Abstract
Cytotoxic T cells secrete perforin to kill virus-infected cells. In this study we show that perforin also plays a role in immune regulation. Perforin-deficient (perf −/−) mice chronically infected with lymphocytic choriomeningitis virus (LCMV) contained greater numbers of antiviral T cells compared to persistently infected +/+ mice. The enhanced expansion was seen in both CD4 and CD8 T cells, but the most striking difference was in the numbers of LCMV-specific CD8 T cells present in infected perf −/− mice. Persistent LCMV infection of +/+ mice results in both deletion and anergy of antigen-specific CD8 T cells, and our results show that this peripheral “exhaustion” of activated CD8 T cells occurred less efficiently in perf −/− mice. This excessive accumulation of activated CD8 T cells resulted in immune-mediated damage in persistently infected perf −/− mice; ∼50% of these mice died within 2 to 4 weeks, and mortality was fully reversed by in vivo depletion of CD8 T cells. This finding highlights an interesting dichotomy between the role of perforin in viral clearance and immunopathology; perforin-deficient CD8 T cells were unable to clear the LCMV infection but were capable of causing immune-mediated damage. Finally, this study shows that perforin also plays a role in regulating T-cell-mediated autoimmunity. Mice that were deficient in both perforin and Fas exhibited a striking acceleration of the spontaneous lymphoproliferative disease seen in Fas-deficient (lpr) mice. Taken together, these results show that the perforin-mediated pathway is involved in downregulating T-cell responses during chronic viral infection and autoimmunity and that perforin and Fas act independently as negative regulators of activated T cells.
Cytotoxic T lymphocytes (CTL) can kill their targets by two distinct mechanisms: (i) a secretory and membranolytic pathway involving perforin and granzymes and (ii) a nonsecretory receptor-mediated pathway involving Fas (CD95) (6, 9, 21). Perforin, a 65-kDa protein with sequence homology to complement components C6 to C9, is stored in cytoplasmic granules of CTL and plays a major role in the secretory pathway. Upon binding of CTL to the target cell and appropriate engagement of the T-cell receptor (TcR), the cytoplasmic granules containing perforin and granzymes (serine proteases) are released vectorially onto the target cell. Perforin monomers assemble into polymeric pore structures that insert into target cell plasma membranes, making the membrane permeable to water and small ions. This “hole punching,” along with the effects of granzymes, eventually leads to apoptotic death of the target cell (6, 19, 21, 26, 47). Studies with perforin-deficient (perf −/−) mice have shown that perforin-mediated cytotoxicity is essential for controlling lymphocytic choriomeningitis virus (LCMV) infection in vivo (21, 57). The importance of perforin has also been shown in Listeria monocytogenes infection (20) and in eliminating certain tumors (22). These studies have clearly established that, at least in certain systems, perforin-mediated cytotoxicity is the dominant killing pathway in vivo.
Similar to the granule exocytosis (perforin) pathway, the Fas-dependent pathway is also initiated by engagement of the TcR by the appropriate antigen (25, 29, 48). This interaction results in upregulation of Fas ligand (FasL) expression on the T cell. Binding and cross-linking of FasL with Fas molecules expressed on the target cells leads to apoptosis of Fas-positive cells. A death-inducing cytoplasmic domain of the Fas protein triggers an intracellular apoptotic program in the target cells involving interleukin-1β-converting enzyme and/or other related proteases (18, 28). Alternative mechanisms of killing, such as cytotoxicity mediated by tumor necrosis factor (TNF) and secreted ATP, have also been postulated, but there is now a general consensus that perforin- and Fas-mediated pathways are the two major killing mechanisms used by CTL (13, 16, 18, 26, 27, 53).
In addition to its proposed role as an effector mechanism, Fas-mediated killing plays an important role in immune regulation (29, 30, 37, 48). Activated T cells express increased levels of Fas, and Fas-mediated apoptosis of effector T cells serves as a mechanism for regulating cell numbers and maintaining homeostasis (25, 29, 37). Thus, it appears that the Fas-mediated pathway has a dual function: both as a potential effector mechanism and as a negative regulator. A role for TNF in regulating T-cell responses, especially of CD8 T cells, has also been demonstrated (61). In contrast, perforin is considered primarily as an effector mechanism (22, 27). In this study, we provide evidence that perforin-mediated killing is involved in the downregulation of T-cell responses in vivo in a viral infection.
MATERIALS AND METHODS
Mice.
Perf −/− mice were made by targeted disruption of the perforin gene (57). Wild-type mice (+/+, strain 129) and C57BL/6J/lpr/lpr mice (B6.MRL-Faslpr) were purchased from The Jackson Laboratory (Bar Harbor, Maine). Cross (F1) and intercross (F2) matings were performed between B6.MRL-Faslpr and perf −/− mice to generate perf −/− mice homozygous for the lpr mutation (lpr/perf −/−). Mice were kept under specific-pathogen-free (SPF) conditions in isolater cages with filter covers.
Virus infection.
The Armstrong CA 1371 strain of LCMV and a spleen cell variant derived from this virus, clone 13 (3), were used in this study. All LCMV stocks used in this study were triple plaque purified on Vero cells, and stocks were grown in BHK-21 cells. Mice were infected with either 2 × 105 PFU of Armstrong strain intraperitoneally (i.p.) or 2 × 106 PFU of clone 13 intravenously (i.v.).
Virus titration.
Infectious LCMV in serum and tissues was quantitated by plaque assay on Vero cell monolayers as previously described (3).
Antisera and T-cell depletion.
The monoclonal antibody (MAb) 2.43 (rat immunoglobulin G2a [IgG2a]) was partially purified from hybridoma culture supernatant by ammonium sulfate precipitation and used for depleting CD8+ T cells in vivo (46). Mice were given three injections of MAb 2.43 (0.3 ml i.p.) on day 0 and days 2 and 4 after the virus infection. This protocol resulted in a ≥90% decrease in the number of CD8+ T cells.
Flow cytometry and cell cycle analysis.
Single-cell suspensions of spleen, lymph node, or bone marrow were prepared, and 106 cells were stained in phosphate-buffered saline (PBS) containing 1% bovine serum albumin and 0.02% sodium azide for 30 min at 4°C. MAbs, phycoerythrin (PE)- or fluorescein isothiocyanate (FITC)-conjugated rat anti-mouse CD8a (53-6.7), PE- or FITC-conjugated rat anti-mouse CD4 (IM7), and FITC-conjugated rat anti-mouse CD44 were purchased from Pharmingen (San Diego, Calif.). Analyses were performed on a FACScan flow cytometer (Becton Dickinson, San Francisco, Calif.). For cell cycle analysis, spleen cells were first surface stained with the appropriate antibody as described above and then fixed for 1 h at 4°C in 2% paraformaldehyde solution. The cells were then washed with PBS, permeabilized at 37°C with 0.2% Tween 20, and stained with 7-amino-actinomycin D (7-AAD; Calbiochem, San Diego, Calif.) at 4°C for 30 min. The data obtained by a FACScan flow cytometer were then analyzed by using the CellFit software (Becton Dickinson) (41).
Immunohistochemistry.
Immunoperoxidase staining of acetone-fixed 6-μm liver sections was done as follows. LCMV antigen was detected by polyclonal anti-LCMV guinea pig serum followed by treatment with mouse-adsorbed biotinylated goat anti-guinea pig IgG (Vector Laboratories, Burlingame, Calif.). For detection of CD8+ T cells, rat monoclonal anti-mouse CD8 (clone 53-6.7; Becton Dickinson) and mouse-adsorbed biotinylated rabbit anti-rat IgG (Vector Laboratories) were used. Positive cells were visualized by the addition of avidin-biotin-peroxidase complexes (Vectastain ABC kit; Vector Laboratories) and with 3-amino-9-ethylcarbazole (AEC) as a substrate. Sections were then counterstained with hematoxylin.
ELISPOT assay to detect gamma interferon (IFN-γ)-producing cells.
