Abstract
Pyrraline, one of advanced glycation end-products, is formed in advanced Maillard reactions. It was reported that the presence of pyrraline was tested to be associated with nephropathy and diabetes. Pyrraline might result in potential health risks because many modern diets are heat processed. In the study, an integrated metabolomics by ultra-high-performance liquid chromatography with mass spectrometry was used to evaluate the effects of pyrraline on metabolism in rats. Thirty-two metabolites were identified as differential metabolites. Linolenic acid metabolism, phenylalanine, tyrosine and tryptophan biosynthesis, arachidonic acid metabolism, tyrosine metabolism and glycerophospholipid metabolism were the main perturbed networks in this pathological process. Differential metabolites and metabolic pathways we found give new insights into studying the toxic molecular mechanisms of pyrraline.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10068-023-01256-7.
Keywords: Mass spectrometry (MS), Food safety, Metabolomic, Untargeted metabolite profiling, Pyrraline
Introduction
Advanced glycation end-products (AGEs) are formed in advanced Maillard reactions, including pyrraline, pentosidine, carboxymethyllysine, and others (Liu et al., 2017). AGEs play a crucial role in process of aging, chronic kidney function harm, diabetes mellitus, atherosclerosis, and Alzheimer’s disease (Li and Yu, 2018). It has been indicated that the level of pyrraline in serum of people with diabetes is higher than that of people with good health (Van Nguyen, 2006). It was reported that pyrraline was shown to be associated with renal failure and diabetes (Aso et al., 2004). As many modern foods are heat processed, they likely have high levels AGEs, which including pyrraline. It might result in potential health risks because of the daily diet (Sharma et al., 2015).
A global metabolomics study, which aims to find some changes in metabolites and pathways, is a powerful tool to study metabolic state of an organism (Chang et al., 2021; Holmes et al., 2008). Analysis of the metabolome has displayed remarkable potential in finding disordered metabolites and pathways, which caused by the endogenous or exogenous disturbance. It gives global insights on the hidden metabolic changes in organism, which can be used to understand living mechanisms better (Hu et al., 2014, 2019; Johnson et al., 2015). Mass spectrometry (MS) and nuclear magnetic resonance (NMR) are two methods to identity and quantify metabolites on a global scale (Goodman et al., 2020; He et al., 2022; Pareek et al., 2020). NMR metabolomics allows quick detection, small sample amounts and gives structural information (Li et al., 2020). However, the sensitivity of NMR is lower than MS.
To this day, there is no study on serum of rats exposed to pyrraline by metabolomics. In the study, integrated metabolomics by ultra-high-performance liquid chromatography with mass spectrometry (UHPLC-MS) was used to evaluate effects of pyrraline on metabolism in rats. The robust global metabolomics method was utilized to find more differential metabolites to comprehensively uncover underlying toxic molecular mechanisms of pyrraline.
Materials and methods
Chemicals and reagents
All standard substances (purity > 98%) for compound identification and 4-chloro-dl-phenylalanine were purchased from Sigma-Aldrich or Sigma (St. Louis, MO, USA). HPLC-grade methanol and formic acid were gotten from Tedia (Fairfield, OH, USA). Pyrraline (purity > 99%) was purchased from J&K scientific. Ultra high purity water was generated by a Milli-Q water system (Millipore Corp., Billerica, MA, USA). Other chemicals were all analytical grade.
Animals and treatments
Thirty male 5- to 6-week-old SD rats were bought from the Laboratory Animal Center of Peking University Health Science Center (Beijing, China). Animals were acclimated for one week before the experiment. They were kept in an environmentally controlled breeding room (temperature 22 ± 1 ℃, humidity 60 ± 5%, and 12-h dark/light cycle). They were offered with standard laboratory food and water. All experimental processes were conducted according to the European Community guidelines for using experimental animals. The study procedure was agreed by the Animal Care and Use Committee of Peking University Health Science Center. All rats were randomly assigned to three groups: high dose group (n = 10, HD) and low dose group (n = 10, LD) were injected 4.5 mg/kg and 0.5 mg/kg of pyrraline dissolved in normal saline each day by tail vein respectively, and the control group (n = 10, ND) received normal saline by the same method. All rats were fed and treated for three days. Body weight was noted every day. At the end of experiment, animals abstained from food for 12 h and then were sacrificed by collecting blood from eye veniplex. Blood was centrifuged at the speed of 3000×g for 10 min at 4 ℃. The supernatant was stored at − 80 ℃ until metabolomics analysis.
Sample processing
Serum samples were thawed at 4 ℃ before analysis. Added eight times methanol to serum and centrifuged at 13,000 rpm for 15 min at 4 ℃ to precipitate the protein. The supernatant was gathered and dried with nitrogen stream. The dried residue was redissolved in solvent, including 80% acetonitrile, and 20% water, and centrifuged at 14,000 rpm, 4 ℃ for 15 min for removing any particulates. After repeated centrifugation three times, the total supernatant was used for metabolomics analysis (Jiang et al., 2020). The quality control (QC) sample was mixed 20 μL of solution from each serum sample.
