L), a mutation of cysteine residue 27 to serine (C27S), a mutation of a lysine residue 41 to alanine (K41A), and replacement of basic amino acids extending from positions 50 to 57 by glycine (K/R[50-57]G). The tat genes containing each of these mutations or a rabbit β-globin gene were inserted downstream of the RSV promoter and transfected into HeLa cells together with HIV-1 LTR-CAT and simian virus 40–β-Gal reporter constructs. At 48 h posttransfection, CAT production was measured by an ELISA with extracts normalized for β-Gal activity. HeLa cells transfected with the HIV-1 LTR-CAT reporter alone (Fig. 2, bar 1) demonstrated low levels of CAT protein, while wild-type tat increased the level of CAT protein to 650 pg/ml (Fig. 2, bar 2). The mutation P3L resulted in a threefold decrease in tat stimulation (Fig. 2, bar 4), while the mutation K41A resulted in a 10-fold reduction in tat stimulation of CAT levels (Fig. 2, bar 8). The levels of CAT protein produced in the presence of the remaining mutants were either at the threshold of detection for the assay (Fig. 2, bars 5 and 7) or below the level of detection (Fig. 2, bars 3, 6, and 9). Similar results were seen in three independent experiments.
FIG. 1.
Schematic of the first exon of the HIV-1 Tat protein. The amino acid changes are shown boxed below the native amino acid sequence. Multiple mutations are indicated by solid lines between boxed amino acids. The mutations in the tat gene product which were constructed are [E2G, D5G, E9G], P3L, P[6, 10]L, P[10, 14]L, C27S, K41A, and K/R[50-57]G.
FIG. 2.
Activation of HIV-1 gene expression by Tat. HeLa cells were cotransfected with the reporter constructs HIV-1 LTR-CAT and pCH110 (β-Gal) together with plasmids (pDex) that expressed either the β-globin gene (bar 1), the wild type tat gene (bar 2), or the mutated tat genes corresponding to [E2G, D5G, E9G] (bar 3), P3L (bar 4), P[6, 10]L (bar 5), P[10, 14]L (bar 6), C27S (bar 7), K41A (bar 8), and K/R[50-57]G (bar 9). The cells were harvested at 48 h posttransfection, and equal amounts of protein were normalized to β-Gal activity and assayed for CAT protein by ELISA. The transfections were performed three times with the standard deviations indicated.
Characterization of 293 cell lines containing tat mutants.
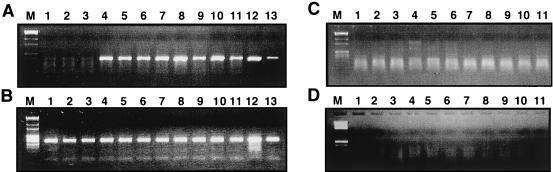
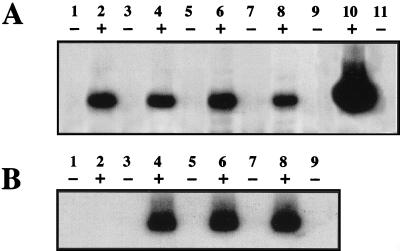
Expression vectors containing each of the different tat genes were transfected into 293 cells containing the HIV-1 Δtat provirus. Stable cell lines containing both HIV-1 Δtat and the tat expression vectors were obtained by G418 selection as previously described (33). The 293 cell lines were then assayed for p24 Ag levels by ELISA and for plasmid-derived tat mRNA by RT-PCR analysis. Western blot analysis of Tat protein from extracts prepared from stably transfected cell lines indicated that Tat was produced at levels of less than 10 ng per sample (32a). No cDNA product for tat was observed with RNA obtained from parental 293 cells or 293 cells containing the Δtat provirus or wild-type virus (Fig. 3A, lanes 1 to 3). In contrast, similar levels of plasmid-derived tat mRNA were present in 293 cells containing the HIV-1 Δtat provirus and either wild-type tat (Fig. 3A, lane 4) or each of the tat mutants (Fig. 3A, lanes 5 to 11). PCR analysis of β-actin cDNA levels indicated that the amounts of RNA in all cDNA synthesis reaction mixtures were similar (Fig. 3B, lanes 1 to 11). No cDNA products were detected in RNA samples produced in the absence of added M-MLV RT (Fig. 3C and D). Following PCR analysis, the tat cDNA product was isolated and sequenced. In each case, the tat genes contained the expected nucleotide changes (data not shown). Chromosomal DNA obtained from each 293 cell line was also subjected to PCR to obtain HIV-1 proviral DNA and to confirm by sequencing that each cell line contained the HIV-1 with a deleted tat gene (data not shown).
FIG. 3.
RT-PCR analysis of wild-type and mutant tat genes. Total RNA was obtained from uninfected 293 cells (lanes 1), 293 cells stably transfected with HIV-1 wild-type (lanes 2) or HIV-1 Δtat (lanes 3), and 293 cells containing both HIV-1 Δtat and wild type tat (lanes 4) or the mutated tat genes corresponding to [E2G, D5G, E9G], P3L, P[6, 10]L, P[10, 14]L, C27S, K41A, and K/R[50-57]G (lanes 5 to 11, respectively). Primers specific for plasmid-derived tat mRNA or cellular β-actin mRNA were annealed to RNA obtained from each of the 293 cell lines, and a reverse transcription reaction was performed in the presence (A and B) or absence (C and D) of M-MLV RT. PCR was performed on each cDNA reaction mixture to detect either the tat (A and C) or β-actin (B and D) gene. PCR products were resolved on a 1.5% agarose gel. Molecular mass markers are shown for each gel (lanes M). PCRs with a plasmid containing the tat gene (panel A, lanes 12 and 13) (equivalent to 0.1 and 0.5 pg) or serially diluted β-globin cDNA (panel B, lanes 12 and 13) are shown.
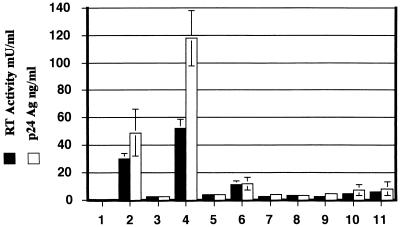
Viral supernatant was obtained from each cell line and assayed for RT activity (Fig. 4). The 293 cells containing the wild-type HIV-1 produced RT and p24 Ag levels of 30 mU/ml and 50 ng/ml, respectively (Fig. 4, bars 2), while the 293 cells harboring the Δtat provirus had 1 mU of RT per ml and 1.2 ng of p24 Ag per ml (Fig. 4, bars 3). Stable transfection of wild-type tat into 293 cells containing HIV-1 Δtat increased expression of RT and p24 Ag levels 50- and 100-fold, respectively (Fig. 4, bars 4). Clonal 293 cell lines containing the different tat genes produced levels of RT and p24 Ag that were slightly greater than those of the parental 293 cell line containing HIV-1 Δtat. The tat mutants [E2G, D5G, E9G], P[6, 10]L, P[10, 14]L, and C27S resulted in RT and p24 Ag levels that were 1.5- to 3-fold greater than those of the parental HIV-1 Δtat cell line (Fig. 4, bars 5, 7, 8, and 9). The proline mutant P3L increased HIV-1 gene expression approximately 11-fold, while the tat mutants K41A and K/R[50-57]G increased RT and p24 Ag 4- and 6-fold, respectively (Fig. 4, bars 6, 10, and 11). This data represents assays performed on four to six independent virus stocks from each 293 cell line. The increase in the amount of HIV-1 produced in the presence of each tat gene correlated with the abilities of these different genes to transactivate the HIV-1 LTR in transient assays of tat activity (Fig. 2).
