Highlights
-
•
Comorbidity status should be incorporated in counseling and selecting oldest old women with endometrial cancer for surgery.
-
•
The CA-CCI scoring system can predict surgical and oncologic outcomes in oldest old women with endometrial cancer.
-
•
Minimally invasive hysterectomy confers less risk of postoperative complications in oldest old women with endometrial cancer.
Keywords: Cancer survival, Comorbidity Index, Older women, Mortality, Postoperative outcomes, Uterine cancer
Abstract
Objective
To describe the surgical and oncologic outcomes in surgically treated oldest old women (≥80 years) with endometrioid endometrial cancer as a function of their comorbidities.
Methods
In this retrospective cohort study, patients aged 80–99 years who underwent surgical management of stage I endometrioid endometrial cancer between 2006 and 2018 were included. Low- and high-intermediate risk disease was defined using the Gynecologic Oncology Group-99 criteria. The validated, Combined Age-Charlson Comorbidity Index (CA-CCI) was used to quantify comorbidity burden. Logistic regression was used to identify the independent predictors of various surgical and oncologic outcomes. Kaplan-Meier survival analysis was performed to compare survival distributions based on mortality cause and comorbidity status.
Results
We identified 64 women who met the eligibility criteria. Median age was 84 years (IQR 80, 94 years). Among oldest old women undergoing a hysterectomy with or without lymph node dissection, women with a CA-CCI score of ≥7 had an 8 times higher risk of postoperative infections compared with oldest old women with a <7 score (95% CI 1.53–48.91, P = 0.015). Women with a CA-CCI score of ≥8 were 45% less likely to survive at 3 years (aRR 0.55, 95% CI 0.004–0.87; P = 0.039) than those with a lower CA-CCI score (three-year overall survival 73% vs 96%).
Conclusion
Surgical and oncologic outcomes in oldest old women with early stage endometrioid endometrial cancer are largely determined by comorbidity status. Less comorbid women (CA-CCI score < 8) had a significantly higher five-year survival at 87% than their more comorbid counterparts. Use of age-comorbidity risk scoring such as CA-CCI, preoperative optimization, and careful selection for and counseling of patients about surgical treatment are paramount in providing optimal recovery and survival advantages in the oldest old.
1. Introduction
To date, uterine cancer remains the most prevalent gynecologic malignancy, with 66,750 estimated new cases and 12,940 estimated deaths in the United States (US) in 2021 as reported by the National Cancer Institute. (Institute et al., 2021) Although most cases (54.8%) are among women < 65 years of age, uterine cancer mortality is disproportionately higher in women ≥ 65 years of age. (Institute et al., 2021) Furthermore, as overall life expectancy is projected to increase, the incidence and mortality of uterine cancer is expected to concomitantly rise in the older groups. (Amant et al., 2005).
Surgical intervention is the cornerstone of uterine cancer treatment, with hysterectomy, salpingo-oophorectomy, and lymphadenectomy, as indicated, being the standard of care for surgical staging. (Colombo et al., 2016) However, older women with endometrial cancer remain suboptimally treated compared with their younger counterparts. (Koual et al., 2018) This disparity in treatment has likely stemmed from studies showing that older patients are at high risk of adverse perioperative outcomes. (Toglia and Nolan, 2003, Bentrem et al., 2009) For instance, women older than 80 years of age who undergo laparotomy for endometrial cancer experience significantly higher rates of postoperative complications, blood transfusions, and mortality as well as longer hospital stays. (Wright et al., 2011) Additionally, in their analysis of perioperative outcomes in surgically-treated oldest (≥80 years) women with endometrial cancer, Wright et al. observed that age, independent from comorbidity status, was a predictor of postoperative complications, which in turn were regarded as the chief determinant of postoperative survival, with complications being most prevalent among patients ≥ 80 years. (Wright et al., 2011) Nevertheless, recent evidence shows that radical pelvic surgery is well-tolerated by older women, (Wright et al., 2004) prompting reconsideration of optimal therapeutic approaches for this age group. Furthermore, surgical treatment for early-stage endometrial cancer, with potential adjuvant therapy, represents the standard of care and accordingly has the greatest curative potential. Excluding surgical intervention as a primary treatment for older women may have unacceptable consequences toward disease-free and overall survival, parameters that remain to be investigated more thoroughly within this group.
Historically, the ideal treatment for older patients with endometrial cancer was largely undefined due to comorbid profiles and questionable survival benefit after aggressive surgery. (Wright et al., 2011) However, with advancements in perioperative and operative care along with favorable data regarding aggressive treatment in older women, these patients may qualify for surgical management. (Wright et al., 2004, Wysham et al., 2015) In this study, we aim to evaluate the surgical and oncologic outcomes among women aged 80–99 years, the oldest old, with stage I endometrioid endometrial cancer based on preoperative comorbidity status and ultimately, risk stratify the oldest old surgical candidates and better inform preoperative counseling based on age and comorbidities.
