Abstract
Chytridiomycosis, caused by the chytrid fungus Batrachochytrium dendrobatidis (Bd), has caused extreme losses in amphibian biodiversity. Finding bacteria that produce metabolites with antifungal properties may turn out to be invaluable in the fight against this devastating disease. The entomopathogenic bacteria, Xenorhabdus szentirmaii and X. budapestensis produce secondary metabolites that are effective against a wide range of fungal plant pathogens. To assess whether they may also be effective against Bd, we extracted cell-free culture media (CFCM) from liquid cultures of X. szentirmaii and X. budapestensis and tested their ability to inhibit Bd growth in vitro. As a second step, using juvenile common toads (Bufo bufo) experimentally infected with Bd we also tested the in vivo antifungal efficacy of X. szentirmaii CFCM diluted to 2 and 10% (v/v), while also assessing possible malign side effects on amphibians. Results of the in vitro experiment documented highly effective growth inhibition by CFCMs of both Xenorhabdus species. The in vivo experiment showed that treatment with CFCM of X. szentirmaii applied at a dilution of 10% resulted in infection intensities reduced by ca. 73% compared to controls and to juvenile toads treated with CFCM applied at a dilution of 2%. At the same time, we detected no negative side effects of treatment with CFCM on toad survival and development. Our results clearly support the idea that metabolites of X. szentirmaii, and perhaps of several other Xenorhabdus species as well, may prove highly useful for the treatment of Bd infected amphibians.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13568-023-01585-0.
Keywords: Amphibian pathogen, Antifungal treatment, Antimicrobial, Bioaugmentation, Chemical-free disinfection, Skin microbiota
Key points
First report of high anti-Bd efficacy of Xenorhabdus metabolites in vitro.
Metabolites of X. szentirmaii can be applied effectively on live toads.
Application of X. szentirmaii metabolites on live toads was safe.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13568-023-01585-0.
Introduction
Amphibians have suffered rapid biodiversity loss over the last decades and became one of the most threatened vertebrate groups (Monastersky 2014). The main causes of this decline are climate change, pollution, habitat loss, and emerging infectious diseases (Wake and Vredenburg 2008; Hof et al. 2011; Campbell Grant et al. 2016). Chytridiomycosis is the most serious emerging disease affecting amphibians caused by the chytrid fungi Batrachochytrium dendrobatidis (Bd) and Batrachochytrium salamandrivorans (Bsal). This disease has already led to the decline or extinction of several hundred species and continues to cause mass mortality events on five continents (Scheele et al. 2019). Because Bsal has only been discovered recently (Martel et al. 2013) and its distribution range is confined to date to the northwest of continental Europe (Spitzen-van der Sluijs et al. 2016; González et al. 2019), here we concentrate on the better known and globally distributed Bd. The fungus infects keratinous epidermal layers of the skin (Berger et al. 1998). The symptoms of the disease are therefore the intensive sloughing or skin shedding, reddening on legs and ventral surfaces even ulcerations or skin lesions. The structural damage of the skin can impair skin-breathing and osmoregulation, provoking shifts in electrolyte balance and finally leading to cardiac asystolic death in metamorphosed amphibians (Voyles et al. 2009). Since its global appearance, several countermeasures against chytridiomycosis have been proposed (Johnson et al. 2003; Woodhams et al. 2003; Harris et al. 2006; Woodward et al. 2014; Hettyey et al. 2019) but finding a widely applicable mitigation method has remained one of the most challenging goals of animal conservation (Garner et al. 2016; Scheele et al. 2019).
Amphibians have a broad repertoire for defence against pathogens via thermoregulatory behaviour (behavioural fever; Sherman et al. 1998; Richards-Zawacki 2010; Murphy et al. 2011) and their highly developed adaptive and innate immune systems (Carey et al. 1999; Grogan et al. 2018). Intrinsic factors of the adaptive immune system, such as the major histocompatibility complex class II (MHCII) can play important roles in determining the susceptibility of the host to pathogens (Barribeau et al. 2008; Bataille et al. 2015; Savage and Zamudio 2016). The innate immune system consists of cells with the function of absorbing and presenting antigens to the adaptive immune system. The complement system (humoral part of the innate immune system) leads the chemotaxis of phagocytes and aids the penetration of prokaryote and fungal cell membranes (Carey et al. 1999; Speth et al. 2008). However, the first line of defence against invading pathogens are the skin-secreted defensive chemicals such as antimicrobial peptides, steroids, alkaloids, and biogenic amines (Daly 1995; Macfoy et al. 2005; Gomes et al. 2007; Tempone et al. 2007; König et al. 2015), and mutualistic skin bacteria, that can prevent infections or disease propagation (Belden and Harris 2007; Krynak et al. 2016). Nonetheless, while their natural defence mechanisms are normally effective against pathogens and parasites, emerging infectious diseases caused by introduced pathogens can have devastating effects on amphibian populations, especially when they act in concert with other stress factors (Koprivnikar 2010; Campbell Grant et al. 2016).
Bioaugmentation, which is the restoration or enrichment of the microbiota to provide additional defences against pathogens, has been found to be useful in agriculture (Patterson and Burkholder 2003; Gentry et al. 2004), aquaculture (Olsson et al. 1992), and in the conservation of corals (Teplitski and Ritchie 2009). Addition or supplementation of mutualistic skin bacteria could be a promising method to mitigate the impact of chytridiomycosis as well (Harris et al. 2009; Bletz et al. 2013; Rebollar et al. 2020). As described in natural habitats, the presence of certain microbial taxa (e.g., species of Janthinobacterium, Lysobacter, Pseudomonas genera) may enhance population resistance to chytridiomycosis in some amphibian species (Woodhams et al. 2007; Lam et al. 2010; Walke et al. 2011; Rebollar et al. 2016a). Bacterial secondary metabolites produced by these microbes associated with the amphibian skin inhibited Bd growth effectively in vitro (Woodhams et al. 2007; Brucker et al. 2008a, b; Myers et al. 2012). Furthermore, the presence of some of the above mentioned bacterial taxa also reduced infection intensities and enhanced the survival of animals in vivo (Becker et al. 2009; Muletz et al. 2012). However, other studies delivered mixed results reporting moderate, or no effect of bioaugmentation (Woodhams et al. 2012; Küng et al. 2014; Rebollar et al. 2016b). Research on bioaugmentation against chytridiomycosis mainly focused on the establishment of certain bacteria producing antifungal compounds or entire bacterial communities on the amphibian skin (Rebollar et al. 2020). However, this approach also has several limitations. The introduction of new bacteria can induce disadvantageous changes in the hosts’ microbiome, they can trigger immune responses in hosts, and environmental conditions varying across time and space can differentially affect microbial community structure and function, and the effectiveness of defence against pathogens (Daskin et al. 2014; Robak and Richards-Zawacki 2018; Woodhams et al. 2018; Rebollar et al. 2020). Additionally, the presence of Bd can also change the structure of the skin microbial community, possibly by suppressing the growth of the beneficial bacteria (Jani and Briggs 2014; Woodhams et al. 2018). Furthermore, the introduction of bacteria to ecosystems where they have not been present before can be hazardous (Simberloff et al. 2013).
Utilizing bacterial metabolites directly against Bd instead of trying to establish live cultures on amphibian hosts has also been tested (Bell et al. 2013; Madison et al. 2017). Without the need for the establishment of live cultures on amphibian skin, this approach may be applied more easily and safely than bioaugmentation. Treatment can be safely controlled and better standardized because the amount of antifungal metabolites can be adjusted so as not to be harmful to amphibians. Finally, the scope of the search for antifungal metabolites with broad-spectrum inhibition capabilities can be widened to cover novel microbial sources, even of non-amphibian origin.
The idea of using wide-spectrum antimicrobials produced by the entomopathogenic nematode-bacterium (EPN-EPB) symbiotic complexes (Akhurst 1982) was first suggested by Bode (2009). Entomopathogenic nematode species belonging to the Steinernema and Heterorhabditis genera are parasites of soil-dwelling insects. Their infective dauer juveniles (IJ, Kaya 1978), carry cells of species-specific obligate entomopathogenic bacterial (EPB) symbionts. Right after the IJ enters the insect body cavity, it releases the EPBs into the hemocoel, where the bacteria start to propagate and synthesize efficient insecticide toxins and various secondary metabolites which suppress the host’s immune response, and accelerate its death. The symbiotic bacteria also produce antimicrobial metabolites which protect the cadaver against microbial food competitors (Forst et al. 1997) keeping pathobiome conditions (Ogier et al. 2020) favourable for the EPN-EPB symbiotic complex in the cadaver and ambient soil. Xenorhabdus szentirmaii and X. budapestensis (Lengyel et al. 2005), the natural symbionts of the EPN species Steinernema rarum (de Doucet 1986) and S. bicornutum (Tallósi et al. 1995) respectively, seem to be the two most potent EPB strains against bacterial, fungal, and protozoan pathogens of plants, livestock, and even humans (Furgani et al. 2008; Böszörményi et al. 2009; Vozik et al. 2015; Wenski et al. 2020; Fodor et al. 2022). Of the antimicrobial peptides (AMPs) produced by these Xenorhabdus species, fabclavine (Fuchs et al. 2012, 2014) and its metabolic derivatives (Wenski et al. 2019, 2020; Watzel et al. 2021) exhibit exceptional antimicrobial potential against different targets (Cimen et al. 2021). This molecular arsenal in combination with the cheap and easy-to-handle cultivation under laboratory conditions, as well as the accessibility for genetic manipulations make Xenorhabdus species and their metabolites great candidates for the fight against diseases of amphibians, including chytridiomycosis.
In this study, we aimed to assess experimentally whether Xenorhabdus szentirmaii and X. budapestensis metabolites may be effective against Bd. We extracted cell-free culture media (CFCM) from their liquid cultures and assessed Bd growth inhibition capabilities in vitro as the practical first step towards finding the range of quantity required for the suppression of Bd. We also tested for possible toxic effects and treatment efficacy of X. szentirmaii CFCM in vivo on juvenile common toads (Bufo bufo) experimentally infected with Bd. Since the integrity of the skin microbiome is crucial for amphibian health and mitigation methods should not disrupt the microbial community present on the amphibian skin (Rebollar et al. 2020), we also observed possible changes in skin microbial community structure caused by treatments. Treatment with CFCM instead of inoculation with living bacterial cells allows to avoid the abovementioned problems arising from interactions with the host’s immune system and from the environment-dependence of the establishment and metabolite production of probiotic bacteria (Rebollar et al. 2020). Thus, the application of antifungal metabolites may minimise undesirable side-effects on the targeted as well as on non-target species and on ecosystem processes (Bletz et al. 2013).
Methods
Culturing of Xenorhabdus
We tested two previously described antimicrobial peptide (AMP)-producing EPB strains: Xenorhabdus budapestensis nov. DSM-16342T from Central Europe, and X. szentirmaii nov. DSM-16338T of South American origin, described in the Department of Genetics of Eötvös University Hungary and deposited in DSMZ, Braunschweig, Germany by Katalin Lengyel and her colleagues (Lengyel et al. 2005).
