Abstract
Objective:
Vascular burden is associated with cognitive deficits and a form of late-life depression, vascular depression (VaDep), which is marked by decreased white matter integrity, executive dysfunction, poor treatment response, and functional disability. Older Black Americans represent a vulnerable population at risk of developing VaDep, but the literature in this group is limited. Thus, the goal of this systematic review is to summarize the existing literature that informs our understanding of VaDep in older Black Americans, including cognitive, functional, and psychosocial outcomes.
Method:
Following Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines, studies were identified that examined the relationship between vascular disease or vascular risk factors and that either had a sample of at least 75% Black participants or conducted race-specific analyses. Thirty studies met all inclusion criterion based on review of both authors.
Results:
Overall, studies support the construct of VaDep in older Black Americans. There is preliminary support for VaDep-related cognitive and functional deficits, and mixed findings regarding racial disparities in prevalence of VaDep.
Conclusion:
This review underscores the need for further neuroimaging and neuropsychological research in Black older adults with comorbid depression and vascular disease. Findings also highlight the importance of screening for depressive symptoms in Black individuals with multiple vascular risk factors.
Keywords: Vascular depression, Black, African American, aging, depression
Late-life depression is associated with a host of negative outcomes such as structural and functional brain changes, dementia, and all-cause and cardiovascular-related mortality (Alexopoulos, 2019; Wei et al., 2019). However, late-life depression consists of multiple endophenotypes, which complicate the ability of healthcare providers to successfully treat these symptoms (Masse-Sibille et al., 2018). Vascular depression (VaDep) represents a major sub-group of late-life depression that is especially treatment resistant (Bella et al., 2010; Gunning-Dixon et al., 2010) and that may have particular relevance for Black Americans given the elevated rates of vascular disease present in this group (Persaud et al., 2012).
Vascular Disease in Black Americans
A wealth of studies document racial and ethnic disparities in vascular diseases, such as stroke and coronary artery disease, and vascular risk factors, including hypertension, diabetes, and obesity. Black Americans have almost twice the rate of obesity and diabetes as well as higher odds of hypertension, heart attack, stroke, and cardiovascular-related mortality compared to White Americans (Tabaei et al., 2019; Zhang & Rodriguez-Monguio, 2012). In fact, Black Americans are reported to have the highest rates of hypertension in the world (Mozaffarian et al., 2016).
In addition to race group differences in individual behavioral and lifestyle choices, social determinants, such as inequalities in health care access, unemployment, and poverty, contribute to racial disparities in VD/VRF (Mensah, 2018). Recent research also highlights the importance of neighborhood characteristics in vascular disparities, including housing quality, environmental pollution, availability of healthy foods, neighborhood walkability, and access to green spaces (Diez Roux, 2016; Havranek et al., 2015). Racial segregation has been shown to contribute to vascular disease risk in Black Americans, with one study showing each standard deviation increase in the degree of neighborhood segregation was associated with a 12% increase in CVD risk (Kershaw & Albrecht, 2015). Moreover, Black Americans are subject to greater degrees of common sources of stress, such as socioeconomic concerns and safety concerns, as well as high levels of perceived discrimination—stressors that are compounded in the time of COVID-19 (Ajilore & Thames, 2020). Chronic stress, including race-related stress, has a direct effect on physiological mechanisms, such as elevated cortisol and chronic inflammation, that increase vascular risk (Kershaw & Albrecht, 2015; Vadiveloo & Mattei, 2017). Racism and discrimination are known to be associated with VD/VRF (Dolezsar et al., 2014; Everson-Rose et al., 2015; Stepanikova, Baker, et al., 2017).
In comparison to other racial groups, older Black Americans with vascular disease and vascular risk factors (VD/VRFs) have increased risk of white matter hyperintensities (WMHs)—neuroimaging indicators of white matter damage—and brain atrophy (Brickman et al., 2008), at least partially due to racial disparities in healthcare access (Divers et al., 2013). Low socioeconomic status (Waldstein et al., 2017) and lifetime racial discrimination (Beatty Moody et al., 2019) have both been linked to greater white matter lesion volume in Black Americans. Black Americans are also more likely to have severe WMH in subcortical regions (Nyquist et al., 2014), greater prevalence of silent lacunar strokes (Prabhakaran et al., 2008), less overall white matter, and greater overall white matter lesion volume (Hsu et al., 2018). Other studies have shown that larger WMH volume predicts worse language, speed, and executive functioning specifically in Black Americans (Zahodne et al., 2015).
Vascular Depression
VD/VRFs are also associated with depression, especially in older adults, though the research in this area is largely based on samples that are predominantly or exclusively White. The vascular depression (VaDep) hypothesis put forth by Alexopoulos et al. (1997) posits that vascular disease may “predispose, precipitate, or perpetuate” (p. 915) some forms of late-life depression. Clinically, VaDep is characterized by co-occurrence of depressive symptoms and VD or VRFs, cognitive impairment, psychomotor retardation, and poor response to antidepressant treatment (Aizenstein et al., 2016; Taylor et al., 2013), although there have been mixed results regarding the symptom profile of VaDep (Naarding et al., 2009). Other common features of VaDep include anhedonia, absence of family history of depression, and functional disability (Chang et al., 2016; Krishnan et al., 2004). Neurocognitive sequelae of VaDep are also common (Johnson et al., 2019); VaDep puts older adults at greater risk of developing mild cognitive impairment (Kim et al., 2016), Alzheimer’s disease, and vascular dementia (Diniz et al., 2013). Relatedly, executive dysfunction is a key feature of VaDep and is linked to more severe WMH and poor treatment response (Culang-Reinlieb et al., 2011; Pimontel et al., 2016). VaDep, therefore, occurs within the context of widespread brain vulnerability.
An imaging-based definition has also been proposed for VaDep using the presence of deep WMH as evidence of cerebrovascular disease (Krishnan et al., 1997). Across both clinical and MRI-defined VaDep, mood symptoms are thought to originate from microstructural insults to the cerebrovascular system, specifically to frontolimbic white matter tracts that underlie both cognitive and emotional functions (Dalby et al., 2010; Sheline et al., 2008; Taylor et al., 2013). Not all older adults with vascular disease develop depression or cognitive changes, thus Taylor et al. (2013) have suggested a threshold model for VaDep such that one must accumulate and surpass an initial level of vascular damage to these neural circuits before frontolimbic compromise is seen in the form of depressive, and often cognitive, symptoms. VaDep accounts for as many as 50% of all older adults with Major Depressive Disorder (MDD; Park et al., 2015). Given the insidious disintegration of white matter pathways over time, individuals who develop VaDep tend to develop first-time depressive symptoms in late life (Krishnan et al., 2004) and experience greater chronicity than non-vascular depression (Barch et al., 2012).
Objective
Since Black Americans have a higher prevalence of vascular disease (Tabaei et al., 2019) and greater WMH burden (Hsu et al., 2018) compared to other racial groups, this population may be at high risk of VaDep. However, there is little research to date that examines the VaDep framework in older Black Americans. VaDep is associated with treatment resistance, cognitive deficits, and functional disability (Aizenstein et al., 2016), therefore synthesizing what is known about VaDep in Black Americans is vital for directing future research and informing successful depression treatment for a vulnerable and underserved population. Thus, the aims of this systematic review are to i) identify and characterize the existing literature on VaDep in Black Americans, including cognitive, functional and psychosocial outcomes, and ii) identify methodological issues and gaps in the literature to inform future research on this topic. VaDep is generally studied in adults over the age of 65; however, given the limited research on VaDep in Black Americans, this review includes all relevant studies with a mean sample age of at least 50.
Methods
This review was performed in accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines (Moher et al., 2010).
Search Strategy
The literature was systematically searched through the PubMed and APA PsycInfo electronic databases to identify academic research articles that examined the relationship between VD/VRFs and later-life depression (i.e., after age 50) in Black Americans. Searches were conducted in December 2020 and were restricted to academic journals and to human research. No publication year restrictions were applied. An example of a search within the APA PsycInfo database is as follows: (african americans or black americans or blacks) AND (cardiovascular disease or heart disease or vascular disease) AND (elderly or aged or older or elder or geriatric or senior) AND (depression or depressive disorder or depressive symptoms or MDD). See Table 1 for a complete list of search terms by database. After relevant articles were identified through database searches, reference lists of the selected articles were also examined to identify studies not identified during initial searches. Reference lists were reviewed for relevant titles, which led to abstract and then full-text review of articles that met eligibility criteria.
Table 1.
Search Terms
| Database | Search Terms |
|---|---|
| PubMed | ((("African Americans"[Mesh]) AND "Cardiovascular Diseases"[Mesh]) AND ("Depression"[Mesh] OR "Depressive Disorder"[Mesh] )) AND "Aged"[Mesh] |
| ((stroke[MeSH Terms]) AND depression[MeSH Terms]) AND african americans[MeSH Terms] | |
| APA PsycInfo | (african americans or black americans or blacks) AND (cardiovascular disease or heart disease or vascular disease) AND ( elderly or aged or older or elder or geriatric or senior ) AND ( depression or depressive disorder or depressive symptoms or major depressive disorder ) |
| stroke AND (depression or depressive disorder or depressive symptoms or major depressive disorder) AND ( african americans or black americans or blacks ) |
Study Selection
Both authors screened each article to determine appropriateness for this review, with disagreements resolved by the senior author. We examined the title, abstract, and full text of each article to determine eligibility.
Eligibility Criteria
Articles identified by the database searches were selected for full-text review if they were in English and the title or abstract indicated that the purpose of the article was to examine both depression (or depressive symptoms) and VD/VRFs. Multi-racial samples were required to either include race-specific analyses or comprise at least 75% Black participants in order to allow the inclusion of studies with majority Black samples, which can provide valuable information given the paucity of studies comprising only Black participants. The bi-directional relationship between depression and vascular disease is well-documented (for a review, see Zhang et al., 2018), however in order to examine how vascular disease can “predispose, precipitate, or perpetuate” depression (Alexopoulos et al., 1997, p. 915) in line with the VaDep hypothesis, we excluded studies in which depression predicted subsequent VD/VRFs. We also excluded studies of post-stroke depression because post-stroke depression and VaDep are considered separate, albeit related, constructs (Newberg et al., 2006). However, studies in which history of stroke was considered as one of multiple VD/VRFs were permitted. Studies in which the target population was recruited for a chronic medical illness that was not vascular in nature (e.g., chronic kidney disease, cognitive impairment/dementia), or that included a psychiatric illness other than depression were excluded.