2. Materials and methods
2.1. Study design and patient cohort
In this single-institution retrospective cohort study, we included women aged 80–99 years who were surgically treated for stage I endometrioid endometrial cancer between 2006 and 2018. Demographic and clinical data were obtained after reviewing medical records over the study and follow up periods. Eligible patients were those with intermediate risk features; low- and high-intermediate risk disease was defined using the Gynecologic Oncology Group (GOG)-99 criteria, wherein high-intermediate risk disease is defined based on age and the presence of three pathologic factors: deep myometrial invasion, grade 2 or 3 histology, and lymphovascular space invasion (LVSI). For women ≥ 70 years, high-intermediate risk disease is ruled in by any one of these pathologic factors. Patients were restricted to those with early-stage cancer as patients > 80 years old with advanced disease are less likely to be offered standard treatment. Patients without complication or follow up data were excluded from the analysis. The study was approved by the Institutional Review Board (IRB) at the Yale University School of Medicine.
2.2. Primary and secondary outcomes
Our primary outcome was a composite of oncologic outcomes, including overall survival at three and five years and progression-free survival at three years. Mortality rate was subdivided into disease- and comorbidity-specific mortality. By contrast, secondary outcomes comprised surgical outcomes, including length of hospitalization and occurrence of postoperative complications within 30 days of surgery, such as postoperative infection, readmissions, step-down unit or intensive care unit admission, and 30-day mortality. For outcomes, such as postoperative infections, length of stay (LOS), and estimated blood loss (EBL), patients were stratified by hysterectomy approach. To quantify the comorbidity burden on oncologic and surgical outcomes, we used the validated Charlson Comorbidity Index (CCI). The CCI, which comprises 17 comorbidities, has been historically used as a prognostic indicator of mortality by weighing comorbid conditions, therefore estimating disease burden. (Charlson et al., 1987, Deyo et al., 1992 Jun) However, with age being an independent predictor of mortality, we used a Combined Age-CCI (CA-CCI) score, which adds 1 point for each decade above 40 years. (Roffman et al., 2016) After determining the CA-CCI, we set two cut-off values for comparison. The first was to dichotomize patient characteristics and perioperative outcomes using the median (CA-CCI score of 7), whereas the second cut-off value was used for dichotomizing oncologic survival using the 90th percentile (CA-CCI score of 8).
A Kaplan-Meier survival analysis was conducted to compare survival outcomes between two groups based on each mortality cause and comorbidity status. Significant differences in survival distributions for each two groups at a time were evaluated using a log-rank test. A Cox proportional hazard model was then used to calculate the hazard ratio (HR) to compare the probability of death in one group compared with the other.
2.3. Covariates
Demographic and clinical data obtained as covariates included patient’s age, hysterectomy approach (abdominal, laparoscopic, robot-assisted, or laparoscopic- or robot-assisted vaginal), EBL, LOS, lymph node dissection status (none, full pelvic only, or full pelvic and paraaortic), 2009 FIGO stage (IA or IB), histologic grade (1, 2, or 3), adjuvant therapy (radiation alone or chemoradiation), and patients’ comorbidities. Patients undergoing sentinel lymph node biopsy were excluded to achieve group homogeneity. Patients' comorbidities comprised the following: history of myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular accident or transient ischemic attack, hemiplegia, dementia, diabetes mellitus, connective tissue disease, peptic ulcer disease, moderate to severe chronic kidney disease, synchronous malignancy, AIDS, liver disease, and chronic obstructive pulmonary disease. Comorbidities in the study population were extracted from electronic medical records by a trained healthcare provider and their presence predated the index surgical intervention in our study.
2.4. Statistical analysis
Descriptive statistics were reported for categorical variables as percentages, and their frequency distributions were compared using χ2 test. A multivariate logistic regression analysis was performed to examine the utility of CA-CCI scoring in predicting the surgical and oncologic outcomes after operative intervention. Results were reported as adjusted and unadjusted risk ratios (RRs) and 95% confidence intervals (CIs). All P values were two-sided, with P < 0.05 considered statistically significant. Statistical analyses were performed using STATA (StataCorp. 2021. Stata Statistical Software: Release 17. College Station, TX: StateCorp LLC), GraphPad Prism version 9.0.0 (GraphPad software, San Diego, California USA), and SPSS statistics v25 (IBM Corporation, Armonk, NY).
3. Results
3.1. Patient characteristics
There were 2,234 patients undergoing surgical staging for uterine cancer from 2006 to 2018. A total of 64 women met eligibility criteria and were followed up for a median of 52 months (Interquartile range (IQR), 3 months to 12 years). The clinical and pathological characteristics of our study population are presented in Table 1. Median age was 84 years (IQR 80 to 94 years). When stratified by disease stage, 54.7% of patients had stage IA and 45.3% had stage IB. After histologic examination, 9.4% had grade 1 disease, 71.9% had grade 2, 18.7% had grades 3, and 23.4% had LVSI. While 20.3% had low-intermediate risk disease, 79.7% had high-intermediate risk disease. Based on comorbidity scoring, 45.3% had a CA-CCI score of < 7 and 54.7% had a score of ≥ 7. Postoperatively, 77.8% of patients were offered radiation therapy and 67.2% received vaginal brachytherapy alone, whereas 1.6% received chemoradiation.