IFN-γ secretion by virus-specific CD8+ T cells was quantitated by enzyme-linked immunospot (ELISPOT) assay (11, 50). Ester-cellulose bottomed plates (Multiscreen-HA; Millipore Corp., Bedford, Mass.) were coated overnight with the capture antibody, rat anti-mouse IFN-γ (clone R4-6A2, Pharmingen) at 2 μg/ml (100 μl/well). The plates were then washed in PBS and blocked for 1 h in RPMI containing 10% fetal bovine serum (FBS; HyClone Laboratories, Inc., Logan, Utah). Threefold dilutions of effector cells in RPMI medium supplemented with 10% fetal calf serum were added to the plates along with 5 × 105 irradiated (1,200 rad) feeder cells (spleen cells from uninfected naive mice). Cultures were stimulated for 24 h with LCMV-specific CTL epitope peptides (NP396-404, GP33-41, and GP276-286). After the culture period, cells were removed by washing the plates in PBS-Tween (0.05%), and then biotinylated anti-mouse IFN-γ (clone XMG1.2, Pharmingen) was added at 4 μg/ml, 100 μl per well. After overnight incubation at 4°C, unbound antibody was removed, and horseradish peroxidase avidin-D (Vector Laboratories) was added. Spots were developed by using the substrate AEC (Sigma, St. Louis, Mo.) with H2O2. The number of virus-specific CD8+ T cells per spleen was determined by multiplying the frequency of IFN-γ-secreting CD8+ T cells by the total number of CD8+ T cells in each spleen. The frequency of LCMV-specific IFN-γ producing CD8 T cells in the spleens of uninfected mice was less than 1 per 5 × 105 cells.
Anti-CD3 stimulation.
Single-cell suspensions of spleens from uninfected mice were cultured in RPMI 1640 medium supplemented with 10% FBS and 5 × 10−5 M 2-mercaptoethanol at 8 × 105 cells per well in 96-well flat-bottomed plates. Cultures were stimulated for various periods of time with anti-mouse CD3 antibody (145-2C11) at a 1 μg/ml concentration. DNA synthesis was measured by pulsing with [3H]thymidine (1 μCi/well) in culture medium for 24 h. At the end of the pulse period, cells were harvested, and the incorporated radioactivity was measured in a Matrix 9600 direct beta counter (Packard, Downers Grove, Ill.).
Analysis of proliferation and apoptosis after restimulation of activated T cells.
Primary stimulation of splenic T cells was done by culturing the spleen cells (8 × 106 cells per well) for 3 to 4 days in RPMI plus 10% FBS containing anti-mouse CD3 antibody (1 μg/ml) in 24-well plates (42). At the end of primary stimulation, activated T cells were washed twice in the culture medium. Cells were then plated at 5 × 105 viable cells/well in 96-well flat-bottomed plates. Cells were restimulated with 1 μg of anti-CD3 antibody per ml for 24 h. Cells were pulsed with [3H]thymidine (1 μCi/well) in culture medium at the time of replating. At the end of 24 h, the incorporated radioactivity was measured as mentioned above. After restimulation as described above, cells were harvested and the number of apoptotic cells in the culture was quantitated by flow cytometry after staining with 7-AAD and FITC-conjugated anti-mouse CD8 antibodies (14). Apoptosis of T cells was also measured by staining with annexin V-FITC (55).
Analysis of the surface expression of Fas and TNF receptor II (p75; TNFR II) on activated T cells.
Spleen cells from perf +/+ and perf −/− mice were stimulated in vitro with anti-mouse CD3 antibody (1 μg/ml) for 48 h in 24-well plates at 8 × 105 cells/well. After stimulation for 48 h, cells were harvested and stained with PE-conjugated anti-mouse Fas (Pharmingen) or hamster anti-mouse TNFR II (kindly provided by Robert Schreiber, Washington University, St. Louis, Mo.) and FITC-conjugated anti-mouse CD4 or anti-mouse CD8 antibodies. To detect TNFR II, a second step staining was performed with PE-conjugated goat anti-hamster antibodies (Caltag Laboratories, San Francisco, Calif.).
Administration of Staphylococcus enterotoxin A (SEA).
SEA was injected i.v. (10 μg/mouse) into +/+ and perf −/− mice (15). Spleens were harvested from the SEA-injected mice at 0, 3, and 10 days postinjection. Spleen cells were double stained with FITC-conjugated anti-mouse Vβ11 antibodies and either PE-conjugated anti-mouse CD4 or CD8 antibodies. The percentages of Vβ11-bearing CD8+ and CD4+ T cells were determined by flow cytometry.
Sensitivity of T cells to TNF-α.
The sensitivity of in vivo-activated T cells to TNF was tested as described previously (39). Spleen cells from LCMV clone 13-infected mice (day 8 postinfection) were cultured in flat-bottomed 96-well plates at 0.8 × 106 cells/well. Cells were treated with mouse recombinant TNF-α (Genzyme, Cambridge, Mass.) at concentrations of 0, 1, 10, and 100 ng/ml for 24 h. Cultures were pulsed with [3H]thymidine (1 μCi/well) for the period of culture. At the end of pulse period, cells were harvested and radioactivity measured as described above.
RESULTS
Enhanced T-cell expansion in vivo in perf −/− mice during chronic viral infection.
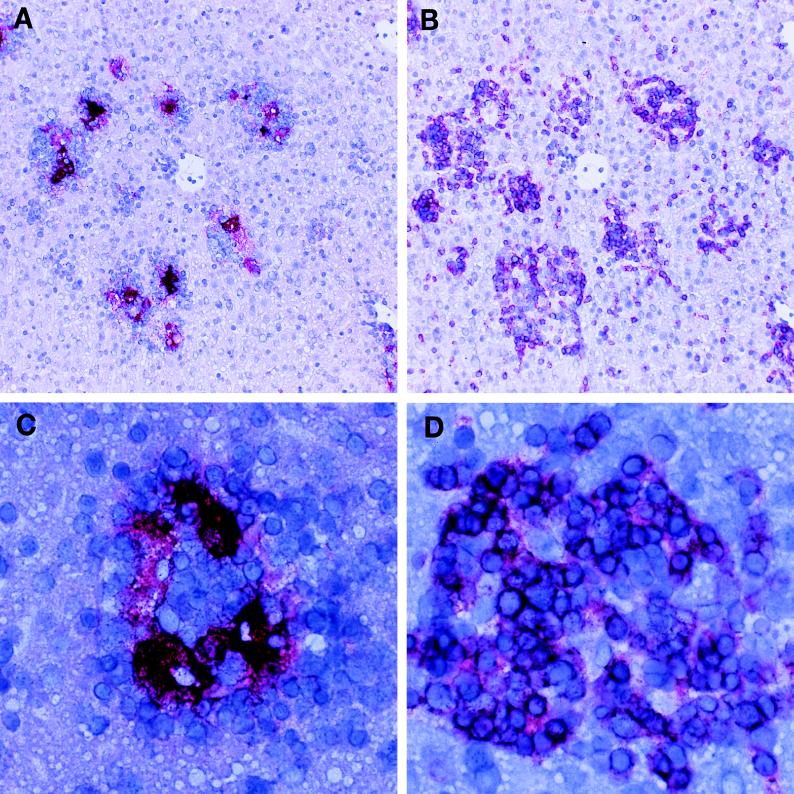
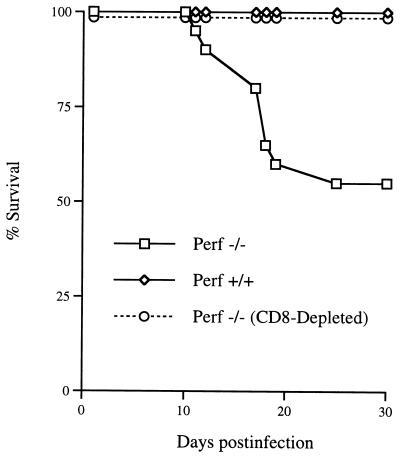
Previous studies have shown that perf −/− mice are unable to control an LCMV infection (21, 57). Wild-type (+/+) mice infected (i.p. or i.v.) with the Armstrong strain of LCMV generate a vigorous antiviral CD8 CTL response and clear the virus within 2 weeks. In contrast, perf −/− mice develop a systemic infection and harbor high levels of infectious virus and viral antigen in several tissues (57). This infection is characterized by splenomegaly and lymphadenopathy due to a huge expansion in the number of activated T cells. The most striking increase was in the number of activated CD8 T cells; at 8 days postinfection there were between 50 × 106 to 80 × 106 CD8 CD44hi cells/spleen (n = 6) compared to ∼5 × 106 CD8 CD44hi cells in the spleens of uninfected perf −/− mice. The LCMV-infected perf −/− mice not only showed an increase in the number of activated CD8 T cells in the spleen and lymph nodes but there were also massive infiltrates of CD8 T cells in the various infected tissues (liver, lung, pancreas, bone marrow, etc.). Figure 1 shows T-cell infiltrates in the liver; note the clusters of CD8 T cells around the foci of LCMV-infected cells. We found that ∼50% of these chronically infected perf −/− mice died between 2 and 4 weeks after infection. To determine if this mortality was due to CD8 T cells, perf −/− mice were depleted of CD8 T cells at the time of infection. As shown in Fig. 2, ∼50% (9 of 20) of LCMV-infected perf −/− mice died between days 10 and 30 postinfection, and this mortality was fully reversed (0 of 11) by in vivo depletion of CD8 T cells. These data also show that CD8-dependent immunopathology can occur by mechanism(s) independent of perforin-mediated cytotoxicity.