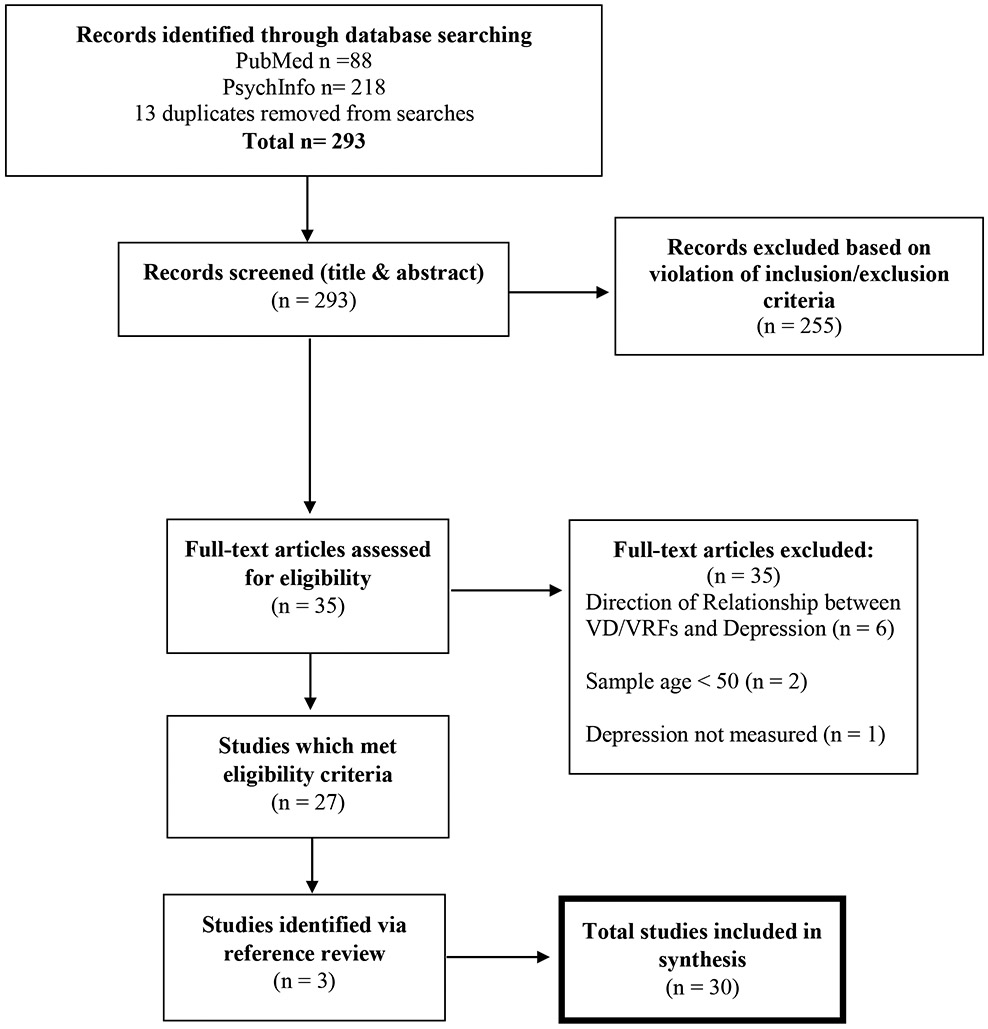
Database searches identified 293 articles, all of which were screened for eligibility based on title and abstract. A total of 35 articles were identified for full-text review, and 27 of these articles met eligibility criteria (see Figure 1). An additional three articles were identified through reference list review.
Figure 1.
Flow diagram of study selection.
Data Extraction
Both authors extracted the following data from the selected studies and organized the data into a matrix in a standardized spreadsheet in order to systematically compare study aims, sample size, methodology, and outcomes: publication details, study characteristics (e.g., study setting and sample design [cross-sectional or longitudinal]), depression measures and method of depression diagnosis (e.g., CES-D vs. clinical interview or medical record review), VD/VRF measures (e.g., self-report or physician diagnosis), age range and mean age, and sample characteristics (e.g., sample size, percent Black, community vs. inpatient).
The senior author assessed bias for each study using a modified JBI Checklist for Analytical Cross Sectional Studies. Each of the items in the checklist was given a score of 0 or 1 based on meeting the specified criteria, for a total of seven possible points (inclusion criterion, description of subjects and setting, objective and standard measurement of condition, identification of confounds, dealing with confounds, valid and reliable outcome measurement, and appropriate statistics). Longitudinal studies had three additional criteria from the Tool to Assess Risk of Bias in Longitudinal Symptom Research Studies Aimed at the General Population from the CLARITY Group at McMaster University: representativeness of the sample, accurate outcome assessment at baseline and follow-up, and missing data. The total JBI bias score for each study, with higher scores indicating better quality, are provided in Table 2. The table also notes the two longitudinal studies where any risk of bias was noted based on the additional criterion.
Table 2.
Studies Investigating the Relationship between Vascular Risk Factors and Depression in Older Black Americans
| Study Design (Bias Rating) |
N | Age Range or M (SD) |
% Black |
Depression Measure |
Depression Criteria |
Vascular Risk Factors (VRF) Examined |
Main Findings | |
|---|---|---|---|---|---|---|---|---|
| Self-Report Measures of VD/VRF | ||||||||
| Azar et al. 2005 | Cross-sectional (7) | 362* | 55+ | 50 | CES-D | CES-D ≥ 18 (highest quartile) | HTN, heart disease, diabetes, arteriosclerosis. High Risk: ≥ 2, Low Risk: ≤ 1 | ≥ 2 VRFs increased risk of depression across race. No race differences in prevalence of VRFs. No race by VRF interaction to predict depression. |
| Baker et al., 1996 | Cross-sectional (5) | 96* | 60+ | 100 | CES-D | CES-D ≥ 16 | HTN, arteriosclerosis, circulatory problems | Depressed group had higher frequency of hypertension, arteriosclerosis, & circulatory problems, but not arthritis. |
| Carmasin et al., 2014 | Longitudinal (7) | 435* |
69 (9) | 100 | CES-D | CES-D ≥ 16 | HTN, diabetes, cardiovascular disease, heart attack, angina, circulatory problems, stroke. High Risk: ≥ 2, Low Risk: ≤ 1 | ≥ 2 VRFs predicted depression at 2.5 year follow-up regardless of baseline depression level. High-risk group was 2.61x more likely to develop depression than low risk group. High baseline vascular risk predicted worsening depression over time. Baseline vascular risk had a small, negative effect on processing speed. |
| Gonzalez & Tarraf, 2013 | Cross-sectional (7) | 4,281* | 50+ | 40 | CIDI Diagnostic Interview for DSM-IV | DSM-IV criteria for MDD | HTN, diabetes, heart disease, stroke, or “other” CVD | Black participants had the highest cardiovascular disease morbidity: 65.4% reported one or more VRF. Black pts were the least likely to meet CVD & MDD criteria (4.7%), but of those who met MDD criteria, Black pts were the most likely to have a comorbid CVD/MDD diagnosis (74.4%). Black pts reported more days of impairment compared to White. |
| Hamm et al., 1993 | Cross-sectional (5) | 710* | 62+ | 100 | CES-D | CES-D Total Score | Self-reported HTN or “cardiac problems” | CVD/HTN group was more depressed and perceived themselves as having less control over their health (health locus of control) compared to the CVD-free or HTN-only groups. |
| Okwumabua et al., 1997 | Cross-sectional (7) | 96* | 60+ | 100 | CES-D | CES-D ≥ 16 | HTN, arteriosclerosis and "circulatory" conditions | Those who screened positive for significant depressive symptoms were more likely to report HTN, arteriosclerosis, and circulatory problems. |
| Yochim et al., 2003 | Cross-sectional (5) | 598* | 60+ | 89 | SF-12 item, “How much of the time during the past 4 weeks have you felt down-hearted and blue?” | Response "all, most, or a good bit of the time" to SF-12 item | HTN, diabetes, “heart problems” such as heart attack, angina/ chest pain, or congestive heart failure. High Risk: ≥ 2, Low Risk: ≤ 1 |
Higher prevalence of depressed mood in those with ≥ 2 VRF (17%) vs. ≤ 1 VRF (10%). The difference in sick days between those with and without depressed mood was greater in those with ≥ 2 VRFs than in those with ≤ 1 VRF. |
| Yochim, Kerkar, et al., 2006 | Cross-sectional (7) | 1,034* | 72(8) | 100 | SF-12 item, “How much of the time during the past 4 weeks have you felt down-hearted and blue?” | Endorsed single item of SF-12 as "all, most, or a good bit of the time" | HTN, diabetes, “heart problem” such as heart attack or atrial fibrillation, high cholesterol. High Risk: ≥ 2, Low Risk: ≤ 1 | High risk group (excluding stroke) was more likely to have depressed mood (13.4%) compared to the low-risk group (7.6%). Greater vascular burden predicted worse physical health, physical health levels predicted depression, but physical health did not mediate the relationship between vascular burden and depression. VRFs predicted depression over and above physical health. |
| Yochim, MacNeill et al., 2006 | Longitudinal (7) | 139† | 73 (9) | 83 | GDS | GDS Total Score | HTN, diabetes, atrial fibrillation. High Risk: ≥ 2, Low Risk: ≤ 1 | At baseline and at 3- and 6-month follow-up visits, the high-risk vascular group had more depressive symptoms. Depression predicted verbal fluency at 3 and 6 months. Vascular burden did not predict verbal fluency. |
| Physician Diagnosis or Objective Measures of VD/VRF | ||||||||
| Andrews et al., 2020 | Cross-sectional (7) | 42* | 59 (12) | 99 | CESD-R | CESD-R Total Score | Interleukins (IL)-1b, IL-6, IL-18 | IL-1 B IL-18) ncreased significantly for every unit increase in depressive symptoms. |
| Boutin-Foster et al., 2008 | Cross-sectional (7) | 571† | 66 (10) | 12 | CES-D | CESD-Total Score and Item Level Scores | Coronary artery disease | Black participants were 1.6 times more likely to have CES-D symptoms above the clinical cut-off score and three times more likely to endorse the item “people were unfriendly” compared to White participants |
| Cummings et al., 2016 | Cross-sectional (7) | 22,003* | 64 (n.r.) | 42 | CESD-4 | CESD-4 ≥ 4 | Diabetes physical exam or self-report) | Those with comorbid diabetes and depression were more likely to be Black |
| Dickson et al., 2013 | Cross-sectional (7) | 30*† | 60 (15) | 100 | PHQ-9 | PHQ-9 ≥ 10 | Heart failure | 40% had significant depressive symptoms and those with depressive symptoms had poorer self-care |
| Freedland et al., 1991 | Cross-sectional (7) | 60† | 60+ | 32 | Modified Diagnostic Interview Schedule | DSM-III-R MDD Diagnosis | Congestive heart failure | 1/6 of the White sample but none of the Black sample met criteria for depression |
| Heard et al., 2011 | Cross-sectional (7) | 997* | 62 (15) | 100 | 11-item version of the CES-D | CES-D Total Score | HTN (objective and self-report) | Higher depressive symptoms were associated with lower blood pressure |
| Hajjar et al., 2009 | Cross-sectional (7) | 580* | 77.8 (0.2) | 14 | CES-D | CES-D Total Score | HTN (objective), self-report of diabetes, heart disease, heart attack, stroke, congestive heart failure, and Framingham cardiovascular risk score | Identified a “vascular aging” phenotype of older adults who have executive function impairment (TMT-B ≥ 262 s.), slow gait (<. 85m/s), and elevated depressive symptoms (CES-D ≥ 8). Members of this phenotype were significantly more likely to be Black. |
| Lamar et al., 2015 | Cross-sectional (7) | 119* | 60 (12) | 50 | 17-item HDRS ≤ 8 for inclusion; CES-D for analyses |
Total CES-D |