For culturing Xenorhabdus, we prepared Mueller–Hinton liquid medium (Mueller and Hinton 1941) by dissolving 21 g powder (obtained from Sigma-Aldrich, St. Louis, USA) in 1000 ml of distilled water and sterilized it by autoclaving at 121 °C for 15 min before use. We alternatively cultured Xenorhabdus species on Luria broth agar (LBA) plates flooded with Luria broth (LB) (10 g casein peptone, 5 g yeast extract, 10 g sodium chloride, and 17 g agar [LB and LBA, respectively] dissolved in 1000 ml distilled water) as described by Ausubel and colleagues (1999). Indicator plates (LBTA) were supplemented with bromothymol blue and 2,3,5-Triphenyltetrazolium chloride, and were used to distinguish AMP producing (phase I) and non-producing (phase II) variants (Leclerc and Boemare 1991). Fresh single phase I colonies derived from frozen bacterial stocks were used for each experiment as previously described (Furgani et al. 2008; Böszörményi et al. 2009; Vozik et al. 2015). Microbiological media were obtained from Biolab Zrt. (Budapest, Hungary).
We cultured EPBs in liquid TGhLY medium (mTGhLY; 8 g tryptone, 2 g gelatine-hydrolysate, 4 g lactose, and 5 g yeast extract in 1000 ml distilled water) with a 7-day old single colony grown on LBA. With the addition of yeast extract, we established this method to provide optimal growth conditions in the same media for both the EPB strains and Bd. In all other respects, this media is equivalent to the TGhL media used for the culturing of Bd (see below). Each Xenorhabdus culture in this study started with 5–10 ml of (either LB or Mueller-Hinton) liquid medium inoculated with a single colony of the respective bacterium picked from an LBTA indicator plate and incubated overnight at 28 °C in a water bath shaker (Lab-Line Orbital Shaker Water Bath, Marshall Scientific, USA). Each late-log phase inoculum was then added to 200 ml mTGhLY into 400 ml tissue culture flasks to create scale-up cultures.
Preparation of cell-free culture media (CFCMs)
We incubated scale-up cultures of both Xenorhabdus species for 7 days at 25 °C on an orbital shaker platform (Gallencamp, UK) and centrifuged cultures at 6000 rpm for 20 min at 4 °C in 400 ml tubes using a JLA-10.500 type rotor (Avanti centrifuge J-26 XPI, Beckman Coulter, Indianapolis, USA). With these preparation conditions, production of antibiotic metabolites in Xenorhabdus cultures reaches a stationary phase in 5–6 days, containing the same amount of metabolites (Furgani et al. 2008; Böszörményi et al. 2009; Vozik et al. 2015). The supernatant was filtered through a sterile 0.22 μm nylon filter and centrifuged again at the same speed. We considered the resulting supernatant to be a cell-free culture medium (CFCM) of the antibiotic-producing Xenorhabdus strains and used it for bioassays. To confirm that CFCM-s were indeed cell-free, we diluted at least two replicates of each with sterile 2× LB, incubated them along with the experimental samples, and checked for bacterial growth on LBA plates. We stored CFCMs at 4 °C in glass bottles until further use.
Maintaining and culturing of Bd
For all experiments, we used the global pandemic lineage (GPL) of Bd. The isolate (Hung_2014) originated from a Bombina variegata collected in 2014 by J. Vörös (Natural History Museum, Budapest, Hungary) in the Bakony Mountains, Hungary, and isolated by M.C. Fisher and colleagues (Imperial College London, London, UK). We maintained cultures in TGhL medium (mTGhL; 8 g tryptone, 2 g gelatine-hydrolysate, and 4 g lactose in 1000 ml distilled water) in 25 cm2 cell culture flasks at 4 °C and passaged them every three months into sterile mTGhL.
One week before the start of in vitro tests, we placed 2–2 ml of Bd stock cultures onto mTGhL agar plates (containing 1% agar w/v) in sterile plastic Petri dishes (90 mm diameter; Biolab Zrt) and incubated them at 20 °C for 7 days. Then we flooded the plates with 2 ml 1% tryptone medium (10 g tryptone in 1000 ml distilled water). After 30 min we collected the liquid media containing the zoospores and rinsed the plates with another 500 µl of 1% tryptone medium which we added to the previously obtained media. We estimated zoospore concentrations in the harvested media (also containing some zoosporangia) using a Bürker chamber at ×400 magnification and adjusted to 107 zoospores (zsp)/ml in 1% tryptone medium.
One week before performing experimental infections in the in vivo experiment, we inoculated 100 ml mTGhLY with 2 ml of Bd stock culture in a 175 cm2 cell culture flask and incubated it for seven days at 21 °C. We assessed the concentration of intact zoospores using a Bürker chamber at ×400 magnification and diluted the zoospore suspension with mTGhLY to a final concentration of 106 zsp/ml and subsequently used this for the inoculation of amphibian individuals.
In vitro experiment
We tested the Bd growth inhibition capability of both Xenorhabdus CFCMs using optical density measurement, which is a semiquantitative test that provides more reliable results than agar diffusion tests (Bell et al. 2013). We prepared a 10-step twofold serial dilution starting with CFCM solution diluted with mTGhLY to 50% (v/v) on two flat bottom 96 well microplates (Orange Scientific, Braine-l’Alleud, Belgium) in a final volume of 50 µl. Then we added 50 µl zoospore suspension in 1% tryptone at a concentration of 107 zsp/ml to the CFCM-containing wells (resulting in a final concentration of 5 × 106 zsp/ml). Each plate also included three positive and three negative control wells containing 50 µl sterile mTGhLY and 50 µl intact or heat-killed (80 °C for 30 min) zoospore suspension in 1% tryptone. We incubated plates at 20 °C for 7 days in closed plastic boxes (30 × 15 × 10 cm). To prevent desiccation, we lined boxes with wet paper towels. After 7 days of incubation, we measured the optical density at 492 nm (OD492) using a Multiskan MS 352 microplate reader (Thermo Fisher Scientific, Waltham, USA).
In vivo experiment
In March 2021 we set up 48 mesocosms by filling plastic boxes (85 × 57 × 51 cm) placed outdoors with 130 l of aged tap water and supplementing them with 50 g beech leaves for spatial complexity, and one litre of pond water containing bacterio-, phyto- and zooplankton. Four weeks later we added another 6 dl pond water to each mesocosm to boost zooplankton density and thereby reduce algal bloom. In April, we collected 200 eggs from each of four freshly laid egg strings of B. bufo from three localities near Budapest (Békás-tó: 47.5763° N, 18.869° E; Ilona-tó: 47.7135° N, 19.0402° E; Jávor-tó: 47.7138° N, 19.0196° E). We transported eggs to the Experimental Station Juliannamajor of the Plant Protection Institute, Centre for Agricultural Research located on the outskirts of Budapest (47.5479° N, 18.9349° E). We placed eggs of each clutch separately (families hereafter) into plastic boxes (32 × 22 × 16 cm) holding 0.7 l of reconstituted soft water (RSW; USEPA 2002) at a constant temperature of 16 °C and a light : dark cycle adjusted weekly to the conditions outside. Nine days after hatching, when all larvae reached development stage 25 (Gosner 1960), we released 50 individuals into each outdoor mesocosm (four mesocosms per family). The self-sustaining environments provided food and other nutrients for tadpoles without the need for any intervention until metamorphosis. For a schematic representation of the course of the experiment please see Fig S1.
Upon metamorphosis, when tadpoles reached development stage 42 (emergence of forelimbs), we monitored mesocosms daily and placed metamorphosing individuals into transparent plastic boxes (52 × 35 × 25 cm) containing 1.5 l of mesocosm water and covered with perforated lids. We tilted these boxes to provide a dry surface as well. When metamorphosis was complete (development stage 46; complete tail resorption), we haphazardly chose 25 toadlets from each family that metamorphosed on the same day and moved them into one rearing container per family (60 × 40 × 30 cm). These plastic containers were lined with 6 l of wet wooden soil, covered with a mixture of wet moss and leaf litter at a height of 6–8 cm. We covered containers with a perforated lid and placed them outdoors in an area shaded by trees. We watered containers weekly using RSW and fed juvenile toads ad libitum with springtails (Folsomia sp.) and small (2–3 mm) crickets (Acheta sp.) sprinkled with a 3:1 mixture of Reptiland 76,280 (Trixie Heimtierbedarf GmbH & Co. KG, Tarp, Germany) and Promotor 43 (Laboratorios Calier S.A., Barcelona, Spain) containing vitamins, minerals, and amino acids.
Fifty days after metamorphosis, we weighed the animals to the nearest mg (Ohaus Pioneer PA-213; Ohaus Europe Gmb, Nanikon, Switzerland), chose 8 medium-sized individuals from each family (Ntotal = 12 families × 8 replicate specimens = 96 individuals), and experimentally infected half of them. We performed experimental infections by placing the juvenile toads for five hours individually into sterile Petri dishes (diameter: 90 mm) containing 19 ml RSW and 1 ml liquid Bd culture in mTGhLY resulting in a final concentration of 50,000 zsp/ml (containing both zoospores and zoosporangia). The other half of the animals were sham-treated with the same amount of sterile mTGhLY. Individuals that were not selected for the experiment were released at their site of origin. Subsequently, we placed juvenile toads individually in covered opaque 2-L plastic boxes lined with wet paper towels as a substrate and a piece of egg carton as a shelter and reared them in the laboratory at 20.3 ± 0.3 °C (mean ± SD) and a light : dark cycle adjusted weekly to outdoor conditions. We arranged boxes on a shelf system in randomized spatial blocks, each containing all treatments from each infection status of a same family. We fed the juvenile amphibians with small crickets as described above.
One week after infection, we assessed the status of the skin microbiota of 12 experimentally infected individuals (‘positive control’ group) by swabbing the belly and hind legs (ten times each) using dry rayon-tipped swabs (MW100, Medical Wire & Equipment, UK). Swabs were stored at -20 °C until DNA isolation. Thereafter, we weighed these individuals (± 1 mg; Ohaus Pioneer PA-213), euthanized them using the “cooling then freezing” method (Shine et al. 2015) and finally preserved them in 96% ethanol at -20 °C until further processing. Subsequently, we exposed seven groups of 12 individuals of the remaining juvenile toads to seven treatments as described in Table 1. We decided to use X. szentirmaii CFCM diluted to 2 and 10% (v/v) based on a pilot experiment, aiming to apply treatments that are not yet harmful to amphibians but are still likely to be effective against Bd (for details see Supplementary text 1; Table S1). We performed these treatments by replacing juvenile toads from rearing boxes to sterile Petri dishes (55 mm diameter, Fig S2) and exposing them for three hours to either RSW, mTGhLY or CFCM solutions (for details see Table 1). We treated animals on four consecutive days. During the first treatment, we changed paper lining and shelter in the rearing boxes. On the last day of treatments, we swabbed, weighed, and preserved individuals as described above (Fig S1). Contaminated water and equipment were disinfected overnight with VirkonS (Johnson et al. 2003) and was disposed of following institutional guidelines on the treatment of dangerous waste.
Table 1.
