Abstract
Background
Distal upper limb tremor during walking (TW) is frequently observed in Parkinson's disease (PD) but its clinical features are unknown.
Objective
To characterize the occurrence and the clinical features of TW in comparison to the other types of tremors in PD.
Methods
Fifty‐one PD patients with rest tremor were evaluated off‐ and on‐treatment. Occurrence, body distribution, severity and latency of TW and of other tremor types were assessed.
Results
TW was present in 78% of the PD patients examined. TW body distribution and severity were similar to those of rest and re‐emergent tremor but different from the postural tremor presented by the same patients. TW latency, observed in 85% of patients, was on average 5.8 s. Dopaminergic treatment significantly improved TW, rest, and re‐emergent tremor severity but left TW latency unaffected.
Conclusions
TW is a frequent motor sign in PD and is likely a clinical variant of rest tremor.
Keywords: tremor during walking, Parkinson's disease, gait, tremor, neurodegenerative disease
Patients with Parkinson's disease (PD) may show various types of distal upper limb tremor. 1 , 2 , 3 , 4 , 5 , 6 Typically, PD tremor is present at rest, and it disappears when the patient performs a voluntary movement (rest tremor). 2 , 7 , 8 , 9 Tremor can also be observed while holding an upper limb posture. In this case tremor can be present after a variable delay (re‐emergent tremor) or can appear immediately after reaching and holding a posture (postural tremor). 10 , 11 , 12 Although both tremors appear during posture holding, re‐emergent and postural tremor differ for the pathophysiological and clinical features. 7 , 12 , 13
In PD, distal upper limb tremor is often observed during walking (TW). 14 In this case, TW appears during automatic upper limbs movements differing therefore from both rest condition and voluntary movement execution. To date, no studies have specifically investigated TW occurrence, clinical features and relationship with other tremors in patients with PD.
The present study aims to characterize the occurrence and clinical features of TW (amplitude, body distribution, latency, response to dopaminergic treatment) compared to other types of tremors present in a cohort of 51 PD patients, tested off‐ and on‐treatment.
Methods
Fifty‐one PD patients were consecutively enrolled. PD patients were diagnosed according to international diagnostic criteria and had rest tremor. 15 The disease stage was assessed using the Hoehn and Yahr scale (H&Y), 16 whereas the severity of motor symptoms through the Movement Disorder Society‐sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS—UPDRS) part III. 17 Patients were tested off‐treatment as well as 60 minutes after taking their usual dopaminergic treatment (on‐treatment evaluation). TW was defined as a tremor appearing immediately or after a variable time interval in the upper limbs of a patient who was asked to walk along a linear path with the arms relaxed for 120 seconds. In all patients, we evaluated the presence of rest, re‐emergent and postural tremor. All patients underwent a standardized video recording and the presence and severity of various forms of tremor were evaluated off‐ and on‐treatment. Other details on the assessment and study protocol are in the Supplementary materials.
Statistical Analysis
We used SPSS software (SPSS Inc., Chicago, IL, USA) version 25 for the statistical analysis. Descriptive analysis techniques were used for demographic and clinical data. The student's t‐test, the Mann Whitney U test or the Wilcoxon signed‐rank test were adopted in the univariate analysis as appropriate tools for comparing clinical characteristics of different types of tremors. (See Supplementary materials).
Results
Demographic and Clinical Features of PD Patients
The patients’ demographic and clinical features are reported in Table S1. and in the Supplementary materials.
TW Occurrence, Body Distribution, Severity, Latency and Response to Dopaminergic Treatment
Occurrence: TW was present in 40/51 (78%) patients when evaluated off treatment and in 31/51 (61%) on treatment (See Table 1, Fig. 1).
TABLE 1.
