Abstract
The E-prM proteins of flaviviruses are unusual complexes which play important roles in virus assembly and fusion modulation and in potential immunity-inducing vaccines. Despite their importance, little is known about the biogenesis and structural organization of E-prM complexes. Pulse-chase radiolabeling of dengue virus-infected Vero cells demonstrated a rapid interassociation of E and prM proteins, and sucrose gradient sedimentation analysis suggested that E-prM complexes progressed from simple heteromers to more densely sedimenting structures indicating increased multimerization. E-prM heteromers of even higher complexity were observed in virus particles, suggesting an intracellular assembly process which results in the networking of E-prM subunits into a lattice-like structure found in virus particles. Trypsin cleavage of E-prM-containing virus particles resulted in the release of a soluble 45-kDa fragment of the E protein which retained cell-binding activity. The results suggest that E-prM interactions in dengue virus particles are largely mediated by domains in the carboxy-terminal anchoring domain of E, while cell-binding activity is retained in a trypsin-releasable ectodomain of the E protein.
The flavivirus E protein is a multifunctional protein which is involved in cell receptor binding (3, 6, 10) and virus entry via fusion with a host cell membrane (26). An unusual feature of the flavivirus E protein is that some of its functional activities, notably membrane fusion, are regulated by interaction with a second viral protein, prM. It is believed that the association of prM with E stabilizes certain pH-sensitive epitopes on the E protein, thereby preventing the conformational changes which normally occur at acidic pH and which activate the fusogenic activity of the E protein (1, 9, 13). In addition to its normal role in flavivirus assembly, the prM protein has also been included in novel recombinant formulations in which it is generally coexpressed with the E protein; the resultant E-prM complexes have been shown to be immunogenic and protective in vaccines against challenge with several flaviviruses, including Japanese encephalitis virus (20), yellow fever virus (22), dengue virus (8), and tick-borne encephalitis virus (11). Despite the importance of the E-prM complex in flavivirus biology and vaccinology, its structure and biosynthesis remain incompletely understood, particularly in relationship to dengue virus infection. The purposes of the present study were to investigate the biogenesis of the E-prM complex in dengue virus-infected cells and to partially localize the biologically important functional activities of prM binding and cell binding in the mature E protein.
Predominance of prM and E proteins in extracellular particles produced by dengue virus-infected Vero cells.
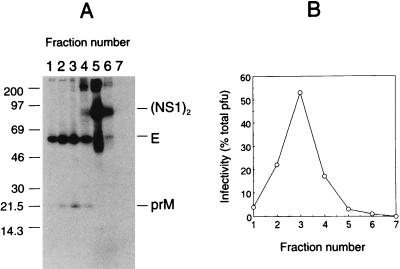
In tick-borne encephalitis virus, the majority of extracellular virus is largely free of prM protein, due to a late intracellular processing event which generates a carboxy-terminal fragment designated M; this fragment and the E and C proteins are believed to constitute the protein components of the mature virus particle (13). In contrast, dengue virus particles purified from cell culture contain a mixture of prM and M proteins (4, 10, 23, 24) in which prM often predominates (4, 10, 23). The preponderance of prM (over M) in extracellular virus particles cannot be explained solely by enhanced radiolabeling (11 methionines in prM versus 5 in M), since dengue virus virions containing prM (and very little M) have also been observed by protein staining (23). Sucrose gradient fractionation of culture fluids from radiolabeled, dengue virus-infected cells clearly shows cosedimentation of infectivity and E-prM-containing particles (Fig. 1). The results indicate a predominance of prM-containing virus particles produced by virus-infected Vero cells, likely as a consequence of relatively inefficient cleavage of prM to M during the late stages of virus maturation. Nevertheless, dengue virus infectivity does not appear to depend on quantitative cleavage of prM to M, as dengue virus particles containing prM but no M (obtained from virus-infected cells cultured in the presence of ammonium chloride, which blocks prM-to-M cleavage) still retain one-eighth to one-sixth the specific infectivity of normal virus particles (24). We have shown previously that virus particles containing E and prM bind to permissive cells and that binding can be blocked with E-specific antibody (10, 29). Virus particles containing mainly E and prM also show antibody-enhanced binding to Fc receptor-bearing K562 cells as well as to platelets (29). Thus, in addition to being requisite precursors to mature virus particles, virus particles containing prM possess many properties associated with mature virus particles. The preponderance of prM-containing virus particles produced by dengue virus-infected Vero cells was exploited in the present study in order to trace the interactions between E and prM which occur during virus particle biogenesis.
