Abstract
Background
Medication adherence improves morbidity and mortality-related outcomes in heart failure, and knowledge of patterns of medication adherence supports patient and clinician decision-making. Routinely collected national data facilitate the exploration of medication adherence and associated factors in older adults with heart failure, including the association between ethnicity and adherence. There are known inequities in access to medicines between Māori (Indigenous People of Aotearoa New Zealand) and non-Māori, yet ethnic variation in medicines adherence in community-dwelling older adults with heart failure has not been explored.
Objective
Here we identify medication adherence rates for community-dwelling older adults diagnosed with heart failure and differences in adherence rates between Māori and non-Māori.
Methods
Cross-sectional analysis of interRAI (comprehensive standardised assessment) data in a continuously recruited national cohort from 2012 to 2019.
Results
Overall, 13,743 assessments (Māori N = 1526) for older community-dwelling adults with heart failure diagnoses were included. The mean age of participants was 74.5 years [standard deviation (SD) 9.1 years] for Māori and 82.3 years (SD 7.8 years) non-Māori. In the Māori cohort, 21.8% did not adhere fully to their medication regimen, whereas in the non-Māori cohort, this figure was 12.8%. After adjusting for confounders, the Māori cohort were more likely to be medication non-adherent than non-Māori [prevalence ratio 1.53, 95% confidence interval (CI) 1.36–1.73].
Conclusions
There was a significant disparity between Māori and non-Māori concerning medication adherence. Given the international use of the interRAI-HC assessment tool, these results have significant transferability to other countries and allow the identification of underserved ethnic groups for which culturally appropriate interventions can be targeted.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40266-023-01044-2.
Key Points
| There is ethnic variation in medication adherence rates for older adults with heart failure in Aotearoa New Zealand. |
| interRAI assessments can be used to assess medication adherence rates at a population level and can be better utilised to target interventions to improve adherence. |
Introduction
As our population ages, it is doing so with increasing levels of often complex comorbidities and competing health needs [1]. This inevitably leads to prescribing a greater number of medications as clinicians endeavour to meet the challenges of supporting clients through quality medication use [2, 3]. Although careful and considered prescribing and dispensing is key, so is medication adherence by the individual concerned so that they receive the benefit of the prescribed medications and their specific health condition or conditions can be managed optimally. Medication adherence can broadly be defined as ‘the process by which patients take their medications as prescribed’ [4]. Medication adherence is the responsibility of both the individual and health practitioners, facilitated by medication optimisation and shared decision-making.
Heart failure (HF) can significantly impact physical function and quality of life even in its early stages [5] and is a leading cause of hospital admissions in older adults [6, 7]. Medication adherence is particularly important in the case of HF, where medicines are a mainstay of therapy and associated with reduced healthcare utilisation and improved quality of life [8]. The clinical management and support of patients with HF in primary care can be complex, with patients often prescribed multiple medications that may need adjusting, titrating and close monitoring. Medication adherence is key to optimising outcomes [6]. Fortunately, there are interventions that have been shown to improve medication adherence and clinical outcomes in those with HF [9, 10], with some data suggesting those with the highest non-adherence benefit to the greatest extent from intervention [11].
Despite advances in clinical management and pathways when treating and diagnosing HF, this condition remains a clinical challenge in Aotearoa New Zealand. Māori have a significantly lower life expectancy than non-Māori [12] and are more likely to experience diseases such as HF at a younger age with worse health outcomes [13, 14]. In addition, significant inequities remain between Māori and non-Māori in terms of both treatment and medicine access concerning the management of HF [15]. Current New Zealand’s Ministry of Health (MoH) data highlight that the mortality rate among Māori with heart failure is more than twice that of non-Māori [relative risk (RR) 2.36, CI 1.76–3.17] [16].
At a population level, Māori medication adherence in Aotearoa New Zealand is lower than that of non-Māori [3]. However, current services designed to support medication adherence, such as medication reviews, are less likely to achieve equitable outcomes for Māori than non-Māori [17]. The significance of this ethnic disparity, specifically to medication adherence in those community-dwelling older adults diagnosed with HF, has not previously been examined at a national level in Aotearoa New Zealand.
Previous research in Aotearoa New Zealand has utilised the international Residential Assessment Instrument (interRAI) as a unique source of big data [18, 19]. interRAI is a suite of standardised assessment tools now used internationally in many clinical settings. They contain questions on demographics, physical function, comorbidities, including heart failure diagnosis, and living conditions. They also include a specific series of questions on medication adherence by the client being assessed [20]. One of the suite of tools, the interRAI Home Care (interRAI-HC) tool, is mandated nationally throughout Aotearoa New Zealand, for those older adults requiring assessment for government-funded home care supports or for assessment for entry into aged residential care. Although the primary purpose of any interRAI assessment is to provide a standardised assessment to create individualised care plans, they also provide a comprehensive dataset at a population level which can be valuable to aid research aimed at improving outcomes for our most vulnerable older population [20, 21].
This research aims to utilise the Aotearoa New Zealand national interRAI-HC database to compare medication adherence between Māori and non-Māori for those older adults with a diagnosis of HF. The same database also allows for considering a suite of confounding factors.
