Abstract
Over the last few years, extracellular vesicles (EVs) have received increasing attention as potential non-invasive diagnostic and therapeutic biomarkers for various diseases. The interest in EVs is related to their structure and content, as well as to their changing cargo in response to different stimuli. One of the potential areas of use of EVs as biomarkers is the central nervous system (CNS), in particular the brain, because EVs can cross the blood–brain barrier, exist also in peripheral tissues and have a diverse cargo. Thus, they may represent “liquid biopsies” of the CNS that can reflect brain pathophysiology without the need for invasive surgical procedures. Overall, few studies to date have examined EVs in neuropsychiatric disorders, and the present evidence appears to lack reproducibility. This situation might be due to a variety of technical obstacles related to working with EVs, such as the use of different isolation strategies, which results in non-uniform vesicular and molecular outputs. Multi-omics approaches and improvements in the standardization of isolation procedures will allow highly pure EV fractions to be obtained in which the molecular cargo, particularly microRNAs and proteins, can be identified and accurately quantified. Eventually, these advances will enable researchers to decipher disease-relevant molecular signatures of the brain-derived EVs involved in synaptic plasticity, neuronal development, neuro-immune communication, and other related pathways. This narrative review summarizes the findings of studies on EVs in major psychiatric disorders, particularly in the field of biomarkers, and discusses the respective therapeutic potential of EVs.
Keywords: Extracellular vesicles, Biomarker, Schizophrenia, Bipolar disorder, Major depressive disorder, Technical limitations
Introduction
Extracellular vesicles (EVs) are nanoparticles derived from endosomes (in which case they are referred to as exosomes) or the plasma membrane (in which case they are referred to as ectosome or micro-vesicles) and are secreted from most cell types [1]. They are released via the cell membrane, either directly or after multi-vesicular bodies (MVBs) merge with the membrane (Fig. 1). Currently, there is a general consensus on EV nomenclature [1]: They can be divided into exosomes and ectosomes, depending on their biogenesis mechanism, or into small EVs (sEVs) and medium and/or large EVs (m/lEVs), depending on their size (diameter of sEVs, < 100 nm or < 200 nm; diameter of m/lEVs, > 200 nm). Because none of the reviewed publications discussed here proves that the EVs are indeed of endocytic origin (i.e., are exosomes), we use the term "EV" throughout the text [1].
Fig. 1.
a Schematic diagram showing how cells release three types of extracellular vesicles: exosomes (30–150 nm), micro-vesicles (50–1000 nm), and apoptotic bodies (500–2000 nm). b Transmission electron microscope image of isolated and purified extracellular vesicles. (Adapted from “Extracellular Vesicle Separation by Density Gradient Ultracentrifugation,” by BioRender.com (2022). Retrieved from https://app.biorender.com/biorender-templates)
EVs are present in various bodily fluids, including serum, cerebral spinal fluid (CSF), urine, saliva, and amniotic fluid, and appear to play a role in a range of biological functions, including cellular communication and maintenance mechanisms, waste removal from cells, immune responses stimulation, tissue repair and regeneration, and tumor progression [2–4]. In the central nervous system (CNS), EVs can be secreted in huge numbers by neurons, microglia, astrocytes, and oligodendrocytes and are involved in neuron–neuron and glial–neuron communication. They also participate in neurite growth; regulation of myelination and stress and immune responses; and modulation of synaptic plasticity and neuronal survival [2, 5].
EVs contain various bioactive compounds, such as proteins, lipids, mRNAs, microRNAs (miRNAs), other non-coding RNAs, metabolites, and DNA. Because of the diversity of their cargo and their ability to cross the blood–brain barrier (BBB) in both directions and diffuse from the site of release, they hold great potential as circulating and non-invasive biomarkers for screening, diagnostics, and treatment in complex brain disorders [4, 6]. The content of EVs may be influenced by disease status. Therefore, in CNS pathologies, their cargo may modify intercellular communication and tissue homeostasis by modulating transcription, neurogenesis, synaptic plasticity, neuronal circuit development, and neuroinflammation [7, 8]. EVs are seen as a promising type of biosignature and as a means of communication between the brain and other organs and tissues [3] (Fig. 2). Because of their effects on brain cell development and function, EVs have been hailed as an intriguing mechanism of cell-to-cell communication, with significant implications for the onset and progression of neurodegenerative and neuropsychiatric disorders [9, 10].
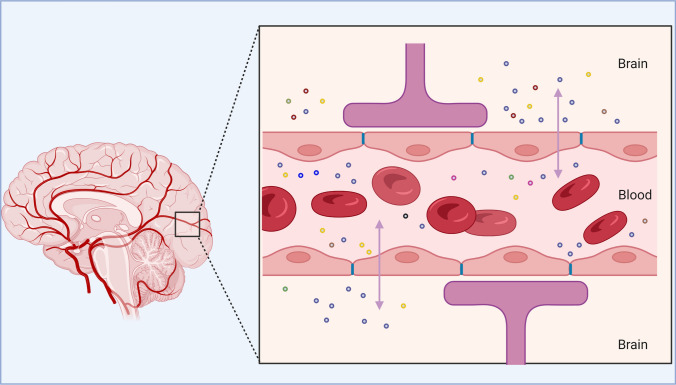
Fig. 2.
Ability of extracellular vesicles to cross the blood–brain barrier in both directions. (Adapted from “Blood Brain Barrier (Simple Longitudinal Zoom),” by BioRender.com (2022). Retrieved from https://app.biorender.com/biorender-templates)
Major psychiatric disorders
In 2019, mental illnesses were the second and seventh leading causes of years lived with disability and disability-adjusted life-years, respectively, worldwide [11]. The most prevalent and severe mental illnesses are schizophrenia (SCZ), bipolar disorder (BD), and major depressive disorder (MDD). All three have a polygenic architecture and partially overlapping genetic and phenotypic features; furthermore, they lack a pathophysiological signature, which represents a fundamental hurdle to their characterization and differentiation [12, 13].
SCZ is a severe, debilitating, incurable, and lifelong psychiatric illness that affects cognitive, behavioral, and emotional functioning and is characterized by symptoms, such as disorganized thinking, hallucinations, and anhedonia. The disease occurs in around 1% of the world population and is one of the top 25 causes of disability globally [14]. BD is characterized by repeated manic, hypomanic, and depressive episodes; it has a lifetime prevalence of around 1%, is associated with a high lifetime risk of suicide and is one of the top ten leading causes of disease burden for people aged 15 to 44 [13]. MDD, the most prevalent neuropsychiatric disorder in the general population (300 million cases globally), is linked to a low quality of life, functional impairment, and morbidity. Globally, it is one of the most significant causes of disability and the leading cause of suicide and is expected to rank as the second most common cause of global disease burden by 2030 [15, 16].
Biomarker potential of EVs in brain disorders
In recent years, multi-omics profiling approaches have been performed in large samples of psychiatric patients with the aim to uncover disease-specific molecular fingerprints, stratify patient subgroups, predict treatment efficacy and develop drug discovery processes [17, 18]. Within this biomarker framework, first steps have been taken to use EVs to identify relevant biomarkers in SCZ, BD, and MDD (Fig. 3), and EVs have been proposed as a novel source of biomarkers and a window into brain cells and their environment [19, 20]
Fig. 3.
Current -omics approaches to analyzing cargos of extracellular vesicles. (Created with BioRender.com). LC–MS/MS, liquid chromatography-tandem mass spectrometry; miRNA, microRNA; qRT-PCR, quantitative real-time polymerase chain reaction; RNA-seq, RNA sequencing
In vitro and in vivo experiments in mouse models have shown that EVs may mediate transmission of neurotoxic protein aggregates between neurons, and consequently, they have been referred to as “Trojan horses” [21]. In addition, they may offer a way to eliminate harmful proteins from the brain [22]. Brain cells are thought to communicate with one another or with other bodily cells by direct cell–cell interactions and soluble mediators, and researchers have suggested that EVs, and specifically their miRNA cargo, may be involved in this communication by providing a finely tuned regulatory system [23]. Changes in the function or composition of EVs from each major type of brain cells may play an important role in neuropsychiatric disorders [22].
