Abstract
Introduction
Although house flies (Musca domestica) do not directly cause disease in humans, they transmit pathogens to them, which provide the basis for many diseases. The main way to deal with this insect is to use insecticides. Due to the resistance from insecticides, the fight against house flies has been hampered. This study aimed to determine the prevalence of knockdown resistance against organochlorine insecticides in house flies worldwide.
Methods
This study was conducted via a systematic review and meta-analysis to investigate the prevalence of knockdown resistance against organochlorine insecticides in house flies. Accordingly, by searching the databases of Web of Science, PubMed, Scopus, Proquest, Bioone, and Embase, all published articles were extracted, and reviewed until the end of May 2022. Statistical data analysis was performed using the random-effects model in the meta-analysis, meta-regression, and I2 index.
Results
Nine studies entered the meta-analysis process. Based on this, the prevalence of knockdown resistance against organochlorine insecticide in house flies was estimated to be 49.1%. Meta-regression showed that the prevalence of knockdown resistance increased with increasing years of study but decreased with increasing sample size.
Conclusion
According to the findings, about 50% of house flies have knockdown resistance against organochlorine insecticide. As a result, it is necessary to adopt effective and combined methods to combat this insect to control it and prevent the transmission of diseases caused by it.
Keywords: Knockdown resistance, Organochlorine insecticide, Housefly, Musca domestica
1. Introduction
Vector-borne diseases are one of the leading causes of disease and mortality in humans, especially in tropical and subtropical regions of the world. One of these vectors that have been present in the human environment for many years and have caused the transmission of many diseases to humans is the housefly (Musca domestica). Domestic flies are the most common and widespread species of flies in the world and are found in urban and rural areas with tropical and temperate climates (Khamesipour et al., 2018; Ommi et al., 2015).
House flies are found in places where humans are active, such as farms, poultry farms, restaurants, hospitals, food markets, slaughterhouses, and food centers, which are a nuisance to humans, poultry, livestock, and other farm animals (Awache and Farouk, 2016). House flies also carry many pathogens that can cause serious and deadly diseases in humans and animals. This insect carries pathogens such as viruses, bacteria, fungi, and parasites (Bahrndorff et al., 2017; Tsagaan and Okado, 2015). House flies feed and reproduce on human feces, animal manure, carrion, and decaying organic matter. Therefore, they are closely related to various microorganisms.
As a result, the constant movement of house flies between breeding sites and human life can lead to the transmission of pathogens to humans and animals. Flies also carry antimicrobial-resistant bacteria and fungi in hospitals and animal farms and have been linked to the transmission of nosocomial infections (Sarwar, 2015; Nazari et al., 2017). Consequently, control of this vector is of particular importance. One of the ways to prevent the transmission of these diseases by vectors is to control them through the use of insecticides and this measure has played a key role in controlling and preventing the transmission of infectious diseases such as dengue fever, malaria, and leishmaniasis (Rivero et al., 2010).
The widespread and improper use of insecticides and incomplete treatment can lead to resistance. It inactivates the insecticide and limits the options available to control disease (Toloza et al., 2014). Resistance to a variety of insecticides has been reported in most species of insects that are major vectors of human diseases. So resistance to insecticides has been considered a serious public health challenge in the world (Organization WH, 2006).
Insecticide resistance is a phenotypic and genotypic feature of insect survival. So far, four types of mechanisms have been identified for resistance to chemical insecticides, including metabolic resistance, resistance at the target site, resistance to penetration, and behavioral resistance (Hemingway and Ranson, 2000). Metabolic resistance involves the isolation, metabolism, or detoxification of insecticides through the overproduction of specific enzymes such as carboxylesterases, glutathione-S-transferases, and cytochrome monooxygenase, which are often overproduced through two non-specific mechanisms. Gene amplification increases the number of copies of a gene and gene expression occurs through changes in the promoter region or mutations in trans-regulatory genes.
