Abstract
Background
The prevalence and clinical profile of asthma with airflow obstruction (AO) remain uncertain. We aimed to phenotype AO in population- and clinic-based cohorts.
Methods
This cross-sectional multicohort study included adults ≥50 years from nine CADSET cohorts with spirometry data (N=69 789). AO was defined as ever diagnosed asthma with pre-BD or post-BD FEV1/FVC <0.7 in population-based and clinic-based cohorts, respectively. Clinical characteristics and comorbidities of AO were compared with asthma without airflow obstruction (asthma-only) and chronic obstructive pulmonary disease (COPD) without asthma history (COPD-only). ORs for comorbidities adjusted for age, sex, smoking status and body mass index (BMI) were meta-analysed using a random effects model.
Results
The prevalence of AO was 2.1% (95% CI 2.0% to 2.2%) in population-based, 21.1% (95% CI 18.6% to 23.8%) in asthma-based and 16.9% (95% CI 15.8% to 17.9%) in COPD-based cohorts. AO patients had more often clinically relevant dyspnoea (modified Medical Research Council score ≥2) than asthma-only (+14.4 and +14.7 percentage points) and COPD-only (+24.0 and +5.0 percentage points) in population-based and clinic-based cohorts, respectively. AO patients had more often elevated blood eosinophil counts (>300 cells/µL), although only significant in population-based cohorts. Compared with asthma-only, AO patients were more often men, current smokers, with a lower BMI, had less often obesity and had more often chronic bronchitis. Compared with COPD-only, AO patients were younger, less often current smokers and had less pack-years. In the general population, AO patients had a higher risk of coronary artery disease than asthma-only and COPD-only (OR=2.09 (95% CI 1.26 to 3.47) and OR=1.89 (95% CI 1.10 to 3.24), respectively) and of depression (OR=1.41 (95% CI 1.19 to 1.67)), osteoporosis (OR=2.30 (95% CI 1.43 to 3.72)) and gastro-oesophageal reflux disease (OR=1.68 (95% CI 1.06 to 2.68)) than COPD-only, independent of age, sex, smoking status and BMI.
Conclusions
AO is a relatively prevalent respiratory phenotype associated with more dyspnoea and a higher risk of coronary artery disease and elevated blood eosinophil counts in the general population compared with both asthma-only and COPD-only.
Keywords: Asthma Epidemiology; COPD epidemiology; Pulmonary Disease, Chronic Obstructive; Asthma; Clinical Epidemiology
WHAT IS ALREADY KNOWN ON THIS TOPIC
Asthma with airflow obstruction (AO) is associated with higher exacerbation rates and mortality compared with asthma without airflow obstruction.
WHAT THIS STUDY ADDS
AO patients show more clinically relevant dyspnoea compared with both asthma without airflow obstruction and COPD without asthma history. Second, AO patients from the general population had more often elevated blood eosinophil counts and are at an increased risk of coronary artery disease.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study may facilitate early detection of (mild) AO and concomitant coronary artery disease in clinical practice.
Introduction
Asthma and chronic obstructive pulmonary disease (COPD) are two prevalent chronic respiratory diseases with overlapping phenotypes and endotypes.1–3 Distinguishing between both diseases may, therefore, be difficult, yet essential as both diseases require different treatment decisions.4–6 Importantly, there is a recognised additional clinical phenotype called asthma with fixed airflow obstruction (AFO), consisting of patients with asthma who develop irreversible airflow obstruction (ie, fixed obstruction) with a reduced response to β2-adrenergic agonists.3 This has been attributed to airway remodelling and persistent inflammation, which is potentially linked to steroid resistance, yet the mechanisms leading to fixed AO and associated comorbidities are not fully understood.7–9 Clinically, these patients show a worse prognosis and are expected to have more frequent and more severe exacerbations compared with patients with asthma with reversible airflow obstruction.7–9 Hence, early recognition of asthma with AO is important as it may affect the patient’s prognosis.7
AO primarily affects severe asthma patients (40%–60% of severe asthmatics are estimated to have airway obstruction) and is more prevalent with older age.8 10–13 However, the prevalence and optimal treatment strategy of AO, including in AFO, have been a subject of debate.1 The target population, seniority and specialisation of physicians undertaking the diagnosis of asthma, and definition of airflow obstruction (FEV1/FVC <0.7 or below lower limit of normal (LLN)) all affect the prevalence of AO.7 14–16 Furthermore, randomised clinical trials in asthma traditionally excluded patients with a rich smoking history while COPD trials excluded patients with a history of asthma.17 18
Altogether, the occurrence and clinical profile of AO patients remain unclear. Hence, our study aimed: (1) to determine the prevalence of AO in population-based and clinic-based cohorts, (2) to compare the clinical characteristics between AO patients and asthma without airflow obstruction (asthma-only) and COPD without asthma history (COPD-only) and (3) to compare the prevalence of comorbidities in patients with AO versus patients with asthma-only or COPD-only.
Methods
Study design and population
This analysis was performed in the framework of CADSET,19 a European Respiratory Society Clinical Research Collaboration. Participants ≥50 years with interpretable spirometry and information on asthma diagnosis were cross-sectionally analysed in nine cohort studies: two asthma-based (OLIN and U-BIOPRED), four COPD/smoker-based (COSYCONET, ECLIPSE, PAC-COPD and Urban Training) and three population-based cohorts (LifeLines, Danish Twin Registry and Rotterdam Study). The design of all cohorts has been published in detail and summarised in online supplemental table S1.20–28
bmjresp-2023-001760supp001.pdf (370.1KB, pdf)
Definitions
AO was defined as ever-diagnosed asthma with airflow limitation (a prebronchodilator FEV1/FVC <0.7 in population-based studies and a postbronchodilator FEV1/FVC <0.7 in clinic-based cohorts). Asthma-only was defined as ever physician-diagnosis of asthma and FEV1/FVC ≥0.7. COPD-only was defined as FEV1/FVC<0.7 without asthma history. Asthma in COPD-based cohorts includes both current asthma, as this was not an exclusion criterium of the included COPD cohorts, and asthma in remission. Additionally, FEV1/FVC <LLN was used to define airflow obstruction. Data collection and definitions are reported in the online supplemental file.