This narrative review gives an overview of studies with the aim to provide an update on the EV approach to major psychiatric disorders.
Methods
We performed literature searches in PubMed and included all articles published by the end of February 2022. The searches were limited to articles written in English and used the following search strings: (i) “schizophren* OR bipolar disorder OR major depressi* AND extracellular vesicle” (99 hits) and (ii) “schizophren* OR bipolar disorder OR major depressi* AND exosom*” (57 hits). After merging and curating the results, we identified 37 duplicate publications and 50 publications that were not related to the topic of interest or were the type of review articles that we did not wish to include. Ultimately, we included 69 publications in our review.
Results
RNA cargo
Among the various types of EV cargo, miRNA has attracted the most attention in the search for reliable and feasible biomarkers for the diagnosis and prognosis of SCZ, BD, and MDD [10, 24]. MiRNAs are significant functional components of EVs and can affect recipient cells by negatively regulating gene expression and acting as ligands at cell receptors [15, 25]. Many miRNAs are expressed in the human brain—neuronal miRNAs account for roughly 70% of all miRNAs in the body [26]—and are involved in the regulation of neuronal development and differentiation [27]. Changes in the miRNA content of EVs could affect transcriptional regulation and neurotransmitter release and function in various brain regions and may eventually contribute to the pathogenesis of major psychiatric disorders, making them potential biomarkers [10, 16, 28–30].
Animal studies
Transplanting serum-derived EVs from patients with SCZ into mice resulted in SCZ-like behavioral and molecular phenotypes, pointing to the importance of EVs in the etiology of this disorder [10]. A comparison of bulk RNA sequencing in the hippocampus and prefrontal cortex (PFC) between mice administered EVs from either patients with SCZ or healthy controls (HCs) showed significant differences in 1887 mRNAs (999 downregulated; 888 upregulated) in the hippocampus samples and 2267 mRNAs (1130 downregulated; 1137 upregulated) in the PFC samples. The genes were significantly enriched in strongly SCZ-associated pathways, such as synaptic transmission, neurodevelopment, and behavior [10].
Peripheral injection of blood-derived EVs from HCs into chronic unpredictable mild stress (CUMS) mice reduced depression-like behaviors, suggesting that EVs have a role in depression pathophysiology [31]. Compared with control mice, CUMS mice showed significantly higher blood levels of EV-derived miR-139-5p. In cell culture assays, overexpression of miR-139-5p decreased the proliferation of neural stem cells and their differentiation into neurons [31].
EVs may be associated with the brain-derived neurotrophic factor (BDNF)/TrkB pathway and may mirror alterations in specific brain regions. Expression levels of BDNF, TrkB, and SYNAPTOTAGMIN 1 in serum-derived EVs were lower in CUMS rats than in controls and were reversed by fluoxetine [32]. Twenty-five of the 152 dysregulated serum EV miRNAs among the three groups (Control + Vehicle, CUMS + Vehicle, and CUMS + Fluoxetine) were identified as being involved in neuroplasticity and the stress response. The MAPK, Wnt, and mTOR pathways are the most notable target pathways for the miRNAs differentially expressed in CUMS rats [32]. In a rat model of depression, miRNA content of EVs from hippocampi and whole brains showed upregulation of miR-29c in hippocampal EVs and downregulation of miR-149 and miR-29c in whole-brain EVs [28]. BDNF, a well-known neurotrophin, is essential for nervous system development and maintenance [29].
Human studies
Schizophrenia
MiRNA assay of PFC-derived EVs revealed significantly higher miR-497 expression in patients with SCZ than in controls [27]. MiR-497 is member of the miR-15/107 gene family, which has been linked to the development of neurodegenerative disorders [27]. Olanzapine treatment resulted in increased DJ-1 and decreased miR203a-3p levels and stopped SCZ-induced overexpression of miR203a-3p in plasma-derived EVs of patients with first-episode SCZ [33]. DJ-1 is a redox-sensitive protein, and research has suggested that miR-203a-3p can be considered as an important mediator of oxidative stress in SCZ because it targets the 3'-UTR of DJ-1 mRNA [33].
Oxidative stress in early psychosis patients (EPP) led to an increase in blood levels of the EV-derived miR-137 and a decrease in the cytochrome c oxidase subunit 6A2 (COX6A2) and changed mitophagy markers. Higher miR-137 and lower COX6A2 levels were associated with a reduction of auditory steady-state response (ASSR) gamma oscillations in an electroencephalogram [34]. Because ASSR relies on the proper functioning of networks related to parvalbumin interneurons, changes in miR-137/COX6A2 plasma EV levels could be used as a proxy marker for dysfunction of the parvalbumin interneuron cortical microcircuit, which is implicated in the psychopathology and cognitive deficits of SCZ [34].
The results of the first genome-wide miRNA expression profiling of serum-derived EVs from patients with SCZ revealed differential expression profiles that may be useful for diagnosing the disease [29]. The top differentially expressed miRNA, miR-206, was shown to influence the expression of BDNF, and miR-206 upregulation was suggested to lead to BDNF malfunction in SCZ [29].
Levels of EV-secreted miRNA-223 were increased in the orbitofrontal cortex of post-mortem brain samples from patients with SCZ and patients with BD with psychosis, and an inverse association was observed with the expression of the targets of miRNA-223, GRIN2B and GRIA2 [9]. The addition of astrocytic EVs to neuronal cultures revealed similar results [9]. Treatment with the antipsychotics olanzapine and haloperidol led to cell-specific regulation of cellular and EV-derived miRNA-233, which regulates neuronal gene expression [9].
The advancement of sequencing technology has led to the discovery of a growing number of human circular RNAs (circRNAs) [35]. These non-coding RNAs can affect gene expression and regulation by acting as miRNA sponges and inhibiting gene silencing, and EV circRNAs, which are linked to early brain development, may represent the amount of circRNAs expressed in cells and tissues [36]. Analyses of plasma-derived EV circRNAs from patients with SCZ revealed the importance of four circRNAs; three of their target miRNAs, miR-34a, miR-34c, and miR-449a, are thought to play a role in the pathogenesis of SCZ [35].
Bipolar disorder
MiRNA assay of PFC-derived EVs revealed significantly higher miR-29c expression in patients with BD than in controls [27]. MiR-29c is induced by Wnt signaling, which is inhibited by GSK-3, a known target of lithium (the first-line treatment for BD) [27].
The miRNA content of EVs from the anterior cingulate cortex (BA24) of patients with BD showed higher levels of miR-149 in post-mortem brain tissue of patients than in that of HCs [28]. Downregulated miR-484, miR-652–3p, and miR-142–3p and upregulated miR-185–5p were shown in plasma-derived EV miRNAs of patients with BD compared with HCs [37].
Major depressive disorder
Expression profiling of genome-wide miRNAs in blood-derived EVs of drug-free patients with MDD and HCs identified miR-139-5p as one of the top differentially expressed miRNAs (area under the curve [AUC], 0.857; good performance) [31]. In addition, miR-139-5p showed good performance in differentiating between MDD and SCZ (AUC, 0.85). The receiver operating characteristics (ROC) curves for a cluster of 10 miRNAs that contributed most to the differentiation between MDD and HC showed excellent performance (AUC, 0.94) in differentiating between MDD and SCZ [31]. Levels of the blood-derived EV miR-139-5p were higher in patients with MDD than in HCs, indicating that miR-139-5p may be a biomarker for MDD [38].
Serum-derived EV miRNA expression profiles of negatively regulating Toll-like receptor 4 (TLR4) signaling, including let-7e, miR-21-5p, miR-145, miR-146a, and miR-155, may be useful for determining whether serum-derived EV miRNAs can be used to predict antidepressant response in MDD [39].
A study on plasma EV miRNAs from patients with treatment-resistant depression identified statistically significant differences in two miRNAs: miR-335 and miR-1292. KEGG analysis of these miRNAs identified the MAPK, Ras, and PI3K-AKT signaling pathways [30].
Protein cargo
EVs have a variety of proteins on the surface and inside, and identifying these proteins could provide important information about the molecular mechanisms associated with their roles in EV biogenesis, structure, trafficking, immunogenicity, mediated synaptic plasticity, and cell targeting in physiological and pathological situations [8]. Thus, proteome analysis of EVs should play a key role in the development of less invasive diagnostic and therapeutic approaches for brain disorders [8].
