Abstract
The biodiversity of aboveground plants and belowground microbes is key for plant communities resisting exotic plant invasion. Whether the soil legacy effects after the invasion are related to the diversity of the invaded community is less studied. Soils from invaded communities were collected and potted to investigate the effects of the invasive community's legacy on the biomass allocation of plants that later grew in these soils. The plots where native plants were present had relatively high nutrient levels (except for available nitrogen) compared to the monodominance communities invaded by Chromolaena odorata. This also indirectly suggests that the severe invasion of C. odorata depleted the nutrients in the soil to a greater extent. When soils were from communities with only C. odorata or one native plant, their biotic legacies showed a significantly positive effect on biomass accumulation of subsequent invasive plants, but this positive effect became negative when more than two native plants were present in the invaded community. This result indicated that the effect of biological resistance increases with the number increase of native species in the invaded communities. The soil legacy effect of the invaded communities on subsequent plants depended on the diversity of native plants. This study can provide insights into the mechanisms of soil biological resistance to exotic plant invasion and provide a theoretical basis for the removal of soil legacy effects after the exotic plant invasion.
Keywords: Chromolaena odorata, biomass allocation, soil biota, invaders, plant community
Introduction
With increasing global economic integration and human interaction, the invasion of exotic species has become a growing problem. Invasive exotic plants often occupy unoccupied ecological niches, and the chances of successful invasion are generally low in plant communities with high diversity.1–3 Accordingly, Elton proposed the diversity-resistance hypothesis (DRH), which states that communities with high diversity are more resistant to invasion, while ecosystems with low species diversity and simple structure are more vulnerable to invasion by exotic plants. 4 In plant communities, the diversity of above-ground plants is closely related to soil microbe diversity, which indicates that soil microbes play an important role in resistance to exotic plant invasion.5–7 However, it is not clear whether the resistance effect of above-ground plant diversity on invasive plants can be transmitted to soil microbes and influence subsequent plant growth.
When exotic plants are introduced, they often alter the physicochemical and biological properties of the invaded soil,8,9 which could influence the dynamics of the soil microbe community and the relationship between plant and soil microbe biodiversity. It has been shown that in the absence of invasive alien plants, native plant community diversity is significantly and positively correlated with soil fungal diversity, whereas when alien plants are introduced, plant community diversity is more closely related to the α-diversity of fungal communities, but plant community diversity is not correlated with the β-diversity of fungal communities. 5 The more invasive plant Smooth Brome has invaded, the higher the bacterial diversity in the soil and the lower the fungal diversity. 10 The response of plant and soil microbe diversity to plant invasion changes with the timing of invasion, 11 and it is not clear whether this change could influence subsequent plant growth.
Changes in the biological and physicochemical properties of soil conditioning by plants continue to affect plants that subsequently grow on the soil. This phenomenon is known as the soil legacy effect, which is importantly regulated by soil microbes. 12 Soil microbes are key factors influencing nutrient uptake, disease defence, phenological traits and interspecific relationships of aboveground plants.13–16 For example, soil microbial taxa are closely related to the plasticity of plant flowering schedules. 13 When Ipomoea purpurea was inoculated with soil from the original field soil, plants flowered significantly earlier and more frequently compared to sterile soil. 17 The effects of soil inheritance are mainly influenced by soil fungi rather than bacteria, which redirect plant community succession. 13 Three months after planting the native plant Lasthenia californica with soil that had been planted with the invasive plant Aegilops triuncialis, the plant L. californica showed a delay in flowering and a marked reduction in above-ground biomass. 18 Does the resistance of exotic plants to invasive forces persist? To date, only a few such studies have been published. An in-depth study of the resistance of soil legacies to invasive alien plants can provide a theoretical basis for uncovering the soil biological mechanisms underlying the resistance effect of species diversity.