Understanding medication adherence rates and identifying differences between Māori and non-Māori patients may give clinicians valuable insight into the extent of non-adherence in older adults living in the community with a diagnosis of HF and identify groups with the most potential to benefit from practices which improve adherence. Given the role of medication in HF treatment, targeted adherence support can improve quality of life and reduce avoidable hospital presentations in a way that promotes equitable outcomes.
Methods
Study Design
Cross-sectional analysis of a continuously recruited national cohort.
Participants
Adults who had an interRAI-HC between 1 July 2012 and 31 December 2019. Individuals aged 65 years and older at the time of assessment were included. In addition, we included Māori aged 50 years and over to account for the earlier onset of ageing or chronic comorbidity. Repeat interRAI assessments for individuals were excluded. Only those with a diagnosis of HF were included. A HF diagnosis is recorded in the interRAI assessment by the trained assessor if there is a HF diagnosis in the clinical record (e.g. hospital discharge summaries, primary care physician/practitioner notes). The severity or classification of HF is not recorded in interRAI.
Primary Outcome
Medication adherence was the primary outcome of interest. Medication adherence was determined from the question in interRAI-HC assessments, which asks patients whether in the last 3 days they were “adherent with medications prescribed by physicians”. Response options were “always adherent”, “adherent 80% of the time or more”, “adherent less than 80% of time, including failure to purchase prescribed medications” and “no medications prescribed”. Responses were compared with available medication and known medication orders by interRAI assessors trained to undertake medicines reconciliation for interRAI assessments. Did the supply remaining seem accurate, considering when the prescription was filled? Did the person and caregiver give accurate information about medication administration? Medication adherence was assessed as a binary outcome with “always adherent” responders deemed adherent, and all other groups as non-adherent as we deemed any level of non-adherence in the last 3 days as significant in the context of HF, with the potential to identify and intervene with those who are non-adherent. People who had no medications prescribed were excluded.
Sociodemographic and Confounding Measures
The primary exposure of interest was ethnicity, which was identified from self-reported ethnicity interRAI-HC assessments. Priority coding was used to categorise ethnicity (Māori, Pacific, Asian and other-including European), whereby patients are counted in one ethnicity only [22]. Multiple sociodemographic and confounding variables were included in the analysis. Sociodemographic variables were age, sex, marital status and lives alone. Confounding variables included: medical diagnoses [coronary heart disease, chronic obstructive pulmonary disease, dementia (Alzheimer’s disease or dementia other than Alzheimer’s disease), stroke, Parkinson’s disease, depression, bipolar disorder, anxiety], self-reported health, physical and lifestyle factors (alcohol intake, dizziness, fatigue, vision, hearing, greatest distance walked, participation in social activities walked in last 3 days, history of falls) and supports (visit by a nurse, enrolment in a palliative care programme, lives with a helper and carer stress). InterRAI HC 9.1 has three questions pertaining to carer stress (that the informal helper was unable to continue caring, the informal helper expressed feelings of distress, anger or depression and family or close friends report being overwhelmed by the person’s illness). As per our previous publications, if any of these were reported the person was reported to have carer stress [19].
All sociodemographic and confounding variables were derived from the interRAI assessments, and the complete list of variables that were adjusted for is presented in Table 1.
Table 1.
Baseline sociodemographic characteristics and prevalence of confounding variables
| Factor | Measure | Māori | Non-Māori | Fisher’s exact test (p-value) | ||
|---|---|---|---|---|---|---|
| Sex | 1. Female | 898 | 58.8% | 6418 | 52.5% | < 0.001 |
| 2. Male | 628 | 41.2% | 5799 | 47.5% | ||
| Married | 1. Married | 501 | 32.8% | 5080 | 41.6% | < 0.001 |
| 2. Not married | 1025 | 67.2% | 7137 | 58.4% | ||
| Lives alone | 1. No | 1042 | 68.3% | 6829 | 55.9% | < 0.001 |
| 2. Yes | 484 | 31.7% | 5388 | 44.1% | ||
| Distance walked | 0. Did not walk | 163 | 10.7% | 876 | 7.2% | < 0.001 |
