Abstract
This study investigated the immune-enhancement effects of Angelica gigas Nakai extract (ANE) and its yeast-fermented extract (FAN) in cyclophosphamide (CPP)-induced immunosuppressed mice. Angelica gigas Nakai (AGN) increased the protein level of inducible nitric oxide synthase (iNOS) and the production of nitric oxide (NO) and immune-related cytokines in mouse splenocytes. AGN also restored CPP-induced suppression of NK cell activity and splenocyte proliferation. Furthermore, AGN activated the ERK and p38 MAPK/NF-κB signaling pathways in mouse splenocytes via phosphorylation of signaling molecules. These findings indicate that upregulation of cytokines and enzymes may be closely associated with the MAPK/NF-κB signaling pathways. In conclusion, AGN can restore CPP-induced immunosuppression in mice, although there was no significant difference in the immune-enhancing effect between ANE and FAN. It is suggested that AGN might have the potential to enhance immunity as an immunostimulant under immunosuppressed conditions. Therefore, it could be used as an effective agent or a dietary supplement for improving immunity.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10068-023-01281-6.
Keywords: Angelica gigas Nakai, Immune enhancement, MAPK, NF-κB signaling, Fermentation
Introduction
The immune system refers to complex networks of cells, molecules, and processes that play important roles in protecting the body from infections caused by foreign antigens such as external viruses, bacteria, and parasites (Brodin and Davis, 2017; Parkin and Cohen, 2001). Recently, due to a global outbreak of infectious disease and aging, immune-enhancement has emerged as one of the most important strategies to protect one’s body. The immune system is tightly regulated via complicated interactions of immune cells with cytokines released by antigenic stimulation. Innate immune cells such as macrophages, natural killer (NK) cells, and dendritic cells (DCs) are activated in response to pathogens, and the activated cells not only directly destroy infected cells but also rapidly trigger the release of distinct cytokines that affect adaptive immune cells such as T and B lymphocytes involved in the acquired or antigen-specific immune response (Belardelli and Ferrantini, 2002). Macrophages produce immune-related mediators such as nitric oxide (NO), tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β), and interleukin-6 (IL-6), which are linked to mitogen-activated protein kinase (MAPK) and nuclear factor-kappa B (NF-κB) signaling pathways (Cavaillon, 1994; Kim et al., 2013). NO exhibits antimicrobial and antitumoral activity, and it plays a role in modulating the production and actions of cytokines and chemokines in the immune system (Bogdan, 2001). The high level of NO in the body is regarded as beneficial for upregulating the immune system. Cytokines take part in a variety of physiological processes, while temporally and locally controlling the duration and amplitude of immune responses. TNF-α is primarily released by activated macrophages and has been recognized as a key mediator in immune cell regulation (Beutler and Cerami, 1989). IL-6 has a variety of biological functions in host defense systems, including B cell maturation, T cell differentiation, and acute response induction (Simpson et al., 1997). IL-1β is related to various cellular activities, such as immune cell differentiation and proliferation (Tulotta and Ottewell, 2018). IL-2 has been known as the T cell growth factor, and it increases the activity of B cells, NK cells, and other T cells. IFN-γ, which is mainly released by activated NK cells and T cells, plays an important role in cellular immunity by activating macrophages and stimulating T cell production (Jorgovanovic et al., 2020). IL-12, secreted by antigen-presenting cells, functions as a key factor in the host defense mechanism as it stimulates both innate and adaptive immune effector cells such as NK cells and T cells (Ma and Trinchieri, 2001). Since activated NK cells can detect infected cells that are not recognized by cytotoxic T cells and directly destroy them, and further induce the secretion of chemokines and cytokines to recruit and activate components associated with both the innate and adaptive immune responses, they play an important role in immune activation in humans (Iannello and Raulet, 2013; Pierce et al., 2020).
Angelica gigas Nakai (AGN), a perennial plant belonging to the Umbelliferae family, is commonly cultivated in South Korea. Its roots have been long utilized as a medicinal herb (Yan et al., 2004). Previous studies have reported that A. gigas has various physiological effects, such as anticancer (Kim et al., 2018), anti-inflammatory, antibacterial (Lee et al., 2003), antioxidant (Noh et al., 2014), antithrombotic (Bravo et al., 2021), and neuroprotective properties (Sowndhararajan and Kim, 2017). Recent studies have reported that a polysaccharide isolated from a water-soluble fraction of A. gigas extracts exerts immunostimulatory effects via activating the innate and adaptive immunity (Kim et al., 2018), and a combined natural product mixture containing A. gigas enhances immunity by modulating cytokine release and immune cell activity in an immunosuppressed mouse model (Han et al., 2022). However, the immune regulatory function of a whole extract of A. gigas containing insoluble components like decursin and decursinol angelate has not been explored. Decursin and decursinol angelate are known as major coumarin compounds obtained from A. gigas roots, but such compounds are not detected in a water-soluble fraction of the extract. In our previous study, decursin treatment increased NO production and cytokine release, such as TNF-α, IL-1β, IL-6, Il-2, and IFN-γ in the RAW264.7 mouse macrophage cell line (unpublished data). In this study, immunostimulatory effects of a whole extract of AGN were investigated using the cyclophosphamide (CPP)-induced immunosuppressed mice model. Additionally, it was examined whether immunostimulatory effects would further increase when the extract was fermented with yeast, because fermentation has offered a variety of health benefits, including stimulation of the immune system, and the production of metabolites that improve human health (Ashraf and Shah, 2014). To determine the immunostimulatory effects of Angelica gigas Nakai extract (ANE) and its fermented extract (FAN), the levels of NO and cytokines were measured in splenocytes isolated from spleen tissues of CPP-induced immunosuppressed mice, and NK cell activity and splenocyte proliferation were calculated. Furthermore, the effects of A. gigas extracts on signal transduction molecules associated with the MAPK/NF-κB signaling cascade were examined to elucidate the mechanism underlying the immunostimulatory activity.