Chromolaena odorata (L.) King & Robinson is native to Central and South America and has spread to tropical Asia and Africa. It is one of the 100 most damaging invasive exotic species in the world. 19 It has a strong dispersal ability, displaces native species through strategies such as allelopathy and pathogen enrichment, occupies native species’ habitats,20–22 forms large mono-optimal communities and threatens local ecological security. Subtropical China is a region with great biodiversity, but it is also a region that suffers from serious invasions of exotic plants, seriously threatening native plant diversity, severely limiting ecological services, and hampering local economic development. There is an urgent need to decipher the mechanisms of invasive exotic plants and to develop reliable and effective control methods. The greater the diversity of plant communities, the more pronounced the effect of resistance to exotic plant invasion. Does plant community diversity, as a legacy of the soil, have a lasting effect on plant invasion? We hypothesized that (1) soil legacy effects affect subsequent plant biomass and biomass allocation depending on changes in soil nutrients and biotic properties in invaded communities and (2) the diversity of invaded communities could alter the subsequent fitness of plants through biotic legacies in the soil.
Materials and methods
Soil sampling and sterilization
This research relied on an artificially constructed platform for long-term monitoring of the dynamics of invaded communities with varying degrees of invasion. This platform is located at the Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences (21°41′N, 101°25′E; 570 m elevation). The artificial plots with invasive plant communities included the following: (i) a single invasive plant community: a single invasive plant, C. odorata; (ii) C. odorata and one native plant; (iii) a combination of C. odorata and two native plants; and (iv) a combination of C. odorata and three native plants. The area of each plot is 3 m × 3 m, with three replicate treatments. The topsoil (0–20 cm) of the above plots was taken and sieved through a 5 cm sieve and the soil was fumigated with Dazomet sterilizer and covered with foil.
Analysis of soil nutrient contents and pH
Soil nutrient content and pH were determined using routine methods described by Lu. 23 Soil pH was measured using a pH meter (FE30, Mettler-Toledo, CH) with a 1:2.5 soil-water suspension. Soil organic carbon (SOC) was measured by K2Cr2O7-H2SO4 oxidation. Total nitrogen and available nitrogen were determined by the Kjeldahl-N method; total P and available P were determined by HF-HClO4 digestion and sodium bicarbonate extraction (Molybdenum Blue method), respectively.
Transplanting and management
A single plant was planted in each pot, including two invasive plants, C. odorata and Bidens pilosa, and four native plants, Desmodium sequax, Neyraudia reynaudiana, Triumfetta rhomboidea and Urena lobata, with three replicates for each treatment, totalling 144 pots. Attention was given to regular hoeing and spraying to reduce the impact of pests, diseases and weeds, while the plants were regularly watered to ensure normal growth.
Phenotypic measurements on the plants
(1) Measurement of plant height: At mid-growth, the height of each plant was measured with a tape measure. (2) Measurement of specific leaf area: At mid to late plant growth, five complete leaves were taken from each individual, and the total leaf area of leaves (cm2) was measured with a leaf area meter (LI-3000C, LI-COR, USA). The leaves were then dried (70 °C, 36 h). Specific leaf area (cm2/g) = total leaf area/total dry weight. (3) Biomass (g) determination: Leaf, stem and root biomass of each pot were harvested at the later stages of plant growth. The total biomass of each pot was calculated, as well as the proportion of the different components in the biomass.
Data analysis
One-way ANOVA was used to analyse the variability in soil nutrients and pH in the invaded communities. Homogeneity and chi-square tests were performed before analysing the data, and Bonferroni's post hoc test was used to compare the invaded communities. This calculation was performed using R software (ver. 3.6.2).
A mixed linear model (MLM) was used to analyse (i) the response variables: total biomass, biomass allocation, specific leaf area and plant height; (ii) soil sources (different richness of invaded communities), sterilization treatment (sterile/live) and potted plant origin (native/invasive) as fixed factors and potted plant species selection as random factors; the legacy effects of invaded community on plant phenotypes were statistically analysed.
We referred to the formula ln(biomassLive/sterile) used by Lekberg et al. 24 to calculate the soil biotic legacy effect, where biomassLive/sterile is the ratio of plant total biomass on live soil to that on sterile soil. A linear mixed model (MLM) was used to analyse the impact of the legacy effect on the accumulation and distribution of biomass of invasive and native plants. A t-test was also used to analyse the significance of the difference between the value of the legacy effect and the value 0. A significant value greater than 0 indicates a positive soil biotic legacy effect and a significant value less than 0 indicates a negative soil biotic legacy effect. The MLM analysis was performed using the ‘lmerTest’ package in R (ver. 3.6.2).