| 1. Less than 5 m | 319 | 20.9% | 1951 | 16.0% | ||
| 2. 5–99 m | 865 | 56.7% | 7581 | 62.1% | ||
| 3. 100+ m | 179 | 11.7% | 1809 | 14.8% | ||
| Participation in social activities | 0. Never | 242 | 15.9% | 1829 | 15.0% | 0.182 |
| 1. More than 30 days ago | 473 | 31.0% | 3619 | 29.6% | ||
| 2. 8–30 days ago | 210 | 13.8% | 1914 | 15.7% | ||
| 3. In the last 7 days | 601 | 39.4% | 4855 | 39.7% | ||
| Lives with helper | 0. Does not live with helper | 559 | 36.6% | 6211 | 50.8% | < 0.001 |
| 1. 6 months or less | 12 | 0.8% | 44 | 0.4% | ||
| 2. More than 6 months | 211 | 13.8% | 644 | 5.3% | ||
| 3. No informal care | 744 | 48.8% | 5318 | 43.5% | ||
| Carer stress | 0. No | 928 | 60.8% | 7394 | 60.5% | 0.846 |
| 1. Yes | 598 | 39.2% | 4823 | 39.5% | ||
| Palliative care input | 0. No | 1481 | 97.1% | 11,841 | 96.8% | 0.875 |
| 1. Yes | 45 | 3.0% | 376 | 3.0% | ||
| Self-related health | 0. Good or excellent | 482 | 31.6% | 4122 | 33.7% | 0.095 |
| 1. Fair or poor | 1044 | 68.4% | 8095 | 66.3% | ||
| Alcohol intake | 0. No | 1383 | 90.6% | 9845 | 80.6% | < 0.001 |
| 1. Yes | 143 | 9.4% | 2372 | 19.4% | ||
| Vision | 0. Adequate or minimal difficulty | 1451 | 95.1% | 11,034 | 90.3% | < 0.001 |
| 1. Moderate difficulty | 59 | 3.9% | 880 | 7.2% | ||
| 2. Severe to no vision | 16 | 1.0% | 303 | 2.5% | ||
| Dizziness | 0. Not present | 999 | 65.5% | 8204 | 67.2% | 0.023 |
| 1. Present but not in last 3 days | 197 | 12.9% | 1720 | 14.1% | ||
| 2. Present in last 3 days | 330 | 21.6% | 2293 | 18.8% | ||
| Hearing | 0. Adequate or minimal difficulty | 1208 | 79.2% | 9533 | 78.0% | 0.527 |
| 1. Moderate difficulty | 251 | 16.4% | 2153 | 17.6% | ||
| 2. Severe or no hearing | 67 | 4.4% | 531 | 4.3% | ||
| Falls | 0. No falls in last 90 days | 986 | 64.6% | 7020 | 57.5% | < 0.001 |
| 1. Fell 31–90 days ago | 139 | 9.1% | 1510 | 12.4% | ||
| 2. Fell within 30 days | 401 | 26.3% | 3687 | 30.2% | ||
| Fatigue | 0. None to minimal | 293 | 19.2% | 2150 | 17.6% | 0.127 |
| 1. Moderate to severe | 1233 | 80.8% | 10,067 | 82.4% | ||
| Swallow | 0. No difficulty | 1363 | 89.3% | 10,754 | 88.0% | 0.153 |
| Some difficulty | 163 | 10.7% | 1463 | 12.0% | ||
| Diabetes | 0. No | 850 | 55.7% | 9134 | 74.8% | < 0.001 |
| 1. Yes | 676 | 44.3% | 3083 | 25.2% | ||
| Parkinson’s | 0. No | 1518 | 99.5% | 11924 | 97.6% | < 0.001 |
| 1. Yes | 8 | 0.5% | 293 | 2.4% | ||
| Dementia | 0. No | 1283 | 84.1% | 10,572 | 86.5% | 0.009 |
| 1. Yes | 243 | 15.9% | 1645 | 13.5% | ||
| Depression | 0. No | 1401 | 91.8% | 10,854 | 88.8% | < 0.001 |
| 1. Yes | 125 | 8.2% | 1363 | 11.2% | ||
| Anxiety | 0. No | 1431 | 93.8% | 11,056 | 90.5% | < 0.001 |
| 1. Yes | 95 | 6.2% | 1161 | 9.5% | ||
| Stroke | 0. No | 1252 | 82.0% | 10,065 | 82.4% | 0.749 |
| 1. Yes | 274 | 18.0% | 2152 | 17.6% | ||
| CHD | 0. No | 693 | 45.4% | 5822 | 47.7% | 0.103 |
| 1. Yes | 833 | 54.6% | 6395 | 52.3% | ||
| Bipolar | 0. No | 1506 | 98.7% | 12,118 | 99.2% | 0.056 |
| 1. Yes | 20 | 1.3% | 99 | 0.8% | ||
| COPD | 0. No | 932 | 61.1% | 9170 | 75.1% | < 0.001 |
| 1. Yes | 594 | 38.9% | 3047 | 24.9% | ||
No adjustment was made for multiple testing
CHD coronary heart disease, COPD chronic obstructive pulmonary disease
Procedure
The interRAI assessments produced algorithm-derived care plans designed to ensure standardisation of care throughout Aotearoa New Zealand, for complex older patients. Further information on interRAI-HC can be found at www.interrai.co.nz. The interRAI assessment forms, modified with permission for New Zealand, are used under license to the Ministry of Health. InterRAI information is stored electronically and is unique national identifier linked, using encryption for data security.
Statistical Analysis
Reporting of analyses conformed to the RECORD guidelines [23]. Descriptive summary statistics of participants’ sociodemographics, medical diagnoses, symptoms and supports are included. Modified Poisson regression was employed to identify factors associated with medication adherence [24, 25]. All analyses were performed using SPSS version 27.0 (IBM Corp. Released 2020. Armonk, NY, USA), and α ≤ 0.05 defined significance.
Ethics
Clearance for this study was provided by Aotearoa New Zealand’s Health and Disability Ethics Committee (14/STH/140). Only those who consented to their deidentified interRAI-HC (approximately 93%) information being used for planning and research purposes were released to the study team [26]. All methods were performed following the ethics committee’s relevant guidelines and regulations.
Funding
This work was supported by a Health Research Council of New Zealand Sir Charles Hercus Research Fellowship (17/106).