FIG. 1.
CD8 T-cell infiltration in liver and colocalization with viral antigen. Parallel liver sections from LCMV-infected (day 8) perf −/− mice were stained for viral antigen (A) and CD8 T cells (B). Note the relationship between the CD8 infiltrates and the viral antigen. LCMV antigen (stained red in panel A) appears in the middle of cellular infiltrates (groups of small cells with blue-staining nuclei in panel A), which consist mostly of CD8 T cells as shown by anti-CD8 antibody staining (red color in panel B). Panel C (viral antigen) and panel D (CD8 T cells) show one of these clusters at a higher magnification. Magnifications: panels A and B, ×100; panels C and D, ×400.
FIG. 2.
Death of LCMV-infected perf −/− mice is mediated by CD8 T cells. Three groups of mice, wild type (perf +/+; n = 20), perf deficient (perf −/−; n = 14), or CD8-depleted perf −/− (perf −/− plus anti-CD8; n = 11), were infected i.p. with 2 × 105 PFU of LCMV-Armstrong, and their survival was monitored for 30 days. All +/+ mice survived. In contrast, 9 of 20 of the LCMV-infected perf −/− mice died by day 25 postinfection. In vivo depletion of CD8 T cells in LCMV-infected perf −/− mice (perf −/− plus anti-CD8 group) resulted in 100% survival of these mice.
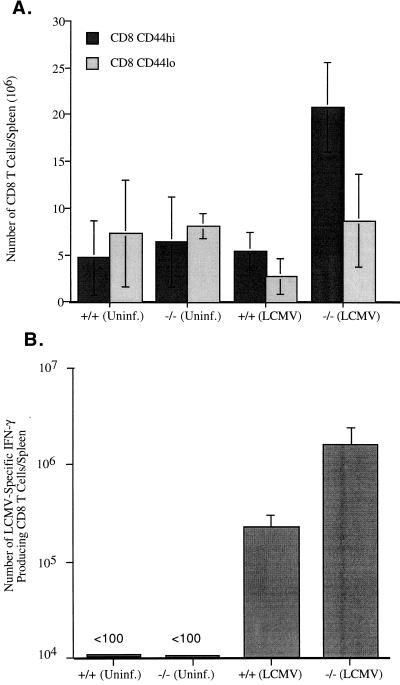
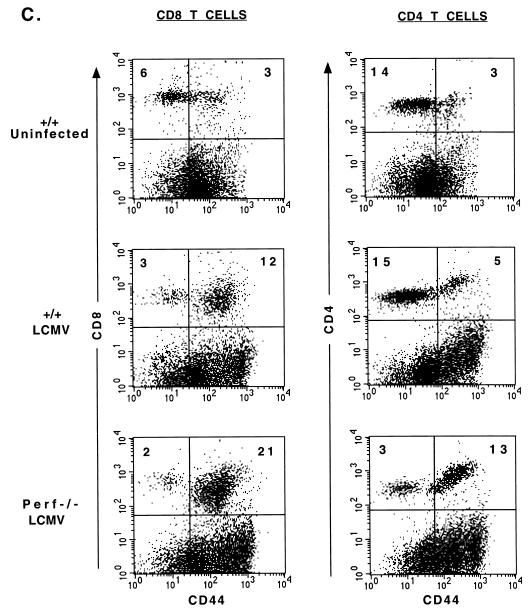
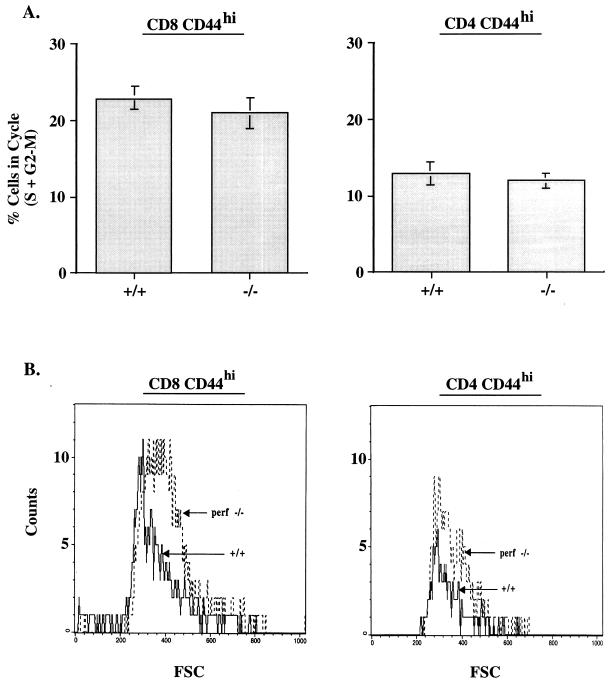
The data presented above (Fig. 1) document the massive expansion of CD8 T cells in LCMV-infected perf −/− mice. Much less overall expansion was seen in LCMV-infected +/+ mice, but this is not a valid comparison since +/+ mice control the infection within 8 to 10 days, whereas perf −/− mice contain large amounts of viral antigen. Thus, it could be argued that the increased T-cell expansion seen in perf −/− mice is the result of a greater antigenic load. To control for this, we next did a series of experiments with a strain of LCMV (LCMV clone 13) that causes a chronic infection in both +/+ and perf −/− mice (3, 32–34). The data in Table 1 show that +/+ and perf −/− mice infected with LCMV clone 13 contain similar levels of virus in all tissues tested. At this time point viral antigen load in various organs as determined by immunohistochemical staining was also comparable between +/+ and perf −/− mice (data not shown). Despite this similar antigenic load there was substantially more activation of T cells in the absence of perforin; perf −/− mice contained ≥4-fold more total CD8 CD44hi T cells in the spleen compared to infected +/+ mice (Fig. 3A). A similar trend was observed in the lymph nodes and in the blood (data not shown). Even more striking differences were noted when we quantitated the number of LCMV-specific CD8 T cells in the spleen by using an IFN-γ ELISPOT assay (Fig. 3B); perf −/− mice contained ∼10-fold more LCMV-specific CD8 T cells than +/+ mice. Differences were also seen in the total number of activated CD4 T cells present in infected +/+ and −/− mice. The spleens and lymph nodes of infected perf −/− mice contained two- to threefold more CD4 CD44hi cells than did infected +/+ mice. A representative fluorescence-activated cell sorter (FACS) analysis is shown in Fig. 3C. Cell cycle analysis of T cells from clone 13-infected +/+ and perf −/− mice showed that similar proportions of CD8 and CD4 T cells were in cycle in both groups of mice (Fig. 4). At 8 days postinfection 23 ± 1.5% of CD8 CD44hi cells were in cycle (S+G2-M) in +/+ mice compared to 21 ± 2% in perf −/− mice. Among CD4 T cells, 13 ± 1.5% of CD44hi T cells were in cycle in +/+ mice versus 12 ± 1% in perf −/− mice (Fig. 4A). It is worth noting that even though the total number of activated (CD44hi) T cells was substantially greater in perf −/− infected mice, the percentage of activated T cells in cycle were similar in +/+ and perf −/− mice. This result suggests that the increased number of activated T cells in LCMV-infected perf −/− mice may be due to decreased death of T cells. In this context it is also of interest that among the activated T cells there was a greater proportion of “large” cells (based on forward scatter) in perf −/− mice than in +/+ mice (Fig. 4B). The finding that the percentages of cycling CD8 and CD4 T cells were similar in both groups but that perf −/− mice contained more “blasting” T cells appears paradoxical. But this result is consistent with an altered regulation of activated T cells in chronically infected perf −/− mice. It is possible that the larger cells comprise end-stage effector cells that are prone to apoptosis and that this end-stage effector population survives longer in perf −/− mice.