Results
Changes in soil nutrients and pH among the invaded communities
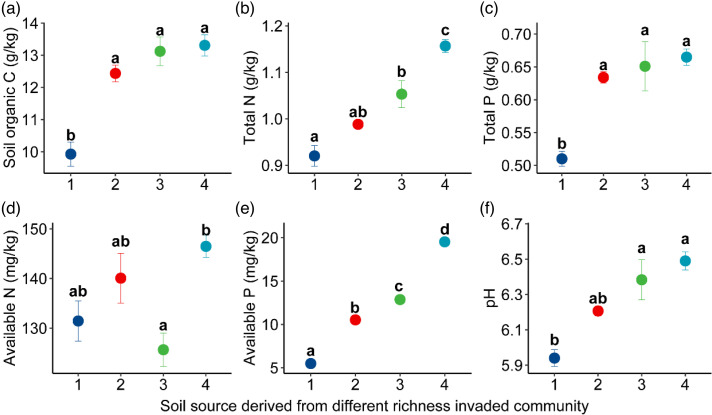
The plots where native plants were present had relatively high nutrient levels (except for available nitrogen) and pH values compared to the monodominant communities invaded by C. odorata (Figure 1). Total nitrogen and available phosphorus levels increased with increasing species diversity in the invaded communities (Figure 1(b) and (e)). This also indirectly suggests that the severe invasion of C. odorata depleted the nutrients in the soil to a greater extent.
Figure 1.
Nutrient content and soil pH (mean ± SE, N = 3) in invaded communities with different richness. Letters indicate significant differences (p < 0.05).
The effects of soil legacy on plant phenotype depend on the diversity of invaded communities
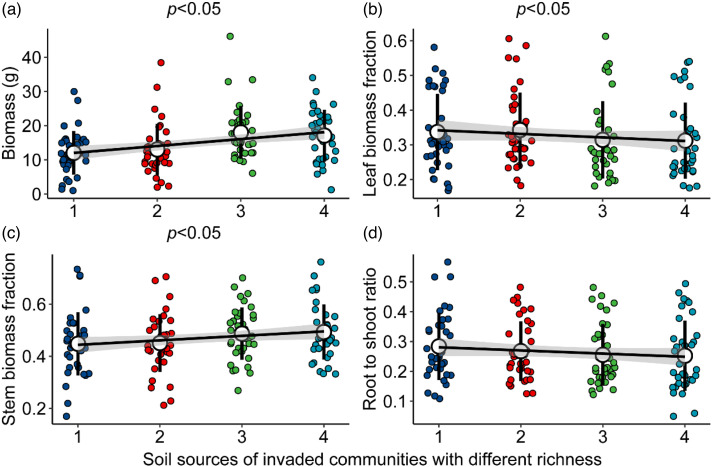
With the increase in the number of native species in the invaded community, the soil legacy effect significantly increased the biomass accumulation of subsequent plants (Figure 2(a)), and the leaf biomass fraction decreased but the stem biomass fraction increased significantly (Figure 2(b) and (c)). The soil legacy effect on subsequent plant height was significantly and positively correlated with community richness (Supplemental Figure S1).
Figure 2.
Soil legacy effects of invaded communities on biomass and its allocations to subsequent plants. p < 0.05 indicates significant regression.