Results
Of 238,883 interRAI-HC assessments, 225,140 were excluded due to young age, being on no medicines, residents of aged residential care, not having a diagnosis of HF or incomplete data. Repeat assessments were also excluded, leaving 13,743 assessments for older community-dwelling adults with heart failure diagnoses included in the analysis (N = 1526 for Māori) (Fig. 1). The mean age of Māori was 74.5 years (SD 9.1 years) and non-Māori was 82.3 years (SD 7.8 years). There was apparent variation between the baseline sociodemographic characteristics for Māori and non-Māori cohorts with Māori being more likely to be unmarried, live with a helper, have diabetes and have COPD. Non-Māori were more likely than Māori to live alone, have a history of falls and use alcohol (Table 1). After adjusting for confounding variables (Supplementary Table 1), it was found that Māori were less likely to be assessed as being medication adherent than non-Māori [adjusted prevalence ratio 1.53 (confidence interval 1.36–1.73)] (Table 2).
Fig. 1.
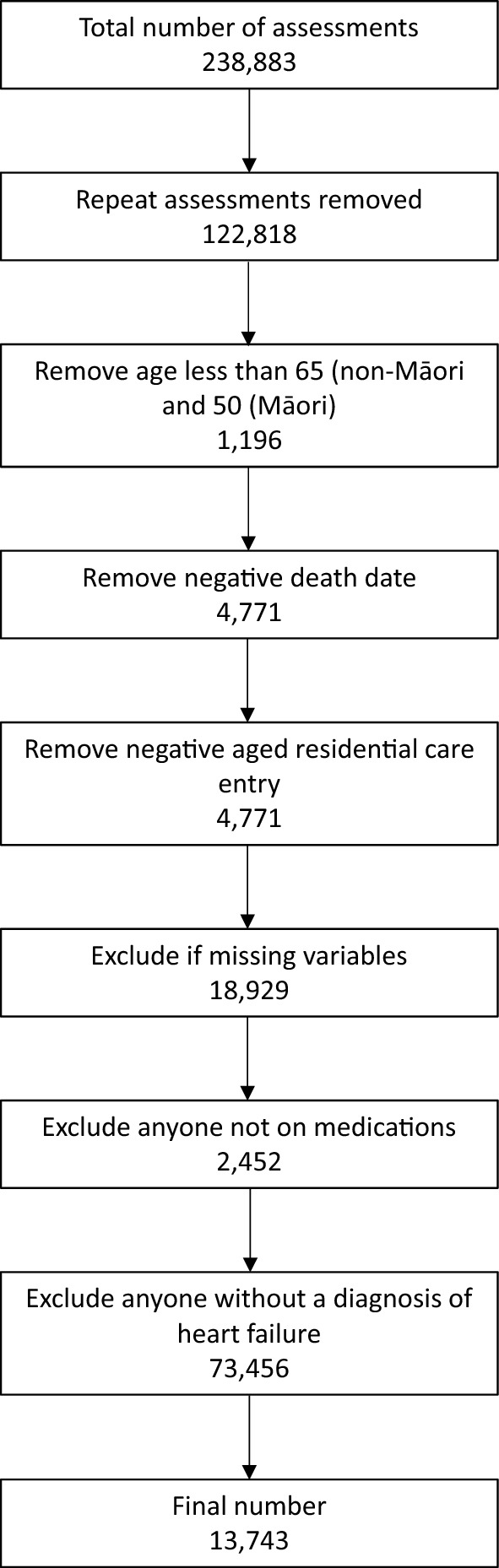
Participant flow diagram
Table 2.
Medication adherence prevalence ratio adjusted for confounding variables
| Adherent to medication (%) | Non-adherent to medication (%) | Unadjusted CI | Adjusted CIa | |
|---|---|---|---|---|
| Māori | 1193 (78.2) | 333 (21.8) | 1.71 (1.53, 1.9) | 1.53 (1.36, 1.73) |
| Non-Māori | 10,657 (87.2) | 1560 (12.8) | 1 | 1 |
CHD coronary heart disease, CI confidence interval, COPD chronic obstructive pulmonary disorder
aAdjusted for: sex, age, married, lives alone, distance walked, partake in social activities, visit by nurse, lives with helper, carer stress, palliative care programme, self reported health, alcohol use, vision, dizziness, hearing, falls, fatigue, swallow, diabetes, Parkinson’s disease, dementia, depression, anxiety, stroke, CHD, bipolar, COPD
Discussion
This large national Aotearoa New Zealand study of 11,850 older community-dwelling adults diagnosed with HF highlighted a significant difference in medication adherence between Māori and non-Māori. In the Māori cohort, 21.8% did not adhere fully to their medication regime, whereas in the non-Māori group, this figure was 12.8%. After adjusting for a significant number of confounding factors, the Māori cohort were 1.53 (CI 1.36–1.73) more likely to be medication non-adherent than non-Māori. The Māori cohort were younger, with a mean age of 74.5 years (SD 9.1 years), whereas the non-Māori cohort had a mean age of 82.3 years (SD 7.8 years).
An Aotearoa New Zealand study identified that ethnicity was a strong predictor of HF-associated hospital admission, particularly for Māori under 75 years [27]. The results of this research are therefore of particular significance given that Māori are already at a higher risk of cardiovascular diseases, which increases the risk of HF [13, 28]. Māori are also diagnosed with associated comorbidities such as atrial fibrillation 10 years earlier than New Zealand Europeans [29]. The earlier onset of these comorbidities among Māori is likely to increase the complexity of medication management as clinicians balance prescribing evidence-based treatment with reducing the risks associated with polypharmacy [30]. In addition to increasing the risk of adverse effects, the increasing number of medications prescribed also increases the likelihood of non-adherence [31].
