Abstract
In this study, we hypothesized that Protaetia brevitarsis larvae (PBL) protein hydrolysates, which have been previously reported to exhibit strong antioxidant activity, might protect liver cells against oxidative stress-induced injury. Thus, the cytoprotective effects and related mechanisms of PBL hydrolysates were investigated in AML12 liver cells. Among PBL hydrolysates prepared by three different proteases, the PBL flavouryzme hydrolysate showed significantly higher protective effect against H2O2-induced cytotoxicity than other hydrolysates in AML12 cells. Further mechanistic studies showed that pretreatment with PFH reduces cellular level of reactive oxygen species through induction of Nrf2-mediated antioxidant enzymes such as catalase and heme oxygenase-1. In addition, the free amino acid analysis revealed that PFH was rich in branched-chain amino acids, aromatic amino acids, and hydrophobic amino acids as compared to other hydrolysates, which could contribute to its hepatoprotective effect. These findings suggest that PFH represents a potential source of nutraceuticals that supports liver functions.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10068-023-01279-0.
Keywords: Antioxidant enzymes, Liver, Nrf2, Protaetia brevitarsis larvae, Protein hydrolysates, Reactive oxygen species
Introduction
The liver exerts various critical functions to maintain homeostasis, and is also the first main organ to be exposed to a large amount of ROS produced during oxygen metabolism and detoxification of xenobiotics (Vargas-Mendoza et al., 2014). Excessive oxidative stress often causes an imbalance between oxidation and reduction and reacts with DNA, lipids, and proteins in hepatocytes, leading to liver injury (Hiraganahalli et al., 2012; Liu et al., 2017). However, liver cells produce two types of antioxidants namely the non-enzymatic antioxidants and enzymatic antioxidants, including glutathione (GSH), thioredoxin, catalase (CAT), heme oxygenase-1 (HO-1), glutathione reductase (GR), glutathione peroxidase (GPx), and superoxide dismutase (SOD), and these cellular antioxidants are known to play a vital role in preventing oxidative stress-related liver disorders, such as hepatic steatosis, hepatitis, liver fibrosis, and liver cancer (Ighodaro and Akinloye, 2018).
Numerous studies have been carried out to develop natural hepatoprotective agents, and most of them have been conducted on plant extracts and plant-derived polyphenolic compounds (Hiraganahalli et al., 2012; Je and Lee, 2015; Udenigwe and Aluko, 2012; Vargas-Mendoza et al., 2014). In addition, food proteins have also been used as raw materials for the development of bioactive products that support liver functions. Bioactive protein hydrolysates prepared from food proteins and the peptides contained therein have been reported to have various physiological activities as well as hepatoprotective effect, and are thus receiving a great deal of attention (Foegeding and Davis, 2011; Undenigwe and Aluko, 2012). Bioactive peptides are derived from diverse protein sources, such as dairy products, fish, cereal, beans, and animal products, and physiological activity of the peptides is source-dependent (Kitts and Weiler, 2003). In addition, insects have recently emerged as protein sources for the production of bioactive peptides (Cho et al., 2019; Lee et al., 2017; Xia et al., 2013; Yu et al., 2017).
Since the Food and Agriculture Organization of the United Nations (FAO) reported that insects are potential protein sources for humans, extracts, protein hydrolysates, and peptides from edible insects with physiological activity have been discovered. And a wide range of their bioactive potential has been found, including antihypertensive, hypolipidemic, antiobesity, anti-inflammatory, antioxidant, and hepatoprotective properties (Bae and Lee, 2020; Cho et al, 2019; Lee et al., 2017; Liu et al., 2019; Nongonierma and FitzGerald, 2017; Sung et al., 2016; Yu et al., 2017). The Korean Ministry of Food and Drug Safety also recently announced seven species of edible insects as new food groups that have nutritional, economic, and environmental value. Among them, larvae of white-spotted flower chafer (Protaetia brevitarsis, PBL), which belongs to the subfamily Cetoniinae, is an excellent nutritional source, as it contains relatively large amounts of essential amino acids, essential fatty acids, and minerals (Ghosh et al., 2017). In Oriental medicine, it has long been used to treat various diseases such as irregular menstruation, strokes, and cataracts, and is known to be particularly effective in treating liver diseases (Yoon et al., 2016). In addition, in several recent studies, PBL was reported to exert a variety of biological functions, including anti-obesity (Ahn et al., 2019), anti-inflammatory (Sung et al., 2016), and angiotensin-converting enzyme inhibitory effects (Bae and Lee, 2020). However, most of these studies have been conducted on aqueous and solvent extracts of PBL, and the bioactive potential of PBL-derived protein hydrolysates or peptides has not been explored in detail.
In our previous studies, we prepared protein hydrolysates containing low molecular weight (LMW) peptides (< 3 kDa) from PBL using a variety of food-grade proteases and determined their direct ROS-scavenging activities (Lee et al., 2017). Therefore, we hypothesized that PBL protein hydrolysates can protect liver cells against oxidative stress-induced cytotoxicity. In this study, we investigated the hepatoprotective effect and its underlying mechanisms of PBL protein hydrolysates in H2O2-treated AML12 mouse hepatocytes.