Table 1.
Clinical and pathological characteristics of the study population.
| Characteristic | Total population (n = 64) |
|---|---|
| Age in years, median (IQR) | 84 (80–94) |
| Cancer stage, no. (%) | |
| IA | 35 (54.7) |
| IB | 29 (45.3) |
| Histologic grade, no. (%) | |
| 1 | 6 (9.4) |
| 2 | 46 (71.9) |
| 3 | 12 (18.7) |
| Lymphovascular invasion (LVSI), no. (%) | |
| Absent | 45 (70.3) |
| Present | 15 (23.4) |
| Indeterminate/unknown | 4 (6.3) |
| Cancer risk stratification, no. (%) | |
| Low intermediate | 13 (20.3) |
| High intermediate | 51 (79.7) |
| Hysterectomy approach, no. (%) | |
| Open | 23 (35.9) |
| Laparoscopic | 12 (18.8) |
| Robotic | 27 (42.2) |
| Vaginal | 2 (3.1) |
| Lymph node dissection, no. (%) | |
| No | 12 (18.8) |
| Pelvic | 14 (21.9) |
| Pelvic and periaortic | 38 (59.4) |
| EBL, no. (%) | |
| < Median (90 mL) | 31 (48.4) |
| ≥ Median |
33 (51.6) |
| Adjuvant therapy, no. (%) | |
| No | 20 (31.2) |
| Radiation alone | 43 (67.2) |
| Chemoradiation | 1 (1.6) |
| Recurrence, no. (%) | |
| Yes | 7 (10.9) |
| No | 57 (89.1) |
| Death, no. (%) | |
| Yes | 26 (40.6) |
| No | 38 (59.4) |
| Cause of death, no. (%) | |
| Disease recurrence | 2 (7.7) |
| Comorbidities | 24 (92.3) |
| Postoperative morbidity*, no. (%) | |
| Infectious morbidity | 16 (25) |
| Pneumonia | 6 (9.4) |
| Cellulitis | 3 (4.7) |
| Urinary tract infection | 5 (7.8) |
| Fever of unknown origin | 2 (3.1) |
| Postoperative transfusion | 2 (3.1) |
| Step-down unit admission | 3 (4.7) |
| Intensive care unit admission | 3 (4.7) |
| Readmission | 3 (4.7) |
| Length of stay, no. (%) | |
| ≤ Median (2 days) | 35 (54.7) |
| > Median | 29 (45.3) |
| CA-CCI score, no. (%) | |
| < Median (7) | 29 (45.3) |
| ≥ Median |
35 (54.7) |
*Not mutually exclusive. EBL, estimated blood loss; CA-CCI, combined age-Charlson comorbidity index.
3.2. Surgical intervention
After dividing patients by surgical approach, 35.9% underwent total abdominal hysterectomy, 42.2% underwent robot-assisted total laparoscopic hysterectomy, 18.8% underwent total laparoscopic hysterectomy, and 3.1% underwent laparoscopic-assisted vaginal hysterectomy with or without robot assistance. Salpingo-oophorectomy was performed in all patients. While 18.8% did not undergo lymph node dissection, 59.4% and 21.9% underwent pelvic and paraaortic lymph node sampling and pelvic lymph node sampling alone, respectively.