The effect of sterilization on the phenotype of subsequent plants
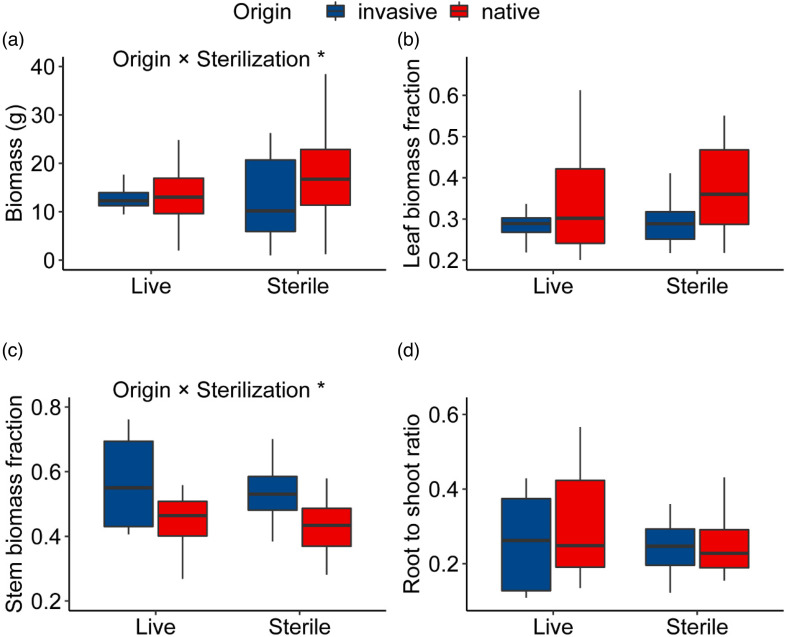
Compared to live soil, sterilization significantly increased plant biomass and leaf biomass fraction (Supplemental Figure S2(a) and (b)); however, sterilization reduced stem biomass fraction and the root to shoot ratio (Supplemental Figure S2(c) and (d)). There was a significant interaction between pot plant origin and soil sterilization on subsequent plant biomass accumulation and stem biomass fraction (Figure 3(a) and (c)).
Figure 3.
Effect of the interaction between potted plant origin and soil sterilization on plant biomass and allocation. * indicates that the interaction effects are significant at the p < 0.05 level.
Soil biotic legacy effects of the invaded community on subsequent plant phenotypes
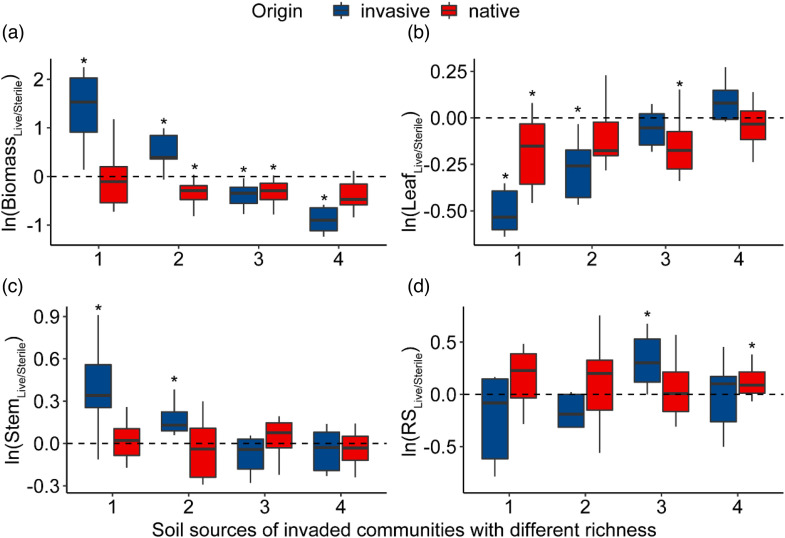
In the communities (plots) with only C. odorata or a native plant, soil legacies showed a significantly positive effect on biomass accumulation and stem biomass fraction of subsequent invasive plants, but this gradually decreased with the number increase of native plants in the field communities (Figure 4(a) and (c)); in contrast, the soil legacy effect on leaf biomass fraction of subsequent invasive plants was significantly negative, but it increased with the number increase of species in the field plots (Figure 4(b)). This suggested that the resistance effect of soil legacy on subsequent alien plant invasion increased with the increase in species richness in the previously invaded community. Soil of the invaded communities had negative legacy effects on subsequent native plant biomass accumulation and the leaf biomass fraction. The biotic legacy showed a significantly positive effect on the root-crown ratio when two native species were present in the field plots, while this positive legacy effect shifted to native plants when three native species were present (Figure 4(d)).
Figure 4.
Soil biotic legacy effects of invaded communities on invasive and native subsequent plants. The RS in panel D refers to the ratio of root to shoot biomass. The box with * indicates that there is a significant difference from 0. If > 0, the effect of biotic legacy is positive; otherwise, the effect of biotic legacy is negative.
