Abstract
Borna disease virus (BDV) is a neurotropic virus with a broad host and geographic range. Lewis rats were immunized against BDV with a recombinant vaccinia virus expressing the BDV nucleoprotein and were later infected with BDV to evaluate protection against Borna disease (BD). Relative to animals that were not immunized, immunized animals had a decreased viral burden after challenge with infectious virus, more marked inflammation, and aggravated clinical disease. These data suggest that a more robust immune response in Borna disease can reduce viral load at the expense of increased morbidity.
Borna disease (BD) is an immune-mediated neurologic disease affecting a wide range of natural and experimental host species, including rodents, ungulates, and primates (13, 24, 28). BD virus (BDV), the etiological agent of BD, differs from other well-known neuropathogens, such as rabies virus and herpes simplex virus, in its slow, low-level replication (6, 8, 10, 13, 24, 25, 28). In experimentally infected Lewis rats, a well-studied model for BDV pathogenesis, the onset of disease corresponds to the accumulation of inflammatory infiltrates in the central nervous system (CNS) (19). Interestingly, the immune response to BDV fails to clear the infection, the inflammation subsides, and the virus persists at constant levels in the CNS for the life of the host (19). This persistence occurs despite the presence of high levels of neutralizing antibodies in serum and cerebrospinal fluid (12, 19, 26), suggesting that antibody-mediated clearance does not contribute significantly to the immunopathogenesis of BD. Persistence of infectious BDV in the absence of apparent immunosuppression is a fascinating feature of BDV molecular biology and immunology. A desire to understand the mechanisms underlying disease severity as well as potential avenues of disease prevention prompted this investigation.
At the most 3′ end of the nonsegmented negative-strand RNA BDV genome is an open reading frame (ORF) encoding the nucleoprotein (N) (1, 4, 7). N is the most abundant BDV protein in infected cells and elicits a strong cellular and humoral immune response in infected hosts (3, 16). Because both cellular and humoral immune responses would be expected to play a role in limitation of viral spread and clearance, a vaccinia virus (VV) was chosen as a vector for immunization. Vaccination with a VV construct expressing a nucleocapsid protein has proven effective in other viral systems (2, 9, 14, 18). The success of other nucleocapsid-based vaccine systems coupled with the abundance and immunogenicity of N supported the selection of N for our first vaccination trial.
A VV construct encoding BDV N (VV-N) was created by introducing the N ORF of BDV strain He/80 (27) into the thymidine kinase gene of wild-type VV by homologous recombination (17). After verification of correct insertion of the N ORF into VV by sequencing, expression was analyzed following infection of HeLa cells. A VV construct containing non-BDV sequence derived from the transfer plasmid pSC11, VVsc, was generated for use as a control for the specificity of the immune response to VV-N (29). Western blot analysis of extracts from HeLa cells infected with VV-N and VVsc demonstrated the presence of an approximately 38-kDa protein that was immunoreactive with rabbit monospecific sera and was seen in cells infected with VV-N, but not in cells infected with VVsc (data not shown).
VV-N and VVsc provided the experimental and control vectors, respectively, for the following immunization strategy. Twelve 4-week-old male Lewis rats (Charles River) were given intraperitoneal inoculations with 2 × 107 PFU of VV-N (immunized [Imm] group). Control animals (not-immunized [NI] group) received either 2 × 107 PFU of VVsc (n = 12) or phosphate-buffered saline (PBS) (n = 12). Six weeks later, all animals were given an intranasal challenge of 5 × 104 focus-forming units (FFU) of BDV. Animals were observed for development of clinical signs of disease. Sera were collected from animals immediately before and 2 and 6 weeks after VV inoculation. Tissue and sera were also collected at the time of sacrifice (14, 21, 31, or 36 days following challenge with BDV).