Previous research has highlighted that non-adherence to medications is a complex and multi-factorial issue [32]. The causes of non-adherence are varied and can involve the consumer, their environment, whānau or family, and the structure and response of the specific health service involved [3, 33, 34]. Reasons for medication non-adherence in Māori have been previously reported, and they include barriers which are intentional (medicines causing adverse effects, not providing therapeutic benefit) and unintentional (forgetting, travelling without medicines, inaccessible supply, cost) [35]. The World Health Organization recognises that providers have a responsibility to support adherence to long-term therapies [36], and Māori have identified factors that health professionals could utilise to facilitate improved adherence, including increasing the provision of relevant information, rationalising and optimising medication therapy and adherence support tools such as alerts and adherence packaging [35]. Cost of medications, and adherence packaging such as dosette boxes, can also impact on adherence and is particularly relevant given best practice HF treatment indicates the prescription of numerous medicines. Previous literature has noted that cost is more of a barrier to medicines access for Māori than non-Māori, and this may have influenced the findings in the current study [37, 38]. Wider determinants of health can also be significant components that all interact and impact on each other to varying degrees to drive medication non adherence [39, 40].
The results of our research show a significant disparity between Māori and non-Māori medication adherence, and monitoring of this allows providers and the health system an opportunity to address these inequities. interRAI assessments are intended to be used to formulate treatment plans, and to our knowledge, there are no methodological approaches currently that flag ‘non-adherence’ responses for action following on from interRAI assessment, which seems a missed opportunity to support patients with their medicine management actively. Much like the discussions about how we improve health literacy [41, 42], rather than purely focussing on the limitations and barriers concerning the patient, we also need to be aware of the limitations of the service itself and how this may need to improve first to serve the needs of specific consumer groups better.
A previous qualitative study investigating the clinical treatment of Māori with ischaemic heart disease highlighted clinicians’ emphasis on non-compliance and the negative connotation that this enforces for both parties [43]. Bissell et al. focussed on the need to consider concordance rather than compliance or adherence so that the relationship is not about clinicians enforcing instructions but about reaching an agreement on the best treatment options for each patient [44]. Including whānau in a consultation may be beneficial in terms of improving health literacy and medication adherence. Although likely to take more time and resources in the short term, the long-term benefits may improve outcomes through the development of trust and understanding, which is particularly relevant as medicine regimens for chronic conditions should be reviewed regularly [45]. As Barker et al. (2017) highlighted, the patient voice is fundamentally important when examining any potential barriers to ongoing medication adherence; otherwise, underlying issues are not understood, and behaviour change from clinicians and patients is unlikely to occur [46].
Inequities in medication adherence highlighted in this study prompt the need for a targeted and tailored response for Māori with HF. We propose a more nuanced, person-centred and culturally appropriate clinical approach which could include improved access to culturally safe medication optimisation reviews by Māori community clinicians (e.g. pharmacists, general practitioners, nurse practitioners). Although foundational work to identify solutions to medicines adherence in Māori has already occurred [35], understanding the issues specific to Māori with HF would also be beneficial, and there is the potential for co-design of interventions to improve adherence. Monitoring new interventions is important as recent interventions post-implementation intentionally designed to improve adherence for those with cardiovascular disease have provided null results [47]. This study demonstrates that interRAI data can be used to monitor adherence and provide a pragmatic approach to an outcome measure for interventions employed in communities regionally, nationally and within aged residential care facilities.
We acknowledge that this study has some limitations. For example, differences in medication adherence can also be associated with socioeconomic status, deprivation [45], or education and health literacy [46]. These factors may be additional confounders that we have been unable to include in our statistical analysis. This additional analysis would add useful information to future studies, particularly given that, at a population level, Māori experience higher levels of poverty and deprivation than non-Māori in Aotearoa New Zealand [48]. We investigated adherence as a binary outcome with people deemed non-adherent if there was any misalignment between what was prescribed and what was taken. This is a higher cut-off than in some other studies, although studies that used less than 80% adherence to define non-adherence identified similar rates of non-adherence as in our study [49]. Furthermore, the medication adherence is self-reported, which may have resulted in some inaccuracies in the data, although any such inaccuracies are likely to be with respect to under-reporting. Although interRAI assessors are trained to reconcile medications when completing the adherence question in interRAI, the extent to which this is done consistently in practice is unclear.
In addition, the recording of individual medications type and number is optional in interRAI through free-text entry, and we therefore did not have access to the numbers and type of medications prescribed. Therefore it is possible that medications being omitted may not necessarily be ones related to heart failure. Severity of HF, HF classifications and clinical outcomes are not recorded in interRAI, and therefore the association of these with adherence could not be examined. Further studies which link other national datasets, such as pharmaceutical dispensing, hospitalisation and mortality records, have the potential to examine this further.
