Abstract
Forensic science is currently fast-growing for the development detection of the latent fingerprint. Currently, chemical dust quickly enters the body through touch or inhalation and will be affected by the user. In this research, a study on the comparison of natural powder from four species of medicinal plants (Zingiber montanum, Solanum Indicum L., Rhinacanthus nasutus, and Euphorbia tirucall) for the detection of latent fingerprints is carried out that has fewer adverse effects on the user's body by using such natural substances instead. In addition, the fluorescence properties of the dust have been found in some natural powder for sample detection and appear on multi-colored surfaces to show that the latent fingerprints are more pronounced than ordinary dust. In this study, medicinal plants have also been applied to detect cyanide, as it has been known that it is hazardous for humans and can be used as a poisonous compound to kill someone. The characteristics of each powder have also been analyzed using naked-eye detection under UV light, Fluorescence spectrophotometer, FIB-SEM, and FTIR. All the powder obtained can then be used for high potential detection of latent fingerprints on the non-porous surface with their specific characteristics and trace amounts of cyanide using turn-on-off fluorescent sensing method.
Keywords: Natural powder, fluorescence, latent fingerprint, forensic science, medicinal plant, biosensor, cyanide detection
Introduction
Cyanide (CN-) is a highly toxic ion to human health because cyanide can inhibit critical biological processes with oxidative metabolism and cellular respiration. Cyanide poisoning usually occurs due to being found in raw or processed foods that contain cyanogenic compounds. Cyanide compounds are also being used intentionally to kill someone because of their toxic properties.1–3 Detection of cyanide in water and foods currently has been developing with many methods such as mass spectrometry, 4 ion chromatograph, 5 flow injection, 6 Raman spectrometry, 7 colorimetry, 8 and others.
Fingerprints are said to be a golden standard for use to identify people, especially in forensic science, and it is also used for biometrics and scientific evidence in the police field. Papillary protrusions on the tips of the fingers, which contain rows of pores connected to the sweat glands, make each person's fingerprints unique. The only exception would be a person who had been in an accident so serious that the papillary tissue on the fingers, palms, soles of the feet, and feet were damaged because fingerprints are unique to each person, cannot be changed, are easy to verify, and leave marks on every object a person touches, they are routinely used as evidence in court and by the police.9–28 To detect latent fingerprints, investigators need some physical or chemical processes (water, amino acids, oils, and some other substances) to enhance the fingerprint residue because latent fingerprints cannot be seen by the naked eye.29–33 Detection of latent fingerprints currently using traditional methods based on chemicals such as ninhydrin, 34 ferric oxide, 35 cyanoacrylate, 36 and DFO. 37 However, various research studies involving the synthesis of fluorescent dust remain limited in many areas, such as the complexity of synthesis. The toxicity of raw materials and dust powder with a high price, for that reason, hinders its practical use. 38 Therefore, the research team is interested in a highly effective method of preparing fluorescent dust powder used in examining latent fingerprints from medicinal plants that have the potential ability to glow under UV light and can detect trace amounts of cyanide using the medicinal plants by using fluorescence turn on-off method, other than having a property that can glow under UV light. The medicinal dust powder can be prepared inexpensively and is environmentally friendly.42,43 Most of the chemical compounds of each medicinal plant are shown in Table 1. It is shown that almost powder compound is antibacterial and anti-inflammatory, meaning the powder is not toxic to the human body. The factors affecting latent fingerprints were also studied, such as different material surfaces, temperature, storage time, and the identification of latent fingerprints from overlapping fingerprints.
Table 1.
Major chemical compounds and their applications in selected four medicinal plants.