Statistical analysis
The prevalence of AO was cross-sectionally meta-analysed by a common effect model using inverse-variance weighting. Clinical characteristics and comorbidities were meta-analysed by a random effects model and logistic regression was performed to adjust the prevalence of comorbidities for age, sex, smoking status and body mass index (BMI). On the cohort level, continuous variables were summarised as means (SD), except for C reactive protein and IgE levels (medians (IQR)). Mean differences (continuous variables) and risk differences (categorical variables) were tested in comparison to the AO group. All comparisons were stratified per cohort type, that is, separately for population-based, asthma-based and COPD-based cohorts. Statistical analysis was performed in R.V.4.1.1 (Vienna, Austria) using the ‘meta’ package.29 30
Results
Prevalence of asthma with AO
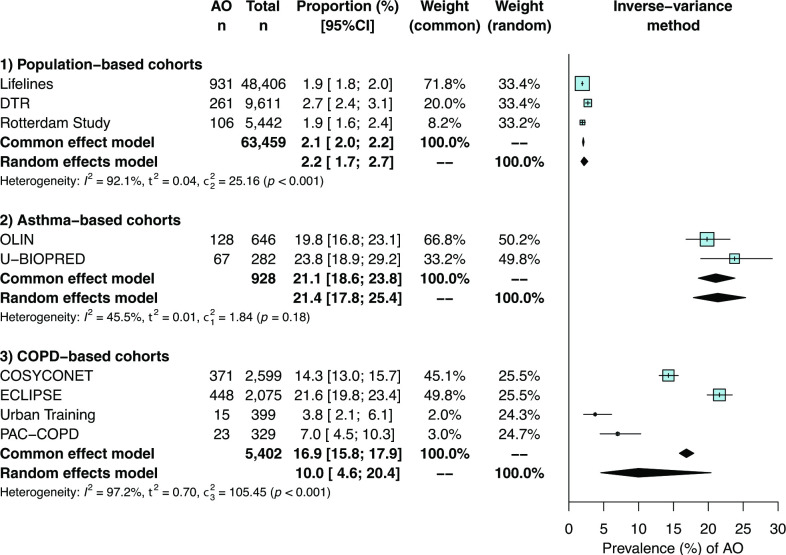
A total of 69 789 participants were included in this study. The prevalence of AO (figure 1) was estimated to be 2.1% (95% CI 2.0% to 2.2%) in three population-based cohorts (n=63 459), 21.1% (95% CI 18.6% to 23.8%) in two asthma-based cohorts (n=928) and 16.9% (95% CI 15.8% to 17.9%) in four COPD-based cohorts (n=5402). The prevalence of AO was highest in U-BIOPRED and ECLIPSE, both showing the lowest mean FEV1% predicted and FEV1/FVC values of their respective cohort types (online supplemental table S2).
Figure 1.
Meta-analysed prevalence of asthma with airflow obstruction (AO) in adults aged 50 years and older in population-based and clinic-based cohorts. COPD, chronic obstructive pulmonary disease. DTR, Danish Twin Registry.
When FEV1/FVC <LLN was used to define AO (online supplemental figure S1), the estimated prevalence of AO was relatively lower in population-based (1.2% vs 2.1%) and asthma-based cohorts (16.4% vs 21.1%). In COPD-based cohorts, the prevalence remained, however, more similar (15.5% vs 16.9%).
Characteristics of patients with AO
Clinical characteristics of patients with AO are presented in table 1 and were compared with asthma-only and COPD-only in population-based and in more symptomatic clinic-based cohorts, reflected by more dyspnoea and chronic bronchitis. AO patients had significantly more often clinically relevant dyspnoea (modified Medical Research Council score ≥2) than asthma-only (+14.4 and +14.7 percentage points) and COPD-only (+24.0 and +5.0 percentage points) in population-based and clinic-based cohorts, respectively.
Table 1.
Meta-analysed characteristics of AO compared with asthma-only and COPD-only in population-based and clinic-based cohorts
| Population-based cohorts | Asthma-based cohorts | COPD-based cohorts | |||||
| AO | Asthma-only | COPD-only | AO | Asthma-only | AO | COPD-only | |
| Characteristics | |||||||
| Age (years), mean (95% CI) | 63.8 (59.4–68.3) | 62.5 (55.8–69.2) | 65.6 (60.1–71.0) | 61.7 (60.7–62.7) | 58.7 (55.9–61.5) | 65.6 (63.3–67.9) | 67.0 (64.7–69.4) |
| Female, prop (95% CI) | 53.3 (50.5–56.0) | 64.4 (59.1–69.7) | 46.9 (42.9–51.0) | 42.3 (34.1–50.6) | 60.6 (57.1–64.1) | 30.7 (6.1–55.3) | 22.3 (8.5–36.1) |
| BMI (kg/m2), mean (95% CI) | 26.7 (26.4–26.9) | 28.5 (27.8–29.2) | 26.2 (26.1–26.4) | 26.4 (24.5–28.4) | 28.3 (26.0–30.6) | 27.7 (26.1–29.3) | 27.3 (26.4–28.2) |
| Underweight, prop (95% CI) | 1.0 (0.5–1.5) | 0.2 (0.0–0.5) | 0.9 (0.2–1.6) | 1.6 (0.0–3.4) | 0.8 (0.0–2.6) | 3.7 (1.7–5.7) | 2.6 (1.0–4.3) |
| Normal weight, prop (95% CI) | 36.4 (32.3–40.4) | 23.4 (19.5–27.2) | 38.7 (35.7–41.6) | 40.4 (23.3–57.4) | 28.3 (21.9–34.7) | 28.4 (14.2–42.5) | 30.3 (22.6–37.9) |
| Overweight, prop (95% CI) | 42.9 (36.9–48.9) | 42.1 (39.2–45.1) | 45.0 (40.8–49.2) | 41.4 (34.3–48.5) | 40.1 (29.5–50.7) | 36.9 (33.6–40.1) | 38.2 (35.3–41.0) |
| Obese, prop (95% CI) | 18.5 (16.4–20.6) | 35.0 (27.6–42.3) | 15.4 (13.7–17.1) | 16.3 (0.0–32.8) | 31.1 (12.3–50.0) | 23.2 (20.1–26.3) | 27.9 (21.4–34.3) |
| Never smoker, prop (95% CI) | 29.7 (24.4–35.0) | 37.9 (34.4–41.5) | 22.3 (17.4–27.1) | 10.2 (5.2–15.1) | 26.2 (0.0–61.1) | 6.4 (0.0–15.2) | 1.7 (0.0–3.9) |
| Former smoker, prop (95% CI) | 52.8 (50.0–55.5) | 52.8 (46.7–59.0) | 49.2 (39.5–59.0) | 43.7 (17.0–70.3) | 34.2 (30.8–37.7) | 66.5 (63.3–69.6) | 61.2 (44.0–78.4) |
