Abstract
Objective
Insomnia is the most prevalent sleep disorder, with few effective pharmacotherapies. Anecdotal reports and recent preclinical research suggest that cannabinol (CBN), a constituent of Cannabis sativa derived from delta-9-tetrahydrocannabinol, could be an effective treatment. Despite this, the isolated effects of CBN on sleep have yet to be systematically studied in humans.
Methods
The present protocol paper describes a randomised, double-blind, placebo-controlled, single-dose, three-arm, cross-over, proof-of-concept study which investigates the effects of CBN on sleep and next-day function in 20 participants with clinician-diagnosed insomnia disorder and an Insomnia Severity Index Score ≥15. Participants receive a single fixed oral liquid dose of 30 mg CBN, 300 mg CBN and matched placebo, in random order on three treatment nights; each separated by a 2-week wash-out period. Participants undergo overnight sleep assessment using in-laboratory polysomnography and next-day neurobehavioural function tests. The primary outcome is wake after sleep onset minutes. Secondary outcomes include changes to traditional sleep staging, sleep-onset latency and absolute spectral power during non-rapid eye movement (NREM) sleep. Tertiary outcomes include changes to sleep spindles during NREM sleep, arousal indices, absolute spectral power during REM sleep and subjective sleep quality. Safety-related and exploratory outcomes include changes to next-day simulated driving performance, subjective mood and drug effects, postural sway, alertness and reaction time, overnight memory consolidation, pre and post-sleep subjective and objective sleepiness; and plasma, urinary, and salivary cannabinoid concentrations. The study will provide novel preliminary data on CBN efficacy and safety in insomnia disorder, which will inform larger clinical trials.
Ethics and dissemination
Human Research Ethics Committee approval has been granted by Bellberry (2021-08-907). Study findings will be disseminated in a peer-reviewed journal and at academic conferences.
Trial registration number
Keywords: sleep medicine, statistics & research methods, clinical trials
STRENGTHS AND LIMITATIONS OF THIS STUDY
This study uses a randomised, double-blind, placebo-controlled, cross-over design to investigate two active doses of cannabinol (CBN) isolate on sleep and neurobehavioural function over ~17 hours using gold-standard objective measures, validated subjective measures and rigorous design methodology.
Study participants undergo extensive screening with a sleep physician to confirm insomnia disorder diagnosis, including an overnight diagnostic polysomnogram to rule out other sleep disorders that commonly co-occur with insomnia.
The study wash-out period (2 weeks) was chosen to minimise participant burden, as participants cannot undergo insomnia treatment before (3 months) or during (~2 months) the protocol; however, this may lead to carry-over effects between doses as a recent cross-over study demonstrated oral cannabinoids can persist in plasma for >4 weeks (1500 mg; single dose).
The study involves a single-dose, in-laboratory proof-of-concept design and results may, therefore, lack ecological validity (repeated CBN dosing may be necessary to effectively treat insomnia).
Background
Insomnia is a sleep disorder characterised by subjective difficulty falling asleep, maintaining sleep and/or achieving restorative sleep, where symptoms persist for ≥3 months and are accompanied by daytime sequelae of dysfunction and/or distress.1 Insomnia disorder is the most prevalent sleep disorder which affects between 10% and 30% of adults2 and persists in 37% of cases at a 5-year follow-up.3 Insomnia disorder increases the risk of mental and physical illnesses such as depression4 and dementia.5 The economic burden of insomnia disorder is estimated at >$A13 billion per annum in Australia.6
Insomnia disorder treatments are typically behavioural or pharmacological in nature. Cognitive behavioural therapy for insomnia (CBTi) is the first-line treatment and its efficacy has been confirmed in recent meta-analyses.7 Nevertheless, significant access barriers to CBTi exist including limited providers, high cost8 and delayed perception of subjective benefits.9 CBTi may also be less effective in individuals with shorter sleep duration (ie, <6 hours/night).10 As such, sedative-hypnotics are commonly used in primary care.7 While effective in the short-term, well-documented side effects include daytime sedation, psychomotor impairment, addiction/dependence and premature mortality.11 There is a clear need for safe pharmacological insomnia disorder treatments.
Cannabis sativa is a complex plant containing numerous potentially therapeutic chemical compounds, including over 140 constituent ‘cannabinoids’.12 As historical legal prohibitions against cannabis are relaxed, cannabinoids are being increasingly used to aid sleep.13 The most well researched cannabinoids are Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD); however, in recent times, cannabinol (CBN) has become recognised as a putative ‘sleep-enhancing’ cannabinoid.14 CBN is produced via the non-enzymatic oxidation of THC and is therefore present at higher concentrations in aged (rather than ‘young’) cannabis plant material.15 This is significant as aged cannabis plant material is anecdotally reported to induce sleep.16 CBN products are also being sold as sleep aids in unregulated markets.17 Importantly, a recent narrative review of CBN highlighted insufficient evidence to support sleep-related claims, despite a plausible mechanism of action.
THC and CBN exert some of their physiological effects via interactions with the endogenous cannabinoid system (ECS),12 comprising signalling molecules termed ‘endocannabinoids’ (eg, anandamide, 2-arachidonoylglycerol (2-AG)), their receptors and enzymes.18 19 The ECS is involved in regulating a myriad of biological processes including circadian rhythmicity.20 Systematic administration of anandamide has been shown to promote sleep in rodents and humans18 21 22 via activation of the cannabinoid receptor 1 (CB1R).21 23 As such, the CB1R is considered a promising therapeutic target.24 The pharmacological effects of THC occur via partial agonism at CB1R25 and can include acute intoxication, psychomotor impairment, sedation and changes in sleep architecture.18 CBN also has agonist actions at CB1R, although approximately 10 times less potent than THC,25 which may potentially account for the low levels of intoxication reported in historic CBN studies.26 27 Minimal intoxication could render CBN a more practical and safer alternative to THC as a sleep aid.
Two recent clinical studies administered small quantities of CBN in multi-cannabinoid formulations.28 29 The first, a cross-over randomised controlled trial (RCT) with a 1-week washout, investigated 2-weeks of an oral liquid containing 20 mg THC, 2 mg CBN, and 1 mg CBD in 20 adults with insomnia disorder. The drug significantly improved insomnia disorder symptoms (Insomnia Severity Index (ISI) −5.1 points, p=0.0001, d=0.94) compared with placebo, with no significant changes to polysomnography (measured on night 14 of dosing).28 In an interventional open-label study, current medicinal cannabis users with subjective sleep difficulty reported improved sleep (assessed via an unvalidated subjective survey and a validated non-contact at-home tracking device) after 3-weeks of nightly administration of an oral capsule containing 10 mg THC and 5 mg CBN.29 Importantly, the effects of CBN cannot be disentangled from those of THC in these studies. We are not aware of any clinical studies to-date that have tested the isolated effects of CBN on objectively measured human sleep.
Importantly, a recently published conference abstract reported on a preclinical study in which CBN isolate (10, 30 and 100 mg/kg intraperitoneally compared with zolpidem 10 mg/kg as a positive control) increased the proportion of non-rapid eye movement (NREM) sleep and sleep bout duration 4-hours post administration in Long-Evan rats.30 With the lowest dose, biphasic effects were observed—CBN (10 mg/kg) initially decreased REM sleep proportion; however, 4-hours after administration, a significant increase in the percentage of REM sleep occurred. These results suggest CBN could reduce wake after sleep onset (WASO), given such delayed effects on sleep.
Study design and aim
Here, we described the protocol for a randomised, double-blinded, placebo-controlled, three-arm, cross-over, single-site, proof-of-concept study design to investigate the acute effects of oral CBN on sleep and next-day function in 20 participants with clinician-diagnosed insomnia disorder. The primary study aim is to investigate the effects of CBN (30 and 300 mg) versus placebo on sleep in insomnia disorder. This study aims to evaluate the safety and efficacy of CBN as a pharmacological therapy and generate preliminary data to inform larger clinical trials. Many outcome measures used in insomnia disorder clinical trials are known to be susceptible to placebo effects. A placebo control is scientifically and ethically defensible choice as the effects of CBN on human sleep are unknown and could not be properly elucidated if an active treatment such as zolpidem was the comparator rather than placebo.
Study outcomes
The primary study outcome is WASO measured in minutes using in-laboratory overnight polysomnography, from the first epoch after lights out until the last epoch, scored as any stage of sleep by an experienced polysomnographic technician in accordance with American Academy of Sleep Medicine (AASM) 2020 Sleep Scoring criteria (Version 2.6).31 Secondary study outcomes include:
Traditional sleep staging: Proportion of the sleep opportunity scored at the five stages (wake, and N1, N2, N3 and REM sleep) between lights out and lights on, measured using overnight in-laboratory polysomnography, scored by a polysomnography technician in accordance with AASM Sleep Scoring criteria.