Materials and methods
Preparation of protein hydrolysates from PBL
PBL hydrolysates were prepared as previously described (Lee et al., 2017). A 4% (w/v) suspension in distilled water of PBL powder (Universal Farm’s Meal, Sunchang, Korea) was prepared, heated at 90 °C for 20 min, and hydrolyzed at 55 °C with each of the three food-grade proteolytic enzymes (flavourzyme, alcalase, and neutrase; an enzyme to PBL ratio was 1:100, v/w) in a shaking incubator at 100 rpm. The hydrolysis was terminated after 8 h of reaction by heating for 20 min at 90 °C. The suspension was centrifuged for 20 min at 13,000 × g and the supernatant was ultrafiltered using a filter device (MWCO, 3 kDa; Merck Millipore Ltd., Burlington, MA) for 2 h at 5,000 × g, followed by additional ultrafiltration with a filter device (MWCO, 1 kDa; Pall Corp., Port Washington, NY). The protein hydrolysates (< 3 kDa and < 1 kDa) were then freeze-dried and stored at –20 °C for further use. A water extract of PBL was also prepared in the same manner without enzyme. The yield was 52% from alcalase hydrolysates, 39% from flavourzyme hydrolysates, 35% from neutrase hydrolysates, and 21% from water extract (Lee et al., 2017).
Cell culture
AML12 mouse liver cell line was purchased from American Type Culture Collection (Manassas, VA) and grown in Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 (Gibco, Grand Island, NY) supplemented with dexamethasone (40 ng/mL; Sigma-Aldrich, St. Louis, MO), insulin-transferrin-selenium (Gibco, Grand Island, NY), and 10% fetal bovine serum. The cells were maintained in a humidified CO2 incubator (5% CO2) at 37 °C.
Cell viability assay
AML12 cells (4 × 104 cells/well in 48 well plates) were seeded, and after 18 h, the cells were treated with each hydrolysate for 24 h. The cells were then treated in a fresh medium with H2O2 (7 mM) for 4 h, alcohol (900 mM) for 24 h, or free fatty acids (4 mM; palmitic acid:oleic acid = 1:1) for 24 h. Cell viability was assessed by an MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay as previously described (Cho et al., 2019).
Detection of intracellular ROS
Intracellular ROS level was measured using 2’,7’-dichlorodihydrofluorescein diacetate (DCF-DA; Sigma-Aldrich, St. Louis, MO) as previously described (Cho and Lee, 2020). In brief, cells (1 × 105 cells/coverslip) were seeded onto glass coverslips (24 × 24 mm) and cultured for 18 h before cells were treated with PFH (PBL flavourzyme hydrolysates) for 24 h. Cells were then treated with H2O2 for 1 h, and then incubated with 25 μM DCF-DA for an additional 30 min. After washing with PBS (pH 7.4), fluorescence was measured using a fluorescence microscope (Carl Zeiss, Oberkochen, Germany).
Determination of enzymatic activity of GPx and CAT
Cells were lysed using reporter lysis buffer (Promega Corp., Madison, WI) and then centrifuged at 15,000 × g for 15 min. The protein content of the resulting supernatant was determined using a BCA assay kit (Thermo Fisher Scientific, Waltham, MA). GPx enzymatic activity was measured using a GPx assay kit (Cayman Chemical, Ann Arbor, MI) according to the manufacturer’s instructions. CAT enzymatic activity was measured according to the method of Cho and Lee (2020). A mixture containing 50 μL of cell lysates, 650 μL of potassium phosphate buffer (50 mM, pH 7.0), and 300 μL of 100 mM H2O2 was incubated at 25 °C. The ultraviolet absorbance of H2O2 was monitored at 240 nm for 1 min. A molar extinction coefficient of H2O2 (0.041/mM/cm) was used to determine the CAT activity. The activity was defined as mmol-reduced H2O2/min/mg protein.
Transfection with small interfering RNA (siRNA)
Cells were transiently transfected with siRNA (100 nM) specific for mouse Nrf2 (siNrf2; Santa Cruz Biotechnology, Inc., Dallas, TX) or scrambled siRNA (100 nM) using Lipofectamine 3000 transfection reagent (Invitrogen Corp., Carlsbad, CA) according to the manufacturer’s instructions.
Analysis of free amino acids
Free amino acid composition was determined by using the Waters AccQ-Fluor Reagent Kit (Waters Co., Milford, MA). AccQ-Fluor reagent was prepared by mixing AccQ-Fluor reagent powder with 1.0 mL of AccQ-Fluor reagent diluent prior to derivatization and heating at 55℃ for 10 min. A 10 µL sample or amino acid standard solution (Thermo Fisher Scientific, Waltham, MA) was transfered to a 1.5 mL micro tube. AccQ-Fulor borate buffer (70 µL) was added to each microtube and briefly vortexed. Then reconstituted AccQ-Fluor reagent (20 µL) was added, vortexed for 10 s, and heated at 55℃ for 10 min. HPLC analysis was carried out using a Shimadzu 06,959 series HPLC system (Kyoto, Japan) with a RF-10AXL fluorescence detector. Mobile phases were solvent A (10% AccQ-Tag Eluent aqueous (v/v)) and solvent B (60% acetonitrile aqueous (v/v)). Gradient flow and other details of the HPLC analysis were as described in Table S2.
Statistical analysis
Statistical significance of differences between groups was determined by either one-way analysis of variance with Duncan’s multiple tests (IBM SPSS 25.0; SPSS Inc., Chicago, IL) or Student t-test (Sigma Plot 10.0; Systat Software, Inc., San Jose, CA). The results are expressed as the mean with standard error of the mean (SEM) of three experiments for each group unless otherwise indicated, and a P value of less than 0.05 was considered statistically significant.
All other Materials and methods are described in the Supplemental materials.