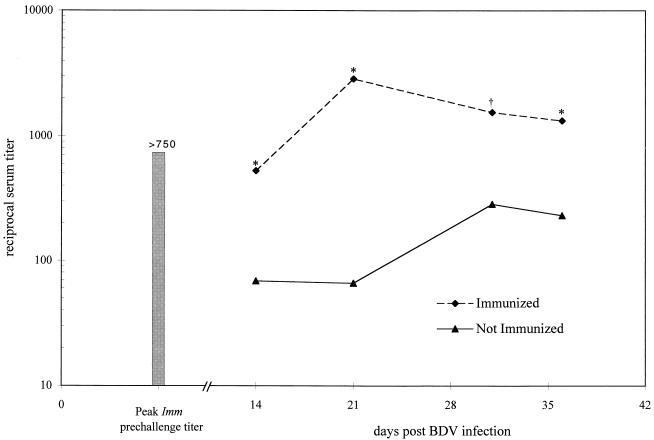
The role of antibodies in the natural progression of BD is unclear. Passive transfer of sera from BDV-infected animals has been unsuccessful in preventing or altering development of disease (19, 21). Following VV-N inoculation, the antibody response to N was determined by enzyme-linked immunosorbent assay (ELISA), as previously described (3), as an indicator of successful immune priming. The majority of VV-N-receiving animals (10 of 13 [Imm]) demonstrated the presence of antibodies to N with a titer greater than or equal to 1:120, with an average titer of 1:750 at 2 weeks after VV-N inoculation (data not shown). Those animals that lacked a significant response (titers of <1/120 at 2 weeks post-VV-N inoculation) were considered not primed and thus were eliminated from further analysis. The anti-N titer in the Imm group declined to less than 1:120 at 6 weeks following VV-N administration, prior to BDV challenge (data not shown). Following infection with BDV, immunized animals showed a rise in serum N antibody titer to levels of greater than 1:2,500. Animals in the NI group, i.e., those receiving VVsc or PBS prior to BDV challenge, showed a slow rise in N-reactive antibody titer consistent with a primary response with peak levels of approximately 1:300 (Fig. 1). These results indicate successful priming of a humoral immune response to N.
FIG. 1.
Infection with VV-N elicits an antibody response. Sera from NI and Imm animals were analyzed by ELISA with recombinant N. Titers of antibody to N were determined at 14, 21, 31, and 36 days following administration of the BDV challenge. A significant difference in titer for those animals immunized compared to those not immunized is indicated by ∗ for P < 0.05 and † for P < 0.005 by Student’s t test.
After verification of successful induction of an immune response by demonstrating antibody production, the effects of immunization on levels of viral RNA and infectious virus were determined by in situ hybridization (ISH) and viral titration, respectively. ISH was performed for detection of viral genomic RNA or mRNAs containing the ORFs for either N or phosphoprotein (P) by using single-stranded RNA probes as previously described (15). ISH with probes for N and P mRNAs and genomic RNAs yielded patterns that differed only in intensity (N>P≫genome), not in distribution. Representative panels are shown in Fig. 2. At day 14, the ISH signal distribution and intensity were similar between the Imm and NI groups. However, later time points showed dramatic decreases in all of the analyzed viral RNAs in the Imm group compared to those in the NI group. (Fig. 2).
FIG. 2.
Immunization results in a decrease in BDV nucleic acids. Brain sections from NI and Imm animals at 14, 21, 31, and 36 days after BDV infection were analyzed by ISH with single-stranded RNA probes for detection of BDV N protein mRNA and genomic RNA. The distribution of signal obtained with each probe was indistinguishable. Results from representative sections probed for genomic RNA are shown.
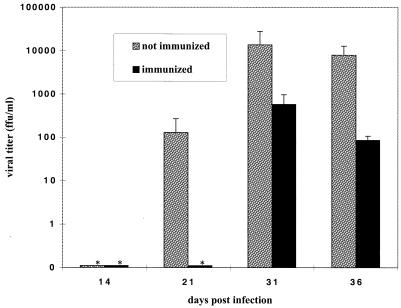
As an additional measure of viral productivity, titers of infectious virus in brain homogenates were determined as previously described (22). Levels of virus determined by direct titration were consistent with ISH results for viral RNA. At all time points at which viral titers were above the limits of detection, titers were greater in the NI group. The peak viral titer in the Imm group was 575 FFU/ml, compared to 14,000 FFU/ml in the NI group (Fig. 3). Decreased levels of viral RNA and infectious virus may be accounted for by two different mechanisms: decreased virus production and spread or increased clearance from or lysis of infected cells. Vaccination may induce these mechanisms alone or in concert to reduce viral load.
FIG. 3.
Infectious virus is reduced in brains of immunized animals. Serial dilutions of a 20% brain homogenate of each NI and Imm animal were added to a monolayer of rabbit fetal glial cells to determine viral infectivity in FFU per milliliter. At each time point, n = 6 for the NI group and n = 2 for the Imm group. An asterisk indicates that no infectious virus was detected. Error bars indicate the standard error of the mean.