Resistance at the target site is created by point mutations that make the real targets of an insecticide less sensitive to the active substance. Most insecticides with a nervous mechanism are used to target one of the following three targets: acetylcholinesterase, gamma-aminobutyric acid (GABA) receptors, or sodium channels. Acetylcholinesterase is the target of organophosphate and carbamate insecticides. GABA receptors are the main target of organochlorine insecticides, and sodium channels are the target of pyrethroid and organochlorine insecticides. As a result, resistance in the target area causes resistance against these insecticides (Rivero et al., 2010).
Penetrance resistance includes resistance to insecticide penetration from the skin and cuticle wall of insects into their lymph and body. Lipophilic insecticides, such as pyrethroids, penetrate the cuticle through the shell and, after passing through this barrier, are transported by the hemolymph to the target organs where they accumulate, metabolize, or excrete (Pan et al., 2009). In resistant insects, cuticle proteins are secreted by the epidermis directly into the cuticle and covalently joined together to form a fixed, sclerotic cuticle. The thickness of the cuticle also changes as the insect grows or ages (Andersen, 2010; Wood et al., 2010). According to one hypothesis, the enzyme laccase is involved in sclerotia of the cuticle in insects and makes the cuticle impermeable to insecticides even at high concentrations. In insects, resistance to insecticides can be attributed to lower penetration rates due to higher protein and fat content of the cuticle or more sclerosis (Pan et al., 2009; Arakane et al., 2005).
Finally, behavioral resistance involves changing the behavior of the vector to reduce exposure to insecticides, such as leaving the site or cutting off feeding (Zalucki and Furlong, 2017).
The mainstay of the vector program is four classes of insecticides, including organochlorines, organophosphates, carbamates, and pyrethroids (Organization WH, 2006). These insecticides, especially organochlorines and pyrethroids, often kill vectors by acting on sodium channels and disrupting the opening and closing of sodium channels (Dong, 2007; Rinkevich et al., 2013). As a result, resistance in these target areas leads to resistance to insecticides. One of the main mechanisms of resistance to organochlorines and pyrethroids is the insensitivity of the target site due to mutations in voltage-sensitive sodium channel gene (VSSC). The first and most important mutation in VSSC is known as knockdown resistance (kdr). So far, more than 50 mutations in the sodium channel or a combination of mutations have been reported that are related to or responsible for kdr allele in vectors of various diseases. Kdr mutations reduce neuronal sensitivity to pyrethroids in insects (Rinkevich et al., 2013; Rinkevich et al., 2012; Firooziyan et al., 2017). Studies have also identified this resistance in house flies. Two mutations, L1014H (kdr-his) and M918T + L1014F (super-kdr), which are also related to kdr, have been identified in house flies that have provided resistance to treatment against pyrethroids (Dong, 2007; Rinkevich et al., 2013).
Considering the importance of resistance of house flies against insecticides to control this disease vector, determining the prevalence of resistance in this insect can be necessary for the control and use of appropriate insecticides to combat it. Therefore, the present study aimed to determine the prevalence of kdr allele in house flies against organochlorine insecticides worldwide via a systematic review and meta-analysis.
2. Material and methods
This study was conducted via a systematic review and meta-analysis on the prevalence of kdr in house flies against organochlorine insecticides worldwide based on the Prism guideline for systematic review and meta-analysis studies (Moher et al., 2015). Two researchers independently performed all stages of the research including scientific databases, selection of studies, quality assessment of articles, and data extraction. This study was registered in the international prospective register of systematic review (PROSPERO) with the code CRD42021231598.
2.1. Search for articles
In the search, all English language articles published without a time limit until the end of May 2022 were extracted during searches in the databases of Web of Science, PubMed, Scopus, Proquest, Bioone, and Embase. All articles with medical subject headings (Mesh) and the keywords knockdown resistance, KDR, Organochlorine, insecticide, Scabecide, chlorophenyl, dichloro, ethane, ethyl phenyl, benzene, chlorobenzene, trichloroethylene, housefly and Musca domestica in the title, abstract and full text of the articles. Single and combined forms were searched using the AND and OR operators.