Results and discussion
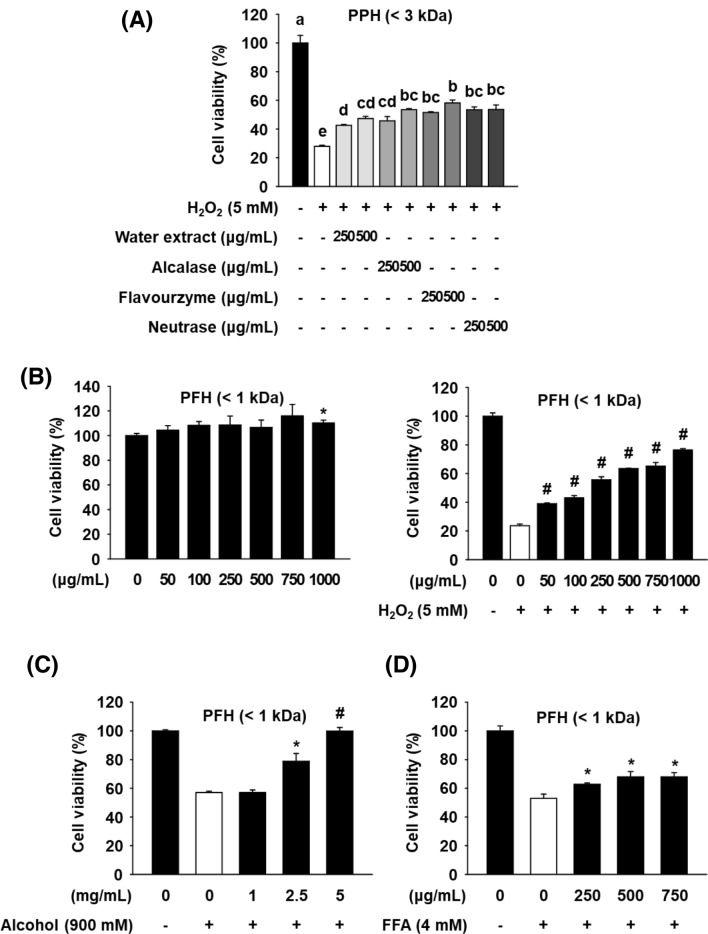
Protective effects of PBL protein hydrolysates (PPHs) on oxidative stress-induced cytotoxicity in AML12 cells
Over the past few years, many studies have demonstrated that enzymatic protein hydrolysates and peptides produced from enzymatically hydrolyzed edible insects exert a variety of biological activities including hepatoprotective effects (Nongonierma and FitzGerald, 2017). Protein hydrolysates and peptides derived from silkworm, for example, have been reported to prevent liver damage caused by oxidative stress through a direct action on ROS and activation of the cellular antioxidant defense system (Xia et al., 2013). More recently, it has also been reported that protein hydrolysates of several other edible insects such as mealworm, the two-spotted cricket (Gryllus bimaculatus) and the Japanese rhinoceros beetle larvae (Allomyrina dichotoma) have direct ROS-scavenging activities (Cho et al., 2019; Ryu et al., 2019; Yu et al., 2017). We observed that the alcalase hydrolysate of mealworm (MAH) and two novel MAH-derived peptides displayed hepatoprotective effect in vitro through activation of the cellular antioxidant system (Cho and Lee, 2020).
In our previous study, PPHs also exhibited a strong scavenging activity toward multiple types of ROS and inhibitory effect on lipid peroxidation (Lee et al., 2017). Since antioxidants are considered as a reasonable strategy to prevent liver injury induced by oxidative stress (Li et al., 2015), in the present study, therefore, we investigated the protective effects of PBL protein hydrolysates against H2O2-induced cytotoxicity in AML12 mouse hepatocytes. We initially performed enzymatic hydrolysis of PBL using five different food-grade proteases (flavourzyme, alcalase, neutrase, papain, and bromelain) and subsequently confirmed that only three proteases, flavourzyme, alcalase, and neutrase, show a high degree of hydrolysis. PPHs prepared using such proteases exert significant ROS scavenging activities (Lee et al., 2017). In the current study, we first compared the protective effects of these three PPHs against H2O2-induced cytotoxicity in AML12 cells. AML12 cells were treated with an aqueous (H2O) extract of PBL (PBLE), the alcalase-PPH (PAH), the flavourzyme-PPH (PFH), or the neutrase-PPH (PNH) at 250 and 500 μg/mL for 24 h; the media were then replaced with fresh media containing H2O2 (5 mM) and the cells cultured for additional 4 h, after which cell viability was measured. Treatment with H2O2 for 4 h caused a significant decrease in cell viability, however, pretreatment with PPHs and aqueous extract of PPL preserved the viability of the H2O2-treated AML12 cells. PFH treatment demonstrated the highest protective effect among all the hydrolysates as well as the aqueous extract (Fig. 1A). In a previous study, an aqueous extract of PBL was reported to exhibit hepatoprotective effects in vitro and in vivo (Chon et al., 2012). However, all the PPHs showed similar or greater protective effects than the aqueous extract on H2O2-treated AML12 cells. The hepatoprotective effect of PFH was also compared to another positive control, MAH, known to have hepatoprotective effects in H2O2-treated AML12 cells (Cho and Lee, 2020). The results indicated that the PFH has a greater protective effect than MAH at all concentrations (Figure S1A).
Fig. 1.
Effect of PFH on H2O2-, alcohol-, or free fatty acids-induced cytotoxicity in AML12 cells. Cells were treated with various concentrations of each hydrolysate for 24 h, the media were replaced with new1 media containing H2O2 (A-B), alcohol (C), or free fatty acids (D), and the cells were incubated for another 4 h. Cell viability was then measured by an MTT assay. The results are presented as mean ± SEM (n ≥ 3) and a different letter indicates a significant difference among groups, according to one-way ANOVA with Duncan’s multiple range test (P < 0.05). *P < 0.05 and #P < 0.001 vs. the H2O2, alcohol, or free fatty acids control. PPH: Protaetia brevitarsis larvae protein hydrolysates
LMW peptides show greater resistance following digestion in the milieu of the gastrointestinal tract than intact proteins and high-molecular weight peptides, and these peptides also exhibit relatively high bioavailability (Udenigwe and Aluko, 2012). Therefore, the effect of PFH (< 3 kDa) was compared with that of PFH (< 1 kDa). First, as a result of confirming the cytotoxicity of the PFHs (< 3 and < 1 kDa) in AML12 cells, both PFHs (100–500 μg/mL) showed no cytotoxicity after 24 h of treatment (Fig. S1C). As shown in Fig. S1B, PFH (< 1 kDa) showed a similar or higher protective effect than PFH (< 3 kDa) at all concentrations, indicating that a large amount of active peptides with hepatoprotective effects are present at LMW (< 1 kDa) fraction. The PFH (< 1 kDa) significantly preserved the viability of the H2O2-treated AML12 cells across all concentration ranges tested and did not display any cytotoxic effects on the cells (Fig. 1B).