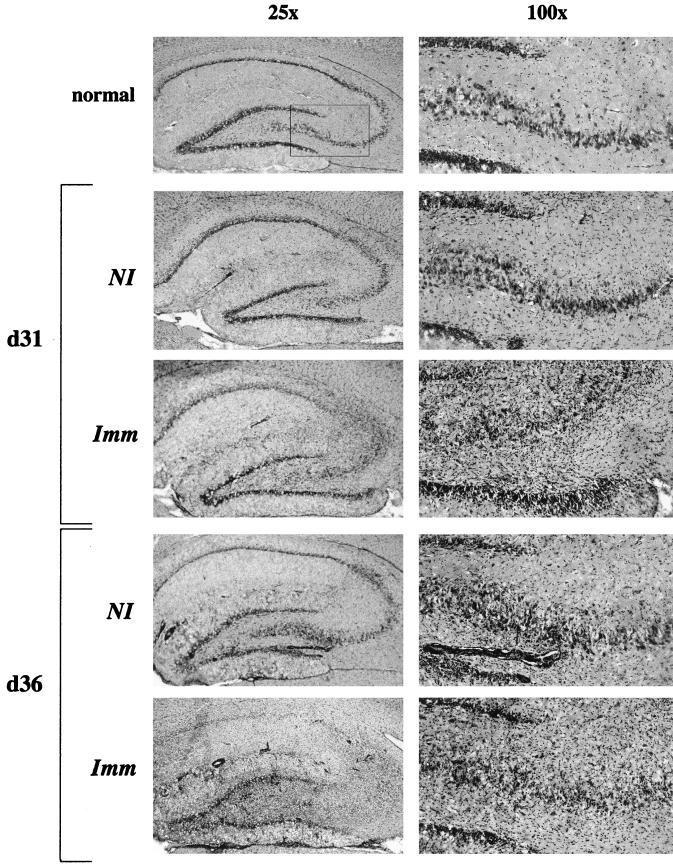
Analysis of hematoxylin-and-eosin-stained brain sections revealed mononuclear cell infiltration in the Imm group in both the parenchyma and around blood vessels, becoming prominent by day 31 (Fig. 4). Although perivascular cuffing was visible in NI group brains at days 31 and 36, parenchymal infiltration was less pronounced. Representative sections of hippocampus in Fig. 4 demonstrate enhanced inflammation and marked distortion of normal hippocampal architecture (Fig. 4, day 31, Imm versus NI).
FIG. 4.
Inflammatory infiltration is increased in Imm brains. Brain sections from NI and Imm animals were stained with hematoxylin and eosin to evaluate inflammatory infiltration. Representative sections from the hippocampus from Imm and NI animals 31 and 36 days post-BDV infection are shown. Photomicrographs were taken of sections of the hippocampal formation at magnifications of ×25 and ×100 as indicated. Note that, in the immunized sections, the hippocampal architecture is disrupted by massive parenchymal infiltration of inflammatory cells.
The pathogenic relevance of T cells to BD has been well established (28). Of particular interest to this investigation was the finding that adoptive transfer of a BDV-N-specific CD4+ cell line can induce prevention or enhancement of the immunopathological disease, with the outcome dependent on the timing of transfer relative to infection (23). The composition of infiltrating inflammatory cells, levels of expression of major histocompatibility, complex (MHC) class I and class II antigens, and the distribution of immunoglobulin G (IgG) were assessed immunohistochemically (11) at days 31 and 36 in the brains of Imm and NI animals (Table 1). In general, total numbers of T cells, CD4 cells, CD8 cells, and NK cells were higher in Imm brains than in NI brains at both days 31 and 36 postinfection. The difference between Imm and NI brains was more marked in parenchymal than in meningeal or perivascular infiltrates. Interestingly, numerous microglia were stained by antibodies to Ox 8, a marker for CD8 cells. The numbers of activated microglia were similar in Imm and NI brains. Higher levels of MHC class I and class II antigen expression were observed in Imm brains than in NI brains at day 31; the levels were similar in Imm and NI brains at day 36. Levels of IgG were higher in neuropil in Imm animals.
TABLE 1.