UHPLC-QTOF-MS analysis
Samples of serum were analyzed by UHPLC-QTOF-MS. UHPLC was performed on an Agilent 1290 UHPLC system (Agilent Corporation) equipped with an ACQUITY UHPLC BEH C18 column (2.1 × 100 mm,1.7 μm, Waters Corporation). The injection volume was 1 μL. The flow was 0.2 mL/min and the column was set at 40 ℃. The optimal mobile phase was made up of 0.1% formic acid in water (A) and methanol (B). The gradient elution program was: 0–5 min, 2–20%B; 5–8 min, 20–75%B; 8–18 min, 75–85%B; 18–33 min, 85–98%B; 33–34 min, 98%B; 34–35 min, 98–2%B. Mass spectrometry (MS) was performed on an Agilent 6530 QTOF-MS with an electrospray ionization (ESI) source in positive and negative modes. The ESI source conditions were as follows: capillary voltage, 3.5 kV (ESI+) and 3.0 kV (ESI−); cone voltage, 60 V; and ion source temperature, 350 ℃. Original data were collected between m/z 50 and 1200 in both modes (Li et al., 2017). QC samples were analyzed in every six samples throughout the whole analytical process to evaluate the stability of the system (Zeng et al., 2021).
Metabolomics data analysis and identification of differential metabolites
Sample data was extracted by Mass Hunter Profinder software (Agilent, USA) for analysis and comparison. Data were normalized and then entered into SIMCA 14.1 (Umetrics, Sweden) for multivariate data analysis. According to variable importance in the projection (VIP) values > 1.0 and p values < 0.05 gained from the Mann–Whitney U test, some differential metabolites were found. Metaboanalyst 5.0 was used to construct pathway. Differential metabolites in serum samples were identified according to accurate data of m/z values, retention time, and MS/MS fragments. All the accurate m/z values were searched in HMDB (http://www.hmdb.ca/) and metlin (https://metlin.scripps.edu/) databases (Zhao et al., 2020). The identified metabolites were verified with authentic standards whenever commercial standard substances were available.
Receiver operator characteristic (ROC) analysis
The ROC analysis was used for assessing the diagnostic ability of the differential metabolites to classify rats into a low or high pyrraline exposure. The area under ROC curve (AUC) from 0.5 to 1.0 showed diagnostic accuracy from no discrimination to good classification (Li et al., 2017). The ROC analysis was completed by SPSS 19.0 software.
Results and discussion
Metabolomics study and metabolite identification
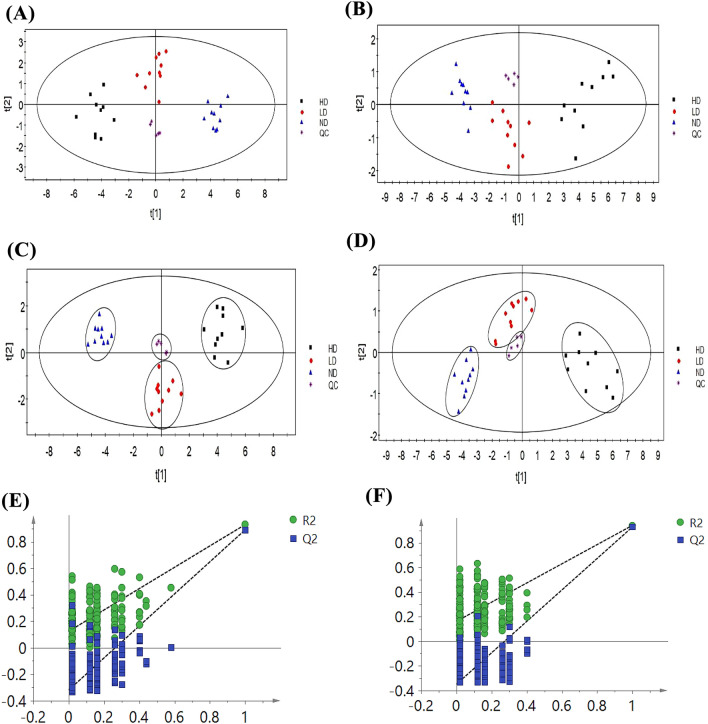
Metabolites in serum samples were profiled by UHPLC-QTOF-MS. Score Plots generated by PCA and PLS-DA presented clear discrimination among them, as shown in Fig. 1.
Fig. 1.