We further investigated whether PFH (< 1 kDa) protects AML12 liver cells from cytotoxicity induced by other types of prooxidants, alcohol and free fatty acids (FFA). As shown in Fig. 1C and 1D, treatment with alcohol (900 mM) and FFA (4 mM) for 24 h caused a decrease in cell viability of AML12 cells; however, pretreatment with PFH at 2.5 and 5 mg/mL, but not 1 mg/mL significantly preserved the viability of the alcohol-treated cells. Similarly, pretreatment with PFH at 250–750 μg/mL also significantly protected cells from FFA-induced cytotoxicity. These results suggest that PFH exhibits hepatoprotective effects against cytotoxicity induced by various prooxidants in AML12 cells although the effective doses are different, depending on the type of prooxidants.
PFH regulates intracellular ROS level and activates antioxidant genes
ROS homeostasis in living cells is maintained through a balance between ROS production and ROS reduction by endogeneous antioxidants (Li et al., 2015). A moderate ROS level is essential to promote cell growth and survival in normal cells whereas an excessive ROS level can be deleterious to the human body due to the damage caused to major cellular components such as proteins, lipids, and DNA (Cichoź-Lach and Michalak, 2014). It is well known that maintaining cellular ROS at safe levels is a good strategy to prevent liver damage. Therefore, we investigated whether PFH regulates cellular ROS level in H2O2-treated AML12 cells using DCF-DA fluorescent probes. Reduced DCFH-DA is non-fluorescent but is oxidized to DCF in the presence of ROS to emit fluorescence, so the intracellular DCF fluorescence is proprotional to the amount of ROS present in the cells (Wang and Joseph, 1999). DCF-DA fluorescence showed that intracellular ROS levels were dramatically increased in H2O2-treated AML12 cells than in untreated control cells (Fig. 2A). However, pretreatment with PFH (0.25–0.75 mg/mL) and SFN (10 μM; a positive control) significantly decreased ROS level in H2O2-treated cells, indicating that PFH protects cells from H2O2-induced cytotoxicity by suppressing intracellular ROS accumulation.
Fig. 2.

Effects of PFH (< 1 kDa) on H2O2-induced ROS production and expression of antioxidant genes in AML12 cells. After treatment with different concentrations of PDH for 24 h, the media were replaced with new media containing H2O2 (5 mM) and the cells were incubated for another 1 h (A). Cells were treated with PFH for 18 h, and mRNA expression levels were determined by qPCR (B). Tbp was used as an internal control and the results are presented as mean ± SEM (n ≥ 3). *P < 0.05 and **P < 0.01 vs. the vehicle control. SFN: sulforaphane
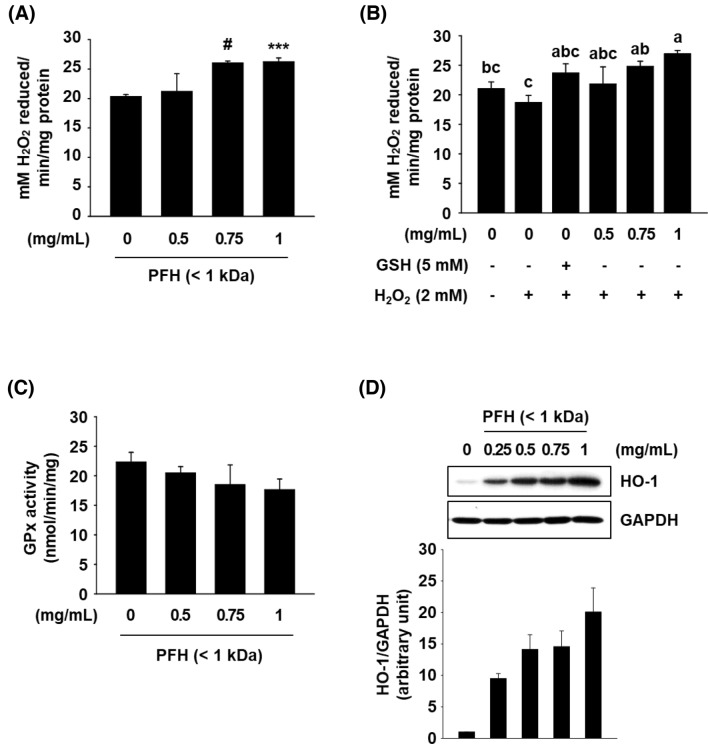
ROS levels in cells are primarily regulated by non-enzymatic and enzymatic antioxidants that make up the intracellular antioxidant system. Thus, we further examined the effect of PFH on mRNA expression of antioxidant genes by real-time quantitative PCR (qPCR) analysis. Genes tested in this study include Cat, Gpx2, Gr, Ho-1, Sod1, Sod2, and GSH synthesis genes (Gss, Gclc, and Gclm). As shown in Fig. 2B, treatment with PFH (0.75 mg/mL) in AML12 cells increased the mRNA levels of Ho-1, Cat, and Gpx2 more than 1.5-fold compared to untreated control cells.