Discussions
The effects of soil legacy on biomass allocation after invasion
Consistent with the first hypothesis, this study shows that the soil legacy effect of the invaded community was mediated by the native plants in the invaded community. There are two possible reasons for this. First, as indicated by the plant community census data, the biomass of the invader C. odorata has a large advantage over native plants in the invaded community, and this advantage of rapid biomass accumulation requires the depletion of more nutrients in the soil, resulting in a lack of available nutrients for subsequent plant growth. This conclusion is supported by trends in community nutrient levels (Figure 1). Second, as the number of native species in the invaded community increases, the diversity of the plant community increases soil biodiversity and soil ecological functions, which promotes plant community resistance to exotic plant invasion 8 and reduces the impact of invasive species on the soil. 25 Therefore, the impact of invasive plants on the soil is reduced when the number of native species in the invasive community increases.
With the increase in species richness in the invaded community, its soil legacy effect facilitates an increase in the stem biomass fraction but a decrease in the leaf biomass fraction (Figure 1(b) and (c)). In invaded communities with high diversity, plants compete not only for belowground nutrients but also for light, plants devote more resources to stems and grow taller, and the invaded community is likely to affect subsequent plant performance through the soil legacy effect.
In this study, we found that soil sterilization significantly increased subsequent plant biomass (Supplemental Figure S2(a)). We also found that soil sterilization increased the specific leaf area (Supplemental Figure S2(f)), an important parameter of photosynthesis. Consistent with our study, some invasive plants rapidly accumulate native pathogens at invaded sites, thereby affecting the competitive relationship between invasive and native plants. 26 For example, invasive populations of the field grass C. odorata significantly increased populations of the soil pathogenic fungus Fusarium semitectum, which significantly inhibited the growth of two native plants. 27 However, sterilization reduced the distribution of biomass in the stem and the ratio of roots to crowns (Supplemental Figure S2(c) and (d)), indirectly suggesting that sterile soils impair root growth and then affect the biomass allocation pattern.
The interaction between soil biotic legacy and plant community diversity on biomass allocation
Consistent with the second hypothesis, a significant soil biological legacy effect occurred in the invaded community. This legacy effect contributes significantly to the accumulation of invasive plant biomass but transforms into a significant suppression effect as the plant diversity in the invaded community increases (Figure 4(a)). The introduction of exotic plants alters the composition of plant root secretions and apoplastic material, which has a selective stimulatory effect on certain soil microorganisms and influences the structure and function of the microbial community. 27 Second, the introduction of exotic plants can alter the vegetation cover, affecting heat radiation and leading to changes in soil evaporation and consequently soil moisture. 28 Both aspects can influence subsequent plant growth through ecological memory in the soil microbial pool. The soil from the community with higher plant diversity has stronger resistance to subsequent plant growth.
In addition, our study found that in the communities with only C. odorata or one native plant, the biological legacy of the soil increased the biomass allocation to the stems of subsequent invasive plants, but this effect decreased with the increase in native species in the invaded community (Figure 4(c)). Consistent with our study, after inoculating the soil microbes of the invaded site, the invasive C. odorata increased its height to obtain more light resources and allocated more photosynthetic carbon to the stem. However, the performance of native Panicum maximum was not affected by inoculation. 29 Dudenhöffer et al. 30 also found that feedback from below-ground organisms to above-ground plants not only affect plant biomass but can also alter the biomass allocation pattern, thereby changing plant fitness. However, contradictory studies also reported that the soil legacy effect of plant diversity showed a weak positive feedback effect on Jacobaea vulgaris, and the soil legacy effect of plant diversity could not be detected in soils inoculated with sterile substrates. 31 This result indicated that soil microbes play a more important role than other factors in the soil legacy effect.
Compared to native plants, introduced exotic plants often have a rapid growth rate and strong allelopathic effect, affecting soil physicochemical properties and microbial community dynamics. 22 These impacts could create a ‘memory’ in the soil that continues to influence the growth of subsequent plants. This study shows that the soil legacy effect is closely linked to the diversity of plants in the invaded community. The diversity of the invaded community had effects on the dynamics of the soil biota, which could affect the biomass allocation pattern among different organs of subsequent plants, which in turn influences plant fitness. However, in this study, the soil was only sterilized, and the structure of the soil microbial community was not measured. It is not clear which groups of soil microorganisms play crucial roles in the soil legacy effect, and in-depth targeted research is needed.