2.2. Inclusion and exclusion criteria
Articles with the following criteria of publications in English language format, on resistance in house flies, kdr survey, resistance to organochlorine insecticides, data acquisition to estimate the prevalence of resistance, and optimal quality, were included in the study. In contrast, articles that were related to other classes of insecticides, review, meta-analysis, case report, or series of cases articles, which did not fulfill the inclusion criteria were excluded from the study.
2.3. Quality assessment
The Strobe checklist (Strengthening the Reporting of Observational Studies in Epidemiology) was used to evaluate the quality of the articles (Von Elm et al., 2014). This checklist has 22 sections in the fields of title, abstract, introduction, objectives, study method, inclusion and exclusion criteria, sample size, study group, results, and limitations, which are scored based on the importance of each section. The minimum and maximum scores that can be obtained by this checklist are 15 and 33. In this study, the minimum acceptable score was 20 (Bazyar et al., 2018).
2.4. Extracting the data
First, the articles were reviewed by two researchers independently by reviewing the title and abstract by considering the inclusion and exclusion criteria. Then, full text articles were reviewed by these researchers and in case of rejection of the articles by two people, the reason was mentioned and in case of disagreement between them, the article was judged by a third person. Data were extracted using a pre-prepared checklist that included study location, study time, study population, the prevalence of kdr resistance, and the proportion of different types of kdr resistance.
2.5. Selection of studies
Based on the search results in scientific databases, 14,536 articles were extracted. The extracted articles were entered into Endnote software and after reviewing the title and abstract, 9012 articles were excluded due to duplication and 4919 articles were excluded due to irrelevance from the title and abstract screening. Then, the full text of the reviewed articles and 596 articles were excluded due to the lack of prevalence of kdr resistance or resistance to organochlorine insecticides, and 9 articles which met the inclusion and exclusion criteria entered the systematic review and meta-analysis process (Fig. 1).
Fig. 1.
The PRISMA flow diagram.
2.6. Statistical analysis
The random-effects model was used to combine the results in heterogeneous studies and the fixed effects model in the meta-analysis was used in homogeneous studies. Heterogeneity of studies using Cochran's test and I2 index, publication bias using funnel plot and Egger test, and investigation of the relationship between the year of study and the prevalence of meta-regression were also used. Statistical analysis of data was conducted using STATA ver.14 software.
3. Results
Nine studies (Cao et al., 2006; Kristensen, 2005; Hamm et al., 2005; Foster et al., 2003; Learmount et al., 2002; Pittendrigh et al., 1997; Williamson et al., 1996; Williamson et al., 1993; Knipple et al., 1994) with a sample size of 961 house flies that were conducted between 1993 and 2006 were included in the meta-analysis process. The specifications of the reviewed articles were presented in Table 1. Apart from a study in China, all the remaining papers were attributed to the western world.
Table 1.
Characteristic of studies included in the systematic review and meta-analysis.