| Plant name | Major compound | Application | Reference |
|---|---|---|---|
| Zingiber montanum | α-Pinene | Anti-inflammatory via PGE1, and is likely antimicrobial activity | 39 |
| β-Pinene | Fungicidal agents, flavors, fragrances, and antiviral and antimicrobial agents | ||
| Terpinen-4-ol | Anti-inflammatory and antioxidant agent | ||
| Rhinacanthus nasutus | 3,4-Dihydro-3,3-dimethyl-2H-naphtho[2,3-b] pyran-5,10-dione | Antitumor activity | 40 |
| Rhinacanthin A | Antimicrobial activity | ||
| 2-Hydroxy-1,4-naphthoquinone | An interconversion of pyridine nucleotides to combat the effects of oxidative stress | ||
| Solanum indicum L. | Luteolin | Anti-inflammation, anti-allergy and anticancer | 39 |
| Apigenin | Strong anti-inflammatory, antioxidant, antibacterial activity | ||
| Gentisic acid | Anti-inflammatory, antigenotoxic, hepatoprotective | ||
| Euphorbia tirucall | Campesterol | Cholesterol-lowering properties | 41 |
| Cyclotirucanenol | Anti-inflammatory, anti-diabetic, anti-cancer | ||
| Diterpene ester | Antimicrobial and anti-inflammatory |
Experimental
Chemicals and reagents
All chemicals used in this research are analytical grade. Potassium cyanide (KCN) was obtained from Spectrum Chemical (USA). Four common species of Thai medicinal plants were selected and used in this study including Zingiber montanum, Euphorbia tirucall, Rhinachantus nasutus, and Solanum indicum collected during a short winter time (November 2021–January 2022) from our flower park of Science Building, Khon Kaen University, Thailand.
Instruments
The pretreatment of the medicinal plant powder was carried out in a heating oven (BINDER ED 115UL-120V), grind by using a high-speed multi-function crusher 2500A, and filtered by using a laboratory test sieve 100 mesh. The dried and ground powder was examined by an optical property using a fluorescence spectrophotometer (RF-5301 PC, Shimadzu), and their characteristics were identified by FTIR (Fourier transform infrared spectrometer, Bruker TENSOR 27), FIB-SEM (Helios NanoLab G3 CX). For the detection application of latent fingerprint, the material surface will be checked using a UV chamber (Trans-illuminator Model TM-26 (302 nm)) and TLC UV Cabinet (365 nm).
Preparation of the medicinal plant powder
The 500-g rhizome of the plant Zingiber montanum and 500 g leaf of Solanum indicum L., Euphorbia tirucall and Rhinacanthus nasutus, each was cut into small sizes and dry in the oven for 48 h at 75°C, after that the plant will be ground in high-speed multi-function crusher 220 V for 5 min, then filtered using laboratory test sieve 100 mesh. The final fine powder was kept in a capped plastic bottle at room temperature for further use.
Method detection of a latent fingerprint
To detect the latent fingerprint using the common brushing method, a volunteer rubbed his forehead with a finger and printed his fingerprint on the different material surfaces for 5 s and check its trace immediately under a UV chamber.
Method detection of cyanide