FIG. 1.
Predominance of E and prM proteins in virus particles produced by dengue 2 (strain 16681) virus-infected Vero cells (multiplicity of infection = 1). Cells were pulse-labeled for 12 h with [35S]methionine-cysteine (400 μCi/ml; ICN, Irvine, Calif.) at 40 h postinfection. Clarified culture fluid was overlaid on a 5 to 55% (wt/wt in phosphate-buffered saline) sucrose gradient and centrifuged for 16 h at 45,000 rpm in a Beckman SW60 rotor. The gradient was fractionated from the bottom, and aliquots were taken for immunoprecipitation with human dengue virus 2 immune serum (1:200 dilution) and for isolation by using protein A-bearing formalin-fixed cells of Staphylococcus aureus. Immunoprecipitates were washed with phosphate-buffered saline (no detergents) in order to recover virus particles as well as immunoreactive soluble proteins. The immunoprecipitates were resolved and revealed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and fluorography (A). The identities of E and prM proteins were confirmed by additional immunoprecipitations with E-specific MAbs 3H5 and 1B7 and prM-specific MAb 2H2 (14) (data not shown). Aliquots from the same sucrose gradient fractions were titrated for virus infectivity by plaque assay; the percentage of total PFU (= 3 × 106 PFU) in each fraction is shown (B).
Time course of intracellular association of dengue virus E and prM proteins.
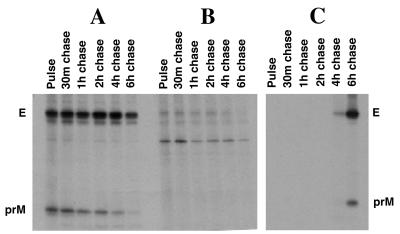
To investigate intracellular protein interactions with newly synthesized E protein, we performed a pulse-chase [35S]methionine-cysteine labeling study with dengue virus-infected Vero cells followed by immunoprecipitation of cytoplasmic extracts with E-specific monoclonal antibody (MAb) 1B7 (14). For this study we found digitonin to give the best results in terms of preservation of E-prM complexes following cell solubilization. Digitonin is a mild cell lysis agent (19) and is known to preserve protein-protein interactions which may be unstable in other detergents, such as Triton X-100 (16). Dengue virus prM protein was found to be associated with newly synthesized E protein (i.e., within the 15-min pulse [Fig. 2A, lane 1]). The prM-E complex persisted in the infected cell for approximately 4 to 6 h, at which time the level of intracellular prM-E complex was observed to decline. The timing of this decline was consistent with the export of E-prM into extracellular progeny virus particles (Fig. 2C).
FIG. 2.
Kinetics of intracellular association of dengue virus E and prM proteins. Dengue virus-infected Vero cells were pulse-labeled for 15 min with [35S]methionine-cysteine at 40 h postinfection. Cells were washed and incubated for various times in 0.5 ml of chase medium containing excess methionine and cysteine. Cells were harvested after being washed in Tris-buffered saline and then solubilized in 100 μl of Tris-buffered saline containing digitonin (1 mg/ml). Extracts were microcentrifuged for 15 min at 4°C, and the supernatants were used for immunoprecipitation with E-specific MAb 1B7 (14) and formalin-fixed protein A-bearing cells of S. aureus. Immunoprecipitates were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and fluorographed (A). (B) Mock immunoprecipitations of the same digitonin extracts with irrelevant antibody (MAb against mouse hepatitis virus N protein). (C) Immunoprecipitations (with MAb 1B7) from culture fluid supernatants from the pulse-chase-labeled cells for detection of the appearance of radiolabeled viral E protein and E protein complexes in the extracellular fluid.