Materials and methods
Chemicals and reagents
Roswell Park Memorial Institute (RPMI) 1640, penicillin/streptomycin, and fetal bovine serum (FBS), and phosphate buffer saline (PBS) were purchased from Gibco (Grand Island, NY, USA). Primary antibodies against extracellular signal-regulated kinase (ERK)1/2, phospho-ERK (p-ERK)1/2, p38, phospho-p38 (p-p38), IκB kinase (IKK), phosphor-IKK (p-IKK), and phosphor-NF-κB (p-NF-κB) were purchased from Cell Signaling (Danvers, MA, USA). Primary antibodies against inhibitor kappa B-alpha (IκB-α), phosphor-IκB-α (p-IκB-α), and β-actin were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-NF-κB and anti-inducible nitric oxide synthase (iNOS) antibodies were purchased from Abcam (Cambridge, MA, US), and Novus Biologicals (Littleton, CO, USA), respectively. Enzyme-linked immunosorbent assay (ELISA) kits for TNF-α, IL-6, IL-1β, interferon-gamma (IFN-γ), interleukin-2 (IL-2), and interleukin-12 (IL-12) were obtained from R&D Systems (Minneapolis, MN, USA). RBC lysis buffer, Griess reagent, dimethyl sulfoxide (DMSO), N-ethylmaleimide, EDTA, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reagent, sodium deoxy cholate, sodium chloride, Triton X-100, CPP, Concanavalin A (ConA), lipopolysaccharides (LPS), and levamisole hydrochloride were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Preparation of aqueous and fermented extracts from roots of Angelica gigas
AGN roots were collected from Gangwon-do, South Korea, and air-dried, followed by pulverizing. The pulverized materials were sterilized at 105 to 115 °C for 1 h after adding 10 times the weight of water. ANE was obtained through filtration with a 60-mesh sieve, and a spray-drying process to produce a dry powder from the sterilized sample. Half of the sterilized sample amount was applied to prepare FAN. The sample was maintained at 30 °C to allow it to cool down, and then inoculated with 0.1% (w/w) of dried yeast (Saccharomyces cerevisiae) culture, and fermented at 30 °C for 20 h. The fermented sample was disinfected at 100 °C for 1 h. The slurry was filtered with a 60-mesh sieve and spray-dried using an atomizer nozzle.
Experimental animals and study design
This study was carried out using 6-week-old male BALB/C mice weighing 18 to 20 g (ORIENT BIO Inc., Seongnam, South Korea). During the experimental period, the animals were kept in standard cages under temperature (23 ± 3 °C), and relative humidity (50 ± 20%) control, with a 12-h light/dark cycle. They were given water and a standard diet with free access. After a week of acclimation, mice were randomly divided into nine groups, consisting of six mice per group. To induce immunosuppression in BALB/c mice, CPP (80 mg/kg) was intraperitoneally given to all experimental mice except for the normal control from Days 1 to 3 of the experiment. For 14 days from the day after CPP administration, mice assigned to each group orally received the following treatments, respectively: the normal control group and the CPP model group were given saline; the positive control group was given 40 mg/kg of levamisole; the experimental groups were given 50, 150, and 300 mg/kg of ANE or FAN, respectively. After all the mice were isoflurane-anesthetized, the blood was collected, and mouse organs such as the spleen, thymus, liver, and kidney were excised on the last day of the experiment, followed by storage at − 70 °C for further experiments. The relative weight of an organ was calculated as follows: Relative weight (%) = (organ weight/body weight) × 100.
Isolation of mouse splenocytes
Each spleen obtained from the mice was pressed using slide glasses and then placed into a 40 μm strainer (SPL, Pocheon, South Korea). It was mashed and filtered through the strainer using a syringe plunger. Filtrated cells were rinsed with RPMI-1640 and spun at 250 × g for 3 min at 4 °C. The supernatant was removed, and the pellet was resuspended in RBC lysis buffer and incubated for 5 min at room temperature. The lysed cells were rinsed with RPMI-1640 and counted using trypan blue reagent and an automated cell counter (NanoEnTek, Seoul, South Korea).
NO assay
The level of NO was indirectly measured by the quantitative determination of nitrite (NO2−) in response to the Griess reagent. Mouse splenocytes isolated from spleen tissue were plated onto 6-well plate (3 × 106 cells) using RPMI 1640 medium containing 10% FBS, streptomycin (100 μg/ml), and penicillin (100 U/ml) and incubated for 48 h at 37 °C in humidified 5% CO2. After 48 h, splenocytes was homogenized with cold PBS including 10 mM N-ethylmaleimide and 2.5 mM EDTA. The homogenized sample was centrifuged, and the supernatant was collected. The obtained supernatant was mixed with an equivalent volume of Griess reagent onto a 96-well plate and incubated for 15 min in the dark at room temperature. It was spectrophotometrically measured at an absorbance of 540 nm (BioTek, Winooski, VT, USA).
Reverse transcription-polymerase chain reaction (RT-PCR)
To isolate total RNA, a portion of spleen tissues derived from immunosuppressed mice was disrupted and homogenized in lysis buffer (RNeasy Kit, Qiagen, Hilden, Germany). The lysate was applied to the RNeasy spin column, the membrane of which total RNA bound to, and was eluted. Using 1 μg of the isolated total RNA, cDNA was synthesized (DiaStar™ RT Series, SolGent, Daejeon, South Korea). The obtained cDNA was amplified through PCR. The reaction was run for 40 cycles at annealing temperatures of 58.5 °C for Il2 and Gapdh, 59.0 °C for Tnfa and Il12, and 59.1 °C for Il6, Il1b, and Ifng. The PCR products were electrophoresed on a 2% agarose gel containing EcoDye™ DNA staining solution (SolGent, Daejeon, South Korea). The following primer sequences (5′˗ 3′) were used to perform PCR: TGT CTA CTC CTC AGA GCC CC for Tnfa_F and GAC CCG TAG GGC GAT TAC AG for Tnfa_R; GCG GCA CTT TTT CCA GAC AG for Il6_F and TTG CAT CTG GCT TTG TTC GC for Il6_R; TCA TTC TGG GTT CAC ACG GG for Il1b_F and GTG ATA ACG GTG GCC TGA CA for Il1b_R; GCC TCC CGT ATG TGT TTG GA for Ifng_F and TGC ATC CTT TTT CGC CTT GC Ifng_R; CTC GCA TCC TGT GTC ACA TTG for Il2_F and TCA AAT CCA GAA CAT GCC GC for Il2_R; ACG GCA GTG TGC TTG TCT AA for Il12_F and ATG GCG AAC CTG GAT GGT TT for Il12_R; ACC ACA GTC CAT GCC ATC AC for Gapdh_F and TCC ACC ACC CTG TTG CTG TA for Gapdh_R.