Sleep-onset latency (SOL): SOL measured in minutes using in-laboratory polysomnography, calculated from the time of lights out to the first sleep epoch as scored by a polysomnographic technician in accordance with AASM Sleep Scoring criteria.
Absolute electroencephalographic (EEG) power during NREM sleep: Spectral power of delta (1–4.5 Hz), theta (4.5–8 Hz), alpha (8–12 Hz), sigma (12–15 Hz), beta (15–25 Hz) and gamma (25–40 Hz) frequency ranges between treatment arms. Power spectral analysis will be applied to EEG signals from polysomnography after artefacts are detected and removed.
Tertiary, safety and exploratory study outcomes are described in box 1 (see ClinicalTrials.gov Identifier: NCT05344170).
Box 1. Tertiary, safety and exploratory study outcomes.
Tertiary outcomes
Sleep spindles during non-rapid eye movement (NREM) sleep: Sleep spindle and slow oscillation events in NREM sleep from in-laboratory overnight polysomnography. A sleep spindle and slow oscillation detection algorithm will be applied to electroencephalography (EEG) signals from polysomnography after artefacts are detected and removed (comparisons between each cannabinol (CBN) dose versus placebo).
EEG Arousal Index: Number of cortical arousals captured via the electroencephalogram per hour of sleep scored by the polysomnographic technician on the polysomnogram in accordance with American Academy of Sleep Medicine Sleep Scoring criteria (comparisons between each CBN dose versus placebo).
Absolute EEG power during REM sleep: Spectral power of delta (1–4.5 Hz), theta (4.5–8 Hz), alpha (8–12 Hz), sigma (12–15 Hz), beta (15–25 Hz) and gamma (25–40 Hz) frequency ranges between treatment arms. Power spectral analysis will be applied to EEG signals from polysomnography after artefacts are detected and removed (comparisons between each CBN dose versus placebo).
Next-day post wake subjective sleep evaluation (Leeds Sleep Evaluation Questionnaire, LSEQ): Richard Campbell Sleep Questionnaire (RCSQ) score. LSEQ score. LSEQ scores range from 0 to 100, with higher scores indicating better subjective experience. Assessed within 1 hour after wake (comparison between each CBN dose vs placebo).
Next-day post wake subjective sleep evaluation (RCSQ): RCSQ score. RCSQ scores range from 0 to 100, with higher scores indicating better subjective experience. Assessed within 1 hour after wake (comparison between each CBN dose vs placebo).
Safety outcomes
SD of lateral position (SDLP) during next-day post-wake simulated drive: SDLP (‘weaving’) is measured across the ‘standard’, ‘car following’ and ‘divided attention’ subsections of a ~30 min simulated driving task. Assessed within 2 hours after wake (comparison between each CBN dose vs placebo).
Speed during next-day post-wake simulated drive: Average speed and SD of speed is measured across the ‘standard’ and ‘divided attention’ subsections of a ~30 min simulated driving task. Assessed within 2 hours after wake (comparison between each CBN dose vs placebo).
Distance headway during next-day post-wake simulated drive: Average distance headway (ie, distance between the driver’s vehicle and vehicle immediately in front) and SD of distance headway is measured across the ‘car following’ subsection of a ~30 min simulated driving task. Assessed within 2 hours after wake at both treatment sessions (comparison between each CBN dose vs placebo).
Subjective mood evaluation: The Abbreviated Profile of Mood States consists of 40 items measuring domains of ‘tension’, ‘depressed’, ‘anger’, ‘vigour’, ‘fatigue’ and ‘concentration’. Participants respond to each item using 5-point Likert scales ranging from 0 (not at all) to 4 (extremely). A total mood disturbance score is calculated by summing negative domains and subtracting positive domains. Administered pre and post drug administration, as well as next-day (comparison between each CBN dose vs placebo).
Subjective drug effects: The Drug Effects Questionnaire assesses the extent to which participants feel a drug effect, feel high, like the effects, dislike the effects, want more of the substance, and feel sedated, on self-rating 100 mm Visual Analogue Scales. A total mood disturbance score is calculated by summing negative domains and subtracting positive domains. Administered pre and post drug administration, as well as next-day (comparison between each CBN dose vs placebo).
Postural Sway: Centre-of-pressure during computerised static posturography. Administered pre and post drug administration, as well as next-day (comparison between each CBN dose vs placebo).
Behavioural alertness and reaction time: Psychomotor Vigilance Test is administered twice the next-day (comparison between each CBN dose vs placebo).
Overnight verbal declarative memory consolidation: Word pair recall scores measured using the computerised Word Pairs Task. Administered predrug administration and next-day (comparison between each CBN dose vs placebo).
Overnight procedural memory consolidation: Motor sequence learning measured using the computerised Finger Tapping Task. Administered predrug administration and next-day (comparison between each CBN dose vs placebo).
Resting wake EEG power after sleep: Resting wake EEG power during the Karolinska Drowsiness Test (KDT) on wake: delta (1–4.5 Hz), theta (4.5–8 Hz), alpha (8–12 Hz), sigma (12–15 Hz), beta (15–25 Hz) and gamma (25–40 Hz) frequency ranges. Power spectral analysis is applied to EEG signals from polysomnography, after artefacts are detected and removed. Comparison between each CBN dose versus placebo.
Subjective sleepiness after sleep: The Karolinska Sleepiness Scale (KSS) is a 10-item measure of subjective drowsiness. Participants respond to each item using a 9-point Likert scale ranging from 1 (extremely alert) to 9 (extremely sleepy). Higher scores are indicative of increased drowsiness. The KSS will be collected in accordance with the KDT protocol but will not be analysed due to insufficient statistical power.
Exploratory outcomes
Resting wake EEG power before sleep (Acute Effects of CBN). Resting wake EEG power during the KDT prior to sleep: delta (1–4.5 Hz), theta (4.5–8 Hz), alpha (8–12 Hz), sigma (12–15 Hz), beta (15–25 Hz) and gamma (25–40 Hz) frequency ranges. Power spectral analysis is applied to EEG signals from polysomnography, after artefacts are detected and removed. Comparison between each CBN dose versus placebo.
Subjective sleepiness before sleep (acute effects): The KSS is a 10-item measure of subjective drowsiness. Participants respond to each item using 9-point Likert scales ranging from 1 (extremely alert) to 9 (extremely sleepy). Higher scores are indicative of higher drowsiness. The KSS will be collected in accordance with the KDT protocol but will not be analysed due to insufficient statistical power.
Plasma cannabinoid concentrations: Presence of cannabinoids (CBN, delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD)) (eg, 11-OH-CBN, 11-COOH-CBN, 11-OH-THC, 11-COOH-THC, 7-OH-CBD, 7-COOH-CBD), and endocannabinoids and related molecules (eg, 2-Arachidonoylglyceroland anandamide) and their metabolites in plasma samples.
Urinary cannabinoid concentrations: Presence of cannabinoids (CBN, THC and CBD) and their metabolites (11-OH-CBN, 11-COOH-CBN, 11-OH-THC, 11-COOH-THC, 7-OH-CBD, 7-COOH-CBD) in urine samples.
Salivary cannabinoid concentrations: Presence of cannabinoids (THC, CBN) and their metabolites (11-OH-CBN, 11-COOH-CBN, 11-OH-THC, 11-COOH-THC) in saliva samples.
Safety
The investigational product, CBN, is currently a Schedule 9 ‘Prohibited Substance’ in Australia.32 The safety profile of CBN can be inferred from human studies conducted between 1973 and 1987. These studies explored oral doses up to 1200 mg CBN with some administering repeated daily doses of CBN for up to 4 weeks.26 33–35 There were no notable safety concerns, aberrations or toxicity concerns from these studies; with no adverse changes in parameters such as heart rate, blood pressure, body temperature, respiratory rate, perception, intoxication and postural stability.26 33–35 None of these studies measured sleep, sedation or residual next-day effects of CBN (such as adverse effects on driving performance and cognitive function). These measures are obtained in the current study. CBN is currently being investigated as an analgesic agent (ClinicalTrials.gov Identifier: NCT03675971) and topical treatment for epidermolysis bullosa (ClinicalTrials.gov Identifier: NCT04908215). An RCT of oral CBN isolate (compared with combination CBN/CBD and placebo doses) on the subjective sleep of healthy adults was retrospectively registered in May 2022 (ClinicalTrials.gov Identifier: NCT05839964). We will monitor and review safety reports from these trials as they become available.