| Authors (Ref.) | Year of study | Place of study | Sample size | Detected kdr allele | Prevalence of kdr (%) |
|---|---|---|---|---|---|
| Cao XM (Cao et al., 2006) | 2006 | China | 33 | 21 | 63.6 |
| Kristensen (Kristensen, 2005) | 2005 | Denmark | 31 | 23 | 74.2 |
| Hamm (Hamm et al., 2005) | 2005 | USA | 86 | 38 | 44.2 |
| Foster SP (Foster et al., 2003) | 2003 | England and mainland Europe | 22 | 12 | 54.5 |
| Learmount (Learmount et al., 2002) | 2002 | England | 19 | 3 | 15.8 |
| Pittendrigh (Pittendrigh et al., 1997) | 1997 | USA | 12 | 6 | 50.0 |
| Williamson (Williamson et al., 1996) | 1996 | England | 14 | 9 | 64.3 |
| Knipple (Knipple et al., 1994) | 1994 | New York | 456 | 228 | 50.0 |
| Williamson (Williamson et al., 1993) | 1993 | England | 288 | 105 | 46.1 |
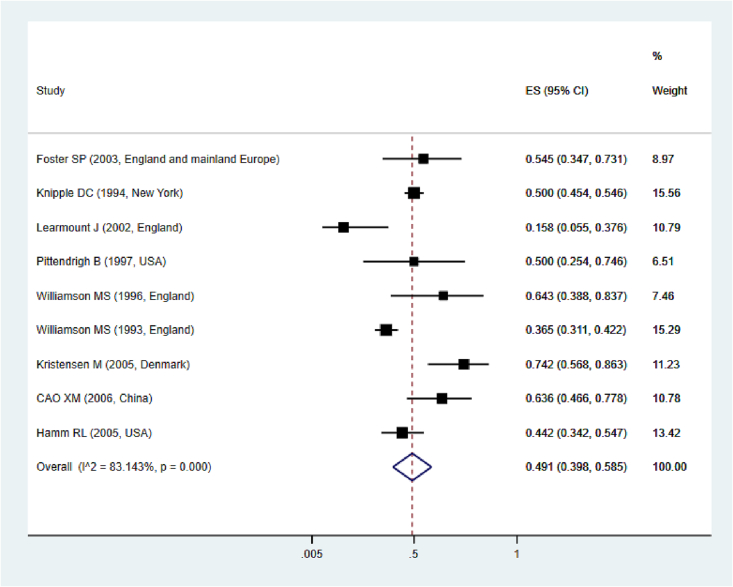
Based on the findings of a meta-analysis of nine studies, the prevalence of kdr allele against organochlorine insecticides in house flies was estimated to be 49.1% (95% CI: 39.8–58.5) (Fig. 2). The prevalence of kdr in these studies showed that the highest prevalence of kdr mutation was related to those in Denmark (2005) (Kristensen, 2005) and then China (2006) (Cao et al., 2006), and the lowest prevalence was related to the study in England (2002) (Learmount et al., 2002). This low prevalence was mainly due to the scarce sample size in the named study. However, in studies conducted in previous years in England, the prevalence was higher, which could be due to changes in the method of diagnosis and treatment or differences in the method of sampling.
Fig. 2.
Pooled prevalence rate of kdr allele based on a random-effects model.
Fig. 2: Pooled prevalence rate of kdr-based mutation on a random-effects model. The midpoint of each line segment shows the prevalence estimate, the length of the line segment indicates the 95% confidence interval in each study, and the diamond mark illustrates the pooled prevalence of kdr allele.
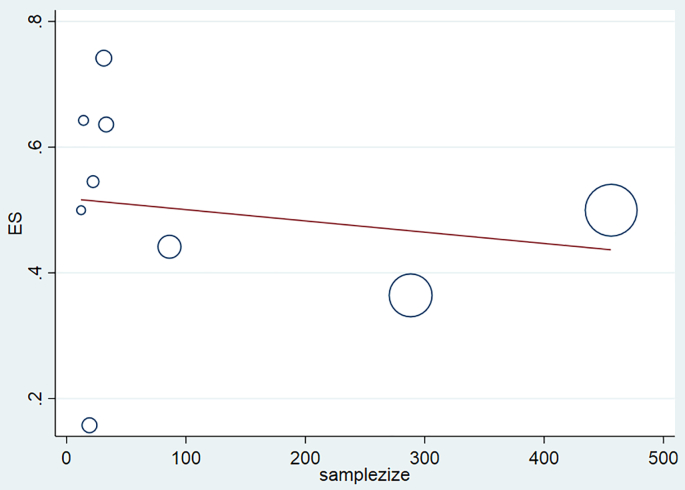
Funnel plot and Egger's test were used to examine the publication bias. Due to the symmetry of the funnel plot and the presence of articles in the range of the plot, it can be mentioned that publication bias did not occur, which was confirmed due to the insignificance of Egger's test (P = 0.1) (Fig. 3). Meta-regression was also used to investigate the relationship between study year and sample size with kdr allele prevalence. Regarding the year of the study, the findings showed that with the increase of the year of the study, the prevalence of kdr mutation had increased, which reflected that the prevalence of kdr allele had increased in recent years compared to the past and resistance was expanding (Fig. 4). Regarding the relationship between prevalence and sample size, it was observed that with increasing sample size, the prevalence decreased, which indicated that increasing the sample size was important in determining the prevalence (Fig. 5).