3.3. Survival outcomes
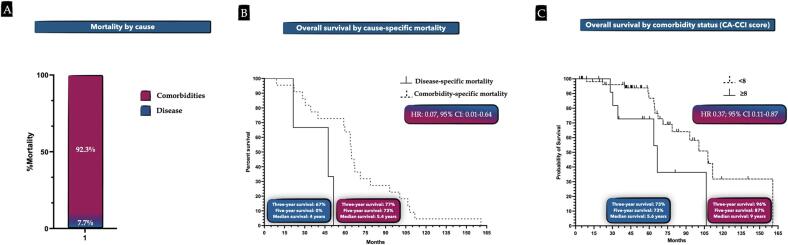
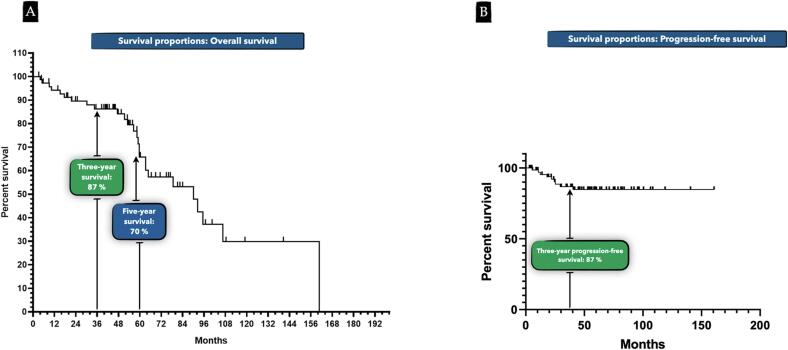
Disease recurrence was noted in 10.9% of patients, and overall mortality rate was 40.6%, with 7.7% of deaths attributed to uterine cancer and 92.3% attributed to comorbidities (Fig. 2A). No mortality was secondary to surgical intervention or any cause within 30 postoperative days. Three- and five-year overall survival rates were 87% and 70%, respectively; three-year progression-free survival was 87% (Fig. 3A and B). Women who suffered death secondary to uterine cancer had a median survival of four years compared with 5.4-year median survival among women dying of their comorbidities (HR 0.07, 95% CI 0.01–0.64: Fig. 2B and C). Patients with a CA-CCI score of ≥ 8 (90th percentile) were 45% less likely to survive at 3 years (aRR 0.55; 95% CI 0.004–0.87; P = 0.039). Median survival in patients with a score of < 8 was 9 years compared with 5.6 years in patients with a score of ≥ 8 (HR 0.37, 95% CI 0.11–0.87). Fig. 2C shows time to death events between patients with a CA-CCI score < 8 versus CA-CCI of ≥ 8. Of note, cancer grade, lymph node dissection status, and EBL did not significantly impact survival.
Fig. 2.
A. Proportion of mortality by cause; B. Probability of overall survival by mortality cause over the follow up period; C. Probability of overall survival by comorbidity status (CA-CCI score) over the follow up period.
Fig. 3.
A. Probability of crude overall survival over the follow up period. B. Probability of crude progression-free survival over the follow up period.
3.4. Surgical outcomes
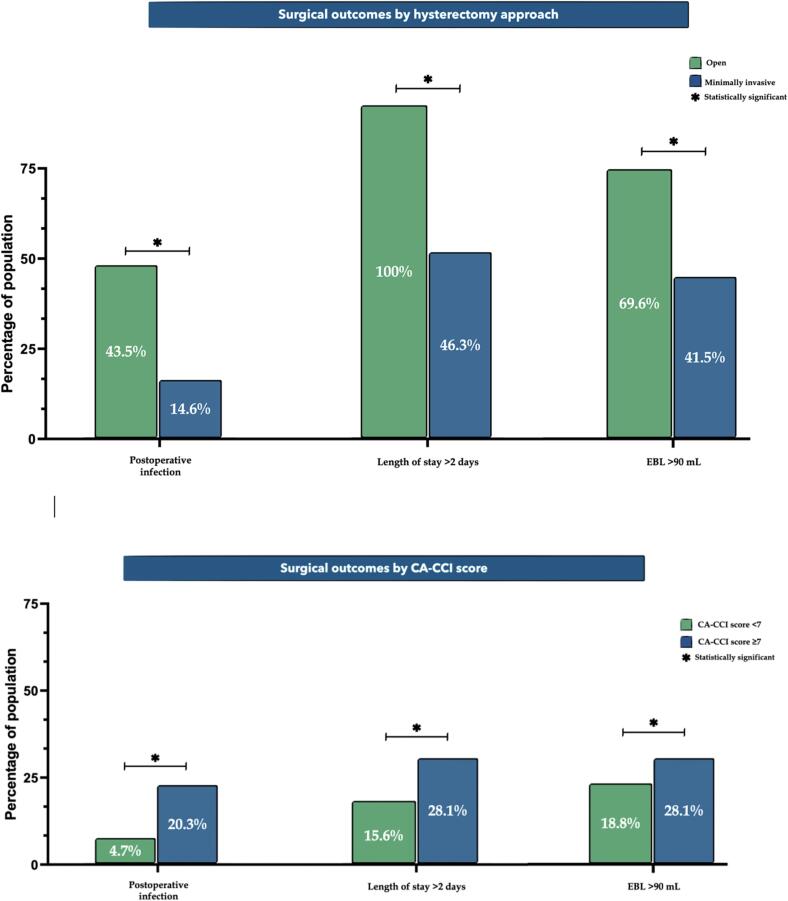
Regarding surgical outcomes, 24 (37.5%) patients developed postoperative complications. Specifically, 16 (25%) patients had a postoperative infection. Of those, 6 (37.5%) had pneumonia, 5 (31.3%) had a urinary tract infection, 3 (18.8%) had cellulitis, and 2 (12.5%) had a fever of unknown origin treated with antibiotics when assessed for primary source. Overall postoperative morbidity as well as infection rate were significantly higher among patients who underwent open surgery compared with the minimally invasive surgery group (43.5% vs 17.1%, P = 0.022; 43.5% vs 14.6%, P = 0.011, respectively; Table 2 and Fig. 1). While only 2 patients (3.1%) required a blood transfusion, the open surgery group was more likely to have an EBL above the median compared with the minimally invasive group (69.6% vs 41.5%, P = 0.031; Table 2 and Fig. 1). Patients undergoing an open surgery were more likely to be hospitalized for > 2 days compared with the minimally invasive group (100% vs 46.3%, P < 0.01; Table 2 and Fig. 1).