Methods
Participants, interventions and outcomes
Trial setting
The trial sponsor and site is the Woolcock Institute of Medical Research, a specialist sleep and respiratory research institute and clinic (Sydney, Australia). The study funder is the Lambert Initiative for Cannabinoid Therapeutics, University of Sydney (Sydney, Australia), a philanthropically funded centre for cannabinoid science. Recruitment commenced in August 2022 and is anticipated to conclude in late 2023. The first participant was enrolled 24 August 2022 and the first participant was randomised on 13 October 2022. The study has ethical approval from Bellberry Human Research Ethics Committee (HREC; 2021-08-907; Version 2.1, 1 August 2022) and is registered on ClinicalTrials.gov (Identifier: NCT05344170; 25 April 2022). The clinical trial protocol is available on request. Significant changes to the study protocol will be documented on ClinicalTrials.gov. The study protocol was developed according to the Standard Protocol Items: Recommendations for Interventional Trials 2013 statement.36 The Therapeutics Goods Administration has acknowledged the use of CBN in this study through the Clinical Trial Notification (CTN) scheme (CTN Number: CT-2022-CTN-00543-1).
Participant eligibility
Eligible participants are aged between 25 and 65 years with physician diagnosed insomnia disorder as per the International Classification of Sleep Disorders-3 (ICSD-3)37 and Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5)38 criteria as determined by the study physician (see box 2). The age range was selected to minimise age-related changes to sleep architecture.39 As cannabinoids have been shown to improve symptoms (eg, anxiety, pain) that commonly impair sleep (ie, causing ‘secondary’ insomnia disorder),40 a ‘primary’ insomnia disorder population is being recruited for this study.
Box 2. Inclusion and exclusion criteria for study participants.
Eligible individuals must fulfil the following criteria:
Between 25 and 65 years of age.
Insomnia Severity Index score ≥15 at eligibility screening.
Insomnia disorder (symptoms occurring at least three times per week and present for longer than 3 months) as determined by the study physician.
Ability to take oral medication.
Provision of signed and dated informed consent form.
Stated willingness to comply with all study procedures and availability for the duration of the study.
Individuals who meet any of the following criteria are ineligible to participate in the study:
Medical condition or medication that is the cause of the insomnia disorder as determined by the study physician.
Known hypersensitivity to cannabis or cannabinoid products (including if this becomes evident during the trial).
Reported use of cannabis or cannabinoid products within the past 3 months as confirmed by at least one negative urine drug screen (or at the study physician’s discretion).
Sleep apnoea (defined as Apnoea Hypopnea Index >15 and Oxygen Desaturation Index >10) as confirmed by polysomnography at screening.
Sleep-related movement disorder as determined by the study physician.
Delayed or advanced sleep phase syndrome (based on actigraphy and sleep diary) as confirmed during screening.
Any medical condition that produces an abnormal electroencephalographic (ie, epilepsy, brain injury).
Clinically relevant cardiovascular abnormalities as determined by the study physician and a 12-lead ECG at screening.
Shift work or trans meridian travel (two time zones) within the last month.
History of major psychiatric disorder in the past 12 months at the study physician’s discretion, except clinically managed mild depression and/or anxiety.
History of suicide attempt or current suicide ideation (score greater than 1 on Q9 of the Patient Health Questionnaire).45
Pregnancy or lactating. Female participants are required to complete a urine pregnancy test at screening and treatment sessions and all participants are instructed to use a reliable form of contraception throughout the study duration.
History of drug or alcohol dependency or abuse within approximately the past 2 years.
Use of central nervous system (CNS)-active drugs (cannabis, amphetamines, cocaine, antidepressants, opioids, benzodiazepines) in the past 3 months as confirmed by a positive urine drug test at screening or at the study physician’s discretion.
Use of medications that may have a clinically significant impact on the metabolism and excretion of cannabinoids as determined by the study physician (eg, CYP450 enzyme inducers/inhibitors).
Excessive caffeine use that in the opinion of the study physician contributes to the participant’s insomnia disorder, or the inability to abstain from caffeine use 24 hours prior to each overnight sleep study.
Inability to refrain from alcohol consumption 24 hours prior to each overnight sleep study.
Individuals with nicotine dependence (ie, daily smokers).
Medical conditions that result in frequent need to get out of bed (eg, sleep walking, nocturia).
Psychological or behavioural treatment for insomnia disorder, including cognitive behavioural therapy for insomnia, within 3 months before screening (excluding sleep hygiene advice).
Occupational or judicially ordered drug screening.
Has held an unrestricted driving license <1 year.
Cannot speak English fluently.
Patient and public involvement
Patients and/or the public were not involved in the design, conduct, reporting, nor dissemination plans of this research.
Intervention
Study doses
Participants receive a single fixed 2 mL oral liquid dose of: (1) 30 mg CBN (‘ECS310’ 1.5% containing 15 mg/mL CBN); (2) 300 mg CBN (‘ECS310’ 15% containing 150 mg/mL CBN) and (3) matched placebo. Treatments are administered across three separate overnight treatment sessions in randomised order 2 hours prior to habitual lights out. The lower dose was selected to match the CBN products available in the USA and supported by customer testimonies.17 The higher dose was informed by a preclinical study of CBN isolate and sleep.30
Investigational product
The investigational drug (‘ECS310’) is a liquid formulation of CBN suspended in medium chain triglyceride (MCT) oil. The placebo contains MCT oil only. The maximum detectable THC content of the active investigational products is ≤150 µg. Neither the study drug nor matched placebo contain any other cannabinoids or terpenes/terpenoids. The study drug and matched placebo are expected to be identical in their visual appearance, taste and smell. The MCT oil contains paprika oleoresin colourant to ensure the double-blind is maintained and prevent oxidation. The sponsor purchased the investigational product from BOD Australia (Sydney, Australia), who sourced investigational products from Medropharm (Zürich, Switzerland). Stability studies supporting the shelf life applied to the drug product were conducted in accordance with the International Conference on Harmonisation (ICH) Guideline Q1A Stability Testing of new Drug Substances and Products and are ongoing. The CBN solution stability is 24 months at 15°C–25°C, 60% humidity and away from sunlight.
Recruitment and retention
We aim to recruit 20 participants over a 12-month period. The sample size was selected with consideration for practical factors such as time, cost and resource allocation (rather than using formal statistical techniques) as it is pilot in nature. Participants are recruited from social media, the Woolcock Institute Volunteer Database and locally displayed physical study advertisements. Participants are reimbursed a fixed amount equivalent to that used in previous clinical trials with similar participant involvement and loss of working hours.41 To encourage retention, participants receive reimbursement on completion of the final Treatment Session. Study advertisements include an embedded link and QR code to the study website (described below).
Eligibility screening
Eligibility assessment is performed in three steps undertaken by the study team at the Woolcock Institute of Medical Research (see figure 1):
Figure 1.
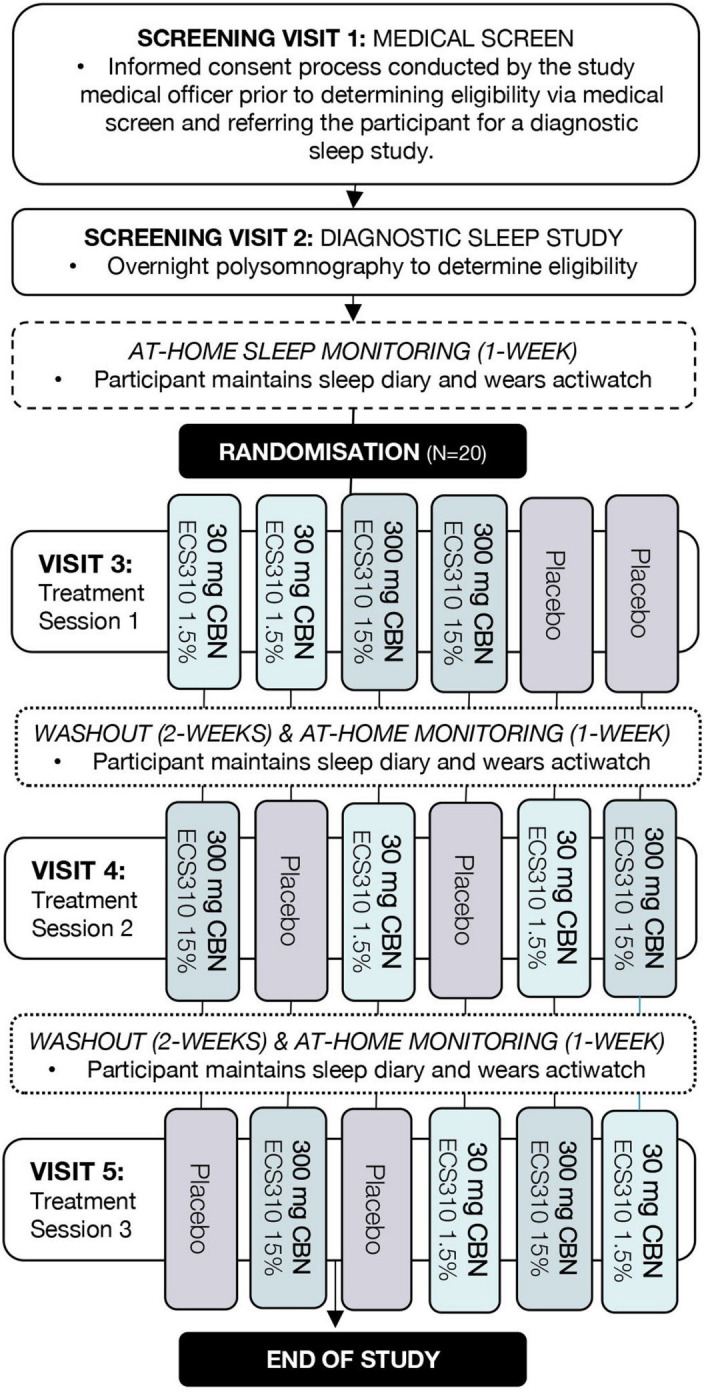
Study flow diagram. CBN, cannabinol.