TABLE 1.
Viral load in the tissues of +/+ and perf −/− mice after LCMV clone 13 infection (day 8 postinfection)
| Group | LCMV titer (log10 PFU/g of tissue or ml of serum)a
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Spleen | Liver | Lung | Lymph nodes | Pancreas | Kidney | Brain | Serum | |
| +/+ | 6.2 ± 0.1 | 8.2 ± 0.1 | 8.3 ± 0.1 | 7.3 ± 0.2 | 7.1 ± 0.6 | 7.1 ± 0.7 | 6.3 ± 0.4 | 5.2 ± 0.2 |
| −/− | 6.5 ± 0.3 | 7.9 ± 0.1 | 8.0 ± 0.1 | 7.6 ± 0.3 | 6.7 ± 0.1 | 6.5 ± 0.1 | 6.0 ± 0.3 | 4.7 ± 0.1 |
Six- to eight-week-old +/+ and −/− mice were infected i.v. with 2 × 106 PFU of LCMV clone 13. Eight days after infection virus titers in serum and various tissues were measured by plaque assay. The data shown are the average of four mice per group.
FIG. 3.
T-cell activation in perf −/− and +/+ mice chronically infected with LCMV clone 13. Spleen cells from uninfected and LCMV clone 13-infected (day 8) perf −/− and +/+ mice were double stained with CD4/CD44 and CD8/CD44. Panel A shows the total numbers of activated (CD44hi) and naive (CD44lo) CD8 T cells in the spleen (average of six mice in each group). Note the increased numbers of activated CD8 T cells in the spleens of perf −/− mice despite the similar viral load in both +/+ and −/− mice (see Table 1). Panel B shows the total number of LCMV-specific IFN-γ producing CD8 T cells in the spleen after infection with LCMV-clone 13 (day 8 postinfection). LCMV-specific CD8 T-cell responses were measured by stimulating the spleen cells with LCMV-specific CTL epitope peptides (NP396-404, GP33-41, and GP276-286) and quantitating the number of IFN-γ-producing CD8 T cells by an ELISPOT assay. Panel C shows a representative FACS profile of spleen cells from LCMV clone 13-infected +/+ and perf −/− mice (day 8). Note the higher percentages of both activated CD8 and CD4 T cells in perf −/− mice.
FIG. 4.
Cell cycle analysis of T cells from LCMV clone 13-infected +/+ and perf −/− mice. Spleen cells from uninfected and LCMV clone 13-infected (day 8) +/+ and −/− mice were stained with CD8/CD44 and CD4/CD44 and then analyzed for DNA content as described in Materials and Methods. Panel A shows the percentage of activated (CD44hi) CD8 and CD4 T cells in cycle (S+G2-M phase) in infected +/+ and −/− mice. Note that similar percentages of activated T cells are in cycle in both +/+ and −/− mice. Approximately 5 to 7% of CD44hi CD8 and CD4 T cells were in cycle in uninfected +/+ and −/− mice (data not shown). In all groups of mice (uninfected and infected +/+ and −/−) <1 to 2% of “naive” CD44lo CD8 and CD4 T cells were in S+G2-M phase; >98% of CD44lo cells were in G0-G1 phase (data not shown). Panel B shows the size (forward scatter) of CD8 CD44hi and CD4 CD44hi T cells from LCMV clone 13-infected +/+ and −/− mice. Note that “activated” T cells from perf −/− mice contain a higher proportion of large cells.
In summary, the results presented in Table 1 and Fig. 3 and 4 show that even under conditions of a similar viral load, there were substantially greater numbers of activated T cells in perf −/− mice than in +/+ mice. The enhanced expansion was seen in both CD4 and CD8 T cells, and the most striking difference (≥10-fold) was seen in the numbers of antigen (LCMV)-specific CD8 T cells present in infected perf −/− mice compared to infected +/+ mice. It should be noted that LCMV clone 13 (high-dose) infection of +/+ mice results in “exhaustion” of antigen-specific CD8 T cells (3, 34, 36), and our results show that this peripheral “deletion” of activated CD8 T cells occurs less efficiently in perf −/− mice.
Differential response of T cells from +/+ and perf −/− mice to continuous TcR stimulation in vitro.
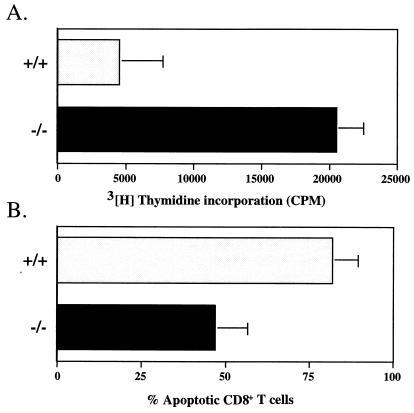
Continuous stimulation of T cells through the TcR by antigen can lead to apoptosis of activated T cells (42, 59). The increased T-cell expansion seen in vivo in perf −/− mice during chronic LCMV infection (Fig. 3) suggested that T cells from perf −/− mice may be less sensitive to activation-induced cell death (AICD). However, enhanced proliferation of T cells can also result in increased T cell numbers. To further address this question, we examined in vitro the effect of stimulation with antibody to CD3 on the proliferation and apoptosis of T cells from perf −/− mice. In these experiments spleen cells from normal (uninfected) +/+ or perf −/− mice were cultured with anti-CD3 antibody, and their proliferation was checked at various times after stimulation. A slight but consistent difference was observed between the proliferative response of T cells from perf −/− and +/+ mice at 48 h poststimulation (35,000 cpm for perf −/− T cells versus 25,000 cpm for perf +/+ T cells). Much more impressive differences were seen upon restimulation of these activated T cells with anti-CD3 antibody. In these experiments spleen cells that had undergone a primary round of anti-CD3 stimulation for 72 to 96 h were then restimulated with anti-CD3 (after adjusting for total number of viable cells). As shown in Fig. 5A, activated T cells from perf −/− mice were still responsive to anti-CD3 stimulation, but T cells from +/+ mice exhibited a very poor proliferative response upon anti-CD3 restimulation. Also there was a higher percentage of apoptotic CD8 T cells in cultures from perf +/+ mice than in perf −/− mice (85 versus 45%) (Fig. 5B). The results presented in Fig. 5 show that, as with the enhanced T-cell expansion seen in vivo in chronically infected perf −/− mice, T cells from −/− mice respond better to continuous TcR stimulation in vitro.
FIG. 5.
Proliferative responses and reactivation-induced apoptosis of T cells from perf +/+ and perf −/− mice after anti-CD3 stimulation. Panel A shows the proliferative responses of splenic T cells from perf +/+ and perf −/− mice to restimulation with anti-CD3. Spleen cells (8 × 106 cells/well in 24-well plates) from uninfected perf +/+ and perf −/− mice were initially stimulated for 72 to 96 h with anti-mouse CD3 antibody (1 μg/ml). After this primary round of stimulation, cells were washed, plated at 5 × 105 viable cells/well (96-well plate), and restimulated with anti-mouse CD3 antibody for another 24 h. Cells were pulsed with [3H]thymidine (1 μCi/well) at the time of restimulation. Panel B shows the relative proportions of apoptotic cells among perf +/+ and perf −/− CD8 T cells after restimulation with anti-CD3 as described above. After restimulation, cells were harvested, and the number of apoptotic cells in the culture was determined as described in Materials and Methods.