| Current smoker, prop (95% CI) | 17.4 (9.2–25.5) | 9.3 (6.4–12.1) | 28.4 (19.7–37.2) | 46.3 (14.7–77.9) | 39.7 (3.7–75.7) | 21.3 (5.1–37.5) | 37.1 (18.6–55.5) |
| Pack-years, mean (95% CI) | 21.2 (16.5–25.9) | 15.4 (11.2–19.5) | 25.3 (21.6–29.0) | 18.2 (11.4–25.0) | 17.5 (13.5–21.5) | 44.1 (34.5–53.8) | 57.2 (47.2–67.2) |
| mMRC score≥2, prop (95% CI) | 38.8 (21.9–55.7) | 24.4 (5.6–43.2) | 14.8 (0.4–29.2) | 54.7 (46.1–63.3) | 40.0 (35.7–44.2) | 51.3 (31.4–71.2) | 46.3 (32.4–60.2) |
| Allergic disease history, prop (95% CI) | 75.7 (73.0–78.5) | 74.9 (72.4–77.4) | 42.9 (41.7–44.0) | 70.3 (47.3–93.3) | 73.2 (61.9–84.4) | 44.1 (21.2–66.9) | 29.9 (24.0–35.8) |
| Chronic bronchitis, prop (95% CI) | 20.3 (10.9–29.8) | 14.4 (9.7–19.2) | 10.4 (5.6–15.2) | 31.7 (1.8–61.5) | 23.3 (0.0–46.9) | 57.2 (31.1–83.2) | 53.3 (34.9–71.7) |
| Emphysema, prop (95% CI) | – | – | – | – | – | 47.8 (4.4–91.1) | 46.5 (4.5–88.6) |
| Spirometry | |||||||
| FEV1 (%) predicted, mean (95% CI) | 75.0 (69.7–80.3) | 95.1 (92.5–97.8) | 81.8 (76.0–87.7) | 54.9 (41.1–68.6) | 80.4 (66.5–94.4) | 51.7 (45.2–58.1) | 51.1 (46.5–55.7) |
| FVC (%) predicted, mean (95% CI) | 93.0 (85.5–100.5) | 95.9 (92.1–99.7) | 97.8 (90.4–105.1) | 78.9 (76.2–81.6) | 88.8 (82.8–94.7) | 77.2 (72.9–81.5) | 77.1 (74.3–79.9) |
| FEV1/FVC (%), mean (95% CI) | 61.6 (59.7–63.6) | 77.0 (76.4–77.6) | 63.9 (63.4–64.5) | 54.2 (41.2–67.2) | 72.8 (57.7–87.8) | 51.6 (46.3–56.9) | 50.2 (46.3–54.1) |
| Biomarkers | |||||||
| Peripheral blood WBC (109 cells/L), mean (95% CI) |
6.7 (5.7–7.8) | 6.6 (5.5–7.7) | 6.9 (5.4–8.4) | 8.8 (7.8–9.7) | 7.6 (7.2–8.0) | 7.9 (7.7–8.1) | 7.7 (7.2–8.2) |
| BEC above 300 cells/µL, prop (95% CI) | 28.3 (25.3–31.2) | 18.0 (15.7–20.2) | 15.7 (14.8–16.5) | 47.0 (34.9–59.0) | 35.9 (29.4–42.4) | 24.0 (20.1–27.9) | 18.6 (11.1–26.0) |
| Serum CRP (mg/dL), median (IQR)* | – | – | – | 2.2 (3.5) | 2.1 (3.8) | 3.0 (3.8) | 3.7 (5.0) |
| Serum IgE (ie,/mL), median (IQR)* | – | – | – | 120 (292) | 110 (221) | 78 (192) | 54 (116) |
*Summary statistics of individual cohorts were meta-analysed, except for CRP and IgE for which only single-study data were available.
AO, asthma with airflow obstruction; BEC, blood eosinophil counts; COPD, chronic obstructive pulmonary disease; CRP, C reactive protein; FEV1, forced expiratory volume in 1 second; FEV1/FVC, ratio of FEV1 to FVC; FVC, forced vital capacity; IgE, immunoglobulin E; mMRC, modified Medical Research Council Dyspnoea; WBC, white blood cell count.
Compared with asthma-only, AO patients were more frequently men, current smokers, had a lower FEV1% predicted and BMI, had less often obesity and had more often chronic bronchitis. Moreover, AO patients had more often elevated blood eosinophil counts (>300 cells/µL), were less frequently never smokers and had more pack-years in population-based cohorts, whereas they had a lower FVC% predicted and higher white blood cell counts in clinic-based cohorts.
Compared with COPD-only, AO patients were significantly younger, less frequently current smokers and had less pack-years. Specifically in population-based cohorts, patients with AO also showed a higher BMI, a lower FEV1% and FVC% predicted, were more frequently never smokers, obese and had more frequently allergic disease history, chronic bronchitis and elevated blood eosinophil counts.
The number of exacerbations in the year prior to spirometry was evaluated in clinic-based cohorts. AO patients showed a higher prevalence of individuals with at least two exacerbations in prior year compared with COPD-only patients (54.0% vs 45.7%, p<0.01) in ECLIPSE. This association remained significant after adjusting for age, sex, smoking status and BMI (OR=1.76 (95% CI 1.44 to 2.15), p<0.01). Furthermore, AO patients showed a modestly higher number of severe exacerbations (β=0.09 (95% CI 0.01 to 0.17), p=0.04) compared with COPD-only in COSYCONET, corrected for age, sex, smoking status and BMI. Compared with asthma-only, AO patients showed a borderline significantly higher risk of having at least one exacerbation in prior year (OR=2.1 (95% CI 1.0 to 4.2), p=0.05) in U-BIOPRED.