Occurrence and Clinical Features of Rest Tremor (REST), Re‐Emergent Tremor (RET), Postural Tremor (PT) and Tremor During Walking (TW) off Treatment and on Treatment
| Off treatment evaluation | On treatment evaluation | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Occurrence (%) | Mean amplitude | Mean latency (s) | Body distribution (%) | Occurrence (%) | Mean Amplitude | Mean latency (s) | Body distribution (%) | |||||
| Right | Left | Bilateral | Right | Left | Bilateral | |||||||
|
REST |
100 | 2.25 ± 0.7 | ‐ | 35.3 | 39.2 | 25.5 | 94 | 1.8 ± 0.8 | ‐ | 38.8 | 42.9 | 18.3 |
| RET | 63 | 2.15 ± 0.8 | 6.2 ± 3.6 | 36.4 | 54.5 | 9.1 | 47 | 1.54 ± 1.1 | 8.6 ± 5.2 | 29.2 | 58.3 | 12.5 |
|
PT |
21 | 1.4 ± 0.5 | ‐ | 18.2 | 18.2 | 63.6 | 18 | 1.2 ± 0.4 | ‐ | 25 | 12.5 | 62.5 |
| TW | 78 | 2.4 ± 0.7 | 5.8 ± 7.7 | 42.5 | 42.5 | 15 | 61 | 1.9 ± 1.1 | 5.5 ± 4.3 | 38.7 | 48.3 | 13 |
Figure 1.
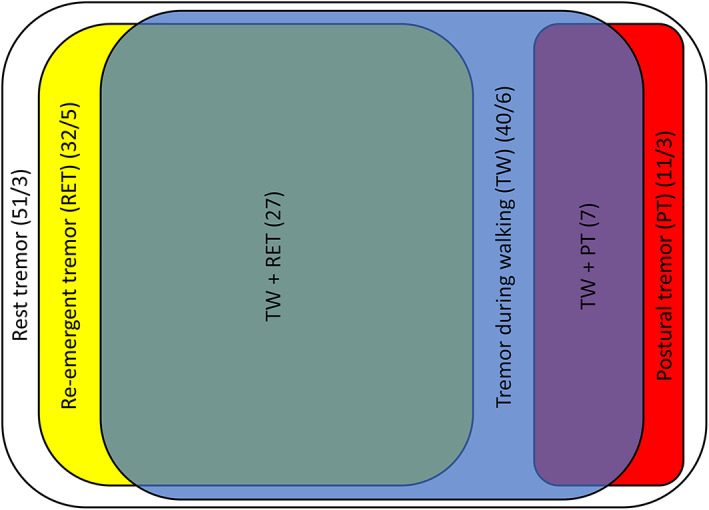
Occurrence of different types of tremors during off‐treatment. Rest tremor was present in all patients. In the white square 51 indicates the total number of patients with rest tremor and 3 indicates the number of patients with isolated rest tremor. In the yellow square 32 indicates the total number of patients with re‐emergent and 5 indicates the number of patients with only re‐emergent. In the blue square, 40 indicates the total number of patients with tremor during walking and 6 indicates the number of patients with only tremor during walking. In red square, 11 indicates the total number of patients with postural tremor and 3 the number of patients with only postural tremor. In the green square, 27 indicates patients with both re‐emergent tremor and tremor during walking. In the purple square, 7 indicates patients with both postural tremor and tremor during walking.
Body distribution: when patients were evaluated off‐treatment, TW was unilateral in 34 patients out of 40 (right side: 17 patients; left side: 17 patients) and bilateral in 6 of 40 patients. TW had the same body distribution (same side when unilateral or bilateral distribution for both tremors) of rest tremor in 95% (38/40) of cases, re‐emergent tremor in 58% (23/40) of patients and postural tremor in 8% (3/40) of cases. In 40 out of 41 patients, TW was found in the most affected body side in terms of bradykinesia and rigidity. In the on‐treatment evaluation, TW was unilateral in 27 patients and bilateral in 4 out of 31 patients (right side: 12 patients; left side: 15 patients). TW had the same body distribution of rest tremor in 94% (29/31) of cases, re‐emergent tremor in 52% (16/31) of patients and postural tremor in 6% (2/31) of cases. In 39 out of 40 patients, TW was found in the most affected body side in terms of bradykinesia and rigidity (See Table 1).