Fig. 3.
Funnel plot of the kdr allele prevalence.
Fig. 4.
Meta-regression plot of the kdr allele prevalence based on the year of study.
Fig. 5.
Meta-regression plot of the kdr allele prevalence based on the sample size.
4. Discussion
The findings of the present meta-analysis showed that about half of the house flies were resistant to organochlorine insecticides. As a result, this resistance leads to a decrease in the effectiveness of controlling this insect. House flies can transmit numerous pathogens including viruses, bacteria, fungi, protozoa and helminths to humans due to their indiscriminate habits of defecation, vomiting and frequent contact with a plethora of other unhygienic matter. Their crucial resistance to almost all organochlorine insecticides is further exacerbated by their cross-resistance to the pyrethroid- and pyrethrin-based insecticides due to their analogical mode of actions on the house fly nerves (Mohammadi et al., 2021).
A study by Wang et al. (2019) in China examined the resistance of house flies to five insecticides of permethrin, deltamethrin, beta-cypermethrin, propoxur, and dichlorvos. Resistance to the insecticide permethrin, deltamethrin, and beta-cypermethrin was found to be common among house flies but was still highly sensitive to dichlorvos. Resistance to deltamethrin and propoxur increased in 2017 compared to 2011 and 2014. It is noteworthy that in this study, resistance to permethrin, deltamethrin, beta-cypermethrin, and propoxur were correlated with each other, and resistance to dichlorvos and beta-cypermethrin were related (Wang et al., 2019).
In a study by Kaufman et al. (2009) in Florida, a significant proportion of house flies were resistant to pyrethroid insecticides. In this study, it was found that resistance to insecticides that were used less was lower (Kaufman et al., 2010). In a study by Li et al. (2018) in China, they studied the resistance of house flies to seven insecticides: propoxur, cypermethrin, imidacloprid, indoxacarb, chlorpyrifos, fipronil, and chlorfenapyr. According to their findings, house flies were resistant to all types of insecticides. The simultaneous use of insecticides increased the toxicity to kill house flies. The mechanism of resistance in house flies was investigated and it was observed that the mechanisms of monooxygenases, esterases, and glutathione-S-transferase were involved in the development of resistance (Li et al., 2018).
Freeman et al. (2019) in the United States investigated the resistance of voltage-sensitive sodium channels to three insecticides permethrin, tetrachlorvinphos, and methomyl, which fall into three categories: pyrethroids, organophosphates, and carbamates. These findings showed that the prevalence of resistance in house flies varied based on the geographical location. New Mexico and Utah had the lowest, while Kansas had the highest resistance to the toxins. Resistance has also increased over the past 10 years and expanded across the United States (Freeman et al., 2019).
A study by Sun et al. (2016) investigated the levels of different types of kdr allele resistance in house flies against 19 types of insecticides. The results showed that kdr-his resistance levels were similar in all 19 insecticides but varied significantly for kdr allele resistance. In 16 types of insecticides, super-kdr resistance levels were higher than kdr allele, and in three types of insecticides, kdr resistance levels were higher than super-kdr, which indicated that super-kdr resistance levels are not always higher. The results of the study of resistance levels in heterozygotes also showed that resistance in hybrids was inherited as a defective trait, but in kdr-his/kdr hybrids, it showed imperfect inheritance to complete resistance (Sun et al., 2016).