PCA score plots of serum samples in the control group and pyrraline-exposed groups at different dosages by UHPLC-MS methods in positive (A) (R2 = 77.9%, Q2 = 61.3%) and negative (B) (R2 = 83.4%, Q2 = 73%) mode. PLS-DA score plots of serum samples in the control group and pyrraline exposed groups at different dosages by UHPLC-MS methods in positive (C) (R2X = 77.7%, R2Y = 87.7%, Q2 = 85.5%) and negative (D) (R2X = 86.8%, R2Y = 77.3%, Q2 = 67.9%) mode. The 200-permutation test demonstrated no overfitting in the PLS-DA model ((E) positive mode (R2 = (0.0, 0.162), Q2 = (0.0, − 0.349) (F) negative mode (R2 = (0.0, 0.124), Q.2 = (0.0, − 0.319))
The PCA score plots showed that the metabolic phenotypes of HD, LD and ND groups were different. As shown in Fig. 1, both positive and negative ion modes of three groups were completely separated in PLS-DA score plots. In the Fig. 1(A), we can find that HD group generally shifts to the left, and the LD group were between HD group and ND group. It indicated that the toxicity of exposing to pyrraline in rats showed a gradual aggravation with an increase of the dose. As shown in the Fig. 1(C) and (D), samples of quality control (QC) were tightly located in the PLS-DA model. It showed that the equipment was stable and analysis was reliable. It verified the repetitiveness of UHPLC-MS for metabolomics analysis. Quality of PLS-DA model was usually estimated by R2X, R2Y and Q2. R2X and R2Y reveal the explanatory ability of the model, and Q2 reveals the predictive power of the model. Figure 1 displayed good predictive ability of the two models. Validation plots in Fig. 1(E) and (F) were performed using a 200 times permutation test. The results indicated that the model was not over-fitted.
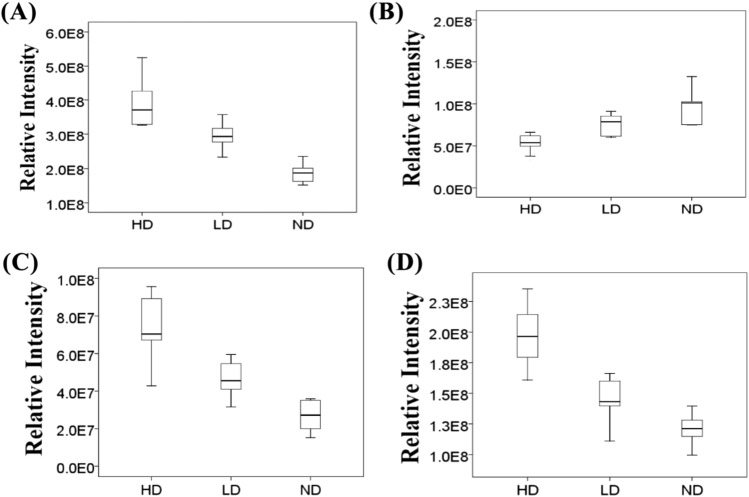
Based on the differential metabolites chosen from VIP values and p values in PLS-DA model, box plots (Fig. 2) were used to describe differences of metabolites among ND, LD and HD groups. The remarkable and dose-dependent decrease in levels of tyrosine was found. Compared with ND group, concentrations of LysoPC (18:3) and PS (22:1) in HD and LD groups increased. The importance of abnormal plasma phospholipid in kidney harm has been proved (Aksenov et al., 2017). In addition, contents of myristic acid were higher in HD group than those in ND and LD groups, showing dose-dependent changes.
Fig. 2.
Box plot of differential metabolites in the study groups. (A) LysoPC (18:3); (B) Tyrosine; (C) Myristic acid; (D) PS (22:1)
Metabolites with VIP values > 1.0 and p value < 0.05 were regarded as differential metabolites. A total of 32 differential metabolites was identified. Detailed information of identified metabolites, including accurate m/z and formula, is shown in Table 1.
Table 1.
Differential metabolites detected in serum of rats from different groups by UHPLC-QTOF-MS
| Metabolite | m/z | Formula | FC (HD/N D) |
VIP | p-value | FDR |
|---|---|---|---|---|---|---|
| Tyrosine | 182.0814 | C9H11NO3 | 0.53 | 1.31 | 6.46 × 10–3 | 2.91 × 10–2 |
| Thymine | 127.0368 | C5H6N2O2 | 1.87 | 1.27 | 7.11 × 10–3 | 2.13 × 10–2 |
| 3-Indolepropionic acid | 190.0858 | C11H11NO2 | 0.23 | 1.30 | 4.67 × 10–6 | 8.41 × 10–5 |
| Tetracosahexaenoic acid | 357.2788 | C24H36O2 | 3.04 | 1.17 | 2.83 × 10–2 | 3.91 × 10–2 |
| γ-CEHC | 279.1591 | C16H22O4 | 0.64 | 1.27 | 4.11 × 10–2 | 4.93 × 10–2 |
| Eicosapentae noic acid | 303.2318 | C20H30O2 | 2.47 | 1.32 | 2.51 × 10–2 | 3.77 × 10–2 |
|
LysoPC (18:2) |
520.3398 |
C26H50NO7 P |
6.23 | 1.41 | 1.74 × 10–3 | 1.57 × 10–2 |
|
LysoPC (20:4) |
544.3398 |
C28H50NO7 P |
3.43 | 1.41 | 2.50 × 10–2 | 4.49 × 10–2 |
|
LysoPC (16:0) |
496.3398 |
C24H50NO7 P |
2.18 | 1.24 | 3.03 × 10–2 | 4.96 × 10–2 |