Schizophrenia
Glucose hypometabolism, systemic insulin resistance (IR), and insulin signaling abnormalities in neuronal cells have been implicated in the pathophysiology of major psychiatric disorders [40–43]. Regarding individual neuronal IR biomarkers, the insulin signal transduction proteins AKT, pGSK3β, and more specifically p70S6K in plasma neuron-derived EVs (NDEVs) showed important group differences between patients with SCZ and controls: Lower neuronal IR biomarker scores were associated with higher brain glucose levels and poorer performance on verbal learning in SCZ [42].
Results from measurements of insulin signal transduction proteins in lysed NDEVs from plasma of drug-naive patients with first-episode SCZ indicated a trend for lower pS312-IRS-1 levels, decreased phosphorylated/total protein ratios of downstream serine-threonine kinases (AKT, GSK3β, mTOR, p70S6K), and lower phosphorylation ratios of mTOR in patients compared with controls, indicating reduced pathway activation [43].
Glial fibrillary acidic protein (GFAP) and α-II-SPECTRIN are two specific cargo proteins of EVs [5]. Elevated EV GFAP may be associated with increased neuro-inflammation and astrocytosis in patients with SCZ, and lower levels of EV α-II-SPECTRIN in SCZ may indicate neuronal loss [5].
According to the mild neuro-inflammation hypothesis of SCZ [which involves hypofunction of glutamatergic signaling via N-methyl-D-aspartate receptors (NMDARs) and hyperactivation of dopamine D2 receptors (D2Rs)] and triplet puzzle theory (in which the code created by the triplet amino acid homologies may help guide the receptors to each other), EV-mediated volume transmission (VT) from glial networks to neuronal networks involving distinct cytokine and chemokine receptors and their agonist ligands can result in the formation of dysfunctional and distinct glutamatergic signaling via NMDAR and D2R heteroreceptor complexes. In such heteroreceptor complexes, new allosteric receptor–receptor interactions created by the effects of agonist ligands may lead to pathological changes in D2R and NMDAR signaling, which may contribute to SCZ-like symptoms [44]. VT enables communication between all cells of the brain by diffusion in the extracellular fluid and flow in the CSF of neurotransmitters, neuromodulators, and trophic factors, where they act as VT signals and enable information handling and trophic intercellular communication, including neuron–glia and glia–glia interactions [45].
Disruption in astrocytes, which are involved in synthesis of neurotransmitters and insulation and maintenance of neural networks, can be considered as a pathophysiological mechanism in SCZ [46]. Aβ aggregates can form plaques, which cause microglial cell activation, inflammation, and neurodegeneration, and plasma levels of amyloid-beta 1–42 (ADEV-Aβ42) from astrocyte-derived extracellular vesicle (ADEVs) were higher in people with SCZ than in HCs [46].
Mitochondrial dysfunction could be one of the most important pathogenic pathways in brain disorders [47, 48]. In patients with first-episode SCZ, mitochondrial ATP production is significantly reduced, as are levels of several proteins required for mitochondrial integrity, immobilization, and endogenous mitochondrial neuroprotection [48]. Mitochondrial ATP synthase activity in ADEVs and the levels of several structurally and functionally important mitochondrial proteins in ADEVs and NDEVs are altered in patients with first-episode SCZ, so plasma NDEVs and ADEVs were hypothesized to alter brain cell metabolism by transferring mitochondrial protein abnormalities [48]. Patients with first-episode SCZ have remarkably low levels of brain mitochondrial proteins with neuroprotective and metabolic regulating functions [48].
Bipolar disorder
Assessments of neuronal insulin signaling at its first node, insulin receptor substrate-1 (IRS-1), and along the canonical (AKT, GSK-3β, and p70S6K) and alternative (ERK1/2, JNK, and p38-MAPK) pathways showed that NDEV biomarkers of the insulin signaling pathway were associated with cognitive dysfunction and brain structural abnormalities in patients with BD [41].
An assessment of the tumor necrosis factor-alpha (TNF-α) receptor/nuclear factor-kappa B (TNFR/NF-κB) neuro-inflammatory pathway that measured NDEV biomarkers in plasma samples of infliximab-treated patients with BD revealed that the antidepressant response to infliximab, an antagonist of TNF-α, was modulated by changes in NDEV TNFR1 levels and showed an association between lower TNFR1 levels in NDEVs and higher overall cortical thickness [49]. The alterations in NDEV cargo seen after treatment with infliximab support the hypothesis that brain insulin signaling is a pathophysiological mechanism involved in BD [41].
Major depressive disorder
Neuro-inflammation is thought to play a role in the pathogenesis of MDD [50]. In the brain, IL34 is secreted by neurons and astrocytes and affects microglial differentiation; when neurons are damaged, high levels of IL34 are produced, resulting in microglial dysfunction and consequent neuro-inflammation and impaired neurogenesis and synaptic plasticity [50]. Increased IL34/CD81 in NDEVs in peripheral blood has been recommended as a biomarker [50].
Baseline BDNF levels in serum-derived EVs were lower in patients with MDD than in controls, and pro-BDNF levels were higher [51]. Furthermore, levels of both BDNF and pro-BDNF in patient serum and EVs changed in the respective opposite direction after antidepressant treatment. EVs in the CNS may aid in the passage of BDNF across the BBB and be involved in modulating BDNF [51].
Serpin Family F Member 1 (SERPINF1), a secreted glycoprotein with a neuroprotective function, was the top differentially expressed protein identified in an analysis of plasma-derived EV proteins in patients with MDD and HCs [52]. SERPINF1 is a target of miR-186-5p, which is elevated in the blood of patients with MDD and suppresses SERPINF1 in the hippocampus of the CUMS mouse model [52].
Brain-enriched EVs have a membrane surface marker, L1 cell adhesion molecule (L1CAM). The mean concentration of IRS-1 in L1CAM + EVs was higher in patients with MDD than in HCs, indicating IRS-1 enrichment and increased turnover; these processes lead to decreased insulin receptor binding sensitivity and corresponding deficits in insulin signaling transduction, key molecular mechanisms of IR [40].
Vascular endothelial growth factor (VEGF) has been linked to the pathophysiology of stress-related psychiatric disorders, and plasma levels of ADEVs were positively correlated with soluble VEGF121 and soluble VEGFtotal levels in patients with stress-induced exhaustion disorder (SED) [53]. More BBB leakage of ADEVs occurs in patients with SED or MDD than in HCs [53, 54].
Measurement of 14 neuron functional mitochondrial proteins in plasma NDEVs of patients with MDD revealed that levels of 11 proteins involved in different processes, such as energy generation, metabolic regulation, and mitochondrial biogenesis, were significantly lower in patients than in HCs [47].
Metabolite cargo
EV-derived metabolites are particularly likely to represent brain pathology because of the unique features of EVs, such as their ability to cross the BBB. Dysregulation of EV-derived metabolites has been proposed to have a role in the pathophysiology of mental illness, and these metabolites were suggested as potential biomarkers for the diagnosis and evaluation of treatment approaches in psychiatric disorders [55, 56].
A panel of 25 serum EV-derived metabolites with good to excellent performance in differentiating patients with SCZ from HCs has been identified [55]. The differentially identified metabolites were shown to have significant KEGG pathway enrichment in pathways linked to glycero-phospholipid metabolism and the production of phenylalanine, tyrosine, and tryptophan. The results from metabolite gene interaction analysis revealed that four out of 264 SCZ risk genes (identified by genome-wide association studies) and two of the differentially expressed metabolites (l-arginine and taurine) are functionally related: L-arginine is associated with nitric oxide synthase 1 (NOS1), dipeptidase 2 (DPEP2), 5-aminolevulinic acid synthase 1 (ALAS1), and glutamic acid decarboxylase 1 (GAD1), and taurine is associated with GAD1 [55].