Fas and TNF-receptor expression and function in perf −/− mice.
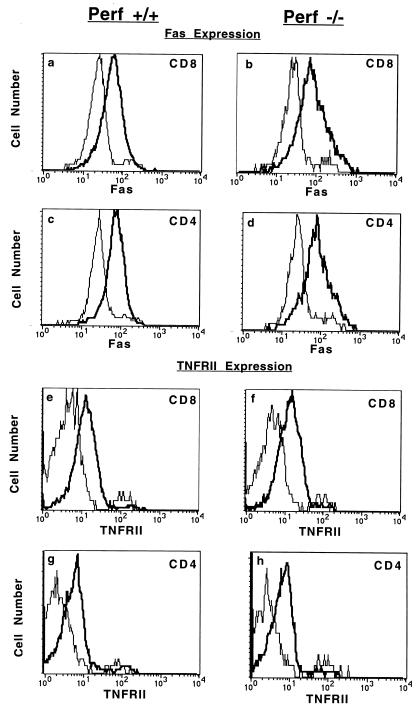
It has been shown that signalling via Fas and TNFR II is involved in the AICD of activated T cells (61). Hence, it is possible that reduced AICD in perf −/− T cells may be due to defects in Fas- and TNF-induced apoptosis. To address this issue, we first determined if the lack of perforin affects the expression of Fas and TNFR II on activated T cells. T cells were cultured in the presence of anti-CD3, and the levels of Fas and TNFR expression were examined by FACS analysis. As shown in Fig. 6, both CD8 and CD4 T cells from +/+ and perf −/− mice expressed elevated levels of Fas and TNFR II on their surface upon activation with anti-CD3. Note that the levels of expression of both Fas and TNFR II on activated T cells were comparable for both perf +/+ and perf −/− mice. Thus, perforin deficiency does not affect the surface expression of either Fas or TNFR II.
FIG. 6.
Upregulation of Fas and TNFR II (p75) expression on activated T cells. Spleen cells from perf +/+ and perf −/− mice were stimulated in vitro with anti-mouse CD3 antibody for 48 h, and the levels of expression of Fas and TNFR II on CD8+ and CD4+ T cells were analyzed by flow cytometry. Histograms show log fluorescence intensities. Thin and bold lines represent unstimulated and anti-CD3-stimulated T cells, respectively.
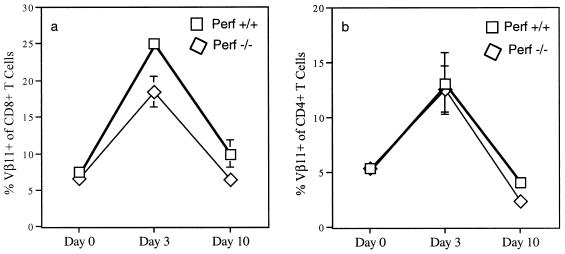
We then asked if perf −/− T cells were sensitive to TNF- and Fas-mediated apoptosis. Sensitivity to TNF was measured according to a method described by Orange et al. (39) with slight modifications. Similar inhibitory effects of TNF were observed on both +/+ and perf −/− T cells (45 versus 41% inhibition of [3H]thymidine incorporation), suggesting that perf −/− T cells were sensitive to TNF-mediated effects. To determine the sensitivity to Fas-mediated apoptosis, we analyzed the in vivo deletion of Vβ11+ T cells after injection with the superantigen SEA. It is well established that deletion of superantigen-reactive T cells in vivo after superantigen injection is mediated via Fas-FasL interaction (1, 2, 48). The administration of SEA into C57BL/6 mice leads to an initial expansion followed by deletion of Vβ11+ T cells in the peripheral lymphoid organs (35), whereas Fas-deficient mice exhibit a defect in the deletion of superantigen-reactive T cells in the periphery (2). In our experiments, minimal to no differences were observed in the kinetics of expansion and deletion of Vβ11+ T cells in perf +/+ and perf −/− mice after SEA injection (Fig. 7). These data show that immune regulation via Fas-FasL interaction was intact in perf −/− mice.
FIG. 7.
Clonal expansion and deletion of Vβ11+ T cells after injection with superantigen SEA. Perf +/+ or perf −/− mice were injected i.v. with SEA (10 μg/mouse). Spleen cells were harvested at the indicated times after injection, and the percentages of Vβ11+ CD8+ (a) and CD4+ (b) cells were determined by flow cytometry. Each value is the average of three mice; the standard deviation is indicated by the bars.
Absence of perforin accelerates lymphoproliferative disease in Fas-deficient (lpr) mice.
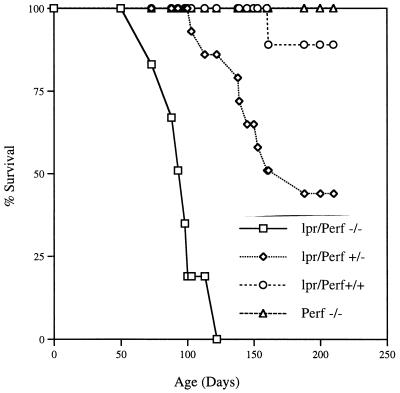
Defects in the Fas pathway result in lymphoproliferative disorders and autoimmune diseases (37). This is best illustrated by mouse strains carrying a mutation in Fas (lpr) or FasL (gld) (30, 58). MRL lpr/lpr and MRL gld/gld mice spontaneously develop lymphadenopathy and splenomegaly (due to accumulation of T cells), produce large quantities of autoantibodies, and develop nephritis and arthritis. B6.MRL lpr/lpr mice develop lymphoadenopathy around 6 months of age and start dying between the ages of 6 months and 1 year (10). A dramatic acceleration of the lymphoproliferative disease and mortality was seen in mice that were deficient in both Fas and perforin (lpr/perf −/−) (see Fig. 8). These mice were made by breeding perf −/− mice with B6.MRL lpr/lpr mice. The double-deficient (lpr/perf −/−) mice were normal at birth but started developing lymphadenopathy as early as 6 to 8 weeks of age and were all dead by 4 months. Death was preceded by wasting, massive lymphadenopathy, and the presence of mononuclear cell infiltrates in several tissues. In the data shown in Fig. 8, eight double-deficient mice were monitored for survival, and all of these mice died between days 73 and 120 after birth. In striking contrast to the rapid lymphoproliferative disease seen in the double-deficient mice, Fas-deficient mice with normal perforin function (lpr/perf +/+) showed ∼90% survival at >200 days; of the nine mice studied in this experiment, only one died at day 160 and the remaining eight were still alive at day 210 (Fig. 8). Of particular interest was the gene dosage effect seen in littermates that were homozygous for the Fas defect but that contained a single copy of the perforin gene (lpr/perf +/−). As shown in Fig. 8, these mice had an intermediate disease phenotype; of the 14 lpr/perf +/− mice monitored for survival, 8 died between days 103 and 188 and the remaining 6 were still alive at day 210. The perforin-deficient mice with normal Fas function (perf −/−) do not exhibit any lymphoproliferative disease or early death. It should be noted that all mice used in these experiments were housed under specific SPF conditions and were negative (by serology) for any of the common mouse pathogens.
FIG. 8.
Mice deficient in both perforin and Fas (lpr) show accelerated lymphoproliferative disease. Fas-deficient mice (lpr/perf +/+) (n = 9), perforin-deficient mice (perf −/−) (n = 10), Fas-deficient mice heterozygous for the perforin gene (lpr/perf +/−) (n = 14), and Fas-deficient mice homozygous for perforin deficiency (lpr/perf −/−) (n = 8) were monitored for lymphoproliferative disease and mortality. The double-deficient (Fas and perforin) mice were normal at birth but rapidly developed lymphadenopathy and died within 4 months. Note the gene dose effect of perforin on the lymphoproliferative disease; Fas-deficient mice heterozygous for perforin showed an intermediate disease phenotype.