The limitations mentioned above allow for additional important research in this field, including surveying those with a diagnosis of HF identified as non-adherent to understand the reasons for this better, investigating adherence specifically to HF medication and impact of non-adherence on clinical outcomes. However, we believe that the findings of this unique study highlight the opportunity for health services to use data to identify groups whose medication management could be better supported, potentially through culturally-tailored services rather than a one size fits all style of communication and clinical management. A recent Aotearoa New Zealand study highlighted the undervalued importance of communication being two-way in a clinical setting, with listening being as important as talking if the interaction is to be effective and positive [45]. Furthermore, the study stressed the importance of clearly disclosing potential medication side effects (e.g. diuretics prescribing) and trialling specific medication regimes that may need to be adjusted to suit the patient response, depending on their feedback. Consideration also needs to be given by clinicians about cultural bias and stereotyping on their part, which may impact interactions and which can unintentionally limit the ability to reach optimal outcomes [50].
This novel study using a standardised assessment tool has presented the significant disparity in medication adherence between Māori and non-Māori for those older community-dwelling adults with a diagnosis of HF. Given the wide use and transferability of the interRAI on which this study was based, it also has significant international importance given that the ethnic disparity in health outcomes for those with HF is common internationally [51, 52]. This study underlines the importance of ensuring that health systems meet the challenges discussed and that services meet all consumers’ needs by responding in a culturally safe manner. The reasons for non-adherence can be varied and complex and warrant further investigation. However, understanding the primary areas of focus is a significant development and allows clinicians to explore this area further when it has been identified following an interRAI assessment. Many potential solutions already exist, such as pharmacy-led medication management visits, but require remodelling to meet different cultural needs and aspirations. Solutions need not necessarily involve significant financial investment, but a realignment of power to centre patients, and to give them control in the medication journey [53]. Suppose our health system can address and successfully meet these challenges. In that case, we have a real opportunity to address health inequity, better support all older adults with HF, improve their quality of life and potentially reduce avoidable hospital presentations and admissions. The use of big data has allowed identification of inequities in medication-related health care access for people with HF and provides evidence to support targeted health care intervention.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Technical Advisory Services provided us with the interRAI data.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Declarations
Funding
This work was supported by a Health Research Council of New Zealand Sir Charles Hercus Research Fellowship (17/106).
Competing Interests
The authors have no conflicts of interest to declare.
Availability of Data and Material
Further data/datasets are not available as we do not have ethics approval to share the data.
Code availability
Not applicable.
Ethics Approval
Clearance for this study was provided by Aotearoa New Zealand’s Health and Disability Ethics Committee (14/STH/140).
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Author Contributions
JH: data interpretation and framing, writing; RA: formal analysis, writing—review & editing; BM: data interpretation and framing, writing—review & editing; PS: writing—review & editing; PN: writing—review & editing; RS: formal analysis, writing—first draft; HJ: conceptualisation, methodology, formal analysis, writing—review & editing, project administration, funding acquisition.
References
- 1.Rechel B, Doyle Y, Grundy E, McKee M. How can health systems respond to population ageing? World Health Organization. Regional Office for Europe. 2009. https://apps.who.int/iris/handle/10665/107941. Accessed 20 June 2023.
- 2.Gorard DA. Escalating polypharmacy. QJM. 2006;99(11):797–800. doi: 10.1093/qjmed/hcl109. [DOI] [PubMed] [Google Scholar]
- 3.Hikaka J, Jones R, Hughes C, Connolly MJ, Martini N. Ethnic variations in the quality use of medicines in older adults: Māori and non-Māori in Aotearoa New Zealand. Drugs Aging. 2021;38(3):205–217. doi: 10.1007/s40266-020-00828-0. [DOI] [PubMed] [Google Scholar]
- 4.Vrijens B, De Geest S, Hughes DA, et al. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol. 2012;73(5):691–705. doi: 10.1111/j.1365-2125.2012.04167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coronel R, De Groot J, Van Lieshout J. Defining heart failure. Elsevier Science; 2001. pp. 419–422. [DOI] [PubMed] [Google Scholar]
- 6.Azad N, Lemay G. Management of chronic heart failure in the older population. J Geriatr Cardiol JGC. 2014;11(4):329. doi: 10.11909/j.issn.1671-5411.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu J-R, Moser DK, Lennie TA, Burkhart PV. Medication adherence in patients who have heart failure: a review of the literature. Nurs Clin N Am. 2008;43(1):133–153. doi: 10.1016/j.cnur.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 8.van der Wal MH, Jaarsma T, van Veldhuisen DJ. Non-compliance in patients with heart failure; how can we manage it? Eur J Heart Fail. 2005;7(1):5–17. doi: 10.1016/j.ejheart.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Granger BB, Ekman I, Hernandez AF, et al. Results of the Chronic Heart Failure Intervention to Improve MEdication Adherence study: a randomised intervention in high-risk patients. Am Heart J. 2015;169(4):539–548. doi: 10.1016/j.ahj.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murray MD, Young J, Hoke S, et al. Pharmacist intervention to improve medication adherence in heart failure: a randomised trial. Ann Intern Med. 2007;146(10):714–725. doi: 10.7326/0003-4819-146-10-200705150-00005. [DOI] [PubMed] [Google Scholar]
- 11.Bouvy ML, Heerdink ER, Urquhart J, Grobbee DE, Hoe AW, Leufkens HG. Effect of a pharmacist-led intervention on diuretic compliance in heart failure patients: a randomised controlled study. J Cardiac Fail. 2003;9(5):404–411. doi: 10.1054/S1071-9164(03)00130-1. [DOI] [PubMed] [Google Scholar]
- 12.Walsh M, Grey C. The contribution of avoidable mortality to the life expectancy gap in Māori and Pacific populations in New Zealand—a decomposition analysis. NZ Med J. 2019;132(1492):46–60. [PubMed] [Google Scholar]
- 13.Mazengarb J, Grey C, Lee M, et al. Inequity in one-year mortality after first myocardial infarction in Māori and Pacific patients: how much is associated with differences in modifiable clinical risk factors?(ANZACS-QI 49) NZ Med J. 2020;133(1521):40–54. [PubMed] [Google Scholar]
- 14.Riddell T. Heart failure hospitalisations and deaths in New Zealand: patterns by deprivation and ethnicity. NZ Med J (Online). 2005;118(1208). [PubMed]
- 15.Metcalfe S, Beyene K, Urlich J, et al. Te Wero tonu-the challenge continues: Maori access to medicines 2006/07–2012/13 update. NZ Med J (Online). 2018;131(1485):27–47. [PubMed] [Google Scholar]
- 16.Cardiovascular Disease [Internet]. 2022 [cited 27/10/22]. Available from: https://www.health.govt.nz/our-work/populations/maori-health/tatau-kahukura-maori-health-statistics/nga-mana-hauora-tutohu-health-status-indicators/cardiovascular-disease. Accessed 16 Oct 2022.