Table 2.
Surgical and oncologic outcomes stratified by hysterectomy approach.
| Characteristic | Open hysterectomy (n = 23) | Minimally invasive hysterectomy (n = 41) | P value |
|---|---|---|---|
| EBL, no. (%) | 0.031 | ||
| <Median | |||
| ≥Median | 16 (69.6) | 17 (41.5) | |
| Recurrence, no. (%) | 1 (4.3) | 6 (14.6) | 0.203 |
| Death, no. (%) | 11 (47.8) | 10 (24.4) | 0.055 |
| Postoperative morbidity*, no. (%) | 10 (43.5) | 7 (17.1) | 0.022 |
| Infectious morbidity | 10 (43.5) | 6 (14.6) | 0.011 |
| Length of stay, no. (%) | <0.001 | ||
| ≤Median | |||
| >Median | 23 (1 0 0) | 19 (46.3) | |
| Three-year survival, no. (%) | 22 (95.7) | 37 (90.2) | 0.404 |
Fig. 1.
Rates of surgical outcomes by hysterectomy approach and comorbidity status (CA-CCI score).
After multivariate regression analysis (Table 3), patients with a CA-CCI score of ≥ 7 were 9 times more likely to have a postoperative complication compared with patients with a lower score (aRR 9.06; 95% CI 1.78–46.25; P = 0.008). Specifically, they were 8 times more likely to have a postoperative infection compared with their less comorbid counterparts (aRR 8.66; 95% CI 1.53–48.91; P = 0.015). These findings were noted after adjusting for patient's age, cancer grade and stage, surgical approach, lymph node dissection status, and EBL. While surgical approach was not a significant predictor of overall complication occurrence, it was significantly associated with postoperative infections. Patients undergoing minimally invasive surgery were 86% less likely to develop a postoperative infection compared with the open surgery group (aRR 0.14; 95% CI 0.02–0.84; P = 0.032).
Table 3.
Logistic regression model* of the surgical and oncologic outcomes in the study population.
| Predictor |
Postoperative morbidity |
|
|---|---|---|
| Adjusted RR (95% CI) | P value | |
| Cancer stage | ||
| 1A | Referent | |
| 1B | 1.10 (0.23–5.21) | 0.904 |
| EBL | ||
| < Median (90 mL) | Referent | |
| ≥ Median | 3.02 (0.64–14.32) | 0.163 |
| Hysterectomy approach | ||
| Open | Referent | |
| Minimally invasive | 0.27 (0.05–1.13) | 0.071 |
| CA-CCI score | ||
| < Median (7) | Referent | |
| ≥ Median | 9.06 (1.78–46.25) | 0.008 |
| Postoperative infection | ||
| Adjusted RR (95% CI) | P value | |
| Cancer stage | ||
| 1A | Referent | |
| 1B | 0.80 (0.15–4.29) | 0.790 |
| EBL | ||
| < Median (90 mL) | Referent | |
| ≥ Median | 2.27 (0.42–12.37) | 0.342 |
| Hysterectomy approach | ||
| Open | Referent | |
| Minimally invasive | 0.14 (0.02–0.84) | 0.032 |
| CA-CCI score | ||
| < Median (7) | Referent | |
| ≥ Median | 8.66 (1.53–48.91) | 0.015 |
| Three-year survival | ||
| Adjusted RR (95% CI) | P value | |
| Cancer stage | ||
| 1A | Referent | |
| 1B | 2.97 (0.16–55.10) | 0.465 |
| EBL | ||
| < Median (90 mL) | Referent | |
| ≥ Median | 0.055 (0.004–6.41) | 0.167 |
| CA-CCI score | ||
| < 90th percentile (8) | Referent | |
| ≥ 90th percentile | 0.06 (0.004–0.87) | 0.039 |
*Model also adjusted for histologic grade and lymph node dissection status. EBL, estimated blood loss; CA-CCI, combined age-Charlson comorbidity index; RR, risk ratio; CI, confidence interval.
4. Discussion
4.1. Summary of the main results
Past studies examined the surgical and oncologic outcomes between younger and older patients undergoing surgical intervention for endometrial cancer. In contrast, we described these outcomes in an exclusively older cohort of women, the oldest old, with early stage endometrioid endometrial cancer as a function of their comorbidities. Using a validated age-weighted comorbidity scoring system, we found that more comorbid oldest old women were at a significantly higher risk of developing postoperative complications compared with their less comorbid oldest old counterparts. We also sought to explore the impact of hysterectomy approach on postoperative outcomes, bringing a new dimension to surgical planning and counseling in the oldest old with endometrial cancer.