Cyanide has been prepared by using KCN, and the KCN was diluted using DI water until varying concentrations 5, 6, 7, 8, 9, and 10 µM. After finishing the preparation, all the reagents were stirred using a magnetic stirrer for 5 min to ensure that KCN was completely dissolved. One milliliter from each solution adding to the vial with 0.25 medicinal solution and 9 mL DI water. The analysis will be using a fluorescence spectrophotometer (RF-5301 PC, Shimadzu) with a maximum wavelength of 380 nm to excite the ground-state electron of the CDs, and then the excited electron undergoes an energy transfer, which is visible as a fluorescence spectrum.44,45
Results and discussion
Characteristics and optical properties of the medicinal plant powder
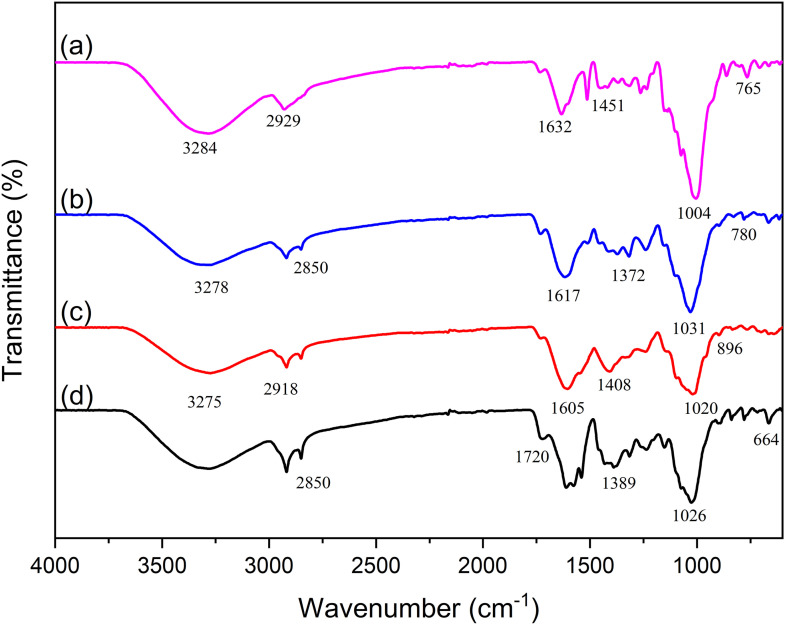
Similar characteristic patterns of four selected medicinal plant powders have been analyzed by using FTIR as shown in Figure 1(a). There are OH/N-H groups on 3284 cm−1, 2929 cm−1 for C-H bonding, 1632 cm−1 for C=C, and C=N, C=C, and C-OH bands were found on 1451 cm−1, 1004 cm−1 was representative for C-H bending peak, and CO2 vibration was found in Zingiber montanum. The FTIR of Solanum indicum L. can be seen in Figure 1(b) having OH/N-H group on 3278 cm−1, 2850 cm−1 for C-H bonding, 1617 cm−1 for hexion vibration N-H, C-H band was found on 1372 cm−1, 1031 was representative on C-O bending, and C-H bonding on 781 cm−1. Figure 1(c) shows the FTIR of Rhinacanthus nasutus OH/N-H group was found on 3275 cm−1, 2918 cm−1 for C-H bounding, 1605 and 1408 cm−1 for C-C band, C-O bonding was found on 1020 cm−1, and C-H bonding on 896 cm−1. The FTIR of Euphorbia tirucall was also shown in Figure 1(d) having a C-H group on 2850 cm−1, 1720 cm−1 for C=O bonding, 1389 cm−1 for C=H bond, and C-O band was found on 1026 cm−1, and C-H bonding was found on 664 cm−1.46–55
Figure 1.
Fourier transform infrared spectra of (a) Zingiber montanum, (b) Solanum Indicum L., (c) Rhinacanthus nasutus, and (d) Euphorbia tirucall.
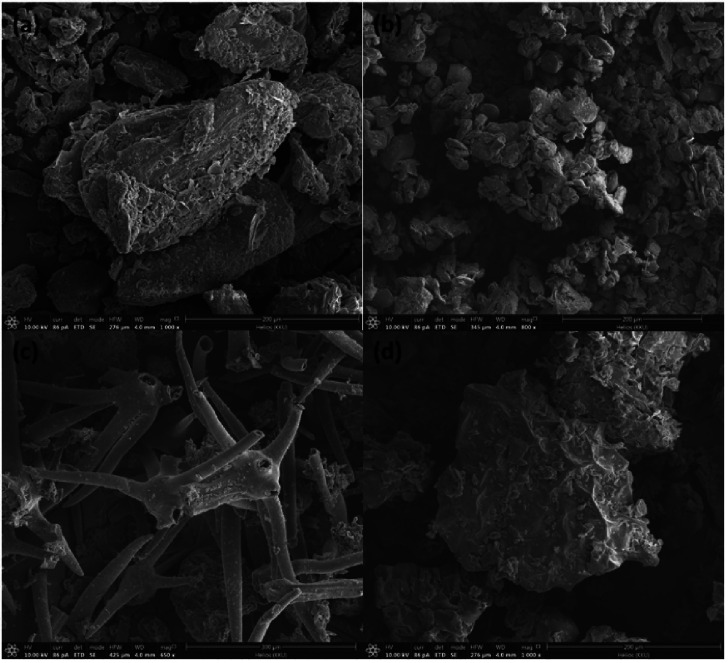
The medicinal powder has been observed for their morphology images with FIB-SEM as shown in Figure 2. It can be seen that the particle of the powder is different from each other depending on the type of plants used. Figure 2(a) shows the characteristic powder from Euphorbia tirucall, it is shown that the powder has a non-smooth surface, and an irregular shape, then by using SEM the average sized of the powder is more than 200 μm. Figure 2(b) gives the characteristic powder from Zingiber montanum, it is shown that the powder has a non-smooth surface and has an oval-shape, and the average size of the powder is less than 100 μm by using SEM. Figure 2(c) shows the characteristic powder from Solanum indicum L., it is evident that the powder has a smooth surface with an elongated part. Figure 2(d) shows the characteristic powder from Rhinacanthus nasutus, indicating the powder has a non-smooth surface with an irregular shape, the average size of the powder is around 200 μm by using SEM.
Figure 2.
Focused ion beam scanning electron microscope of (a) Euphorbia tirucall, (b) Zingiber montanum, (c) Solanum Indicum L., and (d) Rhinacanthus nasutus.