Multimerization of prM-E complexes.
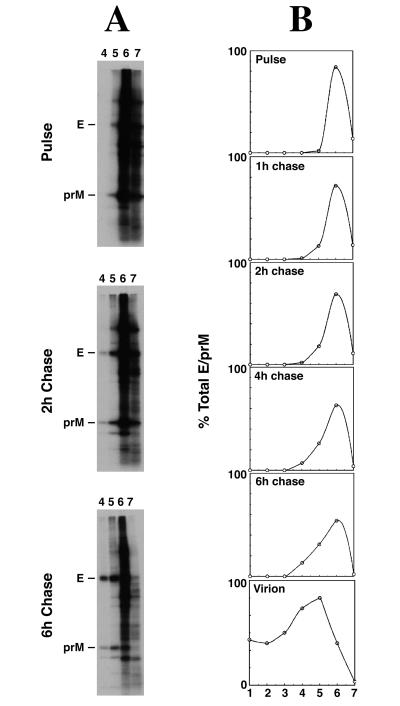
Evidence from studies with immature virions of tick-borne encephalitis virus suggest that E may associate with prM as multimeric complexes (1, 13, 25). To investigate the formation of higher-order E-prM complexes in dengue virus-infected cells, we analyzed pulse-chase-radiolabeled cell extracts on sucrose gradients (Fig. 3). Complexes consisting of prM and E were clearly visible as densely sedimenting structures by 2 h of chase, even in nonimmunoprecipitated total cell extracts (Fig. 3A). Following immunoprecipitation with E-specific MAb 1B7 (14) and semiquantitation of E-prM complexes in all sucrose gradient fractions, the distribution of E-prM complexes throughout each gradient was determined; results are shown in Fig. 3B. In the pulse-labeled sample, the prM-E complex was found to sediment near the top of the gradient (fractions 6 and 7), consistent with the complex being a simple heterodimer. In contrast, by 6 h of chase, the prM-E complex was also found in higher-density fractions (fractions 4 and 5), indicating the formation of more complex, multimeric structures. Similar, densely sedimenting E-prM complexes were also found in dengue virus particles (Fig. 3B, bottom panel), suggesting that the intracellular multimeric E-prM structures may represent preassembly complexes required for incorporation into virus particles. The results imply the existence of an intracellular scaffolding process which occurs within a few hours of E-prM protein synthesis and which leads to the formation of multimeric E-prM complexes. To our knowledge, this is the first evidence for time-dependent expansion of intracellular flavivirus E-prM complexes which likely represent intermediate structures involved in the assembly of heterogeneous, multimeric E-prM structures of the kind reported for immature virions of tick-borne encephalitis virus (13).
FIG. 3.
Multimerization of dengue virus E and prM proteins. Digitonin extracts from infected cells pulse-chase labeled with [35S]methionine-cysteine were overlaid on 10 to 50% (wt/wt) in phosphate-buffered saline) sucrose gradients and centrifuged for 16 h at 45,000 rpm in a Beckman SW60 rotor. Gradient tubes were pierced from the bottom and drained by gravity into seven microcentrifuge tubes. Aliquots of sucrose gradient fractions (without immunoprecipitation [A] or immunoprecipitated with MAb 1B7 [B]) were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and fluorography. With increases in chase time, the E-prM complexes were observed to sediment progressively lower in the gradient, below the bulk of cell proteins which are mainly found near the top (fraction 6) of the gradient (A). The fluorogram was deliberately overexposed to show the densely sedimenting E-prM complexes appearing in fractions 4 and 5 by 2 and 6 h of chase. (B) Relative distribution of radiolabel in each sucrose gradient fraction, as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and fluorography of E-prM complexes immunoprecipitated by MAb 1B7. In the 15-min-pulse sample, predominantly light complexes (presumably heterodimers) of prM-E were found near the top of the gradient (in fractions 6 and 7). With increases of chase time, progressively heavier E-prM complexes (likely representing multimers) were observed, as indicated by a skewing of the distribution of E-prM complexes towards lower fractions in the gradient. Also shown is the distribution of digitonin-released E-prM complexes from purified virus particles, harvested from culture fluids after 12 h of chase. For reference, intact virus particles sedimented in fraction 1, while yeast alcohol dehydrogenase (molecular weight, 150,000) and catalase (molecular weight, 250,000) banded in fraction 6 and fractions 4 and 5, respectively, in these 10 to 50% sucrose gradients.