In a study by Mazzoni et al. (2015) in Italy, bioassays showed the resistance of house flies to pyrethroid insecticides. kdr and s-kdr resistances were observed in them (Mazzoni et al., 2015). In general, in most parts of the world, kdr allele resistance has been observed against various insecticides, especially organochlorine and pyrethroid insecticides. The use of new and effective insecticides is required to replace insecticides that are no longer effective, or the combined use of several types of insecticides, as well as new effective methods of prevention and control.
4.1. Limitations
Limitations of the study included the different times of studies that could affect the prevalence, the heterogeneity between studies, and the low number of studies partly emanating from the inability to use non-English language papers. A major discrepancy involved the survey of various house fly lineages (clades) spread over different geographical regions.
5. Conclusions
Results showed that about half of house flies have kdr allele against organochlorine toxins. The existence of high resistance indicated the inability of organochlorine insecticides to curb this insect. Accordingly, it is recommended to use effective and combined methods or new insecticides to fight and control it. It is also suggested that studies be performed to evaluate the combined effect of organochlorine insecticides and their interactions with resistant house flies.
Declaration of Competing Interest
None is declared.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.parepi.2023.e00310.
Contributor Information
Ebrahim Abbasi, Email: e_abbasie@sums.ac.ir.
Mohammad Djaefar Moemenbellah-Fard, Email: momenbf@sums.ac.ir.
Appendix A. Supplementary data
Supplementary material
References
- Andersen S.O. Insect cuticular sclerotization: a review. Insect Biochem. Mol. Biol. 2010;40(3):166–178. doi: 10.1016/j.ibmb.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Arakane Y., Muthukrishnan S., Beeman R.W., Kanost M.R., Kramer K.J. Laccase 2 is the phenoloxidase gene required for beetle cuticle tanning. Proc. Natl. Acad. Sci. 2005;102(32):11337–11342. doi: 10.1073/pnas.0504982102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awache I., Farouk A. Bacteria and fungi associated with houseflies collected from cafeteria and food Centres in Sokoto. FUW Trends Sci. Technol. J. 2016;1(1):123–125. [Google Scholar]
- Bahrndorff S., De Jonge N., Skovgård H., Nielsen J.L. Bacterial communities associated with houseflies (Musca domestica L.) sampled within and between farms. PLoS One. 2017;12(1) doi: 10.1371/journal.pone.0169753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazyar J., Safarpour H., Daliri S., Karimi A., Keykaleh M.S., Bazyar M. The prevalence of sexual violence during pregnancy in Iran and the world: a systematic review and meta-analysis. J. Inj. Violence Res. 2018;10(2):63. doi: 10.5249/jivr.v10i2.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X., Song F., Zhao T., Dong Y., Sun C.X., Lu B. Survey of deltamethrin resistance in house flies (Musca domestica) from urban garbage dumps in northern China. Environ. Entomol. 2006;35(1):1–9. [Google Scholar]
- Dong K. Insect sodium channels and insecticide resistance. Invertebr. Neurosci. 2007;7(1):17–30. doi: 10.1007/s10158-006-0036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firooziyan S., Sadaghianifar A., Taghilou B., Galavani H., Ghaffari E., Gholizadeh S. Identification of novel voltage-gated sodium channel mutations in human head and body lice (Phthiraptera: Pediculidae) J. Med. Entomol. 2017;54(5):1337–1343. doi: 10.1093/jme/tjx107. [DOI] [PubMed] [Google Scholar]