Splenocyte proliferation assay
Mouse splenocytes were seeded at 1 × 105 cells/well onto 96-well plates, stimulated with LPS or ConA (5 μg/mL, respectively), and then incubated for 48 h at 37 °C. After adding 1:10 volume of MTT solution (5 mg/mL) to each well, the 96-well plate was incubated for 2 h at 37 °C in the dark. MTT formazan crystals were dissolved in DMSO. An absorbance value was measured at 570 nm using a spectrophotometer (BioTek, Winooski, VT, USA). The level of mouse splenocyte proliferation was normalized to that of the control group.
Cell culture
YAC-1 lymphoma cells were obtained from the Korean Cell Line Bank (Seoul, South Korea). Mouse splenocytes and YAC-1 lymphoma cells were cultured with RPMI 1640 medium containing 10% FBS, streptomycin (100 μg/ml), and penicillin (100 U/ml) at 37 °C in humidified 5% CO2.
NK cell activity assay
Splenocytes isolated from homogenized mouse spleen tissues as the effector cells, were seeded at 1 × 106 cells/well onto 96-well plates. Then, YAC-1 lymphoma cells as target cells, were added at 1 × 105 cells/well. A mixture of effector-to-target cells in a ratio of 10:1 was incubated at 37 °C for 24 h. NK cell cytotoxicity was evaluated by MTT assay. NK cell activity was calculated using the formula as follows: NK cell activity (%) = {OD Control target cells − (OD Test samples – OD Control effector cells)} / OD Control target cells × 100.
Measurement of splenic cytokine levels by enzyme-linked immunosorbent assay (ELISA)
Splenocytes were seeded at 3 × 106 cells/well onto 6-well plates, and then treated with ConA or LPS (5 μg/ml, respectively). After 48 h, the concentrations of TNF-α, IL-6, IL-1β, IFN-γ, IL-2, and IL-12 were measured using collected culture supernatants by ELISA kit following the manufacturer’s recommendations (R&D Systems, Minneapolis, MN, USA).
Western blot analysis
Mouse splenic cells were plated at 3 × 106 cells/well onto 6-well plates using RPMI 1640 medium containing 10% FBS, streptomycin (100 μg/ml), and penicillin (100 U/ml) and incubated for 48 h at 37 °C in humidified 5% CO2. Cells were lysed in RIPA lysis buffer including 50 mM Tris (pH 8.0), 0.01% SDS, 0.5% sodium deoxycholate, 150 mM NaCl, and 1% Triton X-100 with phosphatase and protease inhibitor cocktails (Thermo Fisher Scientific, Waltham, MA, USA). Using the bicinchoninic acid assay, total protein levels were quantified (Thermo Fisher Scientific, Waltham, MA, USA). Equal amounts of total proteins were separated on 10% SDS acrylamide gels and transferred to polyvinylidene difluoride (PVDF) membranes (Bio-Rad, Hercules, CA, USA). After being blocked with 5% skimmed milk, the blot was incubated overnight at 4 °C with a specific primary antibody diluted at a ratio of 1:1000. It was labeled for 1 h at room temperature with the pertinent secondary antibody diluted at a ratio of 1:5000 before being visualized on films by enhanced chemiluminescence (ECL) reagent (GE Healthcare, Chicago, IL, USA). For quantitative analysis, band intensities were analyzed by the ImageJ program. Protein levels were normalized to β-actin protein levels.
Statistical analysis
All values were presented as mean ± standard deviation (SD) derived from three independent experiments. The Student’s t-test was used to identify significant differences between the two groups. The statistical differences were regarded as significant when the p-value was below 0.05. All statistical analyses were conducted using the Microsoft Excel program (Microsoft Office Standard 2019, Microsoft Co., WA, USA).
Results and discussion
Marker compounds of AGN have been quantitatively analyzed by HPLC
To determine the amount of decursin and decursinol angelate as marker compounds of AGN, high-performance liquid chromatography (HPLC) was performed (1260 Infinity II LC System, Agilent, Santa Clara, CA, USA). The total contents of decursin and decursinol angelate in ANE and FAN utilized in this study were 86.08 mg/g, and 111.33 mg/g, respectively (Fig. S1). Additionally, in the nutritional composition analysis, the proportion of carbohydrates containing sugars in FAN (63.95%) was lower than that of ANE (72.56%), while the proportion of components such as protein and fat of FAN was higher than that of ANE (18.17%, and 4.43% for FAN vs. 13.88%, and 1.45% for ANE, respectively) (Table S1). Hence, it was revealed that fermentation increased the overall level of decursin and decursinol angelate, and altered nutrient composition.
AGN increases NO levels and immune-related cytokine expressions in mouse splenic tissues
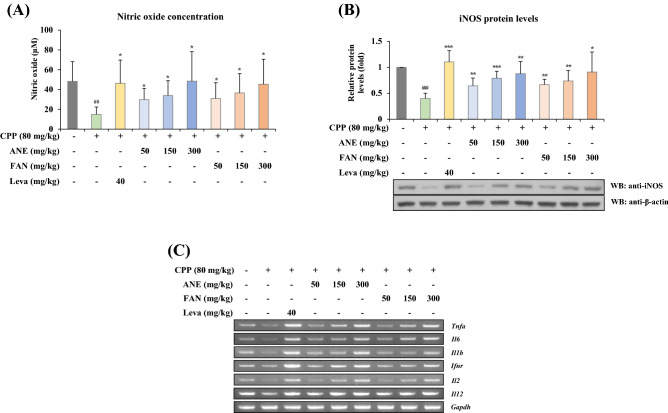
Macrophages are phagocytic cells that eliminate harmful substances, including cancer cells, and microorganisms, and play a vital role in the pathogenesis of numerous immune and degenerative diseases (Hirayama et al., 2017). Effector molecules like NO, TNF-α, IL-1, and IL-6 are released by activated macrophages. In particular, NO is very important as a defense molecule against infection because it regulates the production and functional activity of many immune cells, including natural killer cells, macrophages, neutrophils, and T cells. NO is produced by iNOS, which is synthesized upon cell activation but not expressed in resting cells (Tripathi et al., 2007). iNOS expression during infection or inflammation has been considered a crucial factor in the adaptive immune response to pathogenic organisms or harmful stimuli (Nathan and Xie, 1994). In this study, the immunostimulatory effects of ANE and FAN were evaluated using the immunosuppressed mouse model induced by intraperitoneal administration of CPP, which is an immunosuppressant to repress immune responses by damaging the DNA structure of macrophages and inhibiting cell proliferation and differentiation (Kim et al., 2020). Levamisole hydrochloride, used as a positive control, is an immunostimulatory agent that increase the functions of cellular immunity through macrophage and T-cell activation (Renoux, 1980). To ascertain the effect of ANE and FAN on NO production, we measured the NO concentration in splenic tissues using the Griess reagent. The NO level in the CPP model group was significantly lower than in the normal control group. However, the administration of ANE or FAN significantly restored the decreased NO level by CPP in a concentration-dependent manner (Fig. 1A). Moreover, the protein level of iNOS, which promotes NO production positively related to the phagocytic activity of macrophages, was significantly enhanced in splenocytes isolated from mice administrated with ANE or FAN compared to that of the CPP-treated control mice (Fig. 1B).