Release of a non-prM-associated E protein ectodomain by trypsin cleavage of virus particles.
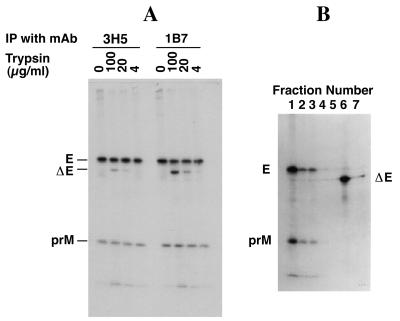
The observation of densely sedimenting, apparently multimeric E-prM complexes in virus particles (Fig. 3) suggests the existence of multiple E-prM interactions, similar to those postulated from studies with immature particles of tick-borne encephalitis virus (1, 13, 25). In order to gain further insight into the intermolecular associations between E and prM within dengue virus particles, radiolabeled virus particles (purified as for Fig. 1) were treated with trypsin to release surface-exposed, trypsin-cleavable proteins. The virus trypsinate was immunoprecipitated with E-specific MAbs 3H5 and 1B7 (14). In addition to immunoprecipitating full-length E protein (as well as prM complexed to E), both antibodies were able to immunoprecipitate an E protein fragment of about 45 kDa, designated ΔE (Fig. 4A). Immunoprecipitation with MAb 1B7 was more effective than with MAb 3H5, perhaps due to the proximity of the MAb 3H5 binding site to the suspected trypsin cleavage site (see below).
FIG. 4.
(A) Cleavage of dengue virus E protein by trypsin. Dengue virus-infected Vero cells were labeled with [35S]methionine-cysteine for 12 h, from 36 to 48 h postinfection. Radiolabeled virus was purified by sucrose gradient centrifugation (as for Fig. 1) and treated with trypsin (at final concentrations of 100, 20, and 4 μg/ml) on ice for 1 h. Reactions were terminated by adding a stop buffer containing bovine serum albumin (30 mg/ml) and 5 mM N-α-tosyl-l-lysyl-chloromethyl ketone (TLCK). Samples were immunoprecipitated (IP) with MAbs 3H5 and 1B7 in the absence of detergents. (B) Release of ΔE from dengue virus particles by trypsin. Radiolabeled dengue virus was treated with trypsin (20 μg/ml) for 1 h at 4°C, overlaid onto a 10 to 50% (wt/wt) sucrose gradient in phosphate-buffered saline, and centrifuged for 16 h at 45,000 rpm in a Beckman SW60 rotor. Samples were collected in seven fractions (from bottom to top) and immunoprecipitated with E-specific MAb 1B7. Aliquots of each sample were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and fluorography.
In order to determine whether the trypsin-cleaved 45-kDa ΔE fragment was virus associated, trypsin-treated dengue virus was centrifuged through a 15 to 50% (wt/wt) sucrose gradient. Fractions of the sucrose gradients were collected and immunoprecipitated with anti-dengue virus E-specific MAb 1B7 (Fig. 4B). In contrast to uncleaved virus, which sedimented near the bottom of the gradient (fractions 1 to 3), the trypsin-generated 45-kDa ΔE fragment was mainly found as a soluble protein near the top of the gradient (fraction 6). There was no detectable prM in this fraction, nor was there any ΔE found in virus particles, suggesting that the major prM binding sites are located in the membrane-anchoring carboxy terminus of the E protein. The results suggest further that the major intermolecular associations which stabilize the E-prM network within the virus particle do not appear to involve the E ectodomain but rather are located in the carboxy-terminal portion of E. These results may be analogous to observations made with tick-borne encephalitis virus in which full-length E protein was shown to form more stable complexes with prM than was anchor-free E protein (2). It is also conceivable that ΔE is derived solely from a subset of E protein molecules which are not associated with prM. This possibility requires further investigation.