4.2. Results in the context of published literature
Regardless of comorbidity profile, open surgery was associated with a significantly higher risk of postoperative infections among older women compared with minimally invasive approaches. These findings are in accordance with Bishop et al. documenting significantly higher rates of pneumonias and antibiotic administration among women ≥ 60 years old undergoing laparotomy for endometrial cancer when compared with laparoscopically operated patients. (Bishop et al., 2018) This observation was noted to span across both benign and malignant, gynecologic and non-gynecologic surgeries, including hepatectomy and appendectomy. (Wang et al., 2019, Chen et al., 2018, Ghezzi et al., 2010) On the contrary to surgical approach, lymph node dissection had no significant impact on postoperative outcomes in our patients. While the sentinel lymph node biopsy technique is a safe, effective alternative, (Garzon et al., 2022, Casarin et al., 2020) older women may tolerate a lymph node dissection should limited resources render sentinel lymph node sampling unfeasible.
Additionally, our study outcomes were extended to include survival at five years after surgery, facilitating a better understanding of the true survival benefit in this age group. Although most women died of their comorbidities, they lived a median of 5.4 years after surgery. Upon stratifying by comorbidity status, less comorbid women (CA-CCI score < 8) had a significantly higher five-year survival (87%) than their more comorbid counterparts (73%). The five-year survival of patients with CA-CCI < 8 is comparable to the five-year survival of 95% in the general population with localized disease. (Institute et al., 2021) Clinical implication of this finding are that the least comorbid oldest old women seem to harbor the most benefits of surgical intervention, particularly women with a CA-CCI score < 8. Preoperative counseling of the oldest old patient group should emphasize the CA-CCI score as a predictor for both postoperative and five-year survival outcomes.
In their population-based study, Wright et al. examined the compounding effect of age and comorbidity status on postoperative morbidity and mortality and utilized CCI to categorize patients based on comorbidity burden. They concluded that increased comorbidities along with advanced age were important indicators of perioperative complications, although their findings were inconsistent for less comorbid advanced-age women. (Wright et al., 2011) In contrast, we observed that for women ≥ 80 years with CA-CCI score of < 8, five-year survival rates were comparable with that of the general population. These findings reiterate that while age may play a role in predicting perioperative events, it should not serve as a sole determinant in selecting women with endometrial cancer for surgery. The findings also emphasize that age with comorbidity profile in an age-weighted comorbidity index (CA-CCI) could be utilized to predict women at risk of perioperative events and shortened survival, especially that physicians may hastily use advanced age as the main criterion for surgical candidacy, disregarding that the older patient cohort includes active, independent, and healthy octogenarians and nonagenarians.
4.3. Implications for practice and future research
Several clinical implications can be inferred from our findings. As advancing age and greater comorbidity burden have additive effects on postoperative outcomes, both factors should be employed for risk stratification and surgical selection of women with endometrial cancer. The CA-CCI has successfully integrated age and comorbidities in predicting postoperative outcomes and can be implemented in practice as a potentially reliable indicator of surgical morbidity. Identifying the oldest old women at highest risk of postoperative morbidity could inform practices aimed at preoperative optimization and facilitate evidence-based counseling regarding candidacy for surgery, although more studies need to be tailored to the older gynecologic population. (Young et al., 2008, Wang et al., 2018, Chen et al., 2010, Gu et al., 2019) In addition, minimally invasive staging approaches are associated with decreased postoperative morbidity in older women and should therefore be increasingly adopted. (Bishop et al., 2018, Guy et al., 2016, Scribner et al., 2001, Uccella et al., 2016).
4.4. Strengths and limitations
Our results should be interpreted with several limitations, including those inherent to retrospective study designs. The small sample size and low complication number may not have sufficiently powered the study to detect significant associations. In addition, while we simultaneously assessed the impact of age and comorbidities on postoperative outcomes, our data did not capture the functional status of our patients, which could presumably influence the occurrence of postoperative complications.
5. Conclusion
Surgically treated oldest old women with stage I endometrioid endometrial cancer were at highest risk of postoperative complications if they harbored a CA-CCI score ≥ 7. Use of scoring systems like CA-CCI, perioperative optimization, and careful selection of patients for surgical intervention may prove substantial in improving postoperative outcomes without depriving the oldest old of the standard of care and informing preoperative patient counseling regarding treatment options and candidacy for surgery. Moreover, in contrast to prior studies investigating surgical intervention in older women with endometrial cancer, our findings convey that surgery is generally well tolerated amongst the oldest old with a low CA-CCI score and thus serves as valuable first-line treatment.