|
LysoPC (18:3) |
518.3241 |
C26H48NO7 P |
2.09 | 1.40 | 7.11 × 10–3 | 2.56 × 10–2 |
|
LysoPC (18:1) |
522.3554 |
C26H52NO7 P |
2.87 | 1.35 | 2.43 × 10–2 | 4.85 × 10–2 |
|
LysoPC (17:0) |
510.3554 |
C25H52NO7 P |
2.16 | 1.25 | 4.57 × 10–2 | 4.84 × 10–2 |
|
LysoPC (18:0) |
524.3711 |
C26H54NO7 P |
1.67 | 1.27 | 1.53 × 10–2 | 3.93 × 10–2 |
|
LysoPC (20:3) |
546.3554 |
C28H52NO7 P |
1.57 | 1.25 | 4.84 × 10–2 | 4.84 × 10–2 |
| Dodecanoic acid | 336.2897 | C21H34O2 | 3.68 | 1.37 | 1.81 × 10–2 | 4.07 × 10–2 |
| 7-Ketochol esterol | 401.3414 | C27H44O2 | 3.21 | 1.43 | 3.68 × 10–3 | 2.21 × 10–2 |
| 3-Deoxyvit amin | 369.3516 | C27H44 | 1.90 | 1.36 | 4.53 × 10–2 | 5.10 × 10–2 |
| Octadecanamide | 360.3261 | C24H41NO | 1.59 | 1.32 | 2.86 × 10–2 | 3.68 × 10–2 |
| Cholic acid | 407.2803 | C24H40O5 | 4.40 | 1.18 | 7.96 × 10–5 | 1.11 × 10–3 |
| 5-HETE | 319.2279 | C20H32O3 | 2.39 | 1.29 | 2.65 × 10–3 | 9.28 × 10–3 |
| Myristic acid | 227.2017 | C14H28O2 | 2.82 | 1.21 | 9.15 × 10–3 | 1.42 × 10–2 |
| Linolenic acid | 277.2173 | C18H30O2 | 7.11 | 1.29 | 6.20 × 10–4 | 2.89 × 10–3 |
| Hexadecenoic acid | 253.2173 | C16H30O2 | 4.03 | 1.23 | 5.43 × 10–4 | 3.80 × 10–3 |
| Arachidonic acid | 303.2330 | C20H32O2 | 2.70 | 1.28 | 7.45 × 10–3 | 1.74 × 10–2 |
| PS (22:1) | 578.3463 |
C28H54NO9 P |
1.67 | 1.21 | 7.84 × 10–3 | 1.57 × 10–2 |
| 2-Hexyldecanoic acid | 255.2329 | C16H32O2 | 1.67 | 1.18 | 2.23 × 10–2 | 2.40 × 10–2 |
| PE (19:0) | 554.3463 |
C24H50NO7 P |
2.02 | 1.04 | 3.13 × 10–2 | 3.13 × 10–2 |
| 11-Octadecenoic acid | 281.2486 | C18H34O2 | 3.31 | 1.29 | 1.57 × 10–2 | 1.84 × 10–2 |
| PS (22:0) | 580.3620 |
C28H56NO9 P |
1.81 | 1.22 | 1.51 × 10–2 | 1.92 × 10–2 |
| Stearic acid | 283.2643 | C18H36O2 | 1.69 | 1.25 | 6.83 × 10–3 | 1.91 × 10–2 |
| PC (18:0) | 582.3776 |
C26H54NO7 P |
4.40 | 1.19 | 8.73 × 10–3 | 1.53 × 10–2 |
| MG (18:0) | 417.3222 | C21H42O4 | 0.52 | 1.14 | 1.18 × 10–2 | 1.65 × 10–2 |
FC fold change, γ-CEHC γ-carboxymethylhydroxychroman, 5-HETE 5-hydroxyeicosatetraenoic acid, PE phosphatidylethanolamine, PS phosphatidylserine, PC phosphatidylcholine, MG methylglyoxal, LysoPC lysophosphatidylcholine
ROC curve
Differential metabolites were subjected to ROC analysis. The ROC value is proportional to the dependability of the diagnostic effect. ROC analysis results with the MS quantitative data of thirty-two metabolites are shown in Table S1, of which metabolites exhibited good diagnostic (AUC > 0.7). These differential metabolites can distinguish between normal diet rats and relatively high exposure rats with pyrraline exposure.
Altered pathways related to pyrraline exposure levels
In order to visualize the disturbed metabolic pathways in rats fed with pyrraline, metabolomics pathway analysis was made with MetaboAnalyst 5.0 which was a powerful method to discover the significantly related pathways (Hu et al., 2021). Summary of pathway study with MetaboAnalyst 5.0 was in the Fig. 3 and listed in Table S2. In this study, the impact-value threshold gotten from pathway topology analysis was 0.10. Metabolic pathways in which impact-values were more than 0.1 were linolenic acid metabolism, phenylalanine, tyrosine and tryptophan biosynthesis, arachidonic acid metabolism, tyrosine metabolism, glycerophospholipid metabolism. These target pathways showed marked perturbations in rats exposed to pyrraline. The metabolic pathway with the largest impact value is linolenic acid metabolism, as shown in Fig. S1. KEGG database was used to understand high level functions and utilities of biological system. Figure 4 was a general overview of the metabolites and metabolic pathway changed by pyrraline intervention according to the KEGG.
Fig. 3.