The optical property of the plant powder has also been characterized using a fluorescence spectrophotometer, UV–Vis spectrophotometer, and UV light chamber. The 0.05-g from each powder has been diluted with 10 mL DI water. 1 and was analyzed by fluorescence spectrophotometer. Figure 3 shows that the fluorescence spectra with excitation 360 nm from Zingiber montanum powder is higher compared with the others, while that of Rhinacanthus nasutus didn’t show the intensity of the fluorescence spectrum.
Figure 3.
Fluorescence spectra of the powder samples from medicinal plants.
Comparison of the medicinal plant powder vs black powder for latent fingerprints detection on a non-porous surface under UV light and daylight
The plant powder was applied to detect latent fingerprints on the non-porous surface, as shown in Figure 4 . All the plant powder can be used for latent fingerprints detection on a non-porous surface, especially in this case using glass material, Zingiber montanum shows a yellow color under lightroom and yellow-green under UV light chamber, Rhinacanthus nasutus gives dark green under UV light chamber and light room, Solanum indicum L. shows the brown color under light room and blue color under UV light chamber, and Euphorbia tirucall also shows the green color under the light room and UV light chamber. That of Zingiber montanum is the brightness than the others. Figure 4 also shows that the comparison of natural powder from medicinal plants with commercial powder (black powder) has been studied in this research. It shows that all medicinal powders give much more detail of latent fingerprint tips compared with that of commercial powder in a lightroom, especially for Zingiber montanum, which shows higher fluorescent intensity under a UV chamber.
Figure 4.
Comparison of the medicinal plant powder vs black powder for latent fingerprints detection on non-porous surface under UV light and day light (The pictures were taken using UV Transilluminator Model TM-26) (a) Zingiber montanum, (b) Rhinacanthus nasutus, (c) Solanum Indicum L., (d) Euphorbia tirucall, and (e) Black powder (commercial powder).
Detection of the latent fingerprints on the non-porous surface has been tried in various surface materials. The plant powder was applied on the surface after the volunteer pressed the fingerprint in the material. The surface was checked under a UV lamp to compare the contrast of fluorescence of latent fingerprints.
Various surface materials have also tried to detect latent fingerprints on the non-porous surface. After the volunteer pressed the fingerprint print on the material, the plant powder was applied on the surface. The surface was checked under a UV lamp to compare the contrast of the fluorophore's emission from the latent fingerprints tracer. However, data shown in Table 2 demonstrated that all the natural plant powder could be used to detect latent fingerprints on many types of surfaces such as plastic, iron, ceramic, and paper. Even the extent of the fade detection could not be differentiated from the visualized details, which can be seen in SI1. The resistance and durability also have been tested in this study. We made comparing on the 1st day after the volunteer pressed the fingerprint with the day 30th. The non-porous material surface was kept in the forensic science laboratory Khon Kaen University, at room temperature (about 27°C). The volunteer pressed the fingerprint on the iron material. In this test, iron material from a knife was applied, and the result is shown in Figure 5. The results showed that all the natural powder could be used to detect the latent fingerprints on the non-porous surfaces, particularly in this case, only after one month the fingertips were pressed on the material surfaces.
Table 2.
Application of four medicinal plants powder on various surface materials.
| Plant name/materials | Plastic | Ceramic | Paper | Iron |
|---|---|---|---|---|
| Zingiber montanum | / | / | / | / |
| Rhinacanthus nasutus | / | / | / | / |
| Solanum indicum L. | / | / | / | / |
| Euphorbia tirucall | / | / | / | / |
/: latent fingerprint detected.
Figure 5.
Comparison of the plant powder on 1st day and 30th day after pressing the fingertips on non-porous surface. (The pictures were taken under TLC UV light cabinet.)