Details of the seven treatments applied on juvenile common toads
| Bd-infection | Treatment | Exposure design | Treatment name | N |
|---|---|---|---|---|
| no | only RSW | 7 ml RSW | RSW control | 12 |
| no | only mTGhLY | 6.3 ml RSW + 0.7 ml mTGhLY | broth control | 12 |
| no | CFCM diluted to 2% (v/v) | 6.3 ml RSW + 0.7 ml CFCM diluted to 20% (v/v) a | low CFCM | 12 |
| no | CFCM diluted to 10% (v/v) | 6.3 ml RSW + 0.7 ml pure CFCM | high CFCM | 12 |
| yes | only mTGhLY | 6.3 ml RSW + 0.7 ml mTGhLY | Bd + broth control | 12 |
| yes | CFCM diluted to 2% (v/v) | 6.3 ml RSW + 0.7 ml CFCM diluted to20% (v/v) a | Bd + low CFCM | 12 |
| yes | CFCM diluted to 10% (v/v) | 6.3 ml RSW + 0.7 ml pure CFCM | Bd + high CFCM | 12 |
aCFCM was diluted with mTGhLY
Assessment of infection intensity
We homogenized toe clips from the hind limbs of the preserved individuals, extracted DNA from samples using PrepMan Ultra Sample Preparation Reagent (Thermo Fisher Scientific, Waltham, Massachusetts, USA) according to previous recommendations (Boyle et al. 2004), and stored extracted DNA at -20°C until further analyses. We assessed infection intensity using real-time quantitative polymerase chain reaction (qPCR) following a standard amplification methodology targeting the ITS-1/5.8S rDNA region (ITS1-3 primer: 5’- CCTTGATATAATACAGTGTGCCATATGTC − 3’; 5.8 S primer: 5’- AGCCAAGAGATCCGTTGTCAAA − 3’; Boyle et al. 2004) on a BioRad CFX96 Touch Real-Time PCR System (BioRad Laboratories, Hercules, USA). To avoid PCR inhibition by ingredients of PrepMan, we diluted samples ten-fold with double-distilled water. We ran samples in duplicate, and in case of equivocal results, we repeated reactions in duplicate. If this again returned an equivocal result, we considered the sample to be Bd positive (Kriger et al. 2006). Genomic equivalent (GE) values were estimated from standard curves based on five dilutions of a standard (1000, 100, 10, 1, and 0.1 zoospore GE; provided by J. Bosch; Museo Nacional de Ciencias Naturales, Madrid, Spain). To assess whether cross-contamination occurred, we investigated one-third of individuals in each one of the uninfected treatment groups.
Analysis of the skin microbiome
We randomly chose three samples from each treatment group, except for the ‘RSW control’ and the ‘low CFCM’ treatment groups, from which we chose five samples for the skin microbiome analyses. We analysed the bacterial community applying Illumina sequencing. DNA was isolated from the swabs using DNeasy® PowerSoil® Pro Kit (Qiagen) according to the manufacturer’s protocol and isolates were stored at -20°C until further use. For PCR amplification we used 16S rDNA specific primers: 341F forward primer with CS1 Illumina adapter sequence and 805R reverse primer with CS2 Illumina adapter sequence, and Phusion® DNA polymerase enzyme. We performed the PCR amplification three times on each sample. The final reaction volume was set to 20 µl. For a 1 µl template we measured 19 µl PCR mix, which contained 4 µl dNTP mix, 4 µl Phusion® HF Buffer, 0.2 µl CS1 341F forward primer (5’- CCTACGGGAGGCAGCAG − 3’), 0.2 µl CS2 805R reverse primer (5’- GACTACHVGGGTATCTAATCC − 3’), 0.4 µl bovine serum albumin (BSA), 0.2 µl Phusion® DNA polymerase and 10 µl PCR-grade water. In each PCR run, we included a positive (random sample that previously worked with the same primers) and a negative control. We applied the following heat profile: initial denaturation at 98 °C for 5 min followed by 25 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s and extension at 72 °C for 2 min. After the 25th cycle samples were kept at 72 °C for 10 min and then cooled to 4 °C. After each amplification, we checked the amplification success by visualizing PCR products on a 1% (w/v) agarose gel dissolved in TBE buffer (Fig S3). We ran samples in triplicates and pooled them after each amplification to reduce the influence of random bias that may occur in single reactions. We measured the DNA content of the pooled samples using a Qubit 2.0 Fluorometer (Invitrogen, Life Technologies, CA, USA). Finally, samples were sequenced on an Illumina MiSeq platform at the Research Technology Supply Facility (RTSF), Michigan State University (East Lansing, Michigan, USA).
Statistical analyses
We ran all statistical analyses in R (version 4.0.5.). In case of the in vivo experiment, ‘RSW control’ and ‘positive control’ groups were only included in the analyses on the skin microbiota. We analysed the data of the in vitro Bd growth inhibitory potential by the two bacterial species separately using general linear models (LM) allowing the variances to differ among CFCM dilutions with the ‘varIdent’ function of the ‘nlme’ package. Models included OD492 as the dependent variable, and CFCM dilution and plate ID as categorical fixed factors. We compared each CFCM dilution step to the negative control by calculating pre-planned linear contrasts (Ruxton and Beauchamp 2008), while correcting the significance threshold for multiple testing using the false discovery rate (FDR) method (Pike 2011). We considered the means of two groups to differ when the relevant 84% confidence intervals (CIs) did not overlap (Payton et al. 2003; Julious 2004). We also identified the minimal inhibitory dilution (MID), which is the smallest dilution which still completely inhibits the growth of the test organism, i.e. the lowest dilution that’s 84% CIs still overlapped with the negative control.
Survival of juvenile toads was not analysed statistically, since only one death occurred (in the ‘Bd + broth control’ treatment). We averaged GE values obtained from qPCR runs for each sample and analysed resulting estimates of infection intensity in treatment groups that were experimentally exposed to Bd using generalized linear mixed models (GLMM) with negative binomial distribution and a log link function using the ‘glmmTMB’ package (Brooks et al. 2017). The model included CFCM dilution (0, 2, or 10%) as a categorical fixed factor and family nested in population as random factors.
We analysed body mass data using linear mixed models (LMM) with the ‘lme’ function of the ‘nlme’ package (Pinheiro et al. 2020). Since graphical model diagnostics indicated heterogeneous variances, we allowed the variances to differ among treatment groups with the ‘varIdent’ function of the ‘nlme’ package. We ran the initial model with infection status at the end of the experiment (yes or no) and CFCM dilution as categorical fixed factors and their interaction, the body mass of individuals measured at the start of the experiment as a numeric covariate, and family nested in population as random factors. We tested the effect of infection within each treatment group by calculating pre-planned linear contrasts (Ruxton and Beauchamp 2008), and corrected the significance threshold for multiple testing using the FDR method (Pike 2011).
During the experiment, we documented intensive skin shedding in infected individuals. To analyse the occurrence of shedding, we applied a generalized linear modelling procedure (GLM) with binomial distribution and logit link function containing infection status and CFCM dilution as categorical fixed factors. All tests were two-tailed, and we checked model fits in the case of all dependent variables by visual inspection of diagnostic plots. In all models containing interactions, we applied a backward stepwise removal procedure removing terms when P > 0.05 (Grafen and Hails 2002) to avoid problems potentially arising from the inclusion of non-significant terms (Engqvist 2005). We obtained statistics for removed variables by re-entering them one by one into the final model.
We processed raw sequencing data of the skin microbiome using the software Seed (version 2.1.1.; Větrovský et al. 2018). First, we joined forward and reverse sequences, then excluded poor quality sequences (mean quality value lower than 30) and sequences that contained ambiguous bases. We removed forward and reverse primers from the sequences. Singleton and short sequences (less than 360 nucleotides) were also excluded. We grouped sequences with at least 97% similarity into operational taxonomic units (OTUs) and removed chimeric sequences using Vsearch. We identified the representative sequences of the OTUs using the Basic Local Alignment Search Tool (BLAST) based on the ARB-SILVA database version 138 (Quast et al. 2013). We searched for archaeal, eukaryotic, and chloroplast sequences in our data and excluded them from the analysis. We set the number of sequences per sample to be the same as the number of sequences in the sample which contained the lowest number of sequences by random sampling. Then we assembled the OTU table which contains the number of OTUs per sample and calculated Chao1 diversity indices since this index is particularly useful for data sets skewed toward the low-abundance classes, as is likely to be the case with microbes (Hughes et al. 2001). To test whether Bd-infection or CFCM treatment and their interaction had a significant effect on the bacterial community living on the skin of the individuals we performed two-way permutational multivariate analysis of variance (PERMANOVA) based on Bray-Curtis similarity indices and non-metric multidimensional scaling (NMDS) analysis implemented in the software Past (version 4.05.). In the analyses on microbial species richness, we included the ‘positive control’ but excluded it from the PERMANOVA and NMDS analyses. To visualise the most abundant genera in treatment groups we used the R package ‘ampvis2’ (Andersen et al. 2018).
Results
In vitro experiment
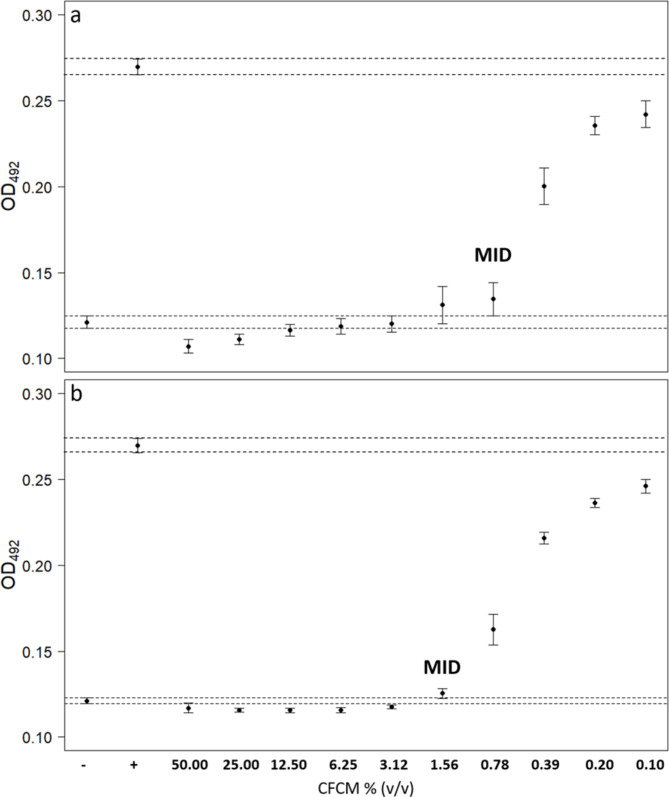
The CFCM of both Xenorhabdus species significantly inhibited Bd growth (X. szentirmaii, LM: F11, 78= 386.74, P < 0.001; X. budapestensis, F11,79 = 751.36, P < 0.001) at all dilutions as neither of the respective 84% CIs of their mean OD492 values overlapped with the 84% CI of the positive control’s mean OD492 (Fig. 1). The MID of X. szentirmaii CFCM was 1.56% and that of X. budapestensis CFCM was 0.78% (Fig. 1; for results of pairwise comparisons see Table S2 and S3).
Fig. 1.