A set of 15 serum EV-derived metabolites (chenodeoxycholic acid, lysoPE 18:0, lysoPE 14:0, N-acetylmethionine, 13-oxoODE, glycine, 1-naphthylacetic acid, 2-aminoethanesulfonic acid, D-2-aminobutyric acid, lysoPC 18:0, lysoPC 20:1, biopterin, phosphoric acid, glucosamine, and PAF C-16) was identified as a potential metabolite biomarker of BD that showed good to excellent performance in different sample sets [56]. According to the KEGG database, these differentially expressed metabolites were enriched in galactose metabolism, amino sugar and nucleotide sugar metabolism, and pentose and glucuronate interconversion pathways, indicating that impairments in sugar metabolism may have a role in the onset or progression of BD. The ROC curve for this set of metabolites resulted in an AUC of 0.886 for discriminating between BD and SCZ and of 0.771 for discriminating between BD and MDD (i.e., good to excellent performance for both) [56]. This study once again demonstrated the importance of blood EV metabolites as powerful potential biomarkers for diagnosing brain disorders.
Table 1 lists some potential biomarkers whose role and effects have been investigated in studies on EVs in major psychiatric disorders in humans.
Table 1.
Potential biomarkers identified in studies on human EVs in major psychiatric disorders
| Author/Year | Disease | Sample size | Exosome source | Potential biomarker | Remarks |
|---|---|---|---|---|---|
| Du et al. 2019 [29] | SCZ | 149 cases, 146 controls | Serum | A set of 11 miRNA (miR-206 was one of the top ones that was validated by qRT-PCR) | Blood EV miRNAs may be potentially considered as a diagnostic biomarker for SCZ |
| Khadimallah et al. 2021 [34] | EPP (SCZ) | 138 cases, 134 controls | Plasma | miR-137 and COX6A2 | Changes in miR-137/COX6A2 plasma EV levels could be used as a proxy marker for dysfunction of PVI cortical microcircuit |
| Tsoporis et al. 2022 [33] | SCZ |
11 cases 10 controls |
Plasma | miR-203a-3p, DJ-1 protein | miR-203a-3p and DJ-1 protein could be promising targets for the treatment of oxidative stress-related SCZ |
| Tan et al. 2021 [35] | SCZ |
5 cases, 5 controls |
Plasma | Four differentially expressed EV circRNAs validated by qRT-PCR (has_circ: chr3_196488683_196483770_ − 4913, has_circ: chr5_69175537_69174877_ + 660, has_circ: chr5_143057747_143054439_ + 3308, has_circ: chr6_130956499_130926605_ − 29,894) | miR-34a, miR-34c, and miR-449a, three of the targets for the four identified circRNAs, are thought to play a role in the pathogenesis of SCZ |
| Banigan et al. 2013 [27] | SCZ and BD |
8 SCZ cases, 6 BD cases, 6 controls |
PFC | miR-497 and miR-29c | Levels of miR-497 and miR-29c were significantly higher in SCZ and BD, respectively, than in controls |
| Amoah et al. 2020 [9] | SCZ and BD |
29 SCZ cases, 26 BD cases, 25 controls |
Orbito-frontal cortex | miR-223 | Antipsychotics exert cell-specific regulation over miRNA-233 abundance |
| Choi et al. 2017 [28] | BD |
4 cases, 6 controls |
BA24 | miR-149 | Significantly higher levels of miR-149 in patients; miRNAs in EVs could be possible biomarkers for BD pathogenesis |
| Ceylan et al. 2020 [37] | BD |
69 cases, 41 controls |
Plasma | miR-484, miR-652–3p, miR-142–3p, miR-185–5p | Downregulated miR-484, miR-652–3p, and miR-142–3p; upregulated miR-185–5p |
| Wei et al. 2020 [31] | MDD |
33 cases, 46 controls |
Serum | hsa-miR-139-5p | miR-139-5p is a negative regulator of neural stem cell proliferation and neuronal differentiation |
| Liang et al. 2020 [38] | MDD |
30 cases, 30 controls |
Serum | miR-139-5p | Patients with MDD had higher blood levels of EVs miR-139-5p, which might be a biomarker for MDD |
| Li et al. 2021 [30] | TRD (MDD) |
4 cases, 4 controls |
Plasma | has-miR-335 and has-miR-1292 | These miRNAs are associated with synaptic function and TRD |
| Hung et al. 2021 [39] | MDD |
52 cases, 31 controls |
Serum | let-7e, miR-21-5p, miR-223, miR-145, miR-146a, and miR-155 | EV negative regulatory miRNAs are useful criteria for antidepressant therapy |
| Lee et al. 2020 [46] | SCZ |
60 cases, 60 controls |
Plasma (NDEVs and ADEVs) | ADEV-Aβ42 |
Higher levels of ADEV-Aβ42 in cases than in controls; EV levels of Aβ and tau may be a stronger indicator of intracellular pathology than peripheral levels |
| Goetzl et al. 2021 [48] | FP (SCZ) |
10 cases, 10 controls |
Plasma (NDEVs and ADEVs) |
ATP synthase, MFN2, CYPD, humanin, MOTS-c, myosin VI, SNPH (mitochondrial proteins) |
Lower ATP synthase activity in ADEVs of patients; lower levels of MFN2, CYPD, humanin, and MOTS-c in ADEVs and NDEVs of patients; higher levels of myosin VI in NDEVs than in ADEVs in patients and controls; higher levels of SNPH in NDEVs of patients |
| Ranganathan et al. 2022 [5] | SCZ | 24 cases, 12 controls | Plasma | GFAP, α-II-SPECTRIN | GFAP concentration was significantly higher and α-II-Spectrin concentration was significantly lower in patients than in controls |
| Mansur et al. 2020; 2021 [41, 49] | BD | 55 cases | Plasma (NDEVs) | TNFR/NF-κB | Biomarker potential of NDEVs in psychiatry; brain insulin signaling is a pathophysiological mechanism involved in BD |
| Rhee et al. 2020 [71] | BD and MDD |
42 BD cases, 30 MDD cases, 36 controls |
Serum (bacteria-derived EVs) |
Prevotella 2 and Ruminococcaceae UCG-002 genera (serum microbiome composition) |
Patients with MDD had significantly higher prevalence of the Prevotella 2 and Ruminococcaceae UCG-002 genera than either patients with BD or controls |
| Kuwano et al. 2018 [50] | MDD |
34 cases, 34 controls |
Plasma (NDEVs) | IL34 | Higher IL34/CD81 was recommended as a diagnostic biomarker for MDD |
| Jiang et al. 2021 [52] | MDD |
10 cases, 10 controls |
Plasma | SERPINF1, miR-186-5p | EVs SERPINF1 may be a valid biomarker for MDD progression; miR-186-5p may be a possible therapeutic target |
| Nasca et al. 2021 [40] | MDD |
64 cases, 29 controls |
Serum (brain-enriched EVs) | L1CAM + EVs, IRS-1 | The mean number of L1CAM + EVs and the mean concentration of IRS-1 in L1CAM + EVs were higher in the MDD group than in the controls |
| Gelle et al. 2021 [51] | MDD |
42 cases, 40 controls |
Serum | BDNF, pro-BDNF | EVs may be involved in modulation of BDNF |
| Wallensten et al. 2021 [54] |
SED (MDD) |
31 SED cases, 31 MDD cases, 61 controls |
Plasma (ADEVs & platelet-derived EVs) | GFAP, AQP4, CD154 | Concentrations of EVs co-expressing AQP4 and GFAP and of CD154-positive EVs were significantly higher in patients with MDD than in controls; BBB leakage of ADEVs may be increased in patients with MDD |
| Wang et al. 2021 [64] | MDD |
6 cases, 6 controls |
Plasma | SIG-1R | SIG-1R was significantly enriched in EVs from patients; in depression, EVs may have an antidepressant-like effect, suggesting a potential strategy for MDD treatment |
| Goetzl et al. 2021 [47] | MDD |
20 cases, 10 controls |
Plasma (NDEVs) | 12 neuron functional mitochondrial proteins | 11 proteins were significantly lower and one was higher in NDEVs of patients compared with controls; numerous processes involved in neuronal mitochondria are likely to be altered in MDD |
| Du et al. 2021 [55] | SCZ |
385 cases, 332 controls |
Serum | 25 EV-derived metabolites with good to excellent performance | L-arginine is connected to NOS1, DPEP2, ALAS1, and GAD1; taurine is connected to GAD1 |
| Du et al. 2022 [56] | BD |
32 cases, 40 controls |
Serum | A set of 15 metabolites as the optimal set for differentiating patients with BD from controls | The importance of blood EVs metabolites as a powerful potential biomarker for BD diagnosis |
ADEV astrocyte-derived extracellular vesicles, ATP adenosine triphosphate, BBB blood–brain barrier, BD bipolar disorder, BDNF brain-derived neurotrophic factor, circRNA circular RNA, EPP early psychosis patients, EV extracellular vesicle, FP first-episode psychosis, GFAP glial fibrillary acidic protein, MDD major depressive disorder, miRNA microRNA, NDEV neuron-derived extracellular vesicles, PVI parvalbumin interneuron, qRT-PCR quantitative real-time polymerase chain reaction, SCZ schizophrenia, SED stress-induced exhaustion disorder, TRD treatment-resistant depression
Potential therapeutic applications
As noted above, the use of EVs as a window into the brain is being studied as a potential strategy for the diagnosis and treatment of brain diseases [57]. Furthermore, analyses of extracted brain-derived EVs provide insight into the brain molecular mechanisms underlying the biological basis of neuropsychiatric disorders [50].