Overall, similar differences in characteristics were observed for LLN-defined AO (online supplemental table S4-S6), while age and sex differences were less pronounced. Compared with asthma-only, LLN-defined AO additionally showed a lower FVC% and more allergic disease history in population-based cohorts and more often elevated blood eosinophil counts in clinic-based cohorts. In contrast, the increased exacerbation risk of AO compared with asthma-only in U-BIOPRED was no longer significant using LLN-defined AO (OR=1.6 (95% CI 0.75 to 3.42), p=0.23).
Comorbidities of AO
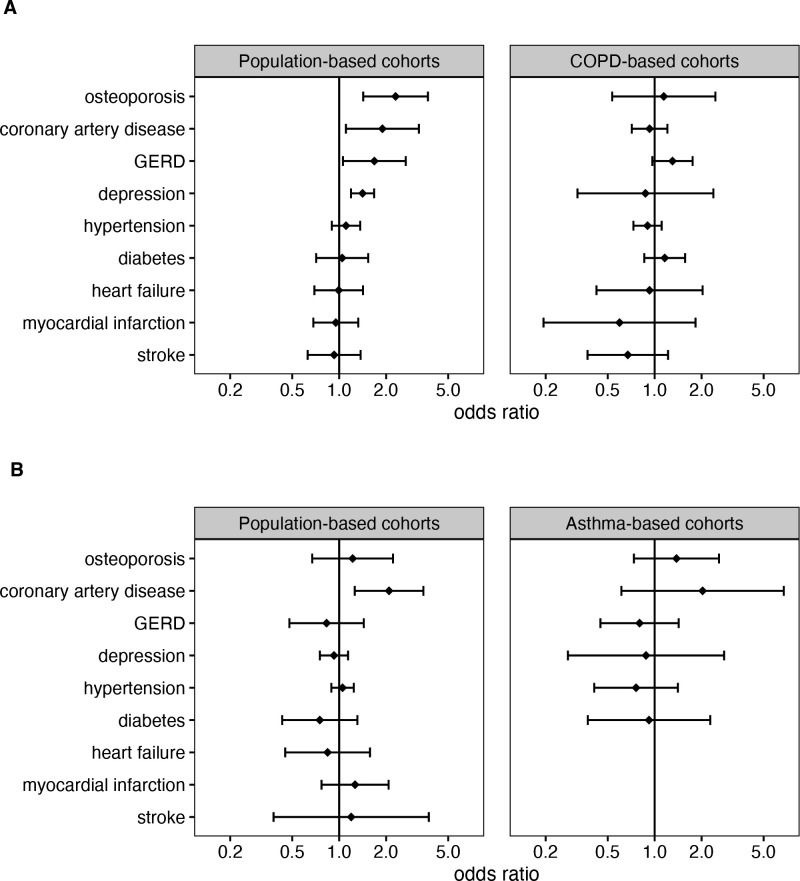
The prevalence of AO comorbidities, adjusted for age, sex, smoking status and BMI, was compared with asthma-only and COPD-only (figure 2). Overall, patients with AO had a significantly higher risk of coronary artery disease (CAD) compared with both asthma-only (OR=2.09 (95% CI 1.26 to 3.47), p<0.01) and COPD-only (OR=1.89 (95% CI 1.10 to 3.24), p=0.02) in population-based cohorts. In clinic-based cohorts, a similar trend was observed compared with asthma-only but not when compared with COPD-only.
Figure 2.
Meta-analysis of comorbidities among patients with asthma with airflow obstruction (AO) compared with COPD without a history of asthma (COPD-only) (A) and compared with asthma without airflow obstruction (asthma-only) (B). ORs were adjusted for age, sex, smoking status and body mass index. The order of comorbidities was based on the effect size compared with COPD-only in population-based cohorts. A detailed meta-analysis for each comorbidity, including individual study effects and statistics, is presented in the supplemental file (online supplemental figures S1.1-S1.9). Osteoporosis and GERD could not be meta-analysed in population-based cohorts and were calculated using data from the Danish Twin Registry (single-cohort data, (online supplemental table S7). Comorbidities in asthma-based cohorts could not be meta-analysed and were calculated using available data from U-BIOPRED (single-cohort data, (online supplemental table S7)). COPD, chronic obstructive pulmonary disease; GERD, gastroesophageal reflux disease.
Additionally, compared with COPD-only, patients with AO showed a higher risk of osteoporosis (OR=2.30 (95% CI 1.43 to 3.72), p<0.01), depression (OR=1.41 (95% CI 1.19 to 1.67), p<0.01) and gastroesophageal reflux disease (GERD) (OR=1.68 (95% CI 1.06 to 2.68), p=0.03) in population-based cohorts. A similar trend was observed for GERD in clinic-based studies. In contrast, the effect size for osteoporosis and depression showed no trend in clinic-based studies, which was due to an opposite direction-of-effect in COSYCONET compared to ECLIPSE (online supplemental figures S2.1 and S2.2, respectively).
Detailed meta-analyses of the OR (online supplemental figures S2.1-S2.9, online supplemental table S7) and crude prevalence (online supplemental figures S3.1-S3.9, online supplemental table S9) of each comorbidity are reported in the online supplemental file, including individual cohort effects. LLN-defined AO showed similar trends for CAD, osteoporosis, depression and GERD in population-based studies, although less pronounced (online supplemental figure S4).
Discussion
In this large multicohort study (N=69 789), we have determined the prevalence of asthma with AO in the general population of adults ≥50 years and in a more symptomatic clinic-based setting. AO affects up to 2% of middle-aged and older adults from the general population, about one in five older patients in asthma cohorts and 4% to 22% of patients in COPD-based cohorts. Our study showed that, irrespective of cohort type, AO patients suffered more often from dyspnoea compared with both asthma subjects without airflow obstruction (asthma-only) and COPD subjects without a history of asthma (COPD-only). Second, AO patients from the general population had higher blood eosinophil levels, a higher risk of CAD compared with asthma-only and COPD-only, and of osteoporosis, depression and GERD compared with COPD-only.