On-line and telephone prescreening: Interested individuals are directed to the study webpage. They provide electronic consent before completing an online prescreening questionnaire to assess initial eligibility (eg, age, location, ISI score, transmeridian travel, shift work). Potentially suitable individuals can enter their contact details and are provided the participant information statement so that the trial coordinator may conduct a brief telephone interview before scheduling screening appointments. Individuals deemed unsuitable are provided with support resources.
Screening visit 1: Willing and potentially suitable participants attend an on-site medical screening. The study physician obtains written informed consent before conducting a screening interview to compile medical history, assess suitability against study eligibility criteria (box 2), and diagnose insomnia disorder (ICSD-337 and DSM-V38). The following validated self-report questionnaires are used to evaluate eligibility: ISI,42 a measure of insomnia disorder symptom severity over the past 2 weeks; Epworth Sleepiness Scale,43 a measure of sleep propensity in daily life; Hospital Anxiety and Depression Scale,44 a measure of anxiety and depression; and, the Patient Health Questionnaire-9,45 an instrument for screening and monitoring the severity of depression and suicidal ideation. An ECG and urinary screen (pregnancy and recent alcohol, cannabis, cocaine, benzodiazepines, opiates or amphetamines, use) are also conducted. Participants must agree to the study protocol, including use of contraception and refraining from driving until the day after Treatment Session discharge.
Screening visit 2: Participants are required to complete a clinical overnight polysomnography to rule out the presence of other sleep disorders (obstructive sleep apnoea or sleep-related movement disorder, see box 2). Diagnostic polysomnography results within the previous 12 months may be used where weight has not changed substantially in that time (at discretion of the study physician). Diagnostic sleep studies are scored by an experienced sleep technician46 and reviewed by the study physician.
Randomisation, allocation concealment and blinding
Participants are allocated to one of six possible treatment orders in a 1:1:1:1:1:1 ratio using a prepopulated randomisation schedule generated by the study epidemiologist (NM). The six orders constitute a balanced Latin square. Sequences were computer generated and are stored in a password-protected system inaccessible to blinded study personnel (centralised computerised randomisation). Allocation concealment is managed by the unblinded study epidemiologist and an independent staff member who do not have any contact with participants, nor involvement in day-to-day trial activities. A study physician may request to be unblinded in the event of a medical emergency. Participants who are enrolled in the study, but not yet randomised, are assigned a unique screening number. Randomisation occurs at the outset of the first treatment session assuming participant eligibility persists. Working under the study physician, the trial coordinator records the decision to randomise the patient. The study database then issues an irrevocable randomisation number for that participant. Investigational product is stored in opaque sequentially numbered containers labelled with the patient randomisation and treatment session number to maintain blinding of all patients, staff and outcome assessors. The drug manufacturer packaged and labelled the investigational product according to the randomisation list generated by the study epidemiologist.
Pretreatment session procedures
Participants are asked to maintain a regular sleep-wake schedule (ie, lights out between 22:00 and 23:00 hours each evening) for 1 week prior to each treatment session. Participants wear a wrist-worn actigraphy monitor (GENEActive, Activinsights, UK) and complete a daily sleep diary (Karolinska Sleepiness Scale (KSS)47 modified to capture additional information, such as naps, alcohol/caffeine consumption and GENEactiv removal times) throughout this period. If the sleep schedule is significantly irregular during the seven-night leadup (eg, >2 hours on >2 nights), the principal medical and non-medical investigators may reschedule the visit. Participants are required to abstain from consuming cannabis, cannabinoids, and other central nervous system (CNS)-active drugs throughout the entire study; and avoid alcohol and caffeine 24 hours prior to treatment sessions. urinary drug, alcohol (DrugCheck NxStep OnSite, USA) and pregnancy (SureStep, Germany) screening occurs at the outset of each treatment session.
Treatment session procedures
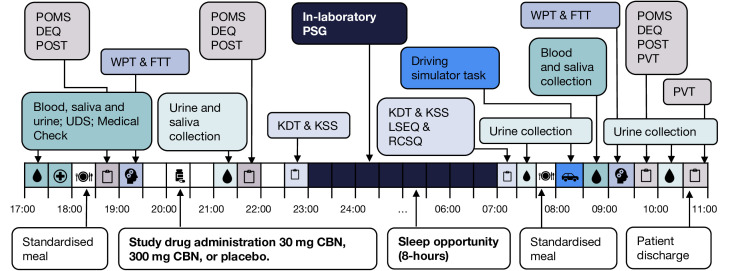
Participants arrive at the Woolcock Sleep Clinic in the early evening (~16:30-17:30 hours, depending on habitual lights out), where they remain until late morning (~10:00–11:00 hours) the following day, as per the treatment session schedule in figure 2. Standardised meals are provided. All treatment sessions are separated by a washout period of ≥2 weeks to minimise carry-over effects between doses.48
Figure 2.
Schedule of events during each treatment session. CBN, cannabinol; DEQ, Drug Effects Questionnaire; EEG, electroencephalography; FTT, Finger Tapping Task; ISI, Insomnia Severity Index; KSD, Karolinska Sleep Diary; KSS, Karolinska Sleepiness Scale; LSEQ, Leeds Sleep Evaluation Questionnaire; POMS, Profile of Mood States; POST, Posturography; PSG, polysomnography; PVT, Psychomotor Vigilance Task; RCSQ, Richard Campbell Sleep Questionnaire; UDS, Urinary Drug Screen; WPT, Word Pairs Task.
Study drug administration
After a brief medical examination, the study physician prescribes and dispenses the study drug, drawing a fixed 2 mL volume of liquid into a prelabelled amber plastic syringe. Participants self-administer the randomly allocated study drug 2-hours prior to lights out. The trial coordinator observes drug administration to ensure accuracy of dosing. The dose is documented in participant records and accountability logs. Timing of administration has been informed by delayed onset of effects observed with oral dosing of other cannabinoids49 and delayed effects observed in a preclinical study of CBN.30
Data collection
Study measures are described below (see table 1).
Table 1.
A summary of assessments completed throughout the investigation
| Measure (ordered first to last) | Screening | Treatment session 1–3 | ||
| At-home sleep monitoring | Night | Day | ||
| Informed consent | · | |||
| Medical examination | · | · | ||
| Baseline questionnaires (ISI, ESS, HADS and PHQ-9) | · | |||
| ECG | · | |||
| Urinary drug and alcohol screen | · | · | ||
| Pregnancy test | · | · | ||
| Overnight polysomnography (clinical EEG) | · | · | ||
| Actigraphy | · | |||
| Sleep diary (KSD) | · | |||
| Oral fluid drug screen (Securetech DrugWipe) | · | · | ||
| Quantisal saliva collection | · | · | ||
| Blood plasma collection | · | · | ||
| Urine collection | · | · | ||
| Mood (POMS) | · | · | ||
| Drug effects (DEQ) | · | · | ||
| Balance (posturography) | · | · | ||
| Memory (FTT and WPT) | · | · | ||
| KDT with EEG | · | · | ||
| Sleep questionnaires (LSEQ, RCSQ and KSS) | · | |||
| Simulated driving performance | · | |||
| PVT | · | |||
DEQ, Drug Effects Questionnaire; ECG, electrocardiogram; EEG, electroencephalography; ESS, Epworth Sleepiness Scale; FTT, Finger Tapping Task; HADS, Hospital Anxiety Depression Scale; ISI, Insomnia Severity Index; KDT, Karolinska Drowsiness Test; KSD, Karolinska Sleep Diary; KSS, Karolinska Sleepiness Scale; LSEQ, Leeds Sleep Evaluation Questionnaire; PHQ-9, Patient Health Questionnaire-9; POMS, Profile of Mood States; PSG, polysomnography; PVT, Psychomotor Vigilance Task; RCSQ, Richard Campbell Sleep Questionnaire; WPT, Word Pairs Task.