Conclusions
Severe invasions (communities with only one exotic plant) led to a severe depletion of soil nutrients and a significant reduction in biomass and biomass allocation to stems of subsequent plants by soil legacy. In the community with only invasive C. odorata or only one native species, biotic legacies in the soil contributed positively to the biomass accumulation of subsequently invasive plants, but this positive effect decreased and even became negative with the increase in species richness in the invaded communities. The most striking aspect of our results is that the soil can ‘inherit’ the resistance of species diversity to exotic plants and resist subsequent exotic plant invasion. The results can provide a theoretical basis for the prevention and control of invasive plants.
Supplemental Material
Supplemental material, sj-docx-1-sci-10.1177_00368504221150060 for Soil legacy effects on biomass allocation depend on native plant diversity in the invaded community by Weitao Li, Xiaoting Bi and Yulong Zheng in Science Progress
Acknowledgements
We thank Wen-bian Bo for taking care of the potted plants and Long Li for their assistance in collecting the biomass data. We are grateful to the Associate Editor and anonymous referee for providing valuable comments.
Author biographies
Weitao Li is an assistant researcher in Xishuangbanna Tropical Botanical Garden (XTBG), Mengla, China. His research focuses on microbial ecology and bioinvasion.
Xiaoting Bi is a student at Puer University in Puer, China, majoring in ecology.
Yulong Zheng is a professor in Xishuangbanna Tropical Botanical Garden (XTBG), Mengla, China. His research focuses on the ecology of bioinvasion.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: this research was supported by Yunnan Fundamental Research Projects (grant number 202101AU070150), the ‘Yunnan Revitalization Talent Support Program’ in Yunnan Province and the National Natural Science Foundation of China (grant number 32171660).
ORCID iD: Weitao Li https://orcid.org/0000-0001-9778-7513
Supplemental material: Supplemental material for this article is available online.
References
- 1.van Ruijven J, De Deyn GB, Berendse F. Diversity reduces invasibility in experimental plant communities: the role of plant species. Ecol Lett 2003; 6: 910–918. [Google Scholar]
- 2.Kennedy TA, Naeem S, Howe KM, et al. Biodiversity as a barrier to ecological invasion. Nature 2002; 417: 636–638. [DOI] [PubMed] [Google Scholar]
- 3.Beaury EM, Finn JT, Corbin JD, et al. Biotic resistance to invasion is ubiquitous across ecosystems of the United States. Ecol Lett 2020; 23: 476–482. [DOI] [PubMed] [Google Scholar]
- 4.Elton CS. The ecology of invasions by animals and plants. London: Chapman and Hall, 1958. [Google Scholar]
- 5.Shen CC, Wang J, He JZ, et al. Plant diversity enhances soil fungal diversity and microbial resistance to plant invasion. Appl Environ Microb 2021; 87: e00251–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang ZJ, Liu YJ, Brunel C, et al. Soil-microorganism-mediated invasional meltdown in plants. Nat Ecol Evol 2020; 4: 1612–1621. [DOI] [PubMed] [Google Scholar]
- 7.Zheng YL, Burns JH, Liao ZY, et al. Species composition, functional and phylogenetic distances correlate with success of invasive Chromolaena odorata in an experimental test. Ecol Lett 2018; 21: 1211–1220. [DOI] [PubMed] [Google Scholar]
- 8.Zhang HY, Goncalves P, Copeland E, et al. Invasion by the weed Conyza canadensis alters soil nutrient supply and shifts microbiota structure. Soil Biol Biochem 2020; 143: 107739. [Google Scholar]
- 9.Gibbons SM, Lekberg Y, Mummey DL, et al. Invasive plants rapidly reshape soil properties in a grassland ecosystem. mSystems 2017; 2: e00178–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martiny JBH, Martiny AC, Weihe C, et al. Microbial legacies alter decomposition in response to simulated global change. ISME J 2017; 11: 490–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luan JW, Liu SR, Li SY, et al. Functional diversity of decomposers modulates litter decomposition affected by plant invasion along a climate gradient. J Ecol 2021; 109: 1236–1249. [Google Scholar]