FIG. 4.
Analysis of HIV-1 gene expression from 293 cells. Culture supernatants were obtained from either 293 cells (bars 1), 293 cells stably infected with HIV-1 wild-type virus (bars 2), 293 cells infected with an HIV-1 Δtat virus (bars 3), or the Δtat cell line stably transfected with pBK-RSV containing the wild-type tat gene (bars 4) or the mutated tat genes corresponding to [E2G, D5G, E9G] (bars 5), P3L (bars 6), P[6, 10]L (bars 7), P[10, 14]L (bars 8), C27S (bars 9), K41A (bars 10), and K/R[50-57]G (bars 11). The amounts of p24 Ag and reverse transcriptase activity in each virus stock were determined as described in Materials and Methods. The data from four to six independent virus stocks were averaged and the standard deviation for each assay indicated.
Next, we assayed the replication of the HIV-1 Δtat viruses produced in the 293 cell lines containing the different tat mutants. Activated PBMCs were infected with cell-free virus containing equivalent amounts of RT activity for wild-type HIV-1, HIV-1 Δtat, or HIV-1 Δtat complemented with either the wild-type or each of the mutated tat genes. The virus was removed from the infected cells at 5 h postinfection, and the PBMCs were cultured and monitored for p24 Ag production for 3 weeks. Small quantities of p24 Ag were present in the PBMCs due to residual virus remaining from the initial infection of HIV-1 Δtat produced in the presence of the different tat mutants. However, only wild-type HIV-1 produced in the 293 cell lines was able to efficiently replicate in PBMCs. No p24 Ag was detected in any of the other cultures (the limit of detection was 10 pg of p24 Ag per ml) after 21 days postinfection (Table 1). These results indicate that no detectable recombination had occurred between the stably transfected tat genes and the HIV-1 provirus.
TABLE 1.
Replication kinetics of wild-type and Δtat HIV-1a
| Day | p24 Ag (pg/ml) with the following virus:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Wild type | Δtat | tat wild type | [E2G, D5G, E9G] | P3L | P[6, 10]L | P[10, 14]L | C275 | K41A | K/R[50-57]G | Mock | |
| 0 | 5 | 15 | 20 | 30 | 20 | 15 | 20 | 20 | 20 | 15 | 0 |
| 3 | 150 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 7 | 750 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10 | 3,598 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 14 | 48,763 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 17 | 24,851 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 21 | 36,891 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Activated PBMCs were infected with virus stocks containing 60 mU of RT activity. The cells were washed and cultured as described in Materials and Methods, and a sample of culture supernatant was removed and assayed for p24 Ag (day 0) by ELISA. The cells were split 1:2 twice weekly, and an aliquot of the supernatant was assayed for p24 Ag. All infections were supplemented with uninfected, activated PBMCs weekly. Each infection was repeated twice with independent virus stocks.
The amino terminus of Tat is critical for modulating HIV-1 reverse transcription.
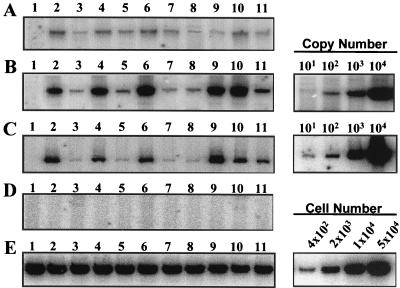
By using similar quantities of HIV-1 virion-associated RT activity, activated PBMCs were infected with either wild-type HIV-1, HIV-1 Δtat, or HIV-1 Δtat produced from 293 cell lines stably expressing wild-type tat or each of the mutated tat genes. Nucleic acids were isolated by Hirt lysis at 2 h (Fig. 5A) and 24 h (Fig. 5B and C) postinfection of PBMCs. PCR analysis of the reverse transcription products corresponding to negative-strand strong-stop HIV-1 DNA (Fig. 5A and B) or full-length HIV-1 DNA (Fig. 5C) was performed. HIV-1 Δtat was very defective for reverse transcription, resulting in a 10- to 30-fold reduction in the levels of both negative-strand strong-stop DNA and full-length cDNA (Fig. 5B and C, lanes 3) compared to wild-type HIV-1 (Fig. 5B and C, lanes 2). The reverse transcription defect in HIV-1 Δtat was fully restored by complementation of the 293 cell lines producing this virus with wild-type tat (Fig. 5B and C, lanes 4) or a tat mutant causing a mutation at proline residue 3 (Fig. 5B and C, lanes 6). Other mutants with mutations in the amino terminus of Tat ([E2G, D5G, E9G], P[6, 10]L, and P[10, 14]L) did not increase either negative-strand strong-stop DNA synthesis (Fig. 5B, lanes 5, 7, and 8) or full-length DNA synthesis (Fig. 5C, lanes 5, 7, and 8).
FIG. 5.
Reverse transcription of HIV-1 lacking tat. Activated PBMCs were infected for 2 h with culture supernatant from transfected 293 cells containing 90 mU of RT activity for either wild-type HIV-1 (lanes 2), Δtat virus (lanes 3), or virus produced from 293 HIV-1 Δtat cells stably transfected with an RSV expression vector containing the wild-type tat gene (lanes 4) or the mutated tat genes corresponding to [E2G, D5G, E9G], P3L, P[6, 10]L, P[10, 14]L, C27S, K41A, and K/R[50-57]G (panels A to C and E, lanes 5 to 11, respectively), and mock supernatant (panels A to C and E, lanes 1). PBMCs were infected with aliquots of the same viruses that were heat inactivated at 60°C (D). At 2 h postinfection, residual virus was removed and Hirt lysates were prepared from half of the infected cells, while the remaining PBMCs were cultured for 24 h before Hirt lysates were prepared. The recovered nucleic acids were assayed for HIV-1 negative-strand strong-stop DNA in 2-h (A) and 24-h (B) lysates and for full-length DNA in 24-h lysates (C) by quantitative PCR. PCR analysis of the Cyt-OxyII content in Hirt lysates was used to standardize the DNA recovery (E). All PCRs were performed within the linear range of the assay as determined by assays of HIV-1 DNA copy number (10, 102, 103, and 104) or cell number (4 × 102, 2 × 103, 1 × 104, and 5 × 104). This analysis is representative of PCRs performed for four separate infections with independently prepared virus stocks.
In contrast to the inability of the majority of the amino-terminal Tat mutants to stimulate gene expression (Fig. 2, bars 3, 4, and 6, and 4, bars 5, 7, and 8) or complement HIV-1 reverse transcription, mutants containing replacements of cysteine residue 27 or lysine residue 41 were able to restore HIV-1 reverse transcription to levels seen with wild-type tat (Fig. 5B and C, lanes 9 and 10). A Tat mutant which has glycine substituted for basic amino acids 50 to 57, which was defective in Tat transcriptional activation, resulted in a four- to eightfold increase in the synthesis of negative-strand strong-stop DNA. These results were consistent for four to six independent virus stocks and suggest that the ability of tat to efficiently initiate HIV-1 reverse transcription is largely dependent on an intact Tat amino terminus. There is an additional requirement for the basic domain of Tat to fully complement HIV-1 reverse transcription. Surprisingly, amino acid residues within the Tat cysteine and core domains that are necessary for tat-mediated activation of HIV-1 gene expression are not required for tat stimulation of HIV-1 reverse transcription. We observed similar patterns of reverse transcription complementation in PBMCs infected with virus stocks produced by transient expression of these genes into 293 Δtat cells, although there was some variability in the overall degree of complementation (data not shown).