Metabolic Syndrome risk factors: elevated blood pressure, glucose, triglycerides, HDL, and body mass index > 30. No Risk = 0, Low Risk = 1-2, High Risk: ≥ 3 | Across race, high-risk pts reported more depressive symptoms than the no-risk and low-risk groups, and incremental metabolic syndrome risk predicted learning and memory scores (no > low = high). No individual VRF predicted depressive symptoms for Black participants. Glucose levels predicted learning scores and systolic blood pressure predicted memory scores in Black participants. |
| Lewis et al., 2009 | Cross-sectional (7) | 508* | 50 (3) | 38 | CES-D | CES-D Total Score | Aortic and coronary calcification | Depressive symptoms were associated with aortic calcification in Black women but not White women. |
| Lu et al., 2017 | Cross-sectional (7) | 611† | 66 (15) | 100 | Depression per medical records | Depression per medical records | Heart failure per medical records | Depression increased the risk for 30-day readmission in heart failure patients. |
| Mast, Neufield et al., 2004 | Longitudinal (7) | 100† | 73 (8) | 82 | GDS-SF | GDS-SF > 5 | ICD-9 codes from treating physicians for HTN, diabetes, Atrial fibrillation. High Risk: ≥ 2, Low Risk: ≤ 1 | High risk group was 5.6x more likely to have a positive depression screen at the 6-month follow-up, 5x more likely at 18 months, and more likely to have persistent depression across timepoints compared to the low risk group. Vascular burden significantly predicted new-onset depression at 6 and 18 months. |
| Mast, MacNeill, et al., 2004 | Cross-sectional (5) | 670† | 76 (8.0) | 68-82 across groups |
GDS | GDS >10 | ICD-9 diagnosis of stroke, HTN, diabetes, atrial fibrillation | There was no difference in prevalence of depression across non-vascular, VRF, and stroke groups. Prevalence of depression was 51% greater in pts with ≥ 2 VRF (excluding stroke) compared to those with one. |
| Mast, Yochim et al., 2004 | Longitudinal (5) | 77† | 72 (8) | 82 | GDS-15 | GDS >5 | ICD-9 diagnosis of HTN, diabetes, atrial fibrillation. High Risk: ≥ 2, Low Risk: ≤ 1 | For the low-performance group on the Mattis Dementia Rating Scale Initiation/Perseveration Subscale at baseline & 18mo, depressive symptoms increased with an increase in vascular risk. Depressive symptoms did not increase with increasing vascular risk in the group with high performance on the DRS-I/P task. Overall, the high-risk vascular group had higher depression scores than low risk group. |
| Mentz et al., 2015 | Cross-sectional (7) | 2,331* | 47-70 | 34 | BDI-II | BDI-II Total Score |
Heart failure | In Black but not White participants, baseline symptoms of depression and worsening of symptoms over time were associated with increased all-cause mortality/hospitalization. |
| Reinlieb et al., 2014 | Cross-sectional (7) | 42* | 62 (9) | 43 | 24-item HDRS | HDRS ≥ 14 | MRI-defined VaDep | Those classified as having VaDep were more likely to be Black, have an earlier age of depression onset, and have psychomotor retardation, and less likely to have a family history of affective illness than the group with non-vascular depression. There were no differences in response rates to antidepressant treatment between groups. |
| Remigio-Baker et al, 2014 | Cross-sectional (7) | 1,944* | 60+ | 16 | CES-D | CES-D ≥ 16 | Visceral adiposity | Higher depressive symptoms were associated with greater visceral adiposity in men but not women. The effect did not differ by race. |
| Rohyans et al., 2009 | Cross-sectional (7) | 150* | 61 (15) | 31 | PHQ-8 | PHQ-8 ≥ 10 | Heart failure severity | Higher depressive symptoms were associated with more severe heart failure, but the effect did not differ by race. |
| Sharma et al., 2009 | Cross-sectional (7) | 134† | 65 (15) | 86 | PHQ-9 | PHQ-9 ≥ 10 | Acute Decompensated Heart Failure (Emergency Room diagnosis) | 45% of pts had elevated depressive symptoms; 60% were functionally impaired. Those with depression had longer hospital stays, more health comorbidities, and were more likely to have severe heart failure compared to those without depression. There were no differences between African Americans and Caribbean Black groups regarding prevalence of depression or quality of life. |
| Sims et al., 2020 | Cross-sectional (7) | 4,806* | 35-84 | 100 | CES-D | Low, medium and high CES-D scores (not defined) | Obesity, HTN prevalence and control, and diabetes prevalence and control | Obesity and HTN predicted higher depressive symptoms in women; diabetes predicted higher depressive symptoms in men. |
| Taylor et al., 2008 | Cross-sectional | 120* | 54 (13) | 100 | CES-D | CES-D Total Score | Blood pressure, body mass index | Higher depressive symptoms were associated with higher systolic and diastolic blood pressure. |
| Waldman et al., 2009 | Cross-sectional | 864* | 62 (n.r.) | 16 | BDI | BDI cutoffs: < 10, 10‘18, and > 18 | Coronary artery disease | Depression prevalence in coronary artery disease patients did not differ by race. |
Note. Study design is based on the analyses that were relevant for this review. In studies where relevant analyses were only performed on a subset of the total sample, the subsample N is reported. n.s. = not significant (p > 0.5), BDI = Beck Depression Inventory, CES-D = Center for Epidemiologic Studies Depression Scale, CIDI Diagnostic Interview for DSM-IV = Composite International Diagnostic Interview for the Diagnostic and Statistical Manual Version IV, CVD = Cardiovascular Disease, DSM-III-R = Diagnostic and Statistical Manual Version III Revised, DSM-IV = Diagnostic and Statistical Manual Version IV, GDS-15= 15-Item Geriatric Depression Scale, GDS-SF = Short Form Geriatric Depression Scale, HDRS = Hamilton Depression Rating Scale, HTN = Hypertension, ICD-9 = International Classification of Diseases, 9th Revision, IL = Interleukins, MDD = Major Depressive Disorder, PHQ = Patient Health Questionnaire, SF-12 = 12-item Short Form Survey, VaDep = Vascular Depression, VRF = Vascular Risk Factor
community sample
inpatient sample
Results
Overall, results of the studies revealed 1) cross-sectional and longitudinal associations between VD/VRFs and depression or depressive symptoms in Black Americans, consistent with the VaDep hypothesis; 2) mixed findings regarding race differences in the prevalence of comorbid depression and VD/VRF; and 3) evidence that comorbid VD/VRF and depression is associated with negative cognitive, functional, and psychosocial outcomes in Black Americans.
Association of Vascular Risk with Depression
Greater vascular risk was consistently associated with depression across all cross-sectional (n = 25) and longitudinal studies (n = 5), as well as across the use of self-report (n = 9) and objective measures of vascular risk (n = 21). The studies included a mix of community (n = 21) and inpatient (n = 9) samples. The majority of studies examined depressive symptoms based on self-report questionnaires, with the exception of four studies that examined the relationship between VD/VRFs and MDD using a diagnostic interview or medical diagnosis to determine depression status. A major limitation of the present literature is that history of depression was not evaluated as a potential confound.
Cross-Sectional Research
A positive relationship between vascular risk and self-reported depression was identified among studies with Black or primarily Black (≥ 83% Black) samples (Baker et al., 1996; Hamm et al., 1993; Okwumabua et al., 1997; Yochim, Kerkar, et al., 2006; Yochim, MacNeill, et al., 2006; Yochim et al., 2003). For example, Yochim et al. (2003) found that 17% of the high-risk vascular group had elevated depressive symptoms compared to 10% in the low-risk (≤ 1 VRF) group. Yochim, Kerkar, et al. (2006) replicated and expanded these findings in 1,034 Black older Americans and found that even when controlling for physical health, the high-risk group was significantly more likely to have depressed mood (13.4%) compared to the low-risk group (7.6%). However, assessment of depression lacked sensitivity in these studies given that both designs utilized one question from the 36-Item Short Form Survey (“How much of the time during the past 4 weeks have you felt down-hearted and blue?”) to categorize depressed and non-depressed groups. In studies using the Center for Epidemiology Studies Depression scale (CES-D), a well-validated self-report measure of depression among older adults, high vascular risk was still significantly related to greater total depressive symptoms as measured by total CES-D score (Hamm et al., 1993) and when a CES-D clinical cut-off score was used to define depressed/non-depressed groups (Baker et al., 1996). Another study found that people who scored above the clinical cutoff on the CES-D were more likely to report hypertension, arteriosclerosis, and circulatory problems compared to those who screened negative for depression (Okwumabua et al., 1997).