Immunohistochemical characterization of immune infiltrates and expression of MHC antigens and intracerebral IgG
| Antibody | Location | Result for groupa
|
||||
|---|---|---|---|---|---|---|
| NL | NI
|
Imm
|
||||
| 31 dpib | 36 dpi | 31 dpi | 36 dpi | |||
| R 7.3 (α/β T-cell receptor) | Meninges | − | ++ | +++ | +++ | +++ |
| Perivascular | − | ++ | ++ | +++ | +++ | |
| Neuropil | − | + | ++ | +++ | +++ | |
| W 3/25 (CD4) | Meninges | − | ++ | ++ | +++ | +++ |
| Perivascular | − | + | ++ | ++ | ++ | |
| Neuropil | − | + | ++ | ++ | ++ | |
| Ox 8 (CD8)c | Meninges | − | ++ | ++ | ++ | +++ |
| Perivascular | − | ++ | ++ | ++ | ++ | |
| Neuropil | − | + | ++ | +++ | +++ | |
| 3.2.3. (NK cells) | Meninges | − | + | ++ | + | ++ |
| Perivascular | − | + | + | + | ++ | |
| Neuropil | − | + | + | ++ | ++ | |
| Anti-IgGd | Meninges | + | ++ | +++ | ++ | +++ |
| Perivascular | + | ++ | +++ | ++ | +++ | |
| Neuropil | − | + | ++ | ++ | +++ | |
| Ox 42 (activated microglia) | − | ++ | +++ | ++ | +++ | |
| I1.69 (MHC class I)e | − | ++ | +++ | +++ | +++ | |
| Ox 6 (MHC class II)f | − | ++ | +++ | +++ | +++ | |
NL, normal (noninfected control); NI, BDV-infected, not vaccinated with BDV protein; Imm, BDV-infected, vaccinated with BDV N.
dpi, days postinfection.
Staining of microglia as well as T cells.
Pronounced meningeal and perivascular staining; focal diffuse parenchymal staining in olfactory cortex, hippocampus, thalamus, and striatum; focal cytoplasmic staining of neurons in hippocampus.
Staining of microglia, astrocytes, endothelial cells, inflammatory cells, and occasional neurons.
Staining of microglia, astrocytes, and inflammatory cells.
Clinical scores were assigned to animals by two independent observers, with one point given for the presence of disheveled fur, dystonia, weakness, or paresis. The score for each animal was the average of the scores of the two observers. Both Imm and NI animals showed evidence of illness after day 25. Following onset of clinical signs, the severity of disease increased more rapidly in the Imm group, with the animals becoming moribund and requiring euthanasia at day 36 (Fig. 5). The aggravation in clinical disease was temporally associated with an increase in mononuclear infiltration (Fig. 4 and 5). It was anticipated that inflammation and clinical signs might appear earlier in the Imm group relative to the NI group; however, the onset of clinical symptoms was unchanged (Fig. 5). The 2- to 4-week latency period for disease onset has been observed for all routes of infection (5). Potential factors influencing latency to disease include the total viral burden, anatomical distribution of virus, and potency of the immune response. In the recombinant VV trial presented here, two impacts were plausible: prolongation secondary to the reduced virus titer and shortening secondary to the enhanced strength of the immune response. The unchanged latency in this study may represent a sum of these opposing effects.
FIG. 5.
BD is exacerbated in Imm animals. Animals were evaluated and given 1 point each for the presence of disheveled fur, dystonia, weakness, and paresis. Scores for animals in each group were averaged. Bars represent 4 to 18 animals/group, with the exception of the final 5 days, at which time, n = 2 for the Imm group.
Disease exacerbation following immune priming has previously been observed with the T-cell-mediated choriomeningitis caused by lymphocytic choriomeningitis virus (LCMV) (20). In the LCMV system, the balance between immune response and viral spread determines protection from versus exacerbation of disease. Similarly, in BDV, it appears that the relative kinetics of viral replication and spread versus the progression and maturation of the immune response determines the latency, expression, and severity of disease.
An ideal vaccine increases the immune response to a pathogen to limit its replication and spread, thereby lessening or eliminating the disease without causing adverse effects. In this experimental paradigm, immune priming with VV-N resulted in a limitation of viral productivity at the expense of enhanced immune cell infiltration of the CNS and an exacerbation of disease.