The trypsin-released ectodomain of E retains cell-binding activity.
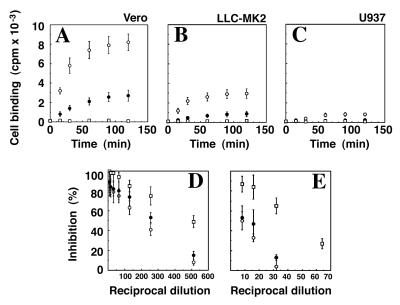
We used the trypsin-cleaved soluble E fragment in a cell binding assay to determine whether this fragment possessed receptor-binding activity. In parallel, cell binding of intact dengue virus (as a positive control) and the NS1 dimer (as a negative control) was monitored. Cell binding was performed with three cell lines commonly used in dengue virus infection studies, i.e., monkey kidney Vero and LLCMK2 cells and human monocyte-like U937 cells. Unequivocal binding of intact virus was observed with Vero and LLCMK2 cells, while much lower binding was seen with U937 cells (Fig. 5A to C). In contrast to Vero and LLCMK2 cells, U937 cells are Fc receptor-bearing monocyte-like cells which support antibody-dependent dengue virus infection but are relatively poorly infectible in the absence of dengue virus-specific antibody (7, 17). The cell binding results with intact virus further support the idea that permissiveness to dengue virus infection is determined at least in part by the degree of virus-cell binding (3), which is a reflection of the relative abundance of virus receptors. As shown in Fig. 5A to C the trypsin-released dengue virus E ectodomain, ΔE, retained partial cell-binding activity. This is the first direct demonstration that the cell receptor-binding portion of any flavivirus E protein can be released in functional form by trypsin cleavage of virus particles. It is noteworthy that ΔE bound to all three cell types apparently less efficiently than did intact virus (Fig. 5A to C). However, this likely reflects the fact that cell binding of intact virus particles would be scored with greater sensitivity in our binding assay than would isolated E proteins. Each cell-bound virus particle contains many E protein molecules (of the order of 90 E dimers as estimated for tick-borne encephalitis virus [27]), only a few of which (per virus particle) would be expected to actually participate in cell attachment. The results therefore indicate that ΔE possesses most if not all of the structural features required for cell binding. Specificity of cell binding for both virus particles and ΔE was shown by blocking studies with E-specific MAbs 3H5 and 1B7 (Fig. 5D and E). For comparison, the virus neutralization activities for each MAb were determined in parallel on the same preparation of radiolabeled virus. Both MAbs blocked binding of virus particles and ΔE to Vero cells (Fig. 5D and E). Blocking of virus particle binding correlated more closely to virus neutralization for MAb 3H5 than for MAb 1B7. This may suggest that MAb 3H5 neutralizes dengue virus predominantly by blocking virus-cell attachment, while MAb 1B7 neutralizes dengue virus largely by a postattachment mechanism.
FIG. 5.
Cell-binding activities of trypsin-released ΔE and purified dengue virus. (A to C) Equal amounts of radiolabeled purified dengue virus (○), trypsin-released E ectodomain (●) (purified by sucrose gradient fractionation as shown in Fig. 4B), and purified NS1 dimer (□) (obtained by sucrose gradient fractionation of culture fluid supernatant from virus-infected Vero cells as for Fig. 1) were incubated with equal numbers (105) of Vero (A), LLC-MK2 (B), and U937 (C) cells for 90 min at 4°C. Cells were washed four times with phosphate-buffered saline, and the amount of cell-bound radiolabel was determined by liquid scintillation counting. (D and E) Blocking of cell binding of purified dengue virus (○) and ΔE (●) was performed as described above, except that virus and ΔE were preincubated with either MAb 3H5 (D) or 1B7 (E) for 60 min at room temperature prior to cell binding. Samples treated with irrelevant MAb showed no significant inhibition of binding (data not shown). For reference, the neutralization activity for each MAb was determined on the same sample of radiolabeled dengue virus (□). Data points and error bars represent the means ± standard deviations for triplicate determinations.