Fig. 1.
Effects of ANE and FAN on NO production and cytokine mRNA expressions in CPP-induced immunosuppressed mice. Total proteins and RNAs from excised mouse splenic tissues were prepared for western blot analysis and RT-PCR, respectively. (A) NO concentrations were detected in mouse splenic tissues by the Griess reagent. (B) The level of iNOS protein expression was normalized to that of β-actin. (C) The mRNA expression levels of Tnfa, Il6, Il1b, Ifnr, Il2, and Il12 were visualized onto 2% agarose gels, and (D-I) each relative mRNA level was presented as normalized to Gapdh. Data were presented as mean ± SD (n = 6 per group). ## p < 0.01, and ### p < 0.001, compared to the normal group; * p < 0.05, ** p < 0.01, and *** p < 0.001, compared to the CPP-induced immunosuppressed group. CPP, cyclophosphamide; ANE, Angelica gigas Nakai extract; FAN, yeast-fermented Angelica gigas Nakai extract; Leva, levamisole hydrochloride; iNOS, inducible nitric oxide synthase; Tnfa, tumor necrosis factor alpha; Il6, interleukin-6; Il1b, interleukin-1 beta; Ifnr, interferon gamma; Il2, interleukin-2; Il12, interleukin-12
To further explore whether ANE and FAN induce changes in immune-related cytokine gene expressions, the mRNA levels of Tnfa, Il6, Il1b, Ifnr, Il2, and Il12 were examined in the splenic tissues separated from immunosuppressed mice administered with ANE or FAN. Cytokines are involved in immune regulatory function, and play a pivotal role in preserving the homeostasis of the immune system mainly through cell-to-cell communication (Hwang, 2013; Wei et al., 2018). As shown in Fig. 1C, CPP administration significantly decreased the mRNA level of each cytokine in comparison with that of the normal control. However, ANE or FAN administration dose-dependently reversed the reduced level. For quantification, the relative mRNA level of each cytokine was normalized to that of GADPH (Fig. 1D–I). We have demonstrated that ANE or FAN promotes NO production by iNOS upregulation, and immune-related cytokine gene expression. However, there was no significant difference in the increase in NO levels and cytokine expression between ANE and FAN. In other words, whether fermented or not, AGN significantly elevated the levels of NO and cytokines in a concentration-dependent manner. These findings imply that AGN may activate the immune system by increasing NO production and cytokine secretion, which in turn stimulates immune cell proliferation and functional activities.
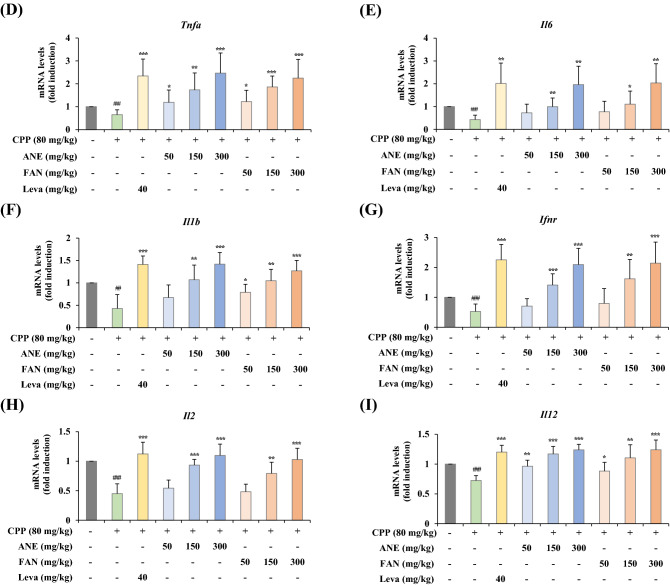
AGN doesn’t induce changes in body weight but it increases the relative weight of the spleen and thymus in CPP-induced immunosuppressed mice
Alterations in the body or organ weights of experimental animals have been commonly considered an indicator of chemical-induced damage or a change in biological functions. To determine whether ANE or FAN has an effect on weight changes in mice, we monitored changes in the body, spleen, and thymus weights of the animals. After inducing immunosuppression with CPP for 3 consecutive days, ANE or FAN was given orally to mice once a day for 14 days. Body weights were measured 5 times during the experimental period. On Day 4, all mice treated with CPP for 3 days showed a significant decrease in body weight compared to that of the normal control. However, on Day 17, there wasn’t any difference in body weight between the normal group and the other groups (Table 1). These findings suggest that CPP administration caused mice to lose weight. Next, we measured the weight of the spleen and thymus excised after the mice were sacrificed. The spleen and thymus are important lymphatic organs in the growth and proliferation of immune cells. Weight alterations in the spleen and thymus are regarded as one of the biomarkers to evaluate immune system activation because they are associated with an increased immunological condition (Slawinska et al., 2014). As a result, the administration of ANE or FAN increased the relative weight of the spleen or thymus to the body (Fig. 2A, B), which is closely related to the promotion of immune cell maturation and proliferation. On the other hand, weight changes in the liver and kidney were not observed in all groups, suggesting that ANE and FAN might not affect organs such as the liver and kidney (Fig. 2C, D).
Table 1.