Priming of the immune response to N resulted in a substantial reduction in viral gene expression without improving the clinical course of BD. Vaccination strategies aimed at pathogens that cause immune-mediated CNS disease present unique challenges for the achievement of enhanced immune responses that lead to protection without exacerbation of disease. A greater understanding of the factors controlling BD latency, viral gene expression, viral spread, and host strain differences will be critical to establishing effective vaccines for BDV.
Acknowledgments
We thank Uyen Ngo for superb technical assistance and Bill Hickey and Marylou Solbrig for valuable comments.
Support for this project was provided by the M.D.-Ph.D. program at the University of California—Irvine (A.J.L.) and NIH awards NS29425 (W.I.L., C.G.H.) and AI-27028 (J.L.W.). H.W. is the recipient of an Erwin Schrödinger Stipend from the Republic of Austria.
REFERENCES
- 1.Banerjee A K, Barik S, De B P. Gene expression of nonsegmented negative strand RNA viruses. Pharmacol Ther. 1991;51:47–70. doi: 10.1016/0163-7258(91)90041-j. [DOI] [PubMed] [Google Scholar]
- 2.Bankamp B, Brinckmann U G, Reich A, Niewiesk S, ter Meulen V, Liebert U G. Measles virus nucleocapsid protein protects rats from encephalitis. J Virol. 1991;65:1695–1700. doi: 10.1128/jvi.65.4.1695-1700.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Briese T, Hatalski C G, Kliche S, Park Y-S, Lipkin W I. Enzyme-linked-immunosorbent assay for detecting antibodies to Borna disease virus-specific proteins. J Clin Microbiol. 1995;33:348–351. doi: 10.1128/jcm.33.2.348-351.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Briese T, Schneemann A, Lewis A J, Park Y-S, Kim S, Ludwig H, Lipkin W I. Genomic organization of Borna disease virus. Proc Natl Acad Sci USA. 1994;91:4362–4366. doi: 10.1073/pnas.91.10.4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carbone K M, Duchala C S, Griffin J W, Kincaid A L, Narayan O. Pathogenesis of Borna disease in rats: evidence that intra-axonal spread is the major route for virus dissemination and the determinant for disease incubation. J Virol. 1987;61:3431–3440. doi: 10.1128/jvi.61.11.3431-3440.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carbone K M, Rubin S A, Sierra-Honigmann A M, Lederman H M. Characterization of a glial cell line persistently infected with Borna disease virus (BDV): influence of neurotrophic factors on BDV protein and RNA expression. J Virol. 1993;67:1453–1460. doi: 10.1128/jvi.67.3.1453-1460.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cubitt B, Oldstone C, de la Torre J C. Sequence and genome organization of Borna disease virus. J Virol. 1994;68:1382–1396. doi: 10.1128/jvi.68.3.1382-1396.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de la Torre J C. Molecular biology of Borna disease virus: prototype of a new group of animal viruses. J Virol. 1994;68:7669–7675. doi: 10.1128/jvi.68.12.7669-7675.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Endo A, Itamura S, Iinuma H, Funahashi S, Shida H, Koide F, Nerome K, Oya A. Homotypic and heterotypic protection against influenza virus infection in mice by recombinant vaccinia virus expressing the haemagglutinin or nucleoprotein of influenza virus. J Gen Virol. 1991;72:699–703. doi: 10.1099/0022-1317-72-3-699. [DOI] [PubMed] [Google Scholar]
- 10.Gosztonyi G, Ludwig H. Borna disease—neuropathology and pathogenesis. Curr Top Microbiol Immunol. 1995;190:39–73. [PubMed] [Google Scholar]
- 11.Hatalski C G, Hickey W F, Lipkin W I. Evolution of the immune response in the central nervous system during experimental Borna disease. J Neuroimmunol. 1998;90:137–142. doi: 10.1016/s0165-5728(98)00076-9. [DOI] [PubMed] [Google Scholar]