The precise location of the trypsin cleavage site on the dengue virus E protein remains uncertain. The E protein of tick-borne encephalitis virus is also cleaved by trypsin, at a site between residues 395 and 408, probably at Lys-408 (12, 25). The corresponding region in the dengue virus E protein similarly contains several basic amino acid residues representing potential trypsin sites. MAb binding sites provide some help in localizing the trypsin cleavage site of the dengue virus E protein. In the present study, both MAbs 3H5 and 1B7 were shown to immunoprecipitate the trypsin-released E protein ectodomain. The MAb 3H5 binding site on the dengue virus E protein has been partly characterized (15, 21, 28) and probably encompasses, at a minimum, residues 383 to 385 (15). This places the trypsin cleavage site on the carboxy side of amino acid 385. Further studies, such as studies involving C-terminal sequencing, will help to define the trypsin cleavage site of the dengue virus E protein.
Elucidation of the precise sites within ΔE which are involved in cell receptor binding is important to understanding cell tropism of dengue virus as well as to designing possible inhibitors of infection. Potential glycosaminoglycan-binding motifs have been identified on the dengue virus E protein at two sites, the best characterized of which appears to be comprised of amino acids 188, 284 to 295, and 305 to 310 and which may also play a role in virus-cell attachment (5). There exists considerable homology among flaviviral E proteins, raising the possibility that different flaviviruses may have similar receptor-binding motifs. For example, many mosquito-borne flaviviruses contain an RGD sequence (e.g., residues 388 to 390 of the Murray Valley encephalitis virus E protein) which has been implicated in virulence (18) and potential receptor binding, by analogy with integrin-binding motifs (25). Further analyses of cell-binding activities and potential receptor-binding activities of proteolytic or recombinantly produced fragments of flaviviral E protein may help to define the sites involved in recognition between flaviviruses and cell surface macromolecules.
Acknowledgments
This work was supported by the Natural Sciences and Engineering Research Council of Canada. R.A. is an associate of the Dalhousie Medical Research Foundation.
We are grateful to Alan King, Walter Reed Army Institute of Research, for generously providing MAbs used in this study.
REFERENCES
- 1.Allison S L, Schalich J, Stiasny K, Mandl C W, Kunz C, Heinz F X. Oligomeric rearrangement of tick-borne encephalitis virus envelope proteins induced by an acidic pH. J Virol. 1995;69:695–700. doi: 10.1128/jvi.69.2.695-700.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allison S L, Stadler K, Mandl C W, Kunz C, Heinz F X. Synthesis and secretion of recombinant tick-borne encephalitis virus protein E in soluble and particulate form. J Virol. 1995;69:5816–5820. doi: 10.1128/jvi.69.9.5816-5820.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson R, King A D, Innis B L. Correlation of E protein binding with cell susceptibility to dengue 4 virus infection. J Gen Virol. 1992;73:2155–2159. doi: 10.1099/0022-1317-73-8-2155. [DOI] [PubMed] [Google Scholar]