Effect of ANE and FAN on body weight changes in CPP-induced immunosuppressed mice
| Groupa | Body weight (g) | |||||
|---|---|---|---|---|---|---|
| Day 1 | Day 4 | Day 7 | Day 10 | Day 13 | Day 17 | |
| Nor | 22.75 ± 1.92 | 22.97 ± 2.01 | 23.15 ± 1.96 | 23.50 ± 2.01 | 24.03 ± 2.13 | 24.32 ± 1.94 |
| CPP | 22.80 ± 1.75 | 19.73 ± 1.36## | 21.20 ± 1.50 | 23.47 ± 1.84 | 23.97 ± 1.70 | 24.30 ± 1.80 |
| Levamisole | 22.73 ± 1.50 | 19.57 ± 1.32## | 20.92 ± 1.45# | 22.62 ± 1.43 | 22.85 ± 1.55 | 23.43 ± 1.48 |
| ANE 50 | 22.68 ± 1.37 | 19.92 ± 1.26# | 21.28 ± 1.16 | 22.97 ± 1.12 | 23.37 ± 1.25 | 23.98 ± 1.53 |
| ANE 150 | 22.68 ± 1.28 | 19.92 ± 1.05## | 21.32 ± 1.09 | 23.15 ± 1.56 | 23.52 ± 1.53 | 24.23 ± 1.33 |
| ANE 300 | 22.73 ± 1.25 | 19.78 ± 1.33## | 21.20 ± 1.28 | 22.87 ± 1.24 | 23.15 ± 1.24 | 23.45 ± 1.30 |
| FAN 50 | 22.67 ± 1.11 | 20.17 ± 1.19# | 21.43 ± 1.14 | 23.18 ± 1.09 | 23.45 ± 1.09 | 23.98 ± 0.97 |
| FAN 150 | 22.68 ± 1.00 | 19.92 ± 1.23## | 21.25 ± 1.21 | 22.87 ± 1.13 | 23.23 ± 1.08 | 23.80 ± 1.16 |
| FAN 300 | 22.68 ± 0.98 | 20.00 ± 1.01## | 21.23 ± 0.88 | 23.03 ± 1.10 | 23.30 ± 0.08 | 23.78 ± 0.95 |
Values were expressed as mean ± SD (n = 6 mice per group). Comparisons between experimental groups were determined by Student’s t-test and statistical significance was set at p < 0.05. # p < 0.05 and ## p < 0.01, compared to the normal group
aNor, normal BALB/c mice; CPP, BALB/c mice intraperitoneally administered with 80 mg/kg cyclophosphamide (CPP); Levamisole, CPP-administered BALB/c mice orally administered with 40 mg/kg levamisole, positive control; ANE 50, CPP-administered BALB/c mice orally administered with 50 mg/kg ANE; ANE 150, CPP-administered BALB/c mice orally administered with 150 mg/kg ANE; ANE 300, CPP-administered BALB/c mice orally administered with 300 mg/kg ANE; FAN 50, CPP-administered BALB/c mice orally administered with 50 mg/kg FAN; FAN 150, CPP-administered BALB/c mice orally administered with 150 mg/kg FAN; FAN 300, CPP-administered BALB/c mice orally administered with 300 mg/kg FAN
Fig. 2.
Effects of ANE and FAN on weight alterations of the spleen, thymus, liver, and kidney in CPP-induced immunosuppressed mice. CPP (80 mg/kg) was administered intraperitoneally to all experimental groups except for the normal group from Day 1 to Day 3 of experiments to induce immunosuppression in BALB/c mice. ANE or FAN was orally administered for 14 days at three different doses (50, 150, and 300 mg/kg). Samples were obtained on the last day of experiments and weighed. The relative weights of (A) spleen, (B) thymus, (C) liver, and (D) kidney were calculated as follows: relative weights (%) = (organ weight/body weight) × 100. Data were presented as mean ± SD (n = 6 per group). ### p < 0.001, compared to the normal group; * p < 0.05, ** p < 0.01, and *** p < 0.001, compared to the CPP-induced immunosuppressed group. CPP, cyclophosphamide; ANE, Angelica gigas Nakai extract; FAN, yeast-fermented Angelica gigas Nakai extract; Leva, levamisole hydrochloride; BW, body weight
AGN increases NK cell activity and splenocyte proliferation in primary cultured splenocytes
NK cells have been recognized as a crucial regulator in the immune system, and a major target for immune enhancement (Pierce et al., 2020). We examined the effect of ANE and FAN on NK cell activity in primary cultured splenocytes isolated from mice using YAC-1 lymphoma cells as target cells. CPP injection significantly reduced splenic NK cell activity compared to that of the normal control, whereas ANE and FAN administration reversely increased its activity to eliminate YAC-1 lymphoma cells (Fig. 3A). In addition, we investigated whether ANE and FAN affect T cell and B cell proliferative responses using cultured splenocytes treated with ConA or LPS. Lymphocyte proliferation has usually been utilized as an alternative marker for cellular and humoral adaptive immunity (Bai et al., 2020). ConA and LPS have been used as representative mitogens to induce T cell and B cell proliferation, respectively (Coutinho et al., 1973). CPP administration inhibited LPS- and ConA-stimulated proliferation in mice, however, administration of ANE or FAN significantly reversed the suppressed splenocyte proliferation in a dose-dependent manner (Fig. 3B). These data indicate that ANE and FAN could enhance humoral and cell-mediated immune responses by stimulating B cell activation and T cell proliferation.
Fig. 3.