Role of Tat in endogenous HIV-1 reverse transcription.
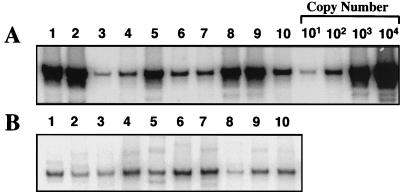
NERT assays were performed as described previously (34). HIV-1 supernatants containing equal amounts of RT were incubated with 50 μM dNTPs in the presence of DNase I, and each of the viruses was then assayed by PCR for the synthesis of negative-strand strong-stop DNA. Both wild-type HIV-1 and HIV-1 Δtat complemented with wild-type tat resulted in 30- to 60-fold more negative-strand strong-stop DNA than seen with HIV-1 Δtat virus alone (Fig. 6, lanes 1 to 3). HIV-1 produced in the presence of amino-terminal mutations of Tat (Fig. 6, lanes 4, 6, and 7) synthesized only three- to fivefold more negative-strand strong-stop DNA than HIV-1 Δtat virus. In contrast, Tat mutants with mutations of proline 3 (Fig. 6, lanes 5), cysteine 27 (Fig. 6, lanes 8), or lysine 41 (Fig. 6, lanes 10) resulted in 20- to 35-fold more negative-strand strong-stop DNA than HIV-1 Δtat. The Tat basic mutant, K/R[50-57]G, (Fig. 6, lanes 10) resulted in approximately 15-fold more negative-strand strong-stop DNA than HIV-1 Δtat (Fig. 6, lanes 3). PCR analysis of molecular standards indicated that all reactions were performed within the linear range of the assay. These NERT assays coupled with our in vivo data indicate a critical role for the amino terminus of Tat in the efficient initiation of HIV-1 reverse transcription. Surprisingly, this effect is not dependent upon cysteine residue 27 or lysine residue 41, both of which are important for Tat-mediated transactivation of HIV-1 transcription.
FIG. 6.
NERT assay for HIV-1 wild-type and tat mutant viruses. Virus stocks for wild-type virus (lanes 1), Δtat virus trans-complemented with wild-type tat (lanes 2), Δtat virus (lanes 3), or Δtat virus produced in the presence of tat mutants [E2G, D5G, E9G], P3L, P[6, 10]L, P[10, 14]L, C27S, K41A, and K/R[50-57]G (lanes 4 to 10, respectively) were analyzed for endogenous reverse transcription. Culture supernatant (200 μl) containing approximately 0.75 mU of RT activity was treated with 100 U of DNase I. Half of each reaction mixture was added to 150 μl of stop solution, incubated at 37°C for 10 min, and then boiled for 10 min (B). The remaining half of each reaction mixture was supplemented with 50 μM dNTPs and incubated at 37°C for 90 minutes before the reaction was terminated as described above. (A) PCR to detect HIV-1 negative-strand strong-stop DNA was performed on NERT reaction mixtures as described in Materials and Methods. All PCRs were performed within the linear range of the assay as determined by assays of HIV-1 DNA copy number (10, 102, 103, and 104).
Tat-associated kinases CDK7 and CDK9 do not complement reverse transcription defects associated with HIV-1 Δtat virions.
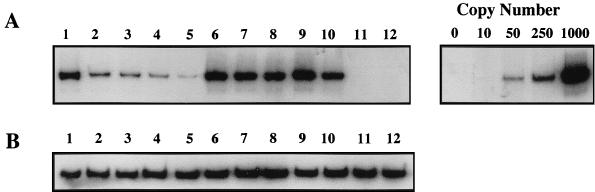
It has been demonstrated that the HIV-1 Tat protein specifically interacts with and activates cyclin-dependent kinases (15, 28, 55, 76, 79) to phosphorylate the C-terminal domain of RNA polymerase II and increase HIV-1 gene expression. Several mutants with mutations in the Tat activation domain, which interacts with cellular kinases to stimulate HIV-1 transcription, were unable to complement the reverse transcription defect in HIV-1 Δtat virions. Thus, it is possible that Tat may interact with a cellular kinase to stimulate reverse transcription. Therefore, we assayed the ability of overexpression of Tat-associated kinases CDK7 and CDK9 to complement the reverse transcription defects seen with the HIV-1 Δtat virions. The 293 cell lines expressing HIV-1 Δtat or wild-type HIV-1 were transiently transfected with expression vectors containing either wild-type cdk7, cdk9, or a control cdc5. Tat does not require cdc5 to activate HIV-1 gene expression. Cell-free supernatants were obtained from 293 cells producing HIV-1 Δtat in the presence or absence of each of these kinases or wild-type tat. Equal amounts of 293 viral supernatants were used to infect PBMCs. Hirt lysates were processed after 24 h and assayed for negative-strand strong-stop DNA synthesis. Neither the Tat-associated kinases nor the unrelated cdc5 were able to complement the reverse transcription defects (Fig. 7, lanes 3 to 5) as compared to the results with wild-type tat (Fig. 7, lanes 1). There was no change in the amount of negative-strand strong-stop DNA synthesized in PBMCs infected with wild-type HIV-1 produced in the presence or absence of these constructs (Fig. 7, lanes 6 to 10).
FIG. 7.
Cyclin-dependent kinases do not complement reverse transcription defects associated with Δtat viruses. (A) Viral supernatants from 293 cells producing Δtat virus (lanes 1 to 5) or wild-type HIV-1 (lanes 6 to 10) following transfection of wild-type tat (lanes 1 and 6), an empty RSV expression vector (lanes 2 and 7), a wild-type cdk7 expression vector (lanes 3 and 8), a wild-type cdk9 expression vector (lanes 4 and 9), a wild-type cdc5 expression vector (lanes 5 and 10), mock supernatant (lane 11), or heat-inactivated wild-type HIV-1 (lane 12) were used to infect 5 × 106 activated PBMCs. At 2 h postinfection, residual virus was removed by washing, and Hirt lysates were prepared at 24 h postinfection. The recovered nucleic acids were assayed for HIV-1 negative-strand strong-stop DNA. (B) Quantitative PCR analysis of Cyt-OxyII content in Hirt lysates was used to standardize the DNA recovery. All PCRs were performed within the linear range as determined by assays of HIV-1 DNA copy number (0, 10, 50, 250, and 1,000). This analysis is representative of PCRs performed for three separate HIV-1 infections with independently prepared virus stocks.
Virion genomic RNA levels are not altered in Δtat viruses.