DISCUSSION
Perforin-mediated killing is known to be an important effector mechanism (6, 20, 22). In this study we show that perforin also plays a role in immune regulation. The first clue that perforin might be involved in regulating T-cell responses came from the observation that some of the perf −/− mice died after LCMV infection. This was an unexpected result since death is rarely seen following an i.p. injection with LCMV. LCMV infection of adult mice by a peripheral route (i.p. or i.v.) usually has two outcomes; either the mice clear the infection or they develop a protracted chronic infection, depending upon the dose and the strain of LCMV used. LCMV is a noncytolytic virus, and any immunopathology and disease seen in this model is usually mediated by T cells (4). Adult +/+ mice chronically infected with LCMV after high-dose challenge with variants such as LCMV clone 13 or LCMV “Docile” exhibit a certain degree of immunopathologic damage, but mortality is rarely seen because many of the activated CD8 T cells are deleted due to chronic antigenic stimulation (3, 34, 36). This overall reduction in the numbers of LCMV-specific CD8 T cells limits the extent of immune-mediated damage. Our finding that some of the chronically infected perf −/− mice were dying suggested that there might be an accumulation of activated T cells in these mice. Indeed, we found that LCMV-infected perf −/− mice contained large numbers of activated CD8 T cells in the lymphoid organs, as well as in many other tissues (Fig. 1) and that in vivo depletion of CD8 T cells completely reversed the mortality (Fig. 2). Enhanced T-cell expansion in perf −/− mice compared to +/+ mice was also seen following chronic infection with LCMV clone 13 (Fig. 3). In these experiments, +/+ and perf −/− mice contained a similar viral load (Table 1) but there was more activation of both CD8 and CD4 T cells in −/− mice. The most striking difference was seen in the number of LCMV-specific CD8 T cells; chronically infected perf −/− mice contained ∼10-fold more antigen-specific CD8 T cells than did chronically infected +/+ mice (Fig. 3). Why were there greater number of antigen-specific CD8 T cells in perf −/− mice? Was this due to increased proliferation or decreased death of activated CD8 T cells? Our data (Fig. 4) showing that similar proportions of T cells were in cycle in both +/+ and perf −/− mice suggests that the increased numbers of activated T cells in chronically infected perf −/− mice may be due to decreased apoptosis of activated T cells. “Exhaustion” of LCMV-specific CD8 T cells has been shown to occur in chronically infected mice (36). Our study now shows that this peripheral “deletion” of activated CD8 T cells occurs less efficiently in perf −/− mice.
The second line of evidence that T cells from perf −/− mice respond differently than T cells from +/+ mice to a continuous antigenic stimulus came from in vitro experiments with anti-CD3 (Fig. 5). Preactivated T cells from perf −/− mice showed higher proliferative responses upon restimulation compared to +/+ mice (Fig. 5). This is consistent with an earlier report showing that in an in vitro allogeneic response, perforin-deficient effector T cells exhibited higher proliferation compared to perforin-intact T cells (45). Stimulation of naive T cells through the TcR induces a series of activation events that result in cell proliferation, cytokine production, and differentiation into killer cells. In contrast, chronic stimulation of activated T cells through the TcR-CD3 complex results in apoptosis, a phenomenon termed AICD (12, 44, 48, 61). This process is critical in clonal downsizing of the T-cell response, and several studies have shown that Fas-mediated (1, 51, 58) and TNF-mediated (61) apoptosis is involved in AICD. It should be pointed out that T cells from perf −/− mice upregulated the surface expression of Fas and TNFR II upon activation and were sensitive to Fas- and TNF-mediated apoptosis (Fig. 6 and 7). Thus, the enhanced T-cell numbers following chronic LCMV infection in perf −/− mice are not due to an intrinsic resistance by perf −/− T cells to Fas- or TNF-mediated apoptosis.
Our third line of evidence that perforin plays a role in regulating T-cell responses comes from the dramatic acceleration of the lymphoproliferative disease that we observed in mice deficient in both Fas and perforin (Fig. 8). Fas-deficient (lpr) mice spontaneously develop lymphoadenopathy and splenomegaly. The lymphocytes that accumulate in lpr mice are Thy-1+ TcR+ CD4− CD8− and are derived from mature CD4+ and CD8+ T cells (24, 31). It is believed that in +/+ mice, Fas-mediated apoptosis maintains homeostasis and eliminates such T cells but that in the absence of Fas these “chronically” activated T cells accumulate in the lymph nodes and spleens of lpr mice. In B6 lpr mice, lymphoadenopathy and splenomegaly start developing around 6 months of age. However, when these lpr mice were also made deficient for perforin (by breeding perf −/− mice with lpr mice) there was a striking acceleration of the lymphoproliferative disease (Fig. 8). These results show that perforin exerts an additional regulatory control on activated T cells. In addition, these experiments revealed a gene dosage effect of perforin; lpr mice that were heterozygous for perforin showed an intermediate disease phenotype. Recently, another report has also shown that mice deficient in both Fas and perforin exhibit accelerated lymphoproliferative disease and autoimmunity compared to perforin-intact lpr mice (40).
How does perforin regulate T-cell responses? There are several possible mechanisms that could account for the results presented in this study. One possibility is that perforin somehow damages the CTL themselves. In this model, the secreted perforin could act in cis (suicide) or in trans (fratricide). There has been considerable debate regarding how CTL escape from self-annihilation during the process of killing their targets (5, 8, 17, 38, 47, 54, 56). It is now well established that T-cell-mediated lysis is primarily vectorial (unidirectional), and there is also some evidence that CTL lines can be partially refractive to killing (6). However, it is possible that at some stage (for example, after a certain number of divisions or a certain period of time) the CTL may become sensitive to the secreted perforin or to the intracellular perforin (nuclear membrane damage?). In this context it is worth noting that the regulatory effects of perforin are seen under conditions of chronic TcR stimulation. Such a mechanism would put an upper limit on the number of times a T cell could divide and for how long it remains an effector in vivo. This could serve as a possible mechanism for controlling the “killers.” The increased numbers of activated CD8 T cells in perf −/− mice chronically infected with LCMV is consistent with a direct effect of perforin on the activated CD8 T cells. The increased activation of CD4 T cells in these mice is more difficult to explain with this model. However, it should be noted that CD4 T cells can also express perforin (23, 60). An alternative mechanism to explain the enhanced T-cell expansion in vivo in perf −/− mice is that in perforin-deficient mice antigen-presenting cells (APC) are not killed and thus there are more APC in these mice for sustaining the response (45). Activated T cells can be rescued from AICD by appropriate costimulatory signals delivered by APC (7, 52). In perf −/− mice the “quality” of such signals may be better than in +/+ mice, where some of the APC are damaged by perforin-mediated killing. Such a mechanism could also account for the enhanced CD8 and CD4 T-cell responses seen in perforin-deficient mice. It should be noted that the mechanisms that we have proposed are not mutually exclusive and that some combination of these could be involved in immune regulation by perforin.
In conclusion, our results show that absence of perforin has a profound impact on T-cell regulation in vivo during a chronic viral infection or during autoimmunity. The finding that perforin plays a role in downregulating T-cell responses in vivo has implications towards developing strategies for adoptive T-cell therapy in the treatment of chronic infections and malignancies (43). A major limitation of these immunotherapy treatments is the poor survival of the adoptively transferred T cells in vivo. Our results suggest that, in instances where control of the viral infection or eradication of the tumor is mediated primarily by cytokine effects and not by direct killing, it might be better to use perforin-negative T cells for the adoptive immunotherapy.
ACKNOWLEDGMENTS
M.M. and M.S. contributed equally to this work.
We thank Rita J. Concepcion and Morry Hsu for excellent technical assistance and Kim Holcombe for help with the manuscript.
This work was supported by National Institutes of Health grants AI-30048 and NS-21496 to R.A. and CA-47307 to W.R.C. M.M. was supported by Medical Scientist Training Program grant GM 08042-08. M.S. was supported by a postdoctoral fellowship from the National Multiple Sclerosis Society.