Effects of ANE and FAN on NK cell activity and splenocyte proliferation in primary cultured mouse splenocytes. (A) Mouse splenocytes and YAC-1 cells were seeded onto a 96-well plate at a ratio of 10:1. Splenocytes and YAC-1 cells were used as effector cells and target cells, respectively. After 24 h, cell viability was evaluated by MTT assay, and NK cell activity was calculated. (B) Splenocytes were seeded onto 96-well plates (1 × 105 cells/well). LPS and ConA were treated, respectively, and incubated for 48 h. Cell viability was evaluated by MTT assay. Data were presented as mean ± SD (n = 6 per group). ## p < 0.01, and ### p < 0.001, compared to the normal group; * p < 0.05, ** p < 0.01, and *** p < 0.001, compared to the CPP-induced immunosuppressed group. CPP, cyclophosphamide; ANE, Angelica gigas Nakai extract; FAN, yeast-fermented Angelica gigas Nakai extract; Leva, levamisole hydrochloride; NK cell, natural killer cell; LPS, lipopolysaccharide; ConA, concanavalin A
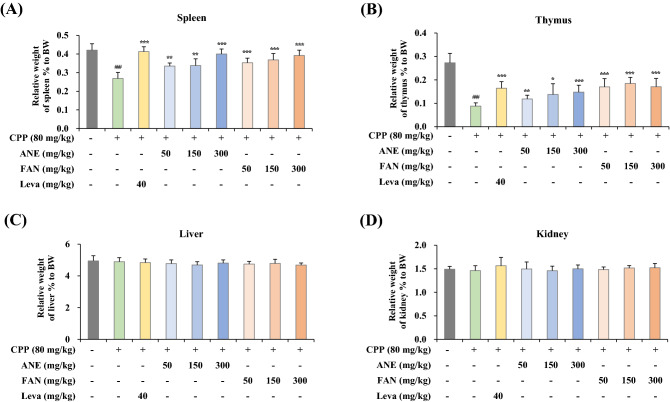
AGN increases the release of cytokines in primary cultured splenocytes
As shown previously in Fig. 1C, ANE and FAN increased the mRNA levels of cytokines such as Tnfa, Il6, Il1b, Ifnr, Il2, and Il12 in splenic tissues. To ascertain the release of these cytokines by administration of ANE or FAN, we performed ELISA using the culture supernatant of mouse splenocytes. Splenocytes were treated with either LPS or ConA for 48 h to induce differentiation into B cells or T cells. As shown in Fig. 4, all cytokine levels in LPS- and ConA-treated splenocytes were significantly reduced in the CPP-administered model group when compared with those of the normal group. However, the decreased levels were reversed in ANE- and FAN-administered groups. These data revealed that ANE and FAN could increase the secretion of cytokines like TNF-α, IL-6, IL-1β, IFN-γ, IL-2, and IL-12 as well as their gene expression.
Fig. 4.
Effects of ANE and FAN on cytokine level changes in primary cultured mouse splenocytes. Splenocytes isolated from the excised spleen were seeded onto a 6-well plate (3 × 106 cells/well) for 48 h, and then the supernatant was collected. The levels of cytokines such as (A) TNF-α, (B) IL-6, (C) IL-1β, (D) IFN-γ, (E) IL-2, and (F) IL-12 were measured using ELISA kits. Data were presented as mean ± SD (n = 6 per group). # p < 0.05, ## p < 0.01, and ### p < 0.001, compared to the normal group; * p < 0.05, ** p < 0.01, and *** p < 0.001, compared to the CPP-induced immunosuppressed group. CPP, cyclophosphamide; ANE, Angelica gigas Nakai extract; FAN, yeast-fermented Angelica gigas Nakai extract; Leva, levamisole hydrochloride; TNF-α, tumor necrosis factor alpha; IL-6, interleukin-6; IL-1β, interleukin-1 beta; IFN-γ, interferon gamma; IL-2, interleukin-2; IL-12, interleukin-12; LPS, lipopolysaccharide; ConA, concanavalin A
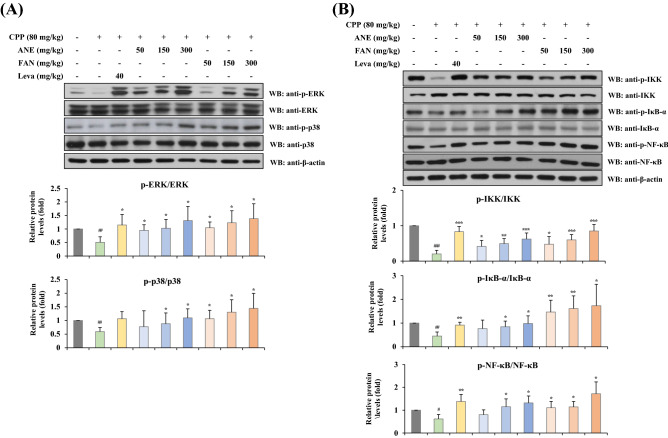
MAPK/NF-κB signaling pathways are related to the immunostimulatory effects of AGN in CPP-induced immunosuppressed mice
Previous studies have reported that MAPK and NF-κB cascade pathways are responsible for stimulating the immune system by upregulating gene expressions of many factors, including cytokines related to immune and inflammatory responses (Yang et al., 2020). To elucidate the underlying mechanism of ANE and FAN for modulating cytokine secretion, we investigated whether the administration of ANE or FAN in immunosuppressed mice affects the phosphorylation of signal transduction molecules associated with the MAPK/NF-κB signaling pathway using primary cultured splenocytes. Western blot analysis was performed to detect the phosphorylated signaling molecules. As shown in Fig. 5, the decreased phosphorylated levels of ERK, p38, IKK, IκB-α, and NF-κB in the CPP model group were significantly restored in the ANE- and FAN-administered groups. The MAPK signaling pathway is associated with the activation of the transcription factor NF-κB, which in turn regulate multifaceted immune functions via the transcriptional expression of immune-related genes. The suppression of ERK and p38 MAPK pathways decreased transactivation potential of the p65 κB subunit in a GAL4 one-hybrid system, although it remains unclear whether MAPKs directly act on the p65 transcriptional complex, or by phosphorylation of intermediate kinases (Berghe et al., 1998). This finding suggests that ERK and p38 MAPK pathways may be cooperative mechanisms required for NF-κB p65 independent from IκB regulation. When not stimulated, NF-κB exists as an inactive complex bound to its inhibitory protein IκB-α in the cytosol. When exposed to extracellular stimuli, IκB-α is phosphorylated by IKKαβγ and rapidly degraded by proteasomal degradation. When IκB-α is degraded and the NF-κB/IκB-α complex is dismantled, NF-κB release as a heterodimer composed of p65 and p50 subunit. Released NF-κB is activated by phosphorylation at serine, threonine, and tyrosine residues of p65. Then, the activated NF-κB is translocated to the nucleus and upregulates gene expression of enzymes and mediators involved in immune responses (Vallabhapurapu and Karin, 2009; Zandi et al., 1997). This study demonstrated that ANE and FAN promote the phosphorylation of ERK, p38, IKK, IκB-α, and NF-κB in mouse splenocytes, and activate the MAPK/NF-κB signaling pathway, followed by leading to upregulation of cytokines and enzymes in response to immune and inflammatory stimulation. It is suggested that the immunostimulatory effect of ANE and FAN might be associated with the activation of the ERK/p38 MAPK and NF-κB signaling pathways.