Nine cross-sectional studies examined the relationship between VRFs and depressive symptoms using medical diagnosis, diagnosis confirmed by blood sample, or blood pressure measurements as opposed to self-report measures of VRFs (Hajjar et al., 2009; Lamar et al., 2015; Mast, MacNeill, et al., 2004a; Sharma et al., 2009). Of two studies that used inpatient populations and medical diagnosis of VRFs, both found that elevated, self-reported depressive symptoms were positively associated with the presence of vascular conditions (Mast, MacNeill, et al., 2004a; Sharma et al., 2009). However, use of convenience sampling based on consecutive admission to each respective medical facility indicates a methodological weakness of both studies. Mast, MacNeill, et al. (2004a) found that geriatric rehabilitation inpatients with two or more VD/VRFs (excluding stroke) were 1.78 times more likely to have depression than individuals with one or fewer VD/VRFs when controlling for impairment in activities of daily living, cognitive impairment, and medical disability. Although the use of a medically frail population may not be generalizable to larger groups of older adults, a strength of this study includes the researchers’ ability to confirm VD/VRFs based on ICD-9 criteria for hypertension, diabetes, and atrial fibrillation in a large sample.
One study included both inpatients and outpatients with confirmed heart failure and found that 40% of the sample had clinically significant depressive symptoms (Dickson et al., 2013). With a single exception, in which greater depressive symptoms were associated with lower rather than higher blood pressure (Heard et al., 2011), all other studies with physician diagnosis or direct measurement of VD/VRFs, including obesity, hypertension, and inflammation, provide further support that depression and depressive symptoms are associated with vascular disease (Andrews et al., 2020; Lamar et al., 2015; Sims et al., 2020; Taylor et al., 2008). Of note, Lamar et al. (2015) provided evidence that individuals with high risk of metabolic syndrome (≥ 3 VRFs) endorsed significantly more depressive symptoms on the CES-D compared to the low (1-2 VRFs) and no-risk groups, which is consistent with the vascular threshold model of VaDep. Although the sample consisted of 50% Black Americans, interpretation of these results is limited because the authors did not report the impact of incremental metabolic syndrome risk on depression specifically for Black Americans.
Longitudinal Research
The potential for high vascular risk (≥ 2 VRFs) to influence depressive symptoms over time was examined by two research groups using self-report measures of both VRFs and depression (Carmasin et al., 2014; Yochim, MacNeill, et al., 2006). Using an odds ratio as a measure of effect size, Carmasin et al. (2014) identified that after 2.5 years, regardless of baseline depression, the high-risk vascular group was 2.61 times more likely than the low-risk group to develop depression as classified by a clinical cut-off score on the CES-D (Carmasin et al., 2014). This finding provides quality evidence that high vascular burden significantly increases one’s risk of depression across time. This relationship was detected even though VRFs were assessed via self-report of lifetime diagnosis (i.e., “have you ever been told by a nurse or doctor that you have…”), which likely limits the accuracy of VRF report. Additional support for a temporal relationship between high vascular burden and depression was found in a small (n = 134) inpatient rehabilitation sample, which revealed that the presence of two or more VRFs predicted higher depressive symptoms after three months (Yochim, MacNeill, et al., 2006). Taken together, these data provide limited evidence that a positive temporal relationship exists between high rates of self-reported VD/VRFs and depression across both community and medically fragile samples of older Black Americans.
Two longitudinal studies were conducted by the same research group within small geriatric inpatient samples (n = 100 and n = 77) (Mast, MacNeill, et al., 2004b; Mast, Neufeld, et al., 2004) and provided evidence that medically-diagnosed VRFs predict depression over time. Findings revealed that the presence of two or more VRFs was associated with higher depressive symptoms after six months (Mast, Neufeld, et al., 2004) and 18 months of follow-up (Mast, Neufeld, et al., 2004; Mast, Yochim, et al., 2004). Specifically, one study reported that high vascular risk resulted in 5.6 times greater likelihood of having a positive depression screen at six months, five times greater likelihood at 18 months, and overall greater likelihood of having persistent depression across timepoints compared to the low-risk group (Mast, Neufeld, et al., 2004). For patients without baseline depression, high vascular burden also predicted new-onset depression at six and 18 months (Mast, Neufeld, et al., 2004). It is important to note that these samples were small and medically frail with significant dropout rates, which impacts the generalizability of the results.
Race Differences in Prevalence of Comorbid Vascular Disease and Depression
Results were mixed in studies that compared the prevalence of VaDep in Black Americans to other racial groups (Azar et al., 2005; González & Tarraf, 2013). González and Tarraf (2013) provided strong support for the relationship between VRFs and depression in Black Americans through findings from a large, nationally representative sample of older adults in which diagnosis of MDD at or after age 50 was determined through a gold-standard diagnostic interview (see Table 2). Black Americans had the highest rate of cardiovascular disease compared to other racial groups (65.4%) but were the least likely racial group to meet criteria for both cardiovascular disease and MDD (4.7%). However, amongst participants diagnosed with MDD, Black Americans were the group most likely group to have a co-occurring diagnosis of cardiovascular disease (74.4%). This is consistent with other studies that found that Black inpatients with coronary artery disease were 1.6 times more likely than their White peers to score above the clinical cutoff on the CES-D (Boutin-Foste, 2008), and that older adults with comorbid diabetes and depression were more likely to be Black (Cummings et al., 2016). Race differences were also notable in a study that found depressive symptoms were associated with aortic calcification in Black women but not White women (Lewis et al., 2009).
In contrast, Azar et al. (2005) found no racial differences in prevalence of VD/VRFs or depression in a community sample (n = 362) of Black and White Americans, and the interaction between race and vascular risk did not predict self-reported depressive symptoms in their sample, similar to four other studies that found no race differences in the association between various VD/VRF and depression (Freedland et al., 1991; Remigio-Baker et al., 2014; Rohyans & Pressler, 2009; Waldman et al., 2009). It is noteworthy that these studies that found no differences primarily used self-report measures of depressive symptoms, which more likely captures subthreshold depressive symptoms and does not control for past history of depression.
Hajjar et al. (2009) also used the CES-D to measure depressive symptoms in a large community sample (n = 580; 14% Black American), and, using latent profile analyses, identified a class of individuals with poor executive function, slow gait speed, and elevated depressive symptoms. In the overall sample, increased rates of vascular conditions were associated with this triad of symptoms, which the authors identified as a “vascular aging” phenotype. Mixed methods were used to identify VRFs; although self-report history was used to identify diabetes, heart disease, stroke, and congestive heart failure, researchers measured participants’ blood pressure on site to provide an objective measure of hypertension. Black Americans made up a minority of the overall sample, however they were more likely to present with this vulnerable phenotype than White Americans.
Reinlieb et al. (2014) also provided tentative evidence of race differences in VaDep. An analysis of a small (n = 42) sample of clinically depressed individuals enrolled in an 8-week trial of antidepressants revealed that Black Americans were more likely than White Americans to have VaDep (61% vs. 10%) (Reinlieb et al., 2014). This represents the only study to examine racial differences in VaDep using the Krishnan et al. (1997) criteria of WMH to define VaDep, however results from this study should be interpreted with caution. The sample of Black Americans was small (n = 18), and only 16 of the 42 individuals enrolled overall were classified as having VaDep.
Overall, six studies found that Black Americans with VD/VRFs, including cardiovascular disease (González & Tarraf, 2013), coronary artery disease (Boutin-Foste, 2008), diabetes (Cummings et al., 2016), and aortic calcification (Lewis et al., 2009) were more likely to have co-occurring depression compared to other racial groups (Hajjar et al., 2009; Reinlieb et al., 2014). Five studies found no racial differences in prevalence of VD/VRFs or depression (Azar et al., 2005; Freedland et al., 1991; Remigio-Baker et al., 2014; Rohyans & Pressler, 2009; Waldman et al., 2009), although these studies used primarily self-report measures of depressive symptoms which are more likely to capture subthreshold depressive symptoms and do not control for past history of depression. The strongest evidence for race differences comes from González and Tarraf (2013), the only study to use a diagnostic interview to identify major depressive symptoms with onset in late life. Lack of standardized matching procedures between racial groups may also account for the lack of significant findings. Further evidence is needed to accurately evaluate the presence of racial differences in VaDep prevalence.
Cognitive Outcomes Associated with Comorbid VD/VRF and Depression
Only two studies with self-report VRF data examined VaDep-related cognitive outcomes (Carmasin et al., 2014; Yochim, MacNeill, et al., 2006). Relative to other cognitive domains, self-reported vascular burden predicted overall lower cognition and mild decreases in processing speed over the course of 2.5 years (Carmasin et al., 2014). Another group identified a more complex relationship with cognition: VRF burden did not independently predict verbal fluency performance, although VRF burden predicted greater depression scores and greater depression scores predicted worse verbal fluency over a six-month period (Yochim, MacNeill, et al., 2006).
A variety of cognitive deficits related to VRFs and depression, including deficits in executive functioning, were identified across studies in which objective measures of VRFs were used. Black Americans were more likely than White Americans to be part of a group with elevated depressive symptoms and worse performance on executive functions as measured by Trail Making Test B (Hajjar et al., 2009). Lamar et al. (2015) also reported that objectively measured elevated systolic blood pressure and fasting blood glucose levels predicted worse memory and learning scores in Black Americans, but not White Americans. Relatedly, an interaction between vascular risk and executive functioning deficits was found to predict depressive symptoms, such that depressive symptoms increased for the high-risk vascular group only when low performance was demonstrated on the Initiation/Perseveration (I/P) subscale of the Mattis Dementia Rating Scale (Mast, Yochim, et al., 2004). Consistent with these findings, Reinlieb et al. (2014) provided limited support that those with MRI-defined VaDep may perform worse on the I/P subscale and Stroop Color/Word Test compared to a non-vascular depression group, as well as had slower reaction times.