Blood collection and plasma cannabinoid levels
Venous blood samples are taken at baseline (~17:00 hours) and approximately 13-hours post drug administration (~08:45 hours). Samples are drawn into EDTA vacutainers (Becton, Dickinson and Company, New Jersey, USA) and centrifuged (2500×g for 15 min at 4°C). Supernatant plasma is aliquoted into 1.5 mL Eppendorf Tubes and stored at −80°C in the Woolcock Institute of Medical Research until study completion when samples will be transferred to the Lambert Initiative Laboratory. Samples will undergo liquid chromatography–mass spectrometry (LC–MS/MS) analysis, using previously published methodology.50–52 Plasma will be analysed for cannabinoids (eg, CBN, THC, CBD), their metabolites (eg, 11-OH-CBN, 11-COOH-CBN, 11-OH-THC, 11-COOH-THC, 7-OH-CBD, 7-COOH-CBD) and endocannabinoids and related molecules (eg, 2-AG and anandamide). THC and CBD and their metabolites are being tested to verify abstinence from common cannabinoids, notwithstanding the trace quantities of THC that the investigational product may contain (≤150 µg/2 mL).
Urine collection and urinary cannabinoid levels
Urine collection occurs at baseline (~17:00 hours), and approximately 90-min (~21:30 hours), 11-hours (~07:00 hours) and 14-hours (~10:00 hours) post drug administration. Urine (~6 mL) is aliquoted into 1.5 mL Eppendorf Tubes and stored at −80°C before being transferred to the Lambert Initiative Laboratory for quantification of cannabinoids (primarily THC, CBD and CBN) and their metabolites (11-OH-CBN, 11-COOH-CBN, 11-OH-THC, 11-COOH-THC, 7-OH-CBD, 7-COOH-CBD) and endocannabinoids at study completion. LC–MS/MS analyses will be conducted using our previously described methods.50 51 It is important to conduct urinary analyses to further characterise the pharmacokinetic profile of CBN, which is currently not well described.
Salivary drug testing
Oral fluid testing occurs at baseline (~17:00 hours), and at approximately 75-min (~21:15 hours) and 13-hours (~08:55 hours) post drug administration using the DrugWipe 5S (DW5s; Securetec, Neubiberg, Germany) and Quantisal collection (Immunalysis, Pomona, California, USA) devices. The DW5s collects oral fluid (approximately 10–20 µL) from the tongue. The DW5s is commonly used in routine roadside drug tests; however, demonstrates variable sensitivity (22%–89%), specificity (50%–100%) and accuracy (53%–94%).53 While the investigational product contains negligible quantities of THC, similarities in chemical structure between CBN and THC may conceivably lead to false-positive test results that would be a concern should CBN become widely used by patients.54 As such, the Quantisal device is used to collect oral fluid from participants (~1 mL) which will be used in LC–MS/MS quantification of cannabinoid (CBN and THC) and metabolite concentrations. Samples are placed in a stabilising buffer and stored at 4°C before being transferred to the Lambert Initiative analytical chemistry laboratory for analysis. Analyses occur within 3 months of collection.
Cognitive and psychomotor performance
Overnight declarative and procedural memory consolidation is assessed at each treatment session using the Word Pairs Task (WPT) and Finger Tapping Task (FTT), respectively. For both tasks, memory encoding during a learning phase occurs prior to drug administration (~19:15–20:00 hours) with next-day retest (~09:00 hours). As THC can acutely impair cognitive function and memory, it is necessary to examine CBN effects in this regard. Contrary to oral dosing studies, in one study, CBN reportedly caused a mild intoxication when administered intravenously at high doses (1.2 mg/min).27 To investigate possible intoxication-induced bodily sway, postural balance (‘posturgraphy’) is being measured using the stabilometric balance platform (Advanced Medical Technologies, AccuSwayPLUS Balance platform, Massachusetts, USA), which measures centre of pressure in anterior-posterior (y-axis) and medial-lateral (x-axis) directions using four strain gauge load cells at the bottom of the device. Centre of pressure is assessed in eyes open and eyes closed conditions. Postural balance assessment takes <5 min to complete and is repeated three times (ie, baseline, 60-min post drug administration and next-day at ~09:30 hours). The psychomotor vigilance test (PVT) is a commonly used reaction time based measure of attention and fatigue related changes in alertness55 involving a hand-held box with a red-light emitting diode display of a three-digit millisecond counter (PVT-192, Ambulatory Monitoring, Adsley, New York, USA). During the 10 min task, participants are instructed to respond as quickly as possible to stimuli appearing at variable intervals (ranging 2–10 s). The PVT is repeated twice during next-day testing (~09:00 and ~10:00 hours).
Self-report questionnaires
Mood is evaluated using the Profile of Mood States (POMS; 40 items with 5-point Likert scales)56 questionnaire, a commonly used rating scale of transient and distinct moods. The Drug Effects Questionnaire (DEQ; 7-items with unipolar 100 mm Visual Analogue Scales (VAS))57 is used to evaluate subjective drug effect; including strength of drug effect, feeling stoned (‘high’), liking/disliking the drug effect, and feeling sedated. The POMS and DEQ are completed at baseline, ~1-hour postdrug administration (~21:00 hours) and the next-day (~09:30 hours). The Leeds Sleep Evaluation Questionnaire (LSEQ; 10 items with 100 mm VAS)58 is a validated measure of the previous night’s sleep quality (sleep onset, maintenance and quality; including behaviour following waking) in comparison to usual. The Richard Campbell Sleep Questionnaire (RCSQ; 5 items with 100 mm VAS)59 is used to measure perceived sleep depth, sleep latency (time to fall asleep), number of awakenings and sleep efficiency and quality. The LSEQ and RCSQ are administered on wake (~06:15 hours).
Subjective and objective sleepiness
KSS and Karolinska Drowsiness Test (KDT) are used to measure subjective and objective sleepiness, respectively.60 The KSS is a single-item rating scale assessing current sleepiness, ranging from 1 (extremely alert) to 9 (extremely sleepy—fighting sleep). KSS scores are typically compared with the objective drowsiness ascertained using the KDT. During the KDT, resting awake EEG is measured while participants focus on a wall marker, with eyes open and closed across 2 min intervals. The KSS and KDT are administered immediately prior to sleep (~22:00 hours) and on wake (~06:00 hours).
Polysomnography
Sleep is assessed using polysomnography (Grael polysomnography, Compumedics, USA), a multiparametric tool used for monitoring and diagnosing sleep disorders. Polysomnography consists of 18 channels recording biophysical changes that occur during sleep, including brain activity (via EEG); muscle activity or skeletal muscle activation (electromyography); eye movement (electrooculogram); cardiac function (ECG); respiratory effort using chest and abdominal belts; and digital pulse oximetry. To improve participant comfort/retention, the pulse oximeter, nasal cannula, and thermistor are only used during screening to detect sleep disordered breathing (visit 2). Polysomnography equipment is fitted by an experienced sleep technician prior to sleep and is removed the next morning once the KDT has been completed. Sleep and associated events are scored according to AASM 2020 criteria in 30 s epochs.46 Each patient’s three polysomnograms are scored by the same sleep technician blinded to treatment allocation. For quantitative EEG analyses, sleep and KDT polysomnography recordings will be processed using a previously validated automated artefact detection programme.61 62 Power spectral and spindle analyses will be derived from artefact-free epochs in central channels using previously published methodology.61 62
Simulated driving performance
Next-day driving performance is measured at ~08:00 using a fixed-based driving simulator (Hyperdrive, Adelaide, Australia) equipped with standard vehicle controls (described elsewhere41 48) and using custom-built scenario developed using the SCANeR Studio Simulation Engine (version 2022.2 r25, AVSimulation, Paris, France). The 30 min driving scenario incorporates three independent epochs: (1) a ‘car following’ drive (~7 min duration) that closely resembles one that has previously demonstrated sensitivity to THC-induced impairment;63 (2) a ‘highway’ drive (~17 min duration) and (3) a novel divided-attention drive (~6 min duration), designed to replicate a casual mobile-phone conversation that does not require mental rehearsal.63 During the divided-attention task, pre-recorded five-word sentences are played every 10 s (through a hands-free speaker). Participants immediately indicate whether the sentence was sensical (eg, ‘the truck delivered the package’) or non-sensical (eg, ‘the octopus burned the onions’) in nature then; and at 7 s, they are asked to recall the last word in the sentence. The main outcome measure for all epochs of the driving task is SDLP, a widely used measure of lateral vehicular control that is sensitive to drug and alcohol effects. Previous reports show a dose-dependent increase in SDLP with THC (higher represents more erratic driving), particularly in occasional cannabis users.64 65 Secondary outcomes for each driving epoch include: (epoch 1) average and SD of car-following headway (ie, distance relative to car in front) and speed coherence (ie, correlation between speed of lead and following car); (epochs 2 and 3) average speed and SD of speed. The driving scenarios were programmed by investigator CI.