Disturbed pathways in response to pyrraline treatment (1) Linolenic acid metabolism. (2) Phenylalanine, tyrosine and tryptophan biosynthesis. (3) Arachidonic acid metabolism. (4) Tyrosine metabolism. (5) Glycerophospholipid metabolism
Fig. 4.
Potential metabolic pathways disturbed in rats induced by pyrraline. “↑”and “↓” indicate changes in compound levels compared to the normal group
Analysis of the metabolic function in serum of all rats
The remarkable decrease in tyrosine concentration may be related to the degradation of protein into amino acids to influence biological functions, including cell cycle progression and inflammatory responses (Drogan et al., 2015; Liang et al., 2016). 3-indolepropionic acid is formed by deamination of tryptophan. In the study, levels of 3-indolepropionic acid significantly decreased in HD group compared with those in ND group. It indicated that exposing to pyrraline can affect the amino acid metabolism, which is consistent with previous study (Wikoff et al., 2009). Hepatotoxicity is related to the enhancement of the endogenous oxygen free radical. 3-indolepropionic acid can protect the cells from oxidative stress and harm. Therefore, decrease in 3-indolepropionic acid level indicates that exposing to pyrraline disturbs the liver metabolism of rats (Wellner et al., 2011).
Levels of octadecanamide were to grow in the pyrraline-exposed group. Fatty acid amides are included in adjustment of different physiological and pathological process, such as inflammation (RoyChoudhury et al., 2016). Octadecanamide is a substrate of fatty acid amide hydrolase (FAAH). Hydrolysis relative rate is affected by the activity of FAAH. Octadecanamide could be a specific biological marker compound for diagnosis between alcohol and non-alcohol caused liver damage (Zhao et al., 2017). Fatty acids of ω-3 and ω-6 are polyunsaturated fatty acids (Guo et al., 2017). Eicosapentaenoic acid (EPA), linolenic acid and arachidonic acid are primary members of ω-6 and ω-3 fatty acids. ω-3 fatty acids activate lipopolysaccharide-stimulated TNF in macrophages by NF-B inhibition, and inflammatory mediators, by competing for metabolic enzymes with arachidonic acid. Arachidonic acid is regarded as the main source of proinflammatory lipid mediators. It is quickly changed into potent inflammatory mediators including leukotrienes, lipoxins and HETEs. Arachidonic acid pathway is linked to some complications of the metabolic syndrome, such as vascular disease (Kwon et al., 2016). Levels of ω-3 and ω-6 fatty acids were improved in pyrraline-exposed group. It is a signal of disturbed lipid metabolism. Fatty acids derivatives are involved in some biological processes, such as inflammation, differentiation and cellular proliferation. It was found that level of 5-HETE was elevated after the pyrraline exposure. 5-HETE gets involved in the formation of leukotrienes and other proinflammatory mediators (Hao and Breyer, 2007). In addition to a necessary structural component of cell membranes, cholesterol acts as a precursor for many important compounds including steroid hormones, oxysterols, and bile acids. Oxysterols are some cholesterol oxidation products. They can be obtained endogenously by autooxidation or by enzymatic reactions, and can be given by food. Oxysterols can pass lipophilic membranes. As a result, if their formation in organisms or their intake with dietary animal fat are too much, oxysterols are helpful for pathogenesis of some diseases (Poli et al., 2013). Oxidation of cholesterol sterol ring is almost finished by the nonenzymatic pathways, resulting in a generation of compounds like 7-ketocholesterol. 7-ketocholesterol has been showed to cause a distinct inflammatory phenotype in endothelial cells and the foam cell production. Thus, these results heavily display a disorder of cholesterol metabolism because of exposing to pyrraline.