Detection of cyanide by using the powder sample of the medicinal plants
Medicinal plant fluorescence turn-off was investigated at different KCN concentrations in the range of 5–10 µM as shown in Figure 6. The quenching effect of the fluorescence intensity may occur due to different reasons such as energy transfer, surface absorption, or surface characteristic. 0.05 g of each medicinal powder was diluted with 10 mL of DI water. For detection using FL spectrophotometer, 0.25 µL of each medicinal liquid, adding 1 mL of KCN and 9 mL DI water with the setting condition of maximum excitation wavelength of 360 nm, after measurement the spectral data were obtained as shown in Figure 6(d). The cyanide detection by using Euphorbia tirucall, powder shows that the powder can be used to detect trace cyanide ion in various concentrations from 5–10 µM with the limitation of detection (LOD) of 1.2 µM as shown in Figure 6(a). Various concentrations of cyanide also can be detected by using Solanum Indicum L. (Figure 6(e)) with 2.8 µM LOD.Figure 6(f) shows that Zingiber montanum can be used to detect cyanide using turn on-off method under the suitable conditions of various concentration titration of cyanide with 1.04 µM LOD. The LOD was calculated by using the following formulation:
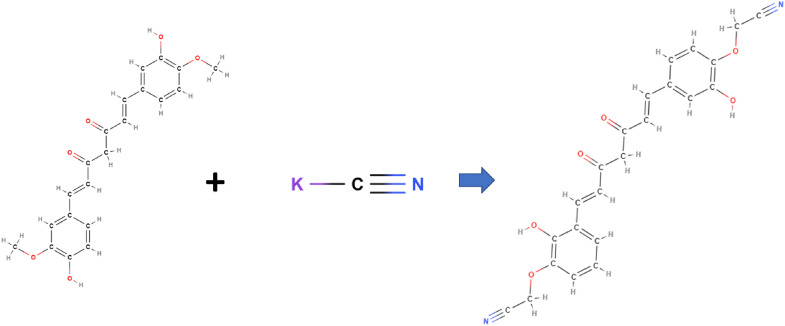
where SD is the standard deviation. The prediction mechanism for the adherence of each powder to the cyanide ion can be assigned by the formation of hydrogen bonds between CN− and the carbonyl or hydroxyl group of organic compounds present in each medicinal powder, for example such as the major chemical compounds of curcumin generally found in Zingiber montanum with cyanide ion (Figure 7). The selectivity test of the selected medicinal powder also has been studied and it was found special for Euphorbia tirucall in Figure 6(g) after adding the cyanide the concentration of fluorescence will decrease compared to others, Figure 6(h)Solanum Indicum and Figure 6(i)Zingiber montanum.
Figure 6.
Detection LOD of (a) Euphorbia tirucall (b) Solanum Indicum L. (c) Zingiber montanum Emission spectra detection KCN of (d) Euphorbia tirucall (e) Solanum Indicum L. (f) Zingiber montanum, Turn-Off methods sensitivity test of (g) Euphorbia tirucall (h) Solanum Indicum L. (i) Zingiber montanum, Wavelength Ex = 360 nm slit width 5/5.
Figure 7.
Prediction Mechanism reaction detection KCN Zingiber Montanum (curcumin).
Conclusion
The natural characteristics of medicinal plants can be simply used for the pronounced detection of latent fingerprints on non-porous surfaces. Some plants exhibit distinct fluorescence under UV excitation, which has quickly been evident under UV light chamber and fluorescence spectrophotometry in the routine analysis. The characteristic powder and its optical properties were observed using fluorescence spectrum, FIB-SEM, and FTIR, demonstrating that the medicinal powder has a different characteristic under FIB-SEM and UV light visualization. Zingiber montanum has the highest intensity fluorescence compared to other powders. The application for latent fingerprint detection was carried out with experiments on various non-porous materials (iron, plastic, etc.). The experimental optimization was also done with the effect of temperature and storage time of the materials used. The effect of storage time of the plant powder could be worked satisfactorily as shelf-life for 30 days. The powder samples of the medicinal plants also have been analyzed for the detection of cyanide. It was found that the Rhinacanthus nasutus can't be used to detect trace cyanide concentrations.
Supplemental Material
Supplemental material, sj-docx-1-sci-10.1177_00368504231156217 for Fluorophores-rich natural powder from selected medicinal plants for detection latent fingerprints and cyanide by David Nugroho, Saksit Chanthai, Won-Chun Oh and Rachadaporn Benchawattananon in Science Progress
Acknowledgements
The authors thank the research financial support to: (1) Research Center for Environmental and Hazardous Substance Management (EHSM), Khon Kaen University; (2) Materials Chemistry Research Center, Department of Chemistry and Center of Excellence for Innovation in Chemistry (PERCH-CIC), Faculty of Science, Khon Kaen University, Thailand.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: David Nugroho https://orcid.org/0000-0002-5628-9283
Supplemental material: Supplemental material for this article is available online.