- 4.Anderson R, Wang S, Osiowy C, Issekutz A C. Activation of endothelial cells via antibody-enhanced dengue virus infection of peripheral blood monocytes. J Virol. 1997;71:4226–4232. doi: 10.1128/jvi.71.6.4226-4232.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y, Maguire T, Hileman R E, Fromm J R, Esko J D, Linhardt R J, Marks R M. Dengue virus infectivity depends on envelope protein binding to target cell heparan sulfate. Nat Med. 1997;3:866–871. doi: 10.1038/nm0897-866. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Maguire T, Marks R M. Demonstration of binding of dengue virus envelope protein to target cells. J Virol. 1996;70:8765–8772. doi: 10.1128/jvi.70.12.8765-8772.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daughaday C C, Brandt W E, McCown J M, Russell P K. Evidence for two mechanisms of dengue virus infection of adherent human monocytes: trypsin-sensitive virus receptors and trypsin-resistant immune complex receptors. Infect Immun. 1981;32:469–473. doi: 10.1128/iai.32.2.469-473.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fonseca B A L, Pincus S, Shope R E, Paoletti E, Mason P W. Recombinant vaccinia viruses co-expressing dengue-1 glycoproteins prM and E induce neutralizing antibodies in mice. Vaccine. 1994;12:279–285. doi: 10.1016/0264-410x(94)90206-2. [DOI] [PubMed] [Google Scholar]
- 9.Guirakhoo F, Bolin R A, Roehrig J T. The Murray Valley encephalitis virus prM protein confers acid resistance to virus particles and alters the expression of epitopes within the R2 domain of E glycoprotein. Virology. 1992;191:921–931. doi: 10.1016/0042-6822(92)90267-S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He R T, Innis B L, Nisalak A, Usawattanakul W, Wang S, Kalayanarooj S, Anderson R. Antibodies that block virus attachment to Vero cells are a major component of the human neutralizing antibody response against dengue virus type 2. J Med Virol. 1995;45:451–461. doi: 10.1002/jmv.1890450417. [DOI] [PubMed] [Google Scholar]
- 11.Heinz F X, Allison S L, Stiasny K, Schalich J, Holzmann H, Mandl C W, Kunz C. Recombinant and virion-derived soluble and particulate immunogens for vaccination against tick-borne encephalitis. Vaccine. 1995;13:1636–1642. doi: 10.1016/0264-410x(95)00133-l. [DOI] [PubMed] [Google Scholar]
- 12.Heinz F X, Mandl C W, Holzmann H, Kunz C, Harris B A, Rey F, Harrison S C. The flavivirus envelope protein E: isolation of a soluble form from tick-borne encephalitis virus and its crystallization. J Virol. 1991;65:5579–5583. doi: 10.1128/jvi.65.10.5579-5583.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heinz F X, Stiasny K, Puschner-Auer G, Holzmann H, Allison S L, Mandl C W, Kunz C. Structural changes and functional control of the tick-borne encephalitis virus glycoprotein E by the heterodimeric association with protein prM. Virology. 1994;198:109–117. doi: 10.1006/viro.1994.1013. [DOI] [PubMed] [Google Scholar]
- 14.Henchal E A, McCown J M, Burke D S, Seguin M C, Brandt W E. Epitopic analysis of antigenic determinants on the surface of dengue 2 virions using monoclonal antibodies. Am J Trop Med Hyg. 1985;34:162–169. doi: 10.4269/ajtmh.1985.34.162. [DOI] [PubMed] [Google Scholar]
- 15.Hiramatsu K, Tadano M, Men R, Lai C-J. Mutational analysis of a neutralization epitope on the dengue type 2 virus (DEN2) envelope protein: monoclonal antibody resistant DEN2/DEN4 chimeras exhibit reduced mouse neurovirulence. Virology. 1996;224:437–445. doi: 10.1006/viro.1996.0550. [DOI] [PubMed] [Google Scholar]
- 16.Hochstenbach F, David V, Watkins S, Brenner M B. Endoplasmic reticulum resident protein of 90 kilodaltons associates with the T- and B-cell antigen receptors and major histocompatibility complex antigens during their assembly. Proc Natl Acad Sci USA. 1992;89:4734–4738. doi: 10.1073/pnas.89.10.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kliks S C, Nisalak A, Brandt W E, Wahl L, Burke D S. Antibody-dependent enhancement of dengue virus growth in human monocytes as a risk factor for dengue hemorrhagic fever. Am J Trop Med Hyg. 1989;40:444–451. doi: 10.4269/ajtmh.1989.40.444. [DOI] [PubMed] [Google Scholar]