- Foster S., Young S., Williamson M., Duce I., Denholm I., Devine G. Analogous pleiotropic effects of insecticide resistance genotypes in peach–potato aphids and houseflies. Heredity. 2003;91(2):98–106. doi: 10.1038/sj.hdy.6800285. [DOI] [PubMed] [Google Scholar]
- Freeman J.C., Ross D.H., Scott J.G. Insecticide resistance monitoring of house fly populations from the United States. Pestic. Biochem. Physiol. 2019;158:61–68. doi: 10.1016/j.pestbp.2019.04.006. [DOI] [PubMed] [Google Scholar]
- Hamm R.L., Shono T., Scott J.G. A cline in frequency of autosomal males is not associated with insecticide resistance in house fly (Diptera: Muscidae) J. Econ. Entomol. 2005;98(1):171–176. doi: 10.1093/jee/98.1.171. [DOI] [PubMed] [Google Scholar]
- Hemingway J., Ranson H. Insecticide resistance in insect vectors of human disease. Annu. Rev. Entomol. 2000;45(1):371–391. doi: 10.1146/annurev.ento.45.1.371. [DOI] [PubMed] [Google Scholar]
- Kaufman P.E., Nunez S.C., Mann R.S., Geden C.J., Scharf M.E. Nicotinoid and pyrethroid insecticide resistance in houseflies (Diptera: Muscidae) collected from Florida dairies. Pest Manag. Sci. 2010;66(3):290–294. doi: 10.1002/ps.1872. [DOI] [PubMed] [Google Scholar]
- Khamesipour F., Lankarani K.B., Honarvar B., Kwenti T.E. A systematic review of human pathogens carried by the housefly (Musca domestica L.) BMC Public Health. 2018;18(1):1–15. doi: 10.1186/s12889-018-5934-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipple D.C., Doyle K.E., Marsella-Herrick P.A., Soderlund D.M. Tight genetic linkage between the kdr insecticide resistance trait and a voltage-sensitive sodium channel gene in the house fly. Proc. Natl. Acad. Sci. 1994;91(7):2483–2487. doi: 10.1073/pnas.91.7.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen M. Glutathione S-transferase and insecticide resistance in laboratory strains and field populations of Musca domestica. J. Econ. Entomol. 2005;98(4):1341–1348. doi: 10.1603/0022-0493-98.4.1341. [DOI] [PubMed] [Google Scholar]
- Learmount J., Chapman P., MacNicoll A. Impact of an insecticide resistance strategy for house fly (Diptera: Muscidae) control in intensive animal units in the United Kingdom. J. Econ. Entomol. 2002;95(6):1245–1250. doi: 10.1603/0022-0493-95.6.1245. [DOI] [PubMed] [Google Scholar]
- Li Q., Huang J., Yuan J. Status and preliminary mechanism of resistance to insecticides in a field strain of housefly (Musca domestica, L) Rev. Bra. Entomol. 2018;62:311–314. [Google Scholar]
- Mazzoni E., Chiesa O., Puggioni V., Panini M., Manicardi G.C., Bizzaro D. Presence of kdr and s-kdr resistance in Musca domestica populations collected in Piacenza province (Northern Italy) Bull. Insectol. 2015;68(1):65–72. [Google Scholar]
- Mohammadi J., Azizi K., Alipour H., Kalantari M., Bagheri M., Shahriari-Namadi M., et al. Frequency of pyrethroid resistance in human head louse treatment: systematic review and meta-analysis. Parasite. 2021;28 doi: 10.1051/parasite/2021083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., Shamseer L., Clarke M., Ghersi D., Liberati A., Petticrew M., et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015;4(1):1–9. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazari M., Mehrabi T., Hosseini S.M., Alikhani M.Y. Bacterial contamination of adult house flies (Musca domestica) and sensitivity of these bacteria to various antibiotics, captured from Hamadan City, Iran. J. Clin. Diagn. Res. 2017;11(4) doi: 10.7860/JCDR/2017/23939.9720. DC04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ommi D., Mohammadreza Hashemian S., Tajbakhsh E., Khamesipour F. Molecular detection and antimicrobial resistance of Aeromonas from houseflies (Musca domestica) in Iran. Rev. MVZ Córdoba. 2015;20:4929–4936. [Google Scholar]
- Organization WH . World Health Organization; 2006. Pesticides and their Application: For the Control of Vectors and Pests of Public Health Importance. [Google Scholar]