In pyrraline-exposed groups, contents of phospholipids including PS (22:1), PE (19:0), PS (22:0), and PC (18:0) were elevated. Phospholipids are lipid compounds including phosphate in their molecular structures. Almost all biological cells have phospholipids. Phospholipids can be classified as glycerol phospholipids and sphingolipids. The glycerol phospholipids include phosphatidylcholine (PC), phosphatidyl inositol (PI), phospholipid acyl ethanol amine (PE), phosphatidylserine (PS), cardiolipin (DG), phosphatidylglycerol (PG), and acetal phospholipids. Phospholipids are irregularly located on the cell membrane. PS is the only one phospholipid that can adjust the function of key proteins in cell membranes. The main function of PS in signal transduction is to transfer the protein kinase C (PKC) to cell membrane by the specific connection of PS and PKC (Zhao et al., 2015). Moreover, PS can influence the activity of other enzymes in signal transduction. Therefore, it was revealed that because of exposing to pyrraline, the related protease activity is declined, signal transduction is barred, and phospholipid metabolism is disrupted. Phosphatidylcholines are structural constituents of cell membranes in animals. It has been reported that phosphatidylcholines are biological marker compounds for chronic glomerulonephritis and diabetic nephropathy. Earlier studies have indicated the prominent role of plasma phosphatidylcholine disorders in renal damage (Quansah et al., 2015), but few studies have been done on plasma phospholipids, such as lysophosphatidylcholine, which might be important factors in pathogenesis of kidney harms. Lysophosphatidylcholines are metabolites of phosphatidylcholines. Level of lysophosphatidylcholine can be a clinical diagnostic marker that shows changes of pathophysiology. Lysophosphatidylcholines have close ties with oxidative stress and immune inflammation (Igarashi and Kashiwagi, 2011). Levels of lysophosphatidylcholines in serum of rats exposed to pyrraline revealed remarkable difference. When body is suffering from some bad conditions, reactive nitrogen species and reactive oxygen species (ROS) are generated unusually, and the rate of production are faster than that of removing oxides from body. The balance between oxidation and antioxidant system is broken, which eventually leads to tissue harm. Unsaturated lysophosphatidylcholines are mainly generated from the hydrolysis of liver phosphatidylcholine by phospholipase A2, while saturated lysophosphatidylcholines is often transferring sn-2 fatty acid from phosphatidylcholine by lecithin cholesterol acyltransferase (Sas et al., 2015). This study revealed that up-regulated lysoPC (16:0), lysoPC (17:0), lysoPC (18:0), lysoPC (18:1), lysoPC (18:2), lysoPC (18:3) and lysoPE (22:4) were obviously found in rats exposed to pyrraline. Clearly, rats suffered high oxidative stress because of decrease in antioxidant defenses and increase in prooxidant factors. When oxidative stress happened, the production of free radical could activate the phospholipase A2, which can hydrolyse phosphatidylcholine to generate lysophosphatidylcholine. The increased lysophosphatidylcholine could generate excessive ROS which results in the imbalance of oxidation-antioxidation. Moreover, it has been reported that lysophosphatidylcholine is included in proinflammatory process of acute damage or chronic diseases (Bereketoglu and Pradhan, 2019).
As we know, this is the first time that UHPLC-QTOF-MS nontargeted metabolomics analysis has been done for study on serum of rats exposed to pyrraline. Thirty-two metabolites were identified as differential metabolites. Linolenic acid metabolism, phenylalanine, tyrosine and tryptophan biosynthesis, arachidonic acid metabolism, tyrosine metabolism and glycerophospholipid metabolism were the main perturbed networks in this pathological process. The study highlights the evidence for the linkage of pyrraline exposure and adverse health risks. Differential metabolites and metabolic pathways we found give new insights into studying the toxic molecular mechanisms of pyrraline. The potential health risk associated with the exposure to pyrraline, should not be ignored.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 31901808, 31972111, 31871772), Beijing Municipal Education Commission common project (KM201810011012), Beijing Excellent Talents Funding for Youth Scientist Innovation Team (201600026833TD01) and Project of High-level Teacher in Beijing Municipal Universities (IDHT20180506).
Author contributions
CH: Investigation, Methodology, Writing-original draft, Conceptualization. JW: Methodology, Formal analysis, Visualization. FQ: Conceptualization. YL: Conceptualization. FZ: Conceptualization. JW: Conceptualization, Supervision, Funding acquisition. BS: Conceptualization. All authors read and approved the final manuscript.
Declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
The animal care and all experimental processes were conducted according to the European Community guidelines for using experimental animals. The study protocol was agreed by the Animal Care and Use Committee of Peking University Health Science Center. This article does not contain any studies with human participants performed by any of the author.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chuanqin Hu, Email: huchuanqin@btbu.edu.cn.
Jiahui Wang, Email: wjh61166@163.com.
Fangyuan Qi, Email: qifoy0518@163.com.
Yingli Liu, Email: liuyingli@th.btbu.edu.cn.
Fen Zhao, Email: zhaofen@btbu.edu.cn.
Jing Wang, Email: wangjing@th.btbu.edu.cn.
Baoguo Sun, Email: sunbg@btbu.edu.cn.