Bd-growth inhibition by serial dilutions of X. budapestensis (a) and X. szentirmaii (b) CFCM determined by measuring optical density at 492 nm (OD492; mean ± 84% CI). The negative control is represented by ‘-‘, the positive control by ‘+’. Dashed lines depict the 84% CI of the controls. Minimal inhibitory dilution (MID) indicates the lowest dilution with complete growth inhibition (84% CI still overlapping with that of the negative control)
In vivo experiment
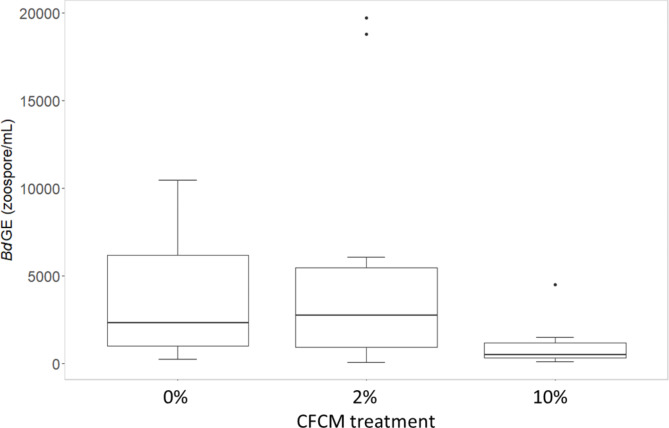
Survival was not affected over the course of the experiment by either treatment since all but one individual survived until the end of the study. We did not observe cross-contamination since all tested individuals from uninfected treatment groups remained Bd negative. Bd prevalence was 100% in all experimentally infected treatment groups. Infection intensity was 2417 ± 1539 GE (mean ± SD) per individual in the ‘positive control’ (treatment names refer for Table 1) right before the start of the CFCM treatments. Among CFCM treated infected groups, the ‘high CFCM’ (10% v/v) treatment resulted in significantly lower infection intensity compared to the ‘Bd + broth control’ treatment (Bd + broth control: 3666 ± 3468 GE [mean ± SD]; high CFCM: 984 ± 1201 GE [mean ± SD]; GLMM: z = -2.98, P = 0.003), however the ‘low CFCM’ (2% v/v) treatment did not affect infection intensity (z = 0.53, P = 0.59; Fig. 2). More concentrated X. szentirmaii CFCM (above 25% v/v) can be lethal for toads (Table S1).
Fig. 2.
Infection load in Bd-exposed juvenile toads after treatment with sterile mTGhLY (0%), 2% or 10% CFCM of X. szentirmaii. Bold lines show medians, boxes show the interquartile range (IQR), bars represent ranges, dots indicate outliers (deviating from the boundary of IQR by more than 1.5 × IQR)
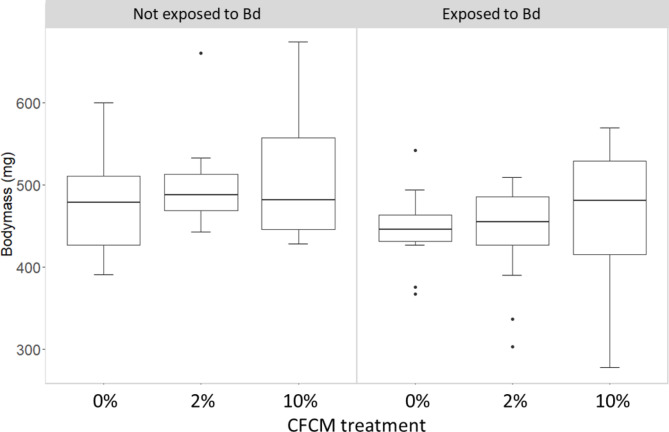
Body mass of the juvenile toads measured after treatments was negatively affected by Bd exposure (LMM: F1,57 = 12.32, P < 0.001), but treatment with CFCM had no significant effect on it, either alone (F2,55 = 0.92, P = 0.40) or in interaction with exposure to Bd (F2,53 = 0.17, P = 0.85; Fig. 3). Body mass measured at the termination of the experiment positively correlated with initial body mass measured at the start of the experiment (B = 0.8, SE = 0.13, F1,57 = 39.52, P < 0.001). Pairwise comparisons revealed that body mass of Bd-exposed individuals in the ‘broth control’ and ‘low CFCM’ was significantly lower compared to their non-infected counterparts (‘Bd + broth control’: t ratio = 2.25, df = 53, P = 0.028; ‘low CFCM’: t ratio = 2.82, df = 53, P = 0.007). However, body mass of individuals in the ‘high CFCM’ group did not differ between infected and non-infected individuals (t ratio = 1.34, df = 53, P = 0.188).
Fig. 3.
Body mass of juvenile toads at the end of the experiment in various treatment combinations. Bold lines show medians, boxes show the interquartile range (IQR), bars represent ranges, dots indicate outliers (deviating from the boundary of the IQR by more than 1.5 × IQR)
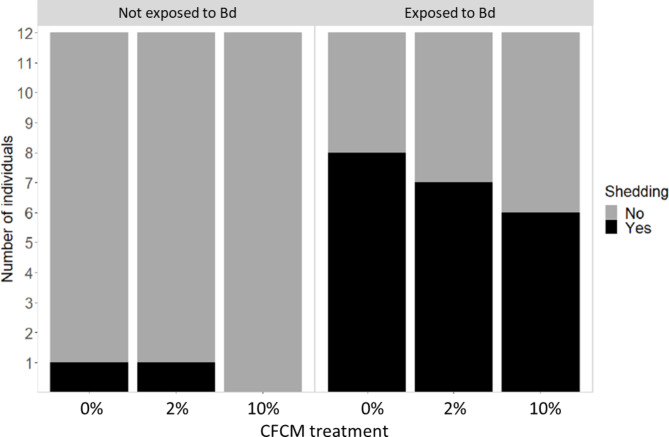
We documented a significantly higher frequency of skin shedding among Bd-exposed individuals compared to controls (GLM: χ2 = 26.3, df = 1, P < 0.001), while exposure to CFCM did not significantly influence the propensity of animals to shed their skin (χ2 = 1.3, df = 2, P = 0.51; Fig. 4).
Fig. 4.
Frequency of skin shedding in various treatment combinations
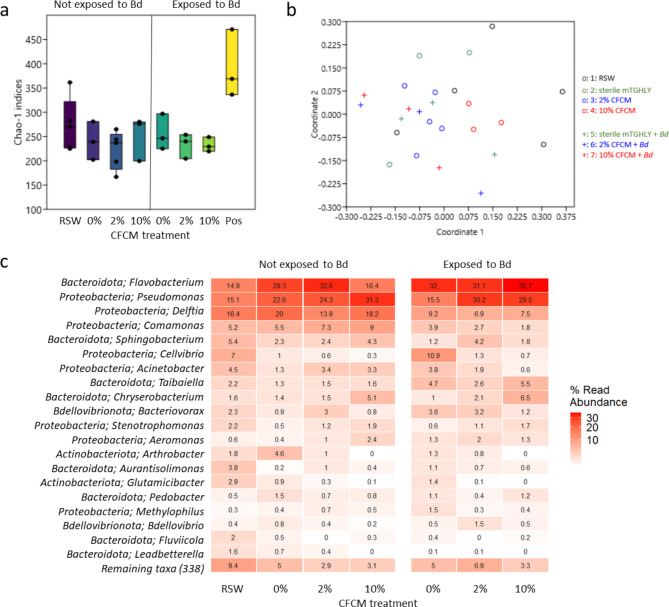
Chao1 diversity indices reflecting species richness varied significantly among treatment groups (2-way ANOVA: F4,27 = 9.08, P < 0.001), where samples from the ‘positive control’ exhibited higher diversity of bacterial taxa than samples from any other treatment group, while the other treatment groups did not differ among each other (Table S4, Fig. 5a). Bd-infection had a marginally significant effect on the community structure of the skin microbiota (PERMANOVA: F1,23 = 1.73, P = 0.07), while the effect of CFCM treatment was non-significant both alone (F6,17 = 0.15, P = 0.57) and in interaction with Bd-infection (F3,17 = 0.35, P = 0.94). NMDS analysis (stress value: 0.27) did not reveal any systematic pattern, ordination appeared to be arbitrary (Fig. 5b). Visual inspection of the relative abundance of the most abundant bacterial genera in the skin microbiota of the juvenile toads based on 16 S rDNA amplicon sequencing or focussing only on the three most abundant bacterial genera (Flavobacterium, Pseudomonas, and Delftia) also did not reveal striking differences among treatments (Fig. 5c).
Fig. 5.
Result on skin microbiota analyses. a: Chao1 bacterial diversity indices of the samples of different treatment groups based on 16 S rDNA amplicon sequencing. Bold lines show medians, boxes show the interquartile range (IQR), bars represent ranges, dots are the original data points. RSW = ‘RSW control’, Pos = ‘positive control’. b: Similarity of bacterial community composition based on 16 S rDNA amplicon sequencing using NMDS ordination with Bray-Curtis distances. c: Most abundant bacterial genera in the skin microbiota of the juvenile toads based on 16 S rDNA amplicon sequencing listed by treatment groups. Shading indicates read abundance
Discussion
The present study is the first to demonstrate Bd growth inhibition by EPB metabolites. In the in vitro experiment both the X. szentirmaii and the X. budapestensis CFCM were highly effective in inhibiting Bd growth, and this effectiveness was confirmed using the X. szentirmaii CFCM in the in vivo experiment. At the same time, measurable adverse effects of the treatment on juvenile common toads did not surface either in terms of lowered survival, lowered body mass or perturbed skin microbiome.
We found that dilutions of both Xenorhabdus CFCMs (as low as 1.56 and 0.78%) fully halted Bd growth in vitro, while CFCM diluted to 0.1% still showed some level of inhibition. This antimicrobial activity of X. szentirmaii CFCM against Bd is much higher compared to former studies that described MID values of 40 and 10% against bacterial pathogens of humans (Ozkan et al. 2019) and plants (Vozik et al. 2015). Consequently, and because EPB CFCMs are already effectively used against several bacterial and fungal pathogens, the application of X. szentirmaii CFCM has great potential in the fight against chytridiomycosis.
The most important finding of this study is that the ‘high CFCM’ treatment (10% dilution) had a striking effect on Bd infection load in experimentally infected juvenile toads. Although the ‘low CFCM’ treatment (2% dilution) did not lower infection intensity in the in vivo experiment, treatment with the 10% dilution of X. szentirmaii CFCM reduced infection load by 73%. Thus, a complete clearance of the infection was not reached, which would prevent the re-growth of the pathogen within individuals and its further spread to other hosts. Nonetheless, the observed high level of infection intensity suppression is also very promising because a complete clearance of infection is not essential for the prevention of mortalities: former studies reported no clinical signs of infection and no mortalities at low levels of infection (Vredenburg et al. 2010; Cheng et al. 2011). Furthermore, amphibian populations can coexist with Bd and maintain large population sizes if infection intensities remain low (Rowley and Alford 2013). Finally, co-existence with enzootic Bd may allow for adaptation to the disease via the spread of alleles providing increased resistance or tolerance on the level of populations (Bataille et al. 2015; Savage and Zamudio 2016; Voyles et al. 2018) and via immunization on the level of individuals (Ramsey et al. 2010; McMahon et al. 2014). To enhance efficiency, CFCM may be applied for extended times or on a larger number of consecutive days, but this would also increase the chances or severity of negative side-effects (see below).
The in vitro MID value of X. szentirmaii CFCM was 1.56% but the ‘low CFCM’ treatment (2%) proved to be ineffective against Bd in the in vivo experiment. These seemingly contradictive results may be partly explained by differences in the duration of exposure, which was continuous for one week in the in vitro experiment versus only three hours on four consecutive days in the in vivo experiment. The experimental microenvironment differed a lot as well: Bd cells were directly exposed to CFCM in the homogeneous mTGhLY in the in vitro experiment, while in the in vivo experiment Bd thalli were to some extent protected inside the keratinized epithelial cells of the amphibian skin. Hence, our study stresses that although in vitro experiments can provide valuable baseline data, in vivo experiments are indispensable when testing the effectiveness of new methods of disease mitigation.