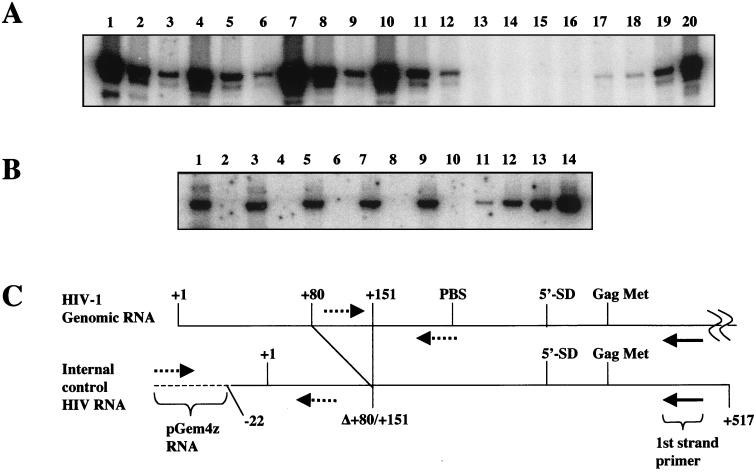
To determine whether the defects in reverse transcription were due to alterations in HIV-1 RNA encapsidation, we performed RT-PCR on RNA obtained from partially purified virions. We had previously used a first-strand cDNA primer that recognized sequences between the primer-binding site and 5′ splice donor site, and we saw no differences in encapsidated RNA (34). In this analysis, we used a first-strand primer directed at sequences located downstream of the Gag initiating methionine (Fig. 8C). Wild-type, Δtat, or complemented Δtat viruses were pelleted through 20% sucrose, suspended in PBS-BSA, and assayed for p24 Ag and RT. Total virion RNA, along with 0.5 pg of an exogenously added IC RNA, was isolated from equivalent amounts of each virus. cDNA was synthesized from the isolated viral RNA and assayed by using PCR primers that could discriminate between HIV-1 cDNA and IC cDNA (Fig. 8C). Each cDNA reaction mixture was serially diluted in fivefold increments and subjected to 30 cycles of PCR as described in Materials and Methods. Equivalent amounts of HIV-1 cDNA were detected for Δtat virus (Fig. 8A, lanes 1 to 3), Δtat virus complemented with [E2G, D5G, E9G] (Fig. 8A, lanes 4 to 6), Δtat virus complemented with wild-type tat (Fig. 8A, lanes 7 to 9), and wild-type virus (Fig. 8A, lanes 10 to 12). No products were observed with mock cDNA (Fig. 8A, lanes 13 to 16) or in reactions performed without M-MLV RT (data not shown). Our analysis showed that other complemented Δtat viruses also had wild-type levels of genomic RNA (data not shown). Finally, PCR analysis of the same cDNA reactions for IC cDNA in either the presence (Fig. 8B, odd-numbered lanes) or absence (Fig. 8B, even-numbered lanes) of M-MLV RT showed that both the RNA recoveries and cDNA synthesis efficiencies were similar in all reactions (Fig. 8B, lanes 1, 3, 5, 7, and 9). Molecular standards showed that the reactions were performed within the linear range of the assays (Fig. 8A, lanes 16 to 20, and B, lanes 11 to 14). These experiments are in agreement with our previous study and indicate that tat does not effect genomic RNA packaging.
FIG. 8.
Analysis of genomic RNA packaging. (A) Supernatants containing wild-type virus (lanes 10 to 12), Δtat virus (lanes 1 to 3), or Δtat virus complemented with wild-type tat (lanes 7 to 9) or [E2G, D5G, E9G] (lanes 4 to 6) or mock complemented (lanes 13 to 16) were pelleted through 20% sucrose and suspended in PBS-BSA buffer. An IC RNA was added to purified virus that contained 100 ng of p24 Ag, and both RNAs were copurified. cDNA reactions were performed in either the presence or absence of M-MLV with a first-strand primer that annealed to sequences located downstream from the Gag initiating methionine shown in panel C. The cDNA was serially diluted in fivefold increments and assayed by PCR for HIV-1 DNA with primers indicated in panel C. PCRs were performed on HIV-1 DNA present at 0, 101, 102, 103, and 104 copies (lanes 16 to 20). (B) The RNA recovery and cDNA synthesis were similar for each cDNA reaction corresponding to Δtat (lanes 1 and 2), Δtat plus [E2G, D5G, E9G] (lanes 3 and 4), Δtat plus wild-type tat (lanes 5 and 6), wild-type virus (lanes 7 and 8), and mock virus (lanes 9 and 10). IC RNA was reverse transcribed in either the presence (lanes 1, 3, 5, 7, and 9) or absence (lanes 2, 4, 6, 8, and 10) of M-MLV and detected by PCR with the primers shown in panel C (dotted lines). IC plasmid DNA standards present at 20, 100, 300, and 1,000 copies are shown (lanes 11 to 14). (C) Model showing HIV-1 RNA and IC RNA. An internal deletion from +80 to +151 in IC RNA allows detection of IC cDNA from HIV-1 cDNA by PCR with the indicated primers. Solid arrow, first-strand cDNA primer; dotted arrows, PCR primers; dotted line, pGem4Z RNA; solid line, HIV-1 RNA.
tRNA3Lys is equally incorporated into wild-type, Δtat, and Δtat complemented viruses.
To determine whether the defect in HIV-1 reverse transcription in the absence of tat was due to a reduction in the packaging of tRNA3Lys, RT-PCR analysis was performed with total RNA isolated from equal amounts of HIV-1 wild-type, Δtat, and Δtat virions produced in the presence of a wild-type tat gene. First-strand synthesis was performed with a primer specific for the 3′ tail of the tRNA3Lys molecule in the presence (Fig. 9, even-numbered lanes) or absence (Fig. 9, odd-numbered lanes) of AMV RT. PCR analysis was then performed with a primer pair specific for the tRNA3Lys molecule, one primer of which was 32P labeled, as described in Materials and Methods. There was no difference in the relative amounts of tRNA for either wild-type or Δtat virions produced in either the presence or absence of a wild-type tat gene (Fig. 9A, lanes 3 to 8). Total HeLa cell RNA and an in vitro-synthesized tRNA3Lys molecule were used as positive controls for the RT-PCRs (Fig. 9A, lanes 1, 2, 10, and 11). As a control for viral RNA recovery, PCR analysis was performed for full-length HIV-1 RNA with the same RNA samples (Fig. 9B) and primers that detect HIV-1 genomic RNA. These results suggest that tat does not play a role in the packaging of the tRNA3Lys primer into HIV-1 virions and support our previous observations that there are no gross biochemical abnormalities in virions produced in the absence of tat.
FIG. 9.
Analysis of tRNA3Lys packaging in wild-type and tat mutant viruses. RNA was extracted from pelleted virus that contained 100 ng of p24 Ag, and cDNA was synthesized in the presence (+) or absence (−) of AMV RT primed with an antisense oligonucleotide that hybridized to either the 3′-terminal 18 nucleotides of the tRNA3Lys molecule (A) or HIV-1 sequences extending from +242 to +219 (B). (A) tRNA3Lys cDNA was detected by PCR with primers that hybridize to internal tRNA3Lys sequences. Total HeLa cell RNA (lanes 1 and 2) or wild-type HIV-1 (lanes 3 and 4), Δtat virus (lanes 5 and 6), and Δtat virus produced following transfection with a wild-type tat expression vector (lanes 7 and 8) contain similar amounts of tRNA3Lys. An in vitro-transcribed tRNA3Lys molecule was added as a positive control for the reactions (lanes 10 and 11). A PCR-negative control is shown in lane 9. (B) As a control for virus load, HIV-1 cDNA was detected by PCR with a nested antisense primer corresponding to HIV-1 sequences +236 to +214 and a sense primer corresponding to +96 to +118 for HeLa cells (lanes 1 and 2), wild-type HIV-1 (lanes 3 and 4), Δtat virus (lanes 5 and 6), or Δtat virus produced following transfection with a wild-type tat expression vector (lanes 7 and 8). A negative PCR control is shown in lane 9.