- 17.Hikaka J, Hughes C, Jones R, Connolly MJ, Martini N. A systematic review of pharmacist-led medicines review services in New Zealand–is there equity for Māori older adults? Res Social Adm Pharm. 2019;15(12):1383–1394. doi: 10.1016/j.sapharm.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 18.Jamieson HA, Schluter PJ, Pyun J, et al. Fecal incontinence is associated with mortality among older adults with complex needs: an observational cohort study. ACG. 2017;112(9):1431–1437. doi: 10.1038/ajg.2017.200. [DOI] [PubMed] [Google Scholar]
- 19.Jamieson H, Abey-Nesbit R, Bergler U, et al. Evaluating the influence of social factors on aged residential care admission in a national home care assessment database of older adults. J Am Med Dir Assoc. 2019;20(11):1419–1424. doi: 10.1016/j.jamda.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Schluter PJ, Ahuriri-Driscoll A, Anderson TJ, et al. Comprehensive clinical assessment of home-based older persons within New Zealand: an epidemiological profile of a national cross-section. Aust N Z J Public Health. 2016;40(4):349–355. doi: 10.1111/1753-6405.12525. [DOI] [PubMed] [Google Scholar]
- 21.Nishtala PS, Jamieson HA. New Zealand's inter RAI: a resource for examining health outcomes in geriatric pharmacoepidemiology. J Am Geriatr Soc. 2017;65(4):876–877. doi: 10.1111/jgs.14778. [DOI] [PubMed] [Google Scholar]
- 22.Mo H. HISO 10001:2017 Ethnicity Data Protocols. Wellington: Ministry of Health; 2017. [Google Scholar]
- 23.Benchimol EI, Smeeth L, Guttmann A, et al. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med. 2015;12(10):e1001885. doi: 10.1371/journal.pmed.1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162(3):199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 25.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 26.Schluter PJ, Askew DA, McKelvey VA, Jamieson HA, Lee M. Oral health among older adults with complex needs living in the community and in aged residential care facilities within New Zealand. J Am Med Dir Assoc. 2021;22(6):1177–83.e1. doi: 10.1016/j.jamda.2020.06.041. [DOI] [PubMed] [Google Scholar]
- 27.Fisher R, Devlin G. Predictors of heart failure hospitalisations. Heart Lung Circ. 2019;28:S57. doi: 10.1016/j.hlc.2019.05.147. [DOI] [Google Scholar]
- 28.Tukuitonga C, Bindman A. Ethnic and gender differences in the use of coronary artery revascularisation procedures in New Zealand. N Z Med J. 2002;115(1152):179. [PubMed] [Google Scholar]
- 29.Poppe KK, Doughty RN, Harwood M, et al. Identification, risk assessment, and management of patients with atrial fibrillation in a large primary care cohort. Int J Cardiol. 2018;254:119–124. doi: 10.1016/j.ijcard.2017.11.045. [DOI] [PubMed] [Google Scholar]
- 30.Sinnott C, Mercer SW, Payne RA, Duerden M, Bradley CP, Byrne M. Improving medication management in multimorbidity: development of the MultimorbiditY COllaborative Medication Review And DEcision making (MY COMRADE) intervention using the Behaviour Change Wheel. Implement Sci. 2015;10(1):1–11. doi: 10.1186/s13012-015-0322-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pasina L, Brucato A, Falcone C, et al. Medication non-adherence among elderly patients newly discharged and receiving polypharmacy. Drugs Aging. 2014;31(4):283–289. doi: 10.1007/s40266-014-0163-7. [DOI] [PubMed] [Google Scholar]
- 32.Ioasa-Martin I, Moore LJ. Problems with non-adherence to antipsychotic medication in Samoan New Zealanders: a literature review. Int J Ment Health Nurs. 2012;21(4):386–392. doi: 10.1111/j.1447-0349.2011.00801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Norris P, Horsburgh S, Lovelock K, et al. Medicalisation or under-treatment? Psychotropic medication use by elderly people in New Zealand. Health Sociol Rev. 2011;20(2):202–218. doi: 10.5172/hesr.2011.20.2.202. [DOI] [Google Scholar]
- 34.Tin ST, Elwood JM, Brown C, et al. Ethnic disparities in breast cancer survival in New Zealand: which factors contribute? BMC Cancer. 2018;18(1):1–10. doi: 10.1186/s12885-017-3797-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hikaka J, Parore N, Haua R, et al. Māori, pharmacists, and medicines adherence—a mixed methods study exploring indigenous experiences of taking medicines ‘as prescribed’ and mechanisms of support. Explor Res Clin Soc Pharm. 2022;7:100175. 10.1016/j.rcsop.2022.100175 [DOI] [PMC free article] [PubMed]