The CFCMs we used likely contain more than one antifungal agent, but it was not our aim to determine their chemical identity and concentration. In this study only the supernatant of the bacterial cultures was used, in which exoenzymes such as chitinases can be also present. Since autoclaved Xenorhabdus CFCMs exerted similar antimicrobial and antifungal effects as the native ones in other experiments involving various pathogens (but not Bd; Fodor et al. 2022, 2023), the activity of the CFCM is most likely effected by thermostable secondary metabolites and not by exoenzymes. Fabclavines are highly effective and thermostable antifungal compounds which are known to be present in Xenorhabdus CFCM (Cimen et al. 2021), but whether these were the compounds which were active against Bd remains to be verified. How the antifungal activity of the EPB CFCMs and their constituents relates to that of metabolites produced by bacteria associated with amphibian skin (Brucker et al. 2008a, b; Myers et al. 2012; Woodhams et al. 2018) remains unknown because we investigated the activity of all metabolites produced by EPBs in their entirety, while the studies using skin bacteria focussed on individual antimicrobial metabolites. Future studies may verify the anti-Bd activity of fabclavines by exposing Bd or Bd-infected animals to CFCM, to fabclavine only, or to CFCM from a fabclavine production deficient Xenorhabdus mutant.
Although the survival of toads can sharply decrease upon infection with Bd (Garner et al. 2009; Bielby et al. 2015; but also see Ujszegi et al. 2021), we found that survival of juvenile toads was not affected by infection with Bd, perhaps because the Bd isolate we used co-occurs with the sampled toad population (also see Kásler et al. 2022). We documented a significant reduction in body mass after eleven days of Bd exposure. Other studies have also shown that exposure to Bd can lead to a decrease in body mass (Parris and Cornelius 2004; Blaustein et al. 2005; Hanlon et al. 2015; Kásler et al. 2022), one of the best predictors of fitness in juvenile amphibians (Semlitsch et al. 1988; Altwegg and Reyer 2003). In the present study, we also documented that shedding was more frequent in the Bd-infected treatment groups than in the non-infected groups. This is in accordance with earlier observations from Berger et al. (2005), Voyles et al. (2009) and Martel et al. (2011). Excessive skin shedding most likely serves as a defence mechanism aiming to remove the infected outer epithelial cell layers (Becker and Harris 2010; Meyer et al. 2012). Finally, PERMANOVA detected a marginally significant effect of Bd-infection (assuming a p-value threshold of 0.05) on the skin bacterial community due to Bd-infection itself, while Chao1 indices did not differ between infected and uninfected juvenile toads, which is in accordance with results of an earlier study (Jani and Briggs 2014).
We expected to detect some harmful effects of CFCM treatment, because EPBs produce toxins that harm the insect that is colonised by the host EPNs and contribute to its death (Forst et al. 1997). However, we did not find this. Treatment with 10% X. szentirmaii CFCM did not cause any surplus mortality nor did it affect body mass adversely. Moreover, the negative effect of Bd exposure on body mass was abolished by the ‘high CFCM’ treatment. CFCM treatment did not affect the frequency of skin shedding in non-infected groups, but it also did not prevent infection-induced skin shedding in the infected groups. Finally, the lack of an effect of CFCM treatment on the skin microbiota we found on the juvenile toads was surprising because of its wide-spectrum antimicrobial activity, including several bacterial taxa. Possibly, the dilutions we applied were too high to affect microbial growth under in vivo conditions in a complex microbial community. Alternatively, changes may not have surfaced right after the treatment and may have appeared later. However, decrease of microbial diversity in all groups compared to the ‘positive control’ group suggests that detectable differences in microbial communities manifest very rapidly (Fig. 5a). Alternatively, the limited sample sizes may have prevented the detection of subtle differences. However, striking immediate effects of CFCM treatment on skin microbiota were clearly absent, and several microbial taxa with anti-Bd properties remained on the juvenile toads’ skin after the CFCM treatments (Supplementary text 2). In sum, X. szentirmaii CFCM diluted to 10% does not appear to harm juvenile common toads. Nonetheless, it remains to be determined whether more concentrated dilutions of EPB CFCM cause harmful effects and to what extent amphibian species and life-stages differ in their susceptibility to CFCM treatment.
We propose that the application of antifungal metabolites without the need for the establishment of probiotic bacteria on the skin of amphibians may be a good solution that would bridge the problems arising from bioaugmentation or the application of conventional antifungals such as itraconazole or amphotericin B (Garner et al., 2009; Holden et al. 2014). Antifungal metabolites of Xenorhabdus bacteria are highly potent candidates because they are easy to cultivate, cheap to produce, highly effective and exceptionally stable even at high temperatures (Fodor et al. 2022). These results of our pioneering study are the first to suggest that EPB CFCMs can be applied effectively and safely to treat Bd-infected amphibians at least under laboratory conditions, where re-application can be performed easily. Currently, 180 endangered amphibian species subsist only thanks to captive breeding programs (Kueneman et al. 2022), and amphibians are increasingly maintained in captivity for research, hobby, public display, and food production (Hadfield and Whitaker 2005; Densmore and Green 2007). These activities require an easily applicable, non-invasive, chemical-free, and relatively cheap mitigation method to halt chytridiomycosis outbreaks and prevent the reintroduction of infected individuals into natural habitats. Our results suggest that the antifungal metabolites of Xenorhabdus bacteria may prove suitable for this task. At the same time, however, this approach also has some limitations, which have to be thoroughly tested and considered before application. Metabolites must be re-applied either until complete clearance or until an effective immune response is mounted by the host. Furthermore, as CFCM contains a culture medium, it can promote the growth of other bacteria (especially in case of prolonged CFCM exposure), which are not sensitive to the contained metabolites and some of these may be facultative or obligate pathogens. Finally, even subtle changes in the skin microbiome may influence the fitness of amphibian hosts. Consequently, to provide the necessary knowledge base, studies will need to identify the antifungal components present in the EPB CFCM and assess their concentration, as well as optimize the method of application to maximize effectiveness. Furthermore, testing for potential malign long-term effects of treatment with CFCM, and investigating its applicability to other amphibian species and pathogens would also be important. Ultimately, CFCM treatment may prove to be an effective and safe approach to the mitigation of chytridiomycosis and perhaps other diseases of amphibians and other vertebrates as well.
In summary, we demonstrated for the first time that metabolites produced by entomopathogenic bacteria of the genus Xenorhabdus can halt Bd growth in vitro already at very low concentrations. We also documented that these metabolites can be safely applied to amphibian juveniles and that the treatment resulted in drastically lowered infection intensities. Hence, our results support the idea that Xenorhabdus metabolites may very well be used in the mitigation of chytridiomycosis in live amphibians. Future studies on this system will likely prove fruitful and will contribute to exploiting the surprising effectiveness of EPB metabolites against this devastating amphibian disease.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank D. Herczeg, D. Holly, A. Kásler, E. Kovács, Zs. Mikó and M. Szederkényi for assistance during the experiment, V. Bókony for help in statistics and the Pilisi Parkerdő Zrt. for allowing us to use their roads. Zoospore genomic equivalent standards were kindly offered by J. Bosch and J. Vörös. T. Vellai provided us with the laboratory capacity at the Department of Genetics, Eötvös Loránd University. Toad paintings were made by B. Bombay.
Author’s contribution
The study was planned and designed by J.U., A.H., and A.F. The experiments and microbial work were carried out by J.U., Z.B. A.F. and A.H. DNA laboratory work and data analyses were carried out by J.U., Z.B. and B.V. The first draft of the manuscript was written by J.U., Z.B. and A.F. All authors have contributed critically to the drafts and gave final approval for publication.
Funding
Research was supported by the Lendület Programme of the Hungarian Academy of Sciences (MTA, LP2012-24/2012), the National Research, Development and Innovation Office (NKFIH) of Hungary (Grant No. K-124375) and the New National Excellence Program of the Ministry for Innovation and Technology from the source of the National Research, Development, and Innovation Fund (ÚNKP-21-4 for U.J., ÚNKP-21-5 and ÚNKP-22-5 Bolyai + Scholarship for A.H.. B.V. and A.H. were supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences (Grant No. BO/00156/21/8 and BO/00677/21/8).
Open access funding provided by Eötvös Loránd University.
Data Availability
All data used in the analyses will be available from Figshare Repository upon publication. 10.6084/m9.figshare.21229439.
Declarations
Ethics approval and consent to participate
The Ethical Commission of the Plant Protection Institute approved experimental procedures and research was carried out according to the permits issued by the Government Agency of Pest County (Department of Environmental Protection and Nature Conservation, PE-06/KTF/8060-1/2018, PE-06/KTF/8060-2/2018, PE-06/KTF/8060-3/2018, PE/EA/295-7/2018).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors have no conflict of interest to declare.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Akhurst RJ. Antibiotic activity of Xenorhabdus spp., bacteria symbiotically associated with insect pathogenic nematodes of the families Heterorhabditidae and Steinernematidae. J Gen Microbiol. 1982;128:3061–3065. doi: 10.1099/00221287-128-12-3061. [DOI] [PubMed] [Google Scholar]
- Altwegg R, Reyer H-U. Patterns of natural selection on size at metamorphosis in water frogs. Evol (N Y) 2003;57:872–882. doi: 10.2307/2423346. [DOI] [PubMed] [Google Scholar]