Taken together, studies using both self-report (Carmasin et al., 2014; Yochim, MacNeill, et al., 2006) and objective measures of VD/VRFs (Hajjar et al., 2009; Lamar et al., 2015; Mast, Yochim, et al., 2004; Reinlieb et al., 2014) support that VaDep in older Black Americans may be associated with cognitive deficits, particularly deficits in executive functioning. Replication in larger samples is required in order to draw accurate conclusions about the relationship between VaDep and cognition in Black Americans.
Functional and Health Outcomes Associated with Comorbid VD/VRF and Depression
Seven studies also examined secondary functional or health outcomes of VaDep (Carmasin et al., 2014; Hamm et al., 1993; Yochim, Kerkar, et al., 2006; Yochim et al., 2003). High vascular risk and presence of depression in community samples of Black Americans predicted a greater number of doctor’s visits and number of days spent sick in bed (Yochim et al., 2003), worse subjective physical health (Hamm et al., 1993; Yochim, Kerkar, et al., 2006), significantly more comorbid medical illnesses (Hamm et al., 1993), and poorer self-care (Dickson et al., 2013) than their non-depressed counterparts. In Black inpatients with heart failure, those who were depressed had an increased risk for 30-day readmission (Lu et al., 2017). Some evidence for racial differences was also identified: compared to White Americans, Black Americans with co-occurring cardiovascular disease and MDD reported more days of functional impairment (González & Tarraf, 2013), and baseline depressive symptoms and worsening of symptoms were associated with increased all-cause mortality and hospitalization in Black but not White participants (Mentz et al., 2015).
Members of the “vascular aging” phenotype defined by Hajjar et al. (2009) were more likely to be Black American compared to other racial groups, impaired in instrumental activities of daily living, and more likely to perform poorly on physical performance measures. Additionally, older Black Americans with acute decompensated heart failure and depression had significantly longer hospital stays, more health comorbidities, and a greater likelihood of having more severe and physically limiting heart failure symptoms than those without depression (Sharma et al., 2009). The depressed group additionally reported high rates of functional impairment and significantly worse quality of life compared to those with low depression scores (Sharma et al., 2009).
The present literature is limited by the heterogeneous methods used to examine functional outcomes related to VaDep in Black Americans, although the variety of methods indicate the multi-faceted functional impact that VaDep may have in this population. For example, it is clear that the co-occurrence of vascular conditions and depression in this population are associated with health factors such as hospitalization rates and mortality (Lu et al., 2017; Mentz et al., 2015; Sharma et al., 2009; Yochim et al., 2003), health behaviors (Dickson et al., 2013; Hamm et al., 1993), and quality of life (González & Tarraf, 2013) in addition to traditional activities of daily living (Hajjar et al., 2009). Further research is required to more clearly determine the impact of VaDep across activities of daily living and other measurements of function and wellness in Black American samples.
Psychological Outcomes Associated with VaDep
While specific depressive symptoms were not well characterized in this review, one study found that urban, socially-isolated Black American elders were likely to have co-occurring VRFs and elevated depression with themes of isolation and loss (Baker et al., 1996). Furthermore, individuals with multiple VRFs and depression perceived themselves as having less control over their health than those with one or fewer VRFs (Hamm et al., 1993). The only study that evaluated psychological characteristics of VaDep among studies that did not rely on self-report VD/VRF data was research performed by Reinlieb et al. (2014). Based on evaluation of 16 participants with MRI-defined VaDep, there is limited evidence that those with VaDep were less likely to have a family history of affective illness, more likely to experience psychomotor retardation, and more likely to have an earlier age of onset than those with non-vascular depression (Reinlieb et al., 2014). Further research and replication are needed to determine the symptom profile of VaDep.
Discussion
The present systematic review broadly supports the validity of the VaDep hypothesis in older Black Americans; specifically, that VD/VRFs increase the likelihood of developing depressive symptoms within this racial minority group. Although the literature is limited in research with strong objective measures of VRFs (e.g., WMH data), there is tentative evidence for a threshold effect of vascular risk in this population such that risk of depression appears to increase when two or more VRFs are present. Preliminary evidence also suggests that a relationship between VaDep, functional disability, and cognitive deficits exists among Black Americans in a way similar to VaDep in the broader literature. However, due to limitations of the available body of research such as reliance on self-report VD/VRF data, lack of substantive neuroimaging data, and longitudinal research limited by short time to follow-up, only limited conclusions can be drawn about the causative nature of VD/VRFs on depression, or about the clinical features of VaDep (e.g., symptom profile or treatment response) in comparison to the broader literature. Despite these limitations, the available data converge with the main tenants of the VaDep hypothesis and support the critical need for further investigation of this construct in Black Americans.
In the broader VaDep literature, vascular disease has been proposed to contribute significantly to a threshold of neurocognitive vulnerability, which, once crossed, increases the likelihood that an older adult will develop depression (Taylor et al., 2013). This review supports the concept of a vascular threshold effect, such that older Black Americans were more likely to have depression in the presence of two or more vascular conditions (Azar et al., 2005; Carmasin et al., 2014; Hamm et al., 1993; Lamar et al., 2015; Mast, MacNeill, et al., 2004a, 2004b; Mast, Neufeld, et al., 2004; Yochim, Kerkar, et al., 2006; Yochim, MacNeill, et al., 2006; Yochim et al., 2003). No individual VD/VRFs were found to independently predict depression in this review (Lamar et al., 2015; Mast, MacNeill, et al., 2004a), therefore increased risk of depression may be due to the cumulative burden of vascular disease in this population. Perhaps in contrast, the disconnection hypothesis of VaDep posits that focal damage to white matter tracts, rather than global severity of WMH, underlies the brain’s vulnerability to VaDep (Taylor et al., 2013). Due to the absence of neuroimaging data in the available literature, the role of focal WMH versus cumulative cerebrovascular disease burden in VaDep onset for older Black Americans is not clear.
Executive dysfunction, as studied in mostly non-Black samples, has been reported as a key feature of VaDep that may underly co-occurring functional disability (Alexopoulos et al., 2002) and poor antidepressant response (Sheline et al., 2010). The present review provides preliminary support for a relationship between executive functioning deficits and VaDep in Black Americans (Hajjar et al., 2009; Mast, Yochim, et al., 2004; Reinlieb et al., 2014; Yochim, MacNeill, et al., 2006). Additionally, in a large sample of Black Americans across 2.5 years, high self-reported vascular burden was found to be linked to overall lower cognition and mild decreases in processing speed (Carmasin et al., 2014), which is consistent with the known impact of VaDep on cognition in mostly non-Black samples (Aizenstein et al., 2016). Some racial differences in cognitive function were also identified: elevated glucose and systolic blood pressure negatively affected learning and memory scores only in Black Americans (Lamar et al., 2015). This early evidence of a potentially race-specific cognitive profile in Black Americans with VaDep highlights the need for comprehensive neuropsychological research.
VaDep has also historically been characterized by functional disability out of proportion to the severity of depressive symptoms (Aizenstein et al., 2016; Chang et al., 2016). The present review provides converging evidence to support that VaDep in Black Americans is also associated with functional disability. Across both inpatient and community samples, those with high vascular risk and depression were more likely to experience worse quality of life, longer hospital stays (Sharma et al., 2009), more health comorbidities (Hamm et al., 1993; Sharma et al., 2009), more doctor’s visits (Yochim et al., 2003), and worse physical function and disability (González & Tarraf, 2013; Hajjar et al., 2009) compared to those with lower vascular risk and depression.
Lastly, although higher prevalence of VaDep in Black Americans was anticipated due to disproportionate rates of vascular disease compared to White Americans, findings from the present review are inconclusive. Evidence from a methodologically strong study with a nationally representative sample supports that Black Americans are the most likely racial group to have comorbid cardiovascular disease when a diagnosis of MDD is present (González & Tarraf, 2013). In contrast, no difference was observed between Black Americans and White Americans in a moderately-sized sample (Azar et al., 2005). Although racial differences in prevalence rates of VaDep were not confirmed, González and Tarraf (2013) also revealed potential race-specific consequences of VaDep: Black Americans with both cardiovascular disease and MDD reported significantly more days of functional impairment compared to White Americans and all other racial/ethnic minorities with these co-occurring conditions. Much larger-scale comparison studies are required to accurately identify whether the prevalence, course, and functional outcomes of VaDep differs between racial groups.
Limitations
The synthesis of the available data concerning VaDep in Black Americans highlights major limitations of the current research, although it is important to note that publication bias is inherently present in this review given that only articles published in academic journals were examined. First and foremost, the “two or more” threshold of vascular risk has yet to be systematically studied against other potential thresholds (e.g., levels of MRI-defined WMH) in Black Americans, and there was great variability in the number of VD/VRFs examined per study. The literature also lacks standardization regarding what should be classified as a VRF when examining the VaDep hypothesis. For example, some studies included history of stroke as a VRF whereas this was exclusionary in others, and across studies there was a mix of potentially well-controlled VRFs (e.g., hypertension) and more severe vascular diseases (e.g., congestive heart failure). Age of onset and severity of those illnesses were also not reported, and the majority of studies with large, community samples relied on self-report data of VRFs, which is known to be unreliable (Cigolle et al., 2018). Given this variability, it is difficult to draw firm conclusions about what threshold of vascular risk increases risk of VaDep in Black Americans.
Limitations in the assessment of depression in Black Americans must also be considered. Evidence suggests the symptom presentation of depression may differ in Black compared to non-Hispanic white individuals (Bailey et al., 2019), and differences in the psychometric properties of depression assessment measures across race have been documented (for a review, see Dotson et al., 2019). This underscores the need for culturally sensitive assessment of depression in both research and clinical settings (Qureshi et al., 2011). A related limitation is potential confounds from comorbid psychological conditions, such as substance use or anxiety disorders, which were not consistently controlled for across previous studies in this area. This raises the possibility that other symptoms mediate or moderate the relationship between VD/VRFs and depression.
Overall, the lack of consistent neuroimaging, neuropsychological, and longitudinal data prevents inferences about the causal nature of vascular risk on depression. Additionally, inherent risk of publication and reporting bias likely affected the review of this literature, given that only primary research studies published in academic journals were reviewed. Despite these limitations, patterns in the data may be interpreted to provide useful directions for future research and educate healthcare practitioners on the need to screen for depression when vascular risk is present in older Black Americans.