References
- 1.Ding M, Wang K. Determination of cyanide in bamboo shoots by microdiffusion combined with ion chromatography–pulsed amperometric detection. R Soc Open sci 2018; 5: 172128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Long L, Yuan X, Cao Set al. et al. Determination of cyanide in water and food samples using an efficient naphthalene-based ratiometric fluorescent probe. ACS Omega 2019; 4: 10784–70790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jo J, Lee D. Turn-on fluorescence detection of cyanide in water: activation of latent fluorophores through remote hydrogen bonds that mimic peptide β-turn motif. J Am Chem Soc 2009; 44: 16283–16291. [DOI] [PubMed] [Google Scholar]
- 4.Argoti D, Liang L, Conteh Aet al. et al. Cyanide trapping of iminium ion reactive intermediates followed by detection and structure identification using liquid chromatography–tandem mass spectrometry (LC–MS/MS). Chem Res Toxicol 2005; 18: 1537–1544. [DOI] [PubMed] [Google Scholar]
- 5.Wu W, Xiao Q, Zhang Pet al. et al. Rapid measurement of free cyanide in liquor by ion chromatography with pulsed amperometric detection. Food Chem 2015; 172: 681–684. [DOI] [PubMed] [Google Scholar]
- 6.Hasan S, Hamza M, Kelany A. A novel spectrophotometric method for batch and flow injection determination of cyanide in electroplating wastewater. Talanta 2007; 71: 1088–1095. [DOI] [PubMed] [Google Scholar]
- 7.Yea K-H, Lee S, Kyong Jet al. et al. Ultra-sensitive trace analysis of cyanide water pollutant in a PDMS microfluidic channel using surface-enhanced Raman spectroscopy. Analyst 2005; 130: 1009–1011. [DOI] [PubMed] [Google Scholar]
- 8.Cheng X, Zhou Y, Qin Jet al. et al. Reaction-based colorimetric cyanide chemosensors: rapid naked-eye detection and high selectivity. ACS Appl Mater Interfaces 2012; 4: 2133–2138. [DOI] [PubMed] [Google Scholar]
- 9.Sari SA, Qalbiah U, Putri IC. Comparison between latent fingerprint identification using black powder and cyanoacrylate glue. Asian J Chem 2018; 30: 2615–2620. [Google Scholar]
- 10.Liu J, Li R, Yang B. Carbon dots: a new type of carbon-based nanomaterial with wide applications. ACS Cent Sci 2020; 6: 2179–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaushal N, Kaushal P. Human identification and fingerprints: a review. J Biomet Biostat 2011; 2: 23. [Google Scholar]
- 12.Vokey JR, Tangen JM, Cole SA. On the preliminary psychophysics of fingerprint identification. Q J Exp Psychol 2009; 62: 1023–1040. [DOI] [PubMed] [Google Scholar]
- 13.Tang M, Ren G, Zhu Bet al. et al. Facile synthesis of orange emissive carbon dots and their application for mercury ion detection and fast fingerprint development. Anal Methods 2019; 11: 2072–2081. [Google Scholar]
- 14.Nayak VC, Rastogi P, Kanchan Tet al. Sex differences from fingerprint ridge density in the Indian population. J Forensic Leg Med 2010; 17: 84–86. [DOI] [PubMed] [Google Scholar]
- 15.Li H, Guo X, Liu Jet al. et al. A synthesis of fluorescent starch based on carbon nanoparticles for fingerprints detection. Opt Mater 2016; 60: 404–410. [Google Scholar]
- 16.Prabakaran E, Pillay K. Nanomaterials for latent fingerprint detection: a review. J Mater Res Technol 2021; 12: 1856–1885. [Google Scholar]
- 17.Chen H, Chang K, Men Xet al. et al. Covalent patterning and rapid visualization of latent fingerprints with photo-cross-linkable semiconductor polymer dots. ACS Appl Mater Interfaces 2015; 7: 14477–14484. [DOI] [PubMed] [Google Scholar]
- 18.Singh K, Sharma S, Garg R. Visualization of latent fingerprints using silica gel G: a new technique. Egypt J Forensic Sci 2013; 3: 20–25. [Google Scholar]
- 19.Abdelwahab W, Phillips E, Patonay G. Preparation of fluorescently labeled silica nanoparticles using an amino acid-catalyzed seeds regrowth technique: application to latent fingerprints detection and hemocompatibility studies. J Colloid Interface Sci 2018; 512: 801–811. [DOI] [PubMed] [Google Scholar]