GPx and CAT are antioxidant enzymes involved in the first line defense by preventing or suppressing ROS formation. These two enzymes can abolish the cytotoxicity of H2O2 by catabolizing H2O2 into water and oxygen (Ighodaro and Akinloye, 2018). As shown in Fig. 3A, CAT activity was increased in PFH-treated cells (at 0.75 and 1 mg/mL, but not 0.5 mg/mL) than in untreated control cells, which correlated well with the qPCR data (Fig. 2B). We further examined the effect of PFH on cellular CAT activity in H2O2-treated cells. CAT activity was slightly decreased in cells treated with H2O2, however, pretreatment with PFH at 0.75 and 1 mg/mL or reduced GSH (a positive control) significantly increased CAT activity in H2O2-treated AML12 cells (Fig. 3B). However, GPx showed no change in activity by PFH treatment (Fig. 3C).
Fig. 3.
Effects of PFH (< 1 kDa) on enzymatic activity and protein expression of cellular antioxidant enzymes in AML12 cells. Cells were treated with PFH for 24 h and enzymatic activity of CAT and GPx was measured (A and C). Cells were treated with PFH for 24 h. The cells were then incubated for another hour with new media containing H2O2, and CAT activity in cells was measured (B). Whole cell lysates were analyzed by western blot analysis, and GAPDH was used as a loading control (D). The western blot band intensity was determined by ImageJ software. The results are presented as mean ± SEM (n ≥ 3) and a different letter indicates a significant difference among groups, according to one-way ANOVA with Duncan’s multiple range test (P < 0.05). *P < 0.05, ***P < 0.005, and #P < 0.001 vs. the vehicle control
HO-1, an inducible enzyme that responds to oxidative stress, catalyzes the degradation of heme to the antioxidant bilirubin which is known to protect cells against oxidative stress (Je and Lee, 2015). Western blot results demonstrated that PFH treatment increased HO-1 protein expression, consistent with the effect of PFH on HO-1 mRNA expression (Fig. 3D). These data suggest that induction of CAT and HO-1 is associated with the hepatoprotective effects of PFH.
On the other hand, a previous study (Lee et al., 2022) has shown the in vivo hepatoprotective activity of PFH in a high-fat diet-fed obese mouse model, however, its underlying mechanisms, such as the effect on the hepatic antioxidant system, have not been elucidated. Therefore, further studies on the effect of PFH on the hepatic antioxidant system in animal models of prooxidant-induced liver damage will be needed.
PFH increases gene expression of CAT and HO-1 via activation of Nrf2
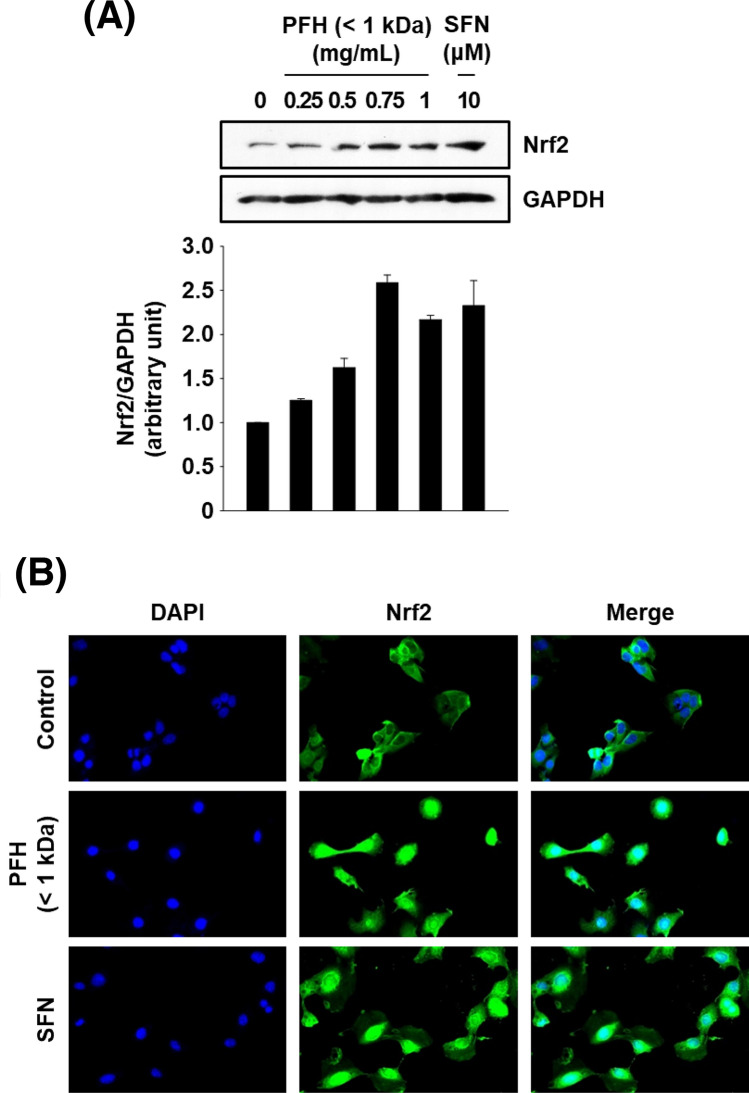
Nrf2 is an essential trascription factor to modulate expression of antioxdiant genes through binding to antioxidant response element (ARE) present in the regulatory regions of various antioxidant genes (Ciamporcero et al., 2018). Under normal physiological conditions, it binds to Kelch-like ECH-associated protein 1 (Keap1) in the cytoplasm and exists in an inactive form; however, when intracellular stress level increases, Nrf2 dissociates from Keap1 by the action of the ubiquitin–proteasome system and then translocates from the cytoplasm to the nucleus. Then it binds to the ARE in the nucleus and activates the expression of ARE-regulated antioxidant genes that include HO-1, CAT, SOD, and GPx (Ciamporcero et al., 2018; Ye et al., 2014).