Severity: TW mean severity was 2.4 ± 0.7 off‐treatment and 1.9 ± 1.1 on‐treatment. TW severity was higher than postural tremor (U = 58.50; P < 0.001) but similar to rest tremor (P = 0.25; t = 1.134, df = 88) and re‐emergent tremor severity (P = 0.12; t = 1.589, df = 71) when evaluated off‐treatment. Similarly, when evaluated on‐treatment, TW severity was similar to the severity of rest tremor (P = 0.58; t = 0.547; df = 89) and re‐emergent tremor (P = 0.15; t = 1.421; df = 71) but higher than for postural tremor (P = 0.003; t = 3.030; df = 49). Dopaminergic treatment induced a significant TW (P = 0.0005; t = 3.779; df = 39), rest tremor (P < 0.0001; t = 4.788; df = 49) and re‐emergent tremor improvement (P = 0.0003; t = 4.070; df = 32), whereas postural tremor amplitude remained unchanged after dopaminergic treatment (W = −15; P = 0.0625) (See Fig. S1.; Table 1).
Latency: TW started immediately after walking in 6 out of 40 and appeared after variable latency (ranging from 1 to 40 seconds) in 34/40 patients (85%). TW mean latency was 5.8 ± 7.7 seconds (range: 0–40 seconds) off‐treatment and 5.5 ± 4.3 seconds (range: 0–42 seconds) on‐treatment. Tremor started with latency in 85% of patients with TW and re‐emergent tremor, and in 57% of patients with TW and postural tremor. In the off‐treatment assessment, in patients with TW and re‐emergent tremor the mean latency of re‐emergent tremor (6.7 ± 3.66 seconds) was similar to TW latency (6.5 ± 5.91 seconds) (P = 0.88; t = 0.146; df = 46). Conversely, in the on‐treatment evaluation on the same group of patients, the mean latency of TW (5.1 ± 3.33 seconds) was significantly lower than that of re‐emergent tremor (9.1 ± 5.54 seconds) (P < 0.0117; t = 2.658; df = 36). Based on the three clinical evaluations, we observed that the coefficient of variation was 9.2% for re‐emergent tremor latency and 28.9% for tremor during walking latency. TW during walking and relative latency were consistently present in every measurement. Dopaminergic treatment did not significantly modify TW latency (W = 96; P = 0.09). Contrarily, re‐emergent tremor latency was significantly prolonged by dopaminergic treatment (W = 136; P = 0.001) (See Fig. S2.).
Discussion
In the present study, TW was the most frequent tremor after rest tremor, being present in 78% of PD patients in the off‐state. TW body distribution and severity were similar to those of rest and re‐emergent tremor and different from those of postural tremor. TW started after a variable delay in 85% of patients. Dopaminergic treatment significantly improved TW as well as rest tremor and re‐emergent tremor but left postural tremor unchanged.
We took several precautions to exclude methodological biases. To minimize selection bias, we consecutively recruited all PD patients who met the international diagnostic criteria and had rest tremor. In addition, video recordings of the different types of tremors were separately evaluated by two different movement disorders specialists and satisfactory inter‐rater reliability was observed for all tremor items. Finally, to exclude any confounding effects due to the patients’ regular dopaminergic treatment, the clinical assessment in off‐condition was performed at least 24 hours after treatment withdrawal.