- 20.Ran X, Wang Z, Zhang Zet al. et al. Nucleic–acid–programmed Ag–nanoclusters as a generic platform for visualization of latent fingerprints and exogenous substances. Chem Commun 2015; 52: 557–560. [DOI] [PubMed] [Google Scholar]
- 21.Spindler X, Hofstetter O, McDonagh Aet al. et al. Enhancement of latent fingermarks on non-porous surfaces using anti-L-amino acid antibodies conjugated to gold nanoparticles. Chem Commun 2011; 47: 5602–5604. [DOI] [PubMed] [Google Scholar]
- 22.Rohatgi R, Kapoor A. Development of latent fingerprints on wet non-porous surfaces with SPR based on basic fuchsin dye. Egypt J Forensic Sci 2016; 6: 179–184. [Google Scholar]
- 23.Jasuja O, Singh GD, Sodhi G. Small particle reagents: development of fluorescent variants. Sci Justice 2008; 48: 141–145. [DOI] [PubMed] [Google Scholar]
- 24.Yuan C, Li M, Wang Met al. et al. Sensitive development of latent fingerprints using rhodamine B-diatomaceous earth composites and principle of efficient image enhancement behind their fluorescence characteristics. Chem Eng J 2020; 383: 123076. [Google Scholar]
- 25.Wang J, Ma Q, Liu Het al. et al. Time-gated imaging of latent fingerprints and specific visualization of protein secretions via molecular recognition. Anal Chem 2017; 89: 12764–12770. [DOI] [PubMed] [Google Scholar]
- 26.Xu C, Zhou R, He Wet al. et al. Fast imaging of eccrine latent fingerprints with nontoxic Mn-doped ZnS QDs. Anal Chem 2014; 86: 3279–3283. [DOI] [PubMed] [Google Scholar]
- 27.Gouiaa M, Bennour I, Haddada LRet al. et al. Spectroscopic characterization of Er,Yb:Y2Ti2O7 phosphor for latent fingerprint detection. Physica B Condens Matter 2020; 582: 412009. [Google Scholar]
- 28.Wang C-F, Cheng R, Ji W-Qet al. et al. Recognition of latent fingerprints and ink-free printing derived from interfacial segregation of carbon dots. ACS Appl Mater Interfaces 2018; 10: 39205–39213. [DOI] [PubMed] [Google Scholar]
- 29.Kumbhakar P, Biswas S, Pandey Pet al. et al. Tailoring of structural and photoluminescence emissions by Mn and Cu co-doping in 2D nanostructures of ZnS for the visualization of latent fingerprints and generation of white light. Nanoscale 2019; 11: 2017–2026. [DOI] [PubMed] [Google Scholar]
- 30.Jin X, Xin R, Wang Set al. et al. A tetraphenylethene-based dye for latent fingerprint analysis. Sens Actuators B Chem 2017; 244: 777–784. [Google Scholar]
- 31.Wang M, Li M, Yu Aet al. et al. Fluorescent nanomaterials for the development of latent fingerprints in forensic sciences. Adv Funct Mater 2017; 27: 1606243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raju GR, Park JY, Nagaraju GPet al. et al. Evolution of CaGd2ZnO5:Eu3+ nanostructures for rapid visualization of latent fingerprints. J Mater Chem C 2017; 5: 4246–4256. [Google Scholar]
- 33.Milenkovic I, Algarra M, Alcoholado C, et al. Fingerprint imaging using N-doped carbon dots. Carbon N Y 2019; 144: 791–797. [Google Scholar]
- 34.Odén S, Hofsten BV. Detection of fingerprints by the ninhydrin reaction. Nature 1954; 173: 449–450. [DOI] [PubMed] [Google Scholar]
- 35.Haque F, Westland A, Milligan Jet al. et al. A small particle (iron oxide) suspension for detection of latent fingerprints on smooth surfaces. Forensic Sci Int 1989; 41: 73–82. [Google Scholar]
- 36.Bumbrah GS. Cyanoacrylate fuming method for detection of latent fingermarks: a review. Egypt J Forensic Sci 2017; 7: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stoilovic M. Improved method for DFO development of latent fingerprints. Forensic Sci Int 1993; 60: 141–153. [Google Scholar]
- 38.Basavaraj R, Nagabhushana H, Darshan Get al. et al. Red and green emitting CTAB assisted CdSiO3:Tb3+/Eu3+ nanopowders as fluorescent labeling agents used in forensic and display applications. Dyes Pigm 2017; 147: 364–377. [Google Scholar]