- 36.Sabaté E, Sabaté E. Adherence to long-term therapies: evidence for action. World Health Organization; 2003. [PubMed] [Google Scholar]
- 37.Jatrana S, Crampton P, Norris P. Ethnic differences in access to prescription medication because of cost in New Zealand. J Epidemiol Community Health. 2011;65(5):454–460. doi: 10.1136/jech.2009.099101. [DOI] [PubMed] [Google Scholar]
- 38.Jatrana S, Richardson K, Norris P, Crampton P. Is cost-related non-collection of prescriptions associated with a reduction in health? Findings from a large-scale longitudinal study of New Zealand adults. BMJ Open. 2015;5(11):e007781. doi: 10.1136/bmjopen-2015-007781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ministry of Health . New Zealand health strategy: future direction. Ministry of Health; 2016. [Google Scholar]
- 40.Holland L, Nelson ML, Westrich K, Campbell PJ, Pickering MK. The patient’s medication access journey: a conceptual framework focused beyond adherence. J Manag Care Spec Pharm. 2021;27(12):1627–1635. doi: 10.18553/jmcp.2021.27.12.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lilo LSu, Tautolo E-S, Smith M. Health literacy, culture and Pacific peoples in Aotearoa, New Zealand: a review. Pac Health (aut.ac.nz). 2020;3.
- 42.Shaw SJ, Huebner C, Armin J, Orzech K, Vivian J. The role of culture in health literacy and chronic disease screening and management. J Immigr Minor Health. 2009;11(6):460–467. doi: 10.1007/s10903-008-9135-5. [DOI] [PubMed] [Google Scholar]
- 43.Penney L, Barnes HM, McCreanor T. The blame game: Constructions of Māori medical compliance. AlterNative. 2011;7(2):73–86. doi: 10.1177/117718011100700201. [DOI] [Google Scholar]
- 44.Bissell P, May CR, Noyce PR. From compliance to concordance: barriers to accomplishing a re-framed model of health care interactions. Soc Sci Med. 2004;58(4):851–862. doi: 10.1016/S0277-9536(03)00259-4. [DOI] [PubMed] [Google Scholar]
- 45.Gu Y, Kennelly J, Warren J, Ahn AB, Harwood M, Neuwelt P, editors. Identifying eHealth opportunities to support medication adherence-findings of a focus group study. eHealth; 2016. [PubMed] [Google Scholar]
- 46.Barker H, Oetzel JG, Scott N, Morley M, Carr PEA, Oetzel KB. Enablers and barriers to secondary prophylaxis for rheumatic fever among Māori aged 14–21 in New Zealand: a framework method study. Int J Equity Health. 2017;16(1):1–10. doi: 10.1186/s12939-017-0700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maddison R, Jiang Y, Stewart R, et al. An intervention to improve medication adherence in people with heart disease (Text4HeartII): randomised controlled trial. JMIR Mhealth Uhealth. 2021;9(6):e24952. doi: 10.2196/24952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bécares L, Cormack D, Harris R. Ethnic density and area deprivation: neighbourhood effects on Māori health and racial discrimination in Aotearoa/New Zealand. Soc Sci Med. 2013;88:76–82. doi: 10.1016/j.socscimed.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ekman I, Andersson G, Boman K, Charlesworth A, Cleland JG, Poole-Wilson P, Swedberg K. Adherence and perception of medication in patients with chronic heart failure during a five-year randomised trial. Patient Educ Counsel. 2006;61(3):348–353. doi: 10.1016/j.pec.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 50.Inkrot S, Chappell D, Gamble G, et al., editors. Comparison of patient reported versus clinician estimated self-care in heart failure: results from the MENSCH-NZ study. Eur Heart J. 2018;39(suppl_1).
- 51.Bahrami H, Kronmal R, Bluemke DA, et al. Differences in the incidence of congestive heart failure by ethnicity: the multi-ethnic study of atherosclerosis. Arch Intern Med. 2008;168(19):2138–2145. doi: 10.1001/archinte.168.19.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bank IE, Gijsberts CM, Teng THK, et al. Prevalence and clinical significance of diabetes in Asian versus white patients with heart failure. JACC Heart Fail. 2017;5(1):14–24. doi: 10.1016/j.jchf.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 53.Hikaka J, Jones R, Hughes C, Martini N. "It is through shared conversation, that I understand" – Maori older adults' experiences of medicines and related services in Aotearoa New Zealand. NZ Med J (Online). 2020;133(1516):33–35. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