- Andersen KS, Kirkegaard RH, Karst SM, Albertsen M (2018) ampvis2: an R package to analyse and visualise 16S rRNA amplicon data. 10.1101/299537. BioRxiv 299537
- Ausubel FM, Brent R, Kingston RE, David D, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Short protocols in Molecular Biology: a compendium of methods from current protocols in Molecular Biology. New York: John Wiley & Sons; 1999. [Google Scholar]
- Barribeau SM, Villinger J, Waldman B. Major histocompatibility complex based resistance to a common bacterial pathogen of amphibians. PLoS ONE. 2008;3:e2692. doi: 10.1371/journal.pone.0002692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bataille A, Cashins SD, Grogan L, Skerratt LF, Hunter D, McFadden M, Scheele B, Brannelly LA, Macris A, Harlow PS, Bell S, Berger L, Waldman B. Susceptibility of amphibians to chytridiomycosis is associated with MHC class II conformation. Proc R Soc B Biol Sci. 2015;282:20143127. doi: 10.1098/rspb.2014.3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker MH, Harris RN. Cutaneous bacteria of the redback salamander prevent morbidity associated with a lethal disease. PLoS ONE. 2010;5:1–6. doi: 10.1371/journal.pone.0010957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker MH, Brucker RM, Schwantes CR, Harris RN, Minbiole KPC. The bacterially produced metabolite violacein is associated with survival of amphibians infected with a lethal fungus. Appl Environ Microbiol. 2009;75:6635–6638. doi: 10.1128/AEM.01294-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belden LK, Harris RN. Infectious diseases in wildlife: the community ecology context. Front Ecol Environ. 2007;5:533–539. doi: 10.1890/060122. [DOI] [Google Scholar]
- Bell SC, Alford RA, Garland S, Padilla G, Thomas AD. Screening bacterial metabolites for inhibitory effects against Batrachochytrium dendrobatidis using a spectrophotometric assay. Dis Aquat Organ. 2013;103:77–85. doi: 10.3354/dao02560. [DOI] [PubMed] [Google Scholar]
- Berger L, Speare R, Daszak P, Green DE, Cunningham AA, Goggin LC, Slocombe R, Ragan MA, Hyatt AD, McDonald KR, Hines HB, Lips KR, Marantelli G, Parkes H. Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proc Natl Acad Sci U S A. 1998;95:9031–9036. doi: 10.1073/pnas.95.15.9031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger L, Hyatt AD, Speare R, Longcore JE. Life cycle stages of the amphibian chytrid Batrachochytrium dendrobatidis. Dis Aquat Organ. 2005;68:51–63. doi: 10.3354/dao068051. [DOI] [PubMed] [Google Scholar]
- Bielby J, Fisher MC, Clare FC, Rosa GM, Garner TW. Host species vary in infection probability, sub-lethal effects, and costs of immune response when exposed to an amphibian parasite. Sci Rep. 2015;5:10828. doi: 10.1038/srep10828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein AR, Romansic JM, Scheessele EA, Han BA, Pessier AP, Longcore JE. Interspecific variation in susceptibility of frog tadpoles to the pathogenic fungus batrachochytrium dendrobatidis. Conserv Biol. 2005;19:1460–1468. doi: 10.1111/j.1523-1739.2005.00195.x. [DOI] [Google Scholar]
- Bletz MC, Loudon AH, Becker MH, Bell SC, Woodhams DC, Minbiole KPC, Harris RN. Mitigating amphibian chytridiomycosis with bioaugmentation: characteristics of effective probiotics and strategies for their selection and use. Ecol Lett. 2013;16:807–820. doi: 10.1111/ele.12099. [DOI] [PubMed] [Google Scholar]
- Bode HB. Entomopathogenic bacteria as a source of secondary metabolites. Curr Opin Chem Biol. 2009;13:224–230. doi: 10.1016/j.cbpa.2009.02.037. [DOI] [PubMed] [Google Scholar]
- Böszörményi E, Érsek T, Fodor A, Fodor AM, Földes LS, Hevesi M, Hogan JS, Katona Z, Klein MG, Kormány A, Pekár S, Szentirmai A, Sztaricskai F, Taylor RAJ. Isolation and activity of Xenorhabdus antimicrobial compounds against the plant pathogens Erwinia amylovora and Phytophthora nicotianae. J Appl Microbiol. 2009;107:746–759. doi: 10.1111/j.1365-2672.2009.04249.x. [DOI] [PubMed] [Google Scholar]
- Boyle DG, Boyle DB, Olsen V, Morgan JAT, Hyatt AD. Rapid quantitative detection of chytridiomycosis (batrachochytrium dendrobatidis) in amphibian samples using real-time Taqman PCR assay. Dis Aquat Organ. 2004;60:141–148. doi: 10.3354/dao060141. [DOI] [PubMed] [Google Scholar]
- Brooks ME, Kristensen K, van Benthem KJ, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Maechler M, Bolker BM. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 2017;9:378–400. doi: 10.32614/RJ-2017-066. [DOI] [Google Scholar]
- Brucker RM, Baylor CM, Walters RL, Lauer A, Harris RN, Minbiole KP. The identification of 2,4-diacetylphloroglucinol as an antifungal metabolite produced by cutaneous bacteria of the salamander Plethodon cinereus. J Chem Ecol. 2008;34:39–43. doi: 10.1007/s10886-007-9352-8. [DOI] [PubMed] [Google Scholar]
- Brucker RM, Harris RN, Schwantes CR, Gallaher TN, Flaherty DC, Lam BA, Minbiole KP. Amphibian chemical defense: antifungal metabolites of the microsymbiont Janthinobacterium lividum on the salamander Plethodon cinereus. J Chem Ecol. 2008;34:1422–1429. doi: 10.1007/s10886-008-9555-7. [DOI] [PubMed] [Google Scholar]
- Campbell Grant EH, Miller DAW, Schmidt BR, Adams MJ, Amburgey SM, Chambert T, Cruickshank SS, Fisher RN, Green DM, Hossack BR, Johnson PTJ, Joseph MB, Rittenhouse TAG, Ryan ME, Waddle HJ, Walls SC, Bailey LL, Fellers GM, Gorman TA, Ray AM, Pilliod DS, Price SJ, Saenz D, Sadinski W, Muths E. Quantitative evidence for the effects of multiple drivers on continental-scale amphibian declines. Sci Rep. 2016;6:25625. doi: 10.1038/srep25625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey C, Cohen N, Rollins-Smith L. Amphibian declines: an immunological perspective. Dev Comp Immunol. 1999;23:459–472. doi: 10.1016/S0145-305X(99)00028-2. [DOI] [PubMed] [Google Scholar]
- Cheng TL, Rovito SM, Wake DB, Vredenburg VT. Coincident mass extirpation of neotropical amphibians with the emergence of the infectious fungal pathogen Batrachochytrium dendrobatidis. Proc Natl Acad Sci U S A. 2011;108:9502–9507. doi: 10.1073/pnas.1105538108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimen H, Touray M, Gulsen SH, Erincik O, Wenski SL, Bode HB, Shapiro-Ilan D, Hazir S. Antifungal activity of different Xenorhabdus and Photorhabdus species against various fungal phytopathogens and identification of the antifungal compounds from X. szentirmaii. Appl Microbiol Biotechnol. 2021;105:5517–5528. doi: 10.1007/s00253-021-11435-3. [DOI] [PubMed] [Google Scholar]
- Daly JW. The chemistry of poisons in amphibian skin. Proc Natl Acad Sci U S A. 1995;92:9–13. doi: 10.1073/pnas.92.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskin JH, Bell SC, Schwarzkopf L, Alford RA. Cool temperatures reduce antifungal activity of symbiotic bacteria of threatened amphibians – implications for disease management and patterns of decline. PLoS ONE. 2014;9:e100378. doi: 10.1371/journal.pone.0100378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Doucet MMA. A new species of Neoaplectana Steiner, 1929 (Nematoda: Steinernematidae) from Córdoba, Argentina. Rev Nématologie. 1986;9:317–323. [Google Scholar]
- Densmore CL, Green DE. Diseases of amphibians. ILAR J. 2007;48:235–254. doi: 10.1093/ilar.48.3.235. [DOI] [PubMed] [Google Scholar]
- Engqvist L. The mistreatment of covariate interaction terms in linear model analyses of behavioural and evolutionary ecology studies. Anim Behav. 2005;70:967–971. doi: 10.1016/j.anbehav.2005.01.016. [DOI] [Google Scholar]
- Fodor A, Gualtieri M, Zeller M, Tarasco E, Klein MG, Fodor AM, Haynes L, Lengyel K, Forst SA, Furgani GM, Karaffa L, Vellai T (2022) Type strains of entomopathogenic Nematode-symbiotic bacterium species, Xenorhabdus szentirmaii (EMC) and X. budapestensis (EMA), are exceptional sources of thermotolerant-antimicrobial peptides (by both), and Iodinin (by EMC). Pathogens 11:342. 10.3390/pathogens11030342 [DOI] [PMC free article] [PubMed]
- Fodor A, Vellai T, Hess C, Makrai L, Dublecz K, Pál L, Molnár A, Klein MG, Tarasco E, Józsa S, Ganas P, Hess M. XENOFOOD—An autoclaved feed supplement containing autoclavable antimicrobial peptides—exerts anticoccidial GI activity, and causes bursa enlargement, but has no detectable harmful effects in broiler cockerels despite in vitro detectable cytotoxicity on LH. Pathogens. 2023;12:458. doi: 10.3390/pathogens12030458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forst S, Nealson KH, Boemare N, Stackebrandt E. Xenorhabdus and Photorhabdus spp.: bugs that kill bugs. Annu Rev Microbiol. 1997;51:47–72. doi: 10.1146/annurev.micro.51.1.47. [DOI] [PubMed] [Google Scholar]
- Fuchs SW, Sachs CC, Kegler C, Nollmann FI, Karas M, Bode HB. Neutral loss fragmentation pattern based screening for arginine-rich natural products in Xenorhabdus and Photorhabdus. Anal Chem. 2012;84:6948–6955. doi: 10.1021/ac300372p. [DOI] [PubMed] [Google Scholar]
- Fuchs SW, Grundmann F, Kurz M, Kaiser M, Bode HB. Fabclavines: bioactive peptide-polyketide-polyamino hybrids from Xenorhabdus. ChemBioChem. 2014;15:512–516. doi: 10.1002/cbic.201300802. [DOI] [PubMed] [Google Scholar]
- Furgani G, Böszörményi E, Fodor A, Máthé-Fodor A, Forst S, Hogan JS, Katona Z, Klein MG, Stackebrandt E, Szentirmai A, Sztaricskai F, Wolf SL. Xenorhabdus antibiotics: a comparative analysis and potential utility for controlling mastitis caused by bacteria. J Appl Microbiol. 2008;104:745–758. doi: 10.1111/j.1365-2672.2007.03613.x. [DOI] [PubMed] [Google Scholar]
- Garner TW, Walker S, Bosch J, Leech S, Rowcliffe JM, Cunningham AA, Fisher MC. Life history tradeoffs influence mortality associated with the amphibian pathogen Batrachochytrium dendrobatidis. Oikos. 2009;118:783–791. doi: 10.1111/j.1600-0706.2008.17202.x. [DOI] [Google Scholar]
- Garner TWJ, Garcia G, Carroll B, Fisher MC. Using itraconazole to clear Batrachochytrium dendrobatidis infection, and subsequent depigmentation of Alytes muletensis tadpoles. Dis Aquat Organ. 2009;83:257–260. doi: 10.3354/dao02008. [DOI] [PubMed] [Google Scholar]
- Garner TW, Schmidt BR, Martel A, Pasmans F, Muths E, Cunningham AA, Weldon C, Fisher MC, Bosch J. Mitigating amphibian chytridiomycoses in nature. Philos Trans R Soc B Biol Sci. 2016;371:20160207. doi: 10.1098/rstb.2016.0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry T, Rensing C, Pepper I. New approaches for bioaugmentation as a remediation technology. Crit Rev Environ Sci Technol. 2004;34:447–494. doi: 10.1080/10643380490452362. [DOI] [Google Scholar]
- Gomes A, Giri B, Saha A, Mishra R, Dasgupta SC, Debnath A, Gomes A. Bioactive molecules from amphibian skin: their biological activities with reference to therapeutic potentials for possible drug development. Indian J Exp Biol. 2007;45:579–593. [PubMed] [Google Scholar]