- 39.Huong L, Huong T, Huong Net al. et al. Chemical composition and larvicidal activity of essential oils from Zingiber montanum (J. Koenig) link ex. A. Dietr. against three mosquito vectors. B Latinoam Caribe Pl 2020; 19: 569–579. [Google Scholar]
- 40.Brimson J, Prasanth M, Malar Det al. et al. Rhinacanthus nasutus “tea” infusions and the medicinal benefits of the constituent phytochemicals. Nutriens 2020; 12: 3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mali P, Panchal S. Euphorbia tirucalli L.: review on morphology, medicinal uses, phytochemistry and pharmacological activities. Asian Pac J Trop Biomed 2017; 7: 603–613. [Google Scholar]
- 42.Al-Amin M, Sultana GN, Hossain CF. Antiulcer principle from Zingiber montanum. J Ethnopharmacol 2012; 141: 57–60. [DOI] [PubMed] [Google Scholar]
- 43.Pattarith K, Chanthai S, Benchawattananon R. Fluorescent labeling of silica gel powder using Zingiber montanum extract for a bright latent fingerprint detection under UV-light. Orient J Chem 2021; 31: 541–546. [Google Scholar]
- 44.Kongsanan N, Pimsin N, Keawprom Cet al. et al. A fluorescence switching sensor for sensitive and selective detections of cyanide and ferricyanide using mercuric cation-graphene quantum dots. ACS Omega 2021; 22: 14379–14393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jaszczak E, Polkowska Ż, Narkowicz Set al. et al. Cyanides in the environment—analysis—problems and challenges. Environ Sci Pollut Res 2017; 24: 15929–15948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chai M, Isa M. The oleic acid composition effect on the carboxymethyl the oleic acid composition effect on the carboxymethyl. JCPT 2013; 3: 1–4. [Google Scholar]
- 47.Zheng J, Xie Y, Wei Yet al. et al. An efficient synthesis and photoelectric properties of green carbon quantum dots with high fluorescent quantum yield. Nanomaterials 2020; 10: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nugroho D, Keawprom C, Chanthai Set al. et al. Highly sensitive fingerprint detection under UV light on non-porous surface using starch-powder based luminol-doped carbon dots (N-CDs) from tender coconut water as a green carbon source. Nanomaterials 2021; 12: 00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Balouch A, Umar AA, Mawarnis ERet al. et al. Synthesis of amorphous platinum nanofibers directly on an ITO substrate and its heterogeneous catalytic hydrogenation characterization. ACS Appl Mater Interfaces 2015; 7: 7776–7785. [DOI] [PubMed] [Google Scholar]
- 50.Anwar M, Nisa K, Indirayati N. Acid–base evaluation of chitosan–ferulic acid conjugate by a free radical grafting method. IOP Conf Ser Earth Environ Sci 2019; 251: 012023. [Google Scholar]
- 51.Gatica N, Alvardo N. Blends of poly(vinyl pyridine)s and two dihydric phenols: thermal and infrared spectroscopic studies. Part II. J Chil Chem Soc 2009; 54: 317–322. [Google Scholar]
- 52.Wahyono T, Astuti DA, Wiryawan IKet al. et al. Fourier transform mid-infrared (FTIR) spectroscopy to identify tannin compounds in the panicle of sorghum mutant lines. IOP Conf Ser: Mater Sci Eng 2019; 546: 042045. [Google Scholar]
- 53.Queiroz MF, Melo KR, Sabry DAet al. et al. Does the use of chitosan contribute to oxalate kidney stone formation? Mar Drugs 2015; 13: 141–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aghaei M, Imani M, Tadjarodi A. Preparation of Cu4SnS4/CuCo2S4 nanoparticles using combustion reaction accelerated by organic driving agents under microwave irradiation †. Chem Proc 2021; 3: 51. [Google Scholar]
- 55.Pijanka J, Sockalingum GD, Kohler Aet al. et al. Synchrotron-based FTIR spectra of stained single cells. Towards a clinical application in pathology. Lab Investig 2010; 90: 797–807. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-sci-10.1177_00368504231156217 for Fluorophores-rich natural powder from selected medicinal plants for detection latent fingerprints and cyanide by David Nugroho, Saksit Chanthai, Won-Chun Oh and Rachadaporn Benchawattananon in Science Progress