First, our estimated prevalence of AO in the general population and in asthma-based cohorts is in line with previous systematic and narrative reviews on so-called asthma-COPD overlap.2 31 Our findings also confirm that a considerable, but variable, percentage of patients with COPD (~17%, ranging from 4% to 22%) in clinic-based studies had a physician diagnosis of asthma. This high variability may be driven by differences in AO and the fact that asthma is an independent risk factor for COPD over time.32 The highest prevalence of AO was found in ECLIPSE, which also showed the highest severity of AO, while the two smallest studies (PAC-COPD and Urban Training) with the lowest AO prevalence comprised of fewer patients with severe AO. Our estimated prevalence is, however, lower than a previous review (~25%)31 and estimates of asthma features in patients with COPD (eg, atopy) ranging up to 50%.33 This may be attributed to the relatively older age of this study population and the potential of underdiagnosis of asthma in the elderly.34 35
Second, defining AO based on the LLN resulted in a lower prevalence of AO in the general population, in line with previous literature.36 Hence, older adults with mild airflow limitation were likely included in the AO and COPD-only groups of the general population. In contrast, both definitions led to a similar prevalence in ECLIPSE, a COPD-cohort, which includes patients with more severe AO. Further studies are needed to identify which patients with mild or borderline AO deteriorate to LLN-defined AO, as they may require additional treatment approaches.
Third, clinically relevant dyspnoea was more common in AO patients than in either asthma-only or COPD-only. This despite AO patients having similar spirometric values than COPD-only in clinic-based cohorts. This suggests that AO patients may have a higher symptomatologic burden for the same spirometric values compared with COPD in a clinic-based setting. Hence, the development of dyspnoea in patients with AO may not be solely explained by AO only and should also be evaluated with other lung function tests (eg, residual lung volume).37 AO patients also showed lower FVC% values compared with COPD-only in the general population and compared with asthma-only in a clinic-based setting. Future studies should investigate whether dyspnoea and low FVC in AO are determined by a concurrent increase in residual volume (eg, due to air trapping as a result of mucus plugging38 and/or small airway collapse39) and investigate its relationship with lung function trajectories (eg, a lower maximally attained vital capacity at young adulthood and accelerated FEV1 and/or FVC decline).40
In addition to the differences in dyspnoea and FVC, AO patients from the general population had more frequently chronic bronchitis and showed more often elevated blood eosinophil levels, in line with a previous study on AO in a population of mild asthmatics.41 It cannot be ruled out, however, that AO patients may predominantly show mixed inflammation, as markers of neutrophilic inflammation were not collected in our study. Furthermore, AO patients showed to be more often current smokers than asthma-only patients, emphasising that smoking is a risk factor for AO in asthmatics.42 Yet still, a third of AO patients were never smokers among the general population as well as in asthma cohorts. The percentage of never smokers among AO patients in clinical COPD cohorts was smaller due to the enrichment of patients with smoking history among these cohorts. Although the causes of obstructive airway disease in never smokers remain unclear, previous studies suggest that other environmental exposures (eg, biomass combustion) are important risk factors, especially in obese women.43 Strikingly, AO patients had a similar prevalence of emphysema compared with clinic-based COPD, despite AO patients having a lower cumulative exposure to smoking. This indicates that emphysema is another potential pathogenic determinant of (fixed) AO in asthma patients next to airway remodelling.44 Our study also contributes further evidence that AO patients in clinic-based studies are more likely to be exacerbators. AO patients had a higher risk for having at least two exacerbations and more severe exacerbations in last year compared with COPD-only, and a borderline higher risk for having at least one exacerbation in last year compared with asthma-only. This is in line with a previous post hoc analysis of the ATLANTIS study, showing that AO patients had more exacerbations during 1 year of follow-up.41 Given the potential of unadjusted confounders such as medication use, this association should, however, be interpreted cautiously. Further longitudinal cohort studies with deep phenotyping and strict definitions of environmental exposure may help disentangle the complex time-dependent interactions leading to (fixed) AO.
Fourth, our data demonstrate that the comorbidity burden in AO from the general population is considerably higher than in asthma-only or COPD-only. AO patients in population-based studies were at a higher risk for coronary artery disease (CAD) compared with asthma- and COPD-only, independent of age, sex, smoking status, and BMI. The pathophysiological link between obstructive lung function and CAD has been previously described and likely relates to systemic (eosinophilic) inflammation.45 46 Furthermore, the higher prevalence of dyspnoea in AO patients may have led to physical inactivity and deconditioning,47 which is an independent risk factor for CAD.48 These results are in line with a previous study showing that patients with late-onset asthma and prebronchodilator FEV1<50% are at the highest risk for CAD among patients with obstructive airway diseases from the general population.49 In clinic-based cohorts, AO patients showed a trend for increased CAD compared with asthma-only but not compared with COPD-only. This may be partly attributed to selection bias, where those with milder AO in the general population may show increased cardiovascular mortality making them less likely to be included in clinic-based cohorts, which primarily consisted of patients with more severe respiratory disease. In addition, the relative difference in FEV1 may partly explain these findings. A previous mendelian randomisation study provided evidence for an inverse relationship between FEV1 and CAD.50 FEV1% was markedly lower in AO compared with COPD-only in population-based studies, but not significantly different compared with COPD-only in a clinical setting.
Finally, AO patients showed a higher risk for depression, osteoporosis and GERD compared with COPD-only in the general population. The increase in depression may be related to the higher dyspnoea burden in AO. Previous studies showed a cross-sectional link between dyspnoea and depression51, as well as a causal link with the development of symptoms of depression.52 Furthermore, previous evidence revealed overlapping genetics for major depressive disorder and asthma related to immunoglobulin gene hypermutation and DNA damage response.53 In a clinic setting, AO patients showed a higher risk for osteoporosis and depression compared with COPD-only in COSYCONET, but an opposite direction of effect in ECLIPSE. These latter results, thus, require further investigation and replication in other clinic-based AO populations. Altogether, these results show the possible importance of dyspnoea and eosinophilic inflammation as potential contributors to the multimorbidity burden in asthma with AO, which may involve cardiovascular disease (coronary artery disease), metabolic disease (osteoporosis), gastrointestinal disease (GERD) and psychological disorders (depression).