DISCUSSION
Previously, we demonstrated that tat plays a role in early steps in the HIV-1 life cycle, specifically in the process of reverse transcription (34). In the studies outlined here, we employed a panel of tat mutants that included activation domain substitution mutants, basic domain substitution mutants, and a variety of point mutants to determine whether we could identify tat mutants that could complement reverse transcription but not viral gene expression. We assayed these tat mutants for their effects on both viral gene expression and ability to complement reverse transcription defects associated with HIV-1 Δtat. Several tat mutants in the amino terminus of Tat were unable to support HIV-1 gene expression or reverse transcription. In contrast, several mutants causing mutations in the activation, core, and the basic domains of Tat, which were defective for viral transcription, were able to complement the reverse transcription defects associated with Δtat virions. Thus, complementation of reverse transcription defects in Δtat virus by exogenously added wild-type tat is not simply the result of tat-modulated increases in HIV-1 gene expression. No differences in the p24 Ag/RT ratios or the amounts of genomic RNA or tRNA3Lys packaged into virions in the presence or absence of a functional tat gene were observed, indicating that these virions were biochemically similar (32).
Since the Tat basic domain substitution mutant and the TAR RNA bulge mutant (35) exhibited little or no defects in the synthesis of negative-strand strong-stop DNA, it is likely that the defects in reverse transcription seen with Δtat virus (34) are due to a process that can be separated from Tat binding to TAR RNA. The fact that overexpression of the cyclin-dependent kinases CDK9 and CDK7, which are believed to be essential for Tat-mediated transcriptional activation (14, 15, 28, 55, 76), had no effect on the process of reverse transcription further serves to distinguish the role of Tat in reverse transcription from its role in transcription. However, these experiments do not rule out the possibility that Tat and TAR RNA might interact with additional viral and/or cellular factors and form a distinct reverse transcription initiation complex.
It is possible that Tat may interact with other cellular kinases to stimulate efficient reverse transcription. Tat has been reported to activate components of signal transduction pathways, including mitogen-activated protein kinases (MAPKs) (7, 13, 26, 48, 58, 59), and it may be involved in regulating signal transduction pathways leading to apoptosis (8, 53, 77). Tat may also act as a cellular growth factor (2, 3, 17, 49, 60, 62), such as in its involvement in the development of Kaposi’s sarcoma (19, 20, 61, 68). Although quiescent T cells can be infected by HIV-1 and reverse transcription can be initiated, full-length proviral DNA cannot be detected and integration does not take place (70, 78). The block in HIV-1 replication in quiescent cells has been reported to involve decreased translocation of the reverse transcription complex and/or the preintegration complex (10) which is regulated by phosphorylation (9, 24). Viral proteins associated with the reverse transcription complex include the heterodimeric RT, integrase, nucleocapsid, Vpr, and matrix protein (11). Studies suggest that phosphorylation of matrix protein on tyrosine (24, 25) by an as-yet-unidentified kinase or on serine residues by a cellular serine/threonine kinase identified as ERK2/MAPK (9, 45) is required to dissociate myristoylated matrix protein from the cell membrane and direct its nuclear import. The latter kinase is induced upon T-cell activation and is specifically incorporated into HIV-1 virions (12, 45). Thus, there is a precedent for alterations in the cellular signal transduction pathways for modulating viral replication.
The effect of Tat on reverse transcription can be distinguished from that found in the studies discussed above, because Tat acts at an earlier step in reverse transcription, perhaps during virion assembly or initiation of reverse transcription. In any event, the defects are present within the virion particles themselves. Like the effects of Tat mutants, mutations in either vif (69) or nef (1, 67) also likely result in reverse transcription defects by different mechanisms. The fact that we cannot identify Tat as a virion-associated protein lends support to the idea that it has an indirect effect on the efficiency of reverse transcription. Because reverse transcription occurs in the absence of Tat, albeit with greatly reduced efficiency, Tat may be able to stimulate this process in a manner parallel to viral transcription. While RT itself has not been demonstrated to be a target of cellular kinases, it may be possible that it can be modified to become a more processive enzyme and that Tat may function to recruit or activate a kinase involved in this process. Future experiments will aim to identify precisely at what point and with what viral and cellular factors Tat functions in the process of HIV-1 reverse transcription.
ACKNOWLEDGMENTS
This work was supported by grants from the National Centre for HIV Virology Research (to D.H., E.P., and A.D.), by the Royal Children’s Hospital project seeding grant (to C.W.H.), by the Department of Veterans Affairs, and by the National Institutes of Health.
We thank León F. Garcia-Martínez for the kind gift of expression plasmids and J. Pata for the tRNA3Lys plasmid.
Footnotes
Publication no. 94 from the Sir Albert Sakzewski Virus Research Centre.
REFERENCES
- 1.Aiken C, Trono D. nef stimulates human immunodeficiency virus type 1 proviral DNA synthesis. J Virol. 1995;69:5048–5056. doi: 10.1128/jvi.69.8.5048-5056.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albini A, Benelli R, Presta M, Rusnati M, Ziche M, Rubartelli A, Paglialunga G, Bussolino F, Noonan D. HIV-tat protein is a heparin-binding angiogenic growth factor. Oncogene. 1996;12:289–297. [PubMed] [Google Scholar]
- 3.Albini A, Soldi R, Giunciuglio D, Giraudo E, Benelli R, Primo L, Noonan D, Salio M, Camussi G, Rockl W, Bussolino F. The angiogenesis induced by HIV-1 tat protein is mediated by the Flk-1/KDR receptor on vascular endothelial cells. Nat Med. 1996;2:1371–1375. doi: 10.1038/nm1296-1371. [DOI] [PubMed] [Google Scholar]
- 4.Baltimore D. RNA-dependent DNA polymerization in virions of RNA tumor viruses. Nature. 1970;226:1209–1211. doi: 10.1038/2261209a0. [DOI] [PubMed] [Google Scholar]
- 5.Barat C, Le Grice S F, Darlix J L. Interaction of HIV-1 reverse transcriptase with a synthetic form of its replication primer, tRNA(Lys,3) Nucleic Acids Res. 1991;19:751–757. doi: 10.1093/nar/19.4.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barat C, Schatz O, Le Grice S, Darlix J L. Analysis of the interactions of HIV-1 replication primer tRNA(Lys3) with nucleocapsid protein and reverse transcriptase. J Mol Biol. 1993;231:185–190. doi: 10.1006/jmbi.1993.1273. [DOI] [PubMed] [Google Scholar]
- 7.Borgatti P, Zauli G, Cantley L C, Capitani S. Extracellular HIV-1 Tat protein induces a rapid and selective activation of protein kinase C (PKC)-alpha, and -epsilon and -zeta isoforms in PC12 cells. Biochem Biophys Res Commun. 1998;242:332–337. doi: 10.1006/bbrc.1997.7877. [DOI] [PubMed] [Google Scholar]