To investigate whether PFH-mediated induction of Cat and Ho-1 is Nrf2-dependent, cells were treated with different concentrations of PFH for 4 h, and cellular localization of Nrf2 were analyzed by immunofluorescence staining. Treatment of cells with PFH (0.5–1.0 mg/mL) or SFN (an Nrf2 activator) markedly increased the protein expression of Nrf2 compared with that of untreated control cells (Fig. 4A). Furthermore, nuclear translocation of Nrf2 was detected after treatment with PFH or SFN (Fig. 4B), and these results indicate that PFH-induced antioxidant genes can be mediated by Nrf2.
Fig. 4.
Effects of PFH (< 1 kDa) on protein expression and activation of Nrf2 in AML12 cells. Cells were treated with PFH for 4 h and whole cell lysates were analyzed by western blot analysis (A). Gapdh was used as a loading control, and the western blot band intensity was determined by ImageJ software. Subcellular localization of Nrf2 (B). Cells were treated with PFH (1 mg/mL) or SFN (10 µM) for 4 h, and endogenous Nrf2 was detected by indirect immunofluorescence staining with anti-Nrf2 antibody. SFN: sulforaphane
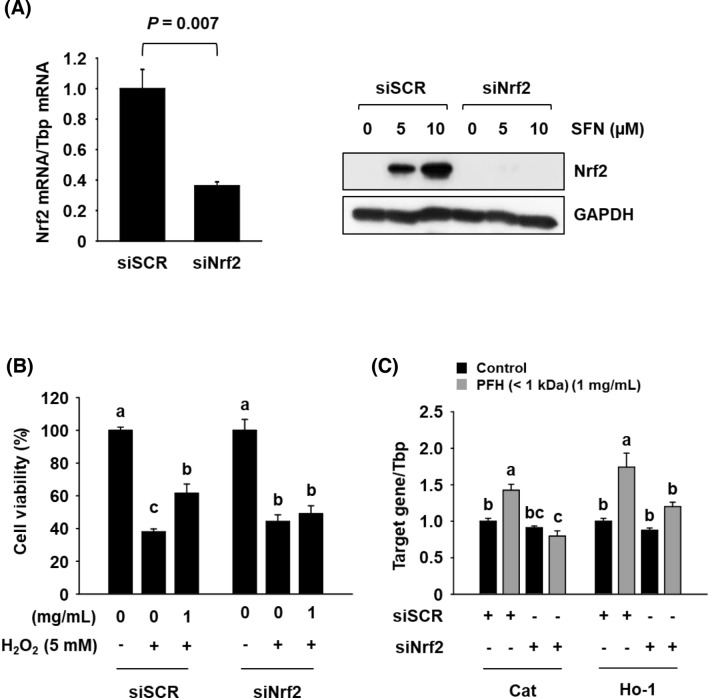
To further confirm that PFH-induced expression of Cat and Ho-1 is regluated mainly by Nrf2 activation, a siRNA-mediated gene silencing assay was employed. Transfection with siNrf2 resulted in efficient knockdown of Nrf2 in AML12 cells (Fig. 5A). Moreover, the PFH-mediated protective effect against H2O2-induced cytotoxicity was significantly decreased in siNrf2-transfected cells (Fig. 5B), and Nrf2 knockdown significantly reduced the PFH-induced expression of Cat and Ho-1 (Fig. 5C). These data indicate that PFH induces gene expression of Cat and Ho-1 primarily through an Nrf2-dependent mechanism.
Fig. 5.
Effects of Nrf2 gene knockdown on PFH (< 1 kDa)-mediated hepatoprotective effects in H2O2-treated AML12 cells. Cells were transfected with siSCR (100 nM), or siNrf2 (100 nM) for 66 h. mRNA expression levels were determined by qPCR (A). Cells were pre-treated with PFH for 24 h and then treated with H2O2 for another 4 h. Cell viability was measured by MTT assay (B). Cells were treated with PFH for 18 h and then mRNA expression levels were determined by qPCR (C). Tbp was used as an internal control. The results are presented as mean ± SEM (n ≥ 3), and a different letter (a-c) indicates a significant difference among groups, according to one-way ANOVA with Duncan’s multiple range test (P < 0.05). SFN: sulforaphane
We previously reported that MAH directly scavenges free radicals and other types of ROS such as H2O2 and reduces H2O2-induced cytotoxicity in AML12 cells through Nrf2-dependent activation of antioxidant genes (Yu et al., 2017; Cho and Lee, 2020). MAH upregulated the expression of Cat and Ho-1 as well as GSH synthesis genes such as Gclc and Gclm; consequently, the total cellular GSH levels were increased. However, PFH did not have a significant effect on the expression of Gclc and Gclm (Fig. 2B) and the total cellular GSH levels (data not shown) in AML12 cells. These results are consistent with previous reports showing that the activities of protein hydrolysates and peptides differ, depending on the primary structure of proteins and types of degrading enzymes (Udenigwe and Aluko, 2012).
Thus, our ongoing studies have focus on identification of hepatoprotective peptides from PFH. Up to now, three fractions were obtained from PFH by C18 solid-phase extraction using variouis concentrations of MeOH (0–60% MeOH). Preliminary data obtained from our study showed that the 10% methanol fraction of PFH displayed a stronger protection against H2O2-mediated cytotoxicity than two other fractions at all the tested concentrations (Fig. S2). The two MAH-derived hepatoprotective peptides were isolated from the aqueous fraction of MAH and exhibited properties of high polarity. Contrarily, it is expected that the active peptides contained in PFH have relatively low polarity, and we are currently conducting experiments to identify active peptides from PFH using LC–MS/MS analysis and de novo sequencing.