Future Research Recommendations
Based on findings from this review, future directions for VaDep research in Black Americans are clear. The relationship between WMH and VaDep is well documented (Salo et al., 2019), yet no research examining WMH and VaDep in Black Americans exists, to our knowledge. Specifically, future research should answer the following questions: Does location and/or burden of WMH predict depression among older Black Americans with vascular disease? Is high vascular burden (≥ 2 VRFs) a useful clinical predictor of VaDep in this population? Are WMH among Black Americans with VaDep associated with functional impairment, executive dysfunction, or other cognitive deficits? Does treatment (with exercise, antidepressants, and/or VRF management) improve integrity of white matter as well as the course of VaDep in Black Americans? Do Black Americans with VaDep have disproportionately severe WMH burden compared to their White counterparts?
In addition, immune dysregulation has been proposed as an underlying factor of late-life depression (Alexopoulos & Morimoto, 2011) given that both the natural aging process and depressive states have separately been linked to inflammatory response (Franceschi & Campisi, 2014; Harrison et al., 2009). This mechanistic theory may have particular relevance for Black Americans since early life adversity and lifetime discrimination have been linked to greater concentrations of systemic inflammatory markers in midlife for Black Americans, but not White Americans (Slopen et al., 2010; Stepanikova, Bateman, et al., 2017). Future research, therefore, should examine the role of inflammation in the development of VaDep in Black Americans.
History of depression was rarely controlled for in this literature and the use of cut-off scores on depression measures limits our understanding of depression as a dimensional construct. Future research should clarify the depressive symptom dimensions (e.g., apathy, sadness, psychomotor slowing) experienced by Black Americans with VaDep and examine racial differences in symptom profiles. For example, religiosity and spirituality have been shown to play a significant role in older Black Americans’ experience of depression and depression care (Turner et al., 2019; Wharton et al., 2018), therefore VaDep symptoms may present uniquely among Black American elders. Under-diagnosis and inadequate management of MDD in Black Americans is already well documented (Agyemang et al., 2014; Das et al., 2006), therefore this research is critical for informing culturally-sensitive diagnosis and treatment of VaDep.
Finally, VaDep and vascular cognitive impairment share common neurobiological substrates and cognitive deficits, such as white matter lesions, neuroinflammation, and executive dysfunction (Luca & Luca, 2019; Taylor et al., 2013; Vinciguerra et al., 2020). Our understanding of and treatments for both disorders would be informed by future studies that examine the interrelationships between these neurobiological changes, mood disruption, and cognitive impairment in Black Americans.
Practice Recommendations
The results of this review have implications for neuropsychologists’ day-to-day practice with Black clients. Although more work needs to be done, the existing literature attests to the importance of screening for depression in late middle-aged (age 50) and older Black clients who have co-occurring vascular illness, particularly when two or more VD/VRFs are present. Neuropsychologists should not only be aware of the potential comorbidity of VD/VRFs and depression, but should also consider the potential impact of this comorbidity on neuropsychological test performance, especially in the domain of executive functioning. Impairment due to VaDep should be ruled out before making a diagnosis of vascular dementia or other neurodegenerative conditions with fronto-subcortical profiles (e.g., considering the severity and breadth of impairment, which are typically greater in dementia than in depression). In addition, reduction of vascular risk, for example through diet and exercise, may be an important intervention for older Black Americans with VaDep and an important preventative strategy for older Black Americans at risk of developing VaDep.
Conclusion
The present review supports the validity of the VaDep framework in aging Black Americans. Specifically, a vascular threshold model may be relevant, such that the risk of depression is higher when two or more VD/VRFs are present. Consistent with the broader VaDep literature, the co-occurrence of VD/VRFs and depressive symptoms is associated with functional disability and cognitive deficits in Black Americans. There is also preliminary support for differing cognitive profiles between Black and White Americans with co-occurring depression and vascular disease. Neuroimaging, neuropsychological, and longitudinal research is required to more fully characterize the psychological and neurobiological profile of VaDep in Black Americans. At this time, the evidence suggests that healthcare providers should screen for depression in Black Americans aged 50 and older, particularly when two or more vascular risk factors are present.
Acknowledgements
VMD is supported by the National Institutes of Health (AG054046-04). VMD is Founder and President of CerebroFit, LLC.
References
- Agyemang AA, Mezuk B, Perrin P, & Rybarczyk B (2014). Quality of depression treatment in Black Americans with major depression and comorbid medical illness. Gen Hosp Psychiatry, 36(4), 431–436. 10.1016/j.genhosppsych.2014.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizenstein HJ, Baskys A, Boldrini M, Butters MA, Diniz BS, Jaiswal MK, … Tene O (2016). Vascular depression consensus report - a critical update. BMC Medicine, 14(1), 161. 10.1186/s12916-016-0720-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajilore O, & Thames AD (2020). The fire this time: The stress of racism, inflammation and COVID-19. Brain Behav Immun, 88, 66–67. 10.1016/j.bbi.2020.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos GS (2019). Mechanisms and treatment of late-life depression. Translational Psychiatry, 9(1), 188. 10.1038/s41398-019-0514-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos GS, Kiosses DN, Klimstra S, Kalayam B, & Bruce M (2002). Clinical presentation of the "Depression-Executive Dysfunction Syndrom" of late life. American Journal of Geriatric Psychiatry, 10(1), 98–106. https://pubmed.ncbi.nlm.nih.gov/11790640/ [PubMed] [Google Scholar]
- Alexopoulos GS, Meyers BS, Young RC, Campbell S, Silbersweig D, & Charlson M (1997). 'Vascular depression' hypothesis. Archives of General Psychiatry, 54, 915–922. 10.1001/archpsyc.1997.01830220033006 [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, & Morimoto SS (2011). The inflammation hypothesis in geriatric depression. International Journal of Geriatric Psychiatry, 26(11), 1109–1118. 10.1002/gps.2672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews MR, Ceasar J, Tamura K, Langerman SD, Mitchell VM, Collins BS, … Powell-Wiley TM (2020). Neighborhood environment perceptions associate with depression levels and cardiovascular risk among middle-aged and older adults: Data from the washington, dc cardiovascular health and needs assessment. Aging & Mental Health. 10.1080/13607863.2020.1793898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azar AR, Murrell SA, & Mast BT (2005). Race and vascular depression risk in community-dwelling older adults. The American Journal of Geriatric Psychiatry, 13(4), 339–342. 10.1176/appi.ajgp.13.4.329 [DOI] [PubMed] [Google Scholar]
- Bailey RK, Mokonogho J, & Kumar A (2019). Racial and ethnic differences in depression: current perspectives. Neuropsychiatr Dis Treat, 15, 603–609. 10.2147/NDT.S128584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker FM, Okwumabua J, Philipose V, & Wong S (1996). Screening African-American elderly for the presence of depressive symptoms: a preliminary investigation. Journal of Geriatric Psychiatry and Neurology, 9, 127–132. 10.1177/089198879600900304. [DOI] [PubMed] [Google Scholar]
- Barch DM, D’Angelo G, Pieper C, Wilkins CH, Welsh-Bohmer K, Taylor W, … Sheline YI (2012). Cognitive improvement following treatment in late-life depression: relationship to vascular risk and age of onset. Am J Geriatr Psychiatry, 20(8), 682–690. 10.1097/JGP.0b013e318246b6cb [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty Moody DL, Taylor AD, Leibel DK, Al-Najjar E, Katzel LI, Davatzikos C, … Waldstein SR (2019). Lifetime discrimination burden, racial discrimination, and subclinical cerebrovascular disease among African Americans. Health Psychology, 38(1), 63–74. 10.1037/hea0000638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bella R, Pennisi G, Cantone M, Palermo F, Pennisi M, Lanza G, … Paolucci S (2010). Clinical presentation and outcome of geriatric depression in subcortical ischemic vascular disease. Gerontology, 56(3), 298–302. 10.1159/000272003 [DOI] [PubMed] [Google Scholar]
- Boutin-Foste C (2008). An item-level analysis of the Center for Epidemiologic Studies Depression Scale (CES-D) by race and ethnicity in patients with coronary artery disease. International Journal of Geriatric Psychiatry, 23(10), 1034–1039. 10.1002/gps.2029 [DOI] [PubMed] [Google Scholar]
- Brickman AM, Schupf N, Manly JJ, Luchsinger JA, Andrews H, Tang MX, … Brown TR (2008). Brain morphology in older African Americans, Caribbean Hispanics, and Whites from northern Manhattan. Archives of Neurology, 65(8), 1053–1061. 10.1001/archneur.65.8.1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmasin JS, Mast BT, Allaire JC, & Whitfield KE (2014). Vascular risk factors, depression, and cognitive change among African American older adults. International Journal of Geriatric Psychiatry, 29(3), 291–298. 10.1002/gps.4007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang KJ, Hong CH, Kim SH, Lee KS, Roh HW, Kang DR, … Son SJ (2016). MRI-defined versus clinically-defined vascular depression; comparison of prediction of functional disability in the elderly. Archives of Gerontology and Geriatrics, 66, 7–12. 10.1016/j.archger.2016.04.010 [DOI] [PubMed] [Google Scholar]