Strengths of our study include that we assessed a wide array of patients in nine population-based and clinic-based cohorts, spanning a multitude of global (mainly European) test sites. We compared clinically relevant characteristics between AO and asthma-only and COPD-only, aiming to single out this important understudied subtype of patients. However, our study also had limitations. We defined AO based on an ever physician-diagnosis of asthma, which could be subjected to recall and misclassification bias. Between-study differences in the diagnosis of asthma may have affected the results. Second, no postbronchodilator spirometry was performed in population-based cohorts, resulting in possible inclusion of asthma patients with reversible airflow obstruction. The use of (long-acting) bronchodilators as part of standard-of-care in general patients with diagnosed asthma may have minimised this; however, it cannot be completely excluded. Given that bronchodilator reversibility in the general population is as least as common in COPD as in asthma, possible inclusion of reversible flow limitation is expected in both groups when comparing AO to COPD-only among the population-based cohorts.54 Third, results from the clinic-based cohorts may not be representative for all clinically diagnosed COPD or patients with asthma as these were mainly recruited from secondary or tertiary care centres. Fourth, each cohort may have had limitations in their data collection methods and some variables were not available in all cohorts. Finally, differences in the cohort populations may have resulted in heterogeneity between patients included in our study. To address this issue, we stratified our analysis on cohort type and used a random effects model. Future longitudinal studies should assess whether the findings presented in this study are more pronounced or limited to AO patients with current asthma and/or chronic persistent AO. Additionally, residual lung volume data may further elucidate the dyspnoea burden and possible FVC reduction in AO patients.
Footnotes
Collaborators: CADSET ERS Clinical Research Collaboration.
Contributors: XB had full access to all summary statistics provided by the individual cohorts and takes responsibility for the integrity of the data and the accuracy of the data analysis as the guarantor. XB, JG-A, RF, HM, PA, NO, NZ and HB performed cohort-specific analyses. XB performed the formal analysis and meta-analysis of summary statistics, and XB and AE drafted the manuscript with guidance from LL. JG-A, RF, HM, TS, PA, CV, NO, NZK, AA, GCD, JAW, GGB, HB, ER, AL, JMV, KFC, IA, MvdB and LL contributed to manuscript revision. All authors have read and approved the final manuscript.
Funding: CADSET ERS Clinical Research Collaboration has been supported by financial and other contributions from the following consortium partners: European Respiratory Society (ERS), AstraZeneca UK Ltd, Chiesi Farmaceutici, GlaxoSmithKline LLC, Menarini and Sanofi-Genzyme.
Competing interests: LL reports consulting fees from AstraZeneca and speaking/lecture fees from Chiesi and IPSA (non-profit) outside the submitted work. CHV reports presentations at symposia and/or served on scientific advisory boards sponsored by Aerogen, AstraZeneca, Boehringer Ingelheim, CSL Behring, Chiesi, GlaxoSmithKline, Grifols, Insmed, Menarini, Novartis, Nuvaira, MedUpdate, Sanofi and Roche outside the submitted work. AA reports grants from GSK, AstraZeneca and Menarini and speaking/lecture fees from GSK, AstraZeneca, Chiesi, Menarini, CIPLA, Zambon, and Sanofi Regeneron outside the submitted work, and is chair of the GOLD board of directors. JAW reports grants from AstraZeneca, Boehringer-Ingelheim, Chiesi, GSK, Novartis, Genentech, and 37Clinical, advisory board fees from AstraZeneca, Epiendo, GSK, Gilead, Novartis, Pieris and Pulmatrix, speaker fees from AstraZeneca, GSK, Boehringer, Recipharm and Novartis, and DSMB chair for Virtus outside the submitted work, and is Editor in Chief of AJRCCM. GGB reports fees for advisory boards and/or lectures from AstraZeneca, Boehringer-Ingelheim, Chiesi, GSK, Merck Sharp & Dohme, Novartis, and Sanofi Regeneron outside the submitted work. HB reports personal fees from AstraZeneca, Boehringer Ingelheim, and GlaxoSmithKline for presentations at scientific meetings outside the submitted work. AL reports speaking/lecture fees from Boehringer-Ingelheim and Novartis, and participation on a advisory board for AstraZeneca, GSK, Novartis, and Boehringer-Ingelheim outside the submitted work. KFC reports advisory board fees from GSK, AstraZeneca, Roche, Novartis, Merck, Nocion, Shionogi and Rickett-Beckinson and has been renumerated for speaking engagements for GSK, AstraZeneca and Merck outside the submitted work. IMA reports investigator-led awards from GSK and Sanofi in addition to travel awards, speakers' fees and advisory board fees from AZ, Chiesi, GSK, Eurodrug, Kineset, Sanofi and Sunovion outside the submitted work. None declared (XB, AE, JG-A, RF, HM, TS, PA, NO, NZK, GCD, ER, JMV and MvdB).
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available. The datasets used and generated in this study are not openly available due to data confidentiality. R scripts are available on reasonable request from the corresponding author.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
All studies were approved by the institutional ethical committees (online supplemental file 1) and informed consent was obtained from all participants. Participants gave informed consent to participate in the study before taking part.