- Pan C., Zhou Y., Mo J. The clone of laccase gene and its potential function in cuticular penetration resistance of Culex pipiens pallens to fenvalerate. Pestic. Biochem. Physiol. 2009;93(3):105–111. [Google Scholar]
- Pittendrigh B., Reenan R., Ganetzky B. Point mutations in the Drosophila sodium channel gene Para associated with resistance to DDT and pyrethroid insecticides. Mol. Gen. Genet. 1997;256(6):602–610. doi: 10.1007/s004380050608. [DOI] [PubMed] [Google Scholar]
- Rinkevich F.D., Hedtke S.M., Leichter C.A., Harris S.A., Su C., Brady S.G., et al. Multiple origins of kdr-type resistance in the house fly, Musca domestica. PLoS One. 2012;7(12) doi: 10.1371/journal.pone.0052761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinkevich F.D., Du Y., Dong K. Diversity and convergence of sodium channel mutations involved in resistance to pyrethroids. Pestic. Biochem. Physiol. 2013;106(3):93–100. doi: 10.1016/j.pestbp.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivero A., Vezilier J., Weill M., Read A.F., Gandon S. Insecticide control of vector-borne diseases: when is insecticide resistance a problem? PLoS Pathog. 2010;6(8) doi: 10.1371/journal.ppat.1001000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarwar M. Insect vectors involving in mechanical transmission of human pathogens for serious diseases. Int. J. Bioinform. Biomed. Eng. 2015;1(3):300–306. [Google Scholar]
- Sun H., Tong K., Kasai S., Scott J. Overcoming super-knock down resistance (super-kdr) mediated resistance: multi-halogenated benzyl pyrethroids are more toxic to super-kdr than kdr house flies. Insect Mol. Biol. 2016;25(2):126–137. doi: 10.1111/imb.12206. [DOI] [PubMed] [Google Scholar]
- Toloza A.C., Ascunce M.S., Reed D., Picollo M.I. Geographical distribution of pyrethroid resistance allele frequency in head lice (Phthiraptera: Pediculidae) from Argentina. J. Med. Entomol. 2014;51(1):139–144. doi: 10.1603/me13138. [DOI] [PubMed] [Google Scholar]
- Tsagaan A., Okado K. Study of pathogenic bacteria detected in fly samples using universal primer-multiplex PCR. Mongolian J. Agric. Sci. 2015;15(2):27–32. [Google Scholar]
- Von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P., et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int. J. Surg. 2014;12(12):1495–1499. doi: 10.1016/j.ijsu.2014.07.013. [DOI] [PubMed] [Google Scholar]
- Wang J.-N., Hou J., Wu Y.-Y., Guo S., Liu Q.-M., Li T.-Q., et al. Resistance of house fly, Musca domestica L.(Diptera: Muscidae), to five insecticides in Zhejiang Province, China: the situation in 2017. Can. J. Infect. Dis. Med. Microbiol. 2019:2019. doi: 10.1155/2019/4851914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson M.S., Denholm I., Bell C.A., Devonshire A.L. Knockdown resistance (kdr) to DDT and pyrethroid insecticides maps to a sodium channel gene locus in the housefly (Musca domestica) Mol. Gen. Genet. 1993;240(1):17–22. doi: 10.1007/BF00276878. [DOI] [PubMed] [Google Scholar]
- Williamson M.S., Martinez-Torres D., Hick C.A., Devonshire A.L. Identification of mutations in the houseflypara-type sodium channel gene associated with knockdown resistance (kdr) to pyrethroid insecticides. Mol. Gen. Genet. 1996;252(1):51–60. doi: 10.1007/BF02173204. [DOI] [PubMed] [Google Scholar]
- Wood O., Hanrahan S., Coetzee M., Koekemoer L., Brooke B. Cuticle thickening associated with pyrethroid resistance in the major malaria vector Anopheles funestus. Parasit. Vectors. 2010;3(1):1–7. doi: 10.1186/1756-3305-3-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalucki M., Furlong M. Behavior as a mechanism of insecticide resistance: evaluation of the evidence. Curr. Opin. Insect Sci. 2017;21:19–25. doi: 10.1016/j.cois.2017.05.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material