With older Americans currently representing one of the fastest growing demographics in the US, indices specific to this subgroup will be important for consistently selecting preeminent interventions most suitable for each individual patient. The current findings are the first to describe surgical and oncologic outcomes in an understudied population of oldest old women as a function of their comorbidities using a combined age-comorbidity scoring system. This study will therefore serve as a benchmark for prospective work as the clinical landscape changes and gynecologic oncologic care continues into the upper limits of age.
Authors’ contribution
Abdelrahman AlAshqar, Emily Webster, and Gary Altwerger have given substantial contributions to the conception and design of the manuscript, acquisition, analysis and interpretation of data, and manuscript preparation. Animesh Upadhyay, Maddie Ghazarian, Masoud Azodi, Peter E. Schwartz, and Elena Ratner have participated in drafting the manuscript. All authors read and approved the final version of the manuscript.
Declaration of Competing Interest
The authors have no conflict of interest to declare.
References
- Amant F., Moerman P., Neven P., Timmerman D., Van Limbergen E., Vergote I. Endometrial cancer. Lancet. 2005;366(9484):491–505. doi: 10.1016/S0140-6736(05)67063-8. [DOI] [PubMed] [Google Scholar]
- Bentrem D.J., Cohen M.E., Hynes D.M., Ko C.Y., Bilimoria K.Y. Identification of specific quality improvement opportunities for the elderly undergoing gastrointestinal surgery. Arch. Surg. 2009;144(11):1013–1020. doi: 10.1001/archsurg.2009.114. [DOI] [PubMed] [Google Scholar]
- Bishop E.A., Java J.J., Moore K.N., Spirtos N.M., Pearl M.L., Zivanovic O., et al. Surgical outcomes among elderly women with endometrial cancer treated by laparoscopic hysterectomy: a NRG/Gynecologic Oncology Group study. Am. J. Obstet. Gynecol. 2018;218(1) doi: 10.1016/j.ajog.2017.09.026. 109 e1- e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casarin J., Multinu F., Tortorella L., Cappuccio S., Weaver A.L., Ghezzi F., Cliby W., Kumar A., Langstraat C., Glaser G., Mariani A. Sentinel lymph node biopsy for robotic-assisted endometrial cancer staging: further improvement of perioperative outcomes. Int. J. Gynecol. Cancer. 2020;30(1):41–47. doi: 10.1136/ijgc-2019-000672. [DOI] [PubMed] [Google Scholar]
- Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic. Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. PMID: 3558716. [DOI] [PubMed] [Google Scholar]
- Chen T.Y., Anderson D.J., Chopra T., Choi Y., Schmader K.E., Kaye K.S. Poor functional status is an independent predictor of surgical site infections due to methicillin-resistant Staphylococcus aureus in older adults. J. Am. Geriatr. Soc. 2010;58(3):527–532. doi: 10.1111/j.1532-5415.2010.02719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K., Pan Y., Maher H., Zhang B., Zheng X.Y. Laparoscopic hepatectomy for elderly patients: Major findings based on a systematic review and meta-analysis. Medicine. (Baltimore). 2018;97(30):e11703. doi: 10.1097/MD.0000000000011703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo N., Creutzberg C., Amant F., Bosse T., González-Martín A., Ledermann J., Marth C., Nout R., Querleu D., Mirza M.R., Sessa C., Abal M., Altundag O., Amant F., van Leeuwenhoek A., Banerjee S., Bosse T., Casado A., de Agustín L.C., Cibula D., Colombo N., Creutzberg C., del Campo J.-M., Emons G., Goffin F., González-Martín A., Greggi S., Haie-Meder C., Katsaros D., Kesic V., Kurzeder C., Lax S., Lécuru F., Ledermann J., Levy T., Lorusso D., Mäenpää J., Marth C., Matias-Guiu X., Morice P., Nijman H.W., Nout R., Powell M., Querleu D., Mirza M.R., Reed N., Rodolakis A., Salvesen H., Sehouli J., Sessa C., Taylor A., Westermann A., Zeimet A.G. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: diagnosis, treatment and follow-up. Ann. Oncol. 2016;27(1):16–41. doi: 10.1093/annonc/mdv484. [DOI] [PubMed] [Google Scholar]
- Deyo R.A., Cherkin D.C., Ciol M.A. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J. Clin. Epidemiol. 1992 Jun;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. PMID: 1607900. [DOI] [PubMed] [Google Scholar]
- Garzon S., Mariani A., Day C.N., Habermann E.B., Langstraat C., Glaser G., Kumar A., Casarin J., Uccella S., Ghezzi F., Larish A. Overall survival after surgical staging by lymph node dissection versus sentinel lymph node biopsy in endometrial cancer: a national cancer database study. Int. J. Gynecol. Cancer. 2022;32(1):28–40. doi: 10.1136/ijgc-2021-002927. [DOI] [PubMed] [Google Scholar]
- Ghezzi F., Cromi A., Siesto G., Serati M., Bogani G., Sturla D., et al. Use of laparoscopy in older women undergoing gynecologic procedures: is it time to overcome initial concerns? Menopause. 2010;17(1):96–103. doi: 10.1097/gme.0b013e3181ade901. [DOI] [PubMed] [Google Scholar]
- Gu A., Malahias M.A., Strigelli V., Nocon A.A., Sculco T.P., Sculco P.K. Preoperative Malnutrition Negatively Correlates With Postoperative Wound Complications and Infection After Total Joint Arthroplasty: A Systematic Review and Meta-Analysis. J. Arthroplasty. 2019;34(5):1013–1024. doi: 10.1016/j.arth.2019.01.005. [DOI] [PubMed] [Google Scholar]
- Guy M.S., Sheeder J., Behbakht K., Wright J.D., Guntupalli S.R. Comparative outcomes in older and younger women undergoing laparotomy or robotic surgical staging for endometrial cancer. Am. J. Obstet. Gynecol. 2016;214(3):350.e1–350.e10. doi: 10.1016/j.ajog.2015.09.085. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Uterine Cancer. Available at: https://seer.cancer.gov/statfacts/html/corp.html. Retrieved Spetember 13, 2021.