- 8.Borgatti P, Zauli G, Colamussi M L, Gibellini D, Previati M, Cantley L L, Capitani S. Extracellular HIV-1 Tat protein activates phosphatidylinositol 3- and Akt/PKB kinases in CD4+ T lymphoblastoid Jurkat cells. Eur J Immunol. 1997;27:2805–2811. doi: 10.1002/eji.1830271110. [DOI] [PubMed] [Google Scholar]
- 9.Bukrinskaya A G, Ghorpade A, Heinzinger N K, Smithgall T E, Lewis R E, Stevenson M. Phosphorylation-dependent human immunodeficiency virus type 1 infection and nuclear targeting of viral DNA. Proc Natl Acad Sci USA. 1996;93:367–371. doi: 10.1073/pnas.93.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bukrinsky M I, Sharova N, Dempsey M P, Stanwick T L, Bukrinskaya A G, Haggerty S, Stevenson M. Active nuclear import of human immunodeficiency virus type 1 preintegration complexes. Proc Natl Acad Sci USA. 1992;89:6580–6584. doi: 10.1073/pnas.89.14.6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bukrinsky M I, Sharova N, McDonald T L, Pushkarskaya T, Tarpley W G, Stevenson M. Association of integrase, matrix, and reverse transcriptase antigens of human immunodeficiency virus type 1 with viral nucleic acids following acute infection. Proc Natl Acad Sci USA. 1993;90:6125–6129. doi: 10.1073/pnas.90.13.6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cartier C, Deckert M, Grangeasse C, Trauger R, Jensen F, Bernard A, Cozzone A, Desgranges C, Boyer V. Association of ERK2 mitogen-activated protein kinase with human immunodeficiency virus particles. J Virol. 1997;71:4832–4837. doi: 10.1128/jvi.71.6.4832-4837.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conant K, Ma M, Nath A, Major E O. Extracellular human immunodeficiency virus type 1 Tat protein is associated with an increase in both NF-kappa B binding and protein kinase C activity in primary human astrocytes. J Virol. 1996;70:1384–1389. doi: 10.1128/jvi.70.3.1384-1389.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cujec T P, Cho H, Maldonado E, Meyer J, Reinberg D, Peterlin B M. The human immunodeficiency virus transactivator Tat interacts with the RNA polymerase II holoenzyme. Mol Cell Biol. 1997;17:1817–1823. doi: 10.1128/mcb.17.4.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cujec T P, Okamoto H, Fujinaga K, Meyer J, Chamberlin H, Morgan D O, Peterlin B M. The HIV transactivator TAT binds to the CDK-activating kinase and activates the phosphorylation of the carboxy-terminal domain of RNA polymerase II. Genes Dev. 1997;11:2645–2657. doi: 10.1101/gad.11.20.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dayton A I, Sodroski J G, Rosen C A, Goh W C, Haseltine W A. The trans-activator gene of the human T cell lymphotropic virus type III is required for replication. Cell. 1986;44:941–947. doi: 10.1016/0092-8674(86)90017-6. [DOI] [PubMed] [Google Scholar]
- 17.Dhawan S, Puri R K, Kumar A, Duplan H, Masson J M, Aggarwal B B. Human immunodeficiency virus-1-tat protein induces the cell surface expression of endothelial leukocyte adhesion molecule-1, vascular cell adhesion molecule-1, and intercellular adhesion molecule-1 in human endothelial cells. Blood. 1997;90:1535–1544. [PubMed] [Google Scholar]
- 18.Dingwall C, Ernberg I, Gait M J, Green S M, Heaphy S, Karn J, Lowe A D, Singh M, Skinner M A, Valerio R. Human immunodeficiency virus 1 Tat protein binds trans-activation-responsive region (TAR) RNA in vitro. Proc Natl Acad Sci USA. 1989;86:6925–6929. doi: 10.1073/pnas.86.18.6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ensoli B, Barillari G, Salahuddin S Z, Gallo R C, Wong S F. Tat protein of HIV-1 stimulates growth of cells derived from Kaposi’s sarcoma lesions of AIDS patients. Nature. 1990;345:84–86. doi: 10.1038/345084a0. [DOI] [PubMed] [Google Scholar]
- 20.Ensoli B, Gendelman R, Markham P, Fiorelli V, Colombini S, Raffeld M, Cafaro A, Chang H K, Brady J N, Gallo R C. Synergy between basic fibroblast growth factor and HIV-1 Tat protein in induction of Kaposi’s sarcoma. Nature. 1994;371:674–680. doi: 10.1038/371674a0. [DOI] [PubMed] [Google Scholar]
- 21.Feng S, Holland E C. HIV-1 Tat trans-activation requires the loop sequence within TAR. Nature. 1988;334:165–167. doi: 10.1038/334165a0. [DOI] [PubMed] [Google Scholar]
- 22.Fisher A G, Feinberg M B, Josephs S F, Harper M E, Marselle L M, Reyes G, Gonda M A, Aldovini A, Debouk C, Gallo R C, Wong-Staal F. The trans-activator gene of HTLV-III is essential for virus replication. Nature. 1986;320:367–371. doi: 10.1038/320367a0. [DOI] [PubMed] [Google Scholar]
- 23.Franke E K, Yuan H E H, Luban J. Specific incorporation of cyclophilin A into HIV-1 virions. Nature. 1994;372:359–362. doi: 10.1038/372359a0. [DOI] [PubMed] [Google Scholar]
- 24.Gallay P, Swingler S, Aiken C, Trono D. HIV-1 infection of nondividing cells: C-terminal tyrosine phosphorylation of the viral matrix protein is a key regulator. Cell. 1995;80:379–388. doi: 10.1016/0092-8674(95)90488-3. [DOI] [PubMed] [Google Scholar]
- 25.Gallay P, Swingler S, Song J, Bushman F, Trono D. HIV nuclear import is governed by the phosphotyrosine-mediated binding of matrix to the core domain of integrase. Cell. 1995;83:569–576. doi: 10.1016/0092-8674(95)90097-7. [DOI] [PubMed] [Google Scholar]
- 26.Ganju R K, Munshi N, Nair B C, Liu Z Y, Gill P, Groopman J E. Human immunodeficiency virus Tat modulates the Flk-1/KDR receptor, mitogen-activated protein kinases, and components of focal adhesion in Kaposi’s sarcoma cells. J Virol. 1998;72:6131–6137. doi: 10.1128/jvi.72.7.6131-6137.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia J A, Harrich D, Pearson L, Mitsuyasu R, Gaynor R B. Functional domains required for tat-induced transcriptional activation of the HIV-1 long terminal repeat. EMBO J. 1988;7:3143–3147. doi: 10.1002/j.1460-2075.1988.tb03181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.García-Martínez L F, Mavankal G, Neveu J, Lane W, Sigman D, Ivanov D, Gaynor R B. Purification of a Tat associated kinase reveals a TFIIH complex that modulates HIV-1 transcription. EMBO J. 1997;16:2836–2850. doi: 10.1093/emboj/16.10.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaynor R. Cellular transcription factors involved in the regulation of HIV-1 gene expression. AIDS. 1992;6:347–363. doi: 10.1097/00002030-199204000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Graham F L, Smiley J, Russell W C, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977;36:59–72. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- 31.Guo J, Henderson L E, Bess J, Kane B, Levin J G. Human immunodeficiency virus type 1 nucleocapsid protein promotes efficient strand transfer and specific viral DNA synthesis by inhibiting TAR-dependent self-priming from minus-strand strong-stop DNA. J Virol. 1997;71:5178–5188. doi: 10.1128/jvi.71.7.5178-5188.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hahn B H, Shaw G M, Arya S K, Popovic M, Gallo R C, Wong S F. Molecular cloning and characterization of the HTLV-III virus associated with AIDS. Nature. 1984;312:166–169. doi: 10.1038/312166a0. [DOI] [PubMed] [Google Scholar]
- 32a.Harrich, D., and R. Gaynor. Unpublished observations.