In certain conditions that involve specific cellular and molecular processes, such as inflammation, physiological control of these processes with the aim to alleviate their harmful consequences might be achieved through EV-mediated communications [58]. For example, the ability of electroconvulsive therapy (ECT) to cause neurons and glia to release EVs harboring harmful proteins has been hypothesized as one mechanism that explains the success of ECT in improving outcomes in treatment-resistant CNS illness [22].
The intranasal route, which is proving to be a reliable and promising approach for delivering therapeutic compounds to the brain, is an area where EVs can be directly used and where they may play a major role in the targeted delivery of medications [57, 59].
As a new strategy for treating MDD, gene engineering may cause particular ligands to be produced on EVs, allowing for targeted delivery of these EVs [59]. By applying this approach, researchers produced rabies virus glycoprotein (RVG)-circDYM EVs in human embryonic kidney 293 T cells (HEK293T cells), which they injected via the tail vein into a mouse model of chronic unpredictable stress [59]. Various experimental and behavioral analyses revealed that circDYM was transported successfully into the mouse brain via the engineered RVG-EVs and that depression-like behaviors improved in the mice through inhibition of microglial activation and remission of astrocyte dysfunction, BBB leakiness, and peripheral immune cell infiltration [59].
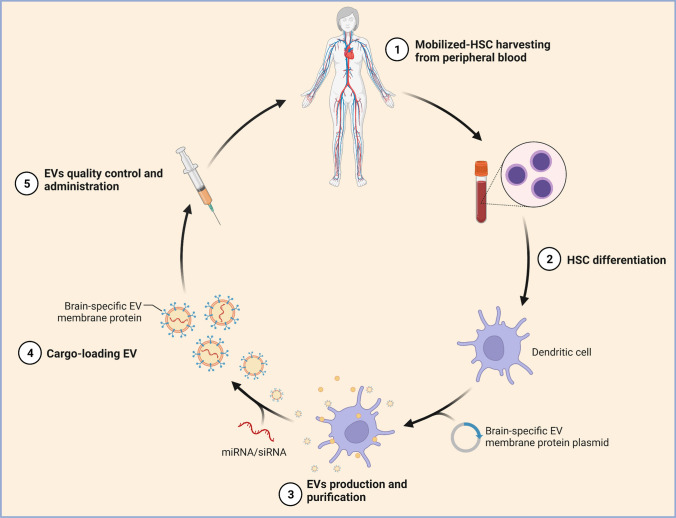
EVs may be an effective system for delivering exogenous genetic material (e.g., miRNA, small interfering RNA) to recipient cells and for supporting gene therapy not only in neuropsychiatric disorders but also in many complex diseases because they are a cell-free, natural mechanism for transporting RNA between cells, they protect the RNA/gene of interest from digestion, and they are quickly taken up by the target cells [60, 61] (Fig. 4).
Fig. 4.
Self-derived extracellular vesicles (EVs) as a novel gene therapy. The figure shows the main steps for obtaining self-derived EVs for use as a novel gene therapy approach. EVs are capable of genetic exchange between cells and can be derived from a patient's differentiated hematopoietic stem cells and used for brain-targeted cargo delivery through expression of brain-specific peptides. By loading microRNA or small interfering RNA of the targeted gene, EVs can selectively regulate gene expression. HSC, hematopoietic stem cells; miRNA, microRNA; siRNA, small interfering RNA. (Reprinted from “Self-Derived Exosomes as a Novel Gene Therapy”, by BioRender.com (2022). Retrieved from https://app.biorender.com/biorender-templates)
Several experimental studies performed in the last decade have provided evidence that mesenchymal stem cell-derived EVs (MSC-EVs) can help with the treatment of neurocognitive disorders [62]. In a study on phencyclidine (PCP)-treated mice (SCZ mouse model), intranasally injected MSC-EVs improved cognitive performance and social interaction and attenuated SCZ-like behavior by increasing the survival of gamma aminobutyric acid-producing neurons and regulating neurotransmitter activity in the CNS [63].
In patients with MDD, EVs were significantly enriched in sigma-1 receptor (SIG-1R), a promising antidepressant target that is a ligand-operated receptor chaperone. When such EVs were injected into lipopolysaccharide (LPS)-challenged mice (a depression-like model), they had beneficial effects on the increased immobility time, anhedonia-like behavior, decreased BDNF expression, and microglia activation [64]. In addition, EVs prevented LPS-induced inflammatory responses of microglial BV2 cells, and their antidepressant-like effect was inhibited when EV SIG-1R was knocked out [64]. These results support a role of EV SIG-1R in the anti-inflammatory effect of EVs in depression, suggesting a potential strategy for MDD treatment [64].
Overall, EVs hold a lot of promise for assisting the diagnosis and treatment of complex brain diseases. Advanced EV-based combinations are gaining traction in research, and more progress in solving the biological and technical difficulties in this field could enable the development of personalized EV-based treatment strategies in psychiatric disorders.
Limitations and future perspective
EVs have emerged as a new theranostic strategy in various domains of molecular medicine because of their low immunogenicity and toxicity, biodegradability, and ability to both cross the BBB and protect their internal active components [59]. To date, EV-based medicines have not been approved by the US Food and Drug Administration in any area of medicine [65]. The successful use of EVs requires the availability of low-cost, large-scale production and high-sensitivity isolation and characterization techniques to evaluate batch-to-batch changes, as well as broadly applicable techniques for drug loading [65].
In psychiatric disorders, EVs may transform biological signals into neuroplastic modifications, leading to changes in specific brain regions and in cognitive and emotional phenotypes, and EVs may represent the missing link between biology and clinical outcome. For instance, as a non-pharmacological intervention in depressive disorders, systemic adaptation to physical exercise may cause a rapid release of EVs into the circulation, which may reduce systemic inflammation and have antidepressant effects [66, 67]. However, the isolation and characterization of EVs remains difficult because of the presence of contaminants with similar properties [68]. No well-established, universally accepted method exists for enriching solutions with total EVs or cell-specific forms of EVs from bodily fluids [69], and the separation of different EV subtypes and co-isolated protein aggregates and lipoproteins will require improvements in isolation techniques [8]. The limited quantities of CNS-derived EVs secreted by disease-relevant cells and transferred into the periphery represents another obstacle in this field [68]. Although many studies have examined EVs in various fields, especially in recent years, research is still in its early stages and is limited by both biological and technological considerations; consequently, new advancements are required [70]. In addition, because EV formation and secretion mechanisms are not completely understood, the related findings should be interpreted with caution [69]. EVs can change as diseases progress, so longitudinal studies may reveal components that better reflect alterations in brain states [69].
The use of new, creative approaches, e.g., analyzing bacteria-derived EVs from the serum microbiome in individuals with psychiatric disorders [71], or the development of a highly specific and sensitive ultra-performance liquid chromatography–tandem mass spectrometry method to detect activity of the enzyme catechol-O-methyltransferase (COMT) in EVs [72] might help to identify differentiating biomarkers. COMT participates in the metabolism of different chemical compounds, e.g., catechol drugs and catecholamine neurotransmitters, and plays a role in psychiatric disorders, and knowledge about COMT activity could be valuable for clinicians and pharmaceutical companies wanting to follow a more simplified personalized medicine approach [72].