In our study, TW represented the most frequent form of tremor observed in PD patients after rest tremor. TW has a similar occurrence to rest tremor, suggesting that TW is a form of rest tremor facilitated by walking, similar to what happens with emotional or cognitive stress. 18 , 19 , 20 , 21 In line with this hypothesis, we observed that TW had a similar body distribution, severity and response to treatment of rest tremor as well as re‐emergent tremor, that is now considered as a clinical variant of rest tremor. 11 , 12 , 22 , 23 TW is unique for the motor context in which it occurs. Human walking, a complex behavior requiring the integration of multiple signals, is accompanied by automatically controlled movement processes, such as the adjustment of postural muscle or arm swings with the motion of the opposite leg. 24 , 25 Then, unlike re‐emergent and postural tremor that occur during a voluntary upper limb movement, TW occurs during automatic upper limb movements. Thus, TW may be a form of rest tremor occurring during automatic movements. In line with our hypothesis, it has been suggested that the simulation of walking in the sitting position facilitates the appearance of rest tremor in parkinsonian patients, possibly by activating common brain areas involved in gait control and tremor generation. 26
In recent years, clinical and neurophysiological studies have pointed out that rest and re‐emergent tremor might represent different manifestations of a single underlying tremor mechanism 11 , 12 , 22 , 23 and configure a clinical spectrum currently defined as “tremor of stability” ie a PD tremor appearing when a stable condition (resting state, posture maintaining) is reached. 13 We now propose that TW may belong to this clinical continuum. In line with this, TW co‐localization with the most bradykinetic side may be due to an abnormal “stability” from reduced arm swing during walking. This hypothesis is, however, speculative and needs to be validated by future neurophysiological studies, providing an objective measurement of TW and arm swing.
Similarly to re‐emergent tremor, TW had a variable latency. As previously demonstrated, dopaminergic treatment increased re‐emergent tremor latency 27 but left unchanged TW. The different responses to treatment may be explained by the tremor‐related activity of non‐dopaminergic brain regions, 28 such as the cerebellum, which plays a critical role in walking. However, further studies are needed to confirm this hypothesis.
We acknowledge some limitations. The major limitation of the present study is the lack of an objective measurement of TW and other PD tremors parameters. This limited the accuracy of the evaluation of clinical features. Future neurophysiological studies are needed to confirm the present findings. In addition, we tested every patient 60 minutes after L‐Dopa intake and all patients showed clinical improvement, reaching their ON state. However, given we performed only one on‐treatment clinical assessment, we cannot fully ensure that they were at their best MDS‐UPDRS score after 60 minutes.
To conclude, this is the first study specifically evaluating the clinical characteristics of TW in PD. Including TW examination may increase the sensitivity to identify and assess distal upper limb tremor in PD since rest tremor is subject to variability due to difficulties in relaxing, especially in the elders, 29 and emotional factors. 17 , 18 , 19 Our clinical evaluation suggests that TW is present in most PD patients with rest tremor and shares common clinical features with rest and re‐emergent tremor. If pathophysiological mechanisms underlying TW and those of rest and re‐emergent tremor are similar remains an open question that will need to be clarified by future neurophysiological and neuroimaging studies.
Author Roles
(1) Research project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the first draft, B. Review and Critique.
M.C.: 1A, 1B, 1C; 2A, 2B, 2C; 3A, 3B
C.C.: 1A, 1B, 1C; 2A, 2B, 2C
G.L.: 1A; 2A, 2B; 3A
M.I.B.: 1B, 1C
A.F.: 1B, 1C
G.V.: 2C; 3B
A.C.: 1A; 2A, 2C; 3B
G.F.: 1A; 2A, 2C; 3B
A.B.: 1A; 2A, 2C; 3B
D.B.: 1A, 1B; 2A, 2B, 2C; 3A, 3B
Disclosures
Ethical Compliance Statement: The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the local institutional review board (Sapienza University of Rome Ethics Committee, No. 5830). All patients were informed of the study purpose and written informed consent was obtained from all participants. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest: The authors report no potential conflict of interest related to the research in this article. This research did not receive any specific grant from funding agencies in the public, commercial or not‐for‐profit sectors.
Financial Disclosures for the Previous 12 Months: The authors declare that there are no disclosures to report.
Supporting information
Supplementary Figure S1. Effect of dopaminergic treatment on clinical severity in different types of tremors. Tremors amplitude has been evaluated according the MDS‐UPDRS item for resting, postural and action tremor (items 3–15, 3–16, 3–17). *Statistically significant differences (P < 0.05).