- 12.Hatalski C G, Kliche S, Stitz L, Lipkin W I. Neutralizing antibodies in Borna disease virus-infected rats. J Virol. 1994;69:741–747. doi: 10.1128/jvi.69.2.741-747.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hatalski C G, Lewis A J, Lipkin W I. Borna disease. Emerg Infect Dis. 1997;3:129–135. doi: 10.3201/eid0302.970205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klavinskis L S, Whitton J L, Oldstone M B A. Molecularly engineered vaccine which expresses an immunodominant T-cell epitope induces cytotoxic T lymphocytes that confer protection from lethal virus infection. J Virol. 1989;63:4311–4316. doi: 10.1128/jvi.63.10.4311-4316.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lipkin W I, Travis G H, Carbone K M, Wilson M C. Isolation and characterization of Borna disease agent cDNA clones. Proc Natl Acad Sci USA. 1990;87:4184–4188. doi: 10.1073/pnas.87.11.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ludwig H, Bode L, Gosztonyi G. Borna disease: a persistent disease of the central nervous system. Prog Med Virol. 1988;35:107–151. [PubMed] [Google Scholar]
- 17.Mackett M, Smith G L, Moss B. General method for production and selection of infectious vaccinia virus recombinants expressing foreign genes. J Virol. 1984;49:857–864. doi: 10.1128/jvi.49.3.857-864.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrison H G, Bauer S P, Lange J V, Esposito J J, McCormick J B, Auperin D D. Protection of guinea pigs from Lassa fever by vaccinia virus recombinants expressing the nucleoprotein or the envelope glycoproteins of Lassa virus. Virology. 1989;171:179–188. doi: 10.1016/0042-6822(89)90525-4. [DOI] [PubMed] [Google Scholar]
- 19.Narayan O, Herzog S, Frese K, Scheefers H, Rott R. Behavorial disease in rats caused by immunopathological responses to persistent Borna virus in the brain. Science. 1983;220:1401–1403. doi: 10.1126/science.6602380. [DOI] [PubMed] [Google Scholar]
- 20.Oehen S, Hengartner H, Zinkernagel R M. Vaccination for disease. Science. 1991;251:195–198. doi: 10.1126/science.1824801. [DOI] [PubMed] [Google Scholar]
- 21.Oldach D, Zink M C, Pyper J M, Herzog S, Rott R, Narayan O, Clements J E. Induction of protection against Borna disease by inoculation with high-dose-attenuated Borna disease virus. Virology. 1995;206:426–434. doi: 10.1016/s0042-6822(95)80058-1. [DOI] [PubMed] [Google Scholar]
- 22.Pauli G, Grunmach J, Ludwig H. Focus-immunoassay for Borna disease virus-specific antigens. Zentbl Vetmed Reihe B. 1984;31:552–557. doi: 10.1111/j.1439-0450.1984.tb01336.x. [DOI] [PubMed] [Google Scholar]
- 23.Richt J A, Stitz L, Wekerle H, Rott R. Borna disease, a progressive meningoencephalomyelitis as a model for CD4+ T cell-mediated immunopathology in the brain. J Exp Med. 1989;170:1045–1050. doi: 10.1084/jem.170.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rott R, Becht H. Natural and experimental Borna disease in animals. Curr Top Microbiol Immunol. 1995;190:17–30. doi: 10.1007/978-3-642-78618-1_2. [DOI] [PubMed] [Google Scholar]
- 25.Schneemann A, Schneider P A, Lamb R A, Lipkin W I. The remarkable coding strategy of Borna disease virus: a new member of the nonsegmented negative strand RNA viruses. Virology. 1995;210:1–8. doi: 10.1006/viro.1995.1311. [DOI] [PubMed] [Google Scholar]
- 26.Schneider P A, Hatalski C G, Lewis A J, Lipkin W I. Biochemical and functional analysis of the Borna disease virus G protein. J Virol. 1997;71:331–336. doi: 10.1128/jvi.71.1.331-336.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schneider P A, Briese T, Zimmermann W, Ludwig H, Lipkin W I. Sequence conservation in field and experimental isolates of Borna disease virus. J Virol. 1994;68:63–68. doi: 10.1128/jvi.68.1.63-68.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stitz L, Dietzschold B, Carbone K M. Immunopathogenesis of Borna disease. Curr Top Microbiol Immunol. 1995;190:75–92. doi: 10.1007/978-3-642-78618-1_5. [DOI] [PubMed] [Google Scholar]
- 29.Whitton J L, Southern P J, Oldstone M B. Analyses of the cytotoxic T lymphocyte responses to glycoprotein and nucleoprotein components of lymphocytic choriomeningitis virus. Virology. 1988;162:321–327. doi: 10.1016/0042-6822(88)90471-0. [DOI] [PubMed] [Google Scholar]