REFERENCES
- 1.Adachi M, Suematsu S, Kondo T, Ogasawara J, Tanaka T, Yoshida N, Nagata S. Targeted mutation in the Fas gene causes hyperplasia in the peripheral lymphoid organs and liver. Nat Genet. 1995;11:294–299. doi: 10.1038/ng1195-294. [DOI] [PubMed] [Google Scholar]
- 2.Adachi M, Suematsu S, Suda T, Watanabe D, Fukuyama H, Ogasawara J, Tanaka T, Yoshida Y, Nagata S. Enhanced and accelerated lymphoproliferation in Fas-null mice. Proc Natl Acad Sci USA. 1996;93:2131–2136. doi: 10.1073/pnas.93.5.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed R, Salmi A, Butler L D, Chiller J M, Oldstone M B A. Selection of genetic varians of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J Exp Med. 1984;160:521–540. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asano M S, Ahmed R. Immune conflicts in lymphocytic choriomeningitis virus. Springer Semin Immunopathol. 1995;17:247–259. doi: 10.1007/BF00196168. [DOI] [PubMed] [Google Scholar]
- 5.Berke G. Lymphocyte-triggered internal target disintegrations. Immunol Today. 1991;12:396–399. doi: 10.1016/0167-5699(91)90138-j. [DOI] [PubMed] [Google Scholar]
- 6.Berke G. The binding and lysis of target cells by cytotoxic T lymphocytes: molecular and cellular aspects. Annu Rev Immunol. 1994;12:735–773. doi: 10.1146/annurev.iy.12.040194.003511. [DOI] [PubMed] [Google Scholar]
- 7.Boise L H, Minn A J, Noel P J, June C H, Accawitti M A, Lindstew T, Thompson C B. CD28 Costimulation can promote T cell survival by enhancing the expression of Bcl-XL. Immunity. 1995;3:87–98. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 8.Burns J, Littlefield K, Gill J, Trotter J. Autoantigen-induced self-lysis of human myelin protein-specific T lymphocytes. J Neuroimmunol. 1991;35:227–236. doi: 10.1016/0165-5728(91)90177-9. [DOI] [PubMed] [Google Scholar]
- 9.Clark W R, Walsh C M, Glass A A, Hayashi F, Matloubian M, Ahmed R. Molecular pathways of CTL-mediated cytotoxicity. Immunol Rev. 1995;146:33–44. doi: 10.1111/j.1600-065x.1995.tb00682.x. [DOI] [PubMed] [Google Scholar]
- 10.Cohen P L, Eisenberg R A. Lpr and gld: single gene models of systemic autoimmunity and lymphoproliferative disease. Annu Rev Immunol. 1991;9:243–269. doi: 10.1146/annurev.iy.09.040191.001331. [DOI] [PubMed] [Google Scholar]
- 11.Czerkinsky C C, Nilsson L A, Nygren H, Ouchterlony O, Tarkowski A. A solid-phase enzyme-linked immunospot (ELISPOT) assay for enumeration of specific antibody-secreting cells. J Immunol Methods. 1983;65:109–121. doi: 10.1016/0022-1759(83)90308-3. [DOI] [PubMed] [Google Scholar]
- 12.Dhein J, Walczak H, Baumler C, Debatin K, Krammer P H. Autocrine T-cell suicide mediated by APO-1 (Fas/CD95) Nature. 1995;343:438–441. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- 13.Di Virgilio F, Pizzo P, Zanovello P, Bronte V, Collavo D. Extracellular ATP as a possible mediator of cell-mediated cytotoxicity. Immunol Today. 1990;11:274–276. doi: 10.1016/0167-5699(90)90111-l. [DOI] [PubMed] [Google Scholar]
- 14.Dillon S R, MacKay V L, Fink P J. A functionally compromised intermediate in extrathymic CD8+ T cell deletion. Immunity. 1995;3:321–333. doi: 10.1016/1074-7613(95)90117-5. [DOI] [PubMed] [Google Scholar]
- 15.Dohlsten M, Bjorklund M, Sundstedt A, Hedlund G, Samson D, Kalland T. Immunopharmacology of the superantigen staphylococcal enterotoxin A in T-cell receptor Vβ3 transgenic mice. Immunology. 1993;79:520–527. [PMC free article] [PubMed] [Google Scholar]
- 16.Filipini A, Traffs R A, Sitovsky M V. Extracellular ATP in T-lymphocyte activation: possible role in effector functions. Proc Natl Acad Sci USA. 1990;87:8267–8271. doi: 10.1073/pnas.87.21.8267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Golstein P. Sensitivity of cytotoxic T cells to T cell-mediated cytotoxicity. Nature. 1974;252:81–83. doi: 10.1038/252081a0. [DOI] [PubMed] [Google Scholar]
- 18.Henkart P A. ICE family proteases: mediators of all apoptotic death? Immunity. 1996;4:195–201. doi: 10.1016/s1074-7613(00)80428-8. [DOI] [PubMed] [Google Scholar]
- 19.Heusel J W, Wesselschmidt R L, Shresta S, Russell J H, Ley T J. Cytotoxic lymphocytes require Granzyme B for the rapid induction of DNA fragmentation and apoptosis in allogeneic target cells. Cell. 1994;76:977–987. doi: 10.1016/0092-8674(94)90376-x. [DOI] [PubMed] [Google Scholar]
- 20.Kagi D, Ledermann B, Burki K, Hengartner H, Zinkernagel R M. CD8+ T cell-mediated protection against an intracellular bacterium by perforin-dependent cytotoxicity. Eur J Immunol. 1994;24:3068–3072. doi: 10.1002/eji.1830241223. [DOI] [PubMed] [Google Scholar]
- 21.Kagi D, Ledermann B, Burki K, Seiler P, Odermatt B, Olsen K J, Podack E R, Zinkernagel R M, Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 22.Kagi D, Ledermann B, Burki K, Zinkernagel R M, Hengartner H. Molecular mechanisms of lymphocyte-mediated cytotoxicity and their role in immunological protection and pathogenesis in vivo. Annu Rev Immunol. 1996;14:207–232. doi: 10.1146/annurev.immunol.14.1.207. [DOI] [PubMed] [Google Scholar]
- 23.Lancki D W, Hsieh C S, Fitch F W. Mechanisms of lysis by cytotoxic T lymphocyte clones. Lytic activity and gene expression in cloned antigen-specific CD4+ and CD8+ T lymphocytes. J Immunol. 1991;146:3242–3249. [PubMed] [Google Scholar]
- 24.Laouar Y, Ezine S. In vivo CD4+ lymph node T cells from lpr mice generate CD4-CD8-B220+TcR-beta low cells. J Immunol. 1994;153:3948–3955. [PubMed] [Google Scholar]
- 25.Lenardo M J. Fas and the art of lymphocyte maintenance. J Exp Med. 1996;183:721–724. doi: 10.1084/jem.183.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu C C, Steffen M, King F, Young J D. Identification, isolation, and characterization of a novel cytotoxin in murine cytolytic lymphocytes. Cell. 1987;51:393–403. doi: 10.1016/0092-8674(87)90635-0. [DOI] [PubMed] [Google Scholar]
- 27.Liu C, Walsh C M, Young J D. Perforin: structure and function. Immunol Today. 1995;16:194–201. doi: 10.1016/0167-5699(95)80121-9. [DOI] [PubMed] [Google Scholar]
- 28.Los M, van de Craen M, Penning L C, Schenk H, Westendorp M, Baeuerle P, Droge W, Krammer P H, Fiers W, Schulze-Osthoff K. Requirement of an ICE/CED-3 protease for Fas/Apo-1-mediated apoptosis. Nature. 1995;375:81–83. doi: 10.1038/375081a0. [DOI] [PubMed] [Google Scholar]
- 29.Lynch D H, Ramsdell F, Alderson M R. Fas and FasL in the homeostatic regulation of immune responses. Immunol Today. 1995;16:569–574. doi: 10.1016/0167-5699(95)80079-4. [DOI] [PubMed] [Google Scholar]
- 30.Lynch D H, Watson M L, Alderson M R, Baum P R, Miller R E, Tough T, Givson M, Davis-Smith T, Smith C A, Hunter K, Bhat D, Din W, Goodwin R G, Seldin M F. The mouse Fas-ligand gene is mutated in gld mice and is part of a TNF family gene cluster. Immunity. 1994;1:131–136. doi: 10.1016/1074-7613(94)90106-6. [DOI] [PubMed] [Google Scholar]