Adverse events
Safety outcome measures are described above. Blood pressure is measured four times during each treatment session (~17:00, 18:45, 21:00 and 09:30 hours). Adverse event collection occurs via standardised interview at three timepoints (prior to discharge as well as 7-hours and 7-days post discharge). Passive adverse event collection occurs throughout the study protocol via open-ended interview and is recorded on paper form. A study physician assesses the severity and causality of each adverse event.
Post-treatment session care
Participants leave the research facility at approximately 10:00–11:00 hours and are contacted by the study co-ordinator that evening (~16:30 hours) and 7-days later, to assess well-being and record adverse events (including changes to sleep). On study completion, if deemed necessary in the study physician’s opinion, a clinical follow-up is arranged for participants on an individual basis.
Data management
Any information obtained for the purpose of this research that could identify participants is treated as confidential and securely stored adhering to guidelines of the Sponsor, HREC, NHMRC National Statement on Ethical Conduct in Human Research (2007) and Note for Guidance on Good Clinical Practice (CPMP/ICH-135/95). All onboarding, consent and questionnaire data are captured through SPARDAC (Single Page Application-Research Data Capture) developed by Wappsystem and hosted on Amazon Web Services in Sydney, NSW. Participant data are identified by unique codes. The code linking participant data to identity (eg, name, date of birth, contact details) is stored securely with password protection, and is not accessible via the internet. Paper files are stored in locked storage cabinets on-site. Digital participant data are stored on a secure electronic data system, that is regularly backed up with disaster recovery features. All data will be stored securely for at least 15 years. Only study investigators have access to participant data. Data monitoring occurs on a regular basis by the study investigators. Data integrity is being enforced through a variety of mechanisms including data rules, range checks and consistency checks against data already stored in the database. Written documentation of changes is available through electronic logs and audit trails. Study safety is internally monitored and evaluated regularly, with adverse events documented and reported according to sponsor and HREC requirements. The decision to terminate the study lies with the principal investigators and will be based on the recruitment target and safety data. The PIs and trial coordinator are monitoring data integrity throughout the trial.
Trial management structure
This is a pilot study and does not require an intricate management structure. The advisory committee provide advice or complete discrete tasks (table 2). The management of day-to-day trial activities is overseen by the principal investigators and the trial coordinator (IL, CMH and BY). This trial does not have an independent data safety monitoring committee because it is a single-site single-night small sample study with a medication that is thought to have a low side effect profile in a group of patients that are known not to be at high risk of life-threatening events.66
Table 2.
Investigator roles and responsibilities
| Investigator | Role |
| Dr Camilla Hoyos | Principal non-medical investigator |
| Professor Brendon Yee | Principal medical investigator |
| Professor Iain McGregor | Advisory committee member (pharmacologist and cannabinoid expert) |
| Professor Ronald Grunstein | Advisory committee member (sleep physician) |
| Professor Bandana Saini | Advisory committee (study pharmacist) |
| Associate Professor Nathaniel Marshall | Advisory committee (study statistician/epidemiologist) |
| Associate Professor Christopher Gordon | Advisory committee member (registered nurse and sleep disorder expert) |
| Dr Angela D'Rozario | Advisory committee member (sleep neurobiology expert/consultant) |
| Dr Danielle McCartney | Driving simulator and cannabinoid expert |
| Dr Chris Irwin | Advisory committee member (driving simulation expert) |
| Dr Anastasia Suraev | Advisory committee member (cannabinoid and sleep expert) |
| Ms Isobel Lavender | Clinical trial coordinator/PhD student |
Roles and responsibilities
The study investigators have led the design and conduct of this trial, will conduct the analyses and make all publication decisions.
Statistical analyses
Statistical analyses will be completed using a commercial statistical package (eg, SPSS (Version 29.0.1.0) and SAS (Version 9.4)). Descriptive statistics will be described using means and SD or counts and percentages as appropriate. Adverse events and serious adverse events will be tabulated with the total number of incidents by each time point. No formal hypothesis testing will be conducted on adverse event and serious adverse event data. Due to insufficient statistical power, the KSS will only be described using a median and IQR. Continuous outcome data will be analysed using linear mixed models due to expected interparticipant variability with repeated measures and ability to handle missing data. Where variables are collected predrug administration (ie, treatment session ‘baseline’) for the outcome being analysed, those scores will also be included in the model as a covariate (eg, posturography, WPT, FTT, DEQ, POMS). Participants will be random factors. Fixed factors will be treatment (placebo, 30 mg CBN and 300 mg CBN) and the arm (first, second and third) of the study. Analysis will be by intention to treat. Difference in least squared means inside the treatment main effect will only be investigated for the hypotheses that the two active treatments are superior to placebo. We will not present a p value comparison for the two active doses. The critical p values for each of these hypotheses is set at 0.05 (two tailed). Effect sizes will be calculated as partial eta squared (ƞp2), Cohen’s d and Hedges’ g, where appropriate. A statistical analysis plan will be finalised before last patient last visit and will be publicly available (eg, Clinical Trial Registry) and/or on request.67 We are not undertaking any interim or stratified analyses.
Ethics and dissemination
Human Research Ethics Committee approval has been granted by Bellberry (2021-08-907). Study findings will be disseminated via scientific peer-reviewed publications, conferences and media, as applicable.
Supplementary Material
Footnotes
Twitter: @lavenderisobel, @NatSleep
Contributors: IL, DM, NM, AS, CI, ALD'R, CJG, RRG, BY, IM and CMH were involved in methodological design and creation of the study protocol. BY and CMH are the principal investigators (medical and non-medical, respectively) who have overall responsibility for the design, conduct and decision to submit for publication. NM is the study epidemiologist and statistician who will design and publish the analysis plan in collaboration with IL. IL is the trial coordinator responsible for collecting trial data. IL drafted the manuscript. Investigator roles and responsibilities are outlined in table 1. All authors have read and approved the final manuscript.
Funding: The study is being funded by the Lambert Initiative for Cannabinoid Therapeutics, within the Brain and Mind Centre at the University of Sydney, a philanthropically funded centre for cannabinoid research. Lambert Initiative for Cannabinoid Therapeutic activities extend from plant science and cannabinoid production, through cellular and preclinical pharmacology, to medicinal chemistry and drug discovery, including human laboratory studies and clinical trials. The decision to fund the study was overseen by a scientific committee known as the Internal Management Group comprising members external to the Lambert Initiative, including the Pro-Vice Chancellor and the Faculty of Science Dean at the University of Sydney. The investigational product was purchased from Medropharm (Zürich, Switzerland) through BOD Australia (Sydney, Australia) who was not involved in the conception, design, nor conduct of the study. IL is supported by a Barry and Joy Lambert Postgraduate Research Scholarship. AS is supported by the Lambert Initiative for Cannabinoid Therapeutics, University of Sydney. CMH is funded by a National Heart Foundation Future Leader Fellowship. ALD'R (2008001) and RRG (1197439) are funded by National Health and Medical Research Council-Australian (NHMRC) Investigator Grants.