Engineered EVs and EV-mediated drug delivery systems for targeted treatments in specific brain cells could be created by genetically or chemically changing EV membrane molecules and cargo content [73]. In EV-based gene therapy, delivery systems with EVs must become more efficient, tissue specific, and non-immunogenic [61]. Integration of EV data from different –omics outputs with other findings from neuroimaging and neuropsychological tests, as well as with genetic variations and other compounds in body fluids, has the potential to increase the precision of biomarker discovery, and the era of EV-based brain "liquid biopsy" could be approaching [5, 69].
While advances in nanotechnology and genetic engineering will aid in the delivery of desired signals to neurons and glia in the brain, EV-based methods and personalized medicine based on biological signatures in EVs have the potential to provide deep understanding of the molecular biology and physiology of behavioral alterations and treatment of brain disorders and support diagnostic, prognostic, and therapeutic approaches in psychiatry [66].
The ability of EVs to move between the CNS and peripheral circulation was recently discovered as a critical property of EVs. Numerous studies cited in our review examined peripheral EVs without any enrichment of brain cell-type specific EVs. Even though peripheral EVs may originate from organs other than the brain, examining them is still very beneficial if they act as or carry diagnostic biomarkers; however, compared with non-enriched total plasma- or serum-derived EVs, EVs enriched for neuronal origin constitute a more sensitive and reliable base for discovering biomarkers for brain disorders [68, 74]. CNS- or neuron-derived EVs in the blood have shown significant promise as "windows into the brain” that will allow alterations in brain biochemistry and intercellular communication linked to CNS diseases to be detected by peripheral blood analysis [68]. More research is needed to analyze individual, brain-derived EVs and their cargos and to create and enhance the processes that allow for the high-yield capture of these specific and high-potential EVs; such EVs may also provide information on specific molecular pathways that are directly connected to the pathophysiology of neurological disorders [68].
Unfortunately, the existing data have not been convincingly replicated, and some challenges remain in the field of EVs, as described above. Nevertheless, future advances may shed light on the precise function of EVs in the physiology and pathology of various brain disorders, such as mental illness. By improving the specific isolation and characterization of brain cell-derived EVs—such as NDEVs, ADEVs, and oligodendrocyte-derived EVs—that more directly reflect brain conditions and by applying more advanced cargo analysis technologies, research will hopefully provide relevant evidence that could be useful in defining practical EV-based diagnostic and treatment strategies for brain disorders.
Acknowledgements
The authors thank Jacquie Klesing, Board-certified Editor in the Life Sciences (ELS), for editing assistance with the manuscript.
Author contributions
MOK designed the review, performed the literature search and prepared the figures. ID and MOK designed the table. TGS supervised and funded the work. All authors contributed to writing and revising the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. Thomas G. Schulze is supported by German Research Foundation (Deutsche Forschungsgemeinschaft; DFG) within the framework of the projects www.kfo241.de and www.PsyCourse.de (SCHU 1603/4-1, 5-1, 7-1; FA241/16-1). He is further supported by the Dr. Lisa Oehler Foundation (Kassel, Germany).
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the international society for extracellular vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doyle LM, Wang MZ (2019) Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells 8(7):727 [DOI] [PMC free article] [PubMed]
- 3.Saeedi S, Israel S, Nagy C, Turecki G. The emerging role of exosomes in mental disorders. Transl Psychiatry. 2019;9:122. doi: 10.1038/s41398-019-0459-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsilioni I, Panagiotidou S, Theoharides TC. Exosomes in neurologic and psychiatric disorders. Clin Ther. 2014;36:882–888. doi: 10.1016/j.clinthera.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Ranganathan M, Rahman M, Ganesh S, D’Souza DC, Skosnik PD, Radhakrishnan R, et al. Analysis of circulating exosomes reveals a peripheral signature of astrocytic pathology in schizophrenia. World J Biol Psychiatry Off J World Fed Soc Biol Psychiatry. 2022;23:33–45. doi: 10.1080/15622975.2021.1907720. [DOI] [PubMed] [Google Scholar]
- 6.Shao H, Im H, Castro CM, Breakefield X, Weissleder R, Lee H. New technologies for analysis of extracellular vesicles. Chem Rev. 2018;118:1917–1950. doi: 10.1021/acs.chemrev.7b00534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanninen KM, Bister N, Koistinaho J, Malm T. Exosomes as new diagnostic tools in CNS diseases. Biochim Biophys Acta. 2016;1862:403–410. doi: 10.1016/j.bbadis.2015.09.020. [DOI] [PubMed] [Google Scholar]
- 8.Mathew B, Mansuri MS, Williams KR, Nairn AC (2021) Exosomes as emerging biomarker tools in neurodegenerative and neuropsychiatric disorders-a proteomics perspective. Brain Sci 11(2):258 [DOI] [PMC free article] [PubMed]
- 9.Amoah SK, Rodriguez BA, Logothetis CN, Chander P, Sellgren CM, Weick JP, et al. Exosomal secretion of a psychosis-altered miRNA that regulates glutamate receptor expression is affected by antipsychotics. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2020;45:656–665. doi: 10.1038/s41386-019-0579-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du Y, Tan W-L, Chen L, Yang Z-M, Li X-S, Xue X, et al. Exosome transplantation from patients with schizophrenia causes schizophrenia-relevant behaviors in mice: an integrative multi-omics data analysis. Schizophr Bull. 2021;47:1288–1299. doi: 10.1093/schbul/sbab039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.GBD 2019 Mental Disorders Collaborators (2022) Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry 9:137–150 [DOI] [PMC free article] [PubMed]
- 12.Zhang Z, Chen G. A logical relationship for schizophrenia, bipolar, and major depressive disorder. Part 1: evidence from chromosome 1 high density association screen. J Comp Neurol. 2020;528:2620–2635. doi: 10.1002/cne.24921. [DOI] [PubMed] [Google Scholar]
- 13.Delalle I. MicroRNAs as candidates for bipolar disorder biomarkers. Psychiatr Danub. 2021;33:451–455. [PubMed] [Google Scholar]
- 14.Correll CU, Howes OD. Treatment-resistant schizophrenia: definition, predictors, and therapy options. J Clin Psychiatry. 2021;82:MY20096AH1C. doi: 10.4088/JCP.MY20096AH1C. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Li S, Li L, Li M, Guo C, Yao J, et al. Exosome and exosomal microRNA: trafficking, sorting, and function. Genom Proteom Bioinform. 2015;13:17–24. doi: 10.1016/j.gpb.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Homorogan C, Enatescu VR, Nitusca D, Marcu A, Seclaman E, Marian C. Distribution of microRNAs associated with major depressive disorder among blood compartments. J Int Med Res. 2021;49:3000605211006633. doi: 10.1177/03000605211006633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwarz E, Bahn S. Biomarker discovery in psychiatric disorders. Electrophoresis. 2008;29:2884–2890. doi: 10.1002/elps.200700710. [DOI] [PubMed] [Google Scholar]
- 18.Schwarz E, Bahn S. The utility of biomarker discovery approaches for the detection of disease mechanisms in psychiatric disorders. Br J Pharmacol. 2008;153(Suppl 1):S133–136. doi: 10.1038/sj.bjp.0707658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fries GR, Lima CNC, Valvassori SS, Zunta-Soares G, Soares JC, Quevedo J. Preliminary investigation of peripheral extracellular vesicles’ microRNAs in bipolar disorder. J Affect Disord. 2019;255:10–14. doi: 10.1016/j.jad.2019.05.020. [DOI] [PubMed] [Google Scholar]