- González DL, Baláž V, Solský M, Thumsová B, Kolenda K, Najbar A, Najbar B, Kautman M, Chajma P, Balogová M, Vojar J. Recent findings of potentially lethal salamander fungus Batrachochytrium salamandrivorans. Emerg Infect Dis. 2019;25:1416–1418. doi: 10.3201/eid2507.181001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosner KL. A simplified table for staging anuran embryos larvae with notes on identification. Herpetologica. 1960;16:183–190. doi: 10.2307/3890061. [DOI] [Google Scholar]
- Grafen A, Hails R. Modern statistics for the life sciences. Oxford; New York: Oxford University Press; 2002. [Google Scholar]
- Grogan LF, Robert J, Berger L, Skerratt LF, Scheele BC, Castley JG, Newell DA, McCallum HI. Review of the amphibian immune response to chytridiomycosis, and future directions. Front Immunol. 2018;9:2536. doi: 10.3389/fimmu.2018.02536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadfield CA, Whitaker BR. Amphibian emergency medicine and care. Semin Avian Exot Pet Med. 2005;14:79–89. doi: 10.1053/j.saep.2005.04.003. [DOI] [Google Scholar]
- Hanlon SM, Lynch KJ, Kerby J, Parris MJ. Batrachochytrium dendrobatidis exposure effects on foraging efficiencies and body size in anuran tadpoles. Dis Aquat Organ. 2015;112:237–242. doi: 10.3354/dao02810. [DOI] [PubMed] [Google Scholar]
- Harris RN, James TY, Lauer A, Simon MA, Patel A. Amphibian pathogen Batrachochytrium dendrobatidis is inhibited by the cutaneous bacteria of amphibian species. EcoHealth. 2006;3:53–56. doi: 10.1007/s10393-005-0009-1. [DOI] [Google Scholar]
- Harris RN, Lauer A, Simon MA, Banning JL, Alford RA. Addition of antifungal skin bacteria to salamanders ameliorates the effects of chytridiomycosis. Dis Aquat Organ. 2009;83:11–16. doi: 10.3354/dao02004. [DOI] [PubMed] [Google Scholar]
- Hettyey A, Ujszegi J, Herczeg D, Holly D, Vörös J, Schmidt BR, Bosch J. Mitigating disease impacts in amphibian populations: capitalizing on the thermal optimum mismatch between a pathogen and its host. Front Ecol Evol. 2019;7:1–13. doi: 10.3389/fevo.2019.00254. [DOI] [Google Scholar]
- Hof C, Araújo MB, Jetz W, Rahbek C. Additive threats from pathogens, climate and land-use change for global amphibian diversity. Nature. 2011;480:516–519. doi: 10.1038/nature10650. [DOI] [PubMed] [Google Scholar]
- Holden WM, Ebert AR, Canning PF, Rollins-Smith LA. Evaluation of amphotericin B and chloramphenicol as alternative drugs for treatment of chytridiomycosis and their impacts on innate skin defenses. Appl Environ Microbiol. 2014;80:4034–4041. doi: 10.1128/AEM.04171-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JB, Hellmann JJ, Ricketts TH, Bohannan BJM. Counting the uncountable: statistical approaches to estimating microbial diversity. Appl Environ Microbiol. 2001;67:4399–4406. doi: 10.1128/AEM.67.10.4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jani AJ, Briggs CJ. The pathogen Batrachochytrium dendrobatidis disturbs the frog skin microbiome during a natural epidemic and experimental infection. Proc Natl Acad Sci U S A. 2014;111:E5049–E5058. doi: 10.1073/pnas.1412752111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ML, Berger L, Philips L, Speare R. Fungicidal effects of chemical disinfectants, UV light, desiccation and heat on the amphibian chytrid Batrachochytrium dendrobatidis. Dis Aquat Organ. 2003;57:255–260. doi: 10.3354/dao057255. [DOI] [PubMed] [Google Scholar]
- Julious SA. Using confidence intervals around individual means to assess statistical significance between two means. Pharm Stat. 2004;3:217–222. doi: 10.1002/pst.126. [DOI] [Google Scholar]
- Kásler A, Ujszegi J, Holly D, Üveges B, Móricz, Ágnes M, Herczeg D, Hettyey A (2022) Metamorphic common toads keep chytrid infection under control, but at a cost. J Zool in press
- Kaya HK. Infectivity of Neoaplectana carpocapsae and heterorhabditis heliothidis to pupae of the parasite Apanteles militaris. J Nematol. 1978;10:241–244. [PMC free article] [PubMed] [Google Scholar]
- König E, Bininda-Emonds ORP, Shaw C. The diversity and evolution of anuran skin peptides. Peptides. 2015;63:96–117. doi: 10.1016/j.peptides.2014.11.003. [DOI] [PubMed] [Google Scholar]
- Koprivnikar J. Interactions of environmental stressors impact survival and development of parasitized larval amphibians. Ecol Appl. 2010;20:2263–2272. doi: 10.1890/09-1558.1. [DOI] [PubMed] [Google Scholar]
- Kriger KM, Hero J-M, Ashton KJ. Cost efficiency in the detection of chytridiomycosis using PCR assay. Dis Aquat Organ. 2006;71:149–154. doi: 10.3354/dao071149. [DOI] [PubMed] [Google Scholar]
- Krynak KL, Burke DJ, Benard MF. Landscape and water characteristics correlate with immune defense traits across Blanchard’s cricket frog (Acris blanchardi) populations. Biol Conserv. 2016;193:153–167. doi: 10.1016/j.biocon.2015.11.019. [DOI] [Google Scholar]
- Kueneman J, Bletz M, Becker M, Gratwicke B, Garcés OA, Hertz A, Holden WM, Ibánez R, Loudon A, McKenzie V, Parfrey L, Sheafor B, Rollins-smith LA, Richards-zawacki C, Voyles J, Woodhams DC. Effects of captivity and rewilding on amphibian skin microbiomes. Biol Conserv. 2022;271:109576. doi: 10.1016/j.biocon.2022.109576. [DOI] [Google Scholar]
- Küng D, Bigler L, Davis LR, Gratwicke B, Griffith E, Woodhams DC. Stability of microbiota facilitated by host immune regulation: informing probiotic strategies to manage amphibian disease. PLoS ONE. 2014;9:e87101. doi: 10.1371/journal.pone.0087101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam BA, Walke JB, Vredenburg VT, Harris RN. Proportion of individuals with anti-Batrachochytrium dendrobatidis skin bacteria is associated with population persistence in the frog Rana muscosa. Biol Conserv. 2010;143:529–531. doi: 10.1016/j.biocon.2009.11.015. [DOI] [Google Scholar]
- Leclerc MC, Boemare NE. Plasmids and phase variation in Xenorhabdus spp. Appl Environ Microbiol. 1991;57:2597–2601. doi: 10.1128/aem.57.9.2597-2601.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengyel K, Lang E, Fodor A, Szállás E, Schumann P, Stackebrandt E (2005) Description of four novel species of Xenorhabdus, family Enterobacteriaceae: Xenorhabdus budapestensis sp. nov., Xenorhabdus ehlersii sp. nov., Xenorhabdus innexi sp. nov., and Xenorhabdus szentirmaii sp. nov. Syst Appl Microbiol 28:115–122. 10.1016/j.syapm.2004.10.004 [DOI] [PubMed]
- Macfoy C, Danosus D, Sandit R, Jones TH, Garraffo MH, Spande TF, Daly JW. Alkaloids of anuran skin: antimicrobial function? Z für Naturforsch. 2005;60:932–937. doi: 10.1515/znc-2005-11-1218. [DOI] [PubMed] [Google Scholar]
- Madison JD, Berg EA, Abarca JG, Whitfield SM, Gorbatenko O, Pinto A, Kerby JL. Characterization of Batrachochytrium dendrobatidis inhibiting bacteria from amphibian populations in Costa Rica. Front Microbiol. 2017;8:1–13. doi: 10.3389/fmicb.2017.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel A, Van Rooij P, Vercauteren G, Baert K, Van Waeyenberghe L, Debacker P, Garner TWJ, Woeltjes T, Ducatelle R, Haesebrouck F, Pasmans F. Developing a safe antifungal treatment protocol to eliminate Batrachochytrium dendrobatidis from amphibians. Med Mycol. 2011;49:143–149. doi: 10.3109/13693786.2010.508185. [DOI] [PubMed] [Google Scholar]
- Martel A, Spitzen-van der Sluijs A, Blooi M, Bert W, Ducatelle R, Fisher MC, Woeltjes A, Bosman W, Chiers K, Bossuyt F, Pasmans F. Batrachochytrium salamandrivorans sp. nov. causes lethal chytridiomycosis in amphibians. Proc Natl Acad Sci U S A. 2013;110:15325–15329. doi: 10.1073/pnas.1307356110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon TA, Sears BF, Venesky MD, Bessler SM, Brown JM, Deutsch K, Halstead NT, Lentz G, Tenouri N, Young S, Civitello DJ, Ortega N, Fites JS, Reinert LK, Rollins-Smith LA, Raffel TR, Rohr JR. Amphibians acquire resistance to live and dead fungus overcoming fungal immunosuppression. Nature. 2014;511:224–227. doi: 10.1038/nature13491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer EA, Cramp RL, Bernal MH, Franklin CE. Changes in cutaneous microbial abundance with sloughing: possible implications for infection and disease in amphibians. Dis Aquat Organ. 2012;101:235–242. doi: 10.3354/dao02523. [DOI] [PubMed] [Google Scholar]
- Monastersky R. Biodiversity: Life a a status report. Nature. 2014;516:158–161. doi: 10.1038/516158a. [DOI] [PubMed] [Google Scholar]
- Mueller HJ, Hinton J. A protein-free medium for primary isolation of the Gonococcus and Meningococcus. Proc Soc Exp Biol Med. 1941;48:330–333. doi: 10.3181/00379727-48-13311. [DOI] [Google Scholar]
- Muletz CR, Myers JM, Domangue RJ, Herrick JB, Harris RN. Soil bioaugmentation with amphibian cutaneous bacteria protects amphibian hosts from infection by Batrachochytrium dendrobatidis. Biol Conserv. 2012;152:119–126. doi: 10.1016/j.biocon.2012.03.022. [DOI] [Google Scholar]
- Murphy PJ, St.-Hilaire S, Corn PS. Temperature, hydric environment, and prior pathogen exposure alter the experimental severity of chytridiomycosis in boreal toads. Dis Aquat Organ. 2011;95:31–42. doi: 10.3354/dao02336. [DOI] [PubMed] [Google Scholar]
- Myers JM, Ramsey JP, Blackman AL, Nichols AE, Minbiole KPC, Harris RN. Synergistic inhibition of the lethal fungal pathogen Batrachochytrium dendrobatidis: the combined effect of symbiotic bacterial metabolites and antimicrobial peptides of the frog Rana muscosa. J Chem Ecol. 2012;38:958–965. doi: 10.1007/s10886-012-0170-2. [DOI] [PubMed] [Google Scholar]
- Ogier JC, Pagès S, Frayssinet M, Gaudriault S. Entomopathogenic nematode-associated microbiota: from monoxenic paradigm to pathobiome. Microbiome. 2020;8:25. doi: 10.1186/s40168-020-00800-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson J, Westerdahl A, Conway P, Kjelleberg S. Intestinal colonization potential of turbot (Scophthalmus maximus)- and dab (Limanda limanda)- associated bacteria with inhibitory effects against Vibrio anguillarum. Appl Environ Microbiol. 1992;58:551–556. doi: 10.1128/aem.58.2.551-556.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozkan HD, Cimen H, Ulug D, Wenski S, Yigit Ozer S, Telli M, Aydin N, Bode HB, Hazir S (2019) Nematode-associated bacteria: production of antimicrobial agent as a presumptive nominee for curing endodontic infections caused by Enterococcus faecalis. Front Microbiol 10. 10.3389/fmicb.2019.02672 [DOI] [PMC free article] [PubMed]
- Parris MJ, Cornelius TO. Fungal pathogen causes competitive and developmental stress in larval amphibian communities. Ecology. 2004;85:3385–3395. doi: 10.1890/04-0383. [DOI] [Google Scholar]
- Patterson JA, Burkholder KM. Application of prebiotics and probiotics in poultry production. Poult Sci. 2003;82:627–631. doi: 10.1093/ps/82.4.627. [DOI] [PubMed] [Google Scholar]