Free amino acid composition of PBLE, PAH, PFH, and PNH
It is generally accepted that many types of amino acids with donor electrons and/or aromatic ring, such as the acidic amino acids, Cys, Met, Phe, and Tyr, exert direct scavenging activity against ROS. Several other hydrophobic amino acids (HAA) and His are also known to act as antioxidants (Xie et al., 2020). Also, amino acids are known to regulate cellular antioxidant enzyme activities, and those include Cys, Ala, Val, Ile, Leu, Trp, His, and Lys (Katayama and Mine, 2007). These reports suggest that the hepatoprotective effect of PFH might be related to its free amino acid composition, thus the free amino acid composition of PBLE, PAH, PFH, and PNH was further analyzed.
As shown in Table 1, the levels of all amino acids except proline were higher in PFH, compared to other samples, and PFH was particularly rich in aromatic amino acids (AAA; Phe and Tyr), branched-chain amino acids (BCAAs; Ile, Leu, and Val), and HAA (Gly, Ala, Pro, Cys, Val, Ile, Leu, and Phe). Cys was detected only in PFH, but not in other hydrolysates. AAA, BCAA, and HAA are all recognized as potent antioxidants, thus suggesting that high levels of these amino acids, along with the type and content of LMW bioactive peptides it contains, may also contribute to the high hepatoprotective effect of PFH.
Table 1.
Free amino acid composition of PBLE, PAH, PFH, and PNH
| Free amino acids | PBLE | PAH | PFH | PNH |
|---|---|---|---|---|
| Aspartic acid | 0.52 | 0.58 | 3.18 | 0.12 |
| Serine | 4.94 | 2.30 | 14.59 | 1.15 |
| Glutamic acid | 1.01 | 30.71 | 36.41 | 23.74 |
| Glycine | 16.11 | 19.25 | 21.33 | 15.10 |
| Histidine | 4.75 | 4.47 | 13.94 | 3.92 |
| Arginine | 0.81 | 3.27 | 13.62 | 3.25 |
| Threonine | 1.48 | 3.50 | 10.37 | 2.77 |
| Alanine | 7.71 | 30.54 | 33.96 | 24.82 |
| Proline | 45.22 | 54.58 | 40.10 | 43.70 |
| Cystine | 0.95 | ND1) | 12.49 | ND |
| Tyrosine | 4.24 | 0.85 | 12.54 | 0.70 |
| Valine | 5.34 | 10.69 | 21.50 | 7.94 |
| Methionine | 0.21 | 0.88 | 3.38 | 0.42 |
| Lysine | 1.54 | 9.94 | 21.76 | 9.73 |
| Isoleucine | 1.84 | 5.11 | 13.15 | 3.77 |
| Leucine | 0.42 | 7.04 | 20.99 | 4.84 |
| Phenylalanine | 0.44 | 2.84 | 11.19 | 1.75 |
| AAA | 4.68 | 3.69 | 23.73 | 2.45 |
| BCAA | 7.60 | 22.84 | 55.64 | 16.55 |
| HAA | 78.23 | 130.92 | 178.09 | 102.34 |
Unit: mg/g
1Not detected
In conclusion, the present study demonstrated that pretreatment with PFH (< 1 kDa) reduces H2O2-mediated cytotoxicity in AML12 cells through Nrf2-mediated induction of cellular antioxidant enzymes such as catalase and HO-1. To the best of our knowledge, this is the first study to show hepatoprotective effects of protein hydrolysates rather than of aqueous or solvent extracts from Protaetia brevitarsis larvae. Overall, these results suggest that PFH represents a potential source of natural supplements or nutraceuticals for healthy liver function, and further studies are planned to confirm the hepatoprotective effect in vivo.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
This work was supported by the National Research Foundation of Korea [NRF-2020R1A2C1005339].
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chae-Eun Park and Syng-Ook Lee contributed equally to this work.
Contributor Information
Chae-Eun Park, Email: parkchaeeun3@kmu.ac.kr.
Syng-Ook Lee, Email: synglee@kmu.ac.kr.
References
- Ahn EM, Myung NY, Jung HA, Kim SJ. The ameliorative effect of Protaetia brevitrasis larvae in HFD-induced obese mice. Food Science and Biotechnology. 2019;28:1177–1186. doi: 10.1007/s10068-018-00553-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae SM, Lee SC. Effect of subcritical water extraction conditions on the activity of alcohol metabolizing enzymes, ACE inhibition, and tyrosinase inhibition in Protaetia brevitarsis larvae. Food Science and Biotechnology. 2020;29:867–872. doi: 10.1007/s10068-019-00728-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HR, Lee SO. Novel hepatoprotective peptides derived from protein hydrolysates of mealworm (Tenebrio molitor) Food Research International. 2020;133:109194. doi: 10.1016/j.foodres.2020.109194. [DOI] [PubMed] [Google Scholar]
- Cho HR, Lee YJ, Hong JE, Lee SO. Enzymatic preparation and antioxidant activities of protein hydrolysates from Gryllus bimaculatus. Journal of Korean Society of Food Science and Nutrition. 2019;51:473–479. [Google Scholar]
- Chon JW, Kweon HY, Jo YY, Yeo JH, Lee HS. Protective effects of extracts of Protaetia brevitarsis on carbon tetrachloride-induced hepatotoxicity in the mice. Journal of Sericultural and Entomological Science. 2012;50:93–100. doi: 10.7852/jses.2012.50.2.93. [DOI] [Google Scholar]
- Ciamporcero E, Daga M, Pizzimenti S, Roetto A, Dianzani C, Compagnone A, Palmieri A, Ullio C, Cangemi L, Pili R, Barrera G. Crosstalk between Nrf2 and YAP contributes to maintaining the antioxidant potential and chemoresistance in bladder cancer. Free Radical Biology & Medicine. 2018;115:447–457. doi: 10.1016/j.freeradbiomed.2017.12.005. [DOI] [PubMed] [Google Scholar]