References
- 1.Maselli DJ, Hardin M, Christenson SA, et al. Clinical approach to the therapy of asthma-COPD overlap. Chest 2019;155:168–77. 10.1016/j.chest.2018.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hosseini M, Almasi-Hashiani A, Sepidarkish M, et al. Global prevalence of asthma-COPD overlap (ACO) in the general population: a systematic review and meta-analysis. Respir Res 2019;20:229. 10.1186/s12931-019-1198-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buist AS. Similarities and differences between asthma and chronic obstructive pulmonary disease: treatment and early outcomes. Eur Respir J Suppl 2003;39:30s–5s. 10.1183/09031936.03.00404903 [DOI] [PubMed] [Google Scholar]
- 4.Criner GJ, Celli BR, Brightling CE, et al. Benralizumab for the prevention of COPD exacerbations. N Engl J Med 2019;381:1023–34. 10.1056/NEJMoa1905248 [DOI] [PubMed] [Google Scholar]
- 5.Tashkin DP, Chipps BE, Trudo F, et al. Fixed airflow obstruction in asthma: a descriptive study of patient profiles and effect on treatment responses. J Asthma 2014;51:603–9. 10.3109/02770903.2014.895012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghebre MA, Bafadhel M, Desai D, et al. “Biological clustering supports both "Dutch" and "British" hypotheses of asthma and chronic obstructive pulmonary disease”. J Allergy Clin Immunol 2015;135:63–72. 10.1016/j.jaci.2014.06.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnes PJ. Asthma-COPD overlap. Chest 2016;149:7–8. 10.1016/j.chest.2015.08.017 [DOI] [PubMed] [Google Scholar]
- 8.Yii ACA, Tan GL, Tan KL, et al. Fixed Airways obstruction among patients with severe asthma: findings from the Singapore general hospital-severe asthma phenotype study. BMC Pulm Med 2014;14:191. 10.1186/1471-2466-14-191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Panizza JA, James AL, Ryan G, et al. Mortality and airflow obstruction in asthma: a 17-year follow-up study. Intern Med J 2006;36:773–80. 10.1111/j.1445-5994.2006.01214.x [DOI] [PubMed] [Google Scholar]
- 10.Tonga KO, King GG, Farah CS, et al. Steroid insensitive fixed airflow obstruction is not related to airway inflammation in older non-Smokers with asthma. Respir Res 2018;19:176. 10.1186/s12931-018-0880-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wenzel SE, Schwartz LB, Langmack EL, et al. Evidence that severe asthma can be divided Pathologically into two inflammatory subtypes with distinct physiologic and clinical characteristics. Am J Respir Crit Care Med 1999;160:1001–8. 10.1164/ajrccm.160.3.9812110 [DOI] [PubMed] [Google Scholar]
- 12.James AL, Wenzel S. Clinical relevance of airway remodelling in airway diseases. Eur Respir J 2007;30:134–55. 10.1183/09031936.00146905 [DOI] [PubMed] [Google Scholar]
- 13.Guerra S, Martinez FD. Epidemiology of the origins of airflow limitation in asthma. Proc Am Thorac Soc 2009;6:707–11. 10.1513/pats.200908-085DP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Çolak Y, Afzal S, Nordestgaard BG, et al. Young and middle-aged adults with airflow limitation according to lower limit of normal but not fixed ratio have high morbidity and poor survival: a population-based prospective cohort study. Eur Respir J 2018;51:1702681. 10.1183/13993003.02681-2017 [DOI] [PubMed] [Google Scholar]
- 15.Sorino C, Pedone C, Scichilone N. Fifteen-year mortality of patients with asthma-COPD overlap syndrome. Eur J Intern Med 2016;34:72–7. 10.1016/j.ejim.2016.06.020 [DOI] [PubMed] [Google Scholar]
- 16.Bonten TN, Kasteleyn MJ, de Mutsert R, et al. Defining asthma-COPD overlap syndrome: a population-based study. Eur Respir J 2017;49:1602008. 10.1183/13993003.02008-2016 [DOI] [PubMed] [Google Scholar]
- 17.Sin DD, Miravitlles M, Mannino DM, et al. What is asthma-COPD overlap syndrome? towards a consensus definition from a round table discussion. Eur Respir J 2016;48:664–73. 10.1183/13993003.00436-2016 [DOI] [PubMed] [Google Scholar]
- 18.Vanfleteren LEGW, Ullman A, Nordenson A, et al. Triple therapy (ICS/LABA/LAMA) in COPD: thinking out of the box. ERJ Open Res 2019;5:00185-2018. 10.1183/23120541.00185-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agusti A, Faner R, Donaldson G, et al. Chronic airway diseases early stratification (CADSET): a new ERS clinical research collaboration. Eur Respir J 2019;53:1900217. 10.1183/13993003.00217-2019 [DOI] [PubMed] [Google Scholar]
- 20.Vestbo J, Anderson W, Coxson HO, et al. Evaluation of COPD Longitudinally to identify predictive Surrogate end-points (ECLIPSE). Eur Respir J 2008;31:869–73. 10.1183/09031936.00111707 [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Aymerich J, Gómez FP, Antó JM, et al. Phenotypic characterization and course of chronic obstructive pulmonary disease in the PAC-COPD study: design and methods. Arch Bronconeumol 2009;45:4–11. 10.1016/j.arbres.2008.03.001 [DOI] [PubMed] [Google Scholar]
- 22.Ikram MA, Brusselle G, Ghanbari M, et al. Objectives, design and main findings until 2020 from the Rotterdam study. Eur J Epidemiol 2020;35:483–517. 10.1007/s10654-020-00640-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scholtens S, Smidt N, Swertz MA, et al. Cohort profile: Lifelines, a three-generation cohort study and Biobank. Int J Epidemiol 2015;44:1172–80. 10.1093/ije/dyu229 [DOI] [PubMed] [Google Scholar]
- 24.Arbillaga-Etxarri A, Gimeno-Santos E, Barberan-Garcia A, et al. Long-term efficacy and effectiveness of a behavioural and community-based exercise intervention (urban training) to increase physical activity in patients with COPD: a randomised controlled trial. Eur Respir J 2018;52:1800063. 10.1183/13993003.00063-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skytthe A, Kyvik KO, Holm NV, et al. The Danish twin Registry. Scand J Public Health 2011;39(7 Suppl):75–8. 10.1177/1403494810387966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karch A, Vogelmeier C, Welte T, et al. The German COPD cohort COSYCONET: aims, methods and descriptive analysis of the study population at baseline. Respir Med 2016;114:27–37. 10.1016/j.rmed.2016.03.008 [DOI] [PubMed] [Google Scholar]