Competing interests: IM is the Academic Director of the Lambert Initiative for Cannabinoid Therapeutics, Brain and Mind Centre, University of Sydney. IM is a consultant for Kinoxis Therapeutics, Psylo, and Emyria and is an inventor on several patents relating to novel cannabinoid and non-cannabinoid therapeutics unrelated to insomnia. He has received consulting fees from the Medicinal Cannabis Industry Australia (MCIA) and acts as an expert witness in legal cases involving cannabis-related issues. AS has received consulting fees from the Medicinal Cannabis Industry Australia (MCIA) and Haleon Australia. RRG has received discounted investigational products for an unrelated clinical trial from Neurim Pharmaceuticals and received investigational product and matched placebo from Teva Pharmaceutical in unrelated clinical trials. He has received funding for lectures for Pfizer, Teva, Jazz and Eisai in the past 3 years. The other authors have no competing interests to disclose. The Woolcock Institute Sleep and Chronobiology Research Group has received research support from Avadel, Nyxoah, Idorsa, ResMed, BOD Australia and Philips.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Medicine, A.A.o.S . International Classification of Sleep Disorders. Darien, IL: American Academy of Sleep Medicine, 2014. [Google Scholar]
- 2.Appleton SL, Gill TK, Lang CJ, et al. Prevalence and Comorbidity of sleep conditions in Australian adults: 2016 sleep health foundation national survey. Sleep Health 2018;4:13–9. 10.1016/j.sleh.2017.10.006 [DOI] [PubMed] [Google Scholar]
- 3.Morin CM, Jarrin DC, Ivers H, et al. Incidence, persistence, and remission rates of insomnia over 5 years. JAMA Netw Open 2020;3:e2018782. 10.1001/jamanetworkopen.2020.18782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riemann D, Krone LB, Wulff K, et al. Sleep, insomnia, and depression. Neuropsychopharmacology 2020;45:74–89. 10.1038/s41386-019-0411-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Almondes KM, Costa MV, Malloy-Diniz LF, et al. Insomnia and risk of dementia in older adults: systematic review and meta-analysis. J Psychiatr Res 2016;77:109–15. 10.1016/j.jpsychires.2016.02.021 [DOI] [PubMed] [Google Scholar]
- 6.Streatfeild J, Smith J, Mansfield D, et al. The social and economic cost of sleep disorders. Sleep 2021;44:zsab132. 10.1093/sleep/zsab132 [DOI] [PubMed] [Google Scholar]
- 7.Grima NA, Bei B, Mansfield D. Insomnia management. Austral J Gen Practit 2019;48:198–202. 10.31128/AJGP-12-18-4780 [DOI] [PubMed] [Google Scholar]
- 8.Koffel E, Bramoweth AD, Ulmer CS. Increasing access to and utilization of cognitive behavioral therapy for insomnia (CBT-I): a narrative review. J Gen Intern Med 2018;33:955–62. 10.1007/s11606-018-4390-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matthews EE, Arnedt JT, McCarthy MS, et al. Adherence to cognitive behavioral therapy for insomnia: a systematic review. Sleep Med Rev 2013;17:453–64. 10.1016/j.smrv.2013.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Troxel WM, Conrad TS, Germain A, et al. Predictors of treatment response to brief behavioral treatment of insomnia (BBTI) in older adults. J Clin Sleep Med 2013;9:1281–9. 10.5664/jcsm.3270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crowe SF, Stranks EK. The residual medium and long-term cognitive effects of benzodiazepine use: an updated meta-analysis. Arch Clin Neuropsychol 2018;33:901–11. 10.1093/arclin/acx120 [DOI] [PubMed] [Google Scholar]
- 12.Lu H-C, Mackie K. An introduction to the endogenous Cannabinoid system. Biol Psychiatry 2016;79:516–25. 10.1016/j.biopsych.2015.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lintzeris N, Mills L, Suraev A, et al. Medical Cannabis use in the Australian community following introduction of legal access: the 2018-2019 online cross-sectional Cannabis as medicine survey (CAMS-18). Harm Reduct J 2020;17:37. 10.1186/s12954-020-00377-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corroon J. Cannabinol and sleep: separating fact from fiction. Cannabis Cannabinoid Res 2021;6:366–71. 10.1089/can.2021.0006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hazekamp A, et al. Chemistry of Cannabis. In: Comprehensive Natural Products II. 2010, Science Direct. The Netherlands: Leiden, 2010: 1033–84. [Google Scholar]
- 16.Hartley ML. The ‘sleepy’ Cannabinoid CBN might not actually be Sedating. 2019. Available: https://www.leafly.com/news/health/is-cbn-cannabinoid-sedating
- 17.Full spectrum CBN oil (60Mg/mL). 2022. Available: https://nuleafnaturals.com/product/full-spectrum-cbn-oil-60mg-ml/#:~:text=NuLeaf%20Naturals%20suggests%20a%20starting,Improved%20sleep%20and%20relaxation
- 18.Kesner AJ, Lovinger DM. Cannabinoids, Endocannabinoids and sleep. Front Mol Neurosci 2020;13:125. 10.3389/fnmol.2020.00125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murillo-Rodríguez E. The role of the Cb1 receptor in the regulation of sleep. Prog Neuropsychopharmacol Biol Psychiatry 2008;32:1420–7. 10.1016/j.pnpbp.2008.04.008 [DOI] [PubMed] [Google Scholar]
- 20.Sanford AE, Castillo E, Gannon RL. Cannabinoids and Hamster circadian activity rhythms. Brain Res 2008;1222:141–8. 10.1016/j.brainres.2008.05.048 [DOI] [PubMed] [Google Scholar]
- 21.Murillo-Rodríguez E, Cabeza R, Méndez-Díaz M, et al. Anandamide-induced sleep is blocked by Sr141716A, a Cb1 receptor antagonist and by U73122, a Phospholipase C inhibitor. Neuroreport 2001;12:2131–6. 10.1097/00001756-200107200-00018 [DOI] [PubMed] [Google Scholar]
- 22.Murillo-Rodríguez E, Sánchez-Alavez M, Navarro L, et al. Anandamide modulates sleep and memory in rats. Brain Res 1998;812:270–4. 10.1016/s0006-8993(98)00969-x [DOI] [PubMed] [Google Scholar]
- 23.Steinberg BA, Cannon CP. Cannabinoid-1 receptor blockade in Cardiometabolic risk reduction: safety, tolerability, and therapeutic potential. Am J Cardiol 2007;100:27P–32P. 10.1016/j.amjcard.2007.10.011 [DOI] [PubMed] [Google Scholar]
- 24.Zou S, Kumar U. Cannabinoid receptors and the Endocannabinoid system: signaling and function in the central nervous system. Int J Mol Sci 2018;19:833. 10.3390/ijms19030833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wallach J. Medicinal Cannabis: an overview for health-care providers, in Remington. 2021:75–101.
- 26.Bird KD, Boleyn T, Chesher GB, et al. Intercannabinoid and Cannabinoid-ethanol interactions and their effects on human-performance. Psychopharmacology (Berl) 1980;71:181–8. 10.1007/BF00434409 [DOI] [PubMed] [Google Scholar]
- 27.Perez-Reyes M, Timmons MC, Davis KH, et al. A comparison of the pharmacological activity in man of intravenously administered Delta9-Tetrahydrocannabinol, Cannabinol, and Cannabidiol. Experientia 1973;29:1368–9. 10.1007/BF01922823 [DOI] [PubMed] [Google Scholar]
- 28.Walsh JH, Maddison KJ, Rankin T, et al. Treating insomnia symptoms with medicinal Cannabis: a randomized, crossover trial of the efficacy of a Cannabinoid medicine compared with placebo. Sleep 2021;44:zsab149. 10.1093/sleep/zsab149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gannon W, et al. Novel formulation of THC and CBN in a repeat-action tablet improves objective and subjective measurements of sleep. Am J Endocan Med 2021;3:12–8. [Google Scholar]
- 30.Occelli Hanbury Brown C. The Phytocannabinoid Cannabinol (Cbn). ENHANCES SLEEP AND ALTERS SLEEP ARCHITECTURE IN RATS, IN 32nd Annual Symposium on the Cannabinoids, International Cannabinoid Research Society; Galway, Ireland: Research Triangle Park, NC, USA, 2022 [Google Scholar]
- 31.Berry RB, Brooks R, Gamaldo CE. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications, version 2.6. 0. American Academy of sleep medicine, Darien, Illinois; 2020. most recent scoring manual from the American Academy of sleep medicine (AASM). 2020.