- Cigolle CT, Nagel CL, Blaum CS, Liang J, & Quiñones AR (2018). Inconsistency in the Self-report of Chronic Diseases in Panel Surveys: Developing an Adjudication Method for the Health and Retirement Study. J Gerontol B Psychol Sci Soc Sci, 73(5), 901–912. 10.1093/geronb/gbw063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culang-Reinlieb ME, Johnert LC, Brickman AM, Steffens DC, Garcon E, & Sneed JR (2011). MRI-defined vascular depression: a review of the construct. International Journal of Geriatric Psychiatry, 26(11), 1101–1108. 10.1002/gps.2668 [DOI] [PubMed] [Google Scholar]
- Cummings DM, Kirian K, Howard G, Howard V, Yuan Y, Muntner P, … Safford MM (2016). Consequences of Comorbidity of Elevated Stress and/or Depressive Symptoms and Incident Cardiovascular Outcomes in Diabetes: Results From the REasons for Geographic And Racial Differences in Stroke (REGARDS) Study. Diabetes Care, 39(1), 101–109. 10.2337/dc15-1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalby RB, Frandsen J, Chakravarty MM, Ahdidan J, Sørensen L, Rosenberg R, … Ostergaard L (2010). Depression severity is correlated to the integrity of white matter fiber tracts in late-onset major depression. Psychiatry Research, 184(1), 38–48. 10.1016/j.pscychresns.2010.06.008 [DOI] [PubMed] [Google Scholar]
- Das AK, Olfson M, McCurtis HL, & Weissman MM (2006). Depression in African Americans: breaking barriers to detection and treatment. Journal of Family Practice, 55(1), 30–39. [PubMed] [Google Scholar]
- Dickson VV, McCarthy MM, & Katz SM (2013). How do depressive symptoms influence self-care among an ethnic minority population with heart failure? Ethn Dis, 23(1), 22–28. [PubMed] [Google Scholar]
- Diez Roux AV (2016). Neighborhoods and Health: What Do We Know? What Should We Do? Am J Public Health, 106(3), 430–431. 10.2105/AJPH.2016.303064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz BS, Butters MA, Albert SM, Dew MA, & Reynolds CF 3rd. (2013). Late-life depression and risk of vascular dementia and Alzheimer's disease: systematic review and meta-analysis of community-based cohort studies. British Journal of Psychiatry, 202(5), 329–335. 10.1192/bjp.bp.112.118307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divers J, Hugenschmidt C, Sink KM, Williamson JD, Ge Y, Smith SC, … Freedman BI (2013). Cerebral white matter hyperintensity in African Americans and European Americans with type 2 diabetes. Journal of Stroke and Cerebrovascular Diseases, 22(7), e46–52. 10.1016/j.jstrokecerebrovasdis.2012.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolezsar CM, McGrath JJ, Herzig AJM, & Miller SB (2014). Perceived racial discrimination and hypertension: a comprehensive systematic review. Health Psychol, 33(1), 20–34. 10.1037/a0033718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotson VM, Levy SA, O’Shea DM, McLaren ME, & Szymkowicz SM (2019). Assessment of mood disorders in ethnic minorities. In Pedraza O (Ed.), Clinical Cultural Neuroscience: An Integrative Approach to Cross-Cultural Neuropsychology. Oxford University Press. [Google Scholar]
- Everson-Rose SA, Lutsey PL, Roetker NS, Lewis TT, Kershaw KN, Alonso A, & Diez Roux AV (2015). Perceived Discrimination and Incident Cardiovascular Events: The Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol, 182(3), 225–234. 10.1093/aje/kwv035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C, & Campisi J (2014). Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. The journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 69 Suppl 1, S4–9. 10.1093/gerona/glu057 [DOI] [PubMed] [Google Scholar]
- Freedland KE, Carney RM, Rich MW, & Caracciolo A (1991). Depression in elderly patients with congestive heart failure. Journal of Geriatric Psychiatry, 24(1), 59–71. https://search.ebscohost.com/login.aspx?direct=true&AuthType=ip,shib&db=psyh&AN=1991-27822-001&site=ehost-live&scope=site&custid=gsu1 [Google Scholar]
- González HM, & Tarraf W (2013). Comorbid cardiovascular disease and major depression among ethnic and racial groups in the United States. International Psychogeriatrics, 25(5), 833–841. 10.1017/S1041610212002062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Walton M, Cheng J, Acuna J, Klimstra S, Zimmerman ME, … Alexopoulos GS (2010). MRI signal hyperintensities and treatment remission of geriatric depression. Journal of Affective Disorders, 126(3), 395–401. 10.1016/j.jad.2010.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjar I, Yang F, Sorond F, Jones RN, Milberg W, Cupples LA, & Lipsitz LA (2009). A novel aging phenotype of slow gait, impaired executive function, and depressive symptoms: Relationship to blood pressure and other cardiovascular risks. The Journals of Gerontology: Series A: Biological Sciences and Medical Sciences, 64(9), 994–1001. 10.1093/gerona/glp075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm VP, Bazargan M, & Barbre AR (1993). Life-style and cardiovascular health among urban Black elderly. Journal of Applied Gerontology, 12(2), 155–169. 10.1177/073346489301200203 [DOI] [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, & Critchley HD (2009). Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biological Psychiatry, 66(5), 407–414. 10.1016/j.biopsych.2009.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havranek EP, Mujahid MS, Barr DA, Blair IV, Cohen MS, Cruz-Flores S, … Stroke C (2015). Social Determinants of Risk and Outcomes for Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation, 132(9), 873–898. 10.1161/CIR.0000000000000228 [DOI] [PubMed] [Google Scholar]
- Heard E, Whitfield KE, Edwards CL, Bruce MA, & Beech BM (2011). Mediating effects of social support on the relationship among perceived stress, depression, and hypertension in African Americans. J Natl Med Assoc, 103(2), 116–122. 10.1016/s0027-9684(15)30260-1 [DOI] [PubMed] [Google Scholar]
- Hsu FC, Sink KM, Hugenschmidt CE, Williamson JD, Hughes TM, Palmer ND, … Freedman BI (2018). Cerebral structure and cognitive performance in African Americans and European Americans with type 2 diabetes. The journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 73(3), 407–414. 10.1093/gerona/glx255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LA, Large SE, Izurieta Munoz H, Hall JR, & O'Bryant SE (2019). Vascular depression and cognition in Mexican Americans. Dementia and Geriatric Cognitive Disorders, 47(1-2), 68–78. 10.1159/000494272 [DOI] [PubMed] [Google Scholar]
- Kershaw KN, & Albrecht SS (2015). Racial/ethnic residential segregation and cardiovascular disease risk. Curr Cardiovasc Risk Rep, 9(3). 10.1007/s12170-015-0436-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Woo SY, Kang HS, Lim SW, Choi SH, Myung W, … Kim DK (2016). Factors related to prevalence, persistence, and incidence of depressive symptoms in mild cognitive impairment: vascular depression construct. International Journal of Geriatric Psychiatry, 31(7), 818–826. 10.1002/gps.4400 [DOI] [PubMed] [Google Scholar]
- Krishnan KRR, Hays JC, & Blazer DG (1997). MRI-defined vascular depression. American Journal of Psychiatry, 154(4), 497–501. 10.1176/ajp.154.4.497 [DOI] [PubMed] [Google Scholar]
- Krishnan KRR, Taylor WD, McQuoid DR, MacFall JR, Payne ME, Provenzale JM, & Steffens DC (2004). Clinical characteristics of magnetic resonance imaging-defined subcortical ischemic depression. Biological Psychiatry, 55(4), 390–397. 10.1016/j.biopsych.2003.08.014 [DOI] [PubMed] [Google Scholar]
- Lamar M, Rubin LH, Ajilore O, Charlton R, Zhang A, Yang S, … Kumar A (2015). What metabolic syndrome contributes to brain outcomes in African American and Caucasian cohorts. Current Alzheimer Research, 12, 640–647. 10.2174/1567205012666150701102325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis TT, Everson-Rose SA, Colvin A, Matthews K, Bromberger JT, & Sutton-Tyrrell K (2009). Interactive effects of race and depressive symptoms on calcification in African American and White women. Psychosomatic Medicine, 71(2), 163–170. 10.1097/PSY.0b013e31819080e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu MLR, De Venecia TA, Goyal A, Rodriguez Ziccardi M, Kanjanahattakij N, Shah MK, … Figueredo VM (2017). Psychiatric conditions as predictors of rehospitalization among African American patients hospitalized with heart failure. Clin Cardiol, 40(11), 1020–1025. 10.1002/clc.22760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luca M, & Luca A (2019). Oxidative stress-related endothelial damage in vascular depression and vascular cognitive impairment: Beneficial effects of aerobic physical exercise. Oxidative Medicine and Cellular Longevity, 2019, 8067045. 10.1155/2019/8067045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masse-Sibille C, Djamila B, Julie G, Emmanuel H, Pierre V, & Gilles C (2018). Predictors of response and remission to antidepressants in geriatric depression: A systematic review. Journal of Geriatric Psychiatry and Neurology, 31(6), 283–302. 10.1177/0891988718807099 [DOI] [PubMed] [Google Scholar]
- Mast BT, MacNeill SE, & Lichtenberg PA (2004a). Post-stroke and clinically-defined vascular depression in geriatric rehabilitation patients. The American Journal of Geriatric Psychiatry, 12(1), 84–92. 10.1097/00019442-200401000-00011 [DOI] [PubMed] [Google Scholar]
- Mast BT, MacNeill SE, & Lichtenberg PA (2004b). Risk factors for geriatric depression: the importance of executive functioning within the vascular depression hypothesis. Journal of Gerontology: Medical Sciences, 59A(12), 1290–1294. 10.1093/gerona/59.12.1290 [DOI] [PubMed] [Google Scholar]
- Mast BT, Neufeld S, MacNeill SE, & Lichtenberg PA (2004). Longitudinal support for the relationship between vascular risk factors and late-life depressive symptoms. The American Journal of Geriatric Psychiatry, 12(1), 93–101. 10.1097/00019442-200401000-00012 [DOI] [PubMed] [Google Scholar]
- Mensah GA (2018). Cardiovascular diseases in African Americans: Fostering community partnerships to stem the tide. American Journal of Kidney Diseases, 72(5 Suppl 1), S37–S42. 10.1053/j.ajkd.2018.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentz RJ, Babyak MA, Bittner V, Fleg JL, Keteyian SJ, Swank AM, … Blumenthal JA (2015). Prognostic significance of depression in blacks with heart failure: insights from Heart Failure: a Controlled Trial Investigating Outcomes of Exercise Training. Circulation: Heart Failure, 8(3), 497–503. 10.1161/circheartfailure.114.001995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, & Group P (2010). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg, 8(5), 336–341. 10.1016/j.ijsu.2010.02.007 [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, … Turner MB (2016). Executive summary: heart disease and stroke statistics--2016 update: A report rrom the American Heart Association. Circulation, 133(4), 447–454. 10.1161/cir.0000000000000366 [DOI] [PubMed] [Google Scholar]