Fig. 5.
Effects of ANE and FAN on the activation of MAPK and NF-κB signaling pathways in CPP-induced immunosuppressed mice. Total proteins were isolated from splenocytes f Leva, levamisole;or western blot analysis. (A) The relative protein expressions of p-ERK and p-p38 were normalized to ERK and p38, respectively. (B) The relative protein expressions of p-IKK, p-IκB-α, and p-NF-κB were normalized to IKK, IκB-α, and NF-κB, respectively. Data were presented as the mean ± SD (n = 4–6 per group). ## p < 0.01 and ### p < 0.001, compared to the normal group; * p < 0.05, ** p < 0.01, and *** p < 0.001, compared to the CPP-induced immunosuppressed group. CPP, cyclophosphamide; ANE, Angelica gigas Nakai extract; FAN, yeast-fermented Angelica gigas Nakai extract; Leva, levamisole hydrochloride; ERK, extracellular signal-regulated kinase; IKK, inhibitor kappa B kinase; IκBα, inhibitor kappa B-alpha; NF-κB, nuclear factor-kappa B
In this study, we demonstrated that AGN administration could restore CPP-induced immunosuppression in mice, but did not find out a significant difference in the immune-enhancement effect between ANE and FAN. In spite of an increase in physiologically active ingredients such as decursin and decursinol angelate during fermentation, FAN did not seem to further enhance the immunostimulatory effects. The reason is presumed to be related to the consumption of saccharides used as an energy source for yeast growth. Since a saccharide derived from A. gigas extracts has been reported to trigger immune responses, its consumption by yeast fermentation may reduce immunological activity, leading to offset the increased immune activity caused by FAN administration in mice.
AGN activated ERK/p38 MAPK and NF-κB signaling pathways, and increased the production of NO and immunostimulatory cytokines like TNF-α, IL-6, IL-1β, IFN-γ, IL-2, and IL-12 in CPP-induced immunosuppressed mice. The activation of MAPK/NF-κB signaling pathways is closely associated with the increased level of NO and cytokines, which plays a critical role in the immunostimulatory function of AGN. Also, it enhanced splenic NK cell activity and splenocyte proliferation, suggesting that AGN might enhance the immune system, in particular, humoral and cell-mediated immune responses by stimulating B cell activation and T cell proliferation as well as the innate immune response. In conclusion, our overall findings revealed the immune-enhancement potential of AGN as an immunostimulant under immunosuppressed conditions. Therefore, we proposed that AGN could be used as an effective agent or a dietary supplement for improving immunity.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the Policy for Upgrading the Competitiveness of Industrial Complexes of the Korea Industrial Complex Corporation (KICOX) Grant funded by the Ministry of Trade, Industry and Energy (MOTIE) (No. PKK21009).
Declarations
Conflict of interest
No competing financial interests exist.
Ethical approval
All animal experimental procedures were carried out based on the guidelines on ethical use and were accomplished with the approval of the guideline of the Institutional Animal Care and Use Committee at WOOJUNGBIO, Inc. (Approval No. IACUC2103-013) (Suwon, South Korea).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jeongho Jeong, Email: jhj@eundan.co.kr.
Mi Kyung Lim, Email: mklim@eundan.co.kr.
Eun Hye Han, Email: ehhan@eundan.co.kr.
Sang Ho Lee, Email: shlee@eundan.co.kr.
Soyeon Lee, Email: solotus22@gmail.com.
References
- Ashraf R, Shah NP. Immune system stimulation by probiotic microorganisms. Critical Reviews in Food Science and Nutrition. 2014;54:938–956. doi: 10.1080/10408398.2011.619671. [DOI] [PubMed] [Google Scholar]
- Bai RB, Zhang YJ, Fan JM, Jia XS, Li D, Wang YP, Zhou J, Yan Q, Hu FD. Immune-enhancement effects of oligosaccharides from Codonopsis pilosula on cyclophosphamide induced immunosuppression in mice. Food & Function. 2020;11:3306–3315. doi: 10.1039/C9FO02969A. [DOI] [PubMed] [Google Scholar]
- Belardelli F, Ferrantini M. Cytokines as a link between innate and adaptive antitumor immunity. Trends in Immunology. 2002;23:201–208. doi: 10.1016/S1471-4906(02)02195-6. [DOI] [PubMed] [Google Scholar]
- Berghe WV, Plaisance S, Boone E, Bosscher KD, Schmitz ML, Fiers W, Haegeman G. p38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways are required for nuclear factor-κB p65 transactivation mediated by tumor necrosis factor. Journal of Biological Chemistry. 1998;273:3285–3290. doi: 10.1074/jbc.273.6.3285. [DOI] [PubMed] [Google Scholar]
- Beutler B, Cerami A. The biology of cachectin/TNF–a primary mediator of the host response. Annual Review of Immunology. 1989;7:625–655. doi: 10.1146/annurev.iy.07.040189.003205. [DOI] [PubMed] [Google Scholar]
- Bogdan C. Nitric oxide and the immune response. Nature Immunology. 2001;2:907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- Bravo PLW, Jin H, Park H, Kim MS, Matsui H, Lee H, Suh JW. Antithrombotic effect of the ethanol extract of Angelica gigas Nakai (AGE 232). Life (Basel). 11 (2021) [DOI] [PMC free article] [PubMed]
- Brodin P, Davis MM. Human immune system variation. Nature Reviews Immunology. 2017;17:21–29. doi: 10.1038/nri.2016.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaillon JM. Cytokines and macrophages. Biomedicine & Pharmacotherapy. 1994;48:445–453. doi: 10.1016/0753-3322(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Coutinho A, Möller G, Andersson J, Bullock WW. In vitro activation of mouse lymphocytes in serum-free medium: effect of T and B cell mitogens on proliferation and antibody synthesis. European Journal of Immunology. 1973;3:299–306. doi: 10.1002/eji.1830030509. [DOI] [PubMed] [Google Scholar]