- Cichoż-Lach H, Michalak A. Oxidative stress as a crucial factor in liver diseases. World Journal of Gastroenterology. 2014;20:8082–8091. doi: 10.3748/wjg.v20.i25.8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foegeding EA, Davis JP. Food protein functionality: A comprehensive approach. Food Hydrocolloids. 2011;25:1853–1864. doi: 10.1016/j.foodhyd.2011.05.008. [DOI] [Google Scholar]
- Ghosh S, Lee SM, Jung C, Meyer-Rochow VB. Nutritional composition of five commercial edible insects in South Korea. Journal of Asia-Pacific Entomology. 2017;20:686–694. doi: 10.1016/j.aspen.2017.04.003. [DOI] [Google Scholar]
- Hiraganahalli BD, Chinampudur VC, Dethe S, Mundkinajeddu D, Pandre MK, Balachandran J, Agarwal A. Hepatoprotective and antioxidant activity of standardized herbal extracts. Pharmacognosy Magazine. 2012;8:116–123. doi: 10.4103/0973-1296.96553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ighodaro OM, Akinloye OA. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria Journal of Medicine. 2018;54:287–293. doi: 10.1016/j.ajme.2017.09.001. [DOI] [Google Scholar]
- Je JY, Lee DB. Nelumbo nucifera leaves protect hydrogen peroxide-induced hepatic damage via antioxidant enzymes and HO-1/Nrf2 activation. Food and Function. 2015;6:1911–1918. doi: 10.1039/C5FO00201J. [DOI] [PubMed] [Google Scholar]
- Kitts DD, Weiler K. Bioactive proteins and peptides from food sources. Applications of bioprocesses used in isolation and recovery. Current Pharmaceutical Design. 9: 1309–1323 (2003) [DOI] [PubMed]
- Lee EH, Chun SY, Yoon BH, Han MH, Chung JW, Ha YS, Lee JN, Kim HT, Kim DH, Beik GY, Jang BI, Kwon TG, Park CE, Lee IS, Kim BS, Lee SO. Antiobesity and hepatoprotective effects of protein hydrolysates derived from Protaetia brevitarsis in an obese mouse model. BioMed Research International. 2022;2022:4492132. doi: 10.1155/2022/4492132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, Ryu HJ, Song HJ, Lee SO. Enzymatic preparation and antioxidant activities of protein hydrolysates from Protaetia brevitarsis larvae. Journal of Korean Society of Food Science and Nutrition. 2017;46:1164–1170. [Google Scholar]
- Liu J, Li D, Zhang T, Tong Q, Ye RD, Lin L. SIRT3 protects hepatocytes from oxidative injury by enhancing ROS scavenging and mitochondrial integrity. Cell Death and Disease. 2017;8:e3158. doi: 10.1038/cddis.2017.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nongonierma AB, FitzGerald RJ. Unlocking the biological potential of proteins from edible insects through enzymatic hydrolysis: A review. Innovative Food Science and Emerging Technologies. 2017;43:293–252. doi: 10.1016/j.ifset.2017.08.014. [DOI] [Google Scholar]
- Ryu HJ, Song HJ, Lee SO. Enzymatic preparation and antioxidant activities of protein hydrolysates from Allomyrina dichotoma larvae. Journal of Korean Society of Food Science and Nutrition. 2019;48:410–417. doi: 10.3746/jkfn.2019.48.4.410. [DOI] [Google Scholar]
- Sung GA, Kim MH, Park SN. Anti-inflammatory and whitening effects of Protaetia brevitarsis seulensis extracts by oriental conversion methods. Journal of Society of Cosmetic Scientists of Korea. 2016;42:421–432. doi: 10.15230/SCSK.2016.42.4.421. [DOI] [Google Scholar]
- Udenigwe CC, Aluko RE. Food protein-derived bioactive peptides: production, processing, and potential health benefits. Journal of Food Science. 2012;77:R11–R24. doi: 10.1111/j.1750-3841.2011.02455.x. [DOI] [PubMed] [Google Scholar]
- Vargas-Mendoza N, Madrigal-Santillán E, Morales-González Á, Esquivel-Soto J, Esquivel-Chirino C, García-Luna y González-Rubio M, A Gayosso-de-Lucio J, A Morales-González J. Hepatoprotective effect of silymarin. World Journal of Hepatology. 6: 144–149 (2014) [DOI] [PMC free article] [PubMed]
- Wang H, Joseph JA. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radical Biology & Medicine. 1999;27:612–616. doi: 10.1016/S0891-5849(99)00107-0. [DOI] [PubMed] [Google Scholar]
- Ye S, Chen M, Jiang Y, Chen M, Zhou T, Wang Y, Hou Z, Ren L. Polyhydroxylated fullerene attenuates oxidative stress-induced apoptosis via a fortifying Nrf2-regulated cellular antioxidant defense system. International Journal of Nanomedicine. 2014;9:2073–2087. doi: 10.2147/IJN.S56973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon CH, Lee BJ, Son D, Jeon SH, Cho YS. Effects of supplementary feeding management on development characteristics of larva Protaetia brevitarsis. Journal of the Korean Society of International Agriculture. 2016;28:414–419. doi: 10.12719/KSIA.2016.28.3.414. [DOI] [Google Scholar]
- Yu MH, Lee HS, Cho HR, Lee SO. Enzymatic preparation and antioxidant activities of protein hydrolysates from Tenebrio molitor larvae (mealworm) Journal of Korean Society of Food Science and Nutrition. 2017;46:435–441. doi: 10.3746/jkfn.2017.46.4.435. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.