- Koual M., Ngo C., Girault A., Lecuru F., Bats A.S. Endometrial cancer in the elderly: does age influence surgical treatments, outcomes, and prognosis? Menopause. 2018;25(9):968–976. doi: 10.1097/GME.0000000000001119. [DOI] [PubMed] [Google Scholar]
- Roffman C.E., Buchanan J., Allison G.T. Charlson Comorbidities Index. J. Physiother. 2016;62(3):171. doi: 10.1016/j.jphys.2016.05.008. [DOI] [PubMed] [Google Scholar]
- Scribner D.R., Jr., Walker J.L., Johnson G.A., McMeekin S.D., Gold M.A., Mannel R.S. Surgical management of early-stage endometrial cancer in the elderly: is laparoscopy feasible? Gynecol. Oncol. 2001;83(3):563–568. doi: 10.1006/gyno.2001.6463. [DOI] [PubMed] [Google Scholar]
- Toglia M.R., Nolan T.E. Morbidity and mortality rates of elective gynecologic surgery in the elderly woman. Am. J. Obstet. Gynecol. 2003;189(6):1584–1587. doi: 10.1016/s0002-9378(03)00940-2. discussion 7–9. [DOI] [PubMed] [Google Scholar]
- Uccella S., Bonzini M., Palomba S., Fanfani F., Malzoni M., Ceccaroni M., Seracchioli R., Ferrero A., Berretta R., Vizza E., Sturla D., Roviglione G., Monterossi G., Casadio P., Volpi E., Mautone D., Corrado G., Bruni F., Scambia G., Ghezzi F. Laparoscopic vs. open treatment of endometrial cancer in the elderly and very elderly: An age-stratified multicenter study on 1606 women. Gynecol. Oncol. 2016;141(2):211–217. doi: 10.1016/j.ygyno.2016.02.029. [DOI] [PubMed] [Google Scholar]
- Wang D., Dong T., Shao Y., Gu T., Xu Y., Jiang Y. Laparoscopy versus open appendectomy for elderly patients, a meta-analysis and systematic review. BMC. Surg. 2019;19(1):54. doi: 10.1186/s12893-019-0515-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.Y., Hu S.F., Ying H.M., Chen L., Li H.L., Tian F., et al. Postoperative tight glycemic control significantly reduces postoperative infection rates in patients undergoing surgery: a meta-analysis. BMC. Endocr. Disord. 2018;18(1):42. doi: 10.1186/s12902-018-0268-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright J.D., Herzog T.J., Powell M.A. Morbidity of cytoreductive surgery in the elderly. Am. J. Obstet. Gynecol. 2004;190(5):1398–1400. doi: 10.1016/j.ajog.2004.01.078. [DOI] [PubMed] [Google Scholar]
- Wright J.D., Lewin S.N., Barrena Medel N.I., Sun X., Burke W.M., Deutsch I., Herzog T.J. Morbidity and mortality of surgery for endometrial cancer in the oldest old. Am. J. Obstet. Gynecol. 2011;205(1):66.e1. doi: 10.1016/j.ajog.2011.02.067. [DOI] [PubMed] [Google Scholar]
- Wysham W.Z., Kim K.H., Roberts J.M., Sullivan S.A., Campbell S.B., Roque D.R., Moore D.T., Gehrig P.A., Boggess J.F., Soper J.T., Huh W.K. Obesity and perioperative pulmonary complications in robotic gynecologic surgery. Am. J. Obstet. Gynecol. 2015;213(1):33.e1. doi: 10.1016/j.ajog.2015.01.033. [DOI] [PubMed] [Google Scholar]
- Young M.H., Washer L., Malani P.N. Surgical site infections in older adults: epidemiology and management strategies. Drugs. Aging. 2008;25(5):399–414. doi: 10.2165/00002512-200825050-00004. [DOI] [PubMed] [Google Scholar]