- 33.Harrich D, Hsu C, Race E, Gaynor R B. Differential growth kinetics are exhibited by human immunodeficiency virus type 1 TAR mutants. J Virol. 1994;68:5899–5910. doi: 10.1128/jvi.68.9.5899-5910.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harrich D, Ulich C, Garcia-Martinez L F, Gaynor R B. Tat is required for efficient HIV-1 reverse transcription. EMBO J. 1997;16:1224–1235. doi: 10.1093/emboj/16.6.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harrich D, Ulich C, Gaynor R B. A critical role for the TAR element in promoting efficient human immunodeficiency virus type 1 reverse transcription. J Virol. 1996;70:4017–4027. doi: 10.1128/jvi.70.6.4017-4027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harrich D, Ulich C, Gaynor R B. A novel role for the human immunodeficiency type 1 TAR element: a critical regulator of viral reverse transcription. J Virol. 1996;70:4017–4027. doi: 10.1128/jvi.70.6.4017-4027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heinzinger N K, Bukinsky M I, Haggerty S A, Ragland A M, Kewalramani V, Lee M A, Gendelman H E, Ratner L, Stevenson M, Emerman M. The Vpr protein of human immunodeficiency virus type 1 influences nuclear localization of viral nucleic acids in nondividing host cells. Proc Natl Acad Sci USA. 1994;91:7311–7315. doi: 10.1073/pnas.91.15.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967;26:365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- 39.Huang L, Joshi A, Willey R, Orenstein J, Jeang K T. Human immunodeficiency viruses regulated by alternative trans-activators: genetic evidence for a novel non-transcriptional function of Tat in virion infectivity. EMBO J. 1994;13:2886–2896. doi: 10.1002/j.1460-2075.1994.tb06583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang Y, Khorchid A, Gabor J, Wang J, Li X, Darlix J L, Wainberg M A, Kleiman L. The role of nucleocapsid and U5 stem/A-rich loop sequences in tRNA3Lys genomic placement and initiation of reverse transcription in human immunodeficiency virus type 1. J Virol. 1998;72:3907–3915. doi: 10.1128/jvi.72.5.3907-3915.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huber H E, McCoy J M, Seehra J S, Richardson C C. Human immunodeficiency virus 1 reverse transcriptase. Template binding, processivity, strand displacement synthesis, and template switching. J Biol Chem. 1989;264:4669–4678. [PubMed] [Google Scholar]
- 42.Isel C, Ehresmann C, Keith G, Ehresmann B, Marquet R. Initiation of reverse transcription of HIV-1: secondary structure of the HIV-1 RNA/tRNA(Lys3) (template primer) complex. J Mol Biol. 1995;247:236–250. doi: 10.1006/jmbi.1994.0136. [DOI] [PubMed] [Google Scholar]
- 43.Isel C, Lanchy J-M, Grice S F J L, Ehresmann B, Marquet R. Specific initiation and switch to elongation of human immunodeficiency virus type 1 reverse transcription require the post-transcriptional modifications of primer tRNALys3. EMBO J. 1996;15:917–924. [PMC free article] [PubMed] [Google Scholar]
- 44.Isel C, Marquet R, Keith G, Ehresmann C, Ehresmann B. Modified nucleotides of tRNA (3Lys) modulate primer/template loop-loop interaction in the initiation complex of HIV-I reverse transcriptase. J Biol Chem. 1993;268:25269–25272. [PubMed] [Google Scholar]
- 45.Jacque J M, Mann A, Enslen H, Sharova N, Brichacek B, Davis R J, Stevenson M. Modulation of HIV-1 infectivity by MAPK, a virion-associated kinase. EMBO J. 1998;17:2607–2618. doi: 10.1093/emboj/17.9.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jones K A, Peterlin B M. Control of RNA initiation and elongation at the HIV-1 promoter. Annu Rev Biochem. 1994;63:717–743. doi: 10.1146/annurev.bi.63.070194.003441. [DOI] [PubMed] [Google Scholar]
- 47.Kiernan R E, Ono A, Englund G, Freed E O. Role of matrix in an early postentry step in the human immunodeficiency virus type 1 life cycle. J Virol. 1998;72:4116–4126. doi: 10.1128/jvi.72.5.4116-4126.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kumar A, Manna S K, Dhawan S, Aggarwal B B. HIV-Tat protein activates c-Jun N-terminal kinase and activator protein-1. J Immunol. 1998;161:776–781. [PubMed] [Google Scholar]
- 49.Lafrenie R M, Wahl L M, Epstein J S, Yamada K M, Dhawan S. Activation of monocytes by HIV-Tat treatment is mediated by cytokine expression. J Immunol. 1997;159:4077–4083. [PubMed] [Google Scholar]
- 50.Lanchy J-M, Ehresmann C, Le Grice S F J, Ehresmann B, Marquet R. Binding and kinetic properties of HIV-1 reverse transcriptase markedly differ during initiation and elongation of reverse transcription. EMBO J. 1996;15:7178–7187. [PMC free article] [PubMed] [Google Scholar]
- 51.Lapadat-Tapolsky M, De Rocoquigny H, Van Gent D, Roques B, Plasterk R, Darlix J L. Interactions between HIV-1 nucleocapsid protein and viral DNA may have important functions in the viral life cycle. Nucleic Acids Res. 1993;21:831–839. doi: 10.1093/nar/21.4.831. . (Erratum, 21:2024.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lapadat-Tapolsky M, Gabus C, Rau M, Darlix J L. Possible roles of HIV-1 nucleocapsid protein in the specificity of proviral DNA synthesis and in its variability. J Mol Biol. 1997;268:250–260. doi: 10.1006/jmbi.1997.0978. [DOI] [PubMed] [Google Scholar]
- 53.Li C J, Friendman D J, Wang C, Meteleve V, Pardee A B. Induction of apoptosis in uninfected lymphocytes by HIV-1 Tat protein. Science. 1995;268:429–431. doi: 10.1126/science.7716549. [DOI] [PubMed] [Google Scholar]
- 54.Li X, Quan Y, Arts E J, Li Z, Preston B D, de Rocquigny H, Roques B P, Darlix J L, Kleiman L, Parniak M A, Wainberg M A. Human immunodeficiency virus type 1 nucleocapsid protein (NCp7) directs specific initiation of minus-strand DNA synthesis primed by human tRNA3Lys in vitro: studies of viral RNA molecules mutated in regions that flank the primer binding site. J Virol. 1996;70:4996–5004. doi: 10.1128/jvi.70.8.4996-5004.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mancebo H S Y, Lee G, Flygare J, Tomassini J, Luu P, Zhu Y, Peng J, Blau C, Hazuda D, Price D, Flores O. P-TEFb kinase is required for HIV Tat transcriptional activation in vivo and in vitro. Genes Dev. 1997;11:2633–2644. doi: 10.1101/gad.11.20.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Masuda T, Planelles V, Krogstad P, Chen I S Y. Genetic analysis of human immunodeficiency virus type 1 integrase and the U3 att site: unusual phenotype of mutants in the zinc finger-like domain. J Virol. 1995;69:6687–6696. doi: 10.1128/jvi.69.11.6687-6696.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mely Y, de Rocquigny H, Sorinas-Jimeno M, Keith G, Roques B P, Marquet R, Gerard D. Binding of the HIV-1 nucleocapsid protein to the primer tRNA(3Lys), in vitro, is essentially not specific. J Biol Chem. 1995;270:1650–1656. doi: 10.1074/jbc.270.4.1650. [DOI] [PubMed] [Google Scholar]