- 18.Lobigs M, Usha R, Nestorowicz A, Marshall I D, Weir R C, Dalgarno L. Host cell selection of Murray Valley encephalitis virus variants altered at an RGD sequence in the envelope protein and in mouse virulence. Virology. 1990;176:587–595. doi: 10.1016/0042-6822(90)90029-q. [DOI] [PubMed] [Google Scholar]
- 19.Mackall J, Meredith M, Lane M D. A mild procedure for the rapid release of cytoplasmic enzymes from cultured animal cells. Anal Biochem. 1979;95:270–274. doi: 10.1016/0003-2697(79)90216-1. [DOI] [PubMed] [Google Scholar]
- 20.Mason P W, Pincus S, Fournier M J, Mason T L, Shope R E, Paoletti E. Japanese encephalitis virus-vaccinia recombinants produce particulate forms of the structural membrane proteins and induce high levels of protection against lethal JEV infection. Virology. 1991;180:294–305. doi: 10.1016/0042-6822(91)90034-9. [DOI] [PubMed] [Google Scholar]
- 21.Megret F, Hugnot J P, Falconar A, Gentry M K, Morens D M, Murray J M, Schlesinger J J, Wright P J, Young P, Van Regenmortel M H V, Deubel V. Use of recombinant fusion proteins and monoclonal antibodies to define linear and discontinuous antigenic sites on the dengue virus envelope glycoprotein. Virology. 1992;187:480–491. doi: 10.1016/0042-6822(92)90450-4. [DOI] [PubMed] [Google Scholar]
- 22.Pincus S, Mason P W, Konishi E, Fonseca B A L, Shope R E, Rice C M, Paoletti E. Recombinant vaccinia virus producing the prM and E proteins of yellow fever virus protects mice from lethal yellow fever encephalitis. Virology. 1992;187:290–297. doi: 10.1016/0042-6822(92)90317-i. [DOI] [PubMed] [Google Scholar]
- 23.Putnak R, Barvir D A, Burrous J M, Dubois D R, D’Andrea V M, Hoke C H, Sadoff J C, Eckels K H. Development of a purified, inactivated, dengue-2 virus vaccine prototype in Vero cells: immunogenicity and protection in mice and rhesus monkeys. J Infect Dis. 1996;174:1176–1184. doi: 10.1093/infdis/174.6.1176. [DOI] [PubMed] [Google Scholar]
- 24.Randolph V B, Winkler G, Stollar V. Acidotropic amines inhibit proteolytic processing of flavivirus prM protein. Virology. 1990;174:450–458. doi: 10.1016/0042-6822(90)90099-d. [DOI] [PubMed] [Google Scholar]
- 25.Rey F A, Heinz F X, Mandl C, Kunz C, Harrison S C. The envelope glycoprotein from tick-borne encephalitis virus at 2A resolution. Nature. 1995;375:291–298. doi: 10.1038/375291a0. [DOI] [PubMed] [Google Scholar]
- 26.Rice C M. Flaviviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, et al., editors. Fields virology. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 931–959. [Google Scholar]
- 27.Schalich J, Allison S L, Stiasny K, Mandl C W, Kunz C, Heinz F X. Recombinant subviral particles from tick-borne encephalitis virus are fusogenic and provide a model system for studying flavivirus envelope glycoprotein functions. J Virol. 1996;70:4549–4557. doi: 10.1128/jvi.70.7.4549-4557.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trirawatanapong T, Chandran B, Putnak R, Padmanabhan R. Mapping of a region of dengue virus type-2 glycoprotein required for binding by a neutralizing monoclonal antibody. Gene. 1992;116:139–150. doi: 10.1016/0378-1119(92)90509-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang S, He R, Patarapotikul J, Innis B L, Anderson R. Antibody-enhanced binding of dengue-2 virus to human platelets. Virology. 1995;213:254–257. doi: 10.1006/viro.1995.1567. [DOI] [PubMed] [Google Scholar]