References
- Aksenov AA, da Silva R, Knight R, Lopes NP, Dorrestein PC. Global chemical analysis of biology by mass spectrometry. Nature Reviews Chemistry. 2017;1:0054. doi: 10.1038/s41570-017-0054. [DOI] [Google Scholar]
- Aso Y, Takanashi K, Sekine K, Yoshida N, Takebayashi K, Yoshihara K, Inukai T. Dissociation between urinary pyrraline and pentosidine concentrations in diabetic patients with advanced nephropathy. Journal of Laboratory and Clinical Medicine. 2004;144:92–99. doi: 10.1016/j.lab.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Bereketoglu C, Pradhan A. Comparative transcriptional analysis of methylparaben and propylparaben in zebrafish. Science of the Total Environment. 2019;671:129–139. doi: 10.1016/j.scitotenv.2019.03.358. [DOI] [PubMed] [Google Scholar]
- Chang HY, Colby SM, Du XX, Gomez JD, Helf MJ, Kechris K, Kirkpatrick CR, Li SZ, Patti GJ, Renslow RS, Subramaniam S, Verma M, Xia JG, Young JD. A Practical Guide to Metabolomics Software Development. Analytical Chemistry. 2021;93:1912–1923. doi: 10.1021/acs.analchem.0c03581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drogan D, Dunn WB, Lin WC, Buijsse B, Schulze MB, Langenberg C, Brown M, Floegel A, Dietrich S, Rolandsson O, Wedge DC, Goodacre R, Forouhi NG, Sharp SJ, Spranger J, Wareham NJ, Boeing H. Untargeted metabolic profiling identifies altered serum metabolites of type 2 diabetes mellitus in a prospective, nested case control study. Clinical Chemistry. 2015;61:487–497. doi: 10.1373/clinchem.2014.228965. [DOI] [PubMed] [Google Scholar]
- Goodman RP, Markhard AL, Shah HD, Sharma R, Skinner OS, Clish CB, Deik A, Patgiri A, Hsu YHH, Masia R, Noh HL, Suk S, Goldberger O, Hirschhorn JN, Yellen G, Kim JK, Mootha VK. Hepatic NADH reductive stress underlies common variation in metabolic traits. Nature. 2020;583:122–127. doi: 10.1038/s41586-020-2337-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C, Ma JF, Zhong QH, Zhao MY, Hu TX, Chen T, Qiu LX, Wen LP. Curcumin improves alcoholic fatty liver by inhibiting fatty acid biosynthesis. Toxicology and Applied Pharmacology. 2017;328:1–9. doi: 10.1016/j.taap.2017.05.001. [DOI] [PubMed] [Google Scholar]
- Hao CM, Breyer MD. Physiologic and pathophysiologic roles of lipid mediators in the kidney. Kidney International. 2007;71:1105–1115. doi: 10.1038/sj.ki.5002192. [DOI] [PubMed] [Google Scholar]
- He Z, Zhang HN, Song YY, Yang Z, Cai ZW. Exposure to ambient fine particulate matter impedes the function of spleen in the mouse metabolism of high-fat diet. Journal of Hazardous Materials. 2022;423:127129. doi: 10.1016/j.jhazmat.2021.127129. [DOI] [PubMed] [Google Scholar]
- Holmes E, Wilson ID, Nicholson JK. Metabolic phenotyping in health and disease. Cell. 2008;134:714–717. doi: 10.1016/j.cell.2008.08.026. [DOI] [PubMed] [Google Scholar]
- Hu CQ, Li R, Wang JH, Liu YL, Wang J, Sun BG. Untargeted metabolite profiling of liver in mice exposed to 2-methylfuran. Journal of Food Science. 2021;86:242–250. doi: 10.1111/1750-3841.15549. [DOI] [PubMed] [Google Scholar]
- Hu CQ, Lin SH, Cai ZW. Fatty acid profiles reveal toxic responses in adipose tissue of C57BL/6J mice exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Analytical Methods. 2014;6:8207–8211. doi: 10.1039/C4AY01479K. [DOI] [Google Scholar]
- Hu CQ, Zhang Y, Liu GR, Liu YL, Wang J, Sun BG. Untargeted metabolite profiling of adipose tissue in hyperlipidemia rats exposed to hawthorn ethanol extracts. Journal of Food Science. 2019;84:717–725. doi: 10.1111/1750-3841.14491. [DOI] [PubMed] [Google Scholar]
- Igarashi K, Kashiwagi K. Use of polyamine metabolites as markers for stroke and renal failure. Methods in Molecular Biology. 2011;720:395–408. doi: 10.1007/978-1-61779-034-8_25. [DOI] [PubMed] [Google Scholar]
- Jiang YC, Li YF, Zhou L, Zhang DP. Comparative metabolomics unveils molecular changes and metabolic networks of syringin against hepatitis B mice by untargeted mass spectrometry. RSC Advances. 2020;10:461–473. doi: 10.1039/C9RA06332C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CH, Dejea CM, Edler D, Hoang LT, Santidrian AF, Felding BH, Ivanisevic J, Cho K, Wick EC, Hechenbleikner EM, Uritboonthai W, Goetz L, Casero RA, Pardoll DM, White JR, Patti GJ, Sears CL, Siuzdak G. Metabolism Links Bacterial Biofilms and Colon Carcinogenesis. Cell Metabolism. 2015;21:891–897. doi: 10.1016/j.cmet.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon HN, Phan HD, Xu WJ, Ko YJ, Park S. Application of a smartphone metabolomics platform to the authentication of Schisandra sinensis. Phytochemical Analysis. 2016;27:199–205. doi: 10.1002/pca.2617. [DOI] [PubMed] [Google Scholar]
- Li H, Wang M, Liang QD, Jin SN, Su XJ, Jiang YQ, Pan XY, Zhou YQ, Peng Y, Zhang B, Zhou AF, Zhang YM, Chen Z, Cao JX, Zhang HL, Xia W, Zheng TZ, Cai ZW, Li YY, Xu SQ. Urinary metabolomics revealed arsenic exposure related to metabolic alterations in general Chinese pregnant women. Journal of Chromatography a. 2017;1479:145–152. doi: 10.1016/j.chroma.2016.12.007. [DOI] [PubMed] [Google Scholar]