Supplementary Figure S2. Effect of dopaminergic treatment on the latency, as expressed in seconds, of tremor during walking (TW) and re‐emergent tremor (RET). ns indicates no statistically significant difference. *Statistically significant differences (P < 0.05).
Supplementary Table S1. Demographic and clinical features of patients.
Supplementary Table S2. Interrater reliability.
Supplementary materials A more detailed description of methods, including clinical assessment and statistical analysis is provided. Additional results on demographic and clinical features of PD patients, interrater reliability and clinical characteristics of tremor during walking are also reported.
Acknowledgment
We thank Laura Centonze for English language editing. Open access funding provided by BIBLIOSAN.
References
- 1. Rajput AH, Rozdilsky B, Ang L. Occurrence of resting tremor in Parkinson's disease. Neurology 1991;41(8): 1298–1299. [DOI] [PubMed] [Google Scholar]
- 2. Deuschl G, Becktepe JS, Dirkx M, et al. The clinical and electrophysiological investigation of tremor. Clin Neurophysiol 2022;136:93–129. [DOI] [PubMed] [Google Scholar]
- 3. Hallett M, Deuschl G. Are we making progress in the understanding of tremor in Parkinson's disease? Ann Neurol 2010;68(6):780–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Helmich RC, Hallett M, Deuschl G, Toni I, Bloem BR. Cerebral causes and consequences of parkinsonian resting tremor: a tale of two circuits? Brain 2012;135(11):3206–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bhatia KP, Bain P, Bajaj N, et al. IPMDS task force on tremor consensus statement. Mov Disord 2018;33(1):75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gupta DK, Marano M, Zweber C, Boyd JT, Kuo SH. Prevalence and relationship of rest tremor and action tremor in Parkinson's disease. Tremor Other Hyperkinet Mov (N Y) 2020;10:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Leodori G, De Bartolo MI, Fabbrini A, Costanzo M, Mancuso M, Belvisi D, et al. The role of the motor cortex in tremor suppression in Parkinson's disease. J Parkinsons Dis 2022. Jul 7;12(6):1957–1963. [DOI] [PubMed] [Google Scholar]
- 8. Naros G, Grimm F, Weiss D, Gharabaghi A. Directional communication during movement execution interferes with tremor in Parkinson's disease. Mov Disord 2018;33(2):251–261. [DOI] [PubMed] [Google Scholar]
- 9. Dirkx MF, Bologna M. The pathophysiology of Parkinson's disease tremor. J Neurol Sci 2022;435:120196. [DOI] [PubMed] [Google Scholar]
- 10. Jankovic J, Schwartz KS, Ondo W. Re‐emergent tremor of Parkinson's disease. J Neurol Neurosurg Psychiatry 1999;67(5):646–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Belvisi D, Conte A, Bologna M, et al. Re‐emergent tremor in Parkinson's disease. Parkinsonism Relat Disord 2017;36:41–46. [DOI] [PubMed] [Google Scholar]
- 12. Belvisi D, Conte A, Cutrona C, Costanzo M, Ferrazzano G, Fabbrini G, Berardelli A. Re‐emergent tremor in Parkinson's disease: the effect of dopaminergic treatment. Eur J Neurol 2018;25(6):799–804. [DOI] [PubMed] [Google Scholar]
- 13. Dirkx MF, Zach H, Bloem BR, Hallett M, Helmich RC. The nature of postural tremor in Parkinson disease. Neurology 2018;90(13):e1095–e1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Uchida K, Hirayama M, Yamashita F, Hori N, Nakamura T, Sobue G. Tremor is attenuated during walking in essential tremor with resting tremor but not parkinsonian tremor. J Clin Neurosci 2011;18(9):1224–1228. [DOI] [PubMed] [Google Scholar]
- 15. Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson's disease: MDS‐PD clinical diagnostic criteria. Mov Disord 2015;30(12):1591–1601. [DOI] [PubMed] [Google Scholar]