- 20.Zhu C-Y, Shen Y, Xu Q. Propagation of dysbindin-1B aggregates: exosome-mediated transmission of neurotoxic deposits. Neuroscience. 2015;291:301–316. doi: 10.1016/j.neuroscience.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 21.Ghidoni R, Benussi L, Binetti G. Exosomes: the Trojan horses of neurodegeneration. Med Hypotheses. 2008;70:1226–1227. doi: 10.1016/j.mehy.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Attili D, Schill DJ, DeLong CJ, Lim KC, Jiang G, Campbell KF, et al. Astrocyte-derived exosomes in an iPSC model of bipolar disorder. Adv Neurobiol. 2020;25:219–235. doi: 10.1007/978-3-030-45493-7_8. [DOI] [PubMed] [Google Scholar]
- 23.Luarte A, Cisternas P, Caviedes A, Batiz LF, Lafourcade C, Wyneken U, et al. Astrocytes at the hub of the stress response: potential modulation of neurogenesis by miRNAs in astrocyte-derived exosomes. Stem Cells Int. 2017;2017:1719050. doi: 10.1155/2017/1719050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raghavan V, Bhomia M, Torres I, Jain S, Wang KK. Hypothesis: Exosomal microRNAs as potential biomarkers for schizophrenia. Med Hypotheses. 2017;103:21–25. doi: 10.1016/j.mehy.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Fabbri M, Paone A, Calore F, Galli R, Gaudio E, Santhanam R, et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci U S A. 2012;109:E2110–2116. doi: 10.1073/pnas.1209414109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kouter K, Paska AV. Biomarkers for suicidal behavior: miRNAs and their potential for diagnostics through liquid biopsy: a systematic review. Epigenomics. 2020;12:2219–2235. doi: 10.2217/epi-2020-0196. [DOI] [PubMed] [Google Scholar]
- 27.Banigan MG, Kao PF, Kozubek JA, Winslow AR, Medina J, Costa J, et al. Differential expression of exosomal microRNAs in prefrontal cortices of schizophrenia and bipolar disorder patients. PLoS ONE. 2013;8:e48814. doi: 10.1371/journal.pone.0048814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi JL, Kao PF, Itriago E, Zhan Y, Kozubek JA, Hoss AG, et al. miR-149 and miR-29c as candidates for bipolar disorder biomarkers. Am J Med Genet Part B Neuropsychiatr Genet Off Publ Int Soc Psychiatr Genet. 2017;174:315–323. doi: 10.1002/ajmg.b.32518. [DOI] [PubMed] [Google Scholar]
- 29.Du Y, Yu Y, Hu Y, Li X-W, Wei Z-X, Pan R-Y, et al. Genome-wide, integrative analysis implicates exosome-derived MicroRNA dysregulation in schizophrenia. Schizophr Bull. 2019;45:1257–1266. doi: 10.1093/schbul/sby191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li L-D, Naveed M, Du Z-W, Ding H, Gu K, Wei L-L, et al. Abnormal expression profile of plasma-derived exosomal microRNAs in patients with treatment-resistant depression. Hum Genomics. 2021;15:55. doi: 10.1186/s40246-021-00354-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei Z-X, Xie G-J, Mao X, Zou X-P, Liao Y-J, Liu Q-S, et al. Exosomes from patients with major depression cause depressive-like behaviors in mice with involvement of miR-139-5p-regulated neurogenesis. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2020;45:1050–1058. doi: 10.1038/s41386-020-0622-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fang K, Xu J-X, Chen X-X, Gao X-R, Huang L-L, Du A-Q, et al. Differential serum exosome microRNA profile in a stress-induced depression rat model. J Affect Disord. 2020;274:144–158. doi: 10.1016/j.jad.2020.05.017. [DOI] [PubMed] [Google Scholar]
- 33.Tsoporis JN, Ektesabi AM, Gupta S, Izhar S, Salpeas V, Rizos IK, et al. A longitudinal study of alterations of circulating DJ-1 and miR203a-3p in association to olanzapine medication in a sample of first episode patients with schizophrenia. J Psychiatr Res. 2022;146:109–117. doi: 10.1016/j.jpsychires.2021.12.049. [DOI] [PubMed] [Google Scholar]
- 34.Khadimallah I, Jenni R, Cabungcal J-H, Cleusix M, Fournier M, Beard E, et al. Mitochondrial, exosomal miR137-COX6A2 and gamma synchrony as biomarkers of parvalbumin interneurons, psychopathology, and neurocognition in schizophrenia. Mol Psychiatry. 2021 doi: 10.1038/s41380-021-01313-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tan G, Wang L, Liu Y, Zhang H, Feng W, Liu Z. The alterations of circular RNA expression in plasma exosomes from patients with schizophrenia. J Cell Physiol. 2021;236:458–467. doi: 10.1002/jcp.29873. [DOI] [PubMed] [Google Scholar]
- 36.Zhuo C-J, Hou W-H, Jiang D-G, Tian H-J, Wang L-N, Jia F, et al. Circular RNAs in early brain development and their influence and clinical significance in neuropsychiatric disorders. Neural Regen Res. 2020;15:817–823. doi: 10.4103/1673-5374.268969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ceylan D, Tufekci KU, Keskinoglu P, Genc S, Özerdem A. Circulating exosomal microRNAs in bipolar disorder. J Affect Disord. 2020;262:99–107. doi: 10.1016/j.jad.2019.10.038. [DOI] [PubMed] [Google Scholar]
- 38.Liang J-Q, Liao H-R, Xu C-X, Li X-L, Wei Z-X, Xie G-J, et al. Serum exosome-derived miR-139-5p as a potential biomarker for major depressive disorder. Neuropsychiatr Dis Treat. 2020;16:2689–2693. doi: 10.2147/NDT.S277392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hung Y-Y, Chou C-K, Yang Y-C, Fu H-C, Loh E-W, Kang H-Y (2021) Exosomal let-7e, miR-21–5p, miR-145, miR-146a and miR-155 in predicting antidepressants response in patients with major depressive disorder. Biomedicines 9(10):1428 [DOI] [PMC free article] [PubMed]
- 40.Nasca C, Dobbin J, Bigio B, Watson K, de Angelis P, Kautz M, et al. Insulin receptor substrate in brain-enriched exosomes in subjects with major depression: on the path of creation of biosignatures of central insulin resistance. Mol Psychiatry. 2021;26:5140–5149. doi: 10.1038/s41380-020-0804-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mansur RB, Delgado-Peraza F, Subramaniapillai M, Lee Y, Iacobucci M, Nasri F, et al. Exploring brain insulin resistance in adults with bipolar depression using extracellular vesicles of neuronal origin. J Psychiatr Res. 2021;133:82–92. doi: 10.1016/j.jpsychires.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wijtenburg SA, Kapogiannis D, Korenic SA, Mullins RJ, Tran J, Gaston FE, et al. Brain insulin resistance and altered brain glucose are related to memory impairments in schizophrenia. Schizophr Res. 2019;208:324–330. doi: 10.1016/j.schres.2019.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kapogiannis D, Dobrowolny H, Tran J, Mustapic M, Frodl T, Meyer-Lotz G, et al. Insulin-signaling abnormalities in drug-naïve first-episode schizophrenia: transduction protein analyses in extracellular vesicles of putative neuronal origin. Eur Psychiatry J Assoc Eur Psychiatr. 2019;62:124–129. doi: 10.1016/j.eurpsy.2019.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Borroto-Escuela DO, Tarakanov AO, Bechter K, Fuxe K. IL1R2, CCR2, and CXCR4 may form heteroreceptor complexes with NMDAR and D2R: relevance for schizophrenia. Front Psychiatry. 2017;8:24. doi: 10.3389/fpsyt.2017.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fuxe K, Dahlström A, Höistad M, Marcellino D, Jansson A, Rivera A, et al. From the Golgi-Cajal mapping to the transmitter-based characterization of the neuronal networks leading to two modes of brain communication: wiring and volume transmission. Brain Res Rev. 2007;55:17–54. doi: 10.1016/j.brainresrev.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 46.Lee EE, Winston-Gray C, Barlow JW, Rissman RA, Jeste DV. Plasma levels of neuron- and astrocyte-derived exosomal amyloid beta1-42, amyloid beta1-40, and phosphorylated tau levels in schizophrenia patients and non-psychiatric comparison subjects: relationships with cognitive functioning and psychopathology. Front Psychiatry. 2020;11:532624. doi: 10.3389/fpsyt.2020.532624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goetzl EJ, Wolkowitz OM, Srihari VH, Reus VI, Goetzl L, Kapogiannis D, et al. Abnormal levels of mitochondrial proteins in plasma neuronal extracellular vesicles in major depressive disorder. Mol Psychiatry. 2021;26:7355–7362. doi: 10.1038/s41380-021-01268-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goetzl EJ, Srihari VH, Guloksuz S, Ferrara M, Tek C, Heninger GR. Neural cell-derived plasma exosome protein abnormalities implicate mitochondrial impairment in first episodes of psychosis. FASEB J Off Publ Fed Am Soc Exp Biol. 2021;35:e21339. doi: 10.1096/fj.202002519R. [DOI] [PubMed] [Google Scholar]