- Payton ME, Greenstone MH, Schenker N. Overlapping confidence intervals or standard error intervals: what do they mean in terms of statistical significance? J Insect Sci. 2003;3:34. doi: 10.1673/031.003.3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike N. Using false discovery rates for multiple comparisons in ecology and evolution. Methods Ecol Evol. 2011;2:278–282. doi: 10.1111/j.2041-210X.2010.00061.x. [DOI] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, Heisterkamp S, Van Willigen B (2020) nlme: Linear and Nonlinear Mixed Effects Models
- Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41:590–596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey JP, Reinert LK, Harper LK, Woodhams DC, Rollins-Smith LA. Immune defenses against Batrachochytrium dendrobatidis, a fungus linked to global amphibian declines, in the south african clawed frog, Xenopus laevis. Infect Immun. 2010;78:3981–3992. doi: 10.1128/IAI.00402-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebollar EA, Hughey MC, Medina D, Harris RN, Ibáñez R, Belden LK. Skin bacterial diversity of panamanian frogs is associated with host susceptibility and presence of Batrachochytrium dendrobatidis. ISME J. 2016;10:1682–1695. doi: 10.1038/ismej.2015.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebollar EA, Simonetti SJ, Shoemaker WR, Harris RN. Direct and indirect horizontal transmission of the antifungal probiotic bacterium Janthinobacterium lividum on Green frog (Lithobates clamitans) tadpoles. Appl Environ Microbiol. 2016;82:2457–2466. doi: 10.1128/AEM.04147-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebollar EA, Martínez-Ugalde E, Orta AH. The amphibian skin microbiome and its protective role against chytridiomycosis. Herpetologica. 2020;76:167–177. doi: 10.1655/0018-0831-76.2.167. [DOI] [Google Scholar]
- Richards-Zawacki CL. Thermoregulatory behaviour affects prevalence of chytrid fungal infection in a wild population of panamanian golden frogs. Proc R Soc B Biol Sci. 2010;277:519–528. doi: 10.1098/rspb.2009.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robak MJ, Richards-Zawacki CL (2018) Temperature-dependent effects of cutaneous bacteria on a frog’s tolerance of fungal infection. Front Microbiol 9. 10.3389/fmicb.2018.00410. :Article 410 [DOI] [PMC free article] [PubMed]
- Rowley JJ, Alford RA. Hot bodies protect amphibians against chytrid infection in nature. Sci Rep. 2013;3:1515. doi: 10.1038/srep01515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruxton GD, Beauchamp G. Time for some a priori thinking about post hoc testing. Behav Ecol. 2008;19:690–693. doi: 10.1093/beheco/arn020. [DOI] [Google Scholar]
- Savage AE, Zamudio KR. Adaptive tolerance to a pathogenic fungus drives major histocompatibility complex evolution in natural amphibian populations. Proc R Soc B Biol Sci. 2016;283:20153115. doi: 10.1098/rspb.2015.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele BC, Pasmans F, Skerratt LF, Berger L, Martel A, Beukema W, Acevedo AA, Burrowes PA, Carvalho T, Catenazzi A, De la Riva I, Fisher MC, Flechas SV, Foster CN, Frías-Álvarez P, Garner TWJ, Gratwicke B, Guayasamin JM, Hirschfeld M, Kolby JE, Kosch TA, La Marca E, Lindenmayer DB, Lips KR, Longo AV, Maneyro R, McDonald CA, Mendelson J, III, Palacios-Rodriguez P, Parra-Olea G, Richards-zawacki CL, Rödel M-O, Rovito SM, Soto-Azat C, Toledo LF, Voyles J, Weldon C, Whitfield SM, Wilkinson M, Zamudio KR, Canessa Amphibian fungal panzootic causes catastrophic and ongoing loss of biodiversity. Sci (80-) 2019;363:1459–1463. doi: 10.1126/science.aav0379. [DOI] [PubMed] [Google Scholar]
- Semlitsch RD, Scott DE, Pechmann JH. Time and size at metamorphosis related to adult fitness in Ambystoma talpoideum. Ecology. 1988;69:184–192. doi: 10.2307/1943173. [DOI] [Google Scholar]
- Sherman E, Baldwin L, Fernandez G, Deurell E. Fever and thermal tolerance in the toad Bufo marinus. J Therm Biol. 1998;16:297–301. doi: 10.1016/0306. [DOI] [Google Scholar]
- Shine R, Amiel J, Munn AJ, Stewart M, Vyssotski AL, Lesku JA. Is “cooling then freezing” a humane way to kill amphibians and reptiles? Biol Open. 2015;4:760–763. doi: 10.1242/bio.012179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simberloff D, Martin JL, Genovesi P, Maris V, Wardle DA, Aronson J, Courchamp F, Galil B, García-Berthou E, Pascal M, Pyšek P, Sousa R, Tabacchi E, Vilà M. Impacts of biological invasions: what’s what and the way forward. Trends Ecol Evol. 2013;28:58–66. doi: 10.1016/j.tree.2012.07.013. [DOI] [PubMed] [Google Scholar]
- Speth C, Rambach G, Wurzner R, Lass-Florl C. Complement and fungal pathogens: an update. Mycoses. 2008;51:477–496. doi: 10.1111/j.1439-0507.2008.01597.x. [DOI] [PubMed] [Google Scholar]
- Spitzen-van der Sluijs A, Martel A, Asselberghs J, Bales EK, Beukema W, Bletz MC, Dalbeck L, Goverse E, Kerres A, Kinet T, Kirst K, Laudelout A, Marin da Fonte LF, Nöllert A, Ohlhoff D, Sabino-Pinto J, Schmidt BR, Speybroeck J, Spikmans F, Steinfartz S, Veith M, Vences M, Wagner N, Pasmans F, Lötters S. Expanding distribution of lethal amphibian fungus Batrachochytrium salamandrivorans in Europe. Emerg Infect Dis. 2016;22:1286–1288. doi: 10.3201/eid2207.160109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallósi B, Peters A, Ehlers R-U. Steinernema bicornutum sp. n. (Rhabditida: Steinernematidae) from Vojvodina, Yugoslavia. Russ J Nematol. 1995;3:71–80. [Google Scholar]
- Tempone AG, de Souza Carvalho Melhem M, Oliveira Prado F, Motoie G, Mitsuyoshi Hiramoto R, Maria Antoniazzi M, Fernando Baptista Haddad C, Jared C. Amphibian secretions for drug discovery studies: a search for new antiparasitic and antifungal compounds. Lett Drug Des Discov. 2007;4:67–73. doi: 10.2174/157018007778992856. [DOI] [Google Scholar]
- Teplitski M, Ritchie K. How feasible is the biological control of coral diseases? Trends Ecol Evol. 2009;24:378–385. doi: 10.1016/j.tree.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Ujszegi J, Ludányi K, Móricz ÁM, Krüzselyi D, Drahos L, Drexler T, Németh MZ, Vörös J, Garner TW, Hettyey A. Exposure to Batrachochytrium dendrobatidis affects chemical defences in two anuran amphibians, Rana dalmatina and Bufo bufo. BMC Ecol Evol. 2021;21:135. doi: 10.1186/s12862-021-01867-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USEPA (2002) Dilution water. In: Methods for Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater and Marine Organisms, 5th edn. Office of Water, U.S. Environmental Protection Agency, Washington, D.C., EPA-821-R-02-012, p 33
- Větrovský T, Baldrian P, Morais D. SEED 2: a user-friendly platform for amplicon high-throughput sequencing data analyses. Bioinformatics. 2018;34:2292–2294. doi: 10.1093/bioinformatics/bty071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voyles J, Young S, Berger L, Campbell C, Voyles WF, Dinudom A, Cook D, Webb R, Alford RA, Skerratt LF, Speare R. Pathogenesis of chytridiomycosis, a cause of catastrophic amphibian declines. Sci (80-) 2009;326:582–585. doi: 10.1126/science.1176765. [DOI] [PubMed] [Google Scholar]
- Voyles J, Woodhams DC, Saenz V, Byrne AQ, Perez R, Rios-Sotelo G, Ryan MJ, Bletz MC, Sobell FA, McLetchie S, Reinert L, Rosenblum EB, Rollins-Smith LA, Ibáñez R, Ray JM, Griffith EJ, Ross H, Richards-Zawacki CL. Shifts in disease dynamics in a tropical amphibian assemblage are not due to pathogen attenuation. Sci (80-) 2018;359:1517–1519. doi: 10.1126/science.aao4806. [DOI] [PubMed] [Google Scholar]
- Vozik D, Bélafi-Bakó K, Hevesi M, Böszörményi E, Fodor A. Effectiveness of a peptide-rich fraction from Xenorhabdus budapestensis culture against fire blight disease on apple blossoms. Not Bot Horti Agrobot Cluj-Napoca. 2015;43:547–553. doi: 10.15835/nbha4329997. [DOI] [Google Scholar]
- Vredenburg VT, Knapp RA, Tunstall TS, Briggs CJ. Dynamics of an emerging disease drive large-scale amphibian population extinctions. Proc Natl Acad Sci. 2010;107:9689–9694. doi: 10.1073/pnas.0914111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake DB, Vredenburg VT. Are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proc Natl Acad Sci. 2008;105:11466–11473. doi: 10.17226/12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walke JB, Harris RN, Reinert LK, Rollins-Smith LA, Woodhams DC. Social immunity in amphibians: evidence for vertical transmission of innate defenses. Biotropica. 2011;43:396–400. doi: 10.1111/j.1744-7429.2011.00787.x. [DOI] [Google Scholar]
- Watzel J, Sarawi S, Duchardt-Ferner E, Bode HB, Wöhnert J. NMR resonance assignments for a docking domain pair with an attached thiolation domain from the PAX peptide-producing NRPS from Xenorhabdus cabanillasii. Biomol NMR Assign. 2021;15:229–234. doi: 10.1007/s12104-021-10010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenski SL, Kolbert D, Grammbitter GL, Bode HB. Fabclavine biosynthesis in X. szentirmaii: shortened derivatives and characterization of the thioester reductase FclG and the condensation domain-like protein FclL. J Ind Microbiol Biotechnol. 2019;46:565–572. doi: 10.1007/s10295-018-02124-8. [DOI] [PubMed] [Google Scholar]
- Wenski SL, Cimen H, Berghaus N, Fuchs SW, Hazir S, Bode HB. Fabclavine diversity in Xenorhabdus bacteria. Beilstein J Org Chem. 2020;16:956–965. doi: 10.3762/bjoc.16.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhams DC, Alford RA, Marantelli G. Emerging disease of amphibians cured by elevated body temperature. Dis Aquat Organ. 2003;55:65–67. doi: 10.3354/dao055065. [DOI] [PubMed] [Google Scholar]
- Woodhams DC, Vredenburg VT, Simon MA, Billheimer D, Shakhtour B, Shyr Y, Briggs CJ, Rollins-Smith LA, Harris RN. Symbiotic bacteria contribute to innate immune defenses of the threatened mountain yellow-legged frog, Rana muscosa. Biol Conserv. 2007;138:390–398. doi: 10.1016/j.biocon.2007.05.004. [DOI] [Google Scholar]
- Woodhams DC, Geiger CC, Reinert LK, Rollins-Smith LA, Lam B, Harris RN, Briggs CJ, Vredenburg VT, Voyles J. Treatment of amphibians infected with chytrid fungus: learning from failed trials with itraconazole, antimicrobial peptides, bacteria, and heat therapy. Dis Aquat Organ. 2012;98:11–25. doi: 10.3354/dao02429. [DOI] [PubMed] [Google Scholar]
- Woodhams DC, LaBumbard BC, Barnhart KL, Becker MH, Bletz MC, Escobar LA, Flechas SV, Forman ME, Iannetta AA, Joyce MD, Rabemananjara F, Gratwicke B, Vences M, Minbiole KP. Prodigiosin, violacein, and volatile organic compounds produced by widespread cutaneous bacteria of amphibians can inhibit two Batrachochytrium fungal pathogens. Microb Ecol. 2018;75:1049–1062. doi: 10.1007/s00248-017-1095-7. [DOI] [PubMed] [Google Scholar]
- Woodward A, Berger L, Skerratt LF. In vitro sensitivity of the amphibian pathogen Batrachochytrium dendrobatidis to antifungal therapeutics. Res Vet Sci. 2014;97:365–367. doi: 10.1016/j.rvsc.2014.06.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used in the analyses will be available from Figshare Repository upon publication. 10.6084/m9.figshare.21229439.