- 12.Heinen R, Hannula SE, De Long JR, et al. Plant community composition steers grassland vegetation via soil legacy effects. Ecol Lett 2020; 23: 973–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wagner MR, Lundberg DS, Coleman-Derr D, et al. Natural soil microbes alter flowering phenology and the intensity of selection on flowering time in a wild Arabidopsis relative. Ecol Lett 2014; 17: 717–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ware IM, Van Nuland ME, Schweitzer JA, et al. Climate-driven reduction of genetic variation in plant phenology alters soil communities and nutrient pools. Global Change Biol 2019; 25: 1514–1528. [DOI] [PubMed] [Google Scholar]
- 15.Zhang FJ, Li Q, Chen FX, et al. Arbuscular mycorrhizal fungi facilitate growth and competitive ability of an exotic species Flaveria bidentis. Soil Biol Biochem 2017; 115: 275–284. [Google Scholar]
- 16.Lau JA, Lennon JT. Rapid responses of soil microorganisms improve plant fitness in novel environments. P Natl Acad Sci USA 2012; 109: 14058–14062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaney L, Baucomn RS. The soil microbial community alters patterns of selection on flowering time and fitness-related traits in Ipomoea purpurea. Am J Bot 2020; 107: 186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Batten KM, Scow KM, Espeland EK. Soil microbial community associated with an invasive grass differentially impacts native plant performance. Microb Ecol 2008; 55: 220–228. [DOI] [PubMed] [Google Scholar]
- 19.Yu XQ, Feng YL, Li QM. Review of research advances and prospects of invasive Chromolaena odorata. Chin J Plant Ecol 2010; 34: 591–600. [Google Scholar]
- 20.Kone AW, Kassi SPAY, Koffi BY, et al. Chromolaena odorata (L.) K&R (Asteraceae) invasion effects on soil microbial biomass and activities in a forest-savanna mosaic. Catena 2021; 207: 105619. [Google Scholar]
- 21.Li WT, Zheng YL, Zhang LK, et al. Post-introduction evolution contributes to the successful invasion of Chromolaena odorata. Ecol Evol 2020; 10: 1252–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qin RM, Zheng YL, Valiente-Banuet A, et al. The evolution of increased competitive ability, innate competitive advantages, and novel biochemical weapons act in concert for a tropical invader. New Phytol 2013; 197: 979–988. [DOI] [PubMed] [Google Scholar]
- 23.Lu RK. Analytical methods for soil and agricultural chemistry. Beijing: China Agricultural Science and Technology Press, 1999. [Google Scholar]
- 24.Lekberg Y, Bever JD, Bunn RA, et al. Relative importance of competition and plant-soil feedback, their synergy, context dependency and implications for coexistence. Ecol Lett 2018; 21: 1268–1281. [DOI] [PubMed] [Google Scholar]
- 25.Chen D, van Kleunen M. Invasional meltdown mediated by plant-soil feedbacks may depend on community diversity. New Phytol 2022; 235: 1589–1598. [DOI] [PubMed] [Google Scholar]
- 26.Eppinga MB, Rietkerk M, Dekker SC, et al. Accumulation of local pathogens: a new hypothesis to explain exotic plant invasions. Oikos 2006; 114: 168–176. [Google Scholar]
- 27.Mangla S, Inderjit, Callaway RM. Exotic invasive plant accumulates native soil pathogens which inhibit native plants. J Ecol 2008; 96: 58–67. [Google Scholar]
- 28.Fiedler AK, Landis DA. Biotic and abiotic conditions in Michigan Prairie fen invaded by Glossy buckthorn (Frangula alnus). Nat Area J 2012; 32: 41–53. [Google Scholar]
- 29.Te Beest M, Stevens N, Olff H, et al. Plant–soil feedback induces shifts in biomass allocation in the invasive plant Chromolaena odorata. J Ecol 2009; 97: 1281–1290. [Google Scholar]
- 30.Dudenhöffer J-H, Ebeling A, Klein A-M, et al. Beyond biomass: soil feedbacks are transient over plant life stages and alter fitness. J Ecol 2018; 106: 230–241. [Google Scholar]
- 31.Kostenko O, Bezemer TM. Abiotic and biotic soil legacy effects of plant diversity on plant performance. Front Ecol Evol 2020; 8: 87. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-sci-10.1177_00368504221150060 for Soil legacy effects on biomass allocation depend on native plant diversity in the invaded community by Weitao Li, Xiaoting Bi and Yulong Zheng in Science Progress