- 49.Mansur RB, Delgado-Peraza F, Subramaniapillai M, Lee Y, Iacobucci M, Rodrigues N et al (2020) Extracellular vesicle biomarkers reveal inhibition of neuroinflammation by infliximab in association with antidepressant response in adults with bipolar depression. Cells 9(4):895 [DOI] [PMC free article] [PubMed]
- 50.Kuwano N, Kato TA, Mitsuhashi M, Sato-Kasai M, Shimokawa N, Hayakawa K, et al. Neuron-related blood inflammatory markers as an objective evaluation tool for major depressive disorder: an exploratory pilot case-control study. J Affect Disord. 2018;240:88–98. doi: 10.1016/j.jad.2018.07.040. [DOI] [PubMed] [Google Scholar]
- 51.Gelle T, Samey RA, Plansont B, Bessette B, Jauberteau-Marchan M-O, Lalloué F, et al. BDNF and pro-BDNF in serum and exosomes in major depression: evolution after antidepressant treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2021;109:110229. doi: 10.1016/j.pnpbp.2020.110229. [DOI] [PubMed] [Google Scholar]
- 52.Jiang M, Gu Y-F, Cai J-F, Wang A, He Y, Feng Y-L. MiR-186-5p dysregulation leads to depression-like behavior by de-repressing SERPINF1 in hippocampus. Neuroscience. 2021;479:48–59. doi: 10.1016/j.neuroscience.2021.10.005. [DOI] [PubMed] [Google Scholar]
- 53.Wallensten J, Mobarrez F, Åsberg M, Borg K, Beser A, Wilczek A, et al. Isoforms of soluble vascular endothelial growth factor in stress-related mental disorders: a cross-sectional study. Sci Rep. 2021;11:16693. doi: 10.1038/s41598-021-96313-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wallensten J, Nager A, Åsberg M, Borg K, Beser A, Wilczek A, et al. Leakage of astrocyte-derived extracellular vesicles in stress-induced exhaustion disorder: a cross-sectional study. Sci Rep. 2021;11:2009. doi: 10.1038/s41598-021-81453-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Du Y, Chen L, Li X-S, Li X-L, Xu X-D, Tai S-B, et al. Metabolomic identification of exosome-derived biomarkers for schizophrenia: a large multicenter study. Schizophr Bull. 2021;47:615–623. doi: 10.1093/schbul/sbaa166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Du Y, Dong J-H, Chen L, Liu H, Zheng G-E, Chen G-Y, et al. Metabolomic identification of serum exosome-derived biomarkers for bipolar disorder. Oxid Med Cell Longev. 2022;2022:5717445. doi: 10.1155/2022/5717445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fan Y, Chen M, Zhang J, Maincent P, Xia X, Wu W. Updated progress of nanocarrier-based intranasal drug delivery systems for treatment of brain diseases. Crit Rev Ther Drug Carrier Syst. 2018;35:433–467. doi: 10.1615/CritRevTherDrugCarrierSyst.2018024697. [DOI] [PubMed] [Google Scholar]
- 58.Ridder K, Keller S, Dams M, Rupp A-K, Schlaudraff J, Del Turco D, et al. Extracellular vesicle-mediated transfer of genetic information between the hematopoietic system and the brain in response to inflammation. PLoS Biol. 2014;12:e1001874. doi: 10.1371/journal.pbio.1001874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu X, Bai Y, Han B, Ju M, Tang T, Shen L, et al. Extracellular vesicle-mediated delivery of circDYM alleviates CUS-induced depressive-like behaviours. J Extracell Vesicles. 2022;11:e12185. doi: 10.1002/jev2.12185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mathiyalagan P, Sahoo S. Exosomes-based gene therapy for MicroRNA delivery. Methods Mol Biol Clifton NJ. 2017;1521:139–152. doi: 10.1007/978-1-4939-6588-5_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Narahari A, Hussain M, Sreeram V. MicroRNAs as biomarkers for psychiatric conditions: a review of current research. Innov Clin Neurosci. 2017;14:53–55. [PMC free article] [PubMed] [Google Scholar]
- 62.Harrell CR, Volarevic A, Djonov V, Volarevic V (2021) Mesenchymal stem cell-derived exosomes as new remedy for the treatment of neurocognitive disorders. Int J Mol Sci 22(3):1433 [DOI] [PMC free article] [PubMed]
- 63.Tsivion-Visbord H, Perets N, Sofer T, Bikovski L, Goldshmit Y, Ruban A, et al. Mesenchymal stem cells derived extracellular vesicles improve behavioral and biochemical deficits in a phencyclidine model of schizophrenia. Transl Psychiatry. 2020;10:305. doi: 10.1038/s41398-020-00988-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang Y, Gao C, Gao T, Zhao L, Zhu S, Guo L. Plasma exosomes from depression ameliorate inflammation-induced depressive-like behaviors via sigma-1 receptor delivery. Brain Behav Immun. 2021;94:225–234. doi: 10.1016/j.bbi.2021.02.004. [DOI] [PubMed] [Google Scholar]
- 65.Herrmann IK, Wood MJA, Fuhrmann G. Extracellular vesicles as a next-generation drug delivery platform. Nat Nanotechnol. 2021;16:748–759. doi: 10.1038/s41565-021-00931-2. [DOI] [PubMed] [Google Scholar]
- 66.Gruzdev SK, Yakovlev AA, Druzhkova TA, Guekht AB, Gulyaeva NV. The missing link: how exosomes and mirnas can help in bridging psychiatry and molecular biology in the context of depression, bipolar disorder and schizophrenia. Cell Mol Neurobiol. 2019;39:729–750. doi: 10.1007/s10571-019-00684-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Soares E, Reis J, Rodrigues M, Ribeiro CF, Pereira FC (2021) Circulating extracellular vesicles: the missing link between physical exercise and depression management? Int J Mol Sci 22(2):542 [DOI] [PMC free article] [PubMed]
- 68.Shi M, Sheng L, Stewart T, Zabetian CP, Zhang J. New windows into the brain: central nervous system-derived extracellular vesicles in blood. Prog Neurobiol. 2019;175:96–106. doi: 10.1016/j.pneurobio.2019.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kano S-I, Yang K, Sawa A. Making sense of extracellular vesicles in body fluids: promise and challenge. Schizophr Bull. 2021;47:586–587. doi: 10.1093/schbul/sbab009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kano S-I, Dohi E, Rose IVL. Extracellular vesicles for research on psychiatric disorders. Schizophr Bull. 2019;45:7–16. doi: 10.1093/schbul/sby127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rhee SJ, Kim H, Lee Y, Lee HJ, Park CHK, Yang J, et al. Comparison of serum microbiome composition in bipolar and major depressive disorders. J Psychiatr Res. 2020;123:31–38. doi: 10.1016/j.jpsychires.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 72.Casal E, Palomo L, Cabrera D, Falcon-Perez JM. A novel sensitive method to measure Catechol-O-Methyltransferase activity unravels the presence of this activity in extracellular vesicles released by rat hepatocytes. Front Pharmacol. 2016;7:501. doi: 10.3389/fphar.2016.00501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liang Y, Duan L, Lu J, Xia J. Engineering exosomes for targeted drug delivery. Theranostics. 2021;11:3183–3195. doi: 10.7150/thno.52570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mustapic M, Eitan E, Werner JKJ, Berkowitz ST, Lazaropoulos MP, Tran J, et al. Plasma extracellular vesicles enriched for neuronal origin: a potential window into brain pathologic processes. Front Neurosci. 2017;11:278. doi: 10.3389/fnins.2017.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]