- Naarding P, Veereschild M, Bremmer M, Deeg D, & Beekman ATF (2009). The symptom profile of vascular depression. International Journal of Geriatric Psychiatry, 24(9), 965–969. 10.1002/gps.2203 [DOI] [PubMed] [Google Scholar]
- Newberg AR, Davydow DS, & Lee HB (2006). Cerebrovascular disease basis of depression: post-stroke depression and vascular depression. International Review of Psychiatry, 18(5), 433–441. 10.1080/09540260600935447 [DOI] [PubMed] [Google Scholar]
- Nyquist PA, Bilgel MS, Gottesman R, Yanek LR, Moy TF, Becker LC, … Vaidya D (2014). Extreme deep white matter hyperintensity volumes are associated with African American race. Cerebrovascular Diseases, 37(4), 244–250. 10.1159/000358117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okwumabua JO, Baker FM, Wong SP, & Pilgram BO (1997). Characteristics of depressive symptoms in elderly urban and rural African Americans. The Journals of Gerontology: Series A: Biological Sciences and Medical Sciences, 52(4), M241–M246. 10.1093/gerona/52A.4.M241 (Series A: Biological Sciences and Medical Sciences) [DOI] [PubMed] [Google Scholar]
- Park JH, Lee SB, Lee JJ, Yoon JC, Han JW, Kim TH, … Kim KW (2015). Epidemiology of MRI-defined vascular depression: A longitudinal, community-based study in Korean elders. Journal of Affective Disordors, 180, 200–206. 10.1016/j.jad.2015.04.008 [DOI] [PubMed] [Google Scholar]
- Persaud AD, Singh D, Whitboume SK, & Sneed JR (2012). Vascular depression and African Americans: A population at risk. In Sullivan JM & Esmail AM (Eds.), African American identity: Racial and cultural dimensions of the Black experience. (pp. 221–246). Lexington Books/Rowman & Littlefield. https://search.ebscohost.com/login.aspx?direct=true&AuthType=ip,shib&db=psyh&AN=2012-12594-009&site=ehost-live&scope=site&custid=gsu1 [Google Scholar]
- Pimontel MA, Rindskopf D, Rutherford BR, Brown PJ, Roose SP, & Sneed JR (2016). A meta-analysis of executive dysfunction and antidepressant treatment response in late-life depression. American Journal of Geriatric Psychiatry, 24(1), 31–41. 10.1016/j.jagp.2015.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakaran S, Wright CB, Yoshita M, Delapaz R, Brown T, DeCarli C, & Sacco RL (2008). Prevalence and determinants of subclinical brain infarction: the Northern Manhattan Study. Neurology, 70(6), 425–430. 10.1212/01.wnl.0000277521.66947.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi A, Collazos F, Revollo HW, & Casas M (2011). S36-04 - Culturally sensitive assessment of depression from a psychometric perspective. European Psychiatry, 26, 2140. https://doi.org/ 10.1016/S0924-9338(11)73843-3 [DOI] [Google Scholar]
- Reinlieb ME, Persaud A, Singh D, Garcon E, Rutherford BR, Pelton GH, … Sneed JR (2014). Vascular depression: overrepresented among African Americans? International Journal of Geriatric Psychiatry, 29(5), 470–477. 10.1002/gps.4029 [DOI] [PubMed] [Google Scholar]
- Remigio-Baker RA, Allison MA, Schreiner PJ, Szklo M, Crum RM, Leoutsakos J-M, … Golden SH (2014). Difference by sex but not by race/ethnicity in the visceral adipose tissue-depressive symptoms association: The Multi-Ethnic Study of Atherosclerosis. Psychoneuroendocrinology, 47, 78–87. 10.1016/j.psyneuen.2014.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohyans LM, & Pressler SJ (2009). Depressive symptoms and heart failure: examining the sociodemographic variables. Clinical Nurse Specialist, 23(3), 138–144. 10.1097/NUR.0b013e3181a443b4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo KI, Scharfen J, Wilden ID, Schubotz RI, & Holling H (2019). Confining the concept of vascular depression to late-onset depression: A meta-analysis of MRI-defined hyperintensity burden in Major Depressive Disorder and Bipolar Disorder. Frontiers in Psychology, 10, 1241. 10.3389/fpsyg.2019.01241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma V, Zehtabchi S, Rojas N, & Birkhahn R (2009). Ethnic variations in quality of life and depressive symptoms among Black Americans with acute decompensated heart failure. Journal of the National Medical Association, 101(10), 985–991. 10.1016/s0027-9684(15)31064-6 [DOI] [PubMed] [Google Scholar]
- Sheline YI, Pieper CF, Barch DM, Welsh-Bohmer K, McKinstry RC, MacFall JR, … Doraiswamy PM (2010). Support for the vascular depression hypothesis in late-life depression: results of a 2-site, prospective, antidepressant treatment trial. Archives of General Psychiatry, 67(3), 277–285. 10.1001/archgenpsychiatry.2009.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Price JL, Vaishnavi SN, Mintun MA, Barch DM, Epstein AA, … McKinstry RC (2008). Regional white matter hyperintensity burden in automated segmentation distinguishes late-life depressed subjects from comparison subjects matched for vascular risk factors. American Journal of Psychiatry, 165(4), 524–532. 10.1176/appi.ajp.2007.07010175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims M, Glover LSM, Gebreab SY, & Spruill TM (2020). Cumulative psychosocial factors are associated with cardiovascular disease risk factors and management among African Americans in the Jackson Heart Study. BMC Public Health, 20(1), 566. 10.1186/s12889-020-08573-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slopen N, Lewis TT, Gruenewald TL, Mujahid MS, Ryff CD, Albert MA, & Williams DR (2010). Early life adversity and inflammation in African Americans and whites in the midlife in the United States survey. Psychosomatic Medicine, 72(7), 694–701. 10.1097/PSY.0b013e3181e9c16f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanikova I, Baker EH, Simoni ZR, Zhu A, Rutland SB, Sims M, & Wilkinson LL (2017). The role of perceived discrimination in obesity among African Americans. American Journal of Preventive Medicine, 52(1S1), S77–S85. 10.1016/j.amepre.2016.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanikova I, Bateman LB, & Oates GR (2017). Systemic inflammation in midlife: Race, socioeconomic status, and perceived discrimination. Am J Prev Med, 52(1, Supplement 1), S63–S76. https://doi.org/ 10.1016/j.amepre.2016.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabaei BP, Chamany S, Perlman S, Thorpe L, Bartley K, & Wu WY (2019). Heart age, cardiovascular disease risk, and disparities by sex and race/ethnicity among New York City adults. Public Health Reports, 134(4), 404–416. 10.1177/0033354919849881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JY, Washington OG, Artinian NT, & Lichtenberg P (2008). Relationship between depression and specific health indicators among hypertensive African American parents and grandparents. Prog Cardiovasc Nurs, 23(2), 68–78. 10.1111/j.1751-7117.2008.08128.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WD, Aizenstein HJ, & Alexopoulos GS (2013). The vascular depression hypothesis: mechanisms linking vascular disease with depression. Molecular Psychiatry, 18(9), 963–974. 10.1038/mp.2013.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner N, Hastings JF, & Neighbors HW (2019). Mental health care treatment seeking among African Americans and Caribbean Blacks: what is the role of religiosity/spirituality? Aging & Mental Health, 23(7), 905–911. 10.1080/13607863.2018.1453484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadiveloo M, & Mattei J (2017). Perceived weight discrimination and 10-year risk of allostatic load among US adults. Annals of Behavioral Medicine, 51(1), 94–104. 10.1007/s12160-016-9831-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinciguerra L, Lanza G, Puglisi V, Fisicaro F, Pennisi M, Bella R, & Cantone M (2020). Update on the neurobiology of vascular cognitive impairment: From lab to clinic. International Journal of Molecular Sciences, 21(8). 10.3390/ijms21082977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman SV, Blumenthal JA, Babyak MA, Sherwood A, Sketch M, Davidson J, & Watkins LL (2009). Ethnic differences in the treatment of depression in patients with ischemic heart disease. Am Heart J, 157(1), 77–83. 10.1016/j.ahj.2008.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldstein SR, Dore GA, Davatzikos C, Katzel LI, Gullapalli R, Seliger SL, … Zonderman AB (2017). Differential associations of socioeconomic status with global brain volumes and white matter lesions in African American and White Adults: the HANDLS SCAN Study. Psychosomatic Medicine, 79(3), 327–335. 10.1097/PSY.0000000000000408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Hou R, Zhang X, Xu H, Xie L, Chandrasekar EK, … Goodman M (2019). The association of late-life depression with all-cause and cardiovascular mortality among community-dwelling older adults: systematic review and meta-analysis. British Journal of Psychiatry, 215(2), 449–455. 10.1192/bjp.2019.74 [DOI] [PubMed] [Google Scholar]
- Wharton T, Watkins DC, Mitchell J, & Kales H (2018). Older, church-going African Americans' attitudes and expectations about formal depression care. Research on Aging, 40(1), 3–26. 10.1177/0164027516675666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yochim BP, Kerkar SP, & Lichtenberg PA (2006). Cerebrovascular risk factors, activity limitations, and depressed mood in African American older adults. Psychology and Aging, 21(1), 186–189. 10.1037/0882-7974.21.1.186 [DOI] [PubMed] [Google Scholar]
- Yochim BP, MacNeill SE, & Lichtenberg PA (2006). "Vascular depression" predicts verbal fluency in older adults. J Clin Exp Neuropsychol, 28(4), 495–508. 10.1080/13803390590949322 [DOI] [PubMed] [Google Scholar]
- Yochim BP, Mast BT, & Lichtenberg PA (2003). Cerebrovascular risk factors and depressed mood in inner city older adults. Clinical Psychologist, 7, 11–20. 10.1080/13284200410001707443 [DOI] [Google Scholar]
- Zahodne LB, Manley JJ, Narkhede A, Griffith EY, DeCarli C, Schupf NS, … Brickman AM (2015). Structural MRI predictors of late-life cognition differ across African Americans, Hispanics, and Whites. Current Alzheimer Research, 12(7), 632–639. 10.2174/1567205012666150530203214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, & Rodriguez-Monguio R (2012). Racial disparities in the risk of developing obesity-related diseases: a cross-sectional study. Ethnicity and Disease, 22(3), 308–316. [PubMed] [Google Scholar]
- Zhang Y, Chen Y, & Ma L (2018). Depression and cardiovascular disease in elderly: Current understanding. Journal of Clinical Neuroscience, 47, 1–5. 10.1016/j.jocn.2017.09.022 [DOI] [PubMed] [Google Scholar]