- Li H, Yu SJ. Review of pentosidine and pyrraline in food and chemical models: formation, potential risks and determination. Journal of the Science of Food and Agriculture. 2018;98:3225–3233. doi: 10.1002/jsfa.8853. [DOI] [PubMed] [Google Scholar]
- Li TT, Xu S, Bi JL, Huang ST, Fan BL, Qian CQ. Metabolomics study of polysaccharide extracts from Polygonatum sibiricum in mice based on H-1 NMR technology. Journal of the Science of Food and Agriculture. 2020;100:4627–4635. doi: 10.1002/jsfa.10523. [DOI] [PubMed] [Google Scholar]
- Liang YH, Tang CL, Lu SY, Cheng B, Wu F, Chen ZN, Song F, Ruan JX, Zhang HY, Song H, Zheng H, Su ZH. Serum metabonomics study of the hepatoprotective effect of Corydalis saxicola Bunting on carbon tetrachloride-induced acute hepatotoxicity in rats by H-1 NMR analysis. Journal of Pharmaceutical and Biomedical Analysis. 2016;129:70–79. doi: 10.1016/j.jpba.2016.06.033. [DOI] [PubMed] [Google Scholar]
- Liu HL, Mu L, Chen XM, Wang J, Wang S, Sun BG. Core-Shell Metal-Organic Frameworks/Molecularly Imprinted Nanoparticles as Absorbents for the Detection of Pyrraline in Milk and Milk Powder. Journal of Agricultural and Food Chemistry. 2017;65:986–992. doi: 10.1021/acs.jafc.6b05429. [DOI] [PubMed] [Google Scholar]
- Pareek V, Tian H, Winograd N, Benkovic SJ. Metabolomics and mass spectrometry imaging reveal channeled de novo purine synthesis in cells. Science. 2020;368:283–290. doi: 10.1126/science.aaz6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli G, Biasi F, Leonarduzzi G. Oxysterols in the pathogenesis of major chronic diseases. Redox Biology. 2013;1:125–130. doi: 10.1016/j.redox.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quansah R, Armah FA, Essumang DK, Luginaah I, Clarke E, Marfoh K, Cobbina SJ, Nketiah-Amponsah E, Namujju PB, Obiri S, Dzodzomenyo M. Association of arsenic with adverse pregnancy outcomes/infant mortality: a systematic review and meta-analysis. Environmental Health Perspectives. 2015;123:412–421. doi: 10.1289/ehp.1307894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RoyChoudhury S, Mishra BP, Khan T, Chattopadhayay R, Lodh I, Ray CD, Bose G, Sarkar HS, Srivastava S, Joshi MV, Chakravarty B, Chaudhury K. Serum metabolomics of Indian women with polycystic ovary syndrome using H-1 NMR coupled with a pattern recognition approach. Molecular Biosystems. 2016;12:3407–3416. doi: 10.1039/C6MB00420B. [DOI] [PubMed] [Google Scholar]
- Sas KM, Karnovsky A, Michailidis G, Pennathur S. Metabolomics and diabetes: analytical and computational approaches. Diabetes. 2015;64:718–732. doi: 10.2337/db14-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma C, Kaur A, Thind SS, Singh B, Raina S. Advanced glycation end-products (AGEs): an emerging concern for processed food industries. Journal of Food Science and Technology. 2015;52:7561–7576. doi: 10.1007/s13197-015-1851-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Nguyen C. Toxicity of the AGEs generated from the Maillard reaction on the relationship of food-AGEs and biological-AGEs. Molecular Nutrition and Food Research. 2006;50:1140–1149. doi: 10.1002/mnfr.200600144. [DOI] [PubMed] [Google Scholar]
- Wellner A, Huettl C, Henle T. Formation of Maillard Reaction Products during Heat Treatment of Carrots. Journal of Agricultural and Food Chemistry. 2011;59:7992–7998. doi: 10.1021/jf2013293. [DOI] [PubMed] [Google Scholar]
- Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, Siuzdak G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:3698–3703. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng T, Liang YS, Chen JY, Cao GD, Yang Z, Zhao XC, Tian JL, Xin X, Lei B, Cai ZW. Urinary metabolic characterization with nephrotoxicity for residents under cadmium exposure. Environment International. 2021;154:106646. doi: 10.1016/j.envint.2021.106646. [DOI] [PubMed] [Google Scholar]
- Zhao DS, Jiang LL, Fan YX, Wang LL, Li ZQ, Shi W, Li P, Li HJ. Investigation of Dioscorea bulbifera rhizome-induced hepatotoxicity in rats by a multisample integrated metabolomics approach. Chemical Research in Toxicology. 2017;30:1865–1873. doi: 10.1021/acs.chemrestox.7b00176. [DOI] [PubMed] [Google Scholar]
- Zhao HZ, Zheng YY, Zhu L, Xiang L, Zhou YQ, Li JF, Fang J, Xu SQ, Xia W, Cai ZW. Paraben exposure related to purine metabolism and other pathways revealed by mass spectrometry-based metabolomics. Environmental Science Technology. 2020;54:3447–3454. doi: 10.1021/acs.est.9b07634. [DOI] [PubMed] [Google Scholar]
- Zhao YY, Vaziri ND, Lin RC. Lipidomics: new insight into kidney disease. Advances in Clinical Chemistry. 2015;68:153–175. doi: 10.1016/bs.acc.2014.11.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.