- 58.Menegon A, Leoni C, Benfenati F, Valtorta F. Tat protein from HIV-1 activates MAP kinase in granular neurons and glial cells from rat cerebellum. Biochem Biophys Res Commun. 1997;238:800–805. doi: 10.1006/bbrc.1997.7393. [DOI] [PubMed] [Google Scholar]
- 59.Milani D, Mazzoni M, Borgatti P, Zauli G, Cantley L, Capitani S. Extracellular human immunodeficiency virus type-1 Tat protein activates phosphatidylinositol 3-kinase in PC12 neuronal cells. J Biol Chem. 1996;271:22961–22964. doi: 10.1074/jbc.271.38.22961. [DOI] [PubMed] [Google Scholar]
- 60.Mitola S, Sozzani S, Luini W, Primo L, Borsatti A, Weich H, Bussolino F. Tat-human immunodeficiency virus-1 induces human monocyte chemotaxis by activation of vascular endothelial growth factor receptor-1. Blood. 1997;90:1365–1372. [PubMed] [Google Scholar]
- 61.New D R, Maggirwar S B, Epstein L G, Dewhurst S, Gelbard H A. HIV-1 Tat induces neuronal death via tumor necrosis factor-alpha and activation of non-N-methyl-d-aspartate receptors by a NFkappaB-independent mechanism. J Biol Chem. 1998;273:17852–17858. doi: 10.1074/jbc.273.28.17852. [DOI] [PubMed] [Google Scholar]
- 62.Opalenik S R, Shin J T, Wehby J N, Mahesh V K, Thompson J A. The HIV-1 TAT protein induces the expression and extracellular appearance of acidic fibroblast growth factor. J Biol Chem. 1995;270:17457–17467. doi: 10.1074/jbc.270.29.17457. [DOI] [PubMed] [Google Scholar]
- 63.Oude Essink B B, Das A T, Berkhout B. Structural requirements for the binding of tRNALys3 to reverse transcriptase of the human immunodeficiency virus type 1. J Biol Chem. 1995;270:23867–23874. doi: 10.1074/jbc.270.40.23867. [DOI] [PubMed] [Google Scholar]
- 64.Panet A C, Haseltine W A, Baltimore D, Peters G, Harada F, Dahlberg J. Specific binding of tRNAtrp to AMV reverse transcriptase. Proc Natl Acad Sci USA. 1975;72:2535–2539. doi: 10.1073/pnas.72.7.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pop M P, Biebricher C K. Kinetic analysis of pausing and fidelity of human immunodeficiency virus type 1 reverse transcription. Biochemistry. 1996;35:5054–5062. doi: 10.1021/bi9530292. [DOI] [PubMed] [Google Scholar]
- 66.Priel E, Showalter S D, Roberts M, Oroszlan S, Segal S, Aboud M, Blair D G. Topoisomerase I activity associated with human immunodeficiency virus (HIV) particles and equine infectious anemia virus core. EMBO J. 1990;9:4167–4172. doi: 10.1002/j.1460-2075.1990.tb07640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schwartz O, Marechal V, Danos O, Heard J M. Human immunodeficiency virus type 1 nef increases the efficiency of reverse transcription in the infected cell. J Virol. 1995;69:4053–4059. doi: 10.1128/jvi.69.7.4053-4059.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shi B, Raina J, Lorenzo A, Busciglio J, Gabuzda D. Neuronal apoptosis induced by HIV-1 Tat protein and TNF-alpha: potentiation of neurotoxicity mediated by oxidative stress and implications for HIV-1 dementia. J Neurovirol. 1998;4:281–290. doi: 10.3109/13550289809114529. [DOI] [PubMed] [Google Scholar]
- 69.Sova P, Volsky D J. Efficiency of viral DNA synthesis during infection of permissive and nonpermissive cells with Vif-negative human immunodeficiency virus type 1. J Virol. 1993;67:6322–6326. doi: 10.1128/jvi.67.10.6322-6326.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stevenson M, Stanwick T L, Dempsey M P, Lamonica C A. HIV-1 replication is controlled at the level of T cell activation and proviral integration. EMBO J. 1990;9:1551–1560. doi: 10.1002/j.1460-2075.1990.tb08274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Temin H M, Mitzutani S. RNA-directed DNA polymerase in virions of Rous sarcoma virus. Nature. 1970;226:1211–1213. doi: 10.1038/2261211a0. [DOI] [PubMed] [Google Scholar]
- 72.Thali M, Bukovsky A, Kondo E, Rosenwirth B, Walsh C T, Sodroski J, Gottlinger H G. Functional association of cyclophilin A with HIV-1 virions. Nature. 1994;372:363–365. doi: 10.1038/372363a0. [DOI] [PubMed] [Google Scholar]
- 73.Ulich C, Harrich D, Estes P, Gaynor R B. Inhibition of human immunodeficiency virus type 1 replication is enhanced by a combination of transdominant Tat and Rev proteins. J Virol. 1996;70:4871–4876. doi: 10.1128/jvi.70.7.4871-4876.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.von Schwedler U, Kornbluth R S, Trono D. The nuclear localization signal of the matrix protein of human immunodeficiency virus type 1 allows the establishment of infection in macrophages and quiescent T-lymphocytes. Proc Natl Acad Sci USA. 1994;91:6992–6996. doi: 10.1073/pnas.91.15.6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.von Schwedler U, Song J, Aiken C, Trono D. vif is crucial for human immunodeficiency virus type 1 proviral DNA synthesis in infected cells. J Virol. 1993;67:4945–4955. doi: 10.1128/jvi.67.8.4945-4955.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wei P, Garber M E, Fang S M, Fischer W H, Jones K A. A novel CDK9-associated C-type cyclin interacts directly with HIV-1 Tat and mediates its high-affinity, loop-specific binding to TAR RNA. Cell. 1998;92:451–462. doi: 10.1016/s0092-8674(00)80939-3. [DOI] [PubMed] [Google Scholar]
- 77.Westendorp M O, Frank R, Ochsenbauer C, Stricker K, Dhein J, Walczak H, Debatin K-M, Krammer P H. Sensitization of T-cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature. 1995;375:497–500. doi: 10.1038/375497a0. [DOI] [PubMed] [Google Scholar]
- 78.Zack J A, Arrigo S J, Weitsman S R, Go A S, Haislip A, Chen I S Y. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990;61:213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]
- 79.Zhu Y, Peery T, Peng J, Ramanathan Y, Marshall N, Marshall T, Amendt B, Mathews M B, Price D H. Transcription elongation factor P-TEFb is required for HIV-1 Tat transactivation in vitro. Genes Dev. 1997;11:2622–2632. doi: 10.1101/gad.11.20.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]