- 31.Maldonado M A, Eisenberg R A, Roper E, Cohen P L, Kotzen B L. Greatly reduced lymphoproliferation in lpr mice lacking major histocompatibility coupled class I. J Exp Med. 1995;181:641–648. doi: 10.1084/jem.181.2.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matloubian M, Somsundaram T, Kohlekar S R, Selvakumar R, Ahmed R. Genetic basis of viral persistence: single amino acid change in the viral glycoprotein affects ability of lymphocytic choriomeningitis virus to persist in adult mice. J Exp Med. 1990;172:1043–1048. doi: 10.1084/jem.172.4.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matloubian M, Concepcion R J, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J Virol. 1994;68:8056–8063. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matloubian M, Kohlekar S R, Somasundaram T, Ahmed R. Molecular determinants of macrophage tropism and viral persistence: importance of single amino acid changes in the polymerase and glycoprotein of lymphocytic choriomeningitis virus. J Virol. 1993;67:7340–7349. doi: 10.1128/jvi.67.12.7340-7349.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCormack J E, Callahan J E, Kappler J, Marrack P C. Profound deletion of mature T cells in vivo by chronic exposure to exogenous superantigen. J Immunol. 1993;150:3785–3792. [PubMed] [Google Scholar]
- 36.Moskophidis D, Lechner F, Pircher H, Zinkernagel R M. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature (London) 1993;362:758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- 37.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 38.Ojcius D M, Jrang S, Persechini P M, Detmers P A, Young J D. Cytoplasts from cytotoxic T lymphocytes are resistant to perforin-mediated lysis. Mol Immunol. 1991;28:1011–1018. doi: 10.1016/0161-5890(91)90187-o. [DOI] [PubMed] [Google Scholar]
- 39.Orange J S, Salazar-Mather T P, Opal S M, Spencer R L, Miller A H, McEwen B S, Biron C B. Mechanism of interleukin 12-mediated toxicities during experimental viral infections. J Exp Med. 1995;181:901–914. doi: 10.1084/jem.181.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peng S L, Moslehi J, Robert M E, Croft J. Perforin protects against autoimmunity in lupus-prone mice. J Immunol. 1998;160:652–660. [PubMed] [Google Scholar]
- 41.Rabinovitch P S, Torres R M, Engel D. Simultaneous cell cycle analysis and two-color surface immunofluorescence using 7-amino-actinomycin D and single laser activation: application to study of cell activation and the cell cycle of murine Ly-1 B cells. J Immunol. 1986;136:2769–2775. [PubMed] [Google Scholar]
- 42.Radvanyi L G, Mills G B, Miller R G. Religation of the T cell receptor after primary activation of mature T cells inhibits proliferation and induces apoptotic cell death. J Immunol. 1993;150:5704–5715. [PubMed] [Google Scholar]
- 43.Riddell S R, Greenberg P D. Principles for adoptive T cell therapy of human viral diseases. Annu Rev Immunol. 1995;13:545–586. doi: 10.1146/annurev.iy.13.040195.002553. [DOI] [PubMed] [Google Scholar]
- 44.Russell J H, Rush B, Weaver C, Wang R. Mature T cells of autoimmune lpr/lpr mice have a defect in antigen-stimulated suicide. Proc Natl Acad Sci USA. 1993;90:4409–4413. doi: 10.1073/pnas.90.10.4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sad S, Kagi D, Mosmann T R. Perforin and Fas killing by CD8+ T cells limits their cytokine synthesis and proliferation. J Exp Med. 1996;184:1543–1547. doi: 10.1084/jem.184.4.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shen H, Slifka M K, Matloubian M, Jensen E R, Ahmed R, Miller J F. Recombinant Listeria monocytogenes as a live vaccine vehicle for the induction of protective anti-viral cell-mediated immunity. Proc Natl Acad Sci USA. 1995;92:3987–3991. doi: 10.1073/pnas.92.9.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shresta S, Heusel J W, Macivor D M, Wesselschmidt R L, Russell J H, Ley T J. Granzyme B plays a critical role in cytotoxic lymphocyte-induced apoptosis. Immunol Rev. 1995;146:211–221. doi: 10.1111/j.1600-065x.1995.tb00690.x. [DOI] [PubMed] [Google Scholar]
- 48.Singer G G, Abbas A K. The Fas antigen is involved in peripheral but not thymic deletion of T lymphocytes in T cell receptor transgenic mice. Immunity. 1994;1:365–371. doi: 10.1016/1074-7613(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 49.Suhrbier A, Burrows S R, Fervan A, Laven M F, Baxter G D, Moss D J. Peptide epitope-induced apoptosis of human cytotoxic T lymphocytes. Implication for peripheral T cell deletion and peptide vaccination. J Immunol. 1993;150:2169–2178. [PubMed] [Google Scholar]
- 50.Taguchi T, McGhee J R, Coffman R L, Beagley K W, Eldridge J H, Takatsu K, Kiyono H. Detection of individual mouse spleen T cells producing IFN-γ and IL-5 using the enzyme-linked immunospot (ELISPOT) assay. J Immunol Methods. 1990;128:65–73. doi: 10.1016/0022-1759(90)90464-7. [DOI] [PubMed] [Google Scholar]
- 51.Takahashi T, Tanaka M, Brannon C L, Jenkins N A, Copeland N A, Sude T, Nagata S. Generalized lymphoproliferative disease in mice caused by a point mutation in the Fas ligand. Cell. 1994;76:969–976. doi: 10.1016/0092-8674(94)90375-1. [DOI] [PubMed] [Google Scholar]
- 52.Thompson C B, Lindsten T, Ledbutter J A, Kunkel S L, Young H A, Emerson S A, Leiden J M, June C H. CD28 activation pathway regulates the production of multiple T-cell-derived lymphokines/cytokines. Proc Natl Acad Sci USA. 1989;86:1333–1337. doi: 10.1073/pnas.86.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tian Q, Streuli M, Saito H, Schlossman S F, Anderson P. A polyadenylate binding protein localized to the granules of cytolytic lymphocytes induces DNA fragmentation in target cells. Cell. 1991;67:629–639. doi: 10.1016/0092-8674(91)90536-8. [DOI] [PubMed] [Google Scholar]
- 54.Tschopp J, Nabholz M. Perforin-mediated target cell lysis by cytotoxic T lymphocytes. Annu Rev Immunol. 1990;8:279–302. doi: 10.1146/annurev.iy.08.040190.001431. [DOI] [PubMed] [Google Scholar]
- 55.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein-labelled annexin V. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 56.Walden P R, Eisen H N. Cognate peptides induce self-destruction of CD8+ cytolytic T lymphocytes. Proc Natl Acad Sci USA. 1990;87:9015–9019. doi: 10.1073/pnas.87.22.9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walsh C M, Matloubian M, Liu C C, Ueda R, Kurahara C G, Christensen J L, Huang M T, Young J D, Ahmed R, Clark W R. Immune function in mice lacking the perforin gene. Proc Natl Acad Sci USA. 1994;91:10854–10858. doi: 10.1073/pnas.91.23.10854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Watanabe-Fukanuga R, Brannon C I, Copeland N G, Jenkins N A, Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that initiate apoptosis. Nature. 1992;356:314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- 59.Wesselborg S, Janssen O, Kabelitz D. Induction of activation-driven death (apoptosis) in activated but not resting peripheral blood T cells. J Immunol. 1993;150:4338–4345. [PubMed] [Google Scholar]
- 60.Yawukawa M, Yakushijin Y, Hasegawa H, Miyake M, Hitsumoto Y, Kimura S, Takeuchi N, Fryrta S. Expression of perforin and membrane bound lymphotoxin (tumor necrosis factor-beta) in virus-specific CD4+ human cytotoxic T-cell clones. Blood. 1993;81:1527–1534. [PubMed] [Google Scholar]
- 61.Zheng L, Fisher G, Miller R E, Peschon J, Lynch D H, Lenardo M J. Induction of apoptosis in mature T cells by tumour necrosis factor. Nature. 1995;377:348–351. doi: 10.1038/377348a0. [DOI] [PubMed] [Google Scholar]