- 16. Hoehn MM, Yahr MD. Parkinsonism: onset, progression, and mortality. Neurology 1967;17(5): 427–442. [DOI] [PubMed] [Google Scholar]
- 17. Antonini A, Abbruzzese G, Ferini‐Strambi L, Tilley B, Huang J, Stebbins GT, et al. Validation of the Italian version of the Movement Disorder Society—unified Parkinson's disease rating scale. Neurol Sci 2013;34(5):683–687. [DOI] [PubMed] [Google Scholar]
- 18. Taylor NL, Müller EJ, Shine JM. Shaking with fear: the role of noradrenaline in modulating resting tremor. Brain 2020;143(5):1288–1291. [DOI] [PubMed] [Google Scholar]
- 19. Dirkx MF, Zach H, van Nuland AJ, Bloem BR, Toni I, Helmich RC. Cognitive load amplifies Parkinson's tremor through excitatory network influences onto the thalamus. Brain 2020;143(5):1498–1511. [DOI] [PubMed] [Google Scholar]
- 20. Kinnerup MB, Sommerauer M, Damholdt MF, et al. Preserved noradrenergic function in Parkinson's disease patients with rest tremor. Neurobiol Dis 2021;152:105295. [DOI] [PubMed] [Google Scholar]
- 21. Zach H, Dirkx MF, Pasman JW, Bloem BR, Helmich RC. Cognitive stress reduces the effect of levodopa on Parkinson's resting tremor. CNS Neurosci Ther 2017;23(3):209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aytürk Z, Yilmaz R, Akbostanci MC. Re‐emergent tremor in Parkinson's disease: clinical and accelerometric properties. J Clin Neurosci 2017;37:31–33. [DOI] [PubMed] [Google Scholar]
- 23. Leodori G, Belvisi D, De Bartolo MI, Fabbrini A, Costanzo M, Vial F, et al. Re‐emergent tremor in Parkinson's disease: the role of the motor cortex. Mov Disord 2020;35(6):1002–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Takakusaki K. Neurophysiology of gait: from the spinal cord to the frontal lobe: neurophysiology of gait. Mov Disord 2013;28(11):1483–1491. [DOI] [PubMed] [Google Scholar]
- 25. Malouin F, Richards CL, Jackson PL, Dumas F, Doyon J. Brain activations during motor imagery of locomotor‐related tasks: a PET study. Hum Brain Mapp 2003;19(1):47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sciacca G, Giliberto C, Luca A, Nicoletti A, Zappia M. Seated man walking: a provocation maneuver for parkinsonian tremor. Mov Disord Clin Pract 2016;3(2):212–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wilken M, Rossi M, Rivero AD, Hallett M, Merello M. Re‐emergent tremor provocation. Parkinsonism Relat Disord 2019;66:241–244. [DOI] [PubMed] [Google Scholar]
- 28. Dirkx MF, Zach H, van Nuland A, Bloem BR, Toni I, Helmich RC. Cerebral differences between dopamine‐resistant and dopamine‐responsive Parkinson's tremor. Brain 2019;142(10):3144–3157. [DOI] [PubMed] [Google Scholar]
- 29. Seraji‐Bzorgzad N, Paulson H, Heidebrink J. Neurologic examination in the elderly. Handb Clin Neurol 2019;167:73–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1. Effect of dopaminergic treatment on clinical severity in different types of tremors. Tremors amplitude has been evaluated according the MDS‐UPDRS item for resting, postural and action tremor (items 3–15, 3–16, 3–17). *Statistically significant differences (P < 0.05).
Supplementary Figure S2. Effect of dopaminergic treatment on the latency, as expressed in seconds, of tremor during walking (TW) and re‐emergent tremor (RET). ns indicates no statistically significant difference. *Statistically significant differences (P < 0.05).
Supplementary Table S1. Demographic and clinical features of patients.
Supplementary Table S2. Interrater reliability.
Supplementary materials A more detailed description of methods, including clinical assessment and statistical analysis is provided. Additional results on demographic and clinical features of PD patients, interrater reliability and clinical characteristics of tremor during walking are also reported.