- 27.Backman H, Hedman L, Stridsman C, et al. A population-based cohort of adults with asthma: mortality and participation in a long-term follow-up. Eur Clin Respir J 2017;4:1334508. 10.1080/20018525.2017.1334508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shaw DE, Sousa AR, Fowler SJ, et al. Clinical and inflammatory characteristics of the European U-BIOPRED adult severe asthma cohort. Eur Respir J 2015;46:1308–21. 10.1183/13993003.00779-2015 [DOI] [PubMed] [Google Scholar]
- 29.Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical Tutorial. Evid Based Ment Health 2019;22:153–60. 10.1136/ebmental-2019-300117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.R: A language and environment for statistical computing [program] 2022. Vienna, Austria, [Google Scholar]
- 31.Mekov E, Nuñez A, Sin DD, et al. Update on asthma-COPD overlap (ACO): A narrative review. Int J Chron Obstruct Pulmon Dis 2021;16:1783–99. 10.2147/COPD.S312560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silva GE, Sherrill DL, Guerra S, et al. Asthma as a risk factor for COPD in a longitudinal study. Chest 2004;126:59–65. 10.1378/chest.126.1.59 [DOI] [PubMed] [Google Scholar]
- 33.Guerriero M, Caminati M, Viegi G, et al. Prevalence and features of asthma-chronic obstructive pulmonary disease overlap in northern Italy general population. J Asthma 2019;56:27–33. 10.1080/02770903.2018.1424190 [DOI] [PubMed] [Google Scholar]
- 34.Enright PL, Mc Clelland RL, Newman AB, et al. Underdiagnosis and Undertreatment of asthma in the elderly. Chest 1999;116:603–13. 10.1378/chest.116.3.603 [DOI] [PubMed] [Google Scholar]
- 35.Parameswaran K, Hildreth AJ, Chadha D, et al. Asthma in the elderly: Underperceived, Underdiagnosed and Undertreated; a community survey. Respir Med 1998;92:573–7. 10.1016/s0954-6111(98)90311-0 [DOI] [PubMed] [Google Scholar]
- 36.Smith LJ. The lower limit of normal versus a fixed ratio to assess airflow limitation: will the debate ever end. Eur Respir J 2018;51:1800403. 10.1183/13993003.00403-2018 [DOI] [PubMed] [Google Scholar]
- 37.Miravitlles M, Soriano JB, Ancochea J, et al. Characterisation of the overlap COPD-asthma phenotype. focus on physical activity and health status. Respir Med 2013;107:1053–60. 10.1016/j.rmed.2013.03.007 [DOI] [PubMed] [Google Scholar]
- 38.Tang M, Elicker BM, Henry T, et al. Mucus plugs persist in asthma, and changes in mucus plugs associate with changes in airflow over time. Am J Respir Crit Care Med 2022;205:1036–45. 10.1164/rccm.202110-2265OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ueda T, Niimi A, Matsumoto H, et al. Role of small Airways in asthma: investigation using high-resolution computed tomography. J Allergy Clin Immunol 2006;118:1019–25. 10.1016/j.jaci.2006.07.032 [DOI] [PubMed] [Google Scholar]
- 40.Agusti A, Faner R. Lung function Trajectories in health and disease. Lancet Respir Med 2019;7:358–64. 10.1016/S2213-2600(18)30529-0 [DOI] [PubMed] [Google Scholar]
- 41.Kole TM, Vanden Berghe E, Kraft M, et al. Predictors and associations of the persistent airflow limitation phenotype in asthma: a post-hoc analysis of the ATLANTIS study. Lancet Respir Med 2023;11:55–64. 10.1016/S2213-2600(22)00185-0 [DOI] [PubMed] [Google Scholar]
- 42.Tan WC, Sin DD, Bourbeau J, et al. Characteristics of COPD in never-Smokers and ever-Smokers in the general population: results from the Cancold study. Thorax 2015;70:822–9. 10.1136/thoraxjnl-2015-206938 [DOI] [PubMed] [Google Scholar]
- 43.Lamprecht B, McBurnie MA, Vollmer WM, et al. COPD in never Smokers: results from the population-based burden of obstructive lung disease study. Chest 2011;139:752–63. 10.1378/chest.10-1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tonga KO, Chapman DG, Farah CS, et al. Reduced lung elastic recoil and fixed airflow obstruction in asthma. Respirology 2020;25:613–9. 10.1111/resp.13688 [DOI] [PubMed] [Google Scholar]
- 45.Rabe KF, Hurst JR, Suissa S. Cardiovascular disease and COPD: dangerous liaisons Eur Respir Rev 2018;27:180057. 10.1183/16000617.0057-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Niccoli G, Cosentino N. Eosinophils: a new player in coronary Atherosclerotic disease. Hypertens Res 2012;35:269–71. 10.1038/hr.2011.221 [DOI] [PubMed] [Google Scholar]
- 47.Ramon MA, Ter Riet G, Carsin A-E, et al. The dyspnoea-inactivity vicious circle in COPD: development and external validation of a conceptual model. Eur Respir J 2018;52:1800079. 10.1183/13993003.00079-2018 [DOI] [PubMed] [Google Scholar]
- 48.Sattelmair J, Pertman J, Ding EL, et al. Dose response between physical activity and risk of coronary heart disease: a meta-analysis. Circulation 2011;124:789–95. 10.1161/CIRCULATIONAHA.110.010710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ingebrigtsen TS, Marott JL, Vestbo J, et al. Coronary heart disease and heart failure in asthma, COPD and asthma-COPD overlap. BMJ Open Respir Res 2020;7:e000470. 10.1136/bmjresp-2019-000470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Au Yeung SL, Borges MC, Lawlor DA. Association of genetic instrumental variables for lung function on coronary artery disease risk: A 2-sample Mendelian randomization study. Circ Genom Precis Med 2018;11:e001952. 10.1161/CIRCGEN.117.001952 [DOI] [PubMed] [Google Scholar]
- 51.Currow DC, Chang S, Reddel HK, et al. Breathlessness, anxiety, depression, and function-the BAD-F study: A cross-sectional and population prevalence study in adults. J Pain Symptom Manage 2020;59:197–205. 10.1016/j.jpainsymman.2019.09.021 [DOI] [PubMed] [Google Scholar]
- 52.Neuman A, Gunnbjörnsdottir M, Tunsäter A, et al. Dyspnea in relation to symptoms of anxiety and depression: A prospective population study. Respir Med 2006;100:1843–9. 10.1016/j.rmed.2006.01.016 [DOI] [PubMed] [Google Scholar]
- 53.Zhu Z, Zhu X, Liu C-L, et al. Shared Genetics of asthma and mental health disorders: a large-scale genome-wide cross-trait analysis. Eur Respir J 2019;54:1901507. 10.1183/13993003.01507-2019 [DOI] [PubMed] [Google Scholar]
- 54.Janson C, Malinovschi A, Amaral AFS, et al. Bronchodilator reversibility in asthma and COPD: findings from three large population studies. Eur Respir J 2019;54:1900561. 10.1183/13993003.00561-2019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjresp-2023-001760supp001.pdf (370.1KB, pdf)
Data Availability Statement
No data are available. The datasets used and generated in this study are not openly available due to data confidentiality. R scripts are available on reasonable request from the corresponding author.