- 32.Therapeutic Goods Administration . Standard for the uniform scheduling of medicines and poisons. Woden, Australia, 2022. [Google Scholar]
- 33.Gong H, Tashkin DP, Simmons MS, et al. Acute and subacute bronchial effects of oral Cannabinoids. Clin Pharmacol Ther 1984;35:26–32. 10.1038/clpt.1984.4 [DOI] [PubMed] [Google Scholar]
- 34.Karniol IG, Shirakawa I, Takahashi RN, et al. Effects of Delta9-Tetrahydrocannabinol and Cannabinol in man. Pharmacology 1975;13:502–12. 10.1159/000136944 [DOI] [PubMed] [Google Scholar]
- 35.Hollister LE. Cannabidiol and Cannabinol in man. Experientia 1973;29:825–6. 10.1007/BF01946311 [DOI] [PubMed] [Google Scholar]
- 36.Chan A-W, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 2013;158:200–7. 10.7326/0003-4819-158-3-201302050-00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.A.A.o.S. Medicine . International Classification of Sleep Disorders, 3rd ed. Darien, IL: American Academy of Sleep Medicine, 2014. [Google Scholar]
- 38.Diagnostic and Statistical Manual of Mental Disorders . Diagnostic and statistical manual of mental disorders: DSM-5, in American Psychiatric Association, 5th edn. Arlington, VA: American Psychiatric Publishing, 22 May 2013. 10.1176/appi.books.9780890425596 [DOI] [Google Scholar]
- 39.Edwards BA, O’Driscoll DM, Ali A, et al. Aging and sleep: physiology and pathophysiology. Semin Respir Crit Care Med 2010;31:618–33. 10.1055/s-0030-1265902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: A systematic review and meta-analysis. JAMA 2015;313:2456–73. 10.1001/jama.2015.6358 [DOI] [PubMed] [Google Scholar]
- 41.Suraev A, Grunstein RR, Marshall NS, et al. “Cannabidiol (CBD) and Delta(9)-Tetrahydrocannabinol (THC) for chronic insomnia disorder ('CANSLEEP' trial): protocol for a randomised, placebo-controlled, double-blinded, proof-of-concept trial”. BMJ Open 2020;10:e034421. 10.1136/bmjopen-2019-034421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bastien CH, Vallières A, Morin CM. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med 2001;2:297–307. 10.1016/s1389-9457(00)00065-4 [DOI] [PubMed] [Google Scholar]
- 43.Johns MW. A new method for measuring daytime Sleepiness: the Epworth Sleepiness scale. Sleep 1991;14:540–5. 10.1093/sleep/14.6.540 [DOI] [PubMed] [Google Scholar]
- 44.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361–70. 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
- 45.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. primary care evaluation of mental disorders. patient health questionnaire. JAMA 1999;282:1737–44. 10.1001/jama.282.18.1737 [DOI] [PubMed] [Google Scholar]
- 46.Berry RB, Gamaldo CE, Harding SM, et al. AASM scoring manual version 2.2 updates: new chapters for scoring infant sleep staging and home sleep apnea testing. J Clin Sleep Med 2015;11:1253–4. 10.5664/jcsm.5176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Akerstedt T, Hume K, Minors D, et al. The subjective meaning of good sleep, an Intraindividual approach using the Karolinska sleep diary. Percept Mot Skills 1994;79:287–96. 10.2466/pms.1994.79.1.287 [DOI] [PubMed] [Google Scholar]
- 48.McCartney D, Suraev AS, Doohan PT, et al. Effects of Cannabidiol on simulated driving and cognitive performance: A dose-ranging randomised controlled trial. J Psychopharmacol 2022;36:1338–49. 10.1177/02698811221095356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huestis MA. Human Cannabinoid pharmacokinetics. Chem Biodivers 2007;4:1770–804. 10.1002/cbdv.200790152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kevin RC, Allsop DJ, Lintzeris N, et al. Urinary Cannabinoid levels during Nabiximols (Sativex®)-Medicated inpatient Cannabis withdrawal. Forensic Toxicol 2017;35:33–44. 10.1007/s11419-016-0330-0 [DOI] [Google Scholar]
- 51.Sahinovic A, Irwin C, Doohan PT, et al. Effects of Cannabidiol on exercise physiology and Bioenergetics: A randomised controlled pilot trial. Sports Med Open 2022;8:27. 10.1186/s40798-022-00417-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kevin RC, Vogel R, Doohan P, et al. A validated method for the simultaneous Quantification of Cannabidiol, Δ(9) -Tetrahydrocannabinol, and their metabolites in human plasma and application to plasma samples from an oral Cannabidiol open-label trial. Drug Test Anal 2021;13:614–27. 10.1002/dta.2947 [DOI] [PubMed] [Google Scholar]
- 53.McCartney D, Kevin RC, Suraev AS, et al. Orally administered Cannabidiol does not produce false-positive tests for Δ9-Tetrahydrocannabinol on the Securetec Drugwipe® 5S or Dräger Drugtest® 5000. Drug Test Anal 2022;14:137–43. 10.1002/dta.3153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McCartney D, Kevin RC, Suraev AS, et al. Orally administered Cannabidiol does not produce false-positive tests for Δ(9) -Tetrahydrocannabinol on the Securetec Drugwipe® 5S or Dräger Drugtest® 5000. Drug Test Anal 2022;14:137–43. 10.1002/dta.3153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Basner M, Mollicone D, Dinges DF. Validity and sensitivity of a brief psychomotor vigilance test (PVT-B) to total and partial sleep deprivation. Acta Astronaut 2011;69:949–59. 10.1016/j.actaastro.2011.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gibson SJ. The measurement of mood States in older adults. J Gerontol B Psychol Sci Soc Sci 1997;52:167–74. 10.1093/geronb/52b.4.p167 [DOI] [PubMed] [Google Scholar]
- 57.Morean ME, de Wit H, King AC, et al. The drug effects questionnaire: Psychometric support across three drug types. Psychopharmacology (Berl) 2013;227:177–92. 10.1007/s00213-012-2954-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shahid A, Wilkinson K, Marcu S, et al. STOP, THAT and one hundred other sleep scales. In: Leeds Sleep Evaluation Questionnaire (LSEQ). New York, NY: Springer, 2012: 211–3. 10.1007/978-1-4419-9893-4 [DOI] [Google Scholar]
- 59.Turci AM, Bevilaqua-Grossi D, Pinheiro CF, et al. The Brazilian Portuguese version of the revised Maastricht upper extremity questionnaire (MUEQ-BR revised): translation, cross-cultural adaptation, reliability, and structural validation. BMC Musculoskelet Disord 2015;16:41. 10.1186/s12891-015-0497-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Putilov AA, Donskaya OG. Construction and validation of the EEG analogues of the Karolinska Sleepiness scale based on the Karolinska drowsiness test. Clin Neurophysiol 2013;124:1346–52. 10.1016/j.clinph.2013.01.018 [DOI] [PubMed] [Google Scholar]
- 61.D’Rozario AL, Dungan GC, Banks S, et al. An automated algorithm to identify and reject Artefacts for quantitative EEG analysis during sleep in patients with sleep-disordered breathing. Sleep Breath 2015;19:607–15. 10.1007/s11325-014-1056-z [DOI] [PubMed] [Google Scholar]
- 62.D’Rozario AL, Hoyos CM, Wong KKH, et al. Improvements in cognitive function and quantitative sleep electroencephalogram in obstructive sleep apnea after six months of continuous positive airway pressure treatment. Sleep 2022;45:zsac013. 10.1093/sleep/zsac013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ogata NG, Daga FB, Jammal AA, et al. Mobile telephone use and reaction time in drivers with glaucoma. JAMA Netw Open 2019;2:e192169. 10.1001/jamanetworkopen.2019.2169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arkell TR, Lintzeris N, Kevin RC, et al. Cannabidiol (CBD) content in Vaporized Cannabis does not prevent Tetrahydrocannabinol (THC)-Induced impairment of driving and cognition. Psychopharmacology (Berl) 2019;236:2713–24. 10.1007/s00213-019-05246-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McCartney D, Arkell TR, Irwin C, et al. Determining the magnitude and duration of acute Δ9-Tetrahydrocannabinol (Δ9-THC)-Induced driving and cognitive impairment: A systematic and meta-analytic review. Neurosci Biobehav Rev 2021;126:175–93. 10.1016/j.neubiorev.2021.01.003 [DOI] [PubMed] [Google Scholar]
- 66.Ellenberg SS, Fleming TR, DeMets DL. Data monitoring in clinical trials: a practical perspective. In: Statistics in practice. 2nd edn. Hoboken, NJ: Wiley, 2019. 10.1002/9781119512684 [DOI] [Google Scholar]
- 67.Gamble C, Krishan A, Stocken D, et al. Guidelines for the content of statistical analysis plans in clinical trials. JAMA 2017;318:2337–43. 10.1001/jama.2017.18556 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.