- Han NR, Kim KC, Kim JS, Ko SG, Park HJ, Moon PD. The immune-enhancing effects of a mixture of Astragalus membranaceus (Fisch.) Bunge, Angelica gigas Nakai, and Trichosanthes Kirilowii (Maxim.) or its active constituent nodakenin. Journal of Ethnopharmacology. 285: 114893 (2022) [DOI] [PubMed]
- Hirayama D, Iida T, Nakase H. The phagocytic function of macrophage-enforcing innate immunity and tissue homeostasis. International Journal of Molecular Sciences. 19 (2017) [DOI] [PMC free article] [PubMed]
- Hwang I. Cell-cell communication via extracellular membrane vesicles and its role in the immune response. Molecules and Cells. 2013;36:105–111. doi: 10.1007/s10059-013-0154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannello A, Raulet DH. Immune surveillance of unhealthy cells by natural killer cells. Cold Spring Harbor Symposia on Quantitative Biology. 2013;78:249–257. doi: 10.1101/sqb.2013.78.020255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgovanovic D, Song M, Wang L, Zhang Y. Roles of IFN-gamma in tumor progression and regression: a review. Biomarker Research. 2020;8:49. doi: 10.1186/s40364-020-00228-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TH, Lee SJ, Rim HK, Shin JS, Jung JY, Heo JS, Kim JB, Lee MS, Lee KT. In vitro and in vivo immunostimulatory effects of hot water extracts from the leaves of Artemisia princeps Pampanini cv. Sajabal. Journal of Ethnopharmacology. 2013;149:254–262. doi: 10.1016/j.jep.2013.06.030. [DOI] [PubMed] [Google Scholar]
- Kim SH, Lee SW, Park HJ, Lee SH, Im WK, Kim YD, Kim KH, Park SJ, Hong S, Jeon SH. Anti-cancer activity of Angelica gigas by increasing immune response and stimulating natural killer and natural killer T cells. BMC Complementary and Alternative Medicine. 2018;18:218. doi: 10.1186/s12906-018-2277-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Shin JS, Chung KS, Han HS, Lee HH, Lee JH, Kim SY, Ji YW, Ha Y, Kang J, Rhee YK, Lee KT. Immunostimulatory effects of live Lactobacillus sakei K040706 on the CYP-induced immuno suppression mouse model. Nutrients. 12 (2020) [DOI] [PMC free article] [PubMed]
- Lee S, Shin DS, Kim JS, Oh KB, Kang SS. Antibacterial coumarins from Angelica gigas roots. Archives of Pharmacal Research. 2003;26:449–452. doi: 10.1007/BF02976860. [DOI] [PubMed] [Google Scholar]
- Ma X, Trinchieri G. Regulation of interleukin-12 production in antigen-presenting cells. Advances in Immunology. 2001;79:55–92. doi: 10.1016/S0065-2776(01)79002-5. [DOI] [PubMed] [Google Scholar]
- Nathan C, Xie QW. Regulation of biosynthesis of nitric oxide. Journal of Biological Chemistry. 1994;269:13725–13728. doi: 10.1016/S0021-9258(17)36703-0. [DOI] [PubMed] [Google Scholar]
- Noh BY, Lee HJ, Do JR, Kim HK. Antioxidant and ACE inhibitory activity of cultivated and wild Angelica gigas nakai extracts prepared using different extraction conditions. Preventive Nutrition and Food Science. 2014;19:274–280. doi: 10.3746/pnf.2014.19.4.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin J, Cohen B. An overview of the immune system. Lancet. 2001;357:1777–1789. doi: 10.1016/S0140-6736(00)04904-7. [DOI] [PubMed] [Google Scholar]
- Pierce S, Geanes ES, Bradley T. Targeting natural killer cells for improved immunity and control of the adaptive immune response. Frontiers in Cellular and Infection Microbiology. 2020;10:231. doi: 10.3389/fcimb.2020.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renoux G. The general immunopharmacology of levamisole. Drugs. 1980;20:89–99. doi: 10.2165/00003495-198020020-00001. [DOI] [PubMed] [Google Scholar]
- Simpson RJ, Hammacher A, Smith DK, Matthews JM, Ward LD. Interleukin-6: structure-function relationships. Protein Science. 1997;6:929–955. doi: 10.1002/pro.5560060501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slawinska A, Siwek M, Zylinska J, Bardowski J, Brzezinska J, Gulewicz KA, Nowak M, Urbanowski M, Plowiec A, Bednarczyk M. Influence of synbiotics delivered in ovo on immune organs development and structure. Folia Biologica-Krakow. 2014;62:277–285. doi: 10.3409/fb62_3.277. [DOI] [PubMed] [Google Scholar]
- Sowndhararajan K, Kim S. Neuroprotective and cognitive enhancement potentials of Angelica gigas nakai root: A review. Scientia Pharmaceutica. 85 (2017) [DOI] [PMC free article] [PubMed]
- Tripathi P, Tripathi P, Kashyap L, Singh V. The role of nitric oxide in inflammatory reactions. FEMS Immunology and Medical Microbiology. 2007;51:443–452. doi: 10.1111/j.1574-695X.2007.00329.x. [DOI] [PubMed] [Google Scholar]
- Tulotta C, Ottewell P. The role of IL-1B in breast cancer bone metastasis. Endocrine-Related Cancer. 2018;25:R421–R434. doi: 10.1530/ERC-17-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annual Review of Immunology. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- Wei B, Zhang R, Zhai J, Zhu J, Yang F, Yue D, Liu X, Lu C, Sun X. Suppression of Th17 cell response in the alleviation of dextran sulfate sodium-induced colitis by ganoderma lucidum polysaccharides. Journal of Immunology Research. 2018;2018:2906494. doi: 10.1155/2018/2906494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan JJ, Kim DH, Moon YS, Jung JS, Ahn EM, Baek NI, Song DK. Protection against beta-amyloid peptide-induced memory impairment with long-term administration of extract of Angelica gigas or decursinol in mice. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2004;28:25–30. doi: 10.1016/S0278-5846(03)00168-4. [DOI] [PubMed] [Google Scholar]
- Yang Y, Wang D, Li Q, He J, Wang B, Li J, Zhang A. Immune-enhancing activity of aqueous extracts from Artemisia rupestris L. via MAPK and NF-kB pathways of TLR4/TLR2 downstream in dendritic cells. Vaccines (Basel). 8 (2020) [DOI] [PMC free article] [PubMed]
- Zandi E, Rothwarf DM, Delhase M, Hayakawa M, Karin M. The IkappaB kinase complex (IKK) contains two kinase subunits, IKKalpha and IKKbeta, necessary for IkappaB phosphorylation and NF-kappaB activation. Cell. 1997;91:243–